Abstract
Observational studies have reported high comorbidity between type 2 diabetes (T2D) and severe COVID‐19. However, the causality between T2D and COVID‐19 has yet to be validated. We performed genetic correlation and Mendelian randomization (MR) analyses to assess genetic relationships and potential causal associations between T2D and three COVID‐19 outcomes (severe acute respiratory syndrome coronavirus 2 [SARS‐CoV‐2] infection, COVID‐19 hospitalization, and critical COVID‐19). Molecular pathways connecting SARS‐CoV‐2 and COVID‐19 were reconstructed to extract insights into the potential mechanisms underlying the connection. We identified a high genetic overlap between T2D and each COVID‐19 outcome (genetic correlations 0.21–0.28). The MR analyses indicated that genetic liability to T2D confers a causal effect on hospitalized COVID‐19 (odds ratio 1.08, 95% confidence interval [CI] 1.04–1.12) and critical COVID‐19 (1.09, 1.03–1.16), while genetic liability to SARS‐CoV‐2 infection exerts a causal effect on T2D (1.25, 1.00–1.56). There was suggestive evidence that T2D was associated with an increased risk for SARS‐CoV‐2 infection (1.02, 1.00–1.03), while critical COVID‐19 (1.06, 1.00–1.13) and hospitalized COVID‐19 (1.09, 0.99–1.19) were associated with an increased risk for T2D. Pathway analysis identified a panel of immunity‐related genes that may mediate the links between T2D and COVID‐19 at the molecular level. Our study provides robust support for the bidirectional causal associations between T2D and COVID‐19. T2D may contribute to amplifying the severity of COVID‐19, while the liability to COVID‐19 may increase the risk for T2D.
Keywords: COVID‐19, Mendelian randomization, type 2 diabetes
1. INTRODUCTION
Since the inception of the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection pandemic, a large number of studies have sought to investigate the risk factors for COVID‐19. 1 , 2 , 3 , 4 , 5 , 6 The shortlist of COVID‐19‐aggravating conditions includes diabetes, cardiovascular diseases, chronic kidney disease, and obesity. 7 , 8 , 9 Meanwhile, a significant subpopulation of individuals infected with SARS‐CoV‐2 suffers from a range of post‐COVID symptoms or consequences of this disease more than a month after an initial infection, which is termed “long‐COVID.” 10 , 11
Metabolic dysfunction can aggravate COVID‐19 syndromes. 12 Type 2 diabetes (T2D) makes up approximately 90% of all diabetes cases and is characterized by peripheral resistance to insulin resulting in elevated blood sugar. Consequently, T2D patients may demonstrate increased thirst, frequent urination, increased hunger, tired feeling, and sores that do not heal. 13 Complications of long‐term T2D may include heart disease, ischemic or hemorrhagic stroke, diabetic retinopathy that can result in low acuity or even blindness, kidney failure, and poor blood flow in the limbs that may lead to amputations. 14 Although T2D primarily occurs as a result of obesity and lack of exercise, genetic predisposition does play a causal role in defining the onset in at least some patients. 15
Multiple studies have suggested that T2D is one of the significant risk factors for severe COVID‐19, often accompanied by hospitalization or death. 16 Hyperglycemia and glycemic fluctuation levels may adversely influence COVID‐19 outcomes. 17 , 18 , 19 , 20 One study indicated that treatment with insulin might contribute to increased mortality in patients with COVID‐19. 21 T2D could also impair nasal immunity and elevate hyposmia risk in patients with mild COVID‐19 pneumonia. 22 Some shared molecular pathways for T2D and coronavirus infections have been described before, with therapeutic implications being suggested. 23 An observational study of 435 504 UK Biobank participants pointed to a strong association between T2D and COVID‐19. 24
In addition, some studies also reported a COVID‐19 association with an increased risk for T2D. 25 , 26 Up to 50% of COVID‐19 deaths are observed in individuals with metabolic and vascular disorders, which suggests a direct link between COVID‐19 and changes in metabolic and endocrine systems. 12 It seems that not only metabolic dysfunctions (e.g., obesity, hypertension, and diabetes) increase the risk of developing severe COVID‐19 but also infection with SARS‐CoV‐2 might lead to new‐onset diabetes or aggravation of pre‐existing metabolic disorders. 12 However, the mechanism behind the T2D‐COVID‐19 association is not clear. Exploring this link may help remove uncertainty in the management of T2D in the context of coronavirus infection.
The Mendelian randomization (MR) framework can be used to infer a potential causative association between a phenotype (exposure) that can be genetically influenced and a disease outcome by utilizing genetic variants as instrumental variables. 27 Previous MR studies have revealed causal risk factors for COVID‐19, including body mass index and smoking intensity. 1 , 2 , 3 Since T2D has been suggested as an additional risk factor for COVID‐19, several previous studies were conducted to investigate the possible causality of this relationship. 1 , 24 , 28 , 29 , 30 However, no causal associations between T2D and COVID‐19 have been identified.
Given the above observations, we hypothesize that T2D may be causally associated with increased severity of COVID‐19, while COVID‐19 may exert a causal effect on T2D, thus promoting diabetes as one of the consequences of COVID‐19. We sought to test genetic correlations and the potential mutual causal associations between T2D and COVID‐19 using large‐sample data sets with three COVID‐19 outcomes: (1) SARS‐CoV‐2 infection; (2) hospitalized COVID‐19, and (3) critical COVID‐19. Moreover, the pathways connecting these two diseases were constructed to explore the potential mechanisms underlying the T2D‐COVID‐19 connection at the molecular level.
