Abstract
The corona virus disease 2019 (COVID‐19) caused by severe acute respiratory syndrome coronavirus type 2 (SARS‐COV‐2) poses a severe threat to human health and still spreads globally. Due to the high mutation ratio and breakthrough infection rate of the virus, vaccines and anti‐COVID‐19 drugs require continual improvements. Drug screening research has shown that some natural active products can target the critical proteins of SARS‐CoV‐2, including 3CLpro, ACE2, FURIN, and RdRp, which could produce great inhibitory effects on SARS‐COV‐2. In addition, some natural products have displayed activities of immunomodulation, antiinflammatory, and antihepatic failure in COVID‐19 clinical trials, which may relate to their non‐monomeric structures. However, further evaluation and high‐quality assessments, including safety verification tests, drug interaction tests, and clinical trials, are needed to substantiate natural products' multi‐target and multi‐pathway effects on COVID‐19. Here, we review the literature on several promising active natural products that may act as vaccine immune enhancers or provide targeted anti‐COVID‐19 drugs. The structures, mechanisms of action, and research progress of these natural products are analyzed, to hopefully provide effective ideas for the development of targeted drugs that possess better structure, potency, and safety.
Keywords: antivirals, coronaviruses, COVID‐19, natural products, SARS‐COV‐2
1. INTRODUCTION
According to the latest World Health Organization (WHO) report, as of May 22, 2022 over 518 million Corona Virus Disease 2019 (COVID‐19) cases have been diagnosed globally, including over 6 million deaths (WHO Coronavirus (COVID‐19) Dashboard, 2022). The global epidemic is still growing. The severe acute respiratory syndrome coronavirus type 2 (SARS‐COV‐2) which causes COVID‐19, shares extremely similar properties with the severe acute respiratory syndrome coronavirus (SARS‐CoV) (Yang, Wang, & Li, 2020), including long latency, high infectiousness, and high severe rates. As a positive‐strand RNA virus, the SARS‐CoV‐2 RNA is almost identical to mRNA, thus it can be directly translated to proteins after invading host cells, with an extremely high recombination rate (Liu et al., 2021). Owing to the above points, SARS‐COV‐2 continues to mutate rapidly, which leads to high transmission, high toxicity, and breakthrough vaccine infection (Chen, Wang, Gilby, & Wei, 2021). At present, WHO has listed five variants of concern (VOC). Most strikingly, the emergence of the Omicron strain has exacerbated the severity of the pandemic. To curb this, more research is urgently needed to develop effective therapeutic drugs and/or vaccines. A number of docking and kinetic studies have been conducted to screen potential anti‐COVID‐19 monomers from natural small molecular libraries. Based on the research results, recent reviews have systematically discussed the possibility of natural active products as a rich source for antiviral drug screening, due to their broad pharmacological activities (Españo, Kim, Lee, & Kim, 2021). They have classified and evaluated the natural products based on their different protein targets, which provides insight for further optimization of small molecular structures for developing COVID‐19‐specific drugs (Singla et al., 2021). While previous reviews have been instructive, there remains a need for more in‐depth discussions on the potential anti‐COVID‐19 mechanisms and clinical applications of natural products. Reviews have thus far focused on the viral protease or host receptor protein and the contribution of natural products to SARS‐COV‐2 has received little attention for other non‐protein targets, though some natural active products may indeed play a key role in the immune system or inflammatory response. As SARS‐COV‐2 causes systemic infection in the human body, we should consider the antiviral potential of natural active products from multiple pathways and multiple targets. In terms of prevention, targeted therapy, and prognosis strategies, we systematically review natural active products, which target or inhibit COVID‐19, regulate the immune system, or improve the potential of inflammatory response. We analyze and list their possible mechanism, structure, clinical/preclinical research progress, and application, to provide a theoretical basis for further development of COVID‐19‐specific drugs. We discuss a selection of typical natural products with good immunomodulatory effects, for example, epigallocatechin gallate (EGCG) as enhancers or supplements of SARS‐COV‐2 vaccine, and natural antiinflammatory products as adjuvants for treating cytokine storms and systemic diseases caused by COVID‐19.
2. CHARACTERISTICS OF SARS‐COV‐2
2.1. Structure and mechanism of SARS‐COV‐2
Coronavirus are single‐stand positive RNA viruses with envelope and spur proteins. They are causative agents of many infectious diseases seen in avians, animals, and humans (Krishnamoorthy, Swain, Verma, & Gunthe, 2020). SARS‐CoV‐2 has four structural proteins: surface spike protein (protein S), an envelope protein (protein E), membrane protein (protein M), and nucleocapsid protein (protein N). In addition, the genomic RNA of SARS‐COV‐2 also encodes multiple non‐structural proteins. It is similar to mRNA and its 5′terminal ORF1a/b encodes two polyprotein precursors 1a and 1b (Ruan et al., 2021). These two protein precursors are cleaved into 16 non‐structural proteins (NSPs), including helicase and RNA‐dependent RNA polymerase (RdRp), which play important roles in virus replication (Ruan et al., 2021). The process by which SARS‐COV‐2 enters and infects host cells is highly dependent on these proteins. First, SARS‐COV‐2 binds to the host angiotensin converting enzyme2 (ACE2) receptor via the receptor binding region (RBD) of the S spike protein (Yan et al., 2020). To preactivate S proteins and promote membrane fusion (Shang et al., 2020), the host furin protein, transmembrane serine protease (TMPRSS2), and Cathepsin B/L (CatB/L) are necessary (Hoffmann et al., 2020; Hoffmann, Kleine‐Weber, & Poehlmann, 2020). Thereafter, the virus RNA is released into the host cell fluid and two superlong polyprotein precursors (1a and 1b) are synthesized. The 3C‐like Proteinase (3CLPro, also called Mpro) (Anand, Ziebuhr, Wadhwani, Mesters, & Hilgenfeld, 2003) and the papain‐like protease (PLpro) are essential for cleaving the precursors 1a and 1b into 16 non‐structural proteins, such as RdRp (which synthesizes RNA) and non‐structural protein 14 (NSP14, which modifies the RNA precursor) (Liu et al., 2021).
2.2. SARS‐COV‐2 mutants and vaccine
SARS‐COV‐2 is prone to subspecies mutation due to its rapid reproduction. Currently, five VOC mutants, including B.1.1.7, P.1, B.1.357, Delta, and Omicron play a significant role in the COVID‐19 pandemic. This is because they mutate at multiple sites of the S protein, and thus exacerbate the viral infection. For example, E484K and N501Y mutations in the RBD of variant strains Alpha, Beta, and Gamma promote the RBD‐ACE2 binding affinity, causing increased viral infectivity and viral load (Khan et al., 2021). L452R and T478K mutations of the Delta variant, which are linked to increased stability of spike proteins, make the virus less responsive to vaccine antibodies and even escape from immune recognition (Deng et al., 2021). As such, these mutations lead to a reduction in the potency of current vaccines and monoclonal antibodies. With six times more mutation sites on the spike (s) protein than the delta strain, the current omicron variant is even more infectious (linked to N440K, T478K, and N501Y mutations in the RBD region) and has vaccine breakthrough (linked to K417N, E484A, and Y505H mutations) (Chen, Wang, Gilby, & Wei, 2021). It has also been revealed that the ACE2 receptor is not the only target for COVID‐19 invading the human body, with observed receptor targets of COVID‐19 and its variants including receptor tyrosine kinase AXL (AXL) (Wang et al., 2021), FURIN (Hoffmann, Kleine‐Weber, & Poehlmann, 2020), 78‐kD glucose‐regulated protein (GRP78) (Gadanec et al., 2021), Basigin (CD147) (Ulrich & Pillat, 2020), and Neuropilin‐1 (NRP1) (Cantuti‐Castelvetri et al., 2020). Therefore, previous COVID‐19 vaccines, monoclonal antibodies, and ACE2 targeted drugs may have limited effect on mutant strains such as Omicron.
2.3. Spillover effect and intermediate host
COVID‐19 has a spillover effect between species It has undergone large‐scale transmission and mutation in many animal species before spreading to humans. As early as 2003, studies showed that SARS‐CoV could transfer between host species (Martina et al., 2003), and the homology of SARS‐CoV‐2 and bat SARS coronavirus (RaTG13) is more than 96% (Zhou et al., 2020). There is also evidence that SARS‐COV‐2 transmits from animal to human. For instance, SARS‐COV‐2 infected staff from a mink breeding farm were detected with animal characteristic SARS‐COV‐2 sequences in vivo (Munnink et al., 2021). A further study on the SARS‐COV‐2 susceptibility of animals also showed that the similarity rate of ACE2 receptor protein between chimpanzees, domestic cats, rhesus monkeys, and humans, was more than 80%, linked to high S‐protein binding affinity (Shen et al., 2020). Spillover effects and adaptive variation between animal–human species will undoubtedly aggravate the speed of virus mutation.
2.4. Clinical manifestations and disease progression of SARS‐COV‐2
Mild symptoms of SARS‐COV‐2 mainly include a cough, runny nose, and fever. However, it is a straightforward process for it to turn into a severe respiratory tract infection or even lead to systemic diseases. One reason is that receptors for SARS‐COV‐2 invasion are distributed across various organs. For instance, ACE2 receptor is found in the heart, kidney, and even digestive tract. AXL has been confirmed in the lungs and bronchus (Wang et al., 2021). In addition, since receptor CD147 is widely expressed in immune‐related cells, COVID‐19 can utilize CD147 to breach the immune barrier throughout the body (Ulrich & Pillat, 2020). When COVID‐19 attacks the body, it quickly leads to an imbalance of the immune system, resulting in the release of proinflammatory cytokines and the inflammatory reaction in many organs.
Based on the SARS‐COV2 clinical and autopsy reports, the cause of the high mortality rate of COVID‐19 is linked to multiple systemic disorders and cytokine storms (Ackermann et al., 2020; Li, Bai, & Hashikawa, 2020). In addition to severe alveolar injury and airway obstruction, most deceased patients also showed multiple organ failures. Some patients otherwise died due to secondary infections and complications such as myocarditis, vasculitis, and renal failure (Pei et al., 2020; Wichmann et al., 2020). Therefore, it is crucial to regulate the immune balance and target to inhibit multiple receptors.
3. MAJOR STRATEGIES FOR TARGETED SCREENING OF COVID‐19 DRUGS
At present, the development of SARS‐COV‐2 drugs mainly involves three strategies: 1) Targeted screen from old drugs, such as Fabiravir and Redcivir. 2) Develop SARS‐COV‐2 specific drugs (mainly nucleic acid analogs and monoclonal antibodies). 3) Take the critical proteins (e.g., 3CLpro, RdRp, and ACE2) as targets, and high throughput screens from the monomer small molecule library. However, most older drugs did not perform well in the clinical treatment of SARS‐COV‐2. Specific drugs, such as BRII‐196/BRII‐198, Molnupiravir, and Proxalutamide have been developed and entered phase III clinical trials, but there are some potential problems that will need greater examination. For example, while it has been announced that Molnupiravir can reduce the mortality or hospitalization rate by 50% for patients with mild to moderate COVID‐19 pneumonia (Jayk Bernal et al., 2022), after blocking virus replication through mismatching RNA Molnupiravir and its active form may be further metabolized to participate in host deoxynucleotide phosphorylation, and then integrate into DNA (Zhou et al., 2021). In addition, specific drugs like Molnupiravir are mainly effective in the early stages of infection and yield little effect on the regulation of inflammation and immune response. Considering the development cost of specific drugs, we still need safer and cheaper ways to treat the comprehensive clinical symptoms caused by COVID‐19.
4. ADVANTAGES OF NATURAL ACTIVE PRODUCTS IN THE TREATMENT OF COVID‐19
Considering the mechanism of COVID‐19 invasion, the S spike pro, 3CL pro, P pro, RdRp, and non‐structural protein 15 (NSP15) of SARS‐COV‐2, ACE2, and FURIN receptors of host cells can serve as drug targets (Saied et al., 2021). Previous reviews have reported that natural products of flavonoids, polyphenols, and alkaloids with complex structures may be more efficient against SARS‐CoV‐2 (Chapman & Andurkar, 2022; Shagufta & Ahmad, 2021). However, with the rapid mutation of the virus and the enhancement of other invasion pathways such as FURIN, GRP78, CD147, NRP1, and AXL, single structure molecular drugs that target only one protein site may not work well. High‐throughput screening and molecular docking technology are the main methods for drug discovery and development because of automation, high specificity, and short development cycle. Recent studies have also used in silico for multiple target screening and development of anti‐SARS‐COV‐2 drugs. In the databases including SCI, PubMed, CNKI, Clinical Trials Gov, and ChiCTR, we use “SARS‐COV‐2” and “natural products” as keywords and restrict the publication time from January 2020 to April 2022 for retrieval. We found that according to the results of multiple in silico screening, molecular docking, and in vitro cell experiments, a variety of natural active products including flavonoids, polyphenols, polyterpenes, flavanols, sterols, and lactones, have high docking scores for the above protein targets. For example, baicalin and quercetin can target 3CLpro and ACE2, EGCG can target GRP78, cordycepin and curcumin can target RdRp, myricetin target to inhibit NSP15, while honokiol targets Furin. Among them, EGCG, Resveratrol, and Caffeic Acid Phenethyl Ester (CPEA) not only have the potential of docking with 3CLpro/ACE2 but also act on multiple targets related to immunomodulation. The potential immune‐enhancing pathways include stimulating natural killer cell (NK) activity, increasing the level of splenic T cells, and regulating the ratio of CD4+/CD8+ and Th1/Th2.
Essentially, this paper seeks to show that natural products may play a vital role in fighting SARS‐COV‐2, by targeting viral proteins and regulating immune‐related pathways or inflammatory responses. The inhibitory effects of natural active products at different stages of SARS‐COV‐2 infection are shown in Figure 1. In addition, we analyze the structure, function, and research progress of these natural products, and propose their development potential as vaccine enhancers, novel coronavirus‐specific drugs or multi‐target compound drugs. However, it is also necessary to further elaborate on drug optimization tests, safety verification tests, and clinical trials, to prove the inhibitory effect of these natural products on COVID‐19.
FIGURE 1.
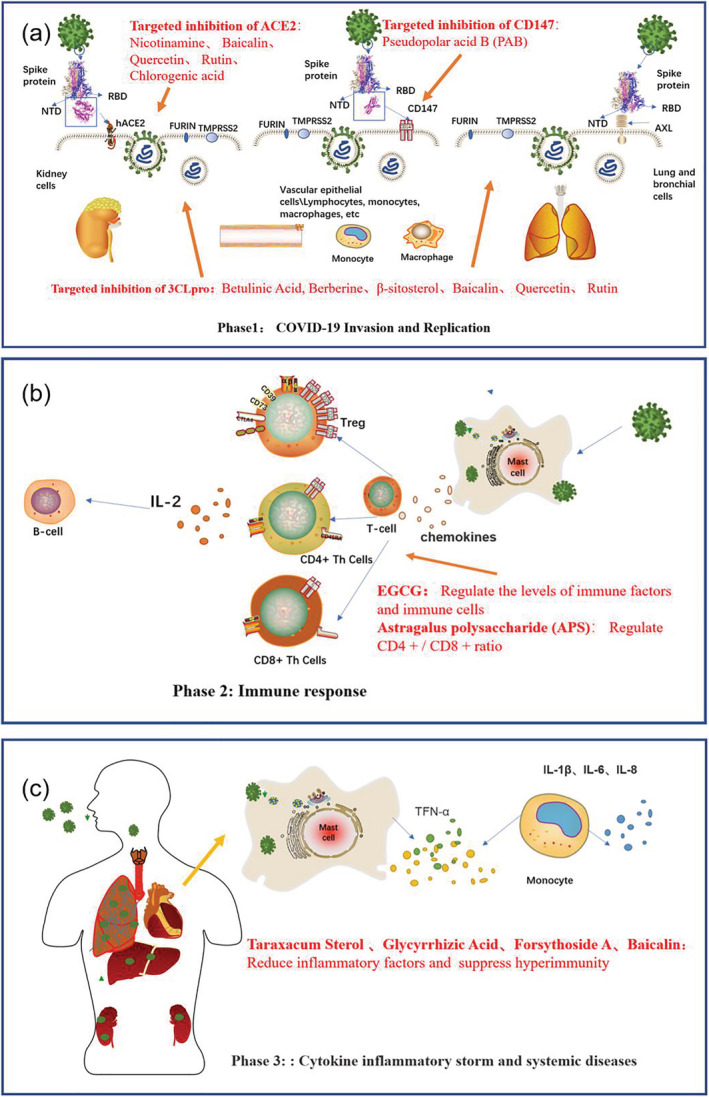
COVID‐19 infection on the human body and the mechanism of natural active products against it. (a) Natural products targeted to inhibit COVID‐19 invasion and replication. (b) Natural products regulate immune balance. (c) Natural products reduce cytokine storms and affect system diseases
5. NATURAL PRODUCTS WITH IMMUNOMODULATORY FUNCTION
The immune system is directly relevant to SARS‐COV‐2 invasion and development in the human body. Infection of SARS‐COV‐2 will lead to immune dysregulation, which significantly affects clinical recovery. Studies have also shown that virus 3CLpro can affect the type I interferon level (Zhang et al., 2021), while type I and III interferons are involved in controlling viremia and regulating the immune system (Sousa & Brites, 2020). In addition, immune disorders caused by SARS‐COV‐2 can also lead to blood disorders and even autoimmune diseases. It was reported that moderate COVID‐19 patients show the clinical symptoms of autoimmune hemolytic anemia (Liput, Jordan, Patadia, & Kander, 2021). Experimental and clinical evidence has shown that natural products, such as flavonoids and polyphenols, can regulate the body's immunity by promoting the secretion of immune factors; increasing the activity and production of immune T cells and B cells which link to the neutralizing antibody release; adjusting the Th1/Th2 balance; and by antagonizing the inflammatory response. This section takes astragalus polysaccharide (APS), caffeic acid (CA), resveratrol, and EGCG as examples for discussion.
