Abstract
Yersinia pestis expresses a set of plasmid-encoded virulence proteins called Yops and LcrV that are secreted and translocated into eukaryotic cells by a type III secretion system. LcrV is a multifunctional protein with antihost and positive regulatory effects on Yops secretion that forms a stable complex with a negative regulatory protein, LcrG. LcrG has been proposed to block the secretion apparatus (Ysc) from the cytoplasmic face of the inner membrane under nonpermissive conditions for Yops secretion, when levels of LcrV in the cell are low. A model has been proposed to describe secretion control based on the relative levels of LcrG and LcrV in the bacterial cytoplasm. This model proposes that under secretion-permissive conditions, levels of LcrV are increased relative to levels of LcrG, so that the excess LcrV titrates LcrG away from the Ysc, allowing secretion of Yops to occur. To further test this model, a mutant LcrG protein that could no longer interact with LcrV was created. Expression of this LcrG variant blocked secretion of Yops and LcrV under secretion permissive conditions in vitro and in a tissue culture model. These results agree with the previously described secretion-blocking activity of LcrG and demonstrate that the interaction of LcrV with LcrG is necessary for controlling Yops secretion.
Yersinia pestis, the causative agent of plague, has a ∼70-kb virulence plasmid that encodes the low-calcium response (LCR) stimulon (35). LCR components include a set of secreted antihost proteins (termed Yops), the V antigen (LcrV), and a specialized type III secretion apparatus (Ysc) for the directed delivery of those proteins to their sites of action (6). Yops expression and secretion are induced by contact between the bacterium and the host cell in tissue culture, which may reflect the in vivo induction that must occur during an infection (26). Induction of Yops expression and secretion is seen in vitro in response to calcium ion concentration after shifting yersiniae to growth at mammalian body temperature (35), i.e., 37°C. In the presence of millimolar concentrations of calcium, expression of Yops and LcrV is downregulated and secretion is blocked. In the absence of calcium, a cessation of growth (termed growth restriction) occurs that is accompanied by maximal Yops expression and secretion (23). In essence, induction of the LCR stimulon is dependent on the activity of the Ysc secretion apparatus; the ability to secrete Yops is required to synthesize Yops. Therefore, an understanding of how the Ysc apparatus is controlled is essential to understanding how yersinae cause disease.
The Ysc secretion apparatus is blocked in the presence of calcium or in the absence of eukaryotic cell contact by several negative regulatory proteins. YopN (also called LcrE) is believed to act at the bacterial surface, along with TyeA, and may act as a calcium sensor (9, 13). LcrG is a negative regulator that has been proposed to block secretion of Yops from the cytoplasmic face of the inner membrane (22). YopN, TyeA, and LcrG are all required for secretion blockage at 37°C under noninducing conditions. Null mutations in each of these genes (lcrG, tyeA, and yopN) result in Yops secretion and growth restriction in the presence and absence of calcium (i.e., “calcium-blind” growth phenotype) (9, 13, 33). LcrQ is an additional secreted negative regulator in this system that is believed to function at the level of transcriptional control. Secretion of LcrQ from the bacterial cell allows induction of the LCR, while retention of LcrQ causes repression of the LCR (26, 28).
LcrV is a secreted antihost protein with strong immunomodulatory effects and is associated with full virulence of Y. pestis (18, 19, 27). Translocation of Yops into eukaryotic cells requires LcrV (7, 21, 25), and LcrV is thought to be exposed at the bacterial surface prior to contact with host cells (7, 25). Recently, LcrV has been shown to form pores in eukaryotic membranes and likely forms the translocation pore in conjunction with YopB and YopD (12). LcrV is also a positive regulatory protein that functions in the LCR to counteract negative regulatory mechanisms (2, 27, 32). An lcrV null mutation results in the loss of Yops secretion under inducing conditions (cell contact or removal of calcium from the growth medium) and does not show the characteristic growth restriction phenotype (32). However, lcrV strains can demonstrate some Yops secretion when, for example, YopM is overexpressed (32). This result is interpreted as evidence that LcrV is involved in counteracting negative regulation of the LCR rather than directly participating in the secretion process.
Recently, LcrV has been shown to form a stable complex with a negative regulator, LcrG (22). The identification of this interaction combined with the phenotypes of single (lcrG or lcrV) and double (lcrG/lcrV) mutation Y. pestis strains led to a speculative model of how the activity of the Ysc could be controlled. The LcrG titration model has the following elements: under noninducing conditions, the Ysc is blocked by LcrG and the other negative regulators (YopN and TyeA). LcrV levels in the bacterial cytoplasm would be relatively low under these conditions. In the presence of secretion-inducing conditions, LcrQ is exported, causing levels of LcrV to increase relative to the levels of LcrG. The excess LcrV titrates LcrG and relieves its secretion-blocking activity, possibly by removing LcrG from the secretion complex, which would allow full induction of the LCR and subsequent secretion of Yops. One aspect of this model is supported by work by Nilles et al. that shows that LcrG blocks secretion when overexpressed in an lcrG strain producing low levels of LcrV (21). Those results support the contention that the ratio of LcrG to LcrV is important in controlling the activity of the Ysc complex. However, to date there is no experimental evidence supporting the role of the LcrG-LcrV interaction in the unblocking of Yops secretion. Specific disruption of the interaction of LcrV and LcrG would allow a direct test of the hypothesis that the interaction of LcrG and LcrV is important in controlling the activity of the Ysc.
