Abstract
The purpose of this study was to identify risk factors for pulmonary involvement by examining the demographic, clinical, and laboratory characteristics of children with COVID‐19. We performed a retrospective single‐center study of COVID‐19 in children treated at a tertiary care hospital in Turkey from December 2020 to June 2021. During the course of the study, 126 patients were evaluated, of whom 70/126 were male. The patients' ages ranged from 1 to 216 (mean, 4.73 ± 81.11) months. Fever (65.9%), cough (52.4%), and shortness of breath (18.3%) were the most common symptoms of COVID‐19. Ten patients required noninvasive mechanical ventilation. Sixty‐nine patients (54.8%) had pneumonia. Longer duration of fever, hospitalization, and the presence of cough were significantly associated with pulmonary involvement. Children with pneumonia had significantly higher levels of C‐reactive protein (CRP), procalcitonin, erythrocyte sedimentation rate (ESR), and viral load, and significantly lower counts of lymphocytes and thrombocytes. The cutoff viral load, CRP, and procalcitonin values for predicting pulmonary involvement were 26.5 cycle threshold (Ct; 95% confidence interval [CI], 0.54–0.74; sensitivity, 0.65; specificity, 0.56; area under curve [AUC]: 0.647, p = 0.005), 7.85 mg/L (95% CI, 0.56–0.75; sensitivity, 0.66; specificity, 0.64; AUC = 0.656; p = 0.003) and 0.105 ng/ml (95% CI, 0.52–0.72; sensitivity, 0.55; specificity, 0.58; AUC = 0.626; p = 0.02), respectively. High CRP, procalcitonin levels, ESR, and viral load, and low lymphocyte and thrombocyte counts can predict pulmonary involvement in children with COVID‐19, so better management may be provided for good prognosis.
Keywords: children, COVID‐19, predictor, pulmonary involvement, viral load
1. INTRODUCTION
Coronavirus disease 2019 (COVID‐19), caused by a novel coronavirus, the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), rapidly spread worldwide and became a major public health problem. By February 2022, almost 420 million people had been infected throughout the world, with more than 5,840,000 deaths. 1 The clinical spectrum can vary widely from asymptomatic infection to acute respiratory distress syndrome in adults, but it appears to result in milder disease in children. 2 However, most patients hospitalized because of COVID‐19 have pulmonary involvement. 3 To date, studies have investigated the association between SARS‐CoV‐2 viral load and disease severity, mortality, age, comorbidities, and outcomes. 4 Nevertheless, there is no information regarding the associations among viral load, inflammatory biomarkers, and pulmonary involvement in children. Therefore, we aimed to investigate the epidemiological, clinical, and laboratory characteristics of children with COVID‐19 and compare the clinical and laboratory features of children with and without pulmonary involvement to identify the factors affecting pulmonary involvement.
2. MATERIALS AND METHODS
2.1. Study design, data collection, and definitions
This prospective, single‐center study involved examinations of 126 children with COVID‐19 at Başakşehir Çam ve Sakura City Hospital between December 2020 and June 2021.
Among the study participants, COVID‐19 diagnosis was confirmed by reverse‐transcriptase polymerase chain reaction (PCR) analysis of samples taken from oropharyngeal and nasopharyngeal swabs. The COVID‐19 patients were divided into two groups: patients with pulmonary involvement and patients without pulmonary involvement according to clinical symptoms, vital signs (especially respiratory rate, body temperature, and oxygen saturation on room air), chest X‐ray (CXR), computed tomography (CT) findings and laboratory tests.
All patients in our study had an initial CXR (posterior‐anterior projection) on the day of admission, and all images were reviewed by the same chest radiologist.
Some patients with respiratory symptoms, including cough, but normal images or suspected opacities on initial CXR were performed a follow‐up CXR, which ruled out pneumonia.
Clinical follow‐up was done for 6 months. None of the patients in the “no pulmonary involvement group” was readmitted with pulmonary symptoms or progressed to pneumonia during this period.
The following demographic information, clinical features, laboratory results, and treatment modalities data were examined: age, gender, underlying medical conditions, duration of symptoms and hospitalization, complete blood count, liver and kidney function, inflammatory biomarkers (procalcitonin, erythrocyte sedimentation rate [ESR], and C‐reactive protein [CRP]), biologic enzyme levels (lactate dehydrogenase and creatine kinase), d‐dimer, coagulation tests, cardiac biomarkers (troponin‐T and pro‐brain natriuretic peptide), SARS‐CoV‐2 PCR, viral load, CXR, and CT scan imaging.