5.1. Astragalus polysaccharide
APS, extracted from the Astragalus membranaceus, can indirectly inhibit the development of tumors or viruses by improving immunity (Li et al., 2017). The structure of APS is shown in Figure 3b. The specific regulation of APS for immune cells is linked to an increase in the number of lymphocyte T cells and B cells and a balance in the proportion of helper T cells. CD4+ and CD8+ subsets of T lymph can promote and inhibit body immunity, respectively. The proportional balance of CD4+ and CD8+ plays a vital role in the recovery of the diseased body, affecting whether the body is immunodeficiency or hyperimmune. APS can promote cancer cell apoptosis by increasing CD3 + T, CD4 + T, CD8 + T, and CD4+/CD8+ ratio in spleen tissue of lung cancer mice, and balance type 1 T‐helper cells (Th1)/type 2 T‐helper cells (Th2) drift to improve thymus index and spleen index (Liu et al., 2021). In addition, APS also improves immune synthesis by regulating the levels of cytokine secretion. For example, APS improves the levels of cytokine interleukin‐2 (IL‐2), interleukin‐4 (IL‐4), interleukin‐10 (IL‐10), and tumor necrosis factor γ (TNF‐γ) in immunosuppressed mice induced by cyclophosphamide (Yan, Li, Li, Yu, & Yu, 2012). According to clinical research results on APS inhibiting Experimental Autoimmune Encephalomyelitis (EAE), high and low doses of APS can significantly inhibit the expression of type M1 macrophage surface marker CD16/32 + L, by regulating the proportion of inflammatory factors and antiinflammatory factors (Liu et al., 2021).
FIGURE 3.
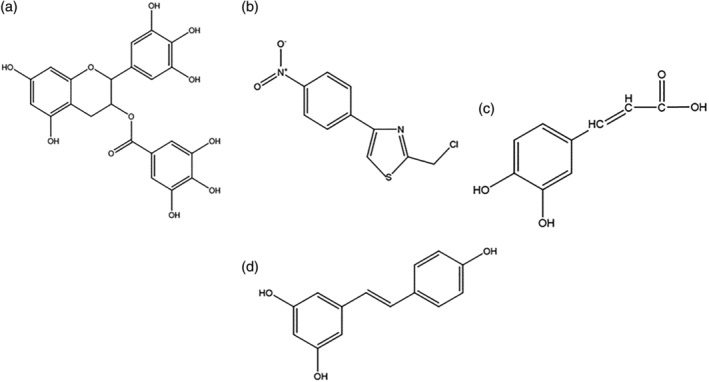
Structure of potential active products in the treatment of COVID‐19 through immunomodulation (a) EGCG, (b) astragalus polysaccharide, (c) caffeic acid, and (d) resveratrol
Clinical evidence of APS has shown good effects on maintaining the balance of immune factors and inflammatory factors. For example, a randomized controlled clinical experiment (RCT) of ovarian epithelial cancer (total sample of 70) showed that the CD4+, CD4+/CD8+ of the experimental group were higher than the control group after APS treatment (Yan & Liu, 2021). In another clinical observation experiment on non‐small cell lung cancer (NSCLC) patients with qi deficiency syndrome (75 patients in total), the observation group was treated with APS injection based on the cytokine‐induced killer (CIK) cells group. The results showed that the observation group was significantly higher in CD3+, CD4+, and CD4+/CD8+ numbers, and the disease control rate was increased to 69.4% compared with 36.1% in the non‐treatment group, suggesting that APS can increase immune cell CD4+ and inhibit fatigue and inflammation (Zhang et al., 2018). However, more clinical evidence for APS‐mediated inhibiting the cytokine storm and enhancing the immunity of COVID‐19 patients is needed.
5.2. Caffeic acid and caffeic acid phenethyl ester
As shown in Figure 2c, caffeic acid belongs to hydroxycinnamic acid. It is a group of catechu polyphenols in tea, fruits, or honey. CA plays a role in antiinflammatory, antitumor, and regulation of the immune system. The antiviral effect of CA is related to its immune regulation ability. A study has shown that 100 μM of CA significantly enhances the lethality of the NK‐cell, which is the main component of the innate immune system as the first defense against pathogens (Kilani‐Jaziri et al., 2017). CA also contributes to specific immunity. CA promotes the proliferation of splenocytes stimulated by lipopolysaccharide (LPS), potentially activates splenic B cells, enhances the host humoral immune response, and significantly promotes the secretion of cytotoxic T lymphocyte (CTL) cells (Kilani‐Jaziri et al., 2017). In a mouse neuritis study, a 30 mg/kg dose of CA reversibly inhibited LPS‐induced oxidative stress by down‐regulating the nuclear factor kappa‐B (NF‐κB)‐dependent proinflammatory gene and reducing levels of TNF‐α and Interleukin‐6 (IL‐6) (Mallik et al., 2016), showing that CA can reversibly regulate the immune system at different concentrations. In addition, studies have shown that CA and CA derivatives can target to M‐pro and Nsp15 of SARS‐COV‐2 (Adem et al., 2021). For example, CPAE is one of the main active components of caffeic acid derivatives. CPAE has been proven to be effective against a variety of viruses and tumor cells. It also showed promising effects on immunity and antiinflammatory. The latest study shows CAPE can bind to SARS‐CoV‐2 Mpro, with binding energies as low as a claimed N3 protease inhibitor (Kumar, Dhanjal, Kaul, Wadhwa, & Sundar, 2021). In addition, CAPE can inhibit NF‐κB and down‐regulate TNF‐α, IL‐1 β (Ma, Xu, Tang, & Ouyang, 2012). At present, caffeic acid and its derivatives are used in the clinical treatment of various diseases, including immune thrombocytopenia (Qin, Wei, Hou, Zhao, & Shen, 2015), oesophageal cancer, antioxidant, and type II diabetes inflammatory response (Kempf et al., 2010). Based on the roles of immunoregulation and antiinflammatory factors, CA and CPAE can be considered an adjunct drugs for COVID‐19 to enhance the innate immunity of the human body, or as an immunomodulating agent for COVID‐19 vaccine.
FIGURE 2.
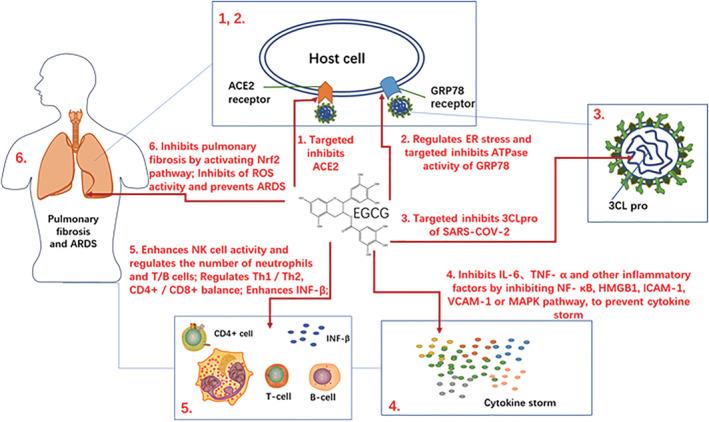
The potential mechanism of EGCG for inhibiting COVID‐19
5.3. Epigallocatechin gallate
EGCG (Figure 3a) is a polyphenol extracted from green tea. It plays a potential role in antioxidant and antiviral applications. The potential inhibitory effect of EGCG on COVID‐19 is explained in Figure 2. On the one hand, EGCG targets to inhibit coronaviruses and binds to 3CLpro of SARS‐CoV with IC50 of 0.874 μM (Du et al., 2021). In a molecular docking experiment, EGCG also had the highest binding score for the SARS‐COV‐2 spike protein out of 11 tested plant polyphenols (Ghosh, Chakraborty, Biswas, & Chowdhuri, 2021; Subbaiyan et al., 2020). On the other hand, EGCG regulates various immune cell activity, such as neutrophils, monocyte macrophages, lymphocyte T cells, and B cells (Feng et al., 2009; Huang et al., 2013; Pae & Wu, 2013). When the body is immune deficient, EGCG can promote the proliferation of T lymphocytes and further activate lymphokines involved in the specific immune response, such as IL‐2 and γ interferon (IFN‐γ) (Feng et al., 2009). It also restores Th1/Th2 balance and adjusts CD4 +/CD8 + ratio in an immunocompromised body (Deng et al., 2010; Lee & Wan, 2000). EGCG can further serve as a promising drug to improve the body’s acquired immunity and specific immunity, to resist COVID‐19 invasion. In addition, the cytokine storm caused by the strong ultra‐immune response is proved to be one of the main reasons for the systemic deterioration in patients. A previous study showed that EGCG acted as a clinical antagonist for various autoimmune diseases, suggesting it may be a potential drug for treating Th cells’ abnormal activation, which leads to the secretion of numerous inflammatory factors (Menegazzi et al., 2020). EGCG can widely inhibit viruses with envelope proteins and significantly inhibit inflammatory factors such as IL‐β, TNF‐α in immune and non‐immune cells (Nance, Siwak, & Shearer, 2009; Peairs et al., 2010; Zhang, Wu, Sang, & Ruan, 2012). Holistically, EGCG promotes the proliferation of active T/B cells and the release of immune factors to counter immune deficiency, whist also inhibiting pro‐inflammatory factors and cytokine storm. Therefore, EGCG can intervene and treat the clinical diseases caused by COVID‐19 in multiple ways (see Figure 2).
At present, the clinical research on EGCG mainly includes its regulatory interventions on the inflammatory response, immune system, energy metabolism, oxidative stress, and cancer treatment. Clinical trials have confirmed the regulatory effect of EGCG on the human immune system. For example, data from a clinical trial showed that when green tea extracts were given to 12 chronic lymphocytic leukemia (CLL) patients and 12 healthy individuals, serum IL‐10 and transforming growth factor‐β (TGF‐β) decreased in both the patients and controls—where 80% of patients showed increased lymphocytosis and a reduction of circulating Treg (D’Arena et al., 2013). This suggests that the green tea extract regulates patient immune levels. In another clinical trial to study the effects of polyphenol supplementation on adipose tissue expression, the obese people who received EGCG and resveratrol as supplements displayed down‐regulated apoptosis. Moreover, the inflammation and immune‐defense‐related pathways were adjusted (Most et al., 2018). Coincidentally, Lynn et al., who mixed flavonoids and EGCG as supplements, successfully induced immune enhancement and antiinflammatory transcriptional changes in overweight women, indicating the inhibition of EGCG and other flavonoids on phagocytosis and inflammatory pathways (Cialdella‐Kam et al., 2016). In addition, there are several lines of evidence showing that EGCG has a significant effect on the clinical treatment of human immunodeficiency virus (HIV) and hepatitis C virus (HCV) virus (Halegoua‐De Marzio et al., 2012; Shiha, Soliman, Elbasiony, Darwish, & Mousa, 2019). As the above two viruses are positive‐chain RNA viruses with similar spike protein and capsule membrane structure to SARS‐COV‐2, EGCG may potentially also apply to COVID‐19 treatment. As shown in Table 1, the clinical evidence of EGCG in immunomodulation and antiinflammatory is relatively sufficient, but its effectiveness in targeted therapy of COVID‐19 needs further study.
TABLE 1.
Mechanism and research progress of EGCG
| Mode of action | Effect | Research phase | System for experiment | Reference |
| EGCG target virus/host cells protein | 1. Inhibits GRP78 receptor and makes it accumulate in ER rather than on cell membrane | Preclinical study (in vivo & in vitro) | Nervous system diseases (MME cells, Ren cells) | (Martinotti, Ranzato, & Burlando, 2018) |
| 2. Inhibits GRP78 expression by enhancing mir‐155‐5p level | Preclinical study | Colon cancer (HCT116) | (La, Zhang, Li, Li, & Yang, 2019) | |
| 3. Inhibits the binding of S‐spike protein to ACE2 receptor and effectively blocks the infection of SARS‐COV‐2 and new variants | Preclinical study | SARS‐CoV‐2 and its variants with D614G, K417N, E484K, and N501Y mutation) | (Liu et al., 2021) | |
| 4. Targets to inhibit 3CLpro | Preclinical study (in vitro) | SARS‐CoV‐2 | (Zhu & Xie, 2020) | |
| Regulate immune level |
|
Preclinical study (animal level) | Ulcerative colitis rat model | (Xue, Liu, Dong, Liang, & Chen, 2017) |
| 2. Increases lymphocytosis and shows a reduction in the absolute number of circulating Treg, and serum IL‐10 and TGF‐β d | Clinical study | 12 CLL patients and 12 healthy person as control | (Most et al., 2018) | |
|
Clinical study | Overweight women | (Cialdella‐Kam et al., 2016) | |
| Inhibits cytokine storm |
|
Preclinical study (animal level) | Lipopolysaccharide‐induced acute lung injury model in mice | (Bae et al., 2010 ) |
|
Preclinical study (animal level) | Lung injury in thermal injury rat model | (Liu et al., 2017) | |
|
Preclinical study (animal level) | Pulmonary fibrosis (radiation‐induced pulmonary fibrosis rat model) | (You et al., 2014) | |
| Broad‐spectrum antivirus function | EGCG has a broad spectrum of antiviral activities against RNA viruses such as HIV, HCV, human immunodeficiency virus, Ebola virus, influenza virus, and respiratory virus | Clinical study (animal level) | HIV and HCV | (Halegoua‐De Marzio et al., 2012; Shiha, Soliman, Elbasiony, Darwish, & Mousa, 2019) |
5.4. Resveratrol
Resveratrol is the main active ingredient of red wine and grapes. Resveratrol has an immunomodulatory effect and can reduce the prevalence of cardiovascular disease. When a host is immunocompromised, resveratrol can improve nonspecific immunity by stimulating the activity of NK‐cell and enhancing the function of macrophages (Leischner et al., 2016). At the same time, it can also activate helper T cells for specific immune regulation (Gao et al., 2003). When the body exhibits hyperimmunity (such as type IV hypersensitivity) it can inhibit the activation of inflammatory factors like IL‐6 (Gao et al., 2003). For example, in LPS‐induced pneumonia mice resveratrol alleviates the symptoms of pneumonia, by inhibiting TNF α, IL‐1β, and IL‐6 (Jin, 2011). Studies have also shown that resveratrol can inhibit human ACE2 receptors (de Ligt et al., 2021; Moran et al., 2017) and so it may play a role in blocking COVID‐19's invasion. The structure of resveratrol is shown in Figure 3d.
In this section, we discuss some natural products that may regulate the immune system. The current research progress is shown in Table 2. When the host is immunocompromised, these natural products can stimulate the activity of NK cells or regulate the balance of Th1/Th2 and increase the ratio of CD4+/CD8+. Therefore, enhancing the specific immune function and potentially preventing COVID‐19 from invading. In addition, EGCG, CPAE can target to dock with COVID‐19's 3CLpro. All the evidence suggests that natural products such as EGCG, APS, CA, and resveratrol can be used in adjuvant therapy for COVID‐19 or be promising immune enhancers for the SARS‐COV‐2 vaccine.
TABLE 2.
