Abstract
Background
To explore type 1 diabetes incidence patterns during the pandemic years 2020 and 2021 in Czechia, to compare them to the trends from the previous decade, and to test its association with indicators of containment measures and of pandemic severity (school closing and the all‐cause excess mortality).
Methods
The Czech Childhood Diabetes Register is a population‐based incidence register recording patients age 0–14.99 years at diabetes onset. Type 1 diabetes incidence in the pandemic period (April 2020–end of observation Dec 2021) was compared by Poisson regression models to the incidence patterns over the past decade 2010–2019.
Results
During the pandemic years 2020–2021, 956 children 0–14.99 years old manifested with type 1 diabetes in Czechia. The observed incidence (27.2/100,000/year) was significantly higher than what was expected from the trends over 2010–2019 (incidence rate ratio, IRR = 1.16, 95%CI 1.06–1.28, p = 0.0022). The incidence had a trough during the first lockdown (March–May 2020), then it rose above expected values with no usual summer decrease. The assessed pandemic indicators (school closing and all‐cause excess mortality) were not associated with the incidence levels.
Conclusions
The COVID‐19 pandemic was associated with a notable upward inflection of the type 1 diabetes incidence curve; the early months of the first lockdown were however hallmarked by a significant dip in new diabetes diagnoses. Long‐term observation will show whether the increased incidence originated only from accelerating an advanced preclinical Stage 2 to overt diabetes, or whether the pandemic triggered new cases of islet autoimmunity.
Keywords: COVID‐19, incidence increase, pandemic, type 1 diabetes
1. INTRODUCTION
Type 1 diabetes (T1D) is an autoimmune disease having several stages. 1 An exposure to infections, in particular to viruses, is known to trigger islet autoimmunity, 2 , 3 as well as to accelerate the transition from the pre‐clinical Stage 2 to overt diabetes (e.g., Reference 4).
The global pandemic of coronavirus disease (COVID‐19) caused by severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2) has severely impacted countries of Central and Eastern Europe. In Czechia the total excess deaths 2020–2021 was twice higher than the global mean (244.8 vs. 120.3/100,000). 5 The pandemic, and containment measures thereof, are capable of affecting the dynamics of pediatric T1D incidence in both directions. Diabetes onset may have been deferred by school closing and restriction of contacts that led to a generally decreased transmission of infectious agents among children population (e.g., Reference 6), and thus may have alleviated the burden on the residual beta cell mass. An increase in incidence may be expected after re‐opening of schools due to a catch‐up in the infection exposure. Moreover, the SARS‐CoV‐2 virus is itself capable of infecting pancreatic endocrine tissue, 7 being a plausible candidate for a trigger or accelerator of islet autoimmunity.
Little is known on the T1D incidence trends during the COVID‐19 pandemic. Several studies suggested that more cases than expected manifested during the first pandemic year, but these mostly lacked exactly defined catchment area and denominator data. Only few formally explored trends originating from long‐term run population based registers. 8 , 9 Recently, studies have been published that utilized big collections of electronic health records or medical claims, reporting conflicting results. 10 , 11 , 12
We therefore explored the data of Czech Childhood Diabetes Register, examining data ascertained over 2020–2021 when Czechia was very severely hit by several consecutive waves of COVID‐19, and compared them to trends from the previous decade, also searching for patterns explainable by the dynamics of the pandemic, or by pandemic contingency measures.
2. METHODS
The Czech Childhood Diabetes Register is a large population‐based register operating since 1989. Its methods of ascertainment have been reported elsewhere, 13 , 14 and are in accord with protocols of the EURODIAB initiative to whom the register regularly contributes. 15 Briefly, it collects incident cases with pediatric diabetes (aged 0–14.99 years), and verifies the completeness using capture‐recapture method. The estimated combined completeness over the pre‐pandemic decade 2010–2019 was 94.4% [95% CI 93.7%–95.1%]. Anonymous patient records include the date of birth, gender, month of onset, reporting center and physician, type of diabetes (type 1 diabetes, type 2 diabetes, other specified type), and status of capturing by primary register and/or the secondary source. Background population sizes on 31st December are taken from the official reports of the Czech Statistics Bureau. The protocols of the register were approved by the Ethical Committee of the Motol University Hospital and data processing registered by the Bureau for Personal Data Protection.