This study extends previous work on the LcrG titration model by examining the effect of specifically disrupting the interaction between LcrG and LcrV. The LcrG-LcrV interaction was found to be a critical factor in the control of Yops secretion by the Ysc. A mutant LcrG that no longer interacted with LcrV was constructed. The LcrV-noninteracting LcrG mutant blocked secretion of Yops and LcrV under secretion-inducing conditions, both in vitro and in a tissue culture model. These results suggested that LcrG has a primary role of blocking Yops secretion when the LCR is not induced. They further demonstrated that the interaction of LcrV with LcrG is required to unblock Yops secretion.
MATERIALS AND METHODS
Bacterial strains, eukaryotic cell lines, and growth conditions.
Y. pestis and Escherichia coli strains used are listed in Table 1. Cells of Y. pestis strains were grown in heart infusion broth or on tryptose blood agar (TBA) base medium (Difco Laboratories, Detroit, Mich.) at 26°C for genetic manipulations. For physiological studies, growth of Y. pestis cells was conducted in a defined medium, TMH, as previously described (34). Y. pestis cultures were grown in exponential phase at 26°C for about eight generations, with shaking at 200 rpm. Cultures were diluted into fresh TMH to an optical density at 620 nm (OD620) of 0.1, initially incubated at 26°C, and shifted to 37°C when cultures had reached an OD620 of ∼0.2. Incubation was continued for 4 or 6 h before the harvesting of cells. Cells of E. coli strains were grown in Luria-Bertani medium or agar (17) at 37°C. When appropriate, bacteria were grown in the presence of antibiotics, which were used at 50 μg/ml for both carbenicillin and kanamycin. The epithelium-derived HeLa cell line (ATCC CC L-2; American Type Culture Collection, Manassas, Va.) was maintained in RPMI (Mediatech, Inc., Herndon, Va.) supplemented with 10% (vol/vol) heat-inactivated fetal bovine serum (FBS; Bio Whittaker, Walkersville, Md.) at 37°C under a 5% CO2 atmosphere. For partitioning experiments that measured distribution of Yops in the culture medium and into HeLa cells, infections were conducted in Leibovitz's L15 medium (Mediatech, Inc.) lacking FBS.
TABLE 1.
Strains and plasmids
| Strain or plasmid | Relevant properties | Source or reference |
|---|---|---|
| Y. pestis | ||
| KIM8-3002 | pCD1 (Lcr+) pMT1 Pla− Smr | 21 |
| KIM8-3002.6 | KIM8-3002 ΔlcrG2 [LcrGΔ5-95]a | 21 |
| KIM8-3002.7 | KIM8-3002 ΔlcrG3 [LcrGΔ6-86]a | 7 |
| KIM8-3002.8 | KIM8-3002 ΔlcrGV2 [LcrGΔ6-95]a [LcrVΔ1-268]a | 7 |
| E. coli | ||
| DH5α | φ80dlacZΔM15 Δ(lacZYA-argF) U169 deoR recA1 endA1 hsdR17 (rK− mK+) phoA supE44 thi-1 gyrA96 relA1 | Life Technologies |
| DH5α/λpir | DH5α λpir | S. Straley |
| Novablue | recA1 endA1 hsdR17 (rK− mK+) supE44 thi-1 gyrA96 relA1 lac [F′ proA+B+lacIqZΔM15::Tn10] | Novagen |
| S. cerevisiae Y190 | MATa ura3-52 his3-200 lys2-801 ade2-101 trp1-901 leu2-3 112 gal4Δ gal80Δ cyhr2 LYS2::GAL1UAS-HIS3TATA-HIS3 URA3::GAL1UAS-GAL1TATA-lacZ | Clontech |
| Plasmids | ||
| pBAD18-Kan | araBADp cloning vector, Kmr | 10 |
| pAraG18K | pBAD18-Kan + lcrG | 22 |
| pAraV18K | pBAD18-Kan + lcrV | 22 |
| pAraGV18K | PBAD18-Kan + lcrGV | 22 |
| pJM89 | pBAD18-Kan + lcrG (A16R) | This study |
| pJM90 | pBAD18-Kan + lcrG (A16D) | This study |
| pACT2 | GAL4(768-881) AD LEU2 Apr | Clontech |
| pACTG | pACT2 + lcrG | This study |
| pJM91 | pACT2 + lcrG (A16R) | This study |
| pJM92 | pACT2 + lcrG (A16D) | This study |
| pAS2-1 | GAL4(1-147) DNA-BD TRP1 AprCYHS2 | Clontech |
| pJM17 | pAS2-1 Δcyh2 | This study |
| pASV | pAS2-1 + lcrV | This study |
| pJM15 | pASV Δcyh2 | This study |
| pLD55 | Apr Tcr suicide vector | 16 |
| pMNΔlcrGV2 | pLD55 + ΔlcrGV2 allele | This study |
| pAra-HT-V | pBAD24 + His6-LcrV, Apr | This study |
Amino acids deleted are indicated.