Immunomodulatory mechanism and research progress of natural products
| Name | Effect | Research phase | System for experiment | Drug dosage regimen | Reference |
| ASP | Increases CD4+/CD8+ ratio in spleen tissue; balances Th1/Th2 drift; improves thymus index and enhances the immune function | Preclinical study (animal level) | Lewis lung cancer cells in model mice | Model group, cisplatin group (3 mg/kg), APS high, medium, and low dose groups (400, 200, and 100 mg/kg), with 10 mice in each group. | (Liu et al., 2021) |
| ASP | Accelerates DTH and NK cells activity, and increased IL‐2, IL‐4, IL‐10, and IFN‐γ level in serum | Preclinical study (animal level) | Immunosuppressed mice induced by cyclophosphamide | Immunosuppressive mice were intraperitoneally injected with high, medium, and low doses (20, 13, and 7 mg/kg) of Lentinan and ASP membranaceus solution (0.5 ml/animal) for 5 consecutive days. | (Yan, Li, Li, Yu, & Yu, 2012) |
| ASP | Inhibits the expression of type M1 macrophage surface marker CD16/32 + L. | Preclinical study (in vitro) | EAE model in C57BL/6 female mice | Splenocytes were taken on the ninth day after immunization and cultured with high (0.2 mg/ml) and low (0.1 mg/ml) doses of ASP for 48 h | (Liu et al., 2021) |
| ASP | Improves the CD4+, and CD4+/CD8+ ratio, as well as immune function. | Clinical study (RCT) | Advanced ovarian epithelial cancer (patients) | 70 patients with advanced epithelial ovarian cancer were treated with paclitaxel albumin binding and lobaplatin in the control group, and ASP in the experimental group for 4 consecutive cycles. | (Yan & Liu, 2021) |
| ASP | Increases CD3+, CD4+, and CD4+/CD8+; inhibits fatigue, and inflammation. | Clinical study (RCT) | NSCLC patients with qi deficiency syndrome | Among 75 patients with NSCLC Qi deficiency syndrome, the combined treatment group was added with Astragalus Polysaccharide for injection (250 mg per day), 10 days as a cycle. | (Zhang et al., 2018). |
| CA | Enhances the lethality of NK cells; promotes the proliferation of splenocytes stimulated by LPS; activates splenic B cells, enhances the host humoral immune response, and significantly promotes the secretion of CTL cells. | Preclinical study (in vitro) | Immunomodulatory effect of phenolic compounds | 0, 100, 300, or 500 mM caffeic acid was added to the complete RPMI medium, and all immune effector cells were incubated at 37C for another 48 h | (Kilani‐Jaziri et al., 2017) |
| CA | Reversibly inhibits LPS‐induced oxidative stress by down‐regulating NF‐κB‐dependent pro‐inflammatory gene, as well as reduce TNF‐α and IL‐6. | Preclinical study (animal level) | LSP‐induced neuroinflammation in mice | Caffeic acid (30 mg/kg) and imipramine (15 mg/kg) were administered orally 1 h before LPS (1.5 mg/kg) test. Behavioral assessment was performed 3 h after LPS injection. | (Mallik et al., 2016) |
| CFDs | Caffeic acid derivatives can be aligned with COVID‐19's M‐pro and Nsp15 target | Preclinical study | COVID‐19 | None | (Adem et al., 2021) |
| CAPE | Binds to SARS‐CoV‐2 Mpro | Preclinical study | COVID‐19 | None | (Kumar, Dhanjal, Kaul, Wadhwa, & Sundar, 2021) |
| CAPE | Inhibit NF kappa B, and down regulate TNF‐α, IL‐1 β, and other proinflammatory cytokines | Preclinical study (animal level) | Liver injury induced by cigarette smoke (in rats) | 21 male Wistar rats, rats in group II were exposed to cigarette smoke and rats in group III were exposed to cigarette smoke and injected with Cape every day. For 60 day experimental period. | (Pekmez et al., 2007) |
| CA | Has good curative effect on ITP, and the incidence of adverse reactions is low and mild | Clinical study (RCT) | Primary immune thrombocytopenia (ITP) | 103 ITP patients in three centers were divided into two groups. PLT patients in group A took 300 mg CA tablets orally every day for 12 weeks. | (Qin, Wei, Hou, Zhao, & Shen, 2015) |
| CA | Coffee consumption seems to have beneficial effects on subclinical inflammation and HDL cholesterol. | Clinical trial | Coffee consumption person | Habitual coffee drinkers (n = 47) avoided drinking coffee for 1 month; they consumed 4 cups of filtered coffee/day in the second month and 8 cups of filtered coffee/day (150 ml/cup) in the third month. | (Kempf et al., 2010) |
| Res | Plays an immunomodulatory role in immunodeficient mice and inhibit type IV hypersensitivity in normal mice. | Preclinical study (animal level) | Immunosuppressive model mice | After sterile intraperitoneal injection of CY 80 mg/kg, the immune function decreased significantly after 5 days, and then gavage according to the high, medium, and low dose groups of res (10, 5, and 2.5 mg per kg body weight per day). | (Gao et al., 2003) |
| Res | Resveratrol supplementation reduced the expression of ACE2 (about 40%) and leptin (about 30%) in human adipose tissue | Clinical study (RCT) | ACE2 expression (SARS‐COV‐2) | 11 obese men were injected with 150 mg/day placebo or trans resveratrol (30 and 150 days) and verified by analyzing free and bound resveratrol in plasma. | (de Ligt et al., 2021) |
| Res | Decreases CSF MMP9, increases MDC, IL‐4, and FGF‐2, regulates neuroinflammation and induces adaptive immunity. | Clinical study (RCT) | Neuroinflammation/Alzheimer’s disease | Mild to moderate Alzheimer’s disease (AD) subjects (n = 119) were treated with SIRT1 activator resveratrol (up to 1 g orally, twice daily) for 52 weeks | (Moussa et al., 2017) |
Abbreviations: DB, double blind; Res: Resveratrol; RCT, randomized controlled trial.
6. NATURAL PRODUCTS THAT TARGET PROTEIN OF SARS‐COV‐2 OR HOST CELLS
According to the types of targets, the screened natural products can be divided into multiple groups, including a targeting viral proteins group and a targeting host receptors group. These natural products, targets, and related preclinical/clinical studies are summarized in Table 3.
TABLE 3.
Potential targets, clinical research, and mechanism of promising anti‐SARS‐COV‐2 natural active products
| Name | Effects | Research progress | Drug dosage regimen | System for experiment | Reference |
| Baicalin/Baicalein (targets:3CLpro & ACE2) | 1.Targeted binds with ACE2. | Preclinical study | None | (silico virtual screen) | (Shakhsi‐Niaei, Soureshjani, & Babaheydari, 2021 ) |
| 2.Targeted inhibits SARS‐COV‐2 3CLpro with IC50 of 1.69 and 10.27 μM respectively. | Preclinical study (in vitro) | Different concentrations of baicalin or baicalein were used to treat COVID‐19 in Vero cells for 48 h | SARS‐COV‐2 in Vero E6 cells | (Su et al., 2020a) | |
| 3. Inhibits 3CLpro in vitro (IC50: 0.39 μM), and in Vero cells (EC50: 2.9 μM). | Preclinical study (in vitro) | Vero cells were pretreated with baicalein for 1 h and then infected with virus for 2 h. The cells were treated with baicalein medium. | SARS‐COV‐2 in Vero E6 cells | (Liu et al., 2021) | |
| 4. SWHD showed better anti influenza virus activity than oseltamivir. | Preclinical study (in vivo) | Use 50 μ L virus suspension containing 10LD50 virus (mice) was inoculated intranasally. Two hours later, the inoculated mice received different concentrations of SWHD extract (5.85, 11.70, or 23.40 g/kg/day) by gavage every day for 5 days. | 10LD50 virus in female BALB/c mice | (Ma et al., 2018) | |
| 5.Improves the inflammatory reaction and lung injury caused by COVID‐19 | Clinical study (RCT) | 235 patients were divided into 4 groups. The experimental group received high (60 ml), medium (40 ml), and low (20 ml) doses of SHL three times a day (in addition to standard treatment). The control group received standard treatment. | COVID‐19 patients | (Ni et al., 2021) | |
| 6.Inhibits inflammatory response, improves immune function and lung function | Clinical study (RCT) | 124 patients with mycoplasma pneumonia were divided into two groups. The control group took azithromycin (once/day, 10 mg/kg/per) and montelukast sodium tablets (once/day, 5 mg per), and the observation group added Shuanghuanglian (3 times/day, 5 g per), 30 days for treatment. | Patients with mycoplasma pneumonia | (Yang, Wang, & Li, 2020) | |
| Keampferol (targets: 3CLpro & ACE2) | 1.Targeted inhibits ACE2 and SARS‐COV‐2 3CLpro | Preclinical study | None | Molecular docking | (Zong, Ding, Jia, Ma, & Ju, 2020) |
| Quercetin (3CLpro and ACE2) | 1.Targeted inhibits ACE2 | Preclinical study | None | Molecular docking | (Shen & Yin, 2021) |
| 2.Targeted inhibits SARS‐COV‐2 3CLpro | Preclinical study | None | Molecular docking | (Zong, Ding, Jia, Ma, & Ju, 2020) | |
| 3.Reduces frequency and length of hospitalization, and invasive oxygen therapy rate | Clinical study (RCT) | Based on the SC (standard of care), the observation group (152 COVID‐19 patients) were given a daily dose of 1,000 mg of QP (quercetin Phy‐tosome ©). For 30 days. | COVID‐19 patients | (Di Pierro et al., 2021). | |
| 4.Reduces EMS, pain and inflammatory factor hs‐TNF α levels in plasma. | Clinical study (RCT plus DB) | 50 female patients were randomized to the quercetin(500 mg/day) or placebo groups for 8 weeks | Women with rheumatoid arthritis | (Javadi et al., 2017) | |
| 5. Quercetin reduces the degree of upper respiratory tract infection. | Clinical study (RCT plus DB) | 1,002 patients with upper respiratory tract infection were divided into 3 groups, respectively received quercetin 500 mg/day, 1,000 mg/day and placebo | Patients with upper respiratory tract infection | (Heinz, Henson, Austin, Jin, & Nieman, 2010). | |
| Rutin (3CLpro and ACE2) | 1.Targeted inhibits ACE2 and SARS‐COV‐2 3CLpro | Preclinical study | None | Molecular docking | (Wu et al., 2021) |
| 2. Decreases MPO level | Clinical study (DB) | Group 1 ate 16.5 mg rutin equivalent/day, group 2 ate 359.7 mg rutin equivalent/day, for 2 weeks. Then change the type of biscuit, they ate for another 2 weeks | Healthy women | (Wieslander et al., 2011) | |
| 3. Improves patients' neurological and inflammatory status (ox‐LDL, NF‐κB p65, TNF‐α and IL‐6) | Clinical study (RCT) | 150 patients with ACI were divided into two groups. Both were given routine treatment. Group 1 were treated with Ginkgo Dharma injection, and patients in the combined treatment group were treated with Ginkgo Dharma injection combined with troxerutin. | ACI (acute cerebral infarction) patients | (Feng, Liu, & Qin, 2021) | |
| Β‐sitosterol (3CL pro) | Targeted binding 3CL pro | Preclinical study | None | Mocular docking | (Narkhede, Pise, Cheke, & Shinde, 2020) |
| Berberine (3CL pro) | 1.Targeted inhibits 3CL pro | Preclinical study | None | Mocular docking | (Liu & Wang, 2020). |
| 2.Decreased interleukins and other inflammatory factors. | Clinical study | 130 ACS patients(received PCI) were divided into two groups. The control group received standard treatment, and 61 patients in the experimental group received erberine treatment(300 similar to mg, t.i.d., for 30 similar to days. | ACS patients | (Meng et al., 2012) | |
| Betulinic acid (3CL pro) | 1.Targeted inhibits 3CL pro (IC50: 5 μM) | Preclinical study | None | Spectrophot‐ometric assay | (Liu & Wang, 2020) |
| 2.Activates the immune response byIncrease the improving CD4+/CD8 + . | Preclinical study (animal trial) | For non‐immunized and red blood cell (SRBC) immunized mice, betulinic acid (50,5,0.5 mg/kg) was orally administered five times every 24 h | SRBC immunized mice | (Jine, Lis, Szczypka, & Obminska‐Mrukowicz, 2012) | |
| Indirubin (3CL pro) | 1.Reduce the expression level of the proinflammatory factor IL‐1β, IL‐6, and TNF‐αmRNA | Preclinical study | 50 μl 0.2 mg/ml LPS was injected into the breast tube and 0.2 ml/20 g 10:1 diluted 25, 50, or 100 μM indirubin were injected by IP at 1 and 12 h. Culture for 24 h | LPS induced mastitis mouse model | (Lai et al., 2017) |
| 2.Targeted binding 3CL pro | Preclinical study | None | Mocular docking | (Narkhede, Pise, Cheke, & Shinde, 2020) | |
| Cordycepin RdRp and 3CLpro | 1.Targeted inhibiting RdRp and 3CL pro (EC50: 2 μ M) | Preclinical study (in vitro) | After Vero E6 was infected with SARS‐COV‐2, cordycepin was administered for 36–48 h (SI > 49.75; EC 50 = 2.0) | (Rabie, 2022) | |
| Curcumin (RdRp/Furin and ACE2) | 1.Targeted inhibiting RdRp protein | Preclinical study | None | Mocular docking | (Singh, Bhardwaj, & Purohit, 2021) |
| 2.Targeted inhibiting Furin and ACE2 protein | Preclinical study | None | Mocular docking | (Verma et al., 2021) | |
| Myricetin NSP15 | Targeted inhibiting NSP15 | Preclinical study | None | Mocular docking | (Sharma et al., 2021) |
| Glycyrrhizin (targets: ACE2& S spike protein) | 1. Inhibits SARS‐COV‐2 infection (EC50: 2.39 μm) | Preclinical study (in vitro) | Vero E6 cells were infected with SARS‐CoV‐2 at 100 μl median tissue culture infectious dose (TCID50), in the presence of glycyrrhizin. | Vero E6 cells infected by SARS‐CoV‐2 | (Zhu et al., 2020) |
| 2.Targeted inhibits ACE2 | Preclinical study | None | Mocular docking | (Ahmad, Waheed, Abro, Abbasi, & Ismail, 2021) | |
| 3.Targeted inhibits Spike protein | Preclinical study | None | Mocular docking | (Li et al., 2021) | |
| 4.Regulates the ex‐pression of HMGB1, NF‐κB, COX‐2, ILS, TNF and other factors | Preclinical study (in vivo) | Glycyrrhizin (2, 4, and 10 mg/kg) was administered by intra‐articular injection for 12 weeks | Male Wistar rats | (Luo et al., 2021) | |
| 5.GF‐ β 1, TNF‐α, liver function and fibrosis were significantly improved | Clinical study | All patients took adefovir dipivoxil orally (10 mg/time, once/day). Study groups were added with compound glycyrrhizin injection (intravenous drip, once/day). Treat for 12 months. | Hepatitis B patients | (WANG, LUO, & CHEN, 2018) | |
| 6.TNF‐α, IL‐6 level of the inflammatory factor in chronic hepatitis B patients were decreased | Clinical study | All patients receive compound glycyrrhizin injection (20 mL/branch, intravenous drip ~2 h), treatment was 2–4 weeks | Hepatitis B patients | (Fang & Shuan‐lin, 2009) (Cai, Zhang, & Zhang, 2004) | |
| 7. Improves chest tightness, cough and pain, protects liver function index | Clinical study | 102 patients with SARS were divided into the control group and the observation group. 27 cases of observation group were treated with compound glycyrrhizin (160 mg·d‐1). | Patients with SARS | ||
| 8. The serum ALT and AST of mild and severe patients tended to return to normal levels after DG treatment | Clinical study | 59 SARS‐COV‐2 patients receive diammonium glycyrrhizinate treatment. | Patients with SARS‐COV‐2 | (Liao et al., 2021) | |
| Nicotinamine (targets: ACE2) | 1.Targeted inhibits ACE2 activity | Preclinical study | None | LC–MS | (Takahashi, Yoshiya, Yoshizawa‐Kumagaye, & Sugiyama, 2015) |
| Chlorogenic acid (targets: ACE2) | Targeted inhibits ACE2 | Preclinical study | None | Mocular docking | (Yu, Wang, & Bao, 2020) |
| Honokiol (targets: Furin) | Targeted inhibits furin protein and SARS‐COV‐2 infection(25 μ M (99.83%) and 50 μ M (99.96%)) | Preclinical study (in vitro) | Vero E6/TMPRSS2 cells were infected with SARS‐COV‐2 (WK‐521) in the presence of honokiol (100, 50, 25, 12.5, 6.25, and 和 3.125 μM) for 24 h | Vero E6/TMPRSS2 cells infected with SARS‐COV‐2 | (Tanikawa, Hayashi, Suzuki, Kitamura, & Inoue, 2021) |
Abbreviations: DB, double blind; RCT, randomized controlled trial.
6.1. Natural products with multiple targets
6.1.1. Baicalin/Baicalein
Baicalin is the main ingredient of traditional chinese medicine (TCM) baicalein and is used for treating fever, upper respiratory tract infection, inflammation, and cough (Li‐Weber, 2009). The structures of Baicalin and Baicalein are shown in Figure 4a, b. During the epidemic in Wuhan, the Lianhua Qingwen Capsule was one of the proprietary Chinese patent medicines used for clinical treatment, for which baicalein is the main component. Preclinical studies reported that baicalin can bind to 3CLpro and ACE2. The molecular docking results showed that baicalin can stably occupy the ACE2's RBD binding region, which provides one of the mechanisms to inhibit SARS‐COV‐2 invasion (Shakhsi‐Niaei, Soureshjani, & Babaheydari, 2021). As for baicalein, all the X‐ray protein crystallography results, in vitro inhibition experiments, and in vivo experiments, confirmed that baicalein can bind to 3CLpro of SARS‐COV‐2 (Liu et al., 2021; Su et al., 2020a). The IC50 of baicalin and baicalein in Vero E6 cells were 1.69 and 10.27 μM, respectively (Su et al., 2020a). Another study showed that baicalein can strongly inhibit the activity of SARS‐CoV‐2 3CLpro with IC50 of 0.39 μM and with EC50 of 2.9 μM in Vero cells (Liu et al., 2021). Baicalein and baicalin work on blocking SARS‐COV‐2 replication by inhibiting 3CLpro and may also bind to the host ACE2 receptor with a unique structure, thus hindering viral invasion.
FIGURE 4.
