Abstract
It is well established that acute moderate-intensity exercise improves cognitive performance. However, the effects of acute high-intensity aerobic exercise on cognitive performance have not been well characterized. In this review, we summarize the literature investigating the exercise-cognition interaction, especially focusing on high-intensity aerobic exercise. We discuss methodological and physiological factors that potentially mediate cognitive performance in response to high-intensity exercise. We propose that the effects of high-intensity exercise on cognitive performance are primarily affected by the timing of cognitive task (during vs. after exercise, and the time delay after exercise). In particular, cognitive performance is more likely to be impaired during high-intensity exercise when both cognitive and physiological demands are high and completed simultaneously (i.e., the dual-task paradigm). The effects may also be affected by the type of cognitive task, physical fitness, exercise mode/duration, and age. Second, we suggest that interactions between changes in regional cerebral blood flow (CBF), cerebral oxygenation, cerebral metabolism, neuromodulation by neurotransmitters/neurotrophic factors, and a variety of psychological factors are promising candidates that determine cognitive performance in response to acute high-intensity exercise. The present review has implications for recreational, sporting, and occupational activities where high cognitive and physiological demands are required to be completed concurrently.
Keywords: cognition, dual task, cerebral blood flow, cerebral oxygenation, cerebral metabolism, neuromodulation
Introduction
A growing body of evidence suggests that acute moderate-intensity exercise improves cognitive performance (Lambourne and Tomporowski, 2010; Chang et al., 2012; Ando et al., 2020; McMorris, 2021). It has been speculated that the relationship between exercise intensity and cognitive performance is inverted-U shaped (Lambourne and Tomporowski, 2010; Chang et al., 2012; McMorris, 2021). In the inverted-U theory, acute exercise gradually increases arousal to an optimal level from rest to moderate intensity and thus improves cognitive performance. A recent review summarized that improvements in cognitive performance following moderate-intensity exercise are frequently accompanied by the changes in brain activation assessed by electroencephalogram (EEG) (Kao et al., 2020), which appears to support the theory that acute exercise alters brain activity and that this is associated with cognitive performance. Acute high-intensity aerobic exercise leads to metabolic, circulatory, and neurohormonal changes at the level of the brain (Ide and Secher, 2000; Meeusen et al., 2001; Nybo and Secher, 2004; Ogoh and Ainslie, 2009; Seifert and Secher, 2011). In contrast to moderate-intensity exercise, theoretically, high-intensity exercise may, therefore, also lead to altered, and potentially impaired cognitive performance. Indeed, the inverted-U theory predicts that high-intensity exercise increases arousal levels beyond the optimal level and leads to a temporary reduction in cognitive performance. However, the current literature base detailing the effects of high-intensity exercise on cognitive performance is not fully supportive of this theory and is somewhat ambiguous and contradictory (Browne et al., 2017; Moreau and Chou, 2019; Cantelon and Giles, 2021; McMorris, 2021; Zheng et al., 2021).
Dietrich and Audiffren (2011) proposed the hypofrontality hypothesis to explain how acute high-intensity exercise affects cognitive performance. The prefrontal cortex (PFC) orchestrates higher-order brain function including cognitive function (Miller and Cohen, 2001; Cools and Arnsten, 2021) and is thought to play a central role in cognitive performance. Acute exercise activates brain regions including motor and sensory cortices, insular cortex, and cerebellum (Williamson et al., 1997; Christensen et al., 2000; Hiura et al., 2014). Hence, the hypofrontality theory speculates that extensive activation of motor and sensory systems during high-intensity exercise likely attenuates higher-order functions of the PFC as the brain has finite metabolic resources (Dietrich and Audiffren, 2011). More recently, McMorris proposed an interoceptive model to explain the effects of high-intensity exercise on cognitive performance (McMorris, 2021). This model offers a more holistic overview of the interaction as it incorporates motivation, perceived effort costs, and perceived availability of resources, together with regional activations and neurotransmitter releases in the brain. Nevertheless, to date, the physiological and psychological mechanism(s) mediating the effects of acute high-intensity exercise on cognitive performance are poorly understood.
In this review, we first summarize the findings of studies investigating the exercise-cognition interaction, especially focusing on high-intensity aerobic exercise. Then, we explore methodological and physiological factors which may alter cognitive performance in response to high-intensity exercise. This review has implications for recreational, sporting, and occupational activities where high cognitive and physiological demands are simultaneously required.
Methodology
A literature search was undertaken using Pubmed to identify studies that examined the effects of high-intensity aerobic exercise on cognitive performance, assessed during and/or after exercise. The reference lists of relevant articles were also searched. The searches were undertaken in February 2022 and relevant articles were obtained. This review focused on healthy adults, and no restrictions were placed on publication date, study design, methodology, or method of assessing cognitive performance. High-intensity aerobic exercise was defined as exercise equating to ≥ 80% maximum power output (Browne et al., 2017), ≥ 80% maximal oxygen uptake () (McMorris, 2016b), or equivalent [e.g., ≥ 80% maximal heart rate (HR)]. Physiological demands are different between continuous and intermittent high-intensity exercise. Thus, we included high-intensity aerobic exercise in this review, and studies incorporating high-intensity interval exercise (HIIE) were considered outside the scope of this review. If specifically interested in this, readers are referred to a recent review that has already explored the effects of HIIE on executive function (Hsieh et al., 2021). In addition, we did not include studies conducted in extreme environments, such as hypoxia and hot/cold environments. However, we referred to evidence from HIIE studies, or studies in extreme environments, for discussion since physiological mechanisms underlying cognitive improvement/impairment in response to HIIE exercise or exercise in extreme environments are, at least partly, shared with those induced by high-intensity aerobic exercise.
Results
Details of the included studies are shown in Table 1, comprising a total of 40 studies (assessed during exercise only, n = 20; assessed both during and after exercise, n = 3; assessed after exercise only, n = 17). In many studies, cognitive performance was impaired during high-intensity exercise (Chmura et al., 1994; McMorris and Keen, 1994; Brisswalter et al., 1997; McMorris et al., 2009; Labelle et al., 2013; Wang et al., 2013; Dutke et al., 2014; Mekari et al., 2015; Schmit et al., 2015; Smith et al., 2016; Gonzalez-Fernandez et al., 2017; Tempest et al., 2017; Komiyama et al., 2020; Stone et al., 2020). In these studies, impairments in both reaction time (RT) and accuracy were frequently observed. Seven studies reported no changes in cognitive performance (Travlos and Marisi, 1995; Fery et al., 1997; Ando et al., 2011; Dutke et al., 2014; Ciria et al., 2019; Tempest and Reiss, 2019; Komiyama et al., 2020). Five studies reported improvement in RT and/or accuracy during high-intensity exercise (McMorris and Graydon, 1997; Huertas et al., 2011; Shields et al., 2011; Davranche et al., 2015; Tempest et al., 2017).
TABLE 1.
Summary of the findings.