DNA methods and plasmid construction.
Plasmid DNA was isolated using a QiaPrep Spin kit (Qiagen, Inc., Studio City, Calif.). Cloning methods were essentially as described previously (29). PCR fragments were purified using the QiaQuick PCR purification kit (Qiagen). Transformation of DNA into E. coli was accomplished by using the calcium-manganese-based transformation protocol (11) or commercially obtained competent cells (Novagen, Madison, Wis.). Electroporation of DNA into Y. pestis cells was done as described previously (24). Gene amplification was performed with Deep Vent (New England Biolabs, Beverly, Mass.) or Taq (Eppendorf Scientific, Westbury, N.Y.) DNA polymerase in a Perkin-Elmer GeneAmp Model 2400 thermocycler (Applied Biosystems, Foster City, Calif.). Site-directed mutagenesis was performed with Pfu Turbo DNA polymerase (Stratagene, La Jolla, Calif.) using the QuikChange Site-directed Mutagenesis Kit (Stratagene) according to the manufacturer's instructions. Oligonucleotide primers were synthesized by Sigma/Genosys (The Woodlands, Tex.) or MWG Biotech (High Point, N.C.).
Plasmids used in this study are described in Table 1. Plasmid pACTG was constructed by cloning a BamHI- and NcoI-cleaved PCR product into pACT2. The primers used to amplify lcrG were GAL4-LcrG (5′ CGC ATG CCA TGG TGA AGT CTT CCC ATT TTG ATG AA 3′) and AraG-Stop (5′ CGC GGA TCC TTA AAT AAT TTG CCC TCG 3′). Plasmids pJM91 and pJM92 were constructed by performing site-directed mutagenesis on pACTG. Complementary oligonucleotides were designed to contain the desired mutation, flanked by unmodified sequence to anneal to the same sequence on opposite strands of the template plasmid. Primers used were A16R (5′ GCT TAA ACA GCG AGA ACTGGC AAT AG 3′) and A16R-Back (5′ CTA TTG CCA GTT CTC GCT GTT TAA GC 3′) to make pJM91 and A16D (5′ CGC TTA AAC AGG ACG AAC TGG CAA TAG 3′) and A16D-Back (5′ CTA TTG CCA GTT CGT CCT GTT TAA GCG 3′) to make pJM92. Plasmids pJM89 and pJM90 were constructed by amplifying the mutated lcrG sequences from pJM91 and pJM92, respectively, by PCR using primers AraG-Start (5′ GGA ATT CAG GAG GAA ACG ATG AAG TCT TCC CAT TTT GAT 3′) and AraG-Stop (see above). The amplified sequences were digested with EcoRI and ligated into EcoRI- and SmaI-cleaved pBAD18-Kan. Mutations were confirmed by double-stranded sequencing (30).
Plasmid pASV was constructed by cloning a BamHI- and NcoI-cleaved PCR product into pAS2-1. The primers used to amplify lcrV were GAL4-LcrV (5′ CGC ATG CCA TGG TGA TTA GAG CCT ACG AAC AAA AC 3′) and AraV-Stop (5′ CGC GGA TCC TTA TCA TTT ACC AGA CGT GTC 3′). Plasmids pJM17 and pJM15 were constructed by deleting cyh2 from pAS2-1 and pASV, respectively. pAS2-1 and pASV were each cleaved with BglII to disrupt cyh2 and then cleaved with NruI and BsaBI to release the entire cyh2 gene from the plasmids. The resulting DNA was blunt-end ligated and transformed into E. coli, and the deletion was verified by restriction digestion.