2. METHODS
2.1. Genome‐wide association study(GWAS) summary data sets
The study utilized publicly available Genome‐wide association study (GWAS) summary results, including those on SARS‐CoV‐2 infection (SARS‐CoV‐2 infection, 112 612 cases, and 2 474 079 controls, with 88.9% participants being of European origins), hospitalized COVID‐19 (24 274 cases and 2 061 529 controls, with 87.7% participants being of European origin), critical COVID‐19 (8779 cases and 1 001 875 controls, with 94.9% participants being of European origin), and T2D (74 124 T2D cases and 824 006 controls, all the participants being of European origin) controls. 31 The COVID‐19 data sets were obtained from the COVID‐19 HGI GWAS round six (release date: June 15, 2021). 32 Ethical approval was obtained from all the original studies. The SARS‐CoV‐2 infection data set mainly reflects the overall susceptibility to the virus, whereas the hospitalized and critical COVID‐19 data sets represent the severity of the disease. Therefore, we collectively called the latter two outcomes “severe COVID‐19.” Single‐nucleotide polymorphisms (SNPs) with conflicting alleles between the T2D and COVID‐19 data sets were excluded. The effects (values and directions) of SNPs were harmonized between the T2D data set and the COVID‐19 data sets.
2.2. Genetic correlation analysis
The genetic correlations between T2D and the COVID‐19 outcomes were calculated using Linkage disequilibrium (LD) score regression. 33 , 34 The 1000 Genome Project Phase 3 was used to estimate the LD structure for European populations. 33 , 34 , 35 SNPs were filtered by 1.1 million variants, a subset of 1000 Genomes and HapMap3, with minor allele frequency above 0.05.
2.3. MR analysis
The main analyses were performed using the inverse‐variance weighted (IVW) method and complemented with the weighted median and MR‐Egger methods implemented in TwoSampleMR. 36 For each MR analysis, SNPs with genome‐wide significance (p < 5 × 10−8) were selected as instrumental variants and further pruned using a clumping r 2 cutoff of 0.01. An odds ratio (OR) from the IVW model significantly different from one indicates that the odds of an outcome are affected by the exposure. The intercept from the MR‐Egger model was used as a measure of directional pleiotropy.
2.4. Knowledge‐based analysis
To explore the potential connection between T2D and COVID‐19 at the molecular level, large‐scale literature data mining was executed in the Pathway Studio (www.pathwaystudio.com) environment, 37 containing approximately 14 million unique associations from >40 million scientific references. Then, a set of molecular pathways connecting T2D and COVID‐19 was constructed. First, the downstream targets and upstream regulators of T2D and COVID‐19 were identified, followed by a manual review of the references and related sentences for quality control of each extracted relationship. The relationships with no polarity or related to COVID‐19 or T2D indirectly were removed. The remaining relationships were employed to build a map of the molecular pathways connecting COVID‐19 and T2D.
For each set of genes, the tissue specificity was measured against each of the differentially expressed gene sets using the hypergeometric test. 38 For each gene set, pathway enrichment analyses of gene ontology molecular functions were conducted using FUMA. 38 A graph of protein–protein interactions (PPIs) was constructed using STRING v11. 39
3. RESULTS
3.1. Genetic correlation analysis
Genetic correlation analyses showed significant positive genetic correlations of T2D with critical COVID‐19 (r g = 0.264 ± 0.052, p = 3.60E−07), hospitalized COVID‐19 (r g = 0.275 ± 0.043, p = 2.23E−10), and SARS‐CoV‐2 infection (r g = 0.207 ± 0.04, p = 2.02E−07).
3.2. MR analysis
In the MR analysis of the causal effects of T2D on the COVID‐19 outcomes, we found that genetically determined liability to T2D confers a causal effect on hospitalized COVID‐19 (OR 1.08, 95% confidence interval [CI]: 1.04–1.12, p = 9.74E−05) and critical COVID‐19 (1.09, 1.03–1.16, p = 3.22E−03), but not on SARS‐CoV‐2 infection (1.02, 1.00–1.03, p = 0.075) (Table 1 and Figure 1).
Table 1.