| References | Participants (F) |
Category of cognitive task | Cognitive task(s) (number of trials, duration) | Exercise modality/intensity | Exercise duration (high intensity only) or until exhaustion | Timing of cognitive task | Physiological variables | Main findings |
| Chmura et al. (1994) | N = 22 (0) Young | Psychomotor task | Choice RT (30 trials, 107 s) | Cycling Near maximal (300 W) | 6 min | During | Blood catecholamine, lactate | RT: impairment |
| McMorris and Keen (1994) | N = 12 (4) Young | Psychomotor task | Simple RT (15 trials) | Cycling 100% maximum workload | Not reported | During | - | RT: impairment |
| McMorris and Graydon (1997) | N = 12 (0) Young | Attentional task executive function | Soccer specific visual search task (30 slides) Soccer specific decision making task (15 trials) | Cycling 100% maximum power output | Not reported | During | - | Visual search: improvement Decision making: improvement |
| McMorris et al. (2009) | N = 24 (0) Young | Executive function | Flanker task (96 trials) | Cycling 80% maximum aerobic power | 15 min or until exhaustion | During | Blood catecholamine, adrenocorticotropic hormone, cortisol | RT: impairment Accuracy: impairment |
| Ando et al. (2011) | N = 12 (0) Young | Executive function | Flanker task (40 trials, 3 min 20 s) | Cycling 80% peak | 6.5 min | During | Cerebral oxygenation | EMG-RT≈ Accuracy≈ |
| Huertas et al. (2011) | N = 18 (0) Young | Executive function | Attention network test (modified flanker task, 480 trials, 25 min) | Cycling 95% LT | 25 min | During | Blood lactate | RT: improvement |
| Shields et al. (2011) | N = 30 (15) Young | Attentional task | Visual threat-detection task (256 trials) | Cycling 80% maximal HR | Not reported | During | - | RT: improvement Accuracy: improvement |
| Labelle et al. (2013) | N = 37 (18) Young High fit = 16 Low fit = 21 | Executive function | Stroop task (80 trials) | Cycling 80% peak power output | 6.5 min | During | - | Accuracy: impairment RT variability: impairment in lower fit |
| Wang et al. (2013) | N = 80 (31) Young | Executive function | Wisconsin card sorting test (128 response cards) | Cycling 80% HRR | 30 min | During | - | Performance: impairment |
| Dutke et al. (2014) | N = 60 (14) Young | Memory task attentional task (simultaneously) | Word comparison (Primary task, 60 trials, 27 min) Interval production (Secondary task, press a button every 2 s, 27 min) | Cycling 120% AT | > 27 min | During | - | Number of correct response≈ Response time≈ Interval production error: impairment |
| Davranche et al. (2015) | N = 14 (3) Young | Executive function | Simon task (200 trials, 4 min) | Cycling 20% above VT | 20 min | During | - | RT: improvement Accuracy≈ |
| Mekari et al. (2015) | N = 19 (12) Young | Executive function | Stoop task (30 trials × 2 blocks) | Cycling 85% peak power output | 9 min | During | Cerebral oxygenation | RT: impairment Accuracy: impairment |
| Schmit et al. (2015) | N = 15 (5) Young | Executive function | Flanker task (40 trials, as many blocks as possible) | Cycling 85% maximal aerobic power | Until exhaustion | During | Cerebral oxygenation | RT≈ Accuracy: impairment |
| Smith et al. (2016) | N = 15 (9) Young | Executive function | Go/No-Go task (100 trials, 2 min) | Running 90% HRR | 10 min | During | - | RT: impairment Accuracy: impairment |
| Gonzalez-Fernandez et al. (2017) | N = 24 (12) Young | Psychomotor task | Psychomotor vigilance task (mean 46.8 trials) | Cycling 100% ventilatory anaerobic threshold | 5 min | During | - | RT: impairment |
| Tempest et al. (2017) | N = 14 (5) Young | Executive function | Flanker task (2 min × 10 blocks) 2-back task (60 trials × 10 blocks, 20 min) | Cycling 10% above VT | 60 min | During | Cerebral oxygenation | RT (flanker task): improvement Accuracy (n-back): impairment |
| Ciria et al. (2019) | N = 20 (0) Young | Attentional task | Oddball task (20 min) | Cycling 80% peak | 20 min | During | EEG | RT≈ Accuracy≈ |
| Tempest and Reiss (2019) | N = 13 (7) Young | Executive function | N-back task (0-back, 60 trials; 2-back, 60 trials) |
Cycling 115% first ventilatory threshold (VT1) | 16 min | During | Cerebral oxygenation (fNIRS) | Response time≈ Accuracy≈ |
| Komiyama et al. (2020) | N = 17 (0) Young | Executive function | Spatial delayed response Go/No-Go tasks (24 trials, ∼5 min) | Cycling 80% peak | 8 min | During | Middle cerebral artery blood velocity Cerebral oxygenation | RT≈ Accuracy: impairment |
| Stone et al. (2020) | N = 13 (5) Young | Executive function | Cedar operator workload assessment tool | Running 100% HRR | Until exhaustion | During | Cerebral oxygenation | Accuracy: impairment |
| Travlos and Marisi (1995) | N = 20 (0) Young High fit = 10 Low fit = 10 | Attentional task psychomotor task | Random number generation test Choice RT (15 trials × 4) | Cycling during 80% max and after volitional exhaustion |
Until exhaustion (> 10 min) |
During/immediately after | - | Random number generation test (during)≈ RT (after)≈ |
| Brisswalter et al. (1997) | N = 20 (0) Young High fit = 10 Low fit = 10 | Psychomotor task | Simple detection RT (20 trials) | Cycling during 80% maximal aerobic power | 10 min | During/1 min after | - | RT (during): impairment in only low fit Accuracy≈ in both groups |
| Fery et al. (1997) | N = 13 (0) Young | Memory task | Short-term memory task (20 trials) | Cycling during 90% max and Volitional exhaustion | Until exhaustion | During/immediately after | - | RT (during)≈ RT (after): impairment |
| Kamijo et al. (2004a; 2004b) | N = 12 (0) Young | Executive function | S1-S2 RT (Go/No-Go) task (60 trials, 10 min) | Cycling Volitional exhaustion | Until exhaustion | Immediately after (< 3 min) | EEG (CNV, P300) | EMG-RT≈ |
| McMorris et al. (2005) | N = 12 (0) Young | Psychomotor task | Whole body choice RT (9 trials) | Cycling 100% maximal power output | Until exhaustion | Immediately after (20 s later) | Blood lactate | RT: impairment |
| Winter et al. (2007) | N = 27 (0) Young | Memory task | Vocabulary learning task (600 training trials + retention) | Running (two sprints, started at 8 km/h, increased every 10 s by 2 km/h) Volitional exhaustion | Until exhaustion | 15 min after | Blood catecholamine, BDNF | Learning speed: improvement RT: improvement (1 week later) |
| Coco et al. (2009) | N = 17 (0) Young | Psychomotor task Attentional task | RT task Attention and concentration task | Cycling Volitional exhaustion Lactate infusion (N = 6) | Until exhaustion | 5 min after | Blood lactate | RT: impairment Accuracy: impairment |
| Luft et al. (2009) | N = 30 (7) Young | Psychomotor task Executive function Memory task Attentional task | Simple RT (35 correct trials, 90 s) Choice RT (30 correct trials, 90 s) Working memory task (one back task, 30 correct trials, 90 s) Short-term memory task (42 trials, 2–3 min) Continuous monitoring task (30 correct trials, 90 s) | Running Volitional exhaustion | Until exhaustion | 10–15 min after | HR variability | Working memory: improvement Others≈ |
| Thomson et al. (2009) | N = 163 (0) Young | Psychomotor task | Speed discrimination (decision-making) | Running Volitional exhaustion | Until exhaustion | 1 min after | - | Time: improvement Accuracy: impairment |
| Griffin et al. (2011) | N = 47 (0) Young | Memory task Executive function | Face-name matching task Stroop task | Running Volitional exhaustion | Until exhaustion | < 30 min After | Blood BDNF, IGF-1 | Face-name matching task: improvement Stroop task≈ |
| Etnier et al. (2016) | N = 16 (7) Young | Memory task | Rey Auditory Verbal Learning Test (15 words × 2) | Running Volitional exhaustion | Until exhaustion | Immediately after (after blood sampling) | Blood BDNF | Memory performance: improvement (24 h later) |
| Hwang et al. (2016) | N = 58 (32) Young | Executive function | Stroop test (100 items × 3 conditions, 4 min) Trail making test (< 2 min) | Running Target HR corresponding to 85–90% max | 10 min | 10 min after | Blood BDNF | Stroop test: improvement Trail making test: improvement |
| Chang et al. (2017) | N = 36 (36) Young | Executive function | Stroop test (neutral 60 s, incongruent 60 s) | Running 80% HRR | 30 min | 15 min after | Cerebral oxygenation | RT≈ |
| Sudo et al. (2017) | N = 32 (0) Young | Executive function | Spatial delayed response task (20 trials) Go/No-Go task (20 trials) | Cycling Volitional exhaustion | Until exhaustion | 2 min after | Cerebral oxygenation, Blood catecholamine, BDNF, IGF-1, lactate | RT≈ Accuracy≈ |
| Zimmer et al. (2017) | N = 119 (41) Young | Executive function | Tower of London | Cycling Volitional exhaustion | Until exhaustion | Immediately after (< 3 min) | Blood lactate | Thinking time: impairment |
| Du Rietz et al. (2019) | N = 29 (0) Young | Executive function Psychomotor task | Cued continuous performance task (modified Go/No-Go task, 80 trials, 11 min) Flanker task (400 trials, 13 min) Choice RT task (72 trials, 10 min) | Cycling 20% delta (difference between gas exchange threshold and peak) | 20 min | 30 min after | EEG | RT≈ Accuracy≈ |
| Hill et al. (2019) | N = 13 (0) Young | Executive function | Flanker task (100 trials, < 3 min) | Cycling and arm cranking Volitional exhaustion | Until exhaustion | Immediately after | - | Cycling: impairment Arm cranking: improvement |
| Coco et al. (2020a) | N = 30 (?) Young = 15 Old = 15 | Psychomotor task Executive function | Simple RT Stroop color word test (50 names, 50 circles, and 50 words) Trail making test | Cycling Volitional exhaustion | Until exhaustion | Immediately after | Blood lactate | Simple RT: impairment Stroop Color Word Test: impairment Trail Making Test: improvement (Young) |
| Loprinzi et al. (2021) | N = 120 (77) Young | Memory task | Word list memory task (15 words) | Running 75% HRR | 20 min | 5 min after | - | Memory: improvement |
| Marin Bosch et al. (2021) | N = 18 (0) Young | Psychomotor task Memory task | Psychomotor vigilance task (RT) Associative memory task (8 series of 6 successive pictures) | Cycling 75% maximal cardiac frequency | 15 min | 24 min after (Psychomotor task) and 69 min after (memory task) |
Neural activity (fMRI), Blood endocannabinoids, BDNF | RT≈ Accuracy≈ |
F, females; N, number of participants; RT, reaction time; W, watts; peak, peak oxygen uptake; EMG, electromyogram; LT, lactate threshold; HR, heart rate; HRR, heart rate reserve; AT, anaerobic threshold; VT, ventilatory threshold; EEG, electroencephalogram; fNIRS, functional near-infrared spectroscopy; CNV, contingent negative variation; P300, positive 300; max, maximal oxygen uptake; BDNF, brain-derived neurotrophic factor; IGF-1, insulin-like growth hormone factor-1; fMRI, functional magnetic resonance imaging. ≈, no effect.