Plasmid pMNΔlcrGV2 was constructed to delete lcrGV in Y. pestis. The ΔlcrGV2 allele results in the mutation of codon 6 in lcrG from a Phe codon to a stop codon (TAA) and the replacement of codons 7 to 95 of lcrG and codons 1 to 268 of lcrV with a KpnI site. pMNΔlcrGV2 was made by cloning two PCR fragments containing DNA upstream and downstream of the desired deletion into SmaI- and BamHI-linearized pLD55 (6). The upstream DNA was obtained using PCR with Deep Vent DNA polymerase (New England Biolabs) and primers ΔlcrG-US (5′CGC GGA TCC GCT ATC TGC TCG AAC AGA 3′) and lcrG1-5-Kpn (5′CGG GGT ACC TTA ATG GGA AGA CTT CAT AAT CTA 3′). The downstream DNA was obtained using PCR with Deep Vent DNA polymerase and primers ΔlcrGV2-Kpn (5′ CGG GGT ACC CAC TTT GCC ACC ACC TGC TCG 3′) and ΔlcrGV-DS (5′ GGA ATT CCA CTG AGG CTA TGG CGC TGA GCC A 3′). The upstream fragment was digested with BamHI and KpnI. The downstream fragment was digested with KpnI. Both fragments were simultaneously ligated into linearized pLD55. The ligation mixture was transformed into DH5α/λpir cells, and transformants were screened by restriction digestion.
pAra-HT-V was constructed by subcloning a His6-tagged lcrV fragment obtained by digesting pHT-V (7) with NcoI and SalI into NcoI- and SalI-digested pBAD24 (10).
Strain construction.
Y. pestis KIM8-3002.8 was constructed as described previously (21). pMNΔlcrGV2 was electroporated into Y. pestis KIM8-3002. Ampicillin-resistant (Apr) colonies were selected on TBA plus ampicillin. Apr colonies were then streak purified on TBA plus ampicillin plus tetracycline to isolate bacteria with a single crossover event that had integrated pMNΔlcrGV2 into the LCR plasmid, pCD1. Four Apr tetracycline-resistant (Tcr) colonies were then streaked onto nonselective medium (TBA) to allow the accumulation of segregants within colonies. Four colonies from each of those four plates (16 colonies total) were streaked onto TBA-TSS agar (21) to counterselect against Tcr bacteria. After 5 to 7 days of growth on TBA-TSS, putative Tc-sensitive (Tcs) colonies were streaked onto nonselective medium and onto TBA plus ampicillin and TBA plus tetracycline to confirm loss of the plasmid markers. Ap-sensitive Tcr colonies were screened for the replacement of lcrGV with ΔlcrGV2 by using PCR analysis with primers ΔlcrG-US and ΔlcrGV-DS. The phenotype of the lcrGV deletion strain was determined by growth in TMH at 37°C as described above.
Yeast two-hybrid assays.
Yeast two-hybrid assays were performed as recommended by the commercial supplier (Clontech, Palo Alto, Calif.). Vectors pAS2-1 (encoding the GAL4 DNA binding domain) and pACT2 (encoding the GAL4 activation domain) were obtained from Clontech Laboratories as part of the Matchmaker Two-Hybrid System 2 Kit. Plasmids were transformed into Saccharomyces cerevisiae strain Y190 by using the S. c. EasyComp Transformation Kit (Invitrogen, Carlsbad, Calif.), and cells were plated on appropriate minimal yeast synthetic-dropout medium plates. Colony lift assays to detect β-galactosidase activity were performed as described by Clontech Laboratories. Liquid β-galactosidase assays were performed using the Yeast β-galactosidase Assay Kit (Pierce Chemical Corp., Rockford, Ill.) according to the manufacturer's instructions.
Cell fractionation, affinity purification, and chemical cross-linking.
Bacterial cells were fractionated as previously described (21). Briefly, bacterial cells were chilled on ice after growth, harvested by centrifugation at 20,800 × g for 5 min at 4°C, and washed in cold phosphate-buffered saline (PBS) (1). Bacterial whole-cell fractions were prepared by resuspending the washed cells in cold PBS and precipitating total proteins with 10% (vol/vol) trichloroacetic acid (TCA) on ice overnight. Secreted proteins were recovered from the bacterial growth medium by centrifuging (20,800 × g for 5 min at 4°C) the spent medium a second time and transferring the supernatant to a clean tube. The total secreted protein was recovered from the medium by precipitation with 10% (vol/vol) TCA on ice overnight. The TCA-precipitated proteins were pelleted by centrifugation (20,800 × g at 4°C) for 20 min and resuspended in 2× sodium dodecyl sulfate (SDS) sample buffer (1). Protein extracts for affinity purification and chemical cross-linking analysis with dithiobis-succinimidyl propionate (DSP; Pierce Chemical Corp.) were prepared by disintegration with a French press. Ice-cold PBS-washed yersiniae were resuspended in ice-cold PBS and passed through a French pressure cell at 20,000 lb/in 2. Subsequent to disintegration, the extracts were clarified by centrifugation at 20,800 × g for 5 min at 4°C. The cross-linking was performed as previously described (22). Affinity purification of His6-LcrV was performed using Talon resin (Clontech) as described by the manufacturer.
Infection assays.