Causal effects of T2D on COVID‐19 outcomes
| Exposure | Outcome | Method | b (SE) | OR [95%CI] | N_IV | Egger_intercept | P_pleiotropy | p |
|---|---|---|---|---|---|---|---|---|
| T2D | Critical COVID‐19 | IVW | 0.090 (0.031) | 1.09 [1.03–1.16] | 300 | 2.83E−03 | 0.509 | 3.22E−03 |
| T2D | Critical COVID‐19 | Weighted median | 0.094 (0.044) | 1.10 [1.01–1.20] | 300 | 2.83E−03 | 0.509 | 0.033 |
| T2D | Critical COVID‐19 | MR Egger | 0.039 (0.084) | 1.04 [0.88–1.23] | 300 | 2.83E−03 | 0.509 | 0.644 |
| T2D | Hospitalized COVID‐19 | IVW | 0.073 (0.019) | 1.08 [1.04–1.12] | 305 | 4.93E−03 | 0.070 | 9.74E−05 |
| T2D | Hospitalized COVID‐19 | Weighted median | 0.083 (0.024) | 1.09 [1.04–1.14] | 305 | 4.93E−03 | 0.070 | 4.98E−04 |
| T2D | Hospitalized COVID‐19 | MR Egger | −0.019 (0.054) | 0.98 [0.88–1.09] | 305 | 4.93E−03 | 0.070 | 0.725 |
| T2D | SARS‐CoV‐2 infection | IVW | 0.016 (0.009) | 1.02 [1.00–1.03] | 303 | 1.29E−03 | 0.303 | 0.075 |
| T2D | SARS‐CoV‐2 infection | Weighted median | 0.006 (0.009) | 1.01 [0.99–1.02] | 303 | 1.29E−03 | 0.303 | 0.516 |
| T2D | SARS‐CoV‐2 infection | MR Egger | −0.008 (0.025) | 0.99 [0.94–1.04] | 303 | 1.29E−03 | 0.303 | 0.733 |
Abbreviations: b, effect size; CI, confidence interval; IVW, inverse variance weighted; MR, Mendelian randomization; N_IV, number of instrumental variables; OR, odds ratio; SARS‐CoV‐2, syndrome coronavirus 2; SE, standard error; T2D, type 2 diabetes.
Figure 1.
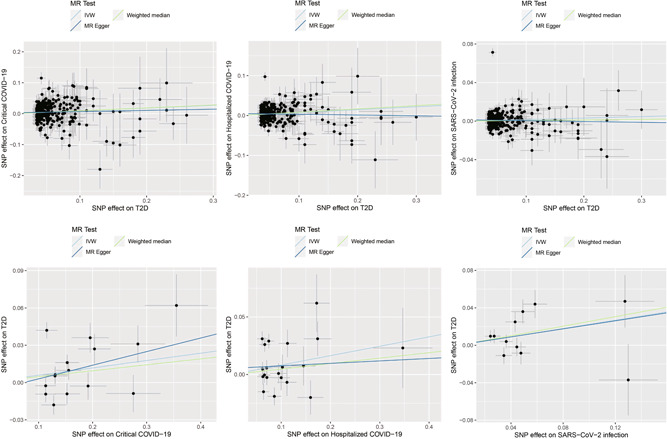
Causal associations between T2D and COVID‐19. The trait on the x‐axis denotes exposure, the trait on the y‐axis denotes outcome, and each cross point represents an instrumental variant. The lines denote b of exposure on outcome. b, effect sizes; IVW, inverse variance weighted.
In the MR analysis of causal effects of the COVID‐19 outcomes on T2D, we found that genetic liability to SARS‐CoV‐2 infection was nominally associated with T2D (1.25, 1.00–1.56, p = 0.046). Genetic liability to critical COVID‐19 (1.06, 1.00–1.13, p = 0.058) and hospitalized COVID‐19 (1.09, 0.99–1.19, p = 0.072) showed suggestive causal effects on T2D (Table 2 and Figure 1).
Table 2.
Causal effects of COVID‐19 outcomes on T2D
| Exposure | Outcome | Method | b (SE) | OR [95%CI] | N_IV | Egger_intercept | P_pleiotropy | p |
|---|---|---|---|---|---|---|---|---|
| Critical COVID‐19 | T2D | IVW | 0.059 (0.031) | 1.06 [1.00–1.13] | 15 | −7.96E−03 | 0.657 | 0.058 |
| Critical COVID‐19 | T2D | Weighted median | 0.047 (0.024) | 1.05 [1.00–1.10] | 15 | −7.96E−03 | 0.657 | 0.049 |
| Critical COVID‐19 | T2D | MR Egger | 0.109 (0.115) | 1.12 [0.89–1.40] | 15 | −7.96E−03 | 0.657 | 0.361 |
| Hospitalized COVID‐19 | T2D | IVW | 0.082 (0.045) | 1.09 [0.99–1.19] | 20 | 5.37E−03 | 0.649 | 0.072 |
| Hospitalized COVID‐19 | T2D | Weighted median | 0.047 (0.036) | 1.05 [0.98–1.12] | 20 | 5.37E−03 | 0.649 | 0.189 |
| Hospitalized COVID‐19 | T2D | MR Egger | 0.021 (0.139) | 1.02 [0.78–1.34] | 20 | 5.37E−03 | 0.649 | 0.880 |
| SARS‐CoV‐2 infection | T2D | IVW | 0.223 (0.112) | 1.25 [1.00–1.56] | 11 | 3.72E−04 | 0.977 | 0.046 |
| SARS‐CoV‐2 infection | T2D | Weighted median | 0.255 (0.114) | 1.29 [1.03–1.61] | 11 | 3.72E−04 | 0.977 | 0.025 |
| SARS‐CoV‐2 infection | T2D | MR Egger | 0.215 (0.306) | 1.24 [0.68–2.26] | 11 | 3.72E−04 | 0.977 | 0.501 |
Abbreviations: b, effect size; CI, confidence interval; IVW, inverse variance weighted; MR, Mendelian randomization; N_IV, number of instrumental variables; OR, odds ratio; SARS‐CoV‐2, syndrome coronavirus 2; SE, standard error; T2D, type 2 diabetes.
The sensitivity analyses revealed that the directions of causal effect estimates across the methods were largely the same (Tables 1 and 2). Notably, tests of MR‐Egger regression did not support the directional pleiotropy of the genetic instrumental variables for the MR analysis (MR‐Egger intercept <0.01, p > 0.05).