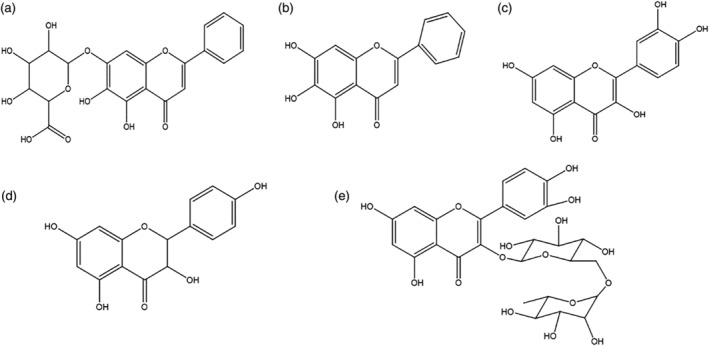
Natural active products target to inhibit the invasion of SARS‐COV‐2 (a) baicalin, (b) baicalein, (c) quercetin, (d) rutin, and (e) kaempferol
Presently, clinical trials with baicalin involve sepsis, respiratory inflammation, SARS‐COV‐2, human influenza, and antitumor treatments. It has been confirmed that baicalin is the effective ingredient of TCM Shuang Huang Lian. In a multi‐central RCT, Li et al. treated 176 COVID‐19 patients with different doses of Shuang Huang Lian (as a control 59 patients were treated with standard therapy). After a 14‐day course of treatment, patients treated with Shuang Huang Lian had a higher negative nucleic acid conversion rate compared with the control group (93.4% vs.73.9%, p = 0.006), and high‐dose Shuang Huang Lian recipients had better absorption of pneumonia and inflammation (Ni et al., 2021). This clinical evidence suggests that Shuang Huang Lian and its main component, baicalein, may act as a promising inhibitor of COVID‐19 and that it may also function within the inflammatory response. In a clinical trial for mycoplasma pneumonia patients (124 in total, RCT, 30 days' course of treatment), the observation group had significant improvement in lung function indicators, inflammatory factors, and immune function indicators, to those of the control group (Yang, Huang, & Yang, 2020). This shows that Shuang Huang Lian may inhibit the inflammatory response and improve immune function. In conclusion, clinical experiments have shown that baicalin may act on SARS‐COV‐2, pneumonia and systemic inflammation, and improve body immunity. However, more clinical evidence regarding baicalein’s potential targeting of 3CLPro and ACE2 to inhibit SARS‐COV‐2 is needed.
6.1.2. Quercetin
As shown in Figure 4c, quercetin is a natural multi‐potent bioactive product found in various plants such as Fagopyrum esculentum Moench, Panax notoginseng, and Ginkgo biloba L., and can also be obtained by rutin deglycosylation. Previous studies have shown that quercetin and its analogs can inhibit multiple RNA viruses (Anusuya & Gromiha, 2017; Choi et al., 2009; Song, Shim, & Choi, 2011). TCM compound, Da Yuan Yin, is used to treat infection and fever caused by SARS‐COV‐2. Zong et al. constructed a compound‐target network of Da Yuan Yin and found that the main component quercetin, had the potential to target and inhibit SARS‐CoV‐2 3CL pro (Zong, Ding, Jia, Ma, & Ju, 2020). According to a mechanism prediction and molecular dynamics study of the Lianhua Qingwen capsule, quercetin as an active ingredient can strongly bind with ACE2 to prevent the invasion of SARS‐COV‐2 (Shen & Yin, 2021). Evidence of quercetin, inhibiting the TNF‐α, IL‐1β, signal NF‐κB, C‐reactive protein, and monocyte chemotactic protein‐1, shows it may also reduce the system disease after SARS‐COV‐2 infection (Gardi et al., 2015; Kim et al., 2005; Zheng, Wu, Wang, Zhan, & Liu, 2021).
Clinical trials of quercetin have shown its potential in response to inflammation caused by SARS‐COV‐2. In a prospective RCT study, 152 COVID‐19 outpatients were divided into two groups. Based on the SC (Standard of Care), the observation group was given 1,000 mg QP (Quercetin Phytosome ©) per day. After 30 days, the results confirmed that the QP group had a significant reduction in the frequency and length of hospitalization (28.9% for SC and 9.2% for QP), as well as invasive oxygen therapy rate (19.7% for SC and 1.3% for QP), and the number of deaths (QP with none) (Di Pierro et al., 2021). At present, the ongoing clinical experiments of quercetin also involve urinary tract infection, lung infection and rheumatoid arthritis. In a clinical study of female rheumatoid arthritis, 50 female patients were randomly designated into quercetin and placebo groups. The results indicated that quercetin significantly reduced pain and inflammatory factor hs‐TNF α levels in plasma (Javadi et al., 2017). In another randomized, double‐blind clinical trial of 72 diabetic patients, the quercetin group had significantly lower high‐density lipoprotein‐c(HDL‐c), TNF‐α, and IL‐6 levels than the placebo group (Dehghani et al., 2021). The above results suggest that the clinical effects of quercetin on inflammation may be linked to the administration dose and that it might be a promising adjuvant in the treatment of COVID‐19. However, new administration methods should be developed to improve the utilization of quercetin (Khursheed, Singh, Wadhwa, Gulati, & Awasthi, 2020).
6.1.3. Rutinum
Rutinum, also known as Ruside, is a flavonol complex naturally found in Ruta graveolens L. and Nicotiana tabacum L. leaves. It can play an antiinflammatory and antioxidant role by promoting the production of antioxidant enzymes and reducing the manifestation of pro‐inflammatory factors (Guruvayoorappan & Kuttan, 2007; Koval’skii et al., 2014). At the same time, rutinum also performs well in antiviral and antithrombotic (Hsu & Yen, 2008). The structure of rutinum is shown in Figure 4d. In China’s drug intervention program against COVID‐19, prescription XG‐1 has shown good treatment effects (Wu et al., 2021). By conducting pharmacological network construction and molecular docking experiments on XG‐1, Wu et al. screened out rutinum as one of the high‐efficiency targets (Wu et al., 2021), which docked to ACE2 and 3CLpro (Wu et al., 2021). Tien’s group designed a more hydrophobic analog of rutin (Rutin‐M) to enhance its target ability to 3CLpro in an aqueous solution (Huynh, Wang, Cornell, & Luan, 2020). This result also provides the potential for drug development of an irreversible combination with 3CLpro.
At present, clinical research on rutin ranges from its potential antioxidant effects and antiinflammatory effects to lowering lipid levels. A double‐blind crossover study on 62 women, which used buckwheat biscuits containing rutin as a marker, showed the myeloperoxidase (MPO, inflammation criteria) serum level of the rutin‐containing biscuit group decreased by 0.84 times after 5 days (Wieslander et al., 2011). In addition, troxerutin, synthesized by hydroxyethylation of rutin, is commonly used to treat blood diseases and inflammation. Feng et al. used troxerutin combined with Ginkgo biloba Damo injection to treat acute cerebral infarction (ACI). The results showed that levels of oxidized low‐density lipoprotein (OX‐LDL), Toll‐likereceptor4 (TLR4), NF‐kappa B p65, TNF‐α, IL‐1 β, and IL‐6 in the observation group were significantly lower than the control group (Feng, Liu, & Qin, 2021). The clinical evidence of rutinum in thrombotic and inflammation suggests its potential as an adjuvant to COVID‐19.
6.1.4. Kaempferol
Kaempferol is mainly found in propolis and functions in antioxidation, antiapoptosis (Yuhang et al., 2021), antitumor (Yao et al., 2016), and bacterial inflammation treatment. The structure of kaempferol is shown in Figure 4e. It is an essential target in the TCM prescription Qing Fei Pai Du Tang, Da Yuan Yin, and Lianhua Qingwen capsule, which have certain effects on the treatment of COVID‐19. According to the network pharmacological analysis of Da Yuan Yin conducted by Zong et al., the binding energy of kaempferol to 3CLpro is the same as that of lopinavir (Zong, Ding, Jia, Ma, & Ju, 2020). Lopinavir combined with other drugs has a better effect in treating SARS‐COV‐2, suggesting the possibility of using kaempferol to treat COVID‐19.
6.2. Natural products that target Mpro (3CLpro)
6.2.1. Betulinic acid (BA)
Some triterpenoid‐active natural products, such as betulinic acid (BA), may interfere with inflammation and target 3CLpro to inhibit SARS‐COV‐2 (Zhou & Huang, 2020). The structure of BA is shown in Figure 5a. BA can improve the body’s immunity and slow down the inflammatory response (Yi et al., 2010). It regulates gene expression by inhibiting the activity of NF‐κb when inflammatory stimulation occurs (Xu, Qin, & Li, 2014). BA also inhibits the influenza virus and membrane fusion of HIV, suggesting a potential role in treating SARS‐COV‐2 (Chen et al., 2020; Hodon, Borkova, Pokorny, Kazakova, & Urban, 2019). It has been reported that the IC50 value of BA in inhibiting 3CLpro is 5 μM (Liu & Wang, 2020). BA can also activate the immune response by increasing the proportion of CD3+, CD4+, and CD4+/CD8 + in thymocytes, splenocytes, and lymphocytes (Jine, Lis, Szczypka, & Obminska‐Mrukowicz, 2012; Yi et al., 2010). At present, there is a lack of clinical studies for BA’s inhibitory effects on SARS‐COV‐2, and further clinical verification will be needed.
FIGURE 5.
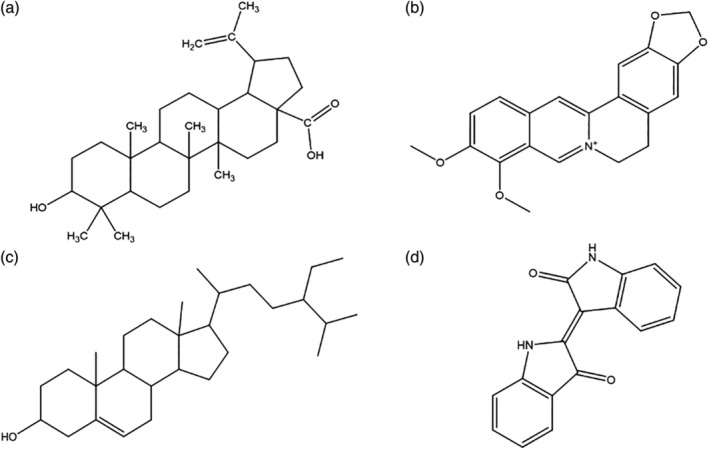
The structure of natural active products which target to inhibit 3CLPro (a) betulinic acid, (b) berberine, (c) β‐sitosterol, and (d) indirubin
6.2.2. Berberine
Berberine is used for antimicrobial, lipid‐lowering, antiantitumor, and antiinflammatory. Berberine has effects on a variety of viruses, including influenza A/FM1/1/47 (H1N1), enterovirus 71 (EV71), and respiratory syncytial virus (Tian & Duan, 2021). The structure is shown in Figure 5b. According to the molecular docking experiments, berberine can bind to 3CLpro of SARS‐COV‐2 (Narkhede, Pise, Cheke, & Shinde, 2020), and can potentially inhibit virus replication. As a broad‐spectrum antiviral and antiinflammatory drug, berberine is widely used in clinical research. In a random clinical trial for the acute coronary syndrome (ACS), 61 patients in the experimental group received berberine treatment based on standard therapy. The results showed that interleukins and other inflammatory factors decreased linearly in the experimental group (Meng et al., 2012). In addition, the clinical manifestations of berberine in the eradication of Helicobacter pylori and Vibrio cholera also indicated an antiinflammation role. (Genovese et al., 2018; Zhang et al., 2020). In conclusion, berberine has potential in treating SARS‐COV‐2 in terms of antiinflammatory and targeting 3CLpro.
6.2.3. β‐Glusterol and indigo red
β‐Glusterol is found in various vegetable oils and nuts. The structure of β‐glusterol is shown in Figure 5c. According to network pharmacology research, glusterol and β‐glusterol are the main targets in TCM Radix Scutellariae, Radix Glycyrrhizae, and Radix Paeoniae Alba (Zong, Ding, Jia, Ma, & Ju, 2020). Molecular docking experiments have proven that β‐glusterol has the potential to target and inhibit 3CLpro (Narkhede, Pise, Cheke, & Shinde, 2020). Another natural product, indirubin, can also bind to 3CLpro (Narkhede, Pise, Cheke, & Shinde, 2020). As shown in Figure 5d, indirubin is a double indole component and is presently found in the TCM Isatis indigotica and Qingdai. Indigo red has significantly decreased the expression of the proinflammatory factor IL‐1β, IL‐6, and TNF‐αmRNA in an LPS‐induced mouse mastitis model (Lai et al., 2017). It is also found that indirubin derivatives have antiviral and antiinflammatory effects on human alveolar epithelial cells (Mok et al., 2014).
6.3. Natural products that targe RdRp
6.3.1. Cordycepin
Isolated from Cordyceps militaris, cordycepin has many functions, such as antiinflammatory (Verma, 2020), antiviral (Panya et al., 2021), and immune regulation (Kang et al., 2015). As shown in Figure 6a, the structure of cordycepin is similar to that of nucleosides. So, it may be incorporated into virus RNA replication as a nucleoside analog. COVID‐19's RNA is identical to mRNA, which is highly polyadenylated at the 3′ terminal. The 3′ terminal of cordycepin has no‐OH, making it easy to incorporate and block the polymerization of adenylated viral RNA, to prevent viral replication (Verma, 2020). Currently, the nucleoside derivative, Molnupiravir, has been applied in phase III clinical trials, indicating the optimistic prospect of such nucleoside analog drugs for anti‐COVID‐19. In addition, cordycepin also has the potential to target RdRp (Bibi, Hasan, Wang, Papadakos, & Yu, 2022) and 3CLpro of COVID‐19, and its EC50 value for anti‐SARS‐COV‐2 in vitro is about 2 μM (Rabie, 2022). Moreover, cordycepin has previously been approved for immune regulation (Kang et al., 2015). In conclusion, cordycepin can be developed and utilized as an anti‐COVID‐19 drug with various functions, such as regulating immunity, targeting to inhibit viral proteins, and acting as nucleotide analogs.
FIGURE 6.
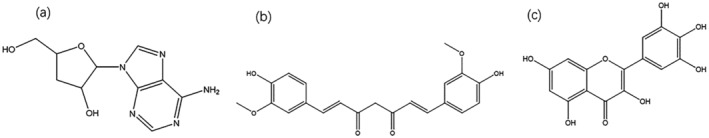
The structure of natural active products which targeted inhibit RdRp and NSP15 (a) cordycepin, (b) curcumin, and (c) myricetin
6.3.2. Curcumin
Curcumin is a diketone natural product extracted from the rhizomes of Curcuma longa L. It has antitumor and antiinflammatory effects and has been utilized to treat liver cancer and a variety of chronic diseases. Molecular docking results have shown that curcumin and its derivatives can stabilize the RdRp protein of COVID‐19 (Singh, Bhardwaj, & Purohit, 2021). It has better potential for anti‐SARS‐COV‐2 development than registered drugs redcivir and fabiravir (Singh, Bhardwaj, & Purohit, 2021). In addition, it also has promising inhibitory effects on host receptors Furin and ACE2 (Verma et al., 2021). The evidence suggests that curcumin is a promising drug for inhibiting COVID‐19 infection and blocking severe inflammation.
6.4. Natural products target non‐structural protein: Myricetin
COVID‐19 RNA encodes a variety of specific non‐structural proteins. Among them, NSP15 is a hexamer ribonuclease, which can specifically cleave 3′ uridine, which helps viruses to evade the host immune recognition mechanism activated by RNA and hinder autophagy formation (Al‐Rashedi, Munahi, & Ah, 2020). Therefore, it is an essential target for inhibiting COVID‐19. Myricetin is a natural product of flavonoids, which exists in grapes, Myrica rubra, and tea. It has the effect of antiinflammatory and inhibiting platelet aggregation. Studies have shown that myricetin targets and inhibits COVID‐19 NSP15 (Sharma et al., 2021). In addition, quercetin and EGCG were also found to potentially inhibit COVID‐19’s NSP15 (Hong et al., 2021).
6.5. Natural products that target host cell receptors ACE2/FURIN
6.5.1. Natural products that target ACE2: Nicotianamine and chlorogenic acid
ACE2 receptors are widely found on the surface of human organs and have an important impact on cardiovascular balance. Herbal medicines and natural products, such as nicotianamine, 1‐methylisoguanosine, and chlorogenic acid can play a role in ACE2 activity (Mohammadi Pour, Farzaei, Soleiman Dehkordi, Bishayee, & Asgary, 2021). Structures of nicotianamine and chlorogenic acid are shown in Figure 7. Nicotianamine is a natural alkaloid obtained from Glycine max extraction. In 2015, a study showed that nicotinamine isolated from soybeans could strongly inhibit the enzymatic activity of ACE2, and that the derived inhibitor ACE2iSB could inhibit ACE2 with an IC50 value of 84 nM (Takahashi, Yoshiya, Yoshizawa‐Kumagaye, & Sugiyama, 2015). Since the SARS‐COV‐2 S spike protein recognizes the ACE2 receptor of the host cell for infection, nicotinamine may have potential anti‐SARS‐COV‐2 effects. Molecular docking results have demonstrated that nicotinamine may block infection of SARS‐COV‐2 by inhibiting ACE2 (Chen & Du, 2020). Chlorogenic acid is the main extract of Lonicera japonica Thunb. There has been evidence that chlorogenic acid presents antiinflammatory and immunomodulatory effects on COVID‐19 and is a promising drug to inhibit SARS‐COV‐2 by targeting the ACE2 site. A molecular docking experiment of ACE2 protein showed that chlorogenic acid binds stably with Gln42/Asp38 of ACE2, such that it can effectively hinder the interaction between the S protein and ACE2 (Yu, Wang, & Bao, 2020).
FIGURE 7.
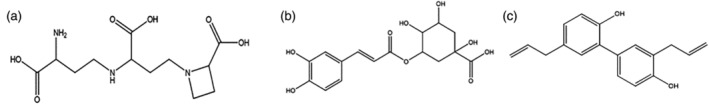
Active natural products that target ACE2 or Furin (a) nicotinamine, (b)chlorogenic acid, and (c) honokiol
6.5.2. Natural products that target Furin: Honokiol
The membrane fusion of COVID‐19 is highly dependent on the Furin protease and TMPRSS2 of the host cell. Therefore, furin protease can be a target for COVID‐19 drug development. Honokiol is the main active ingredient extracted from the root of Houpoea officinalis, which has antiinflammatory and antibacterial effects. A molecular docking experiment showed that honokiol may target and inhibit furin protease (Tanikawa, Hayashi, Suzuki, Kitamura, & Inoue, 2021). An in vitro experiment also indicated that honokiol prevents SARS‐COV‐2 from invading Vero cells (Tanikawa, Hayashi, Suzuki, Kitamura, & Inoue, 2021).