2.2. SARS‐CoV‐2 detection by RT‐qPCR and viral load assessment
A combined nasopharyngeal and oropharyngeal specimen collected with a synthetic fiber swab was inserted into a sterile tube containing 3 ml of vNAT (viral nucleic acid isolation tampon; Bio‐speedy, Bioeksen). Detection of SARS‐CoV‐2 RNA was accomplished by one‐step reverse transcription and real‐time PCR targeting SARS‐CoV‐2‐specific RNA‐dependent RNA polymerase and N gene fragments using the SARS‐CoV‐2 Double Gene RT‐qPCR kit (Bio‐speedy, Bioeksen). 5 For internal control, the kit targets the ribonuclease (RNase) P gene. RT‐qPCR was performed on the Bio‐Rad CFX96 Touch instrument using the following conditions: 52°C for 5 min and 95°C for 10 s, 40 cycles of amplification at 95°C for 1 s and 55°C for 1 s. The cycle threshold (Ct) value represents the number of amplification cycles required for the target gene to exceed a threshold level. A low Ct value is indicative of a high viral load. If the Ct value is below 38, it is considered positive for SARS‐CoV‐2.
2.3. Statistical analysis
Statistical analysis was performed using SPSS version 22.0 (IBM, SPSS). Data are summarized as frequencies, medians, and means with standard deviations. Normally distributed data were assessed using means and the Student's t‐test. The nonparametric data's significance was assessed using the Mann–Whitney U test. The statistical significance of dichotomous outcomes was determined using the chi‐square test, Fisher's exact test, the Fisher–Freeman–Halton test, and Yates's continuity correction. A multivariate logistic regression analysis was performed with the variables found to be statistically significant in the univariate analysis. A receiver operating characteristic (ROC) curve analysis was performed to determine the cutoff levels of viral load, CRP, and procalcitonin to predict pulmonary involvement. A value of p < 0.05 was considered statistically significant.
The Ethics Committee at Basaksehir Cam ve Sakura City Hospital and the Turkish Ministry of Health (Date: August 4, 2021; Decision no: 161) approved this study.
3. RESULTS
3.1. Patient characteristics and treatment modalities
A total of 126 patients were examined between December 2020 and June 2021. Of all the patients, 70 (55.6%) were male. The mean age was 74.73 ± 81.11 months (range, 1–216 months). On admission, the most common symptoms were fever (83 patients, 65.9%) and cough (66 patients, 52.4%), followed by shortness of breath (23 patients, 18.3%) and myalgia (21 patients, 16.7%). Forty‐three patients (34.1%) had underlying medical conditions. All 10 patients (7.9%) required noninvasive mechanical ventilation had pneumonia. Favipiravir was given to 15 patients (11.9%). Ninety‐eight of the patients received broad‐spectrum antibiotics for possible bacterial superinfections. The patients' characteristics and treatment modalities are presented in Table 1. One patient diagnosed with metabolic disease without pulmonary involvement died. All other patients were discharged without any complications.
Table 1.
The comparison of demographic, clinical characteristics, and treatment modalities of the patients according to pulmonary involvement
| Pulmonary involvement | Total | ||||
|---|---|---|---|---|---|
| No (n = 57) | Yes (n = 69) | p | |||
| Mean ± SD (median) | Mean ± SD (median) | Mean ± SD (median) | |||
| Age (months) (median) | 48.1 ± 72.79 (6) | 96.74 ± 81.51 (75) | 74.73 ± 81.11 (24) | 0.000 a , * | |
| Duration of fever (median) (days) | 1.94 ± 1.16 (2) | 3.21 ± 2 (2) | 2.67 ± 1.8 (2) | 0.000 a , * | |
| Duration of cough (median) (days) | 3.06 ± 1.98 (2.5) | 3.85 ± 2.41 (3) | 3.64 ± 2.32 (3) | 0.230a | |