3.3. Knowledge‐based analysis
Mining of the molecular relationships and subsequent analysis of the reconstructed pathways revealed a total of 10 genes mediating the effect of T2D on COVID‐19, including VWF, CRP, OXT, IL6, ALB, ACE, CXCL10, TNF, DPP4, and CXCL8 (Figure 2A), and a total of 19 genes mediating the effect of COVID‐19 on T2D, including IL6, F3, IL10, ICAM1, TNF, IFNG, VEGFA, IL18, CCL2, NPPB, HMGB1, IL17A, TGFB1, VCAM1, TLR4, SERPINE1, ACE, TNNT2, and CRP (Figure 2B). Four genes, CRP, IL6, ACE, and TNF, were shared between the two gene sets. Therefore, T2D and COVID‐19 are connected by at least 25 genes.
Figure 2.
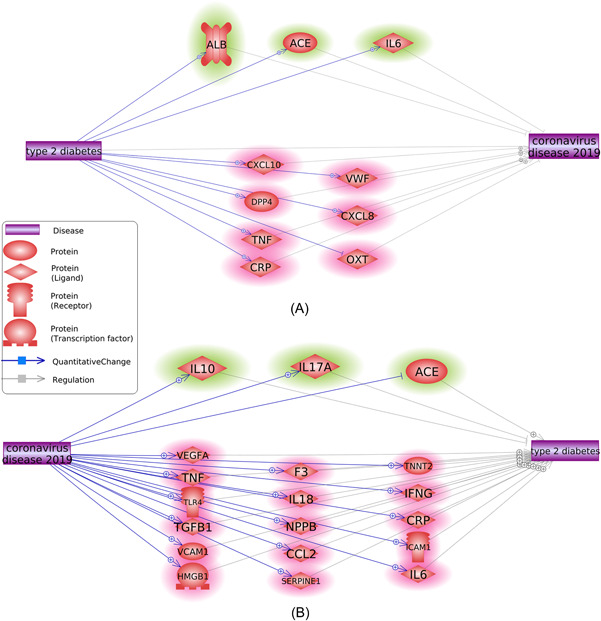
Reconstruction of molecular pathways connecting T2D and COVID‐19. (A) Quantitative genetic changes driven by T2D exert more negative (highlighted in red) than positive (highlighted in green) effects on COVID‐19. (B) Quantitative genetic changes driven by COVID‐19 exert more negative (highlighted in red) than positive (highlighted in green) effects on T2D. T2D, type 2 diabetes.
Gene‐based tissue enrichment analysis showed that this set of 25 genes is expressed predominantly in the lungs, adipose tissue, and kidney (Figure 3A). PPI analysis using the STRING database showed that the 25 proteins encoded by the above mentioned gene set form a tightly interconnected network (Figure 3B) enriched in immunity‐related molecular functions (Figure 3C).
Figure 3.
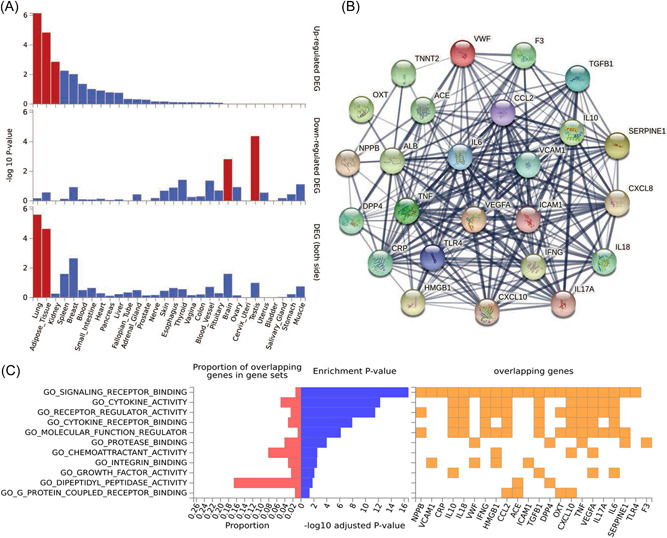
Functional analyses of the 25 genes connecting T2D and COVID‐19. (A) Tissue expression enrichment analyses of the genes. Significantly enriched DEG sets (p Bonferroni < 0.05) are highlighted in red. (B) Protein–protein interactions among the genes. (C) gene ontology pathway analysis of the genes. DEG, differentially expressed gene; T2D, T2D, type 2 diabetes.
4. DISCUSSION
T2D and COVID‐19 have been suggested to be mutual risk factors, with the underlying mechanism(s) being largely unknown. 25 , 40 In this study, we conducted genetic correlation analysis, MR analysis, and knowledge‐based pathway reconstruction to explore the mechanisms underlying the connection between T2D and COVID‐19 in the context of their shared genetics. We detected positive genetic correlations between T2D and all COVID‐19 outcomes. Although the magnitudes of the genetic correlations observed in our study are relatively low (0.207–0.275), the positive relationships support previously observed associations of T2D with COVID‐19 at the genetic level.