We have summarized the promising natural products that can target to inhibit SARS‐COV‐2 and analyzed the related clinical and preclinical studies. The mechanism and research processes are detailed in Figure 8 and Table 3. Among these natural products, Baicalin, Kaempferol, Quercetin, Rutin, Curcumin, Nicotinamine, Chlorogenic acid, Glycyrrhizin, EGCG, and Honokiol, can target ACE2, Furin protease, and GRP78 of host cells, thereby preventing SARS‐COV‐2 from invading host cell and membrane fusion. Betulinic Acid, Berberine, Β‐sitosterol, Indirubin, Cordycepin, and Curcumin can inhibit the activity of 3CLpro or RdRp to block the replication of SARS‐COV‐2. Myricetin and Acid Phenethyl Ester can target to bind to NSP15 and avoid SARS‐COV‐2 escaping from the RNA immune recognition mechanism. In addition, most of these natural products also function as antiinflammatories. In conclusion, further optimization of these natural product structures can be considered to develop specific monomeric drugs against SARS‐COV‐2. To improve upon drug combination therapy, which has already proved effective in treating SARS‐COV‐2, it will be necessary to develop multi‐target drugs for further clinical trials.
FIGURE 8.
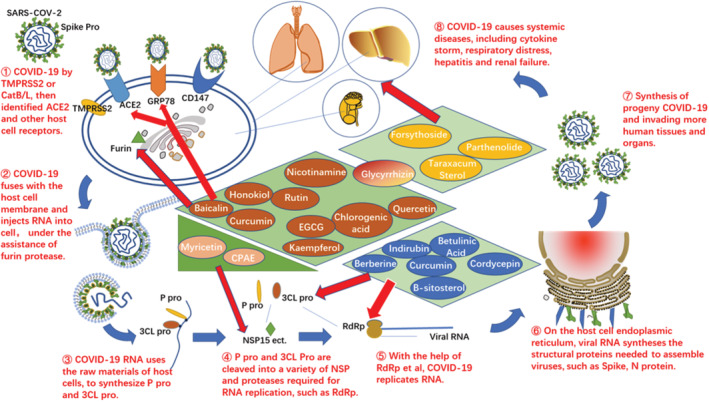
Targeting effects of natural products on COVID‐19 infecting host cells at different stages
7. NATURAL ACTIVE PRODUCTS THAT INHIBIT INFLAMMATION AND CYTOKINE STORM
The high mortality rate of SARS‐COV‐2 is mainly linked to its rapid development of inflammation in multiple organs after infection. The severe manifestation of COVID‐19 pneumonia is a systemic disease, which involves a cytokine storm affecting the respiratory system, liver, kidneys, conjunctiva, and digestive tract. Various Chinese medicines for heart‐clearing and detoxification have been applied in the clinical therapy of inflammatory reactions caused by COVID‐19. The main mechanisms natural products apply to inhibit inflammatory reactions include: inhibiting the release of inflammatory cytokines by restraining vasoactive amines caused by arachidonic acid (AA); decreasing the transcription factors and pro‐inflammatory factors related to the inflammatory signaling pathway; and reducing the activity of mitogen‐activated protein kinase and concentration of nitric oxide in vivo. Among various pro‐inflammatory factors, IL‐6 is recognized as the main cause of cytokine storms. Natural products extracted from licorice, forsythia, honeysuckle, houttuynia, and taraxacum, have antagonistic effects on various pro‐inflammatory factors, including IL‐6. Thus, they are promising antiinflammatory drugs.
7.1. Glycyrrhizin (GA)
7.1.1. Preclinical study of glycyrrhizin inhibiting SARS‐COV‐2
Glycyrrhizin, also known as glycyrrhizic acid, is the main component of Radix Glycyrrhiza extract. Its structure is shown in Figure 9. Glycyrrhizin inhibits pro‐inflammatory factors and inflammatory responses through multiple targets. For example, in a rat osteoarthritis model, glycyrrhizin inhibited high mobility group protein B1 (HMGB1) and TLR4/NF‐κB signaling pathway and reduced the level of inflammatory mediators IL‐1 β, IL‐6, TNF IX, and nitric oxide synthase (iNOS) (Luo et al., 2021). Glycyrrhizin has also shown a specific effect on KSHV, MERS, and other viruses (Sun et al., 2013). In a previous study of COVID‐19 inhibitors, glycyrrhizin was not only found to inhibit the expression levels of inflammatory‐related genes but also to mimic the mechanism of interferon I, and significantly inhibit the replication of SARS‐COV‐2 in Vero cells, with IC50 value of 2.39 μm (Zhu et al., 2020). Glycyrrhizin was also found to dock to ACE2 (Ahmad, Waheed, Abro, Abbasi, & Ismail, 2021) and SARS‐COV‐2's S protein (Li et al., 2021). The evidence indicates that glycyrrhizin may be used as a COVID‐19 inhibitor, by targeting the key proteases that relate to COVID‐19 invasion and replication. Moreover, for the symptoms of critical COVID‐19 patients which have ranged from pulmonary infection to systemic inflammation, glycyrrhizin may also be a promising adjuvant drug to prevent systemic deterioration.
FIGURE 9.
Natural active products inhabit cellular inflammatory storm (a) forsythiaside A, (b) parthenolide, (c) taraxacum sterol, and (d) glycyrrhizin
7.1.2. Clinical study of glycyrrhizin
Severely ill patients with COVID‐19 always have abnormal liver function and acute liver injury. The antiinflammation and anti‐SARS‐COV effects of glycyrrhizin have been verified in clinical studies. In terms of antiinflammatory, glycyrrhizin is used to treat viral hepatitis and chronic hepatitis B. For example, in a controlled clinical trial of 90 patients with hepatitis B, the treatment group with compound glycyrrhizic acid based on adefovir dipivoxil had a significantly higher total effective rate than that of the control group (93.33% vs. 68.89%). Further, the levels of TGF‐β1 and TNF‐α were reduced, while liver function and fibrosis were significantly improved (Wang, Luo, & Chen, 2018). The results of another study also proved that compound glycyrrhizin had a significant inhibitory effect on the TNF‐α and IL‐6 levels of the inflammatory factor in patients with chronic hepatitis B (p < 0.05) (Fang & Shuan‐lin, 2009). In addition, glycyrrhizin also treats other inflammation caused by bacteria, fungi, and viruses (Peng, Li, & Li, 2011)—especially the SARS virus. In a clinical report of 102 patients with SARS, 27 cases of the observation group were treated with compound glycyrrhizin (160 mg·d−1). The results showed that the compound glycyrrhizin had protective effects on the liver and improved chest tightness, cough, and pain (p < 0.01) (Cai, Zhang, & Zhang, 2004). Liao et al. have published clinical reports in which 59 SARS‐COV‐2 patients received diammonium glycyrrhizinate (DG) treatment and the serum ALT and AST of mild and severe patients tended to return to normal levels after DG treatment. In contrast, the serum ALT and AST did not decrease significantly in severe COVID‐19 patients (Liao et al., 2021). In conclusion, glycyrrhizin and its derivatives have the potential to reduce the clinical symptoms of moderate or severe COVID‐19 patients, such as cytokine storm, renal failure, and abnormal liver function.
7.2. Forsythoside
Forsythoside is an active ingredient extracted from Forsythia suspensa with heat‐clearing and detoxification functions. The structure of forsythoside can be seen in Figure 9 a. In a rat model, forsythiaside inhibited cigarette‐induced pneumonia by activating Nrf2 and inhibiting NF kappa from reducing the infiltration of pulmonary inflammatory cells and inflammatory factor TNF‐α, IL‐6, and IL‐1 β (p < 0.01) (Cheng, Li, Ma, & Hu, 2015). Forsythiaside A also effectively inhibited the number of neutrophils and the levels of TNF‐α and IL‐6, by activating NF‐kappa B in the abdominal cavity of peritoneal mice (Zhang, Ding, Kang, Zhang, & Zhang, 2018). In addition, in preclinical studies on avian infectious bronchitis virus, forsythiaside A acted to inhibit the virus and the inflammatory response by regulating the balance of CD3+ CD4+\CD8+ (Li et al., 2011; Wang et al., 2021). These suggest that Forsythoside may play a role against inflammation caused by SARS‐COV‐2.
7.3. Parthenolide and taraxacum sterol
Structures of natural products parthenolide and taraxacum sterol are shown in Figure 9 b and Figure 9 c. Parthenolide is a semiterpene lactone natural product. It is mainly found in Tanacetum vulgare L. Parthenolide can inhibit the activation of the transcription factor NF‐κB (Hui‐qiong, 2009) and the production of IL‐1, IL‐2, IL‐6, and TNF‐α in the human cell line model(Bahrami, Kamalinejad, Latifi, Seif, & Dadmehr, 2020). In addition, the parthenolide‐5 obtained by the epoxidation of parthenolide has a high affinity to 3CLpro (Ouled Aitouna et al., 2021). Since cytokine storm caused by COVID‐19 is the leading cause of complications in the lung and other organs, parthenolide can be used as a potential drug for the therapy of SARS‐COV‐2. The possible mechanism of taraxacum sterol inhibiting COVID‐19 includes blocking the MAPK pathway caused by phosphorylation, preventing pulmonary fibrosis by inhibiting the NF‐κB pathway, and regulating the balance between immune and inflammatory factors. In the treatment of various kinds of infections, taraxacum sterol can significantly reduce the production of the inflammatory factors interleukin‐1 β, TNF‐α,IL‐4, IL‐6, IFN‐γ, as well as regulate the immune levels (Piao, Ma, Li, & Liu, 2015; Xu et al., 2018; Zhang, Xiong, Li, & Cheng, 2014). In liver‐injured mice, taraxacum sterol significantly inhibits CYP2E1, increases Nrf2/HO‐1, and decreases the expression level of NF‐κB p65 in liver tissues (Xu et al., 2018). The above results suggest that taraxerol sterol may have good potential for treating multiple inflammatory responses and liver injury caused by COVID‐19 infection.
In general, natural products such as glycyrrhizin, parthenocissus, and dandelion can inhibit the secondary infection of COVID‐19 by inhibiting inflammatory cytokines such as IL‐6 and regulating inflammatory pathways. These natural products can be used as supplement compounds. However, the specific efficacy needs further clinical verification.
8. IMPORTANT TARGETS AND ACTIVE INGREDIENTS IN TCM PRESCRIPTIONS
During the COVID‐19 pandemic, China first approved six TCM prescriptions and decoction for clinical treatment, which have achieved good results. Several effective TCM prescriptions and their important targets for COVID‐19 are shown in Table 4. For example, Pneumonia No.1 has certain advantages in improving cough and pharyngeal discomfort, antipyretic, and negative nucleic acid. In Pneumonia No.1 plus routine western medicine clinical treatment for COVID‐19, the main symptoms of 69 patients, including fever, cough, and fatigue, significantly improved. The area of the lesions in 60 cases of lung CT showed diminution and thinning (86.96%) (Ba et al., 2020). Molecular docking experiments of Pneumonia No.1 showed that 34 core natural products can be used for treating SARS‐COV‐2. Among them, β‐sitosterol, wogonin, and locustin showed the ability to bind to 3CLpro. Qingfei Paidu Decoction is another effective prescription. It was launched in clinical trials in four provinces, with an initial effective rate of over 90% (Zhao, Tian, Yang, Liu, & Zhang, 2020). The four active natural ingredients with the most targets in Qingfei Paidu Decoction are baicalin, glycyrrhizic acid, hesperidin, and hyperoside. The involved targets mainly included Threonine Kinase 1(AKT1), TNF‐α, IL6, prostaglandin‐Endoperoxide Synthase 2 (PTGS2), heme oxygenase 1 (HMOX1), IL10, and Tumor Protein P53 (TP53). (Zhao et al., 2021), which relates to the immune system and inflammation. Lianhua Qingwen Capsule (LQC) also had a better intervention effect on pneumonia caused by COVID‐19. The natural products kaempferol, quercetin, and luteolin in LQC can bind to 3CLpro. While glycyrrhetinic acid, stigmasterol, and indigo target ACE2 (Ling, Tao, Sun, & Yuan, 2020). Core targets in LQC also bind well to PTGS2, IL6, Caspase 3, Mitogen‐Activated Protein Kinase 1 (MAPK1), epidermal growth factor receptor (EGFR), and TNF targets, suggesting that LQC can treat COVID‐19 via multiple targets (Ling, Tao, Sun, & Yuan, 2020). In 2020, Zhong Nanshan and his team conducted a randomized controlled experiment on 284 COVID‐19 patients, which showed that, compared with conventional treatment, the 14‐day clinical symptom recovery rate of patients receiving LQC was significantly improved to 91% (Su et al., 2020b).
TABLE 4.
The main natural active ingredients and targets of anticoronavirus traditional Chinese medicine prescription
| Name | Elements | Important ingredients | Intervention target/path | Clinical effects |
| Pneumonia No.1 | Radix Bupleuri, Scutellariae, Radix Pinellia Tuber, Codonopsis, Radix Fructus Trichosanthis, Areca catechu L, Fructus Tsaoko, Magnoliae Officmalis, Anemarrhenae Rhizoma, common Peony licorice, Tangerine Peel, and Rhizoma polygoni cuspidat | Glycyrrhizin, Baicalein, β‐Sitosterol, 7‐Acetyloxy‐2‐Methyl Isoflavones, Locustin, and Scutellarin (Liang et al., 2020) | HSP90AA1, CASP8, FASLG, CYP19A1, and MAPK14 (Liang et al., 2020) | Improve the clinical symptoms of SARS‐COV‐2 infection, improve immunity, and reduce inflammation |
| Qingfei‐Paidu Decoction | Ephedra, licorice, Almond, Gypsum, Cassia Twig, Alisma, Polyporus, Atractylodes, Poria, Bupleurum, Scutellaria, Ginger Pinellia, Ginger, Aster Tataricus Coltsfoot Flower, Belamcandae Rhizoma, Asarum, Chinese Yam, Citrus aurantium and Tangerine Peel, Agastache | Baicalin, Glycyrrhizic acid, Hesperidin,Hyperoside. | AKT1, TNF‐α, IL6, PTGS2,HMOX1,IL10 & TP53 (Zhao et al., 2021) | Targeted inhibit virus replication and avoid host cell inflammation storm |
| LQC | Forsythia, Honeysuckle, Roasted Ephedra, Sauteed Bitter Almonds, Gypsum, Radix Isatidis, Mianma Guanzhong, Houttuynia Cordata, Patchouli, Rhubarb, Rhodiola, Menthol, Licorice, Starch | Kaempferol, Quercetin, Luteolin, Glycyrrhetinic Acid, and Stigmasterol, Indigo | PTGS2, IL6, CASP3, MAPK1, EGFR, TNF (Ling, Tao, Sun, & Yuan, 2020) | LQC |
9. SUMMARY AND PROSPECT
In this paper, based on infection and transmission of SARS‐COV‐2, the development of anti‐COVID‐19 drugs has been discussed. Since mutant strains such as Delta and Omicron have become more infectious and yielded breakthrough infections, current vaccines and specific drugs have had limited effect on SARS‐COV‐2 or systemic diseases. Therefore, specific drugs with better potency and safety still need to be developed. In silico screening and molecular docking research have shown that natural active products can bind to COVID‐19's key invasion and replication proteins, which have demonstrated a specific anti‐COVID‐19 effect in vitro. Glycyrrhizic acid can target S‐Spike proteins, where betulinic acid, β‐sitosterol, berberine, and indirubin, have virus 3CLpro targeted docking sites that may play a role in inhibiting virus replication. Curcumin and cordycepin target RdRp, and myricetin may prevent the virus from escaping RNA immunity by inhibiting NSP15 activity. Chlorogenic acid, EGCG, and honokiol can competitively bind to Furin, ACE2, or GRP78 of host cells and inhibit virus invasion and membrane fusion. In addition, polyphenols and natural flavonoid products may serve to be promising multiple‐target drugs. Clinical evidence also hints that these natural products may play a role in inhibiting cytokine storm and immune runaway caused by COVID‐19.
Natural products such as EGCG, APS, caffeic acid, and resveratrol, may intervene in the clinical immune disorder caused by SARS‐COV‐2, by improving the numbers of NK cells and lymphocytes, or by regulating the balance of Th1/Th2 and the ratio of CD4+/CD8+. The critical reactions of COVID‐19 are immune dysfunction, systemic inflammation, and respiratory distress, where the surge of cytokines represented by IL‐6 is one of the main reasons. Natural products such as glycyrrhizin and parthenocissus not only have the potential to inhibit COVID‐19 proteins but can also act on multiple pathways and targets. Glycyrrhizin is particularly effective in treating SARS coronavirus and the treatment of acute inflammation.
