To the Editor,
Many authors recently reported the effect of the severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2) pandemic on patients affected by Inborn Errors of Immunity (IEI). 1 , 2 Although mostly experiencing a mild disease and the course of the infection is comparable or even less symptomatic than the general population, young male IEIs patients were more severely affected and more frequently admitted to intensive care unit compared with the age‐matched healthy peers. 3 Most viral variants were actually first described in immunocompromised patients. 4 , 5 Indeed, impaired B and/or T cell function can be responsible for persisting viral shedding often observed in IEI patients with subsequent higher risk of persistent viral replication and mutation within the host. 6 Noteworthily, in the last 2 years Inborn Errors of type I IFN immunity and autoantibody‐mediated phenocopies of IEIs were identified as responsible for life‐threatening COVID‐19. These conditions were not considered in the studies about IEIs and SARS‐CoV‐2 infection. 7 , 8 Predisposition to increased risk and most severe infection in IEI, despite a lack of clear data in favor of a high risk for progression to severe COVID‐19, led the scientific community to recommend the use of early SARS‐CoV2‐directed treatments to potentially reduce the incidence of long‐lasting infection, as well as morbidity and mortality risk. 9
Casirivimab–imdevimab, also known as REGEN‐COV, is an anti‐spike mAb that has been authorized for the treatment of high‐risk patients (>12 years of age, >40 kg) with mild‐to‐moderate COVID‐19. 10 Bamlanivimab, named LY‐CoV555, then combined with etesevimab, showed a beneficial effect for accelerating the natural decline of the viral load over time. In December 2021, it was approved by FDA for patients<12 years. 10
Sotrovimab, formerly known as VIR‐7831, is an engineered human mAb that neutralizes SARS‐CoV‐2. 10 There are several data confirming the safety and efficacy in both adults and pediatric population. 11
We describe a cohort of 63 SARS‐CoV‐2‐infected IEI patients, providing the first case series report on the early employment of anti‐SARS‐CoV‐2 treatments with antiviral drugs and MAbs in these patients (Table S1). Thirty‐nine patients were younger than 18 years (median age 9.5 years), whereas 24 were adults (median age 33 years). Male‐to‐female ratio was 1:1. Seven individuals have been previously reported. 2 The distribution of SARS‐CoV‐2‐infected IEI patients into International Union of Immunological Societies (IUIS) categories was similar to those described by Meyts et al. 1 and Milito et al. 2 (Figure 1) and also reflected the frequencies of each disease. In fact, 71% of patients belonged to second and third IUIS categories. Interestingly, 58% of IEIs were genetically determined and the most frequently involved pathways were those affecting T cell homeostasis: 22q11.2 deletion, STAT3 and ATM variants were addressed together as responsible for 25% of molecular IEI diagnoses in our cohort. Comorbidities were variable and, in some cases, influenced the clinical course of coronavirus infection and the therapeutic choice.
FIGURE 1.
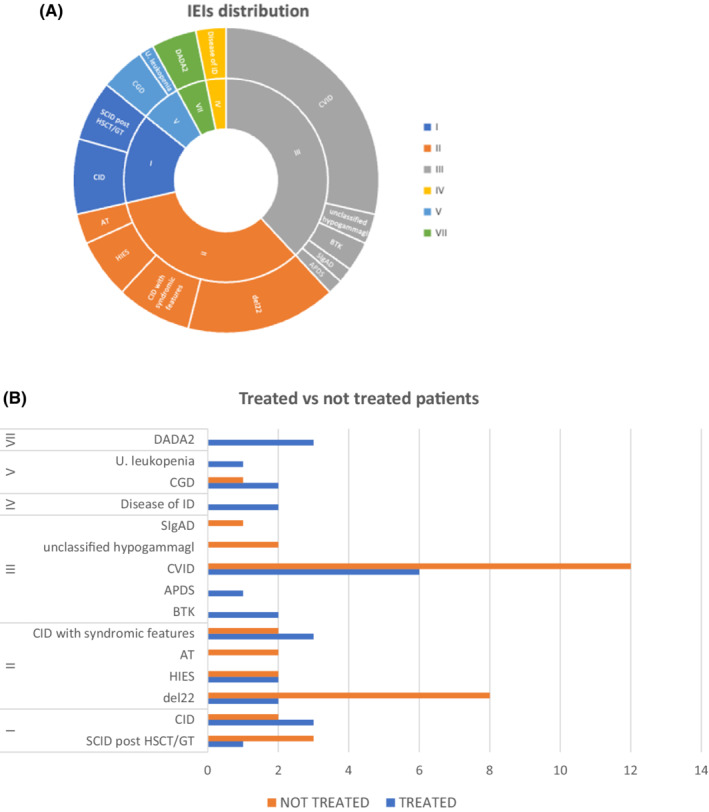
Distribution of SARS‐CoV‐2‐infected patients by (A) IEI entities and (B) treatment
According to current guidelines, all patients affected by IEI are eligible for SARS‐COV2 treatment. At the time of this manuscript, specific guidelines about the choice of the most suitable treatment for each patient were lacking. The criteria for treatment choice were based on the underlying IEI, the patient clinical conditions, the variety of concern (VOC), when available, or the current prevalence of resistant variants and drug's availability in each center and patient's age.