Infection of eukaryotic cells was performed as described previously (21). Prior to infection; eukaryotic cells were subcultured into 35-mm-diameter six-well tissue culture plates in RPMI-FBS and incubated at 37°C under 5% CO2 for 48 to 72 h to a density of 5 × 105 to 8 × 105 cells per well. Cells were washed twice with warm L15 medium lacking FBS immediately prior to infection. Bacteria were cultivated at 26°C in heart infusion broth and used at an OD620 of ∼1.0 for tissue culture infections. Arabinose was added to 0.2% (wt/vol) prior to infection for strains harboring plasmids with inducible promoters. Bacteria were added (at a multiplicity of infection of 5 to 10) directly to prewarmed medium in the wells of the six-well plates (containing arabinose if appropriate). Plates were then centrifuged at 200 × g at room temperature for 5 min to achieve contact between the bacteria and the target cells and were incubated at 37°C for 4 h.
Protein electrophoresis and immunodetection.
Proteins were separated by SDS-polyacrylamide gel electrophoresis (SDS-PAGE), using 12.5 or 15% (wt/vol) polyacrylamide gels as indicated, according to the method described by Laemmli (14). Samples were boiled 3 to 5 min before loading on the gels. Samples were loaded such that lanes containing different culture fractions represented equivalent amounts of the original cultures. Proteins resolved by SDS-PAGE were transferred to Immobilon-P membranes (Millipore Corp., Bedford, Mass.) by using carbonate transfer buffer (pH 9.9) (33). Specific proteins were visualized using rabbit polyclonal antibodies specific for His-tagged LcrV (α-LcrV) (22), glutathione S-transferase (GST)-tagged LcrG (α-LcrG) (22), YopM (α-YopM) (20), GST-tagged LcrQ (α-LcrQ) (37), YopN (also known as LcrE) (α-LcrE) (22), and YopE (α-YopE; gift from S. C. Straley, University of Kentucky, Lexington). Alkaline phosphatase-conjugated secondary antibody (goat anti-rabbit immunoglobulin G; Pierce) was used to visualize proteins by development with nitroblue tetrazolium and 5-bromo-4-chloro-3-indolylphosphate (NBT-BCIP; Fisher Scientific, Fair Lawn, N.J.).
RESULTS
Creation of a stable LcrG mutant that does not interact with LcrV.
A central hypothesis of our LcrG titration model is that LcrV is required to remove an LcrG-mediated secretion block from the Ysc to allow the secretion of Yops. To further characterize the role of the LcrG-LcrV interaction in the LCR, the interaction was disrupted by mutagenesis of lcrG. To this end, site-directed mutagenesis was used to change residues of LcrG and the resulting mutants were screened for interaction with LcrV by using the yeast two-hybrid system. A more detailed analysis of LcrG's interaction with LcrV will be described elsewhere (J. S. Matson and M. L. Nilles, unpublished observation). Two mutant LcrG proteins that no longer interacted with LcrV in yeast were characterized in this study. Both mutants had the alanine at position 16 changed to either arginine or aspartic acid (LcrG A16R and LcrG A16D). Neither mutant LcrG protein demonstrated an interaction with LcrV in yeast, as determined by a colony lift assay (Table 2). Additionally, yeast expressing either mutant protein produced background levels of β-galactosidase activity in a liquid β-galactosidase assay (Table 2). These results demonstrated that the mutant LcrG-GAL4 AD chimeras, containing the A16R and the A16D substitutions, were no longer capable of interacting with the LcrV-GAL4 BD chimeras in a yeast two-hybrid system.
TABLE 2.
Yeast two-hybrid analysis of LcrG and LcrV interactions
| Derivative of pACT2 | Presence of LcrVa | Colony lift assay | β-Gal activity (Miller units) |
|---|---|---|---|
| pACT-LcrG | + | + | 57.7 |
| pACT2 | + | − | 5.2 |
| pACT-LcrG | − | − | 7.3 |
| pJM91 (A16R) | + | − | 4.5 |
| pJM92 (A16D) | + | − | 4.1 |
Vector control (−) or vector with LcrV (+) supplied.
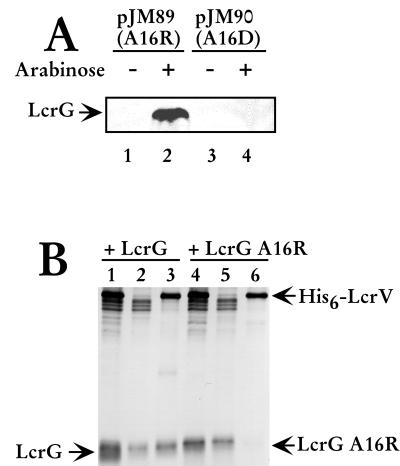
To determine if LcrG A16R and LcrG A16D were stable and recognized by α-LcrG in a prokaryotic background, the mutated lcrG genes were amplified by PCR and cloned into the arabinose-inducible pBAD18-Kan expression vector in E. coli (10). Protein extracts prepared from induced and noninduced cultures of E. coli containing LcrG expression plasmids encoding the mutant proteins were separated by SDS-PAGE, immunoblotted, and probed with α-LcrG. As shown in Fig. 1A, the antibody recognized the induced LcrG A16R (Fig. 1A, lane 2), demonstrating stable expression in E. coli, but did not detect LcrG in any of the other extracts, including extracts containing LcrG A16D. These results indicated that either the LcrG A16D protein was incorrectly folded, thus not recognized by the antibody, or it was unstable and rapidly degraded. LcrG A16R was expressed in Y. pestis, as it was recognized by α-LcrG (Fig. 1B, lane 4) when Y. pestis extracts were analyzed on immunoblots. LcrG A16R is present at levels of approximately 70 to 80% of wild-type LcrG, when steady-state protein levels are compared (Fig. 1b, compare lanes 1 and 4) using densitometry (data not shown).
