Abstract
GABA is a major inhibitory neurotransmitter that regulates the balance between excitatory and inhibitory circuits in the human nervous system. The GABA receptors are divided into three main subtypes, GABAA, GABAB, and GABAC (also termed GABAA rho) receptors. GABAA receptors are pentameric ligand‐gated ion channels widely expressed throughout the central and peripheral nervous system. The activation of GABAA receptors results in opening of an anion‐selective channel that mainly gates chloride ions and allows them to flow into the neuron, causing hyperpolarization of the cell membrane that dampens neural excitability. This makes GABAA receptors critical anaesthetic and analgesic targets for existing as well as for the development of novel drugs. In this review, we first summarize the biochemical properties of GABAA receptors and the clinical anaesthetics and analgesics targeting the receptors. In a forward‐looking section, we summarize the emerging role of GABAergic signalling in treatment of COVID‐19 related infections. Finally, we discuss the opportunities arising from targeting specific and unique subunit interfaces for the development of novel anaesthetics and analgesics leading to more efficient therapies.
Keywords: anaesthetic, analgesic, COVID‐19, GABA receptor, ion channel
1. INTRODUCTION
γ‐Aminobutyric acid (GABA) is an important inhibitory neurotransmitter in the human body and was first discovered in the 1950s. 1 The neurotransmitter GABA is formed from glutamate which again is biosynthesised glutamine (Figure 1). GABA is widely distributed in the mammalian central nervous system (CNS) and in plant tissue. Neurons containing GABA in the brain (GABAergic neurons) account for about 20–30% of the total number of neurons, and most of them are interneurons, which makes GABA one of the most crucial inhibitory neurotransmitters. 1 Insufficient GABAergic activity can cause numerous neuropathological disorders including pain. 1 The GABA receptors discovered so far are divided into three major groups, GABAA, GABAB, and GABAC (also termed GABAA rho) receptors. 2 GABAA receptors are pentameric ligand‐gated ion channels (pLGICs) that mainly mediate rapid synaptic activity. 2 The activation of the receptors can result in opening of an anion‐selective channel that mainly gates chloride ions and allows them to flow into the neuron, causing hyperpolarization of the cell membrane that dampens excitability of GABAergic neurons (Figure 1). GABAB receptors belong to the family C of G‐protein‐coupled receptors, which operate as dimers composed of GABAβ1 and GABAβ2 subunits to transform synaptic neurotransmitter signals into a cellular response through the binding and activation of heterotrimeric G proteins. 3 GABAB receptors signal through three different pathways, voltage‐gated Ca2+ channels, G protein‐activated inwardly‐rectifying K+ channels (GIRK), and adenylyl cyclase, and the effect of this signalling is blockage of neurotransmitter release and hyperpolarization of neurons. 4 GABAC receptors are considered a subclass of GABAARs and are therefore also often referred to as GABAA rho receptors and exist in three different subtypes, ρ1–3. In addition, the GABA transporter (GAT) plays a vital role in GABAergic signalling. It transports GABA against a concentration gradient through the cell membrane and thereby reduces the GABA concentration in the synaptic cleft. 5
FIGURE 1.
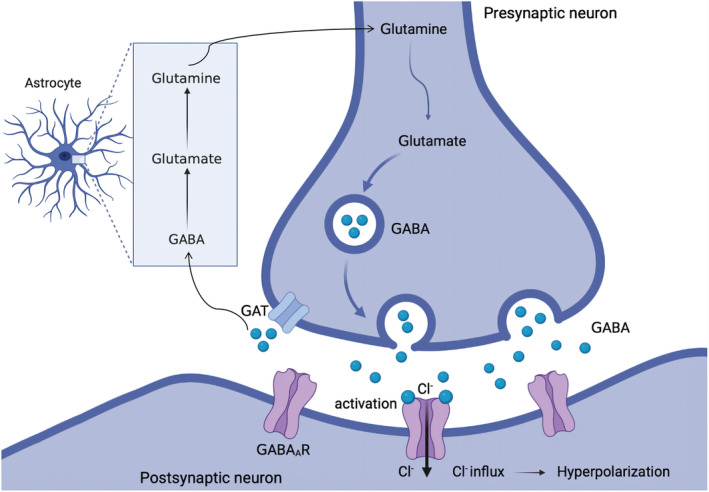
GABA synthesis, release, reuptake, and functional response. GABA is synthesized from glutamine, stored in storage vesicles, and released into the synaptic cleft from the presynaptic neuron. The released GABA can bind to GABA receptors on postsynaptic neurons. GATs clear GABA from the synaptic cleft and transfers it against a concentration gradient back into the presynaptic neuron or into a neighbouring astrocyte where it is converted via Krebs cycle to glutamate and eventually glutamine. Glutamine is then transported back into the presynaptic GABAergic neuron via excitatory amino acid transporters. GABA, γ‐aminobutyric acid; GAT, GABA transporter
In this review, we describe the biochemical properties of GABAA receptors and the clinical anaesthetics and analgesics targeting the receptors, and we discuss the potential additive/synergistic effects of these drugs from a view of their different binding sites. In a forward‐looking section, we summarize the emerging role of GABAergic signalling in relation to COVID‐19 infections. Finally, we discuss the opportunities arising from targeting specific and unique subunit interfaces for the development of novel anaesthetics and analgesics leading to more efficient therapies with fewer adverse effects.