The structure, molecular mechanism, and clinical research results of these natural active products have been reviewed in detail. This not only provided reference to precise screening, structural optimization, and drug‐likeness for developing COVID‐19‐specific drugs but also considered the clinical treatment of COVID‐19 from multiple perspectives such as prevention and prognosis. EGCG, resveratrol, and other natural products with immune regulation, may be used as immune enhancers for the COVID‐19 vaccine. Natural active products have also been seen to perform well in targeting, to inhibit both COVID‐19‐related proteins and inflammatory signal pathway‐related factors. As drug combination therapy has been proved to be more effective in the clinical treatment of SARS‐COV‐2, and the structure of natural active products is complex, it is advisable to consider developing these multi‐target natural products as anti‐COVID‐19 drugs. The performance of Chinese herbal compound decoction in the clinical treatment of COVID‐19 may provide a theoretical basis for this strategy. However, most current experiments are limited to the HST screening and in vitro experiments. There is still a lack of animal and clinical trials to provide evidence for the potential multi‐pathway inhibition effects of these natural products. To better enhance the targeting inhibition effect, new research should make full use of bioinformatic methods to study the safety and clinical potency of these natural products and optimize their structure. We look forward to developing SARS‐COV‐2 drugs/compounds or vaccine immune enhancers with higher potency based on more clinical evidence and careful research.
AUTHOR CONTRIBUTIONS
Conceptualization: X. J., X. H., and Y. Z.; literature collection: Y. Z.; writing—original draft preparation: Y.Z.; writing—review and editing: X. H., J. G., G. K., S. D., Y. Z., and Y. B.; funding acquisition: X. J. and X. H; and supervision: X. J., X. H., J. G., and G. K. All authors have read and agreed to the published version of the manuscript.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
INFORMED CONSENT STATEMENT
None.
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (Nos. 32170119 and 31870135), Sichuan Province Miaozi Program (No. 2020110), and the Fundamental Research Funds for the Central Universities of China (No. 2682020ZT97).
Zhao, Y. , Deng, S. , Bai, Y. , Guo, J. , Kai, G. , Huang, X. , & Jia, X. (2022). Promising natural products against SARS‐CoV‐2: Structure, function, and clinical trials. Phytotherapy Research, 1–26. 10.1002/ptr.7580
Funding information
This review was funded by the National Natural Science Foundation of China (Nos. 32170119 and 31870135), Sichuan Province Miaozi Program (No. 2020110), and the Fundamental Research Funds for the Central Universities of China (No. 2682020ZT97).
Funding information Fundamental Research Funds for the Central Universities of China, Grant/Award Number: No. 2682020ZT97; National Natural Science Foundation of China, Grant/Award Numbers: No. 31870135, No. 32170119; Sichuan Province Miaozi Program, Grant/Award Number: No. 2020110
Contributor Information
Guoyin Kai, Email: guoyinkai1@126.com.
Xinhe Huang, Email: xinhehuang@swjtu.edu.cn.
Xu Jia, Email: jiaxu@cmc.edu.cn.
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- Ackermann, M. , Verleden, S. E. , Kuehnel, M. , Haverich, A. , Welte, T. , Laenger, F. , … Jonigk, D. (2020). Pulmonary vascular Endothelialitis, thrombosis, and angiogenesis in Covid‐19. New England Journal of Medicine, 383(2), 120–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adem, S. , Eyupoglu, V. , Sarfraz, I. , Rasul, A. , Zahoor, A. F. , Ali, M. , … Elfiky, A. A. (2021). Caffeic acid derivatives (CAFDs) as inhibitors of SARS‐CoV‐2: CAFDs‐based functional foods as a potential alternative approach to combat COVID‐19. Phytomedicine, 85, 153310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad, S. , Waheed, Y. , Abro, A. , Abbasi, S. W. , & Ismail, S. (2021). Molecular screening of glycyrrhizin‐based inhibitors against ACE2 host receptor of SARS‐CoV‐2. Journal of Molecular Modeling, 27(7), 206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al‐Rashedi, N. A. M. , Munahi, M. G. , & Ah, A. L. (2020). Prediction of potential inhibitors against SARS‐CoV‐2 endoribonuclease: RNA immunity sensing. Journal of Biomolecular Structure & Dynamics, 40(11), 4879‐4892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand, K. , Ziebuhr, J. , Wadhwani, P. , Mesters, J. R. , & Hilgenfeld, R. (2003). Coronavirus main proteinase (3CL[pro]) structure: Basis for design of anti‐SARS drugs. Science, 300(5626), 1763–1767. [DOI] [PubMed] [Google Scholar]
- Anusuya, S. , & Gromiha, M. M. (2017). Quercetin derivatives as non‐nucleoside inhibitors for dengue polymerase: Molecular docking, molecular dynamics simulation, and binding free energy calculation. Journal of Biomolecular Structure & Dynamics, 35(13), 2895–2909. [DOI] [PubMed] [Google Scholar]
- Ba, Y. , Li, W. , Wang, L. , Zuo, X. , Shi, Q. , Shen, Y. , … Jiang, N. (2020). Clinical efficacy study on Corona virus disease 2019 treated with 'Pneumonia no.1 Formula'. China Journal of Traditional Chinese Medicine and Pharmacy, 35(4), 2178–2181. [Google Scholar]
- Bae, H.‐B. , Li, M. , Kim, J.‐P. , Kim, S.‐J. , Jeong, C.‐W. , Lee, H.‐G. , … Kwak, S.‐H. (2010). The effect of epigallocatechin Gallate on lipopolysaccharide‐induced acute lung injury in a murine model. Inflammation, 33(2), 82–91. [DOI] [PubMed] [Google Scholar]
- Bahrami, M. , Kamalinejad, M. , Latifi, S. A. , Seif, F. , & Dadmehr, M. (2020). Cytokine storm in COVID‐19 and parthenolide: Preclinical evidence. Phytotherapy Research, 34(10), 2429–2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibi, S. , Hasan, M. M. , Wang, Y.‐B. , Papadakos, S. P. , & Yu, H. (2022). Cordycepin as a promising inhibitor of SARS‐CoV‐2 RNA dependent RNA polymerase (RdRp). Current Medicinal Chemistry, 29(1), 152–162. [DOI] [PubMed] [Google Scholar]
- Cai, L. , Zhang, T. , & Zhang, M. (2004). Observation of the efficacy of compound glycyrrhizic acid in treatment of SARS. Chinese Journal New Drugs, 13(9), 842–845. [Google Scholar]
- Cantuti‐Castelvetri, L. , Ojha, R. , Pedro, L. D. , Djannatian, M. , Franz, J. , Kuivanen, S. , … Simons, M. (2020). Neuropilin‐1 facilitates SARS‐CoV‐2 cell entry and infectivity. Science, 370(6518), 856–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman, R. L. , & Andurkar, S. V. (2022). A review of natural products, their effects on SARS‐CoV‐2 and their utility as lead compounds in the discovery of drugs for the treatment of COVID‐19. Medicinal Chemistry Research, 31(1), 40–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., & Du Q. (2020). Potential natural compounds for preventing 2019‐nCoV infection. Preprints. 2020:2020010358. 10.20944/preprints202001.0358.v3 [DOI]
- Chen, J. , Wang, R. , Gilby, N. B. , & Wei, G.‐W. (2021). Omicron (B.1.1.529): Infectivity, vaccine breakthrough, and antibody resistance. ArXiv, 62, 412–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Y. , Wang, X. , Zhu, Y. , Si, L. , Zhang, B. , Zhang, Y. , … Xiao, S. (2020). Synthesis of a hexavalent Betulinic acid derivative as a hemagglutinin‐targeted influenza virus entry inhibitor. Molecular Pharmaceutics, 17(7), 2546–2554. [DOI] [PubMed] [Google Scholar]
- Cheng, L. , Li, F. , Ma, R. , & Hu, X. P. (2015). Forsythiaside inhibits cigarette smoke‐induced lung inflammation by activation of Nrf2 and inhibition of NF‐kappa B. International Immunopharmacology, 28(1), 494–499. [DOI] [PubMed] [Google Scholar]
- Choi, H.‐J. , Kim, J.‐H. , Lee, C.‐H. , Ahn, Y.‐J. , Song, J.‐H. , Baek, S.‐H. , & Kwon, D.‐H. (2009). Antiviral activity of quercetin 7‐rhamnoside against porcine epidemic diarrhea virus. Antiviral Research, 81(1), 77–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cialdella‐Kam, L. , Nieman, D. C. , Knab, A. M. , Shanely, R. A. , Meaney, M. P. , Jin, F. , … Ghosh, S. (2016). A mixed flavonoid‐fish oil supplement induces immune‐enhancing and anti‐inflammatory transcriptomic changes in adult obese and overweight women‐a randomized controlled trial. Nutrients, 8(5), 277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Arena, G. , Simeon, V. , De Martino, L. , Statuto, T. , D’Auria, F. , Volpe, S. , … De Feo, V. (2013). Regulatory T‐cell modulation by green tea in chronic lymphocytic leukemia. International Journal of Immunopathology and Pharmacology, 26(1), 117–125. [DOI] [PubMed] [Google Scholar]
- de Ligt, M. , Hesselink, M. K. C. , Jorgensen, J. , Hoebers, N. , Blaak, E. E. , & Goossens, G. H. (2021). Resveratrol supplementation reduces ACE2 expression in human adipose tissue. Adipocytes, 10(1), 408–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehghani, F. , Jandaghi, S. , Janani, L. , Sarebanhassanabadi, M. , Emamat, H. , & Vafa, M. (2021). Effects of quercetin supplementation on inflammatory factors and quality of life in post‐myocardial infarction patients: A double blind, placebo‐controlled, randomized clinical trial. Phytotherapy Research, 35(4), 2085–2098. [DOI] [PubMed] [Google Scholar]
- Deng, X. , Garcia‐Knight, M. A. , Khalid, M. M. , Servellita, V. , Wang, C. , Morris, M. K. , … Chiu, C. Y. (2021). Transmission, infectivity, and neutralization of a spike L452R SARS‐CoV‐2 variant. Cell, 184(13), 3426–3437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng, Q. , Xu, J. , Yu, B. , He, J. , Zhang, K. , Ding, X. , & Chen, D. (2010). Effect of dietary tea polyphenols on growth performance and cell‐mediated immune response of post‐weaning piglets under oxidative stress. Archives of Animal Nutrition, 64(1), 12–21. [DOI] [PubMed] [Google Scholar]
- Di Pierro, F. , Derosa, G. , Maffioli, P. , Bertuccioli, A. , Togni, S. , Riva, A. , … Ahmed, S. (2021). Possible therapeutic effects of adjuvant quercetin supplementation against early‐stage COVID‐19 infection: A prospective, randomized, controlled, and open‐label study. International Journal of General Medicine, 14, 2359–2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du, A. , Zheng, R. , Disoma, C. , Li, S. , Chen, Z. , Li, S. , … Xia, Z. (2021). Epigallocatechin‐3‐gallate, an active ingredient of traditional Chinese medicines, inhibits the 3CLpro activity of SARS‐CoV‐2. International Journal of Biological Macromolecules, 176, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Españo, E. , Kim, J. , Lee, K. , & Kim, J. K. (2021). Phytochemicals for the treatment of COVID‐19. Journal of Microbiology, 59(11), 959–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang, W. , & Shuan‐lin, J. (2009). Effect of compound glycyrrhizin injection on the treatment of patients with chronic hepatitis B and influence to serum TNF‐a and IL‐6. Chinese Journal of Hospital Pharmacy, 29(1), 49–51. [Google Scholar]
- Feng, Z. , Gui‐wen, Y. , Fu‐miao, Z. , Jing, Z. , Ying‐jie, C. U. I. , & Li‐guo, A. N. (2009). Study on the effects of Green tea extract and EGCG on immune cells. Lishizhen Medicine and Materia Medica Research, 20(5), 1094–1095. [Google Scholar]
- Feng, W. , Liu, G. F. , & Qin, J. B. (2021). Ginkgo biloba Damo injection combined with troxerutin regulates the TLR4/NF‐kappa B pathway and promotes the recovery of patients with acute cerebral infarction. American Journal of Translational Research, 13(4), 3344–3350. [PMC free article] [PubMed] [Google Scholar]
- Gadanec, L. K. , McSweeney, K. R. , Qaradakhi, T. , Ali, B. , Zulli, A. , & Apostolopoulos, V. (2021). Can SARS‐CoV‐2 virus use multiple receptors to enter host cells? International Journal of Molecular Sciences, 22(3), 992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao, L. , Yuan, Y. , Lu, Z. , Fan, G. , Wang, J. , & Ren, H. (2003). The immunomodulatory function of resveratrol. Journal of Xi’an Jiaotong University.Medical Sciences, 24(2), 121–123. [Google Scholar]
- Gardi, C. , Bauerova, K. , Stringa, B. , Kuncirova, V. , Slovak, L. , Ponist, S. , … Russo, G. L. (2015). Quercetin reduced inflammation and increased antioxidant defense in rat adjuvant arthritis. Archives of Biochemistry and Biophysics, 583, 150–157. [DOI] [PubMed] [Google Scholar]
- Genovese, C. , Davinelli, S. , Mangano, K. , Tempera, G. , Nicolosi, D. , Corsello, S. , … Di Marco, R. (2018). Effects of a new combination of plant extracts plus d‐mannose for the management of uncomplicated recurrent urinary tract infections. Journal of Chemotherapy, 30(2), 107–114. [DOI] [PubMed] [Google Scholar]
- Ghosh, R. , Chakraborty, A. , Biswas, A. , & Chowdhuri, S. (2021). Evaluation of green tea polyphenols as novel corona virus (SARS CoV‐2) main protease (Mpro) inhibitors ‐ anin silicodocking and molecular dynamics simulation study. Journal of Biomolecular Structure & Dynamics, 39(12), 4362–4374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guruvayoorappan, C. , & Kuttan, G. (2007). Rutin inhibits nitric oxide and tumor necrosis factor‐alpha production in lipopolysaccharide and concanavalin‐a stimulated macrophages. Drug Metabolism and Drug Interactions, 22(4), 263–278. [DOI] [PubMed] [Google Scholar]
- Halegoua‐De Marzio, D. , Kraft, W. K. , Daskalakis, C. , Ying, X. , Hawke, R. L. , & Navarro, V. J. (2012). Limited sampling estimates of epigallocatechin gallate exposures in cirrhotic and noncirrhotic patients with hepatitis C after single oral doses of green tea extract. Clinical Therapeutics, 34(12), 2279–2285.e2271. [DOI] [PubMed] [Google Scholar]
- Heinz, S. A. , Henson, D. A. , Austin, M. D. , Jin, F. , & Nieman, D. C. (2010). Quercetin supplementation and upper respiratory tract infection: A randomized community clinical trial. Pharmacological Research, 62(3), 237–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodon, J. , Borkova, L. , Pokorny, J. , Kazakova, A. , & Urban, M. (2019). Design and synthesis of pentacyclic triterpene conjugates and their use in medicinal research. European Journal of Medicinal Chemistry, 182, 111653. [DOI] [PubMed] [Google Scholar]
- Hoffmann, M. , Kleine‐Weber, H. , & Poehlmann, S. (2020). A multibasic cleavage site in the spike protein of SARS‐CoV‐2 is essential for infection of human lung cells. Molecular Cell, 78(4), 779–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann, M. , Kleine‐Weber, H. , Schroeder, S. , Krueger, N. , Herrler, T. , Erichsen, S. , … Poehlmann, S. (2020). SARS‐CoV‐2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell, 181(2), 271–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong, S. , Seo, S. H. , Woo, S.‐J. , Kwon, Y. , Song, M. , & Ha, N.‐C. (2021). Epigallocatechin Gallate inhibits the Uridylate‐specific endoribonuclease Nsp15 and efficiently neutralizes the SARS‐CoV‐2 strain. Journal of Agricultural and Food Chemistry, 69(21), 5948–5954. [DOI] [PubMed] [Google Scholar]
- Hsu, C. L. , & Yen, G. C. (2008). Phenolic compounds: Evidence for inhibitory effects against obesity and their underlying molecular signaling mechanisms (vol 52, pg 53, 2008). Molecular Nutrition & Food Research, 52(5), 624–625. [DOI] [PubMed] [Google Scholar]
- Huang, A.‐C. , Cheng, H.‐Y. , Lin, T.‐S. , Chen, W.‐H. , Lin, J.‐H. , Lin, J.‐J. , … Chung, J.‐G. (2013). Epigallocatechin Gallate (EGCG), influences a murine WEHI‐3 leukemia model in vivo through enhancing phagocytosis of macrophages and populations of T‐ and B‐cells. In Vivo, 27(5), 627–634. [PubMed] [Google Scholar]
- Hui‐qiong, Z. (2009). Progress in research of parthenolide. Chinese Journal New Drugs, 18(20), 1954–1957. [Google Scholar]
- Huynh, T., Wang, H. , Cornell, W. , & Luan, B. (2020). In silico exploration of repurposing and optimizing traditional Chinese medicine Rutin for possibly inhibiting SARS‐CoV‐2's Main protease. ChemRxiv, 10.26434/chemrxiv.12281078.v1 [DOI] [Google Scholar]
- Javadi, F. , Ahmadzadeh, A. , Eghtesadi, S. , Aryaeian, N. , Zabihiyeganeh, M. , Foroushani, A. R. , & Jazayeri, S. (2017). The effect of quercetin on inflammatory factors and clinical symptoms in women with rheumatoid arthritis: A double‐blind, randomized controlled trial. Journal of the American College of Nutrition, 36(1), 9–15. [DOI] [PubMed] [Google Scholar]