| Duration of hospitalization (median) (days) | 7.86 ± 3.2 (8) | 9.62 ± 3.99 (9) | 8.83 ± 3.74 (8.5) | 0.004 a , * | |
| n (%) | n (%) | n (%) | |||
| Gender | Female | 22 (38.6%) | 34 (49.3%) | 56 (44.4%) | 0.230b |
| Male | 35 (61.4%) | 35 (50.7%) | 70 (55.6%) | ||
| Household close contact | No | 19 (33.3%) | 27 (39.1%) | 46 (36.5%) | 0.455c |
| Yes | 37 (64.9%) | 42 (60.9%) | 79 (62.7%) | ||
| Underlying condition | No | 40 (70.2%) | 43 (62.3%) | 83 (65.9%) | – |
| Gastroesophageal reflux | 1 (1.8%) | 0 (0%) | 1 (0.8%) | ||
| Hydronephrosis | 1 (1.8%) | 0 (0%) | 1 (0.8%) | ||
| Prematurity | 2 (3.5%) | 1 (1.4%) | 3 (2.4%) | ||
| Congenital heart disease | 4 (7%) | 2 (2.9%) | 6 (4.8%) | ||
| Asthma | 1 (1.8%) | 4 (5.8%) | 5 (4%) | ||
| Type‐1 diabetes mellitus | 3 (5.3%) | 1 (1.4%) | 4 (3.2%) | ||
| Metabolic diseases | 1 (1.8%) | 4 (5.8%) | 5 (4%) | ||
| Congenital neutropenia | 1 (1.8%) | 1 (1.4%) | 2 (1.6%) | ||
| Epilepsy | 0 (0%) | 2 (2.9%) | 2 (1.6%) | ||
| Immune deficiency | 1 (1.8%) | 3 (4.3%) | 4 (3.2%) | ||
| Chronic kidney disease | 0 (0%) | 2 (2.9%) | 2 (1.6%) | ||
| Obesity | 0 (0%) | 2 (2.9%) | 2 (1.6%) | ||
| Autism | 1 (1.8%) | 0 (0%) | 1 (0.8%) | ||
| Down syndrome | 0 (0%) | 1 (1.4%) | 1 (0.8%) | ||
| Acute lymphoblastic leukemia | 0 (0%) | 2 (2.9%) | 2 (1.6%) | ||
| Mental retardation | 0 (0%) | 1 (1.4%) | 1 (0.8%) | ||
| Cerebral palsy | 1 (1.8%) | 0 (0%) | 1 (0.8%) | ||
| Underlying condition | No | 40 (70.2%) | 43 (62.3%) | 83 (65.9%) | 0.461d |
| Yes | 17 (29.8%) | 26 (37.7%) | 43 (34.1%) | ||
| Symptoms | Fever | 35 (61.4%) | 48 (69.6%) | 83 (65.9%) | 0.336b |
| Cough | 18 (3.6%) | 48 (69.6%) | 66 (52.4%) | 0.000 d , * | |
| Runny nose | 2 (3.5%) | 4 (5.8%) | 6 (4.8%) | 0.435e | |
| Sore throat | 1 (1.8%) | 3 (4.3%) | 4 (3.2%) | – | |
| Myalgia | 6 (10.5%) | 15 (21.7%) | 21 (16.7%) | 0.150d | |
| Abdominal pain | 1 (1.8%) | 4 (5.8%) | 5 (4%) | – | |
| Diarrhea | 9 (15.8%) | 5 (7.2%) | 14 (11.1%) | 0.217d | |
| Shortness of breath | 1 (1.8%) | 22 (31.9%) | 23 (18.3%) | – | |
| Favipiravir use | No | 55 (96.5%) | 56 (81.2%) | 111 (88.1%) | 0.018 d , * |
| Yes | 2 (3.5%) | 13 (18.8%) | 15 (11.9%) | ||
| Azithromycin use | No | 49 (86%) | 50 (72.5%) | 99 (78.6%) | 0.105d |
| Yes | 8 (14%) | 19 (27.5%) | 27 (21.4%) | ||
| Antibiotherapy | No | 19 (33.3%) | 9 (13%) | 28 (22.2%) | – |
| Ampicillin‐sulbactam | 3 (5.3%) | 2 (2.9%) | 5 (4%) | ||
| Ampicillin+cefotaxime | 12 (21.1%) | 6 (8.7%) | 18 (14.3%) | ||
| Ceftriaxone | 15 (26.3%) | 14 (20.3%) | 29 (23%) | ||
| Cefotaxime | 4 (7%) | 7 (10.1%) | 11 (8.7%) | ||
| Teicoplanin+piperacillin‐tazobactam | 1 (1.8%) | 1 (1.4%) | 2 (1.6%) | ||
| Teicoplanin+ceftriaxone | 1 (1.8%) | 24 (34.8%) | 25 (19.8%) | ||
| Vancomycin+ceftriaxone | 1 (1.8%) | 4 (5.8%) | 5 (4%) | ||
| Cefepime | 1 (1.8%) | 2 (2.9%) | 3 (2.4%) | ||
| Type of delivery | Spontaneous vaginal delivery | 23 (40.4%) | 42 (60.9%) | 65 (51.6%) | 0.022 b , * |
| Cesarean delivery | 34 (59.6%) | 27 (39.1%) | 61 (48.4%) | ||
| Noninvasive mechanical ventilation | No | 57 (100%) | 59 (85.5%) | 116 (92.1%) | – |
| Yes | 0 (0%) | 10 (14.5%) | 10 (7.9%) | ||
| Neutropenia | No | 42 (73.7%) | 56 (81.2%) | 98 (77.8%) | 0.430d |
| Yes | 15 (26.3%) | 13 (18.8%) | 28 (22.2%) | ||
| Lymphopenia | No | 52 (91.2%) | 49 (71%) | 101 (80.2%) | 0.009 d , * |
| Yes | 5 (8.8%) | 20 (29%) | 25 (1.8%) | ||
| Corticosteroid use | No | 57 (100%) | 58 (84.1%) | 115 (91.3%) | – |
| Yes | 0 (0%) | 11 (15.9%) | 11 (8.7%) | ||
| Low molecular–weight heparin use | No | 53 (93%) | 52 (75.4%) | 105 (83.3%) | 0.016 d , * |
| Yes | 4 (7%) | 17 (24.6%) | 21 (16.7%) | ||
| Mortality | No | 56 (98.2%) | 69 (100%) | 125 (99.2%) | – |
| Yes | 1 (1.8%) | 0 (0%) | 1 (0.8%) | ||
Mann–Whitney U test.