2. BIOCHEMICAL PROPERTIES OF GABAA RECEPTORS
A possible impediment to drug design is a lack of structural information for most GABAA receptors. The discovery of the 3D structure of GABAA receptors marks the beginning of a revolution in developing new drugs. 6 Functional GABAA receptors are assembled of five homologous subunits from a pool of 19 subunits (α1–6, β1–3, γ1–3, δ, ε, π, ρ1–3 and θ) encoded by corresponding genes. 7 The assembled subunits form different types of GABAA receptors, among which the most common receptor subtype is composed of 2α, 2β, and 1γ subunit, in brief α2β2γ. 7 Recent cryogenic electron microscopy structures have confirmed the assembled receptor stoichiometry to be organized as “β‐α‐β‐α‐γ” in a counterclockwise orientation when viewed from the extracellular side 8 (Figure 2A). When viewed perpendicular to the channel direction, each subunit has an extracellular domain (ECD), a transmembrane domain (TMD) and an intracellular domain (ICD, not shown) (Figure 2B). The drugs discussed below bind to the ECD and TMD of the receptor to exert their anaesthetic and analgesic effects.
FIGURE 2.
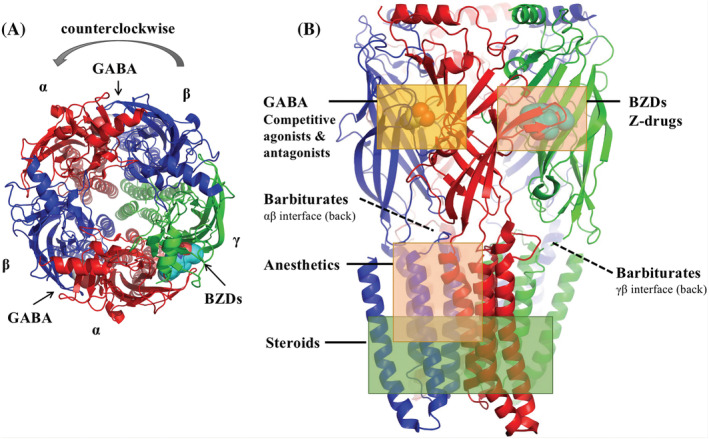
Model of human α1β2γ2 GABAA receptor based on PDB: 6D6U6 to highlight the general structure of GABAA receptors. (A) Top view of the model with surface representation of binding sites for GABA and BZDs; (B) side view of the model with surface representation of binding sites for GABA and BZDs. Binding sites for competitive agonists and antagonists, BZDs and “Z‐drugs,” steroids, anaesthetics and barbiturates are indicated with boxes. The α subunits are shown in red, β in blue and γ in green
The physiological significance of GABAA receptors is reflected in their ability to modulate many different signalling pathways by controlling membrane potentials. They are widely expressed in the central and peripheral nervous system to regulate the afferent excitability and nociception. 9 When GABA binds to GABAA receptors, it results in opening of an anion selective channel that mainly gates chloride ions and allows them to flow into the neuron, causing hyperpolarization of the cell membrane that dampens excitability of GABAergic neurons. Oppositely, antagonizing GABAergic signalling prevents membrane hyperpolarization and causes hyper‐nociceptive effects. For instance, animal tests have shown that treatment with the GABAA antagonist bicuculline enhances nociceptive signalling and behavioural reactivity. 10
3. MERITS AND DEMERITS FOR ANALGESICS AND ANAESTHETICS TARGETING GABAA RECEPTORS
At present, most of the sedative and analgesic drugs that act on GABA receptors can be roughly divided into two major types, agonists and positive allosteric modulators (PAMs). Agonists activate the receptor fully or partially by binding to the GABA binding site while PAMs produce anaesthetic and analgesic effects by enhancing the effects produced by endogenous GABA. These are drugs such as benzodiazepines, barbiturates, volatile anaesthetics, and neurosteroids.
3.1. Benzodiazepines
One of the major drug classes targeting GABAA receptors with analgesic effect commonly used clinically is benzodiazepines (BDZs), a class of psychotropic drugs with a core chemical structure consisting of a phenyl group fused to a diaza heterocyclic ring. The classical high affinity BZD binding site is situated in the receptors ECD in the interface between an α and a γ subunit in a position equivalent to the β‐α interfaces where GABA is binding. The α1, α2, α3, and α5 subunits have a high affinity for BDZs, while α4 and α6 subunits have no affinity for BDZs and are often referred to as being BDZ‐insensitive. 11 Low affinity BZD binding sites have also been reported in the β‐α interfaces and in the γ‐β interface in the TMD. 11 However, much less is known about the influence of these binding sites on the pharmacological profile of BZDs compared to the classical BDZ site. It has been shown that α1βγ2 receptors mediate the sedative, anticonvulsant, and anterograde amnestic actions of BDZs. 12 BDZs have various pain indications (e.g., muscle spasms pain caused by CNS diseases) and can be used for surgical anaesthesia. 13 The neurological effects of BDZs are dose‐dependent. At low doses, they have anxiolytic effects, and as the dose increases, sedation and forgetfulness appear, and finally, anaesthesia is induced. Chlordiazepoxide was the first BDZ drug to be introduced in 1960. It has a sedative, antianxiety, and weak analgesic actions. Since then, extensive research on chlordiazepoxide has led to the introduction of diazepam, midazolam, and remimazolam.