SARS‐CoV‐2 infection prevalence and incidence dramatically increased since the last months of 2021. Indeed, the number of SARS‐CoV‐2‐positive IEI individuals reported in this manuscript is much higher compared with the cases previously reported. This mirrors the current epidemiology of coronavirus disease 2019 (COVID‐19) in general population, characterized by a huge spread due to both the increasing prevalence of Omicron variant over Delta and the gradual softening of social restriction measures.
As reported in the first pandemic phase, most of our IEI patients did not develop severe COVID‐19. In most of our patients, SARS‐CoV‐2 infection dated back no more than October 2021, and 15/17 tested subjects were infected by the Omicron variant. Therefore, it is possible to speculate that most cases were sustained by this variant, which has been reported to be less commonly related to severe complications. 12 Indeed, 90% of our patients experienced a mild clinical course and only 6/63 (9,5%) showed a moderate/severe course: 4/6 were under 18 years and 5/6 presented important comorbidities that may have influenced the clinical course.
5/6 presented a moderate course including a del22 syndrome with associated CID and cardiopathy, a APDS patient with an ongoing lymphoma relapse, a CVID patient with IBD, a patient with CVID and associated osteochondrosis and one post‐HSCT and kidney transplantation for Schimke syndrome with epilepsy and ischemic/hemorrhagical neurological events.
Only a 4‐year‐old male child with an unclassified IEI and a previous history of hemolytic uremic syndrome died 6 days after SARS‐CoV‐2 infection due to CMV‐related acute respiratory distress syndrome, acute renal failure and cerebral edema despite MAbs administration. Remarkably, 3 patients had SARS‐CoV‐2 infection after gene therapy (one after experimental gene therapy for WAS 13 , and two after approved gene therapy for ADA deficiency); all patients experienced a favorable clinical course. A novel issue in our study was the cohort's high anti‐SARS‐CoV‐2 vaccination coverage: 59% of individuals had received at least two vaccine doses, with no significant adverse effects, while 38% had not received any vaccine. Nevertheless, vaccinal campaign did not seem to impact substantially on infection frequency and severity among the studied population, probably due both to Omicron vaccine‐escaping ability and to IEI‐related defective vaccine immunogenicity 14 . Indeed, patients affected by IEI are able to mount a specific antibody response following vaccination although at a lower magnitude compared with healthy peers. On the contrary, the proportion of those who are not capable of Ag‐specific T cells generation at all is higher compared with healthy individuals. Given this evidence, it is getting clearer and clearer the importance of developing vaccination schedules tailored for vulnerable populations such as IEI subjects or patient with secondary immunodeficiencies.
Antivirals or neutralizing monoclonal antibodies were employed in 29/63 patients (46%). Antivirals and MAbs were used in 5/29 and 25/29 cases, respectively. No serious adverse effects were observed. The therapy was proposed to each patient for which the medical staff was aware according to the eligibility criteria. 9 Variability in the therapeutic strategies approach was mainly determined by drugs' availability in each center and patients' age.
Notably, our report is also the first to describe the off‐label use of MAbs in pediatric patients below the age for which they were licensed (8/25 patients treated with MAbs were < 12 years of age).
Brown et al. 15 recently described a cohort of 31 IEI patients, predominantly affected by humoral defects, with chronic or relapsing COVID‐19, and showed a better response to combination of remdesivir plus MAbs. In our study, non‐standardized timing and antigenic/PCR methods determination for SARS‐CoV‐2 testing along with the absence of a long‐term follow‐up did not allow an unbiased impact assessment of the efficacy of these early treatments. However, we did not observe substantial differences in terms of infection clearance (2 days spared) and symptom recovery (1 day spared) between treated and untreated patients (p‐value 0.86); nevertheless, we observed a faster mean time clearance in patients who had received at least 2 vaccination doses respect to the rest of our IEI cohort (17 versus 24 days).
Overall, our experience confirms that COVID‐19 has a mild clinical course in most patients with IEIs, yet a significant minority remains at a risk of severe disease. Early preventive therapies are frequently employed in this population, with good tolerance, even when used off‐label for children under 12 years of age. Standardized protocol specifically set on IEI patients and information on pediatric doses, however, are lacking, possibly hindering access to these treatments.
FUNDING INFORMATION
This research received no external funding.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ETHICAL APPROVAL
All patients or caregivers gave their informed consent prior to their inclusion in the study.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/pai.13833.
Supporting information
TableS1
ACKNOWLEDGMENTS
We would like to thank the patients, caregivers and clinic teams for their participation and kind support of this study.
Associate Editor: Fabio Candotti
Francesca Conti, Lucia Pacillo contributed equally to this work and share first authorship.
Andrea Pession, Andrea Finocchi share last authorship.
REFERENCES
- 1. Meyts I, Bucciol G, Quinti I, et al. Coronavirus disease 2019 in patients with inborn errors of immunity: an international study. J Allergy Clin Immunol. 2021;147(2):520‐531. doi: 10.1016/j.jaci.2020.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Milito C, Lougaris V, Giardino G, et al. Clinical outcome, incidence, and SARS‐CoV‐2 infection‐fatality rates in Italian patients with inborn errors of immunity. J Allergy Clin Immunol Pract. 2021;9(7):2904‐2906. doi: 10.1016/j.jaip.2021.04.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Choi B, Choudhary MC, Regan J, et al. Persistence and evolution of SARS‐CoV‐2 in an immunocompromised host. N Engl J Med. 2020;383(23):2291‐2293. doi: 10.1056/NEJMc2031364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. McCormick KD, Jacobs JL, Mellors JW. The emerging plasticity of SARS‐CoV‐2. Science. 2021;371(6536):1306‐1308. doi: 10.1126/science.abg4493 [DOI] [PubMed] [Google Scholar]
- 5. Jayk Bernal A, Gomes da Silva MM, Musungaie DB, et al. Molnupiravir for Oral treatment of Covid‐19 in nonhospitalized patients. N Engl J Med. 2022;386(6):509‐520. doi: 10.1056/NEJMoa2116044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Haidar G, Mellors JW. Improving the outcomes of immunocompromised patients with coronavirus disease 2019. Clin Infect Dis off Publ Infect Dis Soc Am. 2021;73(6):e1397‐e1401. doi: 10.1093/cid/ciab397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhang Q, Bastard P, Liu Z, et al. Inborn errors of type I IFN immunity in patients with severe COVID‐19. Science. 2020;370(6515):eabd4570. doi: 10.1126/science.abd4570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bastard P, Rosen LB, Zhang Q, et al. Auto‐antibodies against type I IFNs in patients with life‐threatening COVID‐19. Science. 2020;370(6515):eabd4585. doi: 10.1126/science.abd4585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Centers for Disease Control and Prevention . Prioritization of anti‐SARS‐CoV‐2 therapies for the treatment and prevention of COVID‐19 when there are logistical or supply constraints. 2022. Available at: https://www.covid19treatmentguidelines.nih.gov/overview/prioritization‐of‐therapeutics
- 10. Romani L, Calò Carducci FI, Chiurchiù S, et al. Safety of monoclonal antibodies in children affected by SARS‐CoV‐2 infection. Children (Basel). 2022;9(3):369. doi: 10.3390/children9030369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mak G, Dassner AM, Hammer BM, Hanisch BR. Safety and tolerability of monoclonal antibody therapies for treatment of COVID‐19 in pediatric patients. Pediatr Infect Dis J. 2021;40:e507‐e509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang L, Berger NA, Kaelber DC, Davis PB, Volkow ND, Xu R. Comparison of outcomes from COVID infection in pediatric and adult patients before and after the emergence of omicron. MedRxiv Prepr Serv Health Sci. 2022;2021.12.30.21268495. doi: 10.1101/2021.12.30.21268495 [DOI] [Google Scholar]
- 13. Cenciarelli S, Calbi V, Barzaghi F, et al. Mild SARS‐CoV‐2 infection after gene therapy in a child with Wiskott‐Aldrich syndrome: a case report. Front Immunol. 2020;11:603428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Amodio D, Ruggiero A, Sgrulletti M, et al. Humoral and cellular response following vaccination with the BNT162b2 mRNA COVID‐19 vaccine in patients affected by primary immunodeficiencies. Front Immunol. 2021;12:727850. doi: 10.3389/fimmu.2021.727850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Brown LK, Moran E, Goodman A, et al. Treatment of chronic or relapsing COVID‐19 in immunodeficiency. J Allergy Clin Immunol. 2022;149(2):557‐561.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
TableS1