Chi‐square test.
Fisher Freeman Halton test.
Yates's continuity correction.
Fisher's exact test.
p < 0.05.
3.2. Laboratory and radiological findings
The Alpha (B.1.1.7) variant was the predominant circulating variant in Turkey during the study period. On admission, the mean white blood cell count, lymphocyte count, and CRP levels were 7903.49 ± 4751.29 (range, 1030–24,810)/mm3, 3302.94 ± 2918.32 (range, 270–15,890)/mm3, and 24.42 ± 47.65 (range, 0.1–280) mg/dl, respectively. Lymphopenia and neutropenia were present in 25 (19.8%) and 28 (22.2%) patients, respectively. The patients' laboratory findings are presented in Table 2.
Table 2.
The comparison of the laboratory findings of the patients according to pulmonary involvement
| Pulmonary involvement | Total | |||
|---|---|---|---|---|
| Variables | No (n = 57) | Yes (n = 69) | p | |
| Mean ± SD (median) | Mean ± SD (median) | Mean ± SD (median) | ||
| Hemoglobin, g/dl | 11.33 ± 1.76 | 11.77 ± 1.98 | 11.57 ± 1.89 | 0.195a |
| White blood cells, /mm3 | 8578.95 ± 5273.94 (6890) | 7345.51 ± 4230.06 (5920) | 7903.49 ± 4751.29 (6365) | 0.159b |
| Thrombocytes, /mm3 (median) | 337,631.58 ± 122,307.08 | 267,724.64 ± 132,668.08 | 299,349.21 ± 132,275.61 | 0.003 a , * |
| Lymphocyte, /mm3 (median) | 4305.26 ± 3437.42 (3280) | 2474.93 ± 2094.52 (1790) | 3302.94 ± 2918.32 (2345) | 0.000 b , * |
| Neutrophil, /mm3 (median) | 3262.46 ± 3263.51 (2060) | 4090.29 ± 3371.47 (3150) | 3715.79 ± 3335.58 (2600) | 0.075b |
| Monocytes, /mm3 | 1017.02 ± 607.15 (890) | 685.51 ± 502.67 (560) | 835.48 ± 574.5 (710) | 0.000 b , * |
| Eosinophil, /mm3 (median) | 128.25 ± 179.4 (60) | 77.25 ± 121.21 (20) | 100.32 ± 151.85 (40) | 0.004 b , * |
| MCV, fl | 84.2 ± 8.31 (84) | 82.43 ± 7.37 (82) | 83.23 ± 7.83 (83) | 0.272b |
| Erythrocyte sedimentation rate, mm/h (median) | 14.09 ± 9.97 (9) | 27.17 ± 21.69 (24) | 21.1 ± 18.4 (16) | 0.001 b , * |
| C‐reactive protein, mg/L (median) | 12.77 ± 27.23 (2.4) | 33.88 ± 57.78 (9.4) | 24.42 ± 47.65 (4.5) | 0.003 b , * |
| Procalcitonin, ng/ml (median) | 0.31± 1.09 (0.1) | 0.81 ± 2.1 (0.1) | 0.58 ± 1.72 (0.1) | 0.020 b , * |
| Alanine aminotransferase, IU/L (median) | 39.39 ± 84.61 (21.5) | 62.16 ± 220.2 (22) | 51.96 ± 172.9 (22) | 0.626b |
| Aspartate aminotransferase, IU/L (median) | 73.3 ± 197.32 (37.5) | 72.42 ± 221.26 (37) | 72.82 ± 210.04 (37) | 0.794b |
| Lactate dehydrogenase, IU/L (median) | 321.21 ± 229.33 (285) | 350.21 ± 208.74 (291) | 337.01 ± 217.93 (287) | 0.247b |
| INR (median) | 1.09 ± 0.26 (1) | 1.07 ± 0.1 (1.1) | 1.08 ± 0.19 (1) | 0.499b |
| d‐dimer, μgFEU/ml (median) | 1.27 ± 1.57 (0.7) | 1.57 ± 3.79 (0.5) | 1.43 ± 2.99 (0.6) | 0.407b |
| Total bilirubin, mg/dl (median) | 2.55 ± 8.59 (0.4) | 1.18 ± 4.64 (0.3) | 1.79 ± 6.68 (0.3) | 0.025 b , * |
| Indirect bilirubin, mg/dl (median) | 0.8 ± 1.47 (0.2) | 0.48 ± 1.45 (0.2) | 0.62 ± 1.46 (0.2) | 0.600b |
| Total protein, g/L (median) | 60.92 ± 7.69 (60) | 66.08 ± 7.67 (67) | 63.74 ± 8.07 (64) | 0.001 b , * |
| Albumin, g/L | 41.22 ± 4.33 | 41.97 ± 4.72 | 41.62 ± 4.5 | 0.371a |