Although previous MR analyses did not detect causal associations between T2D and COVID‐19, 1 , 24 , 28 , 29 , 30 our study revealed robust causal associations between T2D and severe outcomes of COVID‐19, namely, hospitalized COVID‐19 and critical COVID‐19. A possible explanation for newly discovered significant associations is an increase in the study power achieved by employing larger data sets for both COVID‐19 and T2D. Our results support the hypothesis that T2D may augment the pathophysiology of COVID‐19, thus adding to its severity. The results of our study are in line with previous observations of T2D‐attributed risks for hospitalization and/or death in COVID‐19 patients. 41 The evidence of an increased risk for T2D patients to contract SARS‐CoV‐2 infection was insufficient. As the SARS‐CoV‐2 virus is highly infectious, the observed trend may be negligible or attributed to asymmetric incompleteness of the detection of silent virus carriers or mild infections in relatively healthy, nondiabetic individuals. 42
Our study also detected a causal effect of SARS‐CoV‐2 infection on T2D. However, more severe forms of COVID‐19 showed only suggestive causal effects on T2D, which may be due to reduced power in smaller severe COVID‐19 data sets. Since hospitalized COVID‐19 cases account for approximately 6.8% of all SARS‐CoV‐2 infections, 43 an increase in the available sizes of this type of data set is expected in parallel with the global spread of SARS‐CoV‐2. One may speculate that severe forms of COVID‐19 should be causally associated with an increased risk for T2D, in line with a similar association detected for SARS‐CoV‐2 infection. These results are consistent with previously observed findings that COVID‐19 patients are at risk of T2D 25 and suggest that T2D could be one of the post‐COVID‐19 sequelae. 12 Our study points out that the protocols for post‐acute COVID‐19 care should include the diagnosis and management of diabetes. 26
Knowledge‐based analysis showed that the mutual effects of T2D and COVID‐19 may be partially mediated through the quantitative influence of multiple upstream regulators shared by these two diseases (Figure 2). The map of the pathways built through large‐scale literature data mining shows that T2D is capable of elevating the levels of seven known COVID‐19 promoters while inhibiting one of four known COVID‐19 inhibitors (Figure 2A). Among T2D‐enhanced COVID‐19 promoters is the CXCL10 cytokine, which is upregulated in T2D 44 and serves as a biomarker for worsening the course of COVID‐19 and increasing fatality. 45 Elevated plasma levels of von Willebrand factor are a predictive marker of death and prolonged hospital stay among COVID‐19 inpatients 46 and have also been found to increase in T2D patients in parallel with endothelial dysfunction. 47 , 48 CXCL8 encodes a soluble protein commonly referred to as interleukin‐8 (IL‐8). Along with the (TNF)‐encoded cytokine Tumor necrosis factor (TNF‐α), IL‐8 orchestrates a notorious “cytokine storm” afflicting many hospitalized patients with COVID‐19. 49 Inhibitors of IL‐8 50 and TNF‐α‐dependent 51 pathways have been utilized for attenuating disease progression in severe SARS‐CoV‐2 infection.
Another interesting molecule upregulated in T2D is an angiotensin‐converting enzyme (ACE), which normally counterbalances the effects of ACE2, a functional receptor of SARS‐CoV‐2. An imbalance of the renin–angiotensin system is associated with COVID‐19 progression to its severe form. 52
These and other pathways are shown in Figure 2A and may cumulatively explain the observed links between T2D and the severe forms of COVID‐19.
The molecular pathway in Figure 2B points to the potential mechanism for the effects of COVID‐19 on establishing metabolic dysfunction or its progression to T2D. It seems that COVID‐19 could elevate the expression levels of 16 out of 18 known T2D promoters while boosting only two T2D suppressors, IL‐10 and IL‐17A. In particular, most COVID‐19 patients present with elevated levels of C‐reactive protein (CRP), 53 a widespread biomarker of both acute and systemic inflammation. The latter process also plays an etiologic role in the development of T2D. 54
Similar to T2D influences on COVID‐19 as described above, the role of COVID‐19 in the development of T2D is executed mainly through the elevation of the levels of T2D promoting molecules. Moreover, it seems that COVID‐19 and T2D‐related processes form a vicious cycle, augmenting each other. In physiologically vulnerable states, this augmentation may become even more prominent. For example, a SARS‐CoV‐2‐dependent increase in IL‐6 levels may predispose both to gestational diabetes mellitus and infection‐related hospitalization, 55 with differential liability to severe outcomes of coronavirus disease in pregnancy trimesters 56 being partially attributed to the gradual development of underlying deregulation of glucose metabolism.
Gene‐based tissue enrichment analysis showed that the set of 25 genes forming the molecular pathways presented in Figure 2 was upregulated in the lung, adipose tissue, and kidney (Figure 3A). These tissues are commonly impaired both in COVID‐19 57 , 58 , 59 and in T2D. 60 , 61 , 62 The protein products of these genes form a tightly interconnected network (Figure3B) involving multiple immunity‐related molecular pathways (Figure 3C), which were previously found to be dysfunctional in both COVID‐19 63 and T2D. 64 These results support the validity of the shared pathogenesis model reconstructed in the frame of this study (Figure 2).
The main strength of the study is that MR analysis is generally less affected by causality pitfalls, which are common in traditionally designed observational studies due to confounding factors and reverse causation. The largest available GWAS summary data sets were utilized for tracing the causative association between COVID‐19 and T2D. The vast majority of the participants in the GWAS data sets were of European ancestry, reducing the potential population heterogeneity.