- Jayk Bernal, A. , Gomes da Silva, M. M. , Musungaie, D. B. , Kovalchuk, E. , Gonzalez, A. , Delos, R. V. , … De Anda, C. (2022). Molnupiravir for Oral treatment of Covid‐19 in nonhospitalized patients. The New England Journal of Medicine, 386(6), 509–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin, W. (2011). Protective effect of resveratrol on LPS‐inducing mice acute lung in‐flammation. Chinese Journal of Clinical Pharmacology and Therapeutics, 16(12), 1352–1356. [Google Scholar]
- Jine, Y. , Lis, M. , Szczypka, M. , & Obminska‐Mrukowicz, B. (2012). Influence of betulinic acid on lymphocyte subsets and humoral immune response in mice. Polish Journal of Veterinary Sciences, 15(2), 305–313. [DOI] [PubMed] [Google Scholar]
- Kang, H. J. , Baik, H. W. , Kim, S. J. , Lee, S. G. , Ahn, H. Y. , Park, J. S. , … Lee, S. M. (2015). Cordyceps militaris enhances cell‐mediated immunity in healthy Korean men. Journal of Medicinal Food, 18(10), 1164–1172. [DOI] [PubMed] [Google Scholar]
- Kempf, K. , Herder, C. , Erlund, I. , Kolb, H. , Martin, S. , Carstensen, M. , … Tuomilehto, J. (2010). Effects of coffee consumption on subclinical inflammation and other risk factors for type 2 diabetes: A clinical trial. American Journal of Clinical Nutrition, 91(4), 950–957. [DOI] [PubMed] [Google Scholar]
- Khan, A. , Zia, T. , Suleman, M. , Khan, T. , Ali, S. S. , Abbasi, A. A. , Mohammad, A. , & Wei, D. Q. (2021). Higher infectivity of the SARS‐CoV‐2 new variants is associated with K417N/T, E484K, and N501Y mutants: An insight from structural data. Journal of Cellular Physiology, 236(10), 7045‐7057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khursheed, R. , Singh, S. K. , Wadhwa, S. , Gulati, M. , & Awasthi, A. (2020). Enhancing the potential preclinical and clinical benefits of quercetin through novel drug delivery systems. Drug Discovery Today, 25(1), 209–222. [DOI] [PubMed] [Google Scholar]
- Kilani‐Jaziri, S. , Mokdad‐Bzeouich, I. , Krifa, M. , Nasr, N. , Ghedira, K. , & Chekir‐Ghedira, L. (2017). Immunomodulatory and cellular anti‐oxidant activities of caffeic, ferulic, and p‐coumaric phenolic acids: A structure‐activity relationship study. Drug and Chemical Toxicology, 40(4), 416–424. [DOI] [PubMed] [Google Scholar]
- Kim, H. , Kong, H. S. , Choi, B. , Yang, Y. W. , Kim, Y. , Lim, M. J. , … Jung, Y. J. (2005). Metabolic and pharmacological properties of rutin, a dietary quercetin glycoside, for treatment of inflammatory bowel disease. Pharmaceutical Research, 22(9), 1499–1509. [DOI] [PubMed] [Google Scholar]
- Koval’skii, I. V. , Krasnyuk, I. I. , Krasnyuk, J. I. I. , Nikulina, O. I. , Belyatskaya, A. V. , Kharitonov, Y. Y. , … Lutsenko, S. V. (2014). Pharmacological mechanisms of Rutin action (a review). Khimiko‐Farmatsevticheskii Zhurnal, 48(2), 3–6. [Google Scholar]
- Krishnamoorthy, S. , Swain, B. , Verma, R. S. , & Gunthe, S. S. (2020). SARS‐CoV, MERS‐CoV, and 2019‐nCoV viruses: An overview of origin, evolution, and genetic variations. Virus, 31(4), 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar, V. , Dhanjal, J. K. , Kaul, S. C. , Wadhwa, R. , & Sundar, D. (2021). Withanone and caffeic acid phenethyl ester are predicted to interact with main protease (M‐pro) of SARS‐CoV‐2 and inhibit its activity. Journal of Biomolecular Structure & Dynamics, 39(11), 3842–3854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La, X. , Zhang, L. , Li, Z. , Li, H. , & Yang, Y. (2019). (−)‐epigallocatechin Gallate (EGCG) enhances the sensitivity of colorectal cancer cells to 5‐FU by inhibiting GRP78/NF‐κB/miR‐155‐5p/MDR1 pathway. Journal of Agricultural and Food Chemistry, 67(9), 2510–2518. [DOI] [PubMed] [Google Scholar]
- Lai, J.‐l. , Liu, Y.‐H. , Peng, Y.‐C. , Ge, P. , He, C.‐F. , Liu, C. , … Hu, C.‐M. (2017). Indirubin treatment of lipopolysaccharide‐induced mastitis in a mouse model and activity in mouse mammary epithelial cells. Mediators of Inflammation, 2017, 3082805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, C. Y. J. , & Wan, J. M. F. (2000). Vitamin E supplementation improves cell‐mediated immunity and oxidative stress of Asian men and women. Journal of Nutrition, 130(12), 2932–2937. [DOI] [PubMed] [Google Scholar]
- Leischner, C. , Burkard, M. , Pfeiffer, M. M. , Lauer, U. M. , Busch, C. , & Venturelli, S. (2016). Nutritional immunology: Function of natural killer cells and their modulation by resveratrol for cancer prevention and treatment. Nutrition Journal, 15, 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Y.‐C. , Bai, W.‐Z. , & Hashikawa, T. (2020). The neuroinvasive potential of SARS‐CoV2 may play a role in the respiratory failure of COVID‐19 patients. Journal of Medical Virology, 92(6), 552–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Q. , Hu, J. , Gao, B. , Li, H. , Song, S. , & Yang, L. (2017). Advances on Immunoregulation effect of Astragalus polysaccharides. Chinese Journal of Experimental Traditional Medical Formulae, 23(2), 199–206. [Google Scholar]
- Li, H. , Wu, J. , Zhang, Z. , Ma, Y. , Liao, F. , Zhang, Y. , & Wu, G. (2011). Forsythoside a inhibits the avian infectious bronchitis virus in cell culture. Phytotherapy Research, 25(3), 338–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, J. , Xu, D. , Wang, L. , Zhang, M. , Zhang, G. , Li, E. , & He, S. (2021). Glycyrrhizic acid inhibits SARS‐CoV‐2 infection by blocking spike protein‐mediated cell attachment. Molecules, 26(20), 6090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang, P. , Zhou, Y. , Sun, M. , Huang, J. , Lu, Y. , Shi, J. , & Chang, Q. (2020). Exploring mechanism of Hubei pneumonia no.1 formula in treatment of coronavirus disease 2019 (COVID‐19) based on bioinformatics study. Shanghai Journal of Traditional Chinese Medicine, 54(09), 18–19. [Google Scholar]
- Liao, F. L. , Peng, D. H. , Chen, W. , Hu, H. N. , Tang, P. , Liu, Y. Y. , … Yao, T. (2021). Evaluation of serum hepatic enzyme activities in different COVID‐19 phenotypes. Journal of Medical Virology, 93(4), 2365–2373. [DOI] [PubMed] [Google Scholar]
- Ling, X. , Tao, J. , Sun, X. , & Yuan, B. (2020). Exploring material basis and mechanism of Lianhua Qingwen prescription against coronavirus based on network pharmacology. Chinese Traditional and Herbal Drugs, 51(7), 1723–1730. [Google Scholar]
- Liput, J. R. , Jordan, K. , Patadia, R. , & Kander, E. (2021). Warm autoimmune hemolytic anemia associated with asymptomatic SARS‐CoV‐2 infection. Cureus, 13(3), e14101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, J. , Bodnar, B. H. , Meng, F. , Khan, A. I. , Wang, X. , Saribas, S. , … Ho, W. (2021). Epigallocatechin gallate from green tea effectively blocks infection of SARS‐CoV‐2 and new variants by inhibiting spike binding to ACE2 receptor. Cell & Bioscience, 11(1), 168–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Q. , & Wang, X. (2020). Strategies for the development of drugs targeting novel coronavirus 2019‐nCoV. Acta Pharmaceutica Sinica, 55(2), 181–188. [Google Scholar]
- Liu, R. , Xu, F. , Si, S. , Zhao, X. , Bi, S. , & Cen, Y. (2017). Mitochondrial DNA‐induced inflammatory responses and lung injury in thermal injury rat model: Protective effect of epigallocatechin Gallate. Journal of Burn Care & Research, 38(5), 304–311. [DOI] [PubMed] [Google Scholar]
- Liu, H. , Ye, F. , Sun, Q. , Liang, H. , Li, C. , Li, S. , … Lai, L. (2021). Scutellaria baicalensis extract and baicalein inhibit replication of SARS‐CoV‐2 and its 3C‐like protease in vitro. Journal of Enzyme Inhibition and Medicinal Chemistry, 36(1), 497–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Y. , Yuan, J. , Guo, M. , Zhang, Y. , Lu, J. , & Cao, W. (2021). Effect of astragalus polysaccharides based on TLR4/MyD88/NF‐ κB signaling pathway on immune function of lung cancer mice and its regulatory effect on Th1/Th2. Chinese Journal of Immunology, 37(06), 676–682. [Google Scholar]
- Liu, J. , Zhang, H. , Li, J. , Fan, H. , Chai, Z. , Yu, J. , … Ma, C. (2021). Immunoregulatory effect of astragalus polysaccharide on spleen cells of experi‐ mental autoimmune encephalomyelitis mice. Chinese Journal of Immunology, 37(14), 1701–1705. [Google Scholar]
- Liu, C. , Zhu, X. , Lu, Y. , Zhang, X. , Jia, X. , & Yang, T. (2021). Potential treatment with Chinese and Western medicine targeting NSP14 of SARS‐CoV‐2. Journal of Pharmaceutical Analysis, 11(3), 272–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li‐Weber, M. (2009). New therapeutic aspects of flavones: The anticancer properties of Scutellaria and its main active constituents Wogonin, Baicalein and Baicalin. Cancer Treatment Reviews, 35(1), 57–68. [DOI] [PubMed] [Google Scholar]
- Luo, Y. , Li, J. , Wang, B. , Zhang, Q. , Bian, Y. , & Wang, R. (2021). Protective effect of glycyrrhizin on osteoarthritis cartilage degeneration and inflammation response in a rat model. Journal of Bioenergetics Biomembranes, 53, 285–293. [DOI] [PubMed] [Google Scholar]
- Ma, R. , Xu, X. , Tang, J. , & Ouyang, Z. (2012). Progress in anti‐inflammatory and antineoplastic effect of caffeic acid phenethyl ester by NF‐kappaB pathway. Chinese Journal of Pharmacology and Toxicology, 26(3), 393–396. [Google Scholar]
- Ma, Q. H. , Yu, Q. T. , Xing, X. F. , Liu, S. N. , Shi, C. Y. , & Luo, J. B. (2018). San Wu Huangqin decoction, a Chinese herbal formula, inhibits influenza a/PR/8/34 (H1N1) virus infection in vitro and in vivo. Viruses‐Basel, 10(3), 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallik, S. B. , Mudgal, J. , Nampoothiri, M. , Hall, S. , Anoopkumar‐Dukie, S. , Grant, G. , … Arora, D. (2016). Caffeic acid attenuates lipopolysaccharide‐induced sickness behaviour and neuroinflammation in mice. Neuroscience Letters, 632, 218–223. [DOI] [PubMed] [Google Scholar]
- Martina, B. E. E. , Haagmans, B. L. , Kuiken, T. , Fouchier, R. A. M. , Rimmelzwaan, G. F. , van Amerongen, G. , … Osterhaus, A. (2003). SARS virus infection of cats and ferrets. Nature, 425(6961), 915–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinotti, S. , Ranzato, E. , & Burlando, B. (2018). (−)‐ Epigallocatechin‐3‐gallate induces GRP78 accumulation in the ER and shifts mesothelioma constitutive UPR into proapoptotic ER stress. Journal of Cellular Physiology, 233(10), 7082–7090. [DOI] [PubMed] [Google Scholar]
- Menegazzi, M. , Campagnari, R. , Bertoldi, M. , Crupi, R. , Di Paola, R. , & Cuzzocrea, S. (2020). Protective effect of Epigallocatechin‐3‐Gallate (EGCG) in diseases with uncontrolled immune activation: Could such a scenario be helpful to counteract COVID‐19? International Journal of Molecular Sciences, 21(14), 5171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng, S. , Wang, L. S. , Huang, Z. Q. , Zhou, Q. , Sun, Y. G. , Cao, J. T. , … Wang, C. Q. (2012). Berberine ameliorates inflammation in patients with acute coronary syndrome following percutaneous coronary intervention. Clinical and Experimental Pharmacology & Physiology, 39(5), 406–411. [DOI] [PubMed] [Google Scholar]
- Mohammadi Pour, P. , Farzaei, M. H. , Soleiman Dehkordi, E. , Bishayee, A. , & Asgary, S. (2021). Therapeutic targets of natural products for the management of cardiovascular symptoms of coronavirus disease 2019. Phytotherapy Research, 35(10), 5417–5426. [DOI] [PubMed] [Google Scholar]
- Mok, C. K. P. , Kang, S. S. R. , Chan, R. W. Y. , Yue, P. Y. K. , Mak, N. K. , Poon, L. L. M. , … Chan, M. C. W. (2014). Anti‐inflammatory and antiviral effects of indirubin derivatives in influenza a (H5N1) virus infected primary human peripheral blood‐derived macrophages and alveolar epithelial cells. Antiviral Research, 106, 95–104. [DOI] [PubMed] [Google Scholar]
- Moran, C. S. , Biros, E. , Krishna, S. M. , Wang, Y. , Tikellis, C. , Morton, S. K. , … Golledge, J. (2017). Resveratrol inhibits growth of experimental abdominal aortic aneurysm associated with upregulation of angiotensin‐converting enzyme 2. Arteriosclerosis Thrombosis and Vascular Biology, 37(11), 2195–2203. [DOI] [PubMed] [Google Scholar]
- Most, J. , Warnke, I. , Boekschoten, M. V. , Jocken, J. W. E. , de Groot, P. , Friedel, A. , … Blaak, E. E. (2018). The effects of polyphenol supplementation on adipose tissue morphology and gene expression in overweight and obese humans. Adipocytes, 7(3), 190–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moussa, C. , Hebron, M. , Huang, X. , Ahn, J. , Rissman, R. A. , Aisen, P. S. , & Turner, R. S. (2017). Resveratrol regulates neuro‐inflammation and induces adaptive immunity in Alzheimer’s disease. Journal of Neuroinflammation, 14(1), 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munnink, B. B. O. , Sikkema, R. S. , Nieuwenhuijse, D. F. , Molenaar, R. J. , Munger, E. , Molenkamp, R. , … Koopmans, M. P. G. (2021). Transmission of SARS‐CoV‐2 on mink farms between humans and mink and back to humans. Science, 371(6525), 172–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nance, C. L. , Siwak, E. B. , & Shearer, W. T. (2009). Preclinical development of the green tea catechin, epigallocatechin gallate, as an HIV‐1 therapy. Journal of Allergy and Clinical Immunology, 123(2), 459–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narkhede, R. R. , Pise, A. V. , Cheke, R. S. , & Shinde, S. D. (2020). Recognition of natural products as potential inhibitors of COVID‐19 Main protease (Mpro): In‐silico evidences. Natural Products and Bioprospecting, 10(5), 297–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni, L. , Wen, Z. , Hu, X. , Tang, W. , Wang, H. , Zhou, L. , … Wang, D. W. (2021). Effects of Shuanghuanglian oral liquids on patients with COVID‐19: A randomized, open‐label, parallel‐controlled, multicenter clinical trial. Frontiers of Medicine, 15(5), 704–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouled Aitouna, A. , Belghiti, M. E. , Esme, A. , Anouar, E. , Ouled Aitouna, A. , Zeroual, A. , … Mazoir, N. (2021). Chemical reactivities and molecular docking studies of parthenolide with the main protease of HEP‐G2 and SARS‐CoV‐2. Journal of Molecular Structure, 1243, 130705–130705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pae, M. , & Wu, D. Y. (2013). Immunomodulating effects of epigallocatechin‐3‐gallate from green tea: Mechanisms and applications. Food & Function, 4(9), 1287–1303. [DOI] [PubMed] [Google Scholar]
- Panya, A. , Songprakhon, P. , Panwong, S. , Jantakee, K. , Kaewkod, T. , Tragoolpua, Y. , … Yenchitsomanus, P.‐T. (2021). Cordycepin inhibits virus replication in dengue virus‐infected Vero cells. Molecules, 26(11), 3118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peairs, A. , Dai, R. , Gan, L. , Shimp, S. , Rylander, M. N. , Li, L. , & Reilly, C. M. (2010). Epigallocatechin‐3‐gallate (EGCG) attenuates inflammation in MRL/lpr mouse mesangial cells. Cellular & Molecular Immunology, 7(2), 123–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei, G. , Zhang, Z. , Peng, J. , Liu, L. , Zhang, C. , Yu, C. , … Xu, G. (2020). Renal involvement and early prognosis in patients with COVID‐19 pneumonia. Journal of the American Society of Nephrology, 31(6), 1157–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pekmez, H. , Kus, I. , Colakoglu, N. , Ogeturk, M. , Ozyurt, H. , Turkoglu, A. O. , & Sarsilmaz, M. (2007). The protective effects of caffeic acid phenethyl ester (CAPE) against liver damage induced by cigarette smoke inhalation in rats. Cell Biochemistry and Function, 25(4), 395–400. [DOI] [PubMed] [Google Scholar]
- Peng, Z. , Li, X. , & Li, K. (2011). Glycyrrhizin 18alpha‐assisted treatment for 73 cases of acyclovir‐induced acute renal failure. Chongqing Medicine, 40(36), 3694. [Google Scholar]
- Piao, T. , Ma, Z. , Li, X. , & Liu, J. (2015). Taraxasterol inhibits IL‐1 beta‐induced inflammatory response in human osteoarthritic chondrocytes. European Journal of Pharmacology, 756, 38–42. [DOI] [PubMed] [Google Scholar]