Diazepam is among the most successful BDZ drugs, which is efficacious in treating a wide spectrum of central nervous system disorders, including anxiety and epilepsy. It sets the standard for pharmacotherapy of BDZs in potency, the onset of action, and safety. Nordiazepam, temazepam, and oxazepam are active metabolites of diazepam, which may accumulate after repeated administration, leading to prolonged sedation and posing a significant risk to patients with impaired renal function. 14 Therefore, continuous intravenous diazepam is rarely used to sedate patients in the ICU. Midazolam is a short‐acting BDZ‐derivative with anxiolytic, amnestic, anticonvulsant, hypnotic, and sedative properties. The onset duration of midazolam is short, and it has no continuous analgesic effect on its own, however, continuous application can cause the accumulation of active metabolites (α‐hydroxymidazolam), leading to prolonged sedation. Remimazolam is an ester‐based BDZ derivative with the characteristics of the parent compounds midazolam and remifentanil. The presence of an ester functionality in remimazolam makes it prone to hydrolysis by esterases and produces an inactive metabolite containing a carboxylic acid moiety that is hydrophilic and easily excreted which means less residual drug after end of infusion and this makes remimazolam particularly suitable for surgical sedation. 15 A multicentre, randomized, non‐inferiority, phase III trial established a non‐inferior sedation success rate of remimazolam compared with propofol in patients undergoing upper gastrointestinal endoscopy. 15 The result showed that remimazolam allows faster recovery from sedation compared to propofol. 16
BDZs bind to the interface between α and γ subunits on GABAA receptors and generally do not discriminate significantly between different receptor subtypes. The side effects, such as drowsiness, tolerance, dependence, the risk of withdrawal, and adverse effects on cognition, are inevitable, limiting the potential broader clinical application of BDZs. To reduce the side effects of BDZs, researchers put effort into developing novel drugs that target specific subtypes of GABAA receptors. A class of non‐benzodiazepine hypnotic fast‐acting medicines that act on GABAA receptors has been developed, known as “Z drugs,” including zolpidem, zaleplon, and eszopiclone. 17 It is worth noting that zolpidem, zaleplon, and some other sedative and hypnotic compounds, such as indoplon and abecanide, show preferential selectivity for α1βγ2 receptors, which significantly reduce the side effects. 17 Moreover, several preclinical drugs targeting GABAA receptor α2/3 subunits, such as Saniona's NS11394 and MSD's L838417, have shown sound analgesic effects in animal experiments. 18
3.2. Barbiturates
Barbiturates exhibit a spectrum of GABAA receptor‐mediated activities, including sedative, anxiolytic, hypnotic, and anti‐convulsant effects. The discovery of barbiturates and research into their derivatives have been important for the development of modern anaesthesia. Two important binding sites for barbiturates in the GABAA receptor's TMD have been identified at the α‐β and γ‐β interfaces. 19 Pentothal was a commonly used barbiturate, but it is rarely used today due to its many disadvantages, such as changes of the metabolism of other drugs metabolized in the liver, accumulation of active metabolites in kidneys and associated renal failure, and pain, secondary endothelial damage and inflammation from intravenous injection. Thiopental and methohexital are ultra‐short acting derivatives of barbiturates. 20 They are mainly used in obstetrics for induction of caesarean sections under general anaesthesia. Besides, other barbiturates with longer‐acting effects are available for specialist use, including amobarbital, phenobarbital, and butobarbital. Phenobarbital remains explicitly popular as an anti‐epileptic drug. At low concentrations it potentiates the receptor's response to GABA, while at high concentrations, it evokes direct allosteric activation through binding sites in the TMD. 21 This property makes barbiturates dangerous and potentially lethal as they can cause severe cardiovascular and respiratory depression in overdose. Therefore, barbiturates are only used in patients who cannot tolerate or do not respond to other drugs.