| Creatine kinase, U/L (median) | 99.18 ± 87.93 (75) | 468.52 ± 2922.04 (87.5) | 318.78 ± 2254.25 (80) | 0.400b |
| ProBNP, pg/ml (median) | 873.04 ± 1365.29 (314.5) | 406.81 ± 823.8 (153) | 595.4 ± 1093.32 (217) | 0.127b |
| Troponin T, ng/L (median) | 24.2 ± 24.17 (15) | 11.88 ± 15.07 (4.9) | 17.25 ± 20.41 (5.8) | 0.008 b , * |
| Urea, mg/dl (median) | 16.63 ± 7.93 (16.5) | 20.97 ± 16.22 (18.1) | 19.01 ± 13.27 (17.6) | 0.071b |
| Creatinine, mg/dl (median) | 0.34 ± 0.17 (0.3) | 0.51 ± 0.42 (0.4) | 0.43 ± 0.34 (0.3) | 0.003 b , * |
| Iron, μg/dl (median) | 63.19 ± 46.03 (52) | 50.17 ± 32.53 (36) | 55.94 ± 39.43 (44.5) | 0.058b |
| Ferritin, ng/ml (median) | 325.33 ± 484.97 (167) | 350.85 ± 534.03 (163) | 339.59 ± 510.83 (167) | 0.859b |
| Folate, ng/ml (median) | 16.24 ± 8.49 (15.2) | 12.25 ± 8.4 (10.4) | 14.03 ± 8.63 (12.6) | 0.006 b , * |
| 25‐hydroxyvitamin D, ng/ml (median) | 23.98 ± 14.03 (20.5) | 22.72 ± 18.94 (15) | 23.26 ± 16.93 (19) | 0.225b |
| Vitamin B12, pg/ml (median) | 372.39 ± 178.15 (358) | 480.03 ± 387.23 (349.5) | 432.42 ± 315.8 (353.5) | 0.673b |
| IL‐6, pg/ml (median) | 17.45 ± 24.69 (6.8) | 15.23 ± 22.87 (7.1) | 16.09 ± 23.46 (7) | 0.657b |
| Viral load, Ct | 28 ± 5.2 | 25.4 ± 5.61 | 26.83 ± 5.52 | 0.008 a , * |
Student's t‐test.
Mann–Whitney U test.
p < 0.05.
CXR was performed in all patients. While 57 (45.2%) of the patients had normal CXR findings, 69 (54.8%) patients had potentially pathological findings. Chest CT scans of 51 patients revealed bilateral ground‐glass opacity in 62.7% of them and unilateral ground‐glass opacity in 27.5%. The patients' radiological findings are presented in Table 3.
Table 3.
Radiological findings of the patients with COVID‐19
| n | % | ||
|---|---|---|---|
| Chest X‐ray (n = 126) | Normal | 57 | 45.2 |
| Right paracardiac infiltration | 22 | 17.5 | |
| Bilateral interstitial infiltration | 30 | 23.8 | |
| Peribronchial infiltration | 8 | 6.3 | |
| Bilateral paracardiac infiltration | 6 | 4.8 | |
| Consolidation | 2 | 1.6 | |
| Right pleural effusion | 1 | 0.8 | |
| Chest computed tomography (n = 51) | Bilateral ground‐glass opacity | 32 | 62.7 |
| Right ground‐glass opacity | 10 | 19.6 | |
| Left ground‐glass opacity | 4 | 7.9 | |
| Consolidation | 5 | 9.8 |
3.3. Factors associated with pulmonary involvement
The patients were divided into two groups: patients with pulmonary involvement (Group 1; n = 69) and patients without pulmonary involvement (Group 2; n = 57). We compared the groups' epidemiological, clinical, and laboratory characteristics. Group 1's mean age was significantly higher than that of Group 2. The complaint of cough was significantly more frequent in Group 1. The durations of fever and hospitalization were significantly longer in Group 1 than in Group 2. Lymphopenia was significantly more frequent in Group 1. Favipiravir and low‐molecular‐weight heparin usage were significantly higher in Group 1 than in Group 2. No other epidemiological or clinical characteristics or treatment modalities demonstrated significant differences between the two groups (Table 1).