- Qin, P. , Wei, Y. , Hou, M. , Zhao, C. , & Shen, Z. (2015). A multicenter clinical trial of caffeic acid tablet in treatment of 103 primary immune thrombocytopenia patients. Zhonghua xue ye xue za zhi = Zhonghua Xueyexue Zazhi, 36(2), 103–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabie, A. M. (2022). Potent inhibitory activities of the adenosine analogue cordycepin on SARS‐CoV‐2 replication. Acs Omega, 7(3), 2960–2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan, Z. , Liu, C. , Guo, Y. , He, Z. , Huang, X. , Jia, X. , & Yang, T. (2021). SARS‐CoV‐2 and SARS‐CoV: Virtual screening of potential inhibitors targeting RNA‐dependent RNA polymerase activity (NSP12). Journal of Medical Virology, 93(1), 389–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saied, E. M. , El‐Maradny, Y. A. , Osman, A. A. , Darwish, A. M. G. , Abo Nahas, H. H. , Niedbała, G. , … Abdel‐Azeem, A. M. (2021). A comprehensive review about the molecular structure of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2): Insights into natural products against COVID‐19. Pharmaceutics, 13(11), 1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shagufta , & Ahmad, I. (2021). An update on pharmacological relevance and chemical synthesis of natural products and derivatives with anti SARS‐CoV‐2 activity. ChemistrySelect, 6(42), 11502–11527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakhsi‐Niaei, M. , Soureshjani, E. H. , & Babaheydari, A. K. (2021). In silico comparison of separate or combinatorial effects of potential inhibitors of the SARS‐CoV‐2 binding site of ACE2. Iranian Journal of Public Health, 50(5), 1028–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang, J. , Wan, Y. , Luo, C. , Ye, G. , Geng, Q. , Auerbach, A. , & Li, F. (2020). Cell entry mechanisms of SARS‐CoV‐2. Proceedings of the National Academy of Sciences of the United States of America, 117(21), 11727–11734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma, J. , Bhardwaj, V. K. , Singh, R. , Rajendran, V. , Purohit, R. , & Kumar, S. (2021). An in‐silico evaluation of different bioactive molecules of tea for their inhibition potency against non structural protein‐15 of SARS‐CoV‐2. Food Chemistry, 346, 128933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen, M. , Liu, C. , Xu, R. , Ruan, Z. , Zhao, S. , Zhang, H. , … Jia, X. (2020). Predicting the animal susceptibility and therapeutic drugs to SARS‐CoV‐2 based on spike glycoprotein combined with ACE2. Frontiers in Genetics, 11, 575012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen, X. , & Yin, F. (2021). The mechanisms and clinical application of traditional Chinese medicine Lianhua‐Qingwen capsule. Biomedicine & Pharmacotherapy, 142, 111998–111998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiha, G. , Soliman, R. , Elbasiony, M. , Darwish, N. H. E. , & Mousa, S. A. (2019). Addition of epigallocatechin Gallate 400 mg to Sofosbuvir 400 mg + Daclatisvir 60 mg with or without ribavirin in treatment of patients with chronic hepatitis C improves the safety profile: A pilot study. Scientific Reports, 9(1), 13593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh, R. , Bhardwaj, V. K. , & Purohit, R. (2021). Potential of turmeric‐derived compounds against RNA‐dependent RNA polymerase of SARS‐CoV‐2: An in‐silico approach. Computers in Biology and Medicine, 139, 104965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singla, R. K. , He, X. , Chopra, H. , Tsagkaris, C. , Shen, L. , Kamal, M. A. , & Shen, B. (2021). Natural products for the prevention and control of the COVID‐19 pandemic: Sustainable bioresources. Frontiers in Pharmacology, 12, 758159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, J. H. , Shim, J. K. , & Choi, H. J. (2011). Quercetin 7‐rhamnoside reduces porcine epidemic diarrhea virus replication via independent pathway of viral induced reactive oxygen species. Virology Journal, 8, 460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa, C. P. , & Brites, C. (2020). Immune response in SARS‐CoV‐2 infection: The role of interferons type I and type III. Brazilian Journal of Infectious Diseases, 24(5), 428–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su H., Yao S., Zhao W., Li M., Liu J., Shang W., Xie H., Ke C., Gao M., Yu K., Liu H., Shen J., Tang W., Zhang L., Zuo J., Jiang H., Bai F., Wu Y., Ye Y., & Xu Y. (2020a). Discovery of baicalin and baicalein as novel, natural product inhibitors of SARS‐CoV‐2 3CL protease in vitro. bioRxiv, 10.1101/2020.04.13.038687 [DOI]
- Su, H.‐X. , Yao, S. , Zhao, W.‐F. , Li, M.‐J. , Liu, J. , Shang, W.‐J. , … Xu, Y.‐C. (2020b). Anti‐SARS‐CoV‐2 activities in vitro of Shuanghuanglian preparations and bioactive ingredients. Acta Pharmacologica Sinica, 41(9), 1167–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subbaiyan, A. , Ravichandran, K. , Singh, S. V. , Sankar, M. , Thomas, P. , Dhama, K. , … Chaudhuri, P. (2020). In silico molecular docking analysis targeting SARS‐CoV‐2 spike protein and selected herbal constituents. Journal of Pure and Applied Microbiology, 14, 989–998. [Google Scholar]
- Sun, Y. , Song, M. , Niu, L. , Bai, X. , Sun, N. , Zhao, X. , … Li, H. (2013). Antiviral effects of the constituents derived from Chinese herb medicines on infectious bursal disease virus. Pharmaceutical Biology, 51(9), 1137–1143. [DOI] [PubMed] [Google Scholar]
- Takahashi, S. , Yoshiya, T. , Yoshizawa‐Kumagaye, K. , & Sugiyama, T. (2015). Nicotianamine is a novel angiotensin‐converting enzyme 2 inhibitor in soybean. Biomedical Research‐Tokyo, 36(3), 219–224. [DOI] [PubMed] [Google Scholar]
- Tanikawa, T. , Hayashi, T. , Suzuki, R. , Kitamura, M. , & Inoue, Y. (2021). Inhibitory effect of honokiol on furin‐like activity and SARS‐CoV‐2 infection. Journal of Traditional and Complementary Medicine, 12(1), 69–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian, W. , & Duan, L. (2021). Research status of Berberine on antitumor effect. The Chinese Journal of Clinical Pharmacology, 37(12), 1599–1602. [Google Scholar]
- Ulrich, H. , & Pillat, M. M. (2020). CD147 as a target for COVID‐19 treatment: Suggested effects of azithromycin and stem cell engagement. Stem Cell Reviews and Reports, 16(3), 434–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma, A. K. (2020). Cordycepin: A bioactive metabolite of Cordyceps militaris and polyadenylation inhibitor with therapeutic potential against COVID‐19. Journal of Biomolecular Structure & Dynamics, 40(8), 3745–3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma, A. K. , Kumar, V. , Singh, S. , Goswami, B. C. , Camps, I. , Sekar, A. , … Lee, K. W. (2021). Repurposing potential of Ayurvedic medicinal plants derived active principles against SARS‐CoV‐2 associated target proteins revealed by molecular docking, molecular dynamics and MM‐PBSA studies. Biomedicine & Pharmacotherapy, 137, 111356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, X. , Li, X. , Wang, X. , Chen, L. , Ning, E. , Fan, Y. , … Wang, W. (2021). Experimental study of Forsythoside a on prevention and treatment of avian infectious bronchitis. Research in Veterinary Science, 135, 523–531. [DOI] [PubMed] [Google Scholar]
- Wang, M. J. , Luo, W. G. , & Chen, Z. F. (2018). Effects of compound glycyrrhizin combined with adefovir dipivoxil on the efficacy and serum levels of TGF ‐ β1 and TNF ‐ α in patients with chronic hepatitis B. Journal of Clinical and Experimental Medicine, 17(23), 2504–2508. [Google Scholar]
- Wang, S. , Qiu, Z. Y. , Hou, Y. N. , Deng, X. Y. , Xu, W. , Zheng, T. T. , … Li, X. (2021). AXL is a candidate receptor for SARS‐CoV‐2 that promotes infection of pulmonary and bronchial epithelial cells. Cell Research, 31(2), 126–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO Coronavirus (COVID‐19) Dashboard , 2022. https://covid19.who.int/
- Wichmann, D. , Sperhake, J.‐P. , Luegehetmann, M. , Steurer, S. , Edler, C. , Heinemann, A. , … Kluge, S. (2020). Autopsy findings and venous thromboembolism in patients with COVID‐19. Annals of Internal Medicine, 173(4), 268–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieslander, G. , Fabjan, N. , Vogrincic, M. , Kreft, I. , Janson, C. , Spetz‐Nyström, U. , … Norbäck, D. (2011). Eating buckwheat cookies is associated with the reduction in serum levels of myeloperoxidase and cholesterol: A double blind crossover study in day‐care Centre staffs. The Tohoku Journal of Experimental Medicine, 225(2), 123–130. [DOI] [PubMed] [Google Scholar]
- Wu, H. , Gong, K. , Qin, Y. , Yuan, Z. , Xia, S. , Zhang, S. , … Xie, M. (2021). In silico analysis of the potential mechanism of a preventive Chinese medicine formula on coronavirus disease 2019. Journal of Ethnopharmacology, 275, 114098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, X. , Qin, S. , & Li, Q. (2014). Anti‐inflammatory effects and mechanisms of Betulinic acid. Chinese Journal of Modern Applied Pharmacy, 31(10), 1284–1287. [Google Scholar]
- Xu, L. , Yu, Y. , Sang, R. , Li, J. , Ge, B. , & Zhang, X. (2018). Protective effects of Taraxasterol against ethanol‐induced liver injury by regulating CYP2E1/Nrf2/HO‐1 and NF‐kappa B signaling pathways in mice. Oxidative Medicine and Cellular Longevity, 2018, 8284107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue, B. , Liu, X. , Dong, W. , Liang, L. , & Chen, K. (2017). EGCG maintains Th1/Th2 balance and mitigates ulcerative colitis induced by dextran sulfate sodium through TLR4/MyD88/NF‐kappa B signaling pathway in rats. Canadian Journal of Gastroenterology and Hepatology, 2017, 3057268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan, A. , Li, B. , Li, R. , Yu, K. , & Yu, X. (2012). Effect of Lentinan and Astragalus polysaccharides on immunological function in immunosuppressed mice. Chinese Journal of Immunology, 28(11), 999. [Google Scholar]
- Yan J., & Liu J. (2021). Effect of Astragalus polysaccharide injection on immune function of patients with advanced Epithelial Ovarian Cancer. Drug Evaluation, 18(14), 855–857. [Google Scholar]
- Yan, R. H. , Zhang, Y. Y. , Li, Y. N. , Xia, L. , Guo, Y. Y. , & Zhou, Q. (2020). Structural basis for the recognition of SARS‐CoV‐2 by full‐length human ACE2. Science, 367(6485), 1444–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, R. , Huang, G. , & Yang, C. (2020). Clinical effects of Shuanghuanglian granules combined with montelukast sodium and conventional treatment on patients with mycoplasma pneumoniae. Chinese Traditional Patent Medicine, 42(10), 2631–2634. [Google Scholar]
- Yang, L. , Wang, H. , & Li, Y. (2020). Research progress on therapeutic drugs for corona virus disease 2019. Acta Pharmaceutica Sinica, 55(6), 1081–1090. [Google Scholar]
- Yao, S. , Wang, X. , Li, C. , Zhao, T. , Jin, H. , & Fang, W. (2016). Kaempferol inhibits cell proliferation and glycolysis in esophagus squamous cell carcinoma via targeting EGFR signaling pathway. Tumor Biology, 37(8), 10247–10256. [DOI] [PubMed] [Google Scholar]
- Yi, J. , Obminskamrukowicz, B. , Du, J. , Wei, Y. , Nie, W. , & Yuan, H. (2010). Effects of BETULINIC acid on immune function and antioxidant activity of mouse peritoneal macrophages. Acta Nutrimenta Sinica, 32(3), 281–285. [Google Scholar]
- You, H. , Wei, L. , Sun, W.‐L. , Wang, L. , Yang, Z.‐L. , Liu, Y. , … Zhang, W.‐J. (2014). The green tea extract epigallocatechin‐3‐gallate inhibits irradiation‐induced pulmonary fibrosis in adult rats. International Journal of Molecular Medicine, 34(1), 92–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, J. W. , Wang, L. , & Bao, L. D. (2020). Exploring the active compounds of traditional Mongolian medicine in intervention of novel coronavirus (COVID‐19) based on molecular docking method. Journal of Functional Foods, 71, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuhang, J. , Zhian, Z. , Hai, J. , Jinhua, L. , Xuemeng, S. , & Zhenlong, W. (2021). Kaempferol attenuates diquat‐induced oxidative damage and apoptosis in intestinal porcine epithelial cells. Food & Function, 12(15), 6889‐6899. [DOI] [PubMed] [Google Scholar]
- Zhang, X.‐T. , Ding, Y. , Kang, P. , Zhang, X.‐Y. , & Zhang, T. (2018). Forsythoside a modulates Zymosan‐induced peritonitis in mice. Molecules, 23(3), 593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, J. , Han, C. , Lu, W. Q. , Wang, N. , Wu, S. R. , Wang, Y. X. , … Shi, Y. Q. (2020). A randomized, multicenter and noninferiority study of amoxicillin plus berberine vs tetracycline plus furazolidone in quadruple therapy for helicobacter pylori rescue treatment. Journal of Digestive Diseases, 21(5), 256–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Y. , Jia, Y. , Li, X. , Wang, L. , Du, M. , & Zhang, X. (2018). Clinical observation on Astragalus polysaccharide injection combined with CIK cells treating NSCLC patients with qi deficiency syndrome. Chinese Traditional and Herbal Drugs, 49(7), 1647–1651. [Google Scholar]
- Zhang, W. , Ma, Z. , Wu, Y. , Shi, X. , Zhang, Y. , Zhang, M. , … Liu, W. (2021). SARS‐CoV‐2 3C‐like protease antagonizes interferon‐beta production by facilitating the degradation of IRF3. Cytokine, 148, 155697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, H.‐S. , Wu, T.‐C. , Sang, W.‐W. , & Ruan, Z. (2012). EGCG inhibits tat‐induced LTR transactivation: Role of Nrf2, AKT, AMPK Signaling Pathway. Life Sciences, 90(19–20), 747–754. [DOI] [PubMed] [Google Scholar]
- Zhang, X. , Xiong, H. , Li, H. , & Cheng, Y. (2014). Protective effect of taraxasterol against LPS‐induced endotoxic shock by modulating inflammatory responses in mice. Immunopharmacology and Immunotoxicology, 36(1), 11–16. [DOI] [PubMed] [Google Scholar]
- Zhao, J. , Tian, S. , Lu, D. , Yang, J. , Zeng, H. , Zhang, F. , … Zhang, W. (2021). Systems pharmacological study illustrates the immune regulation, anti‐infection, anti‐inflammation, and multi‐organ protection mechanism of Qing‐Fei‐Pai‐Du decoction in the treatment of COVID‐19. Phytomedicine, 85, 153315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, J. , Tian, S. , Yang, J. , Liu, J. , & Zhang, W. (2020). Investigating mechanism of Qing‐Fei‐Pai‐Du‐Tang for treatment of COVID‐19 by network pharmacology. Chinese Traditional and Herbal Drugs, 51(4), 829–835. [Google Scholar]
- Zheng, W. , Wu, H. , Wang, T. , Zhan, S. , & Liu, X. (2021). Quercetin for COVID‐19 and DENGUE co‐infection: A potential therapeutic strategy of targeting critical host signal pathways triggered by SARS‐CoV‐2 and DENV. Briefings in Bioinformatics, 22(6), bbab199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, S. , Hill, C. S. , Sarkar, S. , Tse, L. V. , Woodburn, B. M. D. , Schinazi, R. F. , … Swanstrom, R. (2021). β‐D‐N 4‐hydroxycytidine (NHC) inhibits SARS‐CoV‐2 through lethal mutagenesis but is also mutagenic to mammalian cells. The Journal of Infectious Diseases, 224(3), 415–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, J. , & Huang, J. (2020). Current findings regarding natural components with potential Anti‐2019‐nCoV activity. Frontiers in Cell and Developmental Biology, 8, 589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, P. , Yang, X. L. , Wang, X. G. , Hu, B. , Zhang, L. , Zhang, W. , … Shi, Z. L. (2020). A pneumonia outbreak associated with a new coronavirus of probable bat origin (vol 579, pg 270, 2020). Nature, 588(7836), E6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J., Deng Y.‐Q., Wang X., Li X.‐F., Zhang N.‐N., Liu Z., Zhang B., Qin C.‐F., & Xie Z. (2020). An artificial intelligence system reveals liquiritin inhibits SARS‐CoV‐2 by mimicking type I interferon .
- Zhu, Y. , & Xie, D.‐Y. (2020). Docking characterization and in vitro inhibitory activity of Flavan‐3‐ols and dimeric Proanthocyanidins against the Main protease activity of SARS‐Cov‐2. Frontiers in Plant Science, 11, 601316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zong, Y. , Ding, M. , Jia, K. , Ma, S. , & Ju, W. (2020). Exploring active compounds of Da‐Yuan‐Yin in treatment of COVID‐19 based on network pharmacology and molecular docking method. Chinese Traditional and Herbal Drugs, 51(4), 836–844. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