FIG. 1.
LcrG A16R is stably expressed in E. coli and does not interact with LcrV in Y. pestis. (A) pJM89 and pJM90 were transformed into E. coli strain Novablue cells. Overnight cultures were diluted 1:100 into fresh media and grown at 37°C for 1 h before the addition of 0.2% (wt/vol) arabinose to induce expression of LcrG A16R (lane 2) and LcrG A16D (lane 4). Samples were harvested by centrifugation after 3 h of growth with arabinose, and bacterial cells were permeabilized with Y-PER Reagent (Pierce) for 20 min. The cell debris was collected by centrifugation, and the supernatant was added to 2× sample buffer. Proteins were resolved by SDS-PAGE in a 15% polyacrylamide gel and analyzed by immunoblotting with α-LcrG. (B) Cells of Y. pestis KIM8-3002.8 (ΔlcrGV2) containing plasmids pAraG18K and pAra-HT-V (lanes 1 to 3) and Y. pestis KIM8-3002.8 (ΔlcrGV2) containing plasmids pJM89 and pAra-HT-V (lanes 4 to 6) were grown in TMH with calcium and induced with 0.2% (wt/vol) arabinose prior to the temperature shift to 37°C. Cultures were harvested after 4 h of growth at 37°C, and cellular extracts were prepared by disintegration in a French press (20,000 lb/in2). Low-speed centrifugation (14,000 × g) for 10 min removed unbroken cells and large debris. After centrifugation, the cleared extracts (lanes 1 and 4) were applied to a Talon column. Proteins that did not bind to the column were collected as the flowthrough fraction (lanes 2 and 5). Proteins eluted from the column with 50 mM imidazole were collected (lanes 3 and 6). Proteins were resolved by SDS-PAGE in a 13.5% polyacrylamide gel after dilution in 2X SDS sample buffer and analyzed by immunoblotting with α-LcrG and α-LcrV. Proteins were visualized by probing with alkaline phosphatase-conjugated secondary antibodies and developing with NBT-BCIP.
Affinity purification and chemical cross-linking were used to examine the interaction of LcrG A16R and LcrV in Y. pestis. Strains of Y. pestis, with deletions of lcrG and lcrV, overexpressing LcrG or LcrG A16K and His6-tagged LcrV were French pressed to obtain whole-cell protein extracts and cleared lysates were applied to Talon columns to purify His6-LcrV. The columns were washed and poly-His6-tagged proteins eluted with imidazole. LcrG was copurified from Y. pestis whole-cell protein extracts along with His6-LcrV (Fig. 1B, lane 3). In contrast, LcrG A16R was not copurified along with His6-LcrV (Fig. 1B, lane 6). These results demonstrated that LcrG A16R and LcrV did not participate in a stable interaction within Y. pestis. To confirm the previous result, protein extracts prepared from LCR-induced yersinae overexpressing either LcrG as a control or LcrG A16R were incubated with 3 mM DSP to determine if LcrG A16R could interact with LcrV. The LcrG-LcrV complex formed by DSP cross-linking was detected on immunoblots with either α-LcrG or α-LcrV while no complex was detected on immunoblots when the extracts contained LcrG A16R (data not shown). The results obtained with these experiments demonstrated that LcrG A16R is a stable mutant of LcrG with a decreased affinity for LcrV that resulted in the inability to form a stable LcrG A16R-LcrV complex in Y. pestis.
LcrG A16R blocks secretion of Yops in vitro.