According to the univariate analysis, total protein, creatinine levels, CRP, procalcitonin, ESR, and viral load were significantly higher in Group 1 than in Group 2 (p < 0.05). Lymphocyte, monocyte, thrombocyte, and eosinophil counts; total bilirubin; troponin T; and folate were significantly lower in Group 1 (p < 0.05; Table 2).
In the multivariate logistic regression analysis, lower lymphocyte counts and higher ESR demonstrated a significant association with pulmonary involvement (Table 4).
Table 4.
Multivariate logistic regression analysis examining factors affecting pulmonary involvement
| OR | 95% CI | p | |
|---|---|---|---|
| Lymphocyte count | 1.000 | 0.999–1.000 | 0.036* |
| Erythrocyte sedimentation rate | 1.061 | 1.015–1.108 | 0.008* |
| Constant | 0.935 | 0.924 |
Abbreviations: CI, confidence interval; OR, odds ratio.
p < 0.05.
3.4. Predictors of pulmonary involvement
According to the ROC curve analysis, the cutoff levels of viral load, CRP, and procalcitonin were 26.5 Ct (95% CI, 0.54–0.74; sensitivity, 0.65; specificity, 0.56; AUC = 0.647, p = 0,005), 7.85 mg/L (95% CI, 0.56–0.75; sensitivity, 0.66; specificity, 0.64; AUC = 0.656, p = 0.003), and 0.105 ng/ml (95% CI, 0.52–0.72; sensitivity, 0.55; specificity, 0.58; AUC = 0.626, p = 0.02), respectively (Figure 1A–C).
Figure 1.
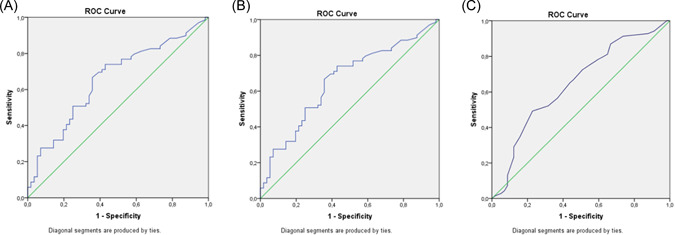
(A–C) ROC curves for viral load, CRP, and procalcitonin. CRP, C‐reactive protein; ROC, receiver operating characteristic. [Color figure can be viewed at wileyonlinelibrary.com]
4. DISCUSSION
This study aimed to evaluate the clinical and laboratory manifestations that can identify the risk of pulmonary involvement in children with COVID‐19. Our study's major findings indicated that increased CRP (>7.85 mg/L), procalcitonin (>0.105 ng/ml), and viral load (<26.5 Ct) threshold values could be a predictor for pulmonary involvement in children with COVID‐19. There is limited research investigating factors associated with pulmonary involvement in children with COVID‐19; hence, we believe our study makes an important contribution to the current literature regarding this challenging issue.
Previous studies have reported that COVID‐19's prevalence in children is lower compared to adults, but individuals of all ages can be infected. 6 , 7 Similarly, we found that the age range was 1–216 months with a median of 24 months.
To date, previous epidemiological studies have reported that most children had milder disease courses in comparison with adult patients. 2 , 6 , 8 , 9 Clinical findings related to COVID‐19 usually resemble other respiratory viral infections, with fever and cough being common in most studies. Our findings were similar to those of previous studies. 2 , 6 , 10
Data from previous studies demonstrate that infants and young children seem to have a high risk of severe disease. 2 , 7 Unlike these reports, we found that older age was associated with increased risk of pulmonary involvement. In a retrospective cohort of adult COVID‐19 patients who progressed to pneumonia, Jung et al. reported that older patients (>45 years of age) developed pneumonia on follow‐up chest radiography. 11 In our study, the durations of fever and hospitalization were significantly longer in patients with pulmonary involvement. No pulmonary involvement group was hospitalized for a median of 8 days. The long stay in the hospital was caused by long‐lasting fever, diarrhea, poor feeding, and underlying medical conditions.