At the time of writing, the secondary source has not been collated for 2021, and likewise the general population data report of 2021 has not been published by the Czech Statistical Bureau. The population size was thus taken from the last year's report, shifted by 1 year to respect the proper age banding, and the youngest year extrapolated from the series of last 5 years.
To inspect the relations between T1D incidence and putative predictors among the containment measures among children population or COVID‐19 pandemic severity, we utilized (a) the School Closing indicator from the open‐access global database of pandemic policies, the Oxford COVID‐19 Government Response Tracker (OxCGRT), 16 (b) the excess all‐cause mortality from data of the World Mortality Dataset. 17
Poisson regression modeling was used for statistical analysis; terms included year as a continuous variable, binary term indicating pandemic period (from April 2020 until the end of observation in December 2021), gender, age bands at diabetes onset (0–4, 5–9, 10–14 completed years), and the two‐way interaction of the latter two variables (Model 1). Indicators of pandemic containment (School Closing index, as factor) and of the overall pandemic severity (excess mortality, continuous term) were tested in separate models (Model 2 and Model 3, respectively). In order to make predictions, we added a factor for months into Model 1 (obtaining Model 4). For testing the changes in monthly incidence distribution in the pandemic, we then changed the pandemic term to a factor with three levels (“before pandemic,” “year 2020,” and “year 2021”) along with two‐way interaction with the months (Model 5).
3. RESULTS
During the pandemic years 2020–2021, 956 patients aged 0–14 years manifested with T1D, resulting in an incidence of 27.2/100,000/year in 2020 and 28.4 in 2021. The incidence was higher in the pandemic period than what would correspond to a log‐linear trend from the preceding decade (2010–2019 and first 3 months of 2020, Model 1), with the incidence rate ratio (IRR) being 1.16, 95%CI 1.06–1.28, p = 0.0022. In the decade preceding the pandemic, the incidence did not rise, but rather fluctuated with a 4‐years periodicity similar to what has been previously described in other European registers 15 —the 2019 would have been at a peak. The long‐term incidence trends are shown in Figure 1.
FIGURE 1.
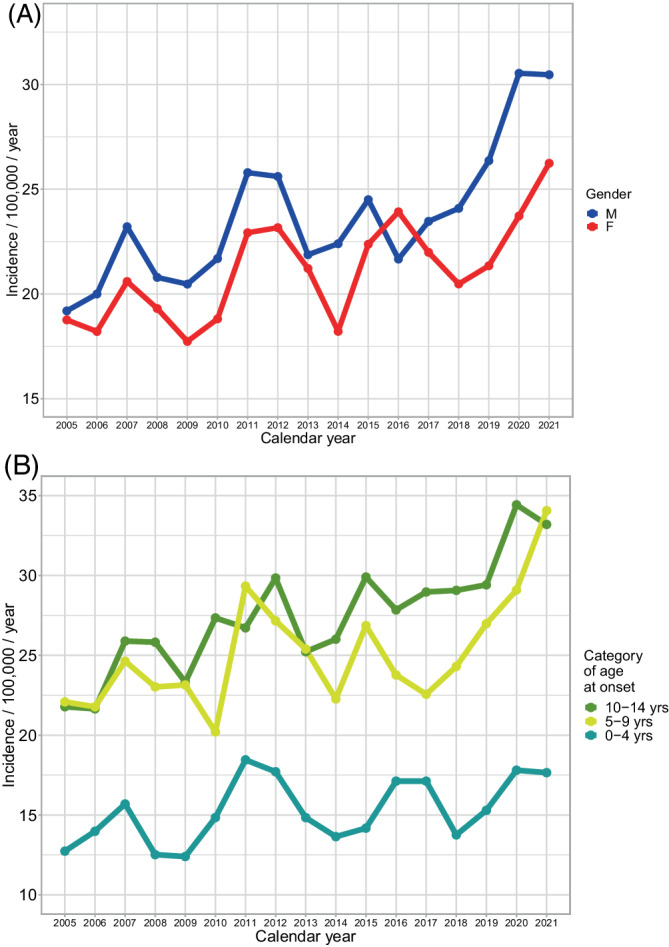
Incidence of type 1 diabetes in Czechia before and during the COVID‐19 pandemic 2020–2021. (A) By gender and (B) By age category
The monthly T1D incidence for 2020–2021 is plotted in Figure 2A, compared to a reference level derived from the pre‐pandemic 2010–2019 decade (testing month‐pandemic year two‐way interaction in Model 5, p = 0.0285). The incidence values are shown along with the School Closing index (which indicates restrictions to school attendance) and the excess mortality data objectifying the pandemic severity (Figure 2B). Here, a clear trough in incidence (March–May 2020) coincides with the introduction of a very strict lockdown, including schools closure, during the first COVID‐19 wave in Czechia (March and May being significantly lower than the baseline, P difference 0.076, 0.135, and 0.038 respectively for the 3 months). This was immediately followed by a rise in incidence, effectively leveling the decrease in incidence otherwise observable over the summer months.
FIGURE 2.
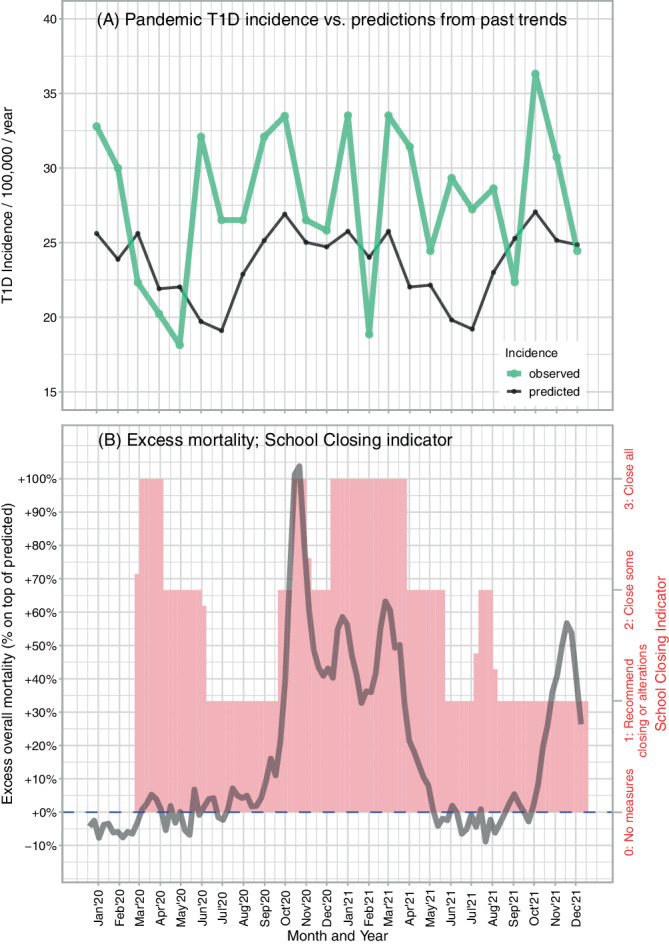
The pandemic waves and type 1 diabetes incidence. (A) Observed type 1 diabetes incidence by month in the pandemic years 2020–2021 (green) versus prediction based on the pre‐pandemic years 2010–2019 (black, from a model that contained year as a continuous variable, gender, age bands at diabetes onset as 0–4, 5–9, 10–14 completed years, two‐way interaction of the latter two variables, and also a factor for calendar month) and (B) Indicators of pandemic severity (excess overall mortality, dark gray line) and pandemic containment (School Closing index, pink bars)
Shortly after the return of children to schools before summer vacation, T1D incidence increased again, and thereafter the incidence kept increased and fluctuated without the usual summer decrease. Modeling of incidence by the School Closing (Model 2, p = 0.2699) indicator and excess mortality as proxy for pandemic severity (Model 3, p = 0.9854) showed no proof of their association with T1D incidence.
Diabetes types other than T1D were and remained rare in the Czech register: in the 2010–2019 decade, 0.87% of the incident cases were of type 2 diabetes, 2.7% had genetically confirmed monogenic diabetes, and 2.1% other types of diabetes. No significant changes were noted between pre‐pandemic years 2018–2019 and the pandemic period 2021–2022: 1.1% versus 1.9% for type 2 diabetes (p = 0.19); 2.4% versus 2.1% for genetically confirmed monogenic diabetes (p = 0.64); and 0.37% versus 0.40% of other diabetes (p = 1.0).
4. DISCUSSION
The 2020–2021 pandemic of COVID‐19 was associated with an unexpected significant upward inflection in the overall pediatric T1D incidence trend, following to a notable decrease in incidence lasting several months during the first lockdown in spring 2020.
The dynamics of incidence started with a sudden dip in incidence during the first lockdown in spring 2020 (Figure 2). Similar temporary decrease in incidence was noted also in Lombardy, 9 and may be indicative of decreased physical activity in children, lower exposure to infections, or delayed diagnoses of already existing cases. In contrast to Lombardy, the first wave in Czechia was very mild as documented by the lack of appreciable excess mortality (Figure 2B). Interestingly, no such dip in incidence was detected in data from neighboring Germany 8 where this wave of infections was likewise mild. The cause for this difference remains speculative, as there was a very complex interplay between the virus, containment measures, and adherence to these measures.
Thereafter, the incidence kept steadily higher than what could be expected from 2010 to 2019 trends. This elevation was almost continuous for 19 months of observation (Figure 2A). Long‐term observation will show whether the excess cases are only accelerations from Stage 2 (islet autoimmunity with dysglycaemia) to Stage 3 (overt type 1 diabetes), or whether new cases of islet autoimmunity may have been triggered ‐ either by the long and massive exposure to SARS‐CoV‐2 Czechia experienced, or by other viruses sweeping through the children population upon their return to schools. An association of common respiratory (presumably viral) infections with triggering islet autoimmunity has been already convincingly documented. 2
The causative roles of COVID‐19 and of the pandemic containment measures are impossible to dissect and our formal testing of the two most plausible indicators did not yield explanations for the T1D incidence fluctuations during the pandemic. Kamrath et al. 8 cautiously suggests that the incidence of T1D followed the peak incidence of COVID‐19 by approximately 3 months, we however do not think this could be interpreted as causation; moreover, neither Kamrath et al 8 nor we have individual COVID‐19 data. Studies using electronic medical records and medical claims might bring more insight ‐ of the published works, some 10 , 12 but not all 11 observed a link between COVID‐19 and an increase in pediatric diabetes incidence.
It has been speculated that the worsened clinical presentation at diagnosis might create false impression of an increasing T1D incidence. 18 Our population‐based data along with data from neighboring Germany 8 , 19 indicate that a true rise in T1D incidence may occur in parallel with worsening of the clinical state at presentation (manuscript of an international study on diabetic ketoacidosis is under review). It is presently unclear whether certain age categories are preferentially hit by the increase—in aforementioned German data the youngest children seem to show especially pronounced increase, whereas in Czechia older children had more pronounced rise.
The strengths of this study is the analysis of two complete pandemic years in a population with a very high burden of COVID‐19, and a very long period of schools closure. The Czech register provides population‐based data and long series were available to assess baseline trend in T1D incidence. Importance of assessment of long‐term regular cyclical trends lies in that they might otherwise be mistaken for COVID‐19 related effect if only last pre‐pandemic years had been used as reference.
Weaknesses include: (a) A minor correction in 2021 incidence rates (max. ~ 1/100 of estimates) may be needed when 2021 general population data become available from the statistical bureau. Such a correction will not affect the patterns of distribution of cases among the months. (b) The design, similarly to so far published register studies, did not involve systematic investigating of serostatus to SARS‐CoV‐2. (c) We could not calculate the completeness of the ascertainment for 2021 as secondary register data had not been collated ‐ completeness however remained stable at ~93%–94% throughout 2017–2020.
In conclusion, we document a clear rise in T1D incidence during the pandemic years 2020–2021 in a large population‐based pediatric diabetes register. An initial temporary decrease in T1D incidence during the first lockdown was later followed by elevated rates with fluctuations not conforming to usual seasonal patterns. Close observation of trends in the following years may help disentangle whether this increase was at least partly caused by triggering new cases of islet autoimmunity.
AUTHOR CONTRIBUTIONS
Ondřej Cinek and Zdeněk Šumník contrived the study, researched data, and led the author team; Matvei Slavenko statistically analyzed data, and contributed to writing. Renata Pomahačová, Petra Venháčová, Lenka Petruželková, Jaroslav Škvor, David Neumann, Jan Vosáhlo, Petra Konečná, Kamila Kocourková, Jiří Strnadel, and Štěpánka Průhová collected and verified the data, interpreted the findings and participed in writing and revisions of the manuscript.
CONFLICT OF INTEREST
The author declares that there is no conflict of interest.
ACKNOWLEDGMENTS
This study was supported by grants from the Czech Diabetes Society ČLS JEP and the Czech Ministry of Health (conceptual support project to research organization 00064203—FN Motol). We thank Michal Kulich from the Faculty of Mathematics and Physics, Charles University, Prague, for helpful advice. O.C. was financed from the National Institute of Virology and Bacteriology (Programme EXCELES, LX22NPO5103, the European Union Recovery Instrument). The authors thank the contributors of the ČENDA Register outside the writing authors group (in alphabetic order of cities)—Drs: J. Vyžrálková, M. Pejchlová (Brno), M. Schubert (Bruntál), L. Kocinová (Česká Lípa), I. Röchlová (Frýdek‐Místek), A. Kudličková (Hodonín), A. Benešová (Chomutov), J. Češek (Chrudim), M. Adam (Jablonec), P. Kracíková (Jičín), P. Vlachý, M. Svojsík (Jihlava), M. Jiřičková (Jilemnice), K. Poločková (Karviná), J. Kytnarová (Prague‐VFN), K. Dimová (Kladno), S. Fialová (Kroměříž), M. Vracovská (Klatovy), J. Sivíčková, P. Pelcová (Karlovy Vary), E. Hlaďáková (Kyjov), J. Bartošová (Liberec), M. Kulinová (Mladá Boleslav), N. Filáková (Ostrava—City Hospital), M. Romanová (Prague‐FNKV), M. Šnajderová, S. Koloušková (Prague—Motol), L. Týce (Náchod), E. Farkaš (Nový Jičín), Z. Ježová (Nové Město na Moravě), M. Honková (Opava), B. Červíčková (Pardubice, Trutnov), T. Farová (Písek), K. Fiklík (Plzeň), J. Malý (Sokolov), M. Gregora (Strakonice), J. Malý (Svitavy), A. Lysáková (Šumperk), J. Chocholová (Tábor), P. Eichl (Teplice), O. MIchálková (Třebíč), M. Struminský (Třinec), M. Procházka (Ústí nad Orlicí), H. Vávrová (Vsetín), P. Gogelová (Zlín), P. Mikyška (Znojmo).
Cinek O, Slavenko M, Pomahačová R, et al. Type 1 diabetes incidence increased during the COVID‐19 pandemic years 2020–2021 in Czechia: Results from a large population‐based pediatric register. Pediatr Diabetes. 2022;1‐5. doi: 10.1111/pedi.13405
The members of the ČENDA Register are listed in Acknowledgements section.
Funding information Czech Diabetes Society; Czech Ministry of Health; National Institute of Virology and Bacteriology
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Becker D, Insel R. Screening, staging, and naming of presymptomatic type 1 diabetes. Pediatr Diabetes. 2018;19:7‐10. [DOI] [PubMed] [Google Scholar]
- 2. Lonnrot M, Lynch KF, Elding Larsson H, et al. Respiratory infections are temporally associated with initiation of type 1 diabetes autoimmunity: the TEDDY study. Diabetologia. 2017;60:1931‐1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yeung WC, Rawlinson WD, Craig ME. Enterovirus infection and type 1 diabetes mellitus: systematic review and meta‐analysis of observational molecular studies. BMJ. 2011;342:d35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nurminen N, Oikarinen S, Hyoty H. Virus infections as potential targets of preventive treatments for type 1 diabetes. Rev Diabet Stud. 2012;9:260‐271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Collaborators C‐EM. Estimating excess mortality due to the COVID‐19 pandemic: a systematic analysis of COVID‐19‐related mortality, 2020‐21. Lancet. 2022;399(10334):1513‐1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brueggemann AB, Jansen van Rensburg MJ, Shaw D, et al. Changes in the incidence of invasive disease due to Streptococcus pneumoniae, Haemophilus influenzae, and Neisseria meningitidis during the COVID‐19 pandemic in 26 countries and territories in the invasive respiratory infection surveillance initiative: a prospective analysis of surveillance data. Lancet digit. Health. 2021;3:e360‐e370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yang L, Han Y, Nilsson‐Payant BE, et al. A human pluripotent stem cell‐based platform to study SARS‐CoV‐2 tropism and model virus infection in human cells and organoids. Cell Stem Cell. 2020;27(125–136):e127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kamrath C, Rosenbauer J, Eckert AJ, et al. Incidence of type 1 diabetes in children and adolescents during the COVID‐19 pandemic in Germany: results from the DPV registry. Diabetes Care. 2022;45:1762‐1771. [DOI] [PubMed] [Google Scholar]
- 9. Mameli C, Scaramuzza A, Macedoni M, et al. Type 1 diabetes onset in Lombardy region, Italy, during the COVID‐19 pandemic: the double‐wave occurrence. EClinicalMedicine. 2021;39:101067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Qeadan F, Tingey B, Egbert J, et al. The associations between COVID‐19 diagnosis, type 1 diabetes, and the risk of diabetic ketoacidosis: a nationwide cohort from the US using the Cerner real‐world data. PLoS One. 2022;17:e0266809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pietropaolo M, Hotez P, Giannoukakis N. Incidence of an insulin‐requiring hyperglycemic syndrome in SARS CoV‐2‐infected young individuals: is it type 1 diabetes? Diabetes. 2022. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Barrett CE, Koyama AK, Alvarez P, et al. Risk for newly diagnosed diabetes >30 days after SARS‐CoV‐2 infection among persons aged <18 years ‐ United States, march 1, 2020‐June 28, 2021. MMWR Morb Mortal Wkly Rep. 2022;71:59‐65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cinek O, Kulich M, Sumnik Z. The incidence of type 1 diabetes in young Czech children stopped rising. Pediatr Diabetes. 2012;13:559‐563. [DOI] [PubMed] [Google Scholar]
- 14. Cinek O, Lanska V, Kolouskova S, et al. Type 1 diabetes mellitus in Czech children diagnosed in 1990‐1997: a significant increase in incidence and male predominance in the age group 0‐4 years. Diabet Med. 2000;17:64‐69. [DOI] [PubMed] [Google Scholar]
- 15. Patterson CC, Harjutsalo V, Rosenbauer J, et al. Trends and cyclical variation in the incidence of childhood type 1 diabetes in 26 European centres in the 25 year period 1989‐2013: a multicentre prospective registration study. Diabetologia. 2019;62:408‐417. [DOI] [PubMed] [Google Scholar]
- 16. Hale T, Angrist N, Goldszmidt R, et al. A global panel database of pandemic policies (Oxford COVID‐19 government response tracker). Nat Hum Behav. 2021;5:529‐538. [DOI] [PubMed] [Google Scholar]
- 17. Karlinsky A, Kobak D. Tracking excess mortality across countries during the COVID‐19 pandemic with the world mortality dataset. Elife. 2021;10:e69336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Salmi H, Heinonen S, Hastbacka J, et al. New‐onset type 1 diabetes in Finnish children during the COVID‐19 pandemic. Arch Dis Child. 2022;107:180‐185. [DOI] [PubMed] [Google Scholar]
- 19. Kamrath C, Monkemoller K, Biester T, et al. Ketoacidosis in children and adolescents with newly diagnosed type 1 diabetes during the COVID‐19 pandemic in Germany. Jama. 2020;324:801‐804. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