Conversely, cognitive performance after high-intensity exercise is heterogeneous; with improvements (Winter et al., 2007; Luft et al., 2009; Thomson et al., 2009; Griffin et al., 2011; Etnier et al., 2016; Hwang et al., 2016; Hill et al., 2019; Coco et al., 2020a; Loprinzi et al., 2021), impairments (Fery et al., 1997; McMorris et al., 2005; Coco et al., 2009, 2020a; Thomson et al., 2009; Zimmer et al., 2017; Hill et al., 2019), and no changes (Travlos and Marisi, 1995; Brisswalter et al., 1997; Kamijo et al., 2004a,b; Luft et al., 2009; Griffin et al., 2011; Chang et al., 2017; Sudo et al., 2017; Du Rietz et al., 2019; Marin Bosch et al., 2021) reported within the literature. These findings suggest that cognitive performance after high-intensity exercise appears to be dependent on experimental design (see below). In the following sections, we discuss the methodological, physiological, and psychological factors that affect cognitive performance “during” and “after” high-intensity exercise.
Methodological factors
Here we discuss the potential methodological and experimental factors that contribute to the inconsistent findings. These include the following: timing of cognitive task, type of cognitive task, physical fitness, exercise mode/duration, and age.
Timing of cognitive task
When participants perform cognitive tasks during exercise, they perform the exercise and cognitive tasks simultaneously (i.e., a dual-task paradigm). However, when cognitive tasks are performed after exercise, participants only perform a single task. A meta-analysis reported higher effect sizes in single-task conditions (after exercise) when compared with dual-task conditions (during exercise) (Lambourne and Tomporowski, 2010), while another meta-analysis reported that effect sizes were not different between single and dual-task conditions (Chang et al., 2012). Furthermore, McMorris and Hale (2012) undertook statistical analyses and found that there were no differences in effect sizes obtained during compared after exercise. Nevertheless, as recently highlighted (McMorris, 2021), the timing of the cognitive tasks is typically less considered within the literature.
Table 1 indicates that the adverse effects are most prominent during high-intensity exercise. These findings are corroborated by a recent review and suggest that impairments in cognitive performance are more likely to occur during high-intensity exercise (Zheng et al., 2021). Based on the assumption that metabolic resources are limited in the brain, extensive activation in several brain regions (e.g., motor and sensory cortices) may attenuate higher-order functions of the PFC and impair cognitive performance (Dietrich and Audiffren, 2011). Furthermore, in the majority of the included studies, cognitive performance was assessed using manual responses where activations of the motor-related areas are required. Given a limited capacity of the brain to simultaneously activate multiple regions involved in cognitive performance and high-intensity exercise, it is plausible that cognitive performance is more likely to be impaired during high-intensity exercise, particularly when both cognitive and physiological demands are high. Indeed, in four studies reporting cognitive improvement during high-intensity exercise (Huertas et al., 2011; Shields et al., 2011; Davranche et al., 2015; Tempest et al., 2017), exercise intensities were relatively less demanding (i.e., HR < 170 bpm). Relatively lower physiological demands may be responsible for cognitive improvements during high-intensity exercise in these studies. Furthermore, McMorris (2021) argued that performance would depend to a large extent on the perception of task costs (demands) and resources available. These two judgments may be difficult to make in the dual-task situation, leading to over-or under-confidence. This could alter motivation, which would affect cognitive performance.
A recent meta-analysis demonstrated that acute high-intensity exercise had a small, significant facilitating effect on cognitive performance after high-intensity exercise (Moreau and Chou, 2019). In the current review, we observed that cognitive performance after high-intensity exercise is inconsistent: and improvements, impairments, and no changes were reported. EEG studies reported reductions in P3 amplitudes after high intensity (Kamijo et al., 2004b) or HIIE (Kao et al., 2017). On the contrary, Du Rietz and colleagues reported improvements in P3 amplitude and delta power reflecting executive and sustained attention after high-intensity exercise (Du Rietz et al., 2019). These findings suggest that brain activity after exercise may be dependent on the experimental design employed (e.g., exercise intensity, time delay after exercise). Indeed, most physiological changes start to recover immediately after high-intensity exercise (Ide et al., 2000; Gonzalez-Alonso et al., 2004; Curtelin et al., 2017; Sudo et al., 2017). Thus, rapid recovery of physiological variables to homeostatic resting levels may, at least in part, explain the contradictory findings related to cognitive performance after high-intensity exercise. To explore this possibility, we summarized the impacts of the timeframe in which cognitive performance was assessed after exercise (Table 2). When multiple cognitive tasks were examined in a single study, or when both improvement and impairment were reported in a cognitive task (e.g., improvement in RT and impairment in accuracy), all results are reported. We observed heterogeneous findings when cognitive performance was assessed within 5 min of completing high-intensity exercise. Intriguingly, however, no impairments were reported when the timing of cognitive tasks was > 6 min after exercise. These findings support the notion that a rapid recovery to homeostatic resting levels is critical for cognitive performance after high-intensity exercise. Taken collectively, we propose that the effects of high-intensity exercise on cognitive performance are closely related to, and impacted by, the timing of cognitive task (during vs. after exercise, and the time delay after exercise).
TABLE 2.
Summary of impacts of time delay after exercise.
| 0–5 min |
6–10 min |
11–20 min |
>20 min |
||||||||
| Improvement (↑) | No change (↔) | Impairment (↓) | ↑ | ↔ | ↓ | ↑ | ↔ | ↓ | ↑ | ↔ | ↓ |
| ⬤⬤⬤⬤⬤ | ⬤⬤⬤⬤⬤ | ⬤⬤⬤⬤⬤⬤⬤⬤⬤ | ⬤⬤ | ⬤⬤ | ⬤⬤⬤⬤⬤ | ⬤ | ⬤⬤⬤⬤⬤⬤ | ||||
Number of black circles indicates the number of studies.
Type of cognitive task
Different brain regions are thought to be activated during different cognitive tasks (Macintosh et al., 2014; Chen A. G. et al., 2016; Won et al., 2019). Thus, we can assume that the type of cognitive task is one of the factors that determine how acute high-intensity exercise impacts cognitive performance. This may be particularly relevant when exercise and cognitive tasks are concurrently performed since multiple brain regions are presumably activated. In this review, we classified the type of cognitive task into executive function, psychomotor, memory, and attentional tasks (Table 3). Executive function encompasses several subdomains and consists of basic components of inhibition, working memory, and cognitive flexibility (Diamond, 2013). Thus, based on the included studies, we classified executive function into response inhibition (Go/No-Go task), interference control (Flanker task, Simon task, and Stroop task), working memory (n-back task and spatial delayed response task), and others (soccer-specific task, Wisconsin card sorting task, Cedar Operator Workload Assessment Tool, Tower of London, and Trail making test).
TABLE 3.
Summary of impacts of the type of cognitive task.
| Executive function |
Psychomotor task |
Memory task |
Attentional task |
|||||||||
| Improvement (↑) | No change (↔) | Impairment (↓) | ↑ | ↔ | ↓ | ↑ | ↔ | ↓ | ↑ | ↔ | ↓ | |
| During | ***⬤ | †*# | †**$$##⬤⬤ | ⬤⬤⬤⬤ | ⬤⬤ | ⬤⬤ | ⬤⬤ | ⬤ | ||||
| After | *$#⬤⬤ | †††*$$# | *$⬤ | ⬤ | ⬤⬤⬤⬤⬤⬤ | ⬤⬤⬤⬤ | ⬤⬤⬤⬤ | ⬤⬤ | ⬤ | ⬤ | ⬤ | |
Number of symbols indicates the number of studies.
†Response inhibition (Go/No-Go task).
*Interference control (Flanker task, Simon task).
$Interference control (Stroop task).
#Working memory (n-back task, spatial delayed response task).
⬤ Others (soccer-specific task, Wisconsin card sorting task, Cedar Operator Workload Assessment Tool, Tower of London, and Trail making test), psychomotor task, memory task, attentional task.
During high-intensity exercise, impairments in cognitive performance were prominent in executive function and psychomotor tasks (Table 3), which is thought to be closely related to dual-task paradigm. Thus, in most cognitive tasks, adverse effects are more likely to occur during high-intensity exercise. Conversely, we found improvements in several studies. In three studies (Huertas et al., 2011; Davranche et al., 2015; Tempest et al., 2017), cognitive improvements were observed in interference control (i.e., Flanker task and Simon task). These findings imply that performances in these cognitive tasks may benefit from high-intensity exercise relative to the other subcomponents of executive function. Furthermore, in the study reporting improvements in soccer-specific cognitive tasks (both soccer-specific and attentional tasks) in college soccer players (McMorris and Graydon, 1997), the cognitive tasks were probably autonomous to the soccer players and therefore less demanding. Hence, cognitive improvements during high-intensity exercise appear to be related to cognitive demands.