Many studies have investigated the association between viral load and disease mortality and severity, but there is no information on the correlation between viral load and pulmonary involvement in SARS‐CoV‐2‐infected children. Previous studies have shown that a higher viral load is associated with increased disease severity and worse outcomes. 4 , 12 , 13 , 14 In addition to these reports, El Zein et al. 15 demonstrated that a high viral load was an independent risk factor for in‐hospital mortality and intubation. A prospective cohort study by Knudtzen et al. 16 conducted on adult patients reported that a higher viral load can predict disease severity in hospitalized patients. Extensive lung involvement was found to be one of the identifying factors associated with increased disease severity. 17 Our study added to this knowledge that a high viral load can predict the risk for pulmonary involvement in children with COVID‐19.
Elevated inflammatory biomarkers suggest excessive cytokine production correlates with COVID‐19 pneumonia and disease severity. Many mechanisms, including inflammatory biomarker release, alveolar tissue macrophage activation, mononuclear cell accumulation, diffuse alveolar edema, and interstitial inflammation, can contribute to pulmonary injury and pneumonia. 17 , 18 , 19 , 20 In this study, we showed that on the one hand, CRP, procalcitonin levels, and ESR were significantly higher and lymphocyte, monocyte, eosinophil, and thrombocyte counts were significantly lower in children with pulmonary involvement than in those without. Our results are consistent with previous studies showing that patients with COVID‐19 had decreased platelet, lymphocyte, monocyte, and eosinophil counts and increased CRP levels. 21 , 22 There are reports investigating factors associated with pulmonary involvement in adult patients with COVID‐19. 23 , 24 In a retrospective study, Damar Çakırca et al. 23 reported that the neutrophil–lymphocyte ratio, the platelet–lymphocyte ratio, and the CRP–lymphocyte ratio were higher and the eosinophil–lymphocyte ratio was lower in adult COVID‐19 patients with pulmonary involvement than in those without. In another study, Abrishami et al. 24 showed that 25 (OH) vitamin D levels were predictive for the amount of lung involvement shown in chest CT. Ferritin was identified as an inflammatory biomarker resulting in immune dysregulation in severe COVID‐19 patients. 25 In addition to this role of ferritin, Carubbi et al. 26 have also demonstrated that higher ferritin levels (above the 25th percentile) were associated with lung involvement severity. Predictors of pulmonary involvement risk in children with COVID‐19 were not clearly determined. In the present study, we detected that high CRP, procalcitonin levels, and ESR, and low lymphocyte and thrombocyte counts may predict pulmonary involvement risk in children with COVID‐19.
5. LIMITATIONS
This study's major limitations were its small sample size and single‐center design. Additionally, we did not perform chest CT for pulmonary involvement in every patient.
6. CONCLUSIONS
The identification of predictors for pulmonary involvement in COVID‐19 is crucial for guiding appropriate management and generating better disease outcomes. The present study determined that high CRP, procalcitonin levels, ESR, and viral load, and low lymphocyte and thrombocyte counts were associated with pulmonary involvement in children with COVID‐19. Procalcitonin, CRP levels, and viral load may predict the risk for pulmonary involvement in COVID‐19 patients. These inflammatory biomarkers can be used to demonstrate pulmonary involvement in children with COVID‐19 when supported by well‐designed different studies. We think our results could make a significant contribution to this field, about which little is currently known.
AUTHOR CONTRIBUTIONS
Nurhayat Yakut: Conceptualization; Investigation; Writing – original draft; Methodology; Validation; Writing – review and editing; Formal analysis; Data curation; Supervision; Resources. Kahraman Yakut: Conceptualization; Investigation; Formal analysis. Zeynep Sarihan: Validation; Investigation. Irem Kabasakal: Investigation; Validation. Murat Aydin: Validation; Investigation. Nuran Karabulut: Investigation; Validation; Supervision; Resources.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ACKNOWLEDGMENT
The authors thank MD Cansaran Tanidir for his editorial support in carrying out the study.
Yakut N, Yakut K, Sarihan Z, Kabasakal I, Aydin M, Karabulut N. Predictors of pulmonary involvement in children with COVID‐19: how strongly associated is viral load? Pediatr Pulmonol. 2022;1‐8. 10.1002/ppul.26165
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1.World Health Organization. Coronavirus Disease (COVID‐19) Situation Reports [Internet]. Accessed February 20, 2022. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports
- 2. Zhu T, Wang Y, Zhou S, Zhang N, Xia L. A comparative study of chest computed tomography features in young and older adults with corona virus disease (COVID‐19). J Thorac Imaging. 2020;35:W97‐W101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID‐19 in the New York City area. JAMA. 2020;323:2052‐2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Maltezou HC, Raftopoulos V, Vorou R, et al. Association between upper respiratory tract viral load, comorbidities, disease severity, and outcome of patients with SARS‐CoV‐2 infection. J Infect Dis. 2021;223:1132‐1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. https://bioeksen.com.tr/sars-cov2-double-gene-rtqpcr-kit.