Our study has several limitations. In particular, we assessed only genetic liability for both diseases with no regard to the effects of the environment, which are critical for both T2D and COVID‐19. However, in these two diseases, a substantial proportion of the variance is heritable. We acknowledge that MR analyses may be biased due to pleiotropy, especially in nonhomogenous data sets. Therefore, we have tested the MR assumptions using various models. Although the results were broadly consistent, some residual uncertainty remained. Additionally, the samples comprising the COVID‐19 data sets were collected before June 2021 and therefore do not include recent waves of SARS‐CoV‐2 infections. The causal effects of severe COVID‐19 on T2D did not reach significance, possibly due to the limited sample sizes. Therefore, validation of the findings in expanded data sets is warranted in the future.
In summary, our study reveals mutual causal associations between T2D and COVID‐19. It seems that COVID‐19 and T2D‐related processes form a vicious cycle, augmenting each other. Patients with T2D may have an increased risk for severe outcomes of COVID‐19, and T2D may be an integral part of the post‐COVID syndrome.
AUTHOR CONTRIBUTIONS
Fuquan Zhang conceived the project, supervised the study, and analyzed the data. Fuquan Zhang, Hongbao Cao, and Ancha Baranova wrote the manuscript. Xuejuan Wei and Chun Wang revised the manuscript for intellectual content. All authors read and approved the final manuscript. All authors critically reviewed and revised the manuscript, and agreed to the published version of the manuscript.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ACKNOWLEDGMENTS
The authors thank all investigators and participants from the COVID‐19 Host Genetics Initiative and the DIAGRAM consortium for sharing these data.
Cao H, Baranova A, Wei X, Wang C, Zhang F. Bidirectional causal associations between type 2 diabetes and COVID‐19. J Med Virol. 2022;1‐9. 10.1002/jmv.28100
Hongbao Cao, Ancha Baranova, and Xuejuan Wei are co‐first authors.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are openly available in the COVID‐19 Host Genetics Initiative, https://www.covid19hg.org/ and Psychiatric Genomics Consortium, https://www.med.unc.edu/pgc/download-results/
REFERENCES
- 1. COVID‐19 Host Genetics Initiative . Mapping the human genetic architecture of COVID‐19. Nature. 2021;600(7889):472‐477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rao S, Baranova A, Cao H, Chen J, Zhang X, Zhang F. Genetic mechanisms of COVID‐19 and its association with smoking and alcohol consumption. Brief Bioinform. 2021;22(6):bbab284. [DOI] [PubMed] [Google Scholar]
- 3. Zhang F, Baranova A. Smoking quantitatively increases risk for COVID‐19. Eur Respir J . Published online July 29, 2021;2101273. 10.1183/13993003.01273-2021 [DOI] [PMC free article] [PubMed]
- 4. Li J, Huang DQ, Zou B, et al. Epidemiology of COVID‐19: a systematic review and meta‐analysis of clinical characteristics, risk factors, and outcomes. J Med Virol. 2021;93(3):1449‐1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Almaghlouth NK, Davis MG, Davis MA, Anyiam FE, Guevara R, Antony SJ. Risk factors for mortality among patients with SARS‐CoV‐2 infection: a longitudinal observational study. J Med Virol. 2021;93(4):2021‐2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Alizadehsani R, Alizadeh Sani Z, Behjati M, et al. Risk factors prediction, clinical outcomes, and mortality in COVID‐19 patients. J Med Virol. 2021;93(4):2307‐2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Docherty AB, Harrison EM, Green CA, et al. Features of 20 133 UK patients in hospital with covid‐19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ. 2020;369:m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yang J, Tian C, Chen Y, Zhu C, Chi H, Li J. Obesity aggravates COVID‐19: an updated systematic review and meta‐analysis. J Med Virol. 2021;93(5):2662‐2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Malik P, Patel U, Patel K, et al. Obesity a predictor of outcomes of COVID‐19 hospitalized patients‐a systematic review and meta‐analysis. J Med Virol. 2021;93(2):1188‐1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Halpin S, O'Connor R, Sivan M. Long COVID and chronic COVID syndromes. J Med Virol. 2021;93(3):1242‐1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Baranova A, Cao H, Zhang F. Severe COVID‐19 increases the risk of schizophrenia. Psychiatry Res. 2022;317:114679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Steenblock C, Schwarz PEH, Ludwig B, et al. COVID‐19 and metabolic disease: mechanisms and clinical management. Lancet Diabetes Endocrinol. 2021;9(11):786‐798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Maruthur NM, Tseng E, Hutfless S, et al. Diabetes medications as monotherapy or metformin‐based combination therapy for type 2 diabetes: a systematic review and meta‐analysis. Ann Intern Med. 2016;164(11):740‐751. [DOI] [PubMed] [Google Scholar]
- 14. Brunton S. Pathophysiology of type 2 diabetes: the evolution of our understanding. J Fam Pract . 2016;65(4 suppl):supp_az_0416 [PubMed] [Google Scholar]