A meta-analysis review reported that facilitating effects of performance were similarly observed in subcomponents of executive function (i.e., working memory, inhibitory control, cognitive flexibility, and attention) after high-intensity exercise (Moreau and Chou, 2019). In the present review, we failed to find clear associations between the type of cognitive task and cognitive performance after high-intensity exercise. It should be noted, however, that improvements in memory performance were often observed after high-intensity exercise (Winter et al., 2007; Griffin et al., 2011; Etnier et al., 2016; Loprinzi et al., 2021). In particular, high-intensity exercise may be beneficial for retention (Winter et al., 2007; Etnier et al., 2016; Loprinzi et al., 2021). Additional studies are necessary to further elucidate potential physiological mechanisms underlying the improvement. In this review, we may not have been sufficiently powered to identify the effects of different cognitive tasks/domains. Further research is required to establish the effects of high-intensity exercise on a variety of cognitive tasks and domains.
Physical fitness level of participants
It has been previously speculated that exercise-cognition interaction is influenced by physical fitness (Lambourne and Tomporowski, 2010; Chang et al., 2012). For example, despite matched relative exercise intensity, individuals with lower physical fitness levels were more susceptible to cognitive impairments during high-intensity exercise, when compared with those who had higher aerobic capacities (Brisswalter et al., 1997; Labelle et al., 2013). Furthermore, choice RT performance gradually improves during incremental exercise until at ∼75% max in young soccer players (Chmura et al., 1994). Aerobic capacity has been suggested to be one of the moderators that affect cognitive performance in response to high-intensity exercise (Browne et al., 2017). McMorris (2021) claimed that fitness levels would affect the individual’s perception of effort costs and, hence, their motivation level. Further, well-powered studies are also necessary to clarify the relationship between cognitive performance and physical fitness before this theory can be confirmed.
Exercise mode
A previous meta-analysis suggested that exercise mode is one of the factors that affect exercise-cognition interaction (Lambourne and Tomporowski, 2010). We summarized the impacts of high-intensity cycling and running in Table 4. The effects of high-intensity exercise on cognitive performance during cycling were inconsistent, but reductions in cognitive performance appeared to be more likely during cycling. Running is kinematically less stable compared to cycling, and thus it may be difficult to complete cognitive performance during high-intensity running. Indeed, only two studies examined cognitive performance during high-intensity running, and both reported impairments in cognitive performance (Smith et al., 2016; Stone et al., 2020). Given the nature of the dual-task paradigm, running may be more detrimental to cognitive performance relative to cycling during high-intensity exercise.
TABLE 4.
Summary of impacts of exercise mode.
| Cycling (+ arm cranking) |
Running |
|||||
| Improvement (↑) | No change (↔) | Impairment (↓) | ↑ | ↔ | ↓ | |
| During | ⬤⬤⬤⬤⬤⬤ | ⬤⬤⬤⬤⬤⬤⬤ | ⬤⬤⬤⬤⬤⬤⬤⬤⬤⬤⬤⬤ | ⬤⬤ | ||
| After | ⬤⬤ | ⬤⬤⬤⬤⬤⬤⬤⬤⬤⬤ | ⬤⬤⬤⬤⬤⬤⬤⬤ | ⬤⬤⬤⬤⬤⬤⬤⬤ | ⬤⬤⬤⬤ | ⬤ |
Number of black circles indicates the number of studies.
After high-intensity cycling, in most cases, we observed no changes or impairments in cognitive performance. Conversely, after high-intensity running, improvements or no changes in cognitive performance were predominant. Since participants perform only a cognitive task after exercise (i.e., single task) regardless of exercise mode, the findings are somewhat unexpected. There are several physiological differences in ventilatory and metabolic responses, HR, and motor unit recruitment between cycling and running (Millet et al., 2009). Hence, the recovery of physiological variables to resting levels could be different between high-intensity cycling and running. Collectively, these findings suggest that the mode of exercise is one of the factors that affect cognitive performance, and these effects are presumably influenced by the timing of cognitive task (during vs. after exercise, and the time delay after exercise). Future studies are required to understand how exercise mode influences cognitive performance after high-intensity exercise.
Exercise duration
Exercise duration also potentially affects cognitive performance in response to high-intensity exercise. We summarized the relationship between exercise duration and cognitive performance in Table 5. During exercise, cognitive impairments were observed when exercise duration was < 10 min. The findings were inconsistent when exercise duration was > 11 min or exhaustive exercise. Thus, it is less likely that exercise duration per se affects cognitive performance. Rather, interactions between exercise duration and intensity would be more important for cognitive performance during high-intensity exercise. In most studies that assessed cognitive performance after high-intensity exercise, exercise was continued until exhaustion (Table 5). Nevertheless, the results were heterogeneous and suggest that exercise duration in isolation is not critical for cognitive performance after exhaustive exercise. Assuming that the duration of high-intensity exercise is likely limited, exercise duration alone is, therefore, not likely to determine cognitive performance.
TABLE 5.
Summary of impacts of exercise duration.
| <10 min |
11–20 min |
>20 min |
Until exhaustion |
|||||||||
| Improvement (↑) |
No change (↔) | Impairment (↓) | ↑ | ↔ | ↓ | ↑ | ↔ | ↓ | ↑ | ↔ | ↓ | |
| During | ⬤⬤ | ⬤⬤⬤⬤⬤⬤⬤ | ⬤ | ⬤⬤ | ⬤⬤ | ⬤ | ⬤⬤⬤ | ⬤⬤ | ⬤⬤⬤ | |||
| After | ⬤⬤ | ⬤ | ⬤ | ⬤⬤⬤⬤⬤ | ⬤ | ⬤⬤⬤⬤⬤⬤⬤ | ⬤⬤⬤⬤⬤⬤⬤⬤⬤ | ⬤⬤⬤⬤⬤⬤⬤⬤⬤ | ||||
Number of black circles indicates the number of studies.
Age
Coco and colleagues compared the effects of exhaustive exercise on the cognitive performance of young and older adults (Coco et al., 2020a). They reported impairments in the performance of simple RT and the Stroop color-word test after exhaustive exercise in both groups. However, improvements in trail making test were observed only in the younger group. These results suggest that the effects of high-intensity exercise on cognitive performance may be different in young and older adults. Cerebral perfusion appears to be lower in older individuals during high-intensity exercise, although the cerebral extraction of glucose, lactate, and oxygen is similar (Braz and Fisher, 2016). Thus, age may be one factor that may influence cognitive performance and reduced cerebral perfusion could affect cognitive performance in older individuals in particular. Given the brevity of studies in this area, definitive conclusion on the impact of age is not yet feasible.
Physiological factors
As noted above, high-intensity exercise induces a variety of physiological effects within the human brain. Here, we summarize and discuss the physiological factors that are linked to cognitive performance during and after high-intensity exercise and we identify some of the potential physiological factors that may contribute to the inconsistent findings. These include the separate and combined effects of cerebral blood flow (CBF), cerebral oxygenation, cerebral metabolism, neuromodulation by neurotransmitters and neurotrophic factors, and various psychological factors.
Cerebral blood flow
During exercise, CBF is regulated by complex interactions between neural activity and metabolism, partial pressure of oxygen, carbon dioxide (CO2), blood pressure, cardiac output, and sympathetic nervous system activity (Ogoh and Ainslie, 2009; Smith and Ainslie, 2017). CBF gradually increases during mild- to moderate-intensity exercise in response to neural activity and metabolism (Ogoh and Ainslie, 2009). However, during high-intensity exercise, hyperventilation-induced hypocapnia constricts the cerebral vessels, thereby reducing CBF (Ogoh and Ainslie, 2009; Smith and Ainslie, 2017). This suggests that brain metabolic demands might be inadequate during high-intensity exercise. Ogoh et al. (2014) reported that an increase in CBF, achieved using CO2 inhalation, did not affect cognitive performance during prolonged moderate-intensity exercise (Ogoh et al., 2014). More recently, Komiyama and colleagues tested the hypothesis that a reduction in CBF is directly linked to impairment in cognitive performance during high-intensity exercise (Komiyama et al., 2020). By restoring CBF via CO2 inhalation, the authors demonstrated that middle cerebral artery (MCA) velocity (a surrogate for CBF) did not prevent impaired cognitive performance during high-intensity exercise. These results suggest that a reduction in CBF per se may not be responsible for impaired cognitive performance during high-intensity exercise. However, given that CBF supplies oxygen and nutrients, the association between cognitive performance and regional CBF (e.g., blood flow to the PFC) in response to high-intensity exercise should be further investigated. In particular, a recent study indicated physiological “uncoupling” between the PFC oxygenation and MCA velocity during high-intensity exercise with CO2 inhalation (Hansen et al., 2020). Follow-up studies are needed to fully understand the association between regional CBF and cognitive performance in response to high-intensity exercise.