- 6. Escosa‐García L, Aguilera‐Alonso D, Calvo C, Mellado MJ, Baquero‐Artigao F. Ten key points about COVID‐19 in children: the shadows on the wall. Pediatr Pulmonol. 2020;55:2576‐2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Parri N, Lenge M, Buonsenso D. Children with Covid‐19 in pediatric emergency departments in Italy. N Engl J Med. 2020;383:187‐190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Castagnoli R, Votto M, Licari A, et al. Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection in children and adolescents: a systematic review. JAMA Pediatrics. 2020;174:882‐889. [DOI] [PubMed] [Google Scholar]
- 9. Hoang A, Chorath K, Moreira A, et al. COVID‐19 in 7780 pediatric patients: a systematic review. EClinicalMedicine. 2020;24:100433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Garazzino S, Montagnani C, Donà D, et al. Multicentre Italian study of SARS‐CoV‐2 infection in children and adolescents, preliminary data as at 10 April 2020. Euro Surveill. 2020;25:2000600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jung HK, Kim JY, Lee MS, et al. Characteristics of COVID‐19 patients who progress to pneumonia on follow‐up chest radiograph: 236 patients from a single isolated cohort in Daegu, South Korea. Korean J Radiol. 2020;21:1265‐1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pujadas E, Chaudhry F, McBride R, et al. SARS‐CoV‐2 viral load predicts COVID‐19 mortality. Lancet Respir Med. 2020;8:e70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bryan A, Fink SL, Gattuso MA, et al. SARS‐CoV‐2 viral load on admission is associated with 30‐day mortality. Open Forum Infect Dis. 2020;7:ofaa535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rao SN, Manissero D, Steele VR, Pareja J. A systematic review of the clinical utility of cycle threshold values in the context of COVID‐19. Infect Dis Ther. 2020;9:573‐586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. El Zein S, Chehab O, Kanj A, et al. SARS‐CoV‐2 infection: initial viral load (iVL) predicts severity of illness/outcome, and declining trend of iVL in hospitalized patients corresponds with slowing of the pandemic. PLoS One. 2021;16:e0255981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Knudtzen FC, Jensen TG, Lindvig SO, et al. SARS‐CoV‐2 viral load as a predictor for disease severity in outpatients and hospitalised patients with COVID‐19: a prospective cohort study. PLoS One. 2021;16:e0258421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gallo Marin B, Aghagoli G, Lavine K, et al. Predictors of COVID‐19 severity: a literature review. Rev Med Virol. 2021;31:1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sun X, Wang T, Cai D, et al. Cytokine storm intervention in the early stages of COVID‐19 pneumonia. Cytokine Growth Factor Rev. 2020;53:38‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jose RJ, Manuel A. COVID‐19 cytokine storm: the interplay between inflammation and coagulation. Lancet Respir Med. 2020;8:e46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shang W, Dong J, Ren Y, et al. The value of clinical parameters in predicting the severity of COVID‐19. J Med Virol. 2020;92:2188‐2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Henry BM, de Oliveira MHS, Benoit S, Plebani M, Lippi G. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID‐19): a meta‐analysis. Clin Chem Lab Med. 2020;58:1021‐1028. [DOI] [PubMed] [Google Scholar]
- 22. Kermali M, Khalsa RK, Pillai K, Ismail Z, Harky A. The role of biomarkers in diagnosis of COVID‐19 a systematic review. Life Sci. 2020;254:117788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Damar Çakırca T, Torun A, Çakırca G, Portakal RD. Role of NLR, PLR, ELR and CLR in differentiating COVID‐19 patients with and without pneumonia. Int J Clin Pract. 2021;75:e14781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Abrishami A, Dalili N, Mohammadi Torbati P, et al. Possible association of vitamin D status with lung involvement and outcome in patients with COVID‐19: a retrospective study. Eur J Nutr. 2021;60:2249‐2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kappert K, Jahić A, Tauber R. Assessment of serum ferritin as a biomarker in COVID‐19: bystander or participant? Insights by comparison with other infectious and non‐infectious diseases. Biomarkers. 2020;25:616‐625. [DOI] [PubMed] [Google Scholar]
- 26. Carubbi F, Salvati L, Alunno A, et al. Ferritin is associated with the severity of lung involvement but not with worse prognosis in patients with COVID‐19: data from two Italian COVID‐19 units. Sci Rep. 2021;11:4863. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