3.3. Anaesthetics
Propofol is the most widely used intravenous general anaesthetic and can directly activate GABAA receptors at high concentrations. It binds to the domain defined by the β(M286) residue at the β(+)/α(−) interface in the TMD and the β(Y143) residue near the β(−) surface in the junction between the extracellular and transmembrane domains. 22 Affinity labelling and mutagenesis results for propofol suggest that in addition to the β‐α sites, there may be additional sites at other subunit interfaces, and/or at the TMD‐ECD junction. 23 Propofol has some characteristics of an ideal sedative, including the advantages of rapid onset and short duration of action, which is especially suitable for patients who need frequent neurological examinations. An open‐label randomized controlled trial revealed that sedation with propofol results in significantly fewer ventilator days compared to lorazepam. 24 However, propofol infusion syndrome from cumulative doses of propofol is a risk factor that should be considered by clinicians. 25 It can often lead to cardiac failure, rhabdomyolysis, metabolic acidosis, and kidney failure and may potentially be fatal. A multicentre ICU database analysis further supports these data: Out of more than 3000 patients, those receiving propofol infusion had lower mortality and shorter ICU stays than those receiving midazolam or lorazepam. 26 Besides, the antioxidant properties of propofol may provide cardioprotection as suggested based on rat studies. 27 However, propofol also has some disadvantages. Up to 30% of patients experience propofol‐related injection pain during intravenous injection of propofol. As a sedative, propofol has a “narrow therapeutic window,” which may cause a sudden transition from sedation to general anaesthesia. Also, propofol reduces the sensitivity of central chemoreceptors to hypercapnia so that dose‐dependent respiratory depression may occur after administration. Also, propofol can cause dose‐related cardiovascular depression, decreased sympathetic tone, decreased vascular resistance, and subsequent hypotension, making propofol unfavourable for non‐specialist applications. In recent years, several water‐soluble analogues of propofol have been developed to avoid the shortcomings of propofol. Fospropofol is a water‐soluble prodrug of propofol, which is activated by endothelial alkaline phosphatase. Fospropofol is suitable for monitored anaesthesia care for adult patients undergoing diagnostic or therapeutic procedures, and for outpatients and day surgery. A single loading dose can endure the entire surgical process. Furthermore, since fospropofol is a water‐soluble preparation, the incidence and severity of injection pain is less, and it is less prone to bacterial growth and contamination than propofol. In an open‐label multicenter trial, 60 patients receiving mechanical ventilation were randomly divided into three groups: fospropofol intravenous injection and infusion for 12 h; fospropofol infusion only for 12 h; and propofol infusion only for 12 h. The results show that fospropofol is effective and well‐tolerated when used for short‐term sedation. 28 Notably, the delay of clinical effects must be considered when using fospropofol (metabolic activation of the prodrug in vivo takes time) to avoid excessive injection leading to more profound sedation than desired. Given propofols binding to a site in the receptor's transmembrane region and distinct from that of benzodiazepines, synergistic/additive effects of propofol and benzodiazepines have been reported and are potentially dangerous and highly dependent on GABA concentrations. 19
Etomidate is another commonly used intravenous general anaesthetic that can rapidly induce general anaesthesia by positively modulating GABAA receptors. 29 It binds to the same site as propofol on GABAA receptors. Etomidate can maintain haemodynamic stability in patients with cardiovascular diseases, and therefore, is commonly substituted for propofol in cases where cardiovascular or respiratory depression is a concern. 30 Recently, a double‐blind, randomized clinical trial showed that in patients undergoing coronary artery bypass graft surgery with low ejection fraction, diazepam is more favourable in terms of haemodynamic stability compared to propofol, and etomidate can be used safely for induction of anaesthesia in patients with impaired ventricular function. 31 The primary defect of etomidate is that administration for rapid sequence intubation is associated with higher rates of adrenal insufficiency and mortality in patients with sepsis. 32 Recently, studies have found that an etomidate analogue methoxycarbonyl etomidate (MOC‐etomidate) does not inhibit the function of the adrenal cortex. It enhances GABAA receptor signalling in rats and constitutes a fast‐metabolism hypnotic with similar haemodynamic stability as etomidate. 33 However, the fly in the ointment is that MOC‐etomidate can be rapidly hydrolyzed by esterases to form carboxylic acid metabolites, which easily accumulate in patients with renal failure. Cyclopropyl methoxycarbonyl metomidate (CPMM or ABP‐700) is a second‐generation etomidate analogue developed after MOC‐etomidate was studied in animals. ABP‐700 aims to solve the problem of accumulation of carboxylic acid metabolites of MOC‐etomidate in the kidneys of patients with renal failure. Animal experiments conducted in tadpoles revealed that ABP‐700 has better potency and a longer half‐life than MOC‐etomidate in rats, long‐term infusion does not cause changes in half‐life, and the carboxylic acid changes metabolites of ABP‐700 are not accumulated in the CNS. 34 Besides, the recovery of sedation after using ABP‐700 is faster than propofol and easier to control. A preliminary phase I study of ABP‐700 showed that a single bolus injection of 0.25 and 0.35 mg/kg of ABP‐700 could provide satisfactory clinical effects, and it is still safe at doses as high as 1 mg/kg. 35
3.4. Neurosteroids
The δ‐containing GABAA receptors are capable of sensing low concentrations of GABA in the intercellular fluid and producing tonic inhibition. The importance of tonic inhibition in regulating states of consciousness and pain transduction is supported by the fact that δ‐containing GABAA receptors are promising targets for anaesthetics and analgesics, for example, propofol is a positive allosteric modulator of α5‐ and δ‐containing GABAA receptors leading to enhanced tonic conductance, which is widely used for anaesthesia and analgesia in bronchoscopy. 36
Neurosteroids are potent positive allosteric modulators of the δ‐containing GABAA receptors to exert their biological effects on the regulation of neuronal excitability. 37 Evidence revealed that the tonic inhibitory neurotransmission mediated by α4βδ GABAA receptors could be modulated by neurosteroids. Some binding sites of neurosteroids on GABAA receptors have been identified by using site‐directed mutagenesis and electrophysiology, including α1V227, T236, Q241, N407, and β3Y284, and it remains to be seen if neurosteroids bind to sites that directly involve γ or δ subunits. 38 Neurosteroids have been found to have a wide range of clinical effects, including sedation, analgesia, anticonvulsant effects, pressure and stress relief, and cognitive improvement, 39 which have prompted the development of synthetic neurosteroids. For instance, alfaxalone (alfaxan) is a progesterone‐derived neuroactive steroid and a non‐competitive GABAA receptor agonist used for general anaesthesia. Its metabolite allopregnanolone has the characteristics of fast onset, fast metabolism, and is relatively safe but may cause mild cardiovascular and respiratory depression. Alfaxalone is used currently in veterinary practice as an induction agent for anaesthesia and as an injectable anaesthetic. A phase I trial compared the efficacy and safety of alfaxalone and propofol and concluded that the new aqueous alfaxalone formulation has similar pharmacodynamic properties to its analogue progesterone, and it has a similar sedative effect compared to propofol. However, a faster cognitive functional recovery is observed. 40 Furthermore, the new aqueous alfaxalone formulation does not cause injection pain and cardiopulmonary inhibition.