Cerebral oxygenation
Cerebral oxygenation reflects the balance between cerebral oxygen availability and utilization (Boushel et al., 2001; Komiyama et al., 2017), which is generally measured from the PFC. Cerebral oxygenation reduces during high-intensity exercise close to maximal intensity (Rooks et al., 2010). Some studies suggest that a reduction in cerebral oxygenation is not associated with impairments in cognitive performance during high-intensity exercise (Ando et al., 2011; Schmit et al., 2015; Tempest et al., 2017). In contrast, others have indicated that impairments in cognitive performance were accompanied by reduction in cerebral oxygenation during high-intensity exercise (Mekari et al., 2015; Stone et al., 2020). The latter studies suggest that impairments in cognitive performance may be associated with attenuated PFC oxygenation. However, several studies have shown that cognitive performance improved during acute moderate-intensity exercise in hypoxia despite substantial reductions in cerebral oxygenation (Ando et al., 2013; Komiyama et al., 2015, 2017). Hence, it is likely that a reduction in cerebral oxygenation, in isolation, does not result in impaired cognitive performance. However, reduction in cerebral oxygenation during high-intensity exercise may impair cognitive performance in concert with other physiological factors.
Cerebral oxygenation starts to recover immediately after maximal exercise (Gonzalez-Alonso et al., 2004; Sudo et al., 2017). Notably, the degree of recovery of cerebral oxygenation following maximal exercise may be associated with cognitive performance (Sudo et al., 2017). This finding suggests that the recovery of cerebral oxygenation after high-intensity exercise may, at least in part, account for the differential effects of high-intensity exercise on cognitive performance between single (i.e., after) and dual (i.e., during) conditions.
Cerebral metabolism
It is generally accepted that blood glucose is the primary energy source for the brain at rest (Gold, 1995). Komiyama et al. (2016) reported that cognitive performance improves during moderate-intensity exercise after skipping breakfast. This suggests that substrates other than glucose may compensate for the reduced availability of blood glucose during moderate-intensity exercise. It is plausible that the same would be true for high-intensity exercise. Indeed, blood glucose uptake is thought to be reduced in the brain during high-intensity exercise (Kemppainen et al., 2005). In contrast, blood lactate substantially increases during/after high-intensity exercise, and it is taken up by the brain (Ide et al., 2000; Gonzalez-Alonso et al., 2004; Quistorff et al., 2008; Siebenmann et al., 2021). Several studies suggested that blood lactate would provide energy that contributes to improvements in cognitive performance following HIIE (Tsukamoto et al., 2016; Hashimoto et al., 2018; Herold et al., 2022). In particular, Hashimoto et al. (2018) directly measured lactate uptake in the brain after HIIE and suggested that lactate production in extra-cerebral tissues supports brain function. On the contrary, Coco et al. (2020b) suggested that high levels of blood lactate have detrimental effects on cognitive performance. Interestingly, Coco et al. (2009) indicated that intravenous lactate infusion of a lactate solution impaired attentional performance. Hence, further studies are warranted to investigate how blood lactate acts as a mediator of exercise-induced alterations in cognitive performance (Ando et al., 2022).
Neuromodulation by neurotransmitters and neurotrophic factors
In humans, it is less clear how acute exercise alters central neurotransmitter release due to technical and methodological challenges. Nevertheless, given that rodent studies indicate that acute exercise releases neurotransmitters in the brain (Meeusen et al., 2001; Hasegawa et al., 2011; Goekint et al., 2012; Chen C. et al., 2016), acute exercise is likely to influence brain circuits involving a number of neurotransmitters including dopamine and noradrenaline (McMorris, 2016a; Ando et al., 2020). Dopamine and noradrenaline modulate the strength of the PFC network connections, and regulation of dopamine and noradrenaline is required for appropriate prefrontal cognitive function (Arnsten, 2011). Furthermore, excess noradrenaline and dopamine appear to weaken the signal-to-noise ratio, which may result in impairments in the PFC function (Arnsten, 2011; Cools and Arnsten, 2021). Hence, the available literature suggests that excess neuromodulators in the brain may have adverse effects on cognitive performance during/after high-intensity exercise. High-intensity exercise also seems to increase brain-derived neurotrophic factors (BDNFs) (Ferris et al., 2007; Winter et al., 2007; Fernandez-Rodriguez et al., 2021) and insulin-like growth hormone factor-1 (IGF-1) (Sudo et al., 2017). Several studies have implicated that changes in BDNF are associated with cognitive improvement induced by acute exercise (Winter et al., 2007; Lee et al., 2014; Skriver et al., 2014; Hwang et al., 2016). However, BDNF and IGF-1 are known to play a crucial role in angiogenesis, synaptogenesis, and neurogenesis following long-term exercise (Cotman and Berchtold, 2002; Voss et al., 2011; Nieto-Estevez et al., 2016). High concentrations of dopamine, noradrenaline, and BDNF are necessary for long-term potentiation, which is essential for long-term memory (McMorris, 2021). A couple of studies have shown positive effects of high-intensity exercise on long-term memory (Winter et al., 2007; Griffin et al., 2011). At present, it is premature to conclude that changes in BDNF and IGF-1 play a role in cognitive performance during/after high-intensity exercise.
Psychological factors
In most studies, psychological factors are typically not well considered when attempting to elucidate the effects of high intensity on cognitive performance. However, as suggested by McMorris (2021), psychological factors such as the motivation and perception of effort may affect the acute exercise–catecholamine–cognition interaction. Cantelon and Giles also suggested that psychological factors are moderating factors that affect exercise–cognition interaction (Cantelon and Giles, 2021). At present, and given the lack of empirical evidence, further investigations are needed to investigate the association between psychological factors and cognitive performance during and after high-intensity exercise.
Integration of physiological and psychological factors
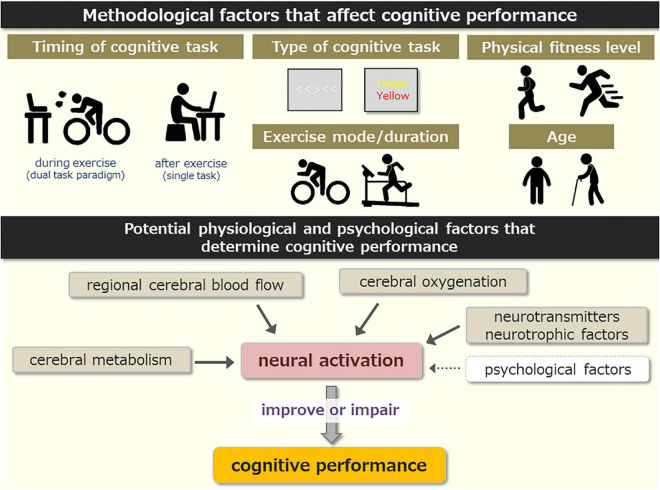
It is likely that the effects of high-intensity exercise on cognitive performance are multifactorial and determined by the integration of several physiological and psychological factors (Figure 1). We propose that interactions of these factors influence neural activity associated with cognitive performance and that this determines cognitive performance during and after high-intensity exercise. This is consistent with McMorris (2021) claiming that the perception of physiological stress affects the motivation and perception of effort costs. However, the current literature base is insufficient to substantiate this speculation and this should be the focus of future research in this area. A recent fNIRS study detected cognitive task-related hemodynamic changes from the left PFC during high-intensity exercise (Tempest and Reiss, 2019). Furthermore, an fMRI study indicated that HIIE decreased brain activation associated with cognitive performance (Mehren et al., 2019). Thus, future studies using sophisticated neuroimaging methods (e.g., fNIRS, fMRI, and PET) are required to fully understand how a single bout of high-intensity exercise affects cognitive performance.
FIGURE 1.
(Upper) Summary of methodological factors that affect cognitive performance in response to high-intensity exercise. (Lower) Potential physiological and psychological factors that mediate cognitive performance.
Conclusion
This narrative review summarized the literature examining the effects of acute high-intensity exercise on cognitive performance. We propose that the effects of high-intensity exercise on cognitive performance are primarily affected by a variety of methodological, physiological, and psychological factors. Specifically, these include the timing of cognitive task (during vs. after exercise, and the time delay after exercise), cognitive task(s), fitness level, exercise mode/duration, and age. It is also likely that a complex interaction between changes in regional CBF, cerebral oxygenation, cerebral metabolism, neurotransmitters/neurotrophic factors, and a variety of psychological factors contributes to the heterogeneous findings reported. The review is likely to have implications for recreational, sporting, and occupational activities where high cognitive and physiological demands are required simultaneously.