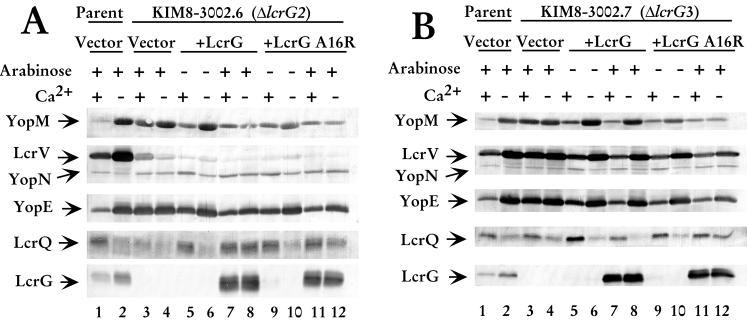
To determine the effect of overexpressing LcrG A16R on Yops secretion, the supernatant of LCR-induced (absence of Ca2+) and LCR-noninduced (presence of Ca2+) bacteria were examined for the presence of secreted proteins as previously described (21). Cells were harvested 4 h after a temperature shift to 37°C, and the culture was fractionated by centrifugation to obtain whole cells and the cell-free culture supernatant. The cell-free culture supernatant was examined for YopM, YopN, YopE, LcrV, LcrG, and LcrQ secretion. Secretion profiles were determined in two previously described lcrG deletion strains. Y. pestis KIM8-3002.6 (ΔlcrG2) has a deletion of lcrG as well as the RBS of the downstream lcrV and thus produces no LcrG and only a small amount of LcrV (21). The other strain used in this study, Y. pestis KIM8-3002.7 (ΔlcrG3), contains a nonpolar in-frame deletion in lcrG that does not affect LcrV levels (7).
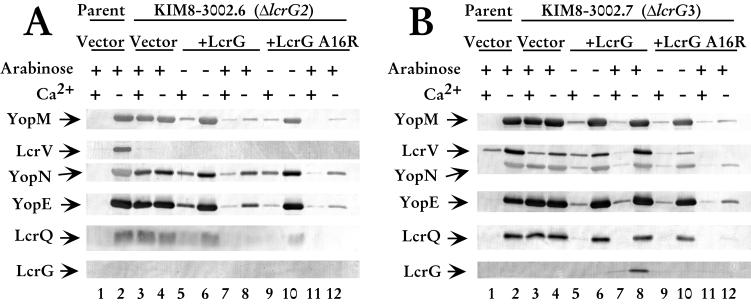
The ΔlcrG2 strain was used in this study because LcrV levels remained low after LCR induction, allowing us to determine if LcrG A16R retained the previously described Yops secretion-blocking activity of LcrG when LcrV levels in the cell were low. Overexpression of LcrG in the ΔlcrG2 strain blocked Yops secretion in the presence of calcium and caused a dramatic decrease in Yops secretion in the absence of calcium, confirming previous results (21). We observed the same decreased secretion of YopM, YopN, and YopE, as well as the negative regulator LcrQ when LcrG was overexpressed in the ΔlcrG2 strain (Fig. 2A, compare lanes 7 and 8 with lanes 3 and 4). In this study, overexpression of LcrG incompletely blocked Yops secretion in the presence of calcium. This result contrasts with previous work where Yops secretion was blocked (21). The difference in the effect of LcrG reflects the timing of arabinose induction (M. L. Nilles and S. C. Straley, unpublished data). In the previous study, arabinose was added prior to the temperature shift, while in this study, arabinose was added at the temperature shift. When LcrG A16R was overexpressed in the ΔlcrG2 strain, Yops secretion was blocked in the presence and absence of calcium, similar to wild-type LcrG. Secretion was blocked in the presence of calcium (Fig. 2A, lane 11) and greatly reduced when calcium was absent from the medium (Fig. 2A, lane 12). The amounts of YopM and YopN secreted when LcrG A16R was overexpressed were similar to the amounts secreted by the LcrG-overexpressing strain (Fig. 2A, compare lanes 12 and 8). The amount of YopE secreted by the mutant may be reduced compared to that of the wild-type LcrG-complemented strain (Fig. 2A, compare lanes 12 and 8). LcrQ was not secreted when either form of LcrG was overexpressed (Fig. 2A, lanes 7, 8, 11, and 12). Neither LcrG nor LcrV were secreted by the ΔlcrG2 strain, consistent with previous results (21). These data show that LcrG A16R functions to block secretion in a background with low LcrV levels, thus retaining one if its two previously described functions while no longer interacting with LcrV, suggesting proper folding of LcrG A16R. LcrG A16R was able to mediate a decrease in Yops secretion even when the expression plasmid was not induced (Fig 2A, lane 9). This is consistent with previous work that showed that little LcrG is required to transcomplement the LcrG defect (7, 21).
FIG. 2.