- 15. Wu Y, Ding Y, Tanaka Y, Zhang W. Risk factors contributing to type 2 diabetes and recent advances in the treatment and prevention. Int J Med Sci. 2014;11(11):1185‐1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Koh H, Moh AMC, Yeoh E, et al. Diabetes predicts severity of COVID‐19 infection in a retrospective cohort: a mediatory role of the inflammatory biomarker C‐reactive protein. J Med Virol. 2021;93(5):3023‐3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chai C, Chen K, Li S, et al. Effect of elevated fasting blood glucose level on the 1‐year mortality and sequelae in hospitalized COVID‐19 patients: a bidirectional cohort study. J Med Virol. 2022;94(7):3240‐3250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Xiao F, Zhou YC, Zhang MB, et al. Hyperglycemia and blood glucose deterioration are risk factors for severe COVID‐19 with diabetes: a two‐center cohort study. J Med Virol. 2022;94(5):1967‐1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Leng Y, Chen M, Dai M, et al. Minimized glycemic fluctuation decreases the risk of severe illness and death in patients with COVID‐19. J Med Virol. 2021;93(7):4060‐4062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Smith SM, Boppana A, Traupman JA, et al. Impaired glucose metabolism in patients with diabetes, prediabetes, and obesity is associated with severe COVID‐19. J Med Virol. 2021;93(1):409‐415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yu B, Li C, Sun Y, Wang DW. Insulin treatment is associated with increased mortality in patients with COVID‐19 and type 2 diabetes. Cell Metab. 2021;33(1):65‐77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhao Y, Liu Y, Yi F, et al. Type 2 diabetes mellitus impaired nasal immunity and increased the risk of hyposmia in COVID‐19 mild pneumonia patients. Int Immunopharmacol. 2021;93:107406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Drucker DJ. Coronavirus infections and type 2 diabetes‐shared pathways with therapeutic implications. Endocr Rev. 2020;41:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gao M, Wang Q, Piernas C, et al. Associations between body composition, fat distribution and metabolic consequences of excess adiposity with severe COVID‐19 outcomes: observational study and Mendelian randomisation analysis. Int J Obes (Lond). 2022;46:943‐950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ho G, Ali A, Takamatsu Y, Wada R, Masliah E, Hashimoto M. Diabetes, inflammation, and the adiponectin paradox: therapeutic targets in SARS‐CoV‐2. Drug Discov Today. 2021;26(8):2036‐2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Xie Y, Al‐Aly Z. Risks and burdens of incident diabetes in long COVID: a cohort study. Lancet Diabetes Endocrinol. 2022;10:311‐321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lawlor DA, Harbord RM, Sterne JA, Timpson N, Smith GD. Mendelian randomization: using genes as instruments for making causal inferences in epidemiology. Stat Med. 2008;27(8):1133‐1163. [DOI] [PubMed] [Google Scholar]
- 28. Qu HQ, Qu J, Glessner J, Hakonarson H. Mendelian randomization study of obesity and type 2 diabetes in hospitalized COVID‐19 patients. Metabolism. 2022;129:155156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Au Yeung SL, Zhao JV, Schooling CM. Evaluation of glycemic traits in susceptibility to COVID‐19 risk: a Mendelian randomization study. BMC Med. 2021;19(1):72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lorincz‐Comi N, Zhu X. Cardiometabolic risks of SARS‐CoV‐2 hospitalization using Mendelian randomization. Sci Rep. 2021;11(1):7848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mahajan A, Taliun D, Thurner M, et al. Fine‐mapping type 2 diabetes loci to single‐variant resolution using high‐density imputation and islet‐specific epigenome maps. Nat Genet. 2018;50(11):1505‐1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. COVID‐19 Host Genetics Initiative . The COVID‐19 host genetics initiative, a global initiative to elucidate the role of host genetic factors in susceptibility and severity of the SARS‐CoV‐2 virus pandemic. Eur J Hum Genet. 2020;28(6):715‐718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bulik‐Sullivan BK, Loh PR, Finucane HK, et al. LD score regression distinguishes confounding from polygenicity in genome‐wide association studies. Nat Genet. 2015;47(3):291‐295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bulik‐Sullivan B, Finucane HK, Anttila V, et al. An atlas of genetic correlations across human diseases and traits. Nat Genet. 2015;47(11):1236‐1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Finucane HK, Bulik‐Sullivan B, Gusev A, et al. Partitioning heritability by functional annotation using genome‐wide association summary statistics. Nat Genet. 2015;47(11):1228‐1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hemani G, Zheng J, Elsworth B, et al. The MR‐Base platform supports systematic causal inference across the human phenome. eLife. 2018;7:e34408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nikitin A, Egorov S, Daraselia N, Mazo I. Pathway studio—the analysis and navigation of molecular networks. Bioinformatics. 2003;19(16):2155‐2157. [DOI] [PubMed] [Google Scholar]
- 38. Watanabe K, Taskesen E, van Bochoven A, Posthuma D. Functional mapping and annotation of genetic associations with FUMA. Nat Commun. 2017;8(1):1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Szklarczyk D, Gable AL, Lyon D, et al. STRING v11: protein–protein association networks with increased coverage, supporting functional discovery in genome‐wide experimental datasets. Nucleic Acids Res. 2019;47(D1):D607‐D613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Brandão SCS, Ramos JOX, Dompieri LT, et al. Is Toll‐like receptor 4 involved in the severity of COVID‐19 pathology in patients with cardiometabolic comorbidities? Cytokine Growth Factor Rev. 2021;58:102‐110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Longo M, Caruso P, Maiorino MI, Bellastella G, Giugliano D, Esposito K. Treating type 2 diabetes in COVID‐19 patients: the potential benefits of injective therapies. Cardiovasc Diabetol. 2020;19(1):115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chatterjee S, Datey A, Sengupta S, et al. Clinical, virological, immunological, and genomic characterization of asymptomatic and symptomatic cases with SARS‐CoV‐2 infection in India. Front Cell Infect Microbiol. 2021;11:725035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Simpson CR, Robertson C, Vasileiou E, et al. Temporal trends and forecasting of COVID‐19 hospitalisations and deaths in Scotland using a national real‐time patient‐level data platform: a statistical modelling study. Lancet Digit Health. 2021;3(8):e517‐e525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Schulthess FT, Paroni F, Sauter NS, et al. CXCL10 impairs beta cell function and viability in diabetes through TLR4 signaling. Cell Metab. 2009;9(2):125‐139. [DOI] [PubMed] [Google Scholar]
- 45. Rizzi M, Costanzo M, Tonello S, et al. Prognostic markers in hospitalized COVID‐19 patients: the role of IP‐10 and C‐reactive protein. Dis Markers. 2022;2022:3528312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Cotter AH, Yang ST, Shafi H, Cotter TM, Palmer‐Toy DE. Elevated von Willebrand factor antigen is an early predictor of mortality and prolonged length of stay for coronavirus disease 2019 (COVID‐19) inpatients. Arch Pathol Lab Med. 2022;146(1):34‐37. [DOI] [PubMed] [Google Scholar]
- 47. Domingueti CP, Dusse LM, Carvalho M, Gomes KB, Fernandes AP. Hypercoagulability and cardiovascular disease in diabetic nephropathy. Clin Chim Acta. 2013;415:279‐285. [DOI] [PubMed] [Google Scholar]
- 48. Persson F, Rossing P, Hovind P, et al. Endothelial dysfunction and inflammation predict development of diabetic nephropathy in the Irbesartan in patients with type 2 diabetes and microalbuminuria (IRMA 2) study. Scand J Clin Lab Invest. 2008;68(8):731‐738. [DOI] [PubMed] [Google Scholar]
- 49. Cesta MC, Zippoli M, Marsiglia C, et al. The role of interleukin‐8 in lung inflammation and injury: implications for the management of COVID‐19 and hyperinflammatory acute respiratory distress syndrome. Front Pharmacol. 2021;12:808797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Landoni G, Zangrillo A, Piersanti G, Scquizzato T, Piemonti L. The effect of reparixin on survival in patients at high risk for in‐hospital mortality: a meta‐analysis of randomized trials. Front Immunol. 2022;13:932251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Guo Y, Hu K, Li Y, et al. Targeting TNF‐alpha for COVID‐19: recent advanced and controversies. Front Public Health. 2022;10:833967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Tepasse PR, Vollenberg R, Steinebrey N, Konig S. High angiotensin‐converting enzyme and low carboxypeptidase N serum activity correlate with disease severity in COVID‐19 patients. J Pers Med. 2022;12(3):406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Luo X, Zhou W, Yan X, et al. Prognostic value of C‐reactive protein in patients with coronavirus 2019. Clin Infect Dis. 2020;71(16):2174‐2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wolford JK, Gruber JD, Ossowski VM, et al. A C‐reactive protein promoter polymorphism is associated with type 2 diabetes mellitus in pima Indians. Mol Genet Metab. 2003;78(2):136‐144. [DOI] [PubMed] [Google Scholar]
- 55. Fedullo AL, Schiattarella A, Morlando M, et al. Mediterranean diet for the prevention of gestational diabetes in the Covid‐19 era: implications of Il‐6 in diabesity. Int J Mol Sci. 2021;22:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Sahin D, Tanacan A, Erol SA, et al. Management of pregnant women with COVID‐19: a tertiary pandemic center experience on 1416 cases. J Med Virol. 2022;94(3):1074‐1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Iacobellis G, Secchi F, Capitanio G, et al. Epicardial fat inflammation in severe COVID‐19. Obesity (Silver Spring). 2020;28(12):2260‐2262. [DOI] [PubMed] [Google Scholar]
- 58. Liu H, Liu F, Li J, Zhang T, Wang D, Lan W. Clinical and CT imaging features of the COVID‐19 pneumonia: focus on pregnant women and children. J Infect. 2020;80(5):e7‐e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Post A, Dullaart RPF, Bakker SJL. Sodium status and kidney involvement during COVID‐19 infection. Virus Res. 2020;286:198034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Szekacs B, Vajo Z, Varbiro S, et al. Postmenopausal hormone replacement improves proteinuria and impaired creatinine clearance in type 2 diabetes mellitus and hypertension. BJOG. 2000;107(8):1017‐1021. [DOI] [PubMed] [Google Scholar]
- 61. Koivula FNM, McClenaghan NH, Harper AGS, Kelly C. Islet‐intrinsic effects of CFTR mutation. Diabetologia. 2016;59(7):1350‐1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Giannopoulou I, Ploutz‐Snyder LL, Carhart R, et al. Exercise is required for visceral fat loss in postmenopausal women with type 2 diabetes. J Clin Endocrinol Metab. 2005;90(3):1511‐1518. [DOI] [PubMed] [Google Scholar]
- 63. Donma MM, Donma O. The effects of allium sativum on immunity within the scope of COVID‐19 infection. Med Hypotheses. 2020;144:109934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Simar D, Versteyhe S, Donkin I, et al. DNA methylation is altered in B and NK lymphocytes in obese and type 2 diabetic human. Metabolism. 2014;63(9):1188‐1197. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are openly available in the COVID‐19 Host Genetics Initiative, https://www.covid19hg.org/ and Psychiatric Genomics Consortium, https://www.med.unc.edu/pgc/download-results/