Author contributions
MS, JC, TM, and SA drafted the manuscript. All authors approved the final version of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- Ando S., Hatamoto Y., Sudo M., Kiyonaga A., Tanaka H., Higaki Y. (2013). The effects of exercise under hypoxia on cognitive function. PLoS One 8:e63630. 10.1371/journal.pone.0063630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ando S., Kokubu M., Yamada Y., Kimura M. (2011). Does cerebral oxygenation affect cognitive function during exercise? Eur. J. Appl. Physiol. 111 1973–1982. [DOI] [PubMed] [Google Scholar]
- Ando S., Komiyama T., Sudo M., Higaki Y., Ishida K., Costello J. T., et al. (2020). The interactive effects of acute exercise and hypoxia on cognitive performance: a narrative review. Scand. J. Med. Sci. Sports 30 384–398. 10.1111/sms.13573 [DOI] [PubMed] [Google Scholar]
- Ando S., Komiyama T., Tanoue Y., Sudo M., Costello J. T., Uehara Y., et al. (2022). Cognitive improvement after aerobic and resistance exercise is not associated with peripheral biomarkers. Front. Behav. Neurosci. 16:853150. 10.3389/fnbeh.2022.853150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten A. F. (2011). Catecholamine influences on dorsolateral prefrontal cortical networks. Biol. Psychiatry 69 e89–e99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boushel R., Langberg H., Olesen J., Gonzales-Alonzo J., Bulow J., Kjaer M. (2001). Monitoring tissue oxygen availability with near infrared spectroscopy (NIRS) in health and disease. Scand. J. Med. Sci. Sports 11 213–222. [DOI] [PubMed] [Google Scholar]
- Braz I. D., Fisher J. P. (2016). The impact of age on cerebral perfusion, oxygenation and metabolism during exercise in humans. J. Physiol. 594 4471–4483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brisswalter J., Arcelin R., Audiffren M., Delignieres D. (1997). Influence of physical exercise on simple reaction time: Effect of physical fitness. Percept Mot. Skills 85 1019–1027. [DOI] [PubMed] [Google Scholar]
- Browne S. E., Flynn M. J., O’neill B. V., Howatson G., Bell P. G., Haskell-Ramsay C. F. (2017). Effects of acute high-intensity exercise on cognitive performance in trained individuals: a systematic review. Prog. Brain Res. 234 161–187. [DOI] [PubMed] [Google Scholar]
- Cantelon J. A., Giles G. E. (2021). A review of cognitive changes during acute aerobic exercise. Front. Psychol. 12:653158. 10.3389/fpsyg.2021.653158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang H., Kim K., Jung Y. J., Kato M. (2017). Effects of acute high-intensity resistance exercise on cognitive function and oxygenation in prefrontal cortex. J. Exerc. Nutr. Biochem. 21 1–8. 10.20463/jenb.2017.0012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y. K., Labban J. D., Gapin J. I., Etnier J. L. (2012). The effects of acute exercise on cognitive performance: a meta-analysis. Brain Res 1453 87–101. [DOI] [PubMed] [Google Scholar]
- Chen A. G., Zhu L. N., Yan J., Yin H. C. (2016). Neural basis of working memory enhancement after acute aerobic exercise: fMRI study of preadolescent children. Front. Psychol. 7:1804. 10.1016/j.ijpsycho.2020.09.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C., Nakagawa S., Kitaichi Y., An Y., Omiya Y., Song N., et al. (2016). The role of medial prefrontal corticosterone and dopamine in the antidepressant-like effect of exercise. Psychoneuroendocrinology 69 1–9. 10.1016/j.psyneuen.2016.03.008 [DOI] [PubMed] [Google Scholar]
- Chmura J., Nazar K., Kaciuba-Uscilko H. (1994). Choice reaction time during graded exercise in relation to blood lactate and plasma catecholamine thresholds. Int. J. Sports Med. 15 172–176. 10.1055/s-2007-1021042 [DOI] [PubMed] [Google Scholar]
- Christensen L. O. D., Johannsen P., Sinkjaer T., Petersen N., Pyndt H. S., Nielsen J. B. (2000). Cerebral activation during bicycle movements in man. Exp. Brain Res. 135 66–72. [DOI] [PubMed] [Google Scholar]
- Ciria L. F., Luque-Casado A., Sanabria D., Holgado D., Ivanov P. C., Perakakis P. (2019). Oscillatory brain activity during acute exercise: Tonic and transient neural response to an oddball task. Psychophysiology 56:e13326. 10.1111/psyp.13326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coco M., Buscemi A., Guerrera C. S., Di Corrado D., Cavallari P., Zappala A., et al. (2020a). Effects of a bout of intense exercise on some executive functions. Int. J. Environ. Res. Public Health 17:898. 10.3390/ijerph17030898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coco M., Buscemi A., Ramaci T., Tusak M., Corrado D. D., Perciavalle V., et al. (2020b). Influences of blood lactate levels on cognitive domains and physical health during a sports stress. Brief review. Int. J. Environ. Res. Public Health 17:9043. 10.3390/ijerph17239043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coco M., Di Corrado D., Calogero R. A., Perciavalle V., Maci T., Perciavalle V. (2009). Attentional processes and blood lactate levels. Brain Res. 1302 205–211. [DOI] [PubMed] [Google Scholar]
- Cools R., Arnsten A. F. T. (2021). Neuromodulation of prefrontal cortex cognitive function in primates: THE powerful roles of monoamines and acetylcholine. Neuropsychopharmacology 47 309–328. 10.1038/s41386-021-01100-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotman C. W., Berchtold N. C. (2002). Exercise: a behavioral intervention to enhance brain health and plasticity. Trends Neurosci. 25 295–301. 10.1016/s0166-2236(02)02143-4 [DOI] [PubMed] [Google Scholar]
- Curtelin D., Morales-Alamo D., Torres-Peralta R., Rasmussen P., Martin-Rincon M., Perez-Valera M., et al. (2017). Cerebral blood flow, frontal lobe oxygenation and intra-arterial blood pressure during sprint exercise in normoxia and severe acute hypoxia in humans. J. Cereb. Blood Flow Metab. 38 136–150. 10.1177/0271678X17691986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davranche K., Brisswalter J., Radel R. (2015). Where are the limits of the effects of exercise intensity on cognitive control? J. Sport Health Sci. 4 56–63. [Google Scholar]
- Diamond A. (2013). Executive functions. Annu. Rev. Psychol. 64 135–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich A., Audiffren M. (2011). The reticular-activating hypofrontality (RAH) model of acute exercise. Neurosci. Biobehav. Rev. 35 1305–1325. 10.1016/j.neubiorev.2011.02.001 [DOI] [PubMed] [Google Scholar]
- Du Rietz E., Barker A. R., Michelini G., Rommel A. S., Vainieri I., Asherson P., et al. (2019). Beneficial effects of acute high-intensity exercise on electrophysiological indices of attention processes in young adult men. Behav. Brain Res. 359 474–484. 10.1016/j.bbr.2018.11.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutke S., Jaitner T., Berse T., Barenberg J. (2014). Acute physical exercise affected processing efficiency in an auditory attention task more than processing effectiveness. J. Sport Exerc. Psychol. 36 69–79. 10.1123/jsep.2013-0044 [DOI] [PubMed] [Google Scholar]
- Etnier J. L., Wideman L., Labban J. D., Piepmeier A. T., Pendleton D. M., Dvorak K. K., et al. (2016). The effects of acute exercise on memory and brain-derived neurotrophic factor (BDNF). J. Sport Exerc. Psychol. 38 331–340. [DOI] [PubMed] [Google Scholar]
- Fernandez-Rodriguez R., Alvarez-Bueno C., Martinez-Ortega I. A., Martinez-Vizcaino V., Mesas A. E., Notario-Pacheco B. (2021). Immediate effect of high-intensity exercise on brain-derived neurotrophic factor in healthy young adults: a systematic review and meta-analysis. J. Sport Health Sci. 11 367–375. 10.1016/j.jshs.2021.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris L. T., Williams J. S., Shen C. L. (2007). The effect of acute exercise on serum brain-derived neurotrophic factor levels and cognitive function. Med. Sci. Sports Exerc. 39 728–734. [DOI] [PubMed] [Google Scholar]
- Fery Y. A., Ferry A., Vom Hofe A., Rieu M. (1997). Effect of physical exhaustion on cognitive functioning. Percept Mot. Skills 84 291–298. [DOI] [PubMed] [Google Scholar]
- Goekint M., Bos I., Heyman E., Meeusen R., Michotte Y., Sarre S. (2012). Acute running stimulates hippocampal dopaminergic neurotransmission in rats, but has no influence on brain-derived neurotrophic factor. J. Appl. Physiol (1985) 112 535–541. 10.1152/japplphysiol.00306.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold P. E. (1995). Role of glucose in regulating the brain and cognition. Am. J. Clin. Nutr. 61 987S–995S. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Alonso J., Dalsgaard M. K., Osada T., Volianitis S., Dawson E. A., Yoshiga C. C., et al. (2004). Brain and central haemodynamics and oxygenation during maximal exercise in humans. J. Physiol. 557 331–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Fernandez F., Etnier J. L., Zabala M., Sanabria D. (2017). Vigilance performance during acute exercise. Inter. J. Sport Psychol. 48 435–447. [Google Scholar]
- Griffin E. W., Mullally S., Foley C., Warmington S. A., O’mara S. M., Kelly A. M. (2011). Aerobic exercise improves hippocampal function and increases BDNF in the serum of young adult males. Physiol. Behav. 104 934–941. [DOI] [PubMed] [Google Scholar]
- Hansen R. K., Nielsen P. S., Schelske M. W., Secher N. H., Volianitis S. (2020). CO2 supplementation dissociates cerebral oxygenation and middle cerebral artery blood velocity during maximal cycling. Scand. J. Med. Sci. Sports 30 399–407. 10.1111/sms.13582 [DOI] [PubMed] [Google Scholar]
- Hasegawa H., Takatsu S., Ishiwata T., Tanaka H., Sarre S., Meeusen R. (2011). Continuous monitoring of hypothalamic neurotransmitters and thermoregulatory responses in exercising rats. J. Neurosci. Methods 202 119–123. 10.1016/j.jneumeth.2011.05.024 [DOI] [PubMed] [Google Scholar]
- Hashimoto T., Tsukamoto H., Takenaka S., Olesen N. D., Petersen L. G., Sorensen H., et al. (2018). Maintained exercise-enhanced brain executive function related to cerebral lactate metabolism in men. FASEB J. 32 1417–1427. 10.1096/fj.201700381RR [DOI] [PubMed] [Google Scholar]
- Herold F., Behrendt T., Meißner C., Müller N., Schega L. (2022). The influence of acute sprint interval training on cognitive performance of healthy younger adults. Int. J. Environ. Res. Public Health 19:613. 10.3390/ijerph19010613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill M., Walsh S., Talbot C., Price M., Duncan M. (2019). Exercise intensity-dependent effects of arm and leg-cycling on cognitive performance. PLoS One 14:e0224092. 10.1371/journal.pone.0224092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiura M., Nariai T., Ishii K., Sakata M., Oda K., Toyohara J., et al. (2014). Changes in cerebral blood flow during steady-state cycling exercise: a study using oxygen-15-labeled water with PET. J. Cereb. Blood Flow Metab. 34 389–396. 10.1038/jcbfm.2013.220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh S. S., Chueh T. Y., Huang C. J., Kao S. C., Hillman C. H., Chang Y. K., et al. (2021). Systematic review of the acute and chronic effects of high-intensity interval training on executive function across the lifespan. J. Sports Sci. 39 10–22. 10.1080/02640414.2020.1803630 [DOI] [PubMed] [Google Scholar]
- Huertas F., Zahonero J., Sanabria D., Lupianez J. (2011). Functioning of the attentional networks at rest vs. During acute bouts of aerobic exercise. J. Sport Exerc. Psychol. 33 649–665. 10.1123/jsep.33.5.649 [DOI] [PubMed] [Google Scholar]
- Hwang J., Brothers R. M., Castelli D. M., Glowacki E. M., Chen Y. T., Salinas M. M., et al. (2016). Acute high-intensity exercise-induced cognitive enhancement and brain-derived neurotrophic factor in young, healthy adults. Neurosci. Lett. 630 247–253. [DOI] [PubMed] [Google Scholar]
- Ide K., Secher N. H. (2000). Cerebral blood flow and metabolism during exercise. Prog. Neurobiol. 61 397–414. [DOI] [PubMed] [Google Scholar]
- Ide K., Schmalbruch I. K., Quistorff B., Horn A., Secher N. H. (2000). Lactate, glucose and O2 uptake in human brain during recovery from maximal exercise. J. Physiol. 522(Pt 1) 159–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamijo K., Nishihira Y., Hatta A., Kaneda T., Kida T., Higashiura T., et al. (2004a). Changes in arousal level by differential exercise intensity. Clin. Neurophysiol. 115 2693–2698. [DOI] [PubMed] [Google Scholar]
- Kamijo K., Nishihira Y., Hatta A., Kaneda T., Wasaka T., Kida T., et al. (2004b). Differential influences of exercise intensity on information processing in the central nervous system. Eur. J. Appl. Physiol. 92 305–311. 10.1007/s00421-004-1097-2 [DOI] [PubMed] [Google Scholar]
- Kao S. C., Cadenas-Sanchez C., Shigeta T. T., Walk A. M., Chang Y. K., Pontifex M. B., et al. (2020). A systematic review of physical activity and cardiorespiratory fitness on P3b. Psychophysiology 57:e13425. 10.1111/psyp.13425 [DOI] [PubMed] [Google Scholar]
- Kao S. C., Westfall D. R., Soneson J., Gurd B., Hillman C. H. (2017). Comparison of the acute effects of high-intensity interval training and continuous aerobic walking on inhibitory control. Psychophysiology 54 1335–1345. 10.1111/psyp.12889 [DOI] [PubMed] [Google Scholar]
- Kemppainen J., Aalto S., Fujimoto T., Kalliokoski K. K., Langsjo J., Oikonen V., et al. (2005). High intensity exercise decreases global brain glucose uptake in humans. J. Physiol. 568 323–332. 10.1113/jphysiol.2005.091355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komiyama T., Katayama K., Sudo M., Ishida K., Higaki Y., Ando S. (2017). Cognitive function during exercise under severe hypoxia. Sci. Rep. 7:10000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komiyama T., Sudo M., Higaki Y., Kiyonaga A., Tanaka H., Ando S. (2015). Does moderate hypoxia alter working memory and executive function during prolonged exercise? Physiol. Behav. 139 290–296. 10.1016/j.physbeh.2014.11.057 [DOI] [PubMed] [Google Scholar]
- Komiyama T., Sudo M., Okuda N., Yasuno T., Kiyonaga A., Tanaka H., et al. (2016). Cognitive function at rest and during exercise following breakfast omission. Physiol. Behav. 157 178–184. 10.1016/j.physbeh.2016.02.013 [DOI] [PubMed] [Google Scholar]
- Komiyama T., Tanoue Y., Sudo M., Costello J. T., Uehara Y., Higaki Y., et al. (2020). Cognitive impairment during high-intensity exercise: Influence of cerebral blood flow. Med. Sci. Sports Exerc. 52 561–568. 10.1249/MSS.0000000000002183 [DOI] [PubMed] [Google Scholar]
- Labelle V., Bosquet L., Mekary S., Bherer L. (2013). Decline in executive control during acute bouts of exercise as a function of exercise intensity and fitness level. Brain Cogn. 81 10–17. 10.1016/j.bandc.2012.10.001 [DOI] [PubMed] [Google Scholar]
- Lambourne K., Tomporowski P. (2010). The effect of exercise-induced arousal on cognitive task performance: a meta-regression analysis. Brain Res. 1341 12–24. 10.1016/j.brainres.2010.03.091 [DOI] [PubMed] [Google Scholar]
- Lee J. K., Koh A. C., Koh S. X., Liu G. J., Nio A. Q., Fan P. W. (2014). Neck cooling and cognitive performance following exercise-induced hyperthermia. Eur. J. Appl. Physiol. 114 375–384. 10.1007/s00421-013-2774-9 [DOI] [PubMed] [Google Scholar]
- Loprinzi P. D., Day S., Hendry R., Hoffman S., Love A., Marable S., et al. (2021). The effects of acute exercise on short- and long-term memory: considerations for the timing of exercise and phases of memory. Eur. J. Psychol. 17 85–103. 10.5964/ejop.2955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luft C. D., Takase E., Darby D. (2009). Heart rate variability and cognitive function: effects of physical effort. Biol. Psychol. 82 164–168. [DOI] [PubMed] [Google Scholar]
- Macintosh B. J., Crane D. E., Sage M. D., Rajab A. S., Donahue M. J., Mcilroy W. E., et al. (2014). Impact of a single bout of aerobic exercise on regional brain perfusion and activation responses in healthy young adults. PLoS One 9:e85163. 10.1371/journal.pone.0085163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin Bosch B., Bringard A., Logrieco M. G., Lauer E., Imobersteg N., Thomas A., et al. (2021). A single session of moderate intensity exercise influences memory, endocannabinoids and brain derived neurotrophic factor levels in men. Sci. Rep. 11:14371. 10.1038/s41598-021-93813-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMorris T. (2016b). “History of research into the acute exercise–cognition interaction,” in Exercise-Cognition Interaction: Neuroscience Perspective, ed. Mcmorris T. (Amsterdam: Elsevier; ). [Google Scholar]
- McMorris T. (2016a). Developing the catecholamines hypothesis for the acute exercise-cognition interaction in humans: Lessons from animal studies. Physiol. Behav. 165 291–299. 10.1016/j.physbeh.2016.08.011 [DOI] [PubMed] [Google Scholar]
- McMorris T. (2021). The acute exercise-cognition interaction: From the catecholamines hypothesis to an interoception model. Int. J. Psychophysiol. 170 75–88. 10.1016/j.ijpsycho.2021.10.005 [DOI] [PubMed] [Google Scholar]
- McMorris T., Graydon J. (1997). The effect of exercise on cognitive performance in soccer-specific tests. J. Sports Sci. 15 459–468. [DOI] [PubMed] [Google Scholar]