LcrG A16R blocks secretion regardless of the presence of calcium. (A) Cells of Y. pestis KIM8-3002 containing pBAD18-Kan (vector; lanes 1 and 2) and Y. pestis KIM8-3002.7 (ΔlcrG3) containing plasmids pBAD18-Kan (vector; lanes 3 and 4), pAraG (+LcrG; lanes 5 to 8), and pJM89 (+LcrG A16R; lanes 9 to 12) were grown in TMH with or without calcium. (B) Cells of Y. pestis KIM8-3002 containing pBAD18-Kan (vector; lanes 1 and 2) and Y. pestis KIM8-3002.6 (ΔlcrG2) containing plasmids pBAD18-Kan (vector; lanes 3 and 4), pAraG (+LcrG; lanes 5 to 8), and pJM89 (+LcrG A16R; lanes 9 to 12) were grown in TMH with or without calcium. Arabinose was added at 0.2% (wt/vol) to the cultures immediately prior to the temperature shift to 37°C to induce the expression of LcrG or LcrG A16R from the plasmids. Cultures were harvested after 4 h of growth at 37°C, and samples were fractionated into whole-cell and cell-free culture supernatants. Cell-free culture supernatant samples were separated by SDS-PAGE in a 12.5% polyacrylamide gel and analyzed by immunoblotting with an antibody cocktail containing α-LcrV, α-YopN, and α-YopE. Duplicate immunoblots were probed with α-YopM. Samples were separated by SDS-PAGE in a 15% polyacrylamide gel and analyzed by immunoblotting with α-LcrQ and α-LcrG on separate immunoblots. Proteins were visualized by probing with alkaline phospatase-conjugated secondary antibodies and developing with NBT-BCIP.
The ability of LcrG A16R to complement the ΔlcrG2 strain of Y. pestis led us to investigate the role of this noninteracting LcrG mutant in the presence of wild-type levels of LcrV. In contrast to ΔlcrG2 used above, the ΔlcrG3 Y. pestis strain described by Fields et al. (7) produces wild-type levels of LcrV and is not blocked for secretion when LcrG is overexpressed. The overexpression of LcrG in the ΔlcrG3 strain results in a calcium-dependent growth phenotype accompanied by a wild-type secretion pattern. In agreement with results published by Fields et al. (7), transcomplementation of the ΔlcrG3 strain with LcrG resulted in little or no secretion of Yops and LcrV in the presence of calcium and full secretion of Yops, LcrV, and LcrQ in the absence of calcium (Fig. 2B, lanes 7 and 8). Overexpression of LcrG A16R in the ΔlcrG3 strain caused a dramatic decrease in secretion compared to overexpression of wild-type LcrG in the same strain (Fig. 2B, compare lanes 7 and 8 with 11 and 12). In the absence of calcium, the level of YopM, YopN, and YopE secreted was greatly reduced (Fig. 2B, compare lanes 12 and 8). Secretion of LcrV and LcrQ was blocked when LcrG A16R was overexpressed (Fig. 2B, lane 12), whereas they were secreted at wild-type levels in the presence of LcrG (Fig. 2B, lane 8). These data indicate that in the presence of wild-type LcrV levels, LcrG A16R functions as a secretion block regardless of the presence of calcium. This is consistent with the LcrG titration model that proposes that high relative LcrV levels are required to remove the LcrG secretion block through a stable LcrG-LcrV interaction when the LCR is induced. In the absence of interaction between LcrG and LcrV, the secretion apparatus remains blocked regardless of the levels of LcrV in the bacterial cytoplasm.
LcrG A16R allows repression of Yops expression.
To determine the effect of overexpressing LcrG A16R on Yops expression, bacteria were harvested from LCR-induced (absence of Ca2+) and LCR-noninduced (presence of Ca2+) cultures at 4 h after the temperature shift. The whole-cell fraction was examined by immunoblotting for YopM, YopN, YopE, LcrV, LcrQ, and LcrG. As previously described for Y. pestis strain KIM8-3002.6 (ΔlcrG2) (21), the expression of Yops was elevated regardless of the calcium concentration in the medium (Fig. 3A, lanes 3 and 4). When LcrG was overexpressed in the ΔlcrG2 strain, decreased expression of Yops was observed in the presence and absence of calcium (Fig. 3A, lanes 7 and 8). The negative regulator LcrQ was retained inside the cell in increased amounts (Fig. 3A, lanes 7 and 8). Retention of LcrQ could account for the lack of LCR induction seen in ΔlcrG2 transcomplemented with LcrG. Overexpression of LcrG A16R in the ΔlcrG2 strain resulted in the same pattern of Yops expression. The levels of Yops were decreased irrespective of the presence of calcium (Fig. 3A, lanes 11 and 12). LcrQ levels were elevated inside the cell, presumably due to the inability of this strain to secrete LcrQ when LcrG was overexpressed (Fig. 3A, lanes 11 and 12). These results showed that the expression patterns of Yops were similar when either LcrG or LcrG A16R was overexpressed in the presence of low LcrV levels inside the cell.
FIG. 3.
LcrG A16R does not fully repress expression of Yops. (A) Cells of Y. pestis KIM8-3002 containing pBAD18-Kan (vector; lanes 1 and 2) and Y. pestis KIM8-3002.7 (ΔlcrG3) containing plasmids pBAD18-Kan (vector; lanes 3 and 4), pAraG (+LcrG; lanes 5 to 8), and pJM89 (+LcrG A16R; lanes 9 to 12) were grown in TMH with or without calcium. (B) Cells of Y. pestis KIM8-3002 containing pBAD18-Kan (vector; lanes 1 and 2) and Y. pestis KIM8-3002.6 (ΔlcