Abstract
Post–coronavirus disease 2019 (COVID-19) cholangiopathy (PCC) is a new entity observed in patients recovering from severe COVID-19 pneumonia. Most patients recover with cholestasis improving over a period of time. In some patients, cholestasis is severe and persists or progresses to liver failure necessitating liver transplant. We present a previously healthy 50-year-old man who developed PCC with peak total bilirubin of 42.4 mg/dl and did not improve with medical management. He underwent living donor auxiliary right lobe liver transplantation. He recovered well after transplant and remains asymptomatic at 6 months follow-up with good graft function and recovering function in native liver remnant.
KEYWORDS: cholestasis, COVID-19 pneumonia, liver regeneration, living donor liver transplantation, subacute liver failure, therapeutic plasma exchange
Abbreviations: APOLT, auxiliary partial orthotopic liver transplantation; COVID-19, coronavirus disease 2019; PCC, post–coronavirus disease 2019 cholangiopathy; LT, liver transplant; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2
1. INTRODUCTION
Post-coronavirus disease 2019 (COVID-19) cholangiopathy (PCC) is a new disease described in patients recovering from severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. Initially considered as a form of secondary sclerosing cholangitis of critically ill patients, it has now been accepted as a new entity.1 Literature regarding this recently described disease is sparse. The incidence and the natural course are not entirely clear. Most patients with PCC make an uneventful recovery from cholestasis, while some have a protracted and/or fulminant course necessitating a liver transplant (LT).1 , 2 Herein, we discuss a patient of severe PCC who was successfully treated with living donor auxiliary partial orthotopic liver transplantation (APOLT) with the hope of future functional recovery of the native liver remnant allowing for an immunosuppression-free life.
2. CASE PRESENTATION
A 50-year-old previously healthy gentleman was referred to our institute with progressively worsening cholestasis in May 2021. Six weeks prior to this presentation he had recovered from severe COVID-19 pneumonia which necessitated mechanical ventilation. His treatment included steroids and remdesivir. Though, he recovered from most of the COVID-19–associated symptoms, he was readmitted with severe jaundice [serum bilirubin: total: 22.9 mg/dl (N: 0.6–1.2 mg/dl) and direct 12 mg/dl (N: 0.1–0.4 mg/dl)]. He had no prior history of liver disease. Etiological workup for viral, metabolic, and autoimmune liver diseases was negative. There was no history of hepatotoxic drug intake or other medical comorbidity. Magnetic resonance cholangiography showed mild prominence of the central intrahepatic, common hepatic, and common bile ducts with minimal beading of the right posterior sectoral and segment 2 ducts. Bilirubinostasis with ductopenia was noted on percutaneous liver biopsy. He was initially treated with steroids and subsequently underwent 8 cycles of therapeutic plasma exchange over a 12-week period for severe jaundice with pruritus. During this period, he was hospitalized once in intensive care for sepsis. Following a multidisciplinary meeting, in view of his persistent cholestasis with a total bilirubin of 26.8 mg/dl (peak total bilirubin: 42.4 mg/dl), an episode of sepsis, and poor quality of life due to intractable pruritus, the option of APOLT was discussed with the family. His daughter came forward to donate a part of her liver. She underwent robotic right lobe donor hepatectomy after a thorough donor assessment and counseling. The patient underwent right lobe APOLT 12 weeks from the index presentation with Model for End-stage Liver Disease score of 22. Intraoperatively, the recipient’s liver appeared extremely cholestatic without evidence of cirrhosis or portal hypertension. Right trisectionectomy with caudate lobectomy was performed in the recipient and the right lobe from his daughter (graft-to-recipient weight ratio: 0.94) was implanted in the orthotopic position ( Figure 1A). Right hepatic vein and right portal vein of the right lobe graft were anastomosed to the recipient’s right hepatic vein orifice and right portal vein, respectively. After reperfusion, right hepatic artery and right hepatic duct were anastomosed to the right hepatic artery and duct of the recipient, respectively. Standard thromboprophylaxis with low-molecular weight heparin was followed in the postoperative period. The donor’s and recipient’s postoperative recovery were uneventful and they were discharged home on the 4th and 9th postoperative days, respectively. Explant histology demonstrated diffuse ductopenia with focal inflammatory infiltrate in the portal tracts with lymphocytes, histiocytes, and eosinophils. The lobular parenchyma showed marked hepatocanalicular bilirubinostasis and patchy inflammation (Figure 1B). Hepatocytes demonstrated granular positivity for SARS-CoV-2.
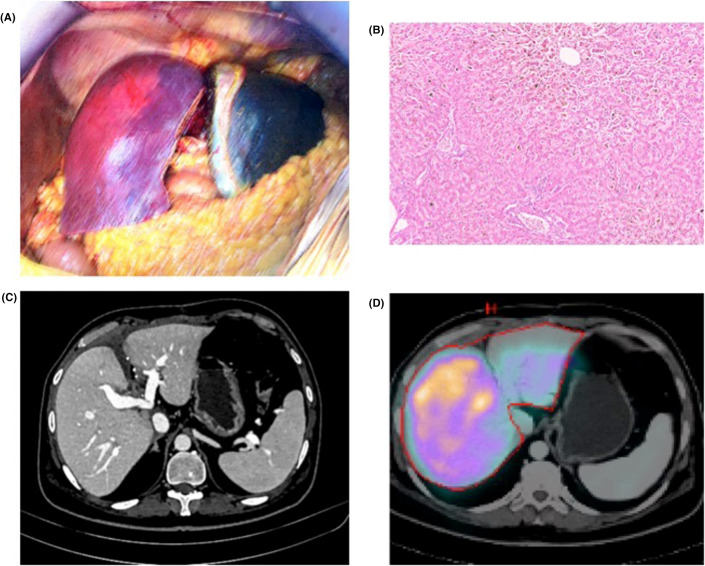
FIGURE 1.
(A) Well-perfused right lobe graft after implantation and extremely cholestatic native liver remnant. (B) Diffuse ductopenia and hepatocanalicular bilirubinostasis in the liver explant. (C) Well-perfused right lobe graft and liver remnant at 6 months follow-up. (D) HIDA scintigraphy demonstrating 90% and 10% activity in the right lobe graft and remnant native liver, respectively
At 6-month follow-up, patient remains well with unremarkable liver function tests. Imaging showed good contrast enhancement of both native and graft livers with no significant change in the volumes compared to immediate postoperative scans (Figure 1C). Hepatobiliary iminodiacetic acid scintigraphy demonstrated 90% and 10% function in the graft and native livers respectively (Figure 1D).
3. DISCUSSION
PCC is a recently described non-pulmonary complication of COVID-19 infection and is increasingly reported in patients requiring mechanical ventilation and prolonged hospitalization.1, 2, 3 With the pandemic continuing unabated, it is likely that this disease will be more commonly encountered in the clinic. Its pathophysiology, clinical course, and natural history remains unclear. Various purported causes for this disorder include critical-illness cholangiopathy, microthromboses, and autoimmune etiology among others. Direct viral damage to cholangiocytes could also be the cause as they express angiotensin-converting enzyme-2 the host receptor for SARS-CoV-2.1, 2, 3 Recently, LT has been proposed as a therapeutic option for PCC in patients with liver failure.4 , 5 Durazo et al reported a 47-year-old man who underwent deceased donor liver transplantation for COVID-19 associated end-stage liver disease with features of secondary sclerosing cholangitis.4 APOLT is an accepted treatment alterative to orthotopic LT in patients with acute liver failure and in certain metabolic liver diseases. In APOLT, the allograft acts as a bridge till native-liver regeneration occurs.6 Since the natural history of PCC remains unknown, the possibility of spontaneous liver recovery cannot be excluded. There have been reports of spontaneous recovery in liver function with time. Furthermore, unlike published reports of LT for PCC, there were no hepatic duct strictures in the expected native liver in our patient.1 , 2 , 4 Hence, replacing the entire liver and subjecting the patient to life-long immunosuppression could potentially be avoided with APOLT. On follow-up, there is no atrophy or collapse of the native liver in our case even 6 months after APOLT and demonstrated at least 10% of the liver function in the native liver suggesting that further regeneration is likely to take place. In patients with subacute liver failure, the duration of liver regeneration is likely to be protracted.
PCC is a newly described, yet unclear disease which may have a myriad of presentations. Furthermore, its natural history and pathology have not been fully elucidated. We propose that in patients with symptomatic PCC, APOLT may be a pragmatic option. Should the native liver recover from the cholangiopathic insult, this operation provides the patient with a realistic possibility of becoming immunosuppression free.
Acknowledgments
FUNDING INFORMATION
None.
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Footnotes
A 50-year-old man who underwent living-related right lobe auxiliary partial orthotopic liver transplantation for severe post–coronavirus disease cholangiopathy is having a gradual recovery in native left liver remnant function.
REFERENCES
- 1.Roth NC, Kim A, Vitkovski T, et al. Post-COVID-19 cholangiopathy: a novel entity. Am J Gastroenterol. 2021;116:1077–1082. doi: 10.14309/ajg.0000000000001154. [DOI] [PubMed] [Google Scholar]
- 2.Faruqui S, Okoli FC, Olsen SK, et al. Cholangiopathy after severe COVID-19: clinical features and prognostic implications. Am J Gastroenterol. 2021;116:1414–1425. doi: 10.14309/ajg.0000000000001264. [DOI] [PubMed] [Google Scholar]
- 3.Deltenre P, Moreno C, Trépo E. Progressive cholangiopathy in COVID-19 patients: other possible diagnoses than ketamine-induced cholangiopathy should be considered. J Hepatol. 2021;75:989–990. doi: 10.1016/j.jhep.2021.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Durazo FA, Nicholas AA, Mahaffey JJ, et al. Post–Covid-19 cholangiopathy—a new indication for liver transplantation: a case report. Transplant Proc. 2021;53:1132–1137. doi: 10.1016/j.transproceed.2021.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee A, Wein AN, Doyle MBM, Chapman WC. Liver transplantation for post-COVID-19 sclerosing cholangitis. BMJ Case Rep. 2021;14:1–5. doi: 10.1136/bcr-2021-244168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rela M, Kaliamoorthy I, Reddy MS. Current status of auxiliary partial orthotopic liver transplantation for acute liver failure. Liver Transplant. 2016;22:1265–1274. doi: 10.1002/lt.24509. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.