- McMorris T., Hale B. J. (2012). Differential effects of differing intensities of acute exercise on speed and accuracy of cognition: a meta-analytical investigation. Brain Cogn. 80 338–351. 10.1016/j.bandc.2012.09.001 [DOI] [PubMed] [Google Scholar]
- McMorris T., Keen P. (1994). Effect of exercise on simple reaction times of recreational athletes. Percept Mot. Skills 78 123–130. [DOI] [PubMed] [Google Scholar]
- McMorris T., Davranche K., Jones G., Hall B., Corbett J., Minter C. (2009). Acute incremental exercise, performance of a central executive task, and sympathoadrenal system and hypothalamic-pituitary-adrenal axis activity. Int. J. Psychophysiol. 73 334–340. 10.1016/j.ijpsycho.2009.05.004 [DOI] [PubMed] [Google Scholar]
- McMorris T., Delves S., Sproule J., Lauder M., Hale B. (2005). Effect of incremental exercise on initiation and movement times in a choice response, whole body psychomotor task. Br. J. Sports Med. 39 537–541. 10.1136/bjsm.2004.014456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeusen R., Piacentini M. F., De Meirleir K. (2001). Brain microdialysis in exercise research. Sports Med. 31 965–983. [DOI] [PubMed] [Google Scholar]
- Mehren A., Diaz Luque C., Brandes M., Lam A. P., Thiel C. M., Philipsen A., et al. (2019). Intensity-dependent effects of acute exercise on executive function. Neural Plast. 2019:8608317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mekari S., Fraser S., Bosquet L., Bonnery C., Labelle V., Pouliot P., et al. (2015). The relationship between exercise intensity, cerebral oxygenation and cognitive performance in young adults. Eur. J. Appl. Physiol. 115 2189–2197. [DOI] [PubMed] [Google Scholar]
- Miller E. K., Cohen J. D. (2001). An integrative theory of prefrontal cortex function. Annu. Rev. Neurosci. 24 167–202. [DOI] [PubMed] [Google Scholar]
- Millet G. P., Vleck V. E., Bentley D. J. (2009). Physiological differences between cycling and running: lessons from triathletes. Sports Med. 39 179–206. 10.2165/00007256-200939030-00002 [DOI] [PubMed] [Google Scholar]
- Moreau D., Chou E. (2019). The acute effect of high-intensity exercise on executive function: a meta-analysis. Perspect Psychol. Sci. 14 734–764. [DOI] [PubMed] [Google Scholar]
- Nieto-Estevez V., Defterali C., Vicario-Abejon C. (2016). IGF-I: A key growth factor that regulates neurogenesis and synaptogenesis from embryonic to adult stages of the brain. Front. Neurosci. 10:52. 10.3389/fnins.2016.00052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nybo L., Secher N. H. (2004). Cerebral perturbations provoked by prolonged exercise. Prog. Neurobiol. 72 223–261. [DOI] [PubMed] [Google Scholar]
- Ogoh S., Ainslie P. N. (2009). Cerebral blood flow during exercise: Mechanisms of regulation. J. Appl. Physiol. 107 1370–1380. [DOI] [PubMed] [Google Scholar]
- Ogoh S., Tsukamoto H., Hirasawa A., Hasegawa H., Hirose N., Hashimoto T. (2014). The effect of changes in cerebral blood flow on cognitive function during exercise. Physiol. Rep. 2:e12163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quistorff B., Secher N. H., Van Lieshout J. J. (2008). Lactate fuels the human brain during exercise. FASEB J. 22 3443–3449. [DOI] [PubMed] [Google Scholar]
- Rooks C. R., Thom N. J., Mccully K. K., Dishman R. K. (2010). Effects of incremental exercise on cerebral oxygenation measured by near-infrared spectroscopy: a systematic review. Prog. Neurobiol. 92 134–150. 10.1016/j.pneurobio.2010.06.002 [DOI] [PubMed] [Google Scholar]
- Schmit C., Davranche K., Easthope C. S., Colson S. S., Brisswalter J., Radel R. (2015). Pushing to the limits: The dynamics of cognitive control during exhausting exercise. Neuropsychologia 68 71–81. 10.1016/j.neuropsychologia.2015.01.006 [DOI] [PubMed] [Google Scholar]
- Seifert T., Secher N. H. (2011). Sympathetic influence on cerebral blood flow and metabolism during exercise in humans. Prog. Neurobiol. 95 406–426. [DOI] [PubMed] [Google Scholar]
- Shields M. R., Larson C. L., Swartz A. M., Smith J. C. (2011). Visual threat detection during moderate- and high-intensity exercise. Emotion 11 572–581. 10.1037/a0021251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebenmann C., Sorensen H., Bonne T. C., Zaar M., Aachmann-Andersen N. J., Nordsborg N. B., et al. (2021). Cerebral lactate uptake during exercise is driven by the increased arterial lactate concentration. J. Appl. Physiol. 131 1824–1830. 10.1152/japplphysiol.00505.2021 [DOI] [PubMed] [Google Scholar]
- Skriver K., Roig M., Lundbye-Jensen J., Pingel J., Helge J. W., Kiens B., et al. (2014). Acute exercise improves motor memory: exploring potential biomarkers. Neurobiol. Learn Mem. 116 46–58. 10.1016/j.nlm.2014.08.004 [DOI] [PubMed] [Google Scholar]
- Smith K. J., Ainslie P. N. (2017). Regulation of cerebral blood flow and metabolism during exercise. Exp. Physiol. 102 1356–1371. [DOI] [PubMed] [Google Scholar]
- Smith M., Tallis J., Miller A., Clarke N. D., Guimaraes-Ferreira L., Duncan M. J. (2016). The effect of exercise intensity on cognitive performance during short duration treadmill running. J. Hum. Kinet. 51 27–35. 10.1515/hukin-2015-0167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone B. L., Beneda-Bender M., Mccollum D. L., Sun J., Shelley J. H., Ashley J. D., et al. (2020). Understanding cognitive performance during exercise in reserve officers’. training corps: Establishing the executive function-exercise intensity relationship. J. Appl. Physiol. 129 846–854. 10.1152/japplphysiol.00483.2020 [DOI] [PubMed] [Google Scholar]
- Sudo M., Komiyama T., Aoyagi R., Nagamatsu T., Higaki Y., Ando S. (2017). Executive function after exhaustive exercise. Eur. J. Appl. Physiol. 117 2029–2038. [DOI] [PubMed] [Google Scholar]
- Tempest G. D., Reiss A. L. (2019). The utility of functional near-infrared spectroscopy for measuring cortical activity during cycling exercise. Med. Sci. Sports Exerc. 51 979–987. [DOI] [PubMed] [Google Scholar]
- Tempest G. D., Davranche K., Brisswalter J., Perrey S., Radel R. (2017). The differential effects of prolonged exercise upon executive function and cerebral oxygenation. Brain Cogn. 113 133–141. 10.1016/j.bandc.2017.02.001 [DOI] [PubMed] [Google Scholar]
- Thomson K., Watt A. P., Liukkonen J. (2009). Differences in ball sports athletes speed discrimination skills before and after exercise induced fatigue. J. Sports Sci. Med. 8 259–264. [PMC free article] [PubMed] [Google Scholar]
- Travlos A. K., Marisi D. Q. (1995). Information processing and concentration as a function of fitness level and exercise-induced activation to exhaustion. Percept Mot. Skills 80 15–26. 10.2466/pms.1995.80.1.15 [DOI] [PubMed] [Google Scholar]
- Tsukamoto H., Suga T., Takenaka S., Tanaka D., Takeuchi T., Hamaoka T., et al. (2016). Repeated high-intensity interval exercise shortens the positive effect on executive function during post-exercise recovery in healthy young males. Physiol. Behav. 160 26–34. 10.1016/j.physbeh.2016.03.029 [DOI] [PubMed] [Google Scholar]
- Voss M. W., Nagamatsu L. S., Liu-Ambrose T., Kramer A. F. (2011). Exercise, brain, and cognition across the life span. J. Appl. Physiol. 111 1505–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C. C., Chu C. H., Chu I. H., Chan K. H., Chang Y. K. (2013). Executive function during acute exercise: the role of exercise intensity. J. Sport Exerc. Psychol. 35 358–367. [DOI] [PubMed] [Google Scholar]
- Williamson J. W., Nobrega A. C., Mccoll R., Mathews D., Winchester P., Friberg L., et al. (1997). Activation of the insular cortex during dynamic exercise in humans. J. Physiol. 503(Pt 2) 277–283. 10.1111/j.1469-7793.1997.277bh.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter B., Breitenstein C., Mooren F. C., Voelker K., Fobker M., Lechtermann A., et al. (2007). High impact running improves learning. Neurobiol. Learn Mem. 87 597–609. 10.1016/j.nlm.2006.11.003 [DOI] [PubMed] [Google Scholar]
- Won J., Alfini A. J., Weiss L. R., Callow D. D., Smith J. C. (2019). Brain activation during executive control after acute exercise in older adults. Int. J. Psychophysiol. 146 240–248. 10.1016/j.ijpsycho.2019.10.002 [DOI] [PubMed] [Google Scholar]
- Zheng K., Zou L., Wei G., Huang T. (2021). Concurrent performance of executive function during acute bouts of exercise in adults: a systematic review. Brain Sci. 11:1364. 10.3390/brainsci11101364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer P., Binnebossel S., Bloch W., Hubner S. T., Schenk A., Predel H. G., et al. (2017). Exhaustive exercise alters thinking times in a tower of London task in a time-dependent manner. Front. Physiol. 7:694. 10.3389/fphys.2016.00694 [DOI] [PMC free article] [PubMed] [Google Scholar]