4. GABAA RECEPTORS STIMULATION PREVENT DISEASE PROGRESSION IN COVID‐19
The role of GABAergic signalling in relation to immune response has regained interest during the COVID‐19 pandemic. 41 , 42 GABAA receptors are expressed in antigen‐presenting cells (APCs) and activation of these receptors can hyperpolarize APCs, thereby reducing their activity. 43 This may lead to a reduction in the body's inflammatory response. Further, GABAA receptors have been found in alveolar macrophages, and the application of GABAA receptor agonists and PAMs (i.e., GABA and diazepam) reduce the production of pro‐inflammatory molecules after lipopolysaccharide (LPS) stimulation. 44 The specific type of GABAA receptors expressed on APCs and alveolar macrophages is not determined yet. However, polymerase chain reaction (PMR) was used to screen human peripheral blood mononuclear cells (PBMC) and Jurkat cells for the presence of GABAA receptor subunit mRNAs. Positive signals were detected for the α1, α3, β2, β3, δ and ε subunit mRNAs in both cell populations. 45 In addition, a study of the bronchial asthma mouse model showed that the inhibition of GABAA receptor subunit π (GABRP) could reduce the differentiation of airway epithelial progenitor cells into goblet cells, leading to reduced inflammation. 46
Coronavirus mouse hepatitis virus‐1 (MHV‐1) causes pneumonia in mice and shares pathological characteristics with human COVID‐19 infection. A recent study administered GABA to MHV‐1‐infected mice and found that mice that received GABA immediately after inoculation with MHV‐1 had mild pneumonia symptoms and eventually all recovered. When GABA treatment was started 3 days after MHV‐1 infection, the severity of the disease was also significantly reduced. 47 The study revealed that the activation of GABA receptors prevents the development of severe pneumonia and death in mice caused by MHV‐1 infection and suggests that GABAA receptors stimulation potentially could help prevent disease progression in COVID‐19 and other coronavirus strains infections in humans. Another study performed animal experiments in susceptible inbread A/J mice to test whether oral treatment with GABA could modulate the MHV‐1 induced pneumonitis. The research revealed that MHV‐1‐inoculated control mice became severely ill (over 60% succumbed to the infection), whereas mice that received GABA immediately after MHV‐1 inoculation became only mildly ill and all of them recovered. 48 In addition to GABA, similar experiments were conducted using the GABAA agonist homotaurine, and led to similar results. Treatment with the GABAAR‐specific agonist homotaurine significantly reduced the severity of pneumonitis and death rates in MHV‐1‐infected mice. However, the similar effect was not observed in the mice treated with GABABR‐specific agonist baclofen, indicating that the therapeutic effects were mediated primarily through GABAA receptors. 49
In addition to animal tests, a recent clinical study revealed that GABA levels were significantly reduced in COVID‐19 patients and GABA plasma levels allowed for stratification of COVID‐19 patients with high sensitivity and specificity. 50 These results strongly suggest a correlation between GABA and COVID‐19. However, the anti‐inflammatory mechanism by which GABAA receptor stimulation prevents disease progression in COVID‐19 should be further explored.
5. CONCLUSIONS AND PERSPECTIVES
The GABAergic system is a critical target for anaesthetics and analgesics. Since GABAA receptors are widely distributed in the central and peripheral nervous system, many drugs targeting GABAA receptors are currently used in clinical practice. However, their side effects (i.e., addictive effect and drug depence) due to lack of true subtype selectivity limit their potential broader clinical application. Therefore, the development of more selective drugs is imminent. Although this is promising in theory, the complexity and a plethora of subtypes have hampered significant progress so despite knowing about the roles of different subtypes for many years, the successful development of receptor truly selective drugs that can exert the desired efficacy without causing side effects has not yet to be realized. There is currently a structural revolution happening 3D structures of GABAA receptors are becoming available. This may guide development of novel analgesics and anaesthetics that target specific and unique subunits or subunit interfaces for the development of novel anaesthetics and analgesics. In addition, more and more studies have proven the relationship between the GABAergic system and the prognosis of COVID‐19. Considering that drugs targeting GABAA receptors are proven to be safe from many years of clinical applications, we should further explore their mechanism of action in relation to immune response.
CONFLICTS OF INTEREST
The authors declare no competing interests.
ACKNOWLEDGEMENTS
This work was supported by funding from the Australian Research Council (LP160100560). Open access publishing facilitated by The University of Sydney, as part of the Wiley ‐ The University of Sydney agreement via the Council of Australian University Librarians.
Luo Y, Balle T. GABAA receptors as targets for anaesthetics and analgesics and promising candidates to help treat coronavirus infections: A mini‐review. Basic Clin Pharmacol Toxicol. 2022;1‐9. doi: 10.1111/bcpt.13798
REFERENCES
- 1. Bak LK, Schousboe A, Waagepetersen HS. The glutamate/GABA‐glutamine cycle: aspects of transport, neurotransmitter homeostasis and ammonia transfer. J Neurochem. 2006;98(3):641‐653. doi: 10.1111/j.1471-4159.2006.03913.x [DOI] [PubMed] [Google Scholar]
- 2. Mann EO, Kohl MM, Paulsen O. Distinct roles of GABA(a) and GABA(B) receptors in balancing and terminating persistent cortical activity. J Neurosci off J Soc Neurosci. 2009;29(23):7513‐7518. doi: 10.1523/jneurosci.6162-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Papasergi‐Scott MM, Robertson MJ, Seven AB, Panova O, Mathiesen JM, Skiniotis G. Structures of metabotropic GABA(B) receptor. Nature. 2020;584(7820):310‐314. doi: 10.1038/s41586-020-2469-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bettler B, Kaupmann K, Mosbacher J, Gassmann M. Molecular structure and physiological functions of GABA(B) receptors. Physiol Rev. 2004;84(3):835‐867. doi: 10.1152/physrev.00036.2003 [DOI] [PubMed] [Google Scholar]
- 5. Sałat K, Kulig K. GABA transporters as targets for new drugs. Future Med Chem. 2011;3(2):211‐222. doi: 10.4155/fmc.10.298 [DOI] [PubMed] [Google Scholar]
- 6. Zhu S, Noviello CM, Teng J, Walsh RM Jr, Kim JJ, Hibbs RE. Structure of a human synaptic GABA(a) receptor. Nature. 2018;559(7712):67‐72. doi: 10.1038/s41586-018-0255-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Martenson JS, Yamasaki T, Chaudhury NH, Albrecht D, Tomita S. Assembly rules for GABAA receptor complexes in the brain. Elife. 2017;6. doi: 10.7554/eLife.27443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhu S, Noviello CM, Teng J, Walsh RM Jr, Kim JJ, Hibbs RE. Structure of a human synaptic GABAA receptor. Nature. 2018;559(7712):67‐72. doi: 10.1038/s41586-018-0255-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yowtak J, Wang J, Kim HY, Lu Y, Chung K, Chung JM. Effect of antioxidant treatment on spinal GABA neurons in a neuropathic pain model in the mouse. Pain. 2013;154(11):2469‐2476. doi: 10.1016/j.pain.2013.07.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sorkin LS, Puig S, Jones DL. Spinal bicuculline produces hypersensitivity of dorsal horn neurons: effects of excitatory amino acid antagonists. Pain. 1998;77(2):181‐190. doi: 10.1016/s0304-3959(98)00094-3 [DOI] [PubMed] [Google Scholar]
- 11. Sigel E, Ernst M. The benzodiazepine binding sites of GABA(a) receptors. Trends Pharmacol Sci. 2018;39(7):659‐671. doi: 10.1016/j.tips.2018.03.006 [DOI] [PubMed] [Google Scholar]
- 12. McKernan RM, Rosahl TW, Reynolds DS, et al. Sedative but not anxiolytic properties of benzodiazepines are mediated by the GABA(a) receptor alpha1 subtype. Nat Neurosci. 2000;3(6):587‐592. doi: 10.1038/75761 [DOI] [PubMed] [Google Scholar]
- 13. O'Connell M, Sandgren M, Frantzen L, Bower E, Erickson B. Medical cannabis: effects on opioid and benzodiazepine requirements for pain control. Ann Pharmacother. 2019;53(11):1081‐1086. doi: 10.1177/1060028019854221 [DOI] [PubMed] [Google Scholar]
- 14. Luk S, Atayee RS, Ma JD, Best BM. Urinary diazepam metabolite distribution in a chronic pain population. J Anal Toxicol. 2014;38(3):135‐142. doi: 10.1093/jat/bku001 [DOI] [PubMed] [Google Scholar]
- 15. Doi M, Morita K, Takeda J, Sakamoto A, Yamakage M, Suzuki T. Efficacy and safety of remimazolam versus propofol for general anesthesia: a multicenter, single‐blind, randomized, parallel‐group, phase IIb/III trial. J Anesth. Aug 2020;34(4):543‐553. doi: 10.1007/s00540-020-02788-6 [DOI] [PubMed] [Google Scholar]
- 16. Chen SH, Yuan TM, Zhang J, et al. Remimazolam tosilate in upper gastrointestinal endoscopy: a multicenter, randomized, non‐inferiority, phase III trial. J Gastroenterol Hepatol. 2020;36:474‐481. doi: 10.1111/jgh.15188 [DOI] [PubMed] [Google Scholar]
- 17. Brandt J, Leong C. Benzodiazepines and Z‐drugs: an updated review of major adverse outcomes reported on in epidemiologic research. Drugs R&D. 2017;17(4):493‐507. doi: 10.1007/s40268-017-0207-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hofmann M, Kordás KS, Gravius A, et al. Assessment of the effects of NS11394 and L‐838417, α2/3 subunit‐selective GABA(a) [corrected] receptor‐positive allosteric modulators,in tests for pain, anxiety, memory and motor function. Behav Pharmacol. 2012;23(8):790‐801. doi: 10.1097/FBP.0b013e32835a7c7e [DOI] [PubMed] [Google Scholar]
- 19. Chiara DC, Jayakar SS, Zhou X, et al. Specificity of intersubunit general anesthetic‐binding sites in the transmembrane domain of the human α1β3γ2 γ‐aminobutyric acid type a (GABAA) receptor. J Biol Chem. 2013, 27;288:19343‐19357. doi: 10.1074/jbc.M113.479725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dumps C, Halbeck E, Bolkenius D. Drugs for intravenous induction of anesthesia: barbiturates. Medikamente zur intravenösen Narkoseinduktion: Barbiturate. Der Anaesthesist. 2018;67(7):535‐552. doi: 10.1007/s00101-018-0440-7 [DOI] [PubMed] [Google Scholar]
- 21. Löscher W, Rogawski MA. How theories evolved concerning the mechanism of action of barbiturates. Epilepsia. 2012;53(Suppl 8):12‐25. doi: 10.1111/epi.12025 [DOI] [PubMed] [Google Scholar]
- 22. Shin DJ, Germann AL, Johnson AD, Forman SA, Steinbach JH, Akk G. Propofol is an allosteric agonist with multiple binding sites on Concatemeric ternary GABA(a) receptors. Mol Pharmacol. 2018;93(2):178‐189. doi: 10.1124/mol.117.110403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jayakar SS, Zhou X, Chiara DC, et al. Identifying drugs that bind selectively to Intersubunit general anesthetic sites in the α1β3γ2 GABA(a)R transmembrane domain. Mol Pharmacol. 2019;95(6):615‐628. doi: 10.1124/mol.118.114975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Carson SS, Kress JP, Rodgers JE, et al. A randomized trial of intermittent lorazepam versus propofol with daily interruption in mechanically ventilated patients. Crit Care Med. 2006;34(5):1326‐1332. doi: 10.1097/01.Ccm.0000215513.63207.7f [DOI] [PubMed] [Google Scholar]
- 25. Hemphill S, McMenamin L, Bellamy MC, Hopkins PM. Propofol infusion syndrome: a structured literature review and analysis of published case reports. Br J Anaesth. 2019;122(4):448‐459. doi: 10.1016/j.bja.2018.12.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lonardo NW, Mone MC, Nirula R, et al. Propofol is associated with favorable outcomes compared with benzodiazepines in ventilated intensive care unit patient. Am J Resp Crit Care Med. 2014;189(11):1383‐1394. doi: 10.1164/rccm.201312-2291OC [DOI] [PubMed] [Google Scholar]
- 27. Chen K, Yu J, Wang Q, et al. The timing of propofol administration affects the effectiveness of remote ischemic preconditioning induced cardioprotection in rats. J Cell Biochem. 2020;121(11):4535‐4541. doi: 10.1002/jcb.29671 [DOI] [PubMed] [Google Scholar]
- 28. Candiotti KA, Gan TJ, Young C, et al. A randomized, open‐label study of the safety and tolerability of fospropofol for patients requiring intubation and mechanical ventilation in the intensive care unit. Anesth Analg. 2011;113(3):550‐556. doi: 10.1213/ANE.0b013e31821d7faf [DOI] [PubMed] [Google Scholar]
- 29. McGrath M, Yu Z, Jayakar SS, et al. Etomidate and etomidate analog binding and positive modulation of γ‐aminobutyric acid type a receptors: evidence for a state‐dependent cutoff effect. Anesthesiology. 2018;129(5):959‐969. doi: 10.1097/aln.0000000000002356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shah SB, Chowdhury I, Bhargava AK, Sabbharwal B. Comparison of hemodynamic effects of intravenous etomidate versus propofol during induction and intubation using entropy guided hypnosis levels. J Anaesthesiol Clin Pharmacol. 2015;31(2):180‐185. doi: 10.4103/0970-9185.155145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Soleimani A, Heidari N, Habibi MR, et al. Comparing hemodynamic responses to diazepam, Propofol and etomidate during anesthesia induction in patients with left ventricular dysfunction undergoing coronary artery bypass graft surgery: a double‐blind, randomized clinical trial. Med Arch (Sarajevo, Bosnia and Herzegovina). 2017;71(3):198‐203. doi: 10.5455/medarh.2017.71.198-203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chan CM, Mitchell AL, Shorr AF. Etomidate is associated with mortality and adrenal insufficiency in sepsis: a meta‐analysis*. Crit Care Med. 2012;40(11):2945‐2953. doi: 10.1097/CCM.0b013e31825fec26 [DOI] [PubMed] [Google Scholar]
- 33. Cotten JF, Husain SS, Forman SA, et al. Methoxycarbonyl‐etomidate: a novel rapidly metabolized and ultra‐short‐acting etomidate analogue that does not produce prolonged adrenocortical suppression. Anesthesiology. 2009;111(2):240‐249. doi: 10.1097/ALN.0b013e3181ae63d1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pejo E, Liu J, Lin X, Raines DE. Distinct hypnotic recoveries after infusions of Methoxycarbonyl etomidate and Cyclopropyl Methoxycarbonyl Metomidate: the role of the metabolite. Anesth Analg. 2016;122(4):1008‐1014. doi: 10.1213/ane.0000000000001146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Struys M, Valk BI, Eleveld DJ, et al. A phase 1, single‐center, double‐blind, placebo‐controlled study in healthy subjects to assess the safety, tolerability, clinical effects, and pharmacokinetics‐pharmacodynamics of intravenous Cyclopropyl‐methoxycarbonylmetomidate (ABP‐700) after a single ascending bolus dose. Anesthesiology. 2017;127(1):20‐35. doi: 10.1097/aln.0000000000001662 [DOI] [PubMed] [Google Scholar]
- 36. Eleveld DJ, Colin P, Absalom AR, Struys M. Pharmacokinetic‐pharmacodynamic model for propofol for broad application in anaesthesia and sedation. Br J Anaesth. 2018;120(5):942‐959. doi: 10.1016/j.bja.2018.01.018 [DOI] [PubMed] [Google Scholar]
- 37. Belelli D, Lambert JJ. Neurosteroids: endogenous regulators of the GABA(a) receptor. Nat Rev Neurosci. 2005;6(7):565‐575. doi: 10.1038/nrn1703 [DOI] [PubMed] [Google Scholar]
- 38. Li Y, Song D, Bo F, Deng M, Tang X. Diazepam inhibited lipopolysaccharide (LPS)‐induced pyroptotic cell death and alleviated pulmonary fibrosis in mice by specifically activating GABA(a) receptor α4‐subunit. Biomed Pharmacother Biomed Pharmacother. 2019;118:109239. doi: 10.1016/j.biopha.2019.109239 [DOI] [PubMed] [Google Scholar]
- 39. Reddy DS. Neurosteroids: endogenous role in the human brain and therapeutic potentials. Prog Brain Res. 2010;186:113‐137. doi: 10.1016/b978-0-444-53630-3.00008-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Monagle J, Siu L, Worrell J, Goodchild CS, Serrao JM. A phase 1c trial comparing the efficacy and safety of a new aqueous formulation of alphaxalone with propofol. Anesth Analg. 2015;121(4):914‐924. doi: 10.1213/ane.0000000000000856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Nájera‐Martínez M, López‐Tapia BP, Aguilera‐Alvarado GP, et al. Sub‐basal increases of GABA enhance the synthesis of TNF‐α, TGF‐β, and IL‐1β in the immune system organs of the Nile tilapia. Journal of Neuroimmunol. 2020;348:577382. doi: 10.1016/j.jneuroim.2020.577382 [DOI] [PubMed] [Google Scholar]
- 42. Pomeranz Krummel DA, Nasti TH, Kaluzova M, et al. Melanoma cell intrinsic GABA(a) receptor enhancement potentiates radiation and immune checkpoint inhibitor response by promoting direct and T cell‐mediated antitumor activity. Int J Radiat Oncol Biol Phys. 2021;109(4):1040‐1053. doi: 10.1016/j.ijrobp.2020.10.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bhat R, Axtell R, Mitra A, et al. Inhibitory role for GABA in autoimmune inflammation. Proc Natl Acad Sci USA. 2010;107(6):2580‐2585. doi: 10.1073/pnas.0915139107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wang YY, Sun SP, Zhu HS, et al. GABA regulates the proliferation and apoptosis of MAC‐T cells through the LPS‐induced TLR4 signaling pathway. Res Vet Sci. 2018;118:395‐402. doi: 10.1016/j.rvsc.2018.04.004 [DOI] [PubMed] [Google Scholar]
- 45. Alam S, Laughton DL, Walding A, Wolstenholme AJ. Human peripheral blood mononuclear cells express GABAA receptor subunits. Mol Immunol. 2006;43(9):1432‐1442. doi: 10.1016/j.molimm.2005.07.025 [DOI] [PubMed] [Google Scholar]
- 46. Wang A, Zhang Q, Wang Y, et al. Inhibition of Gabrp reduces the differentiation of airway epithelial progenitor cells into goblet cells. Exp Ther Med. 2021;22(1):720. doi: 10.3892/etm.2021.10152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tian J, Milddleton B, Kaufman DL. GABA administration prevents severe illness and death following coronavirus infection in mice. bioRxiv: the preprint server for biology. 2020. doi: 10.1101/2020.10.04.325423 [DOI]
- 48. Tian J, Middleton B, Kaufman DL. GABA administration prevents severe illness and death following coronavirus infection in mice. bioRxiv: the preprint server for biology. Oct 4, 2020. doi: 10.1101/2020.10.04.325423 [DOI]
- 49. Tian J, Middleton B, Kaufman DL. GABA(a)‐receptor agonists limit pneumonitis and death in murine coronavirus‐infected mice. Viruses. 2021;13(6). doi: 10.3390/v13060966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Masoodi M, Peschka M, Schmiedel S, et al. Disturbed lipid and amino acid metabolisms in COVID‐19 patients. J Mol Med (Berl). 2022;100(4):555‐568. doi: 10.1007/s00109-022-02177-4 [DOI] [PMC free article] [PubMed] [Google Scholar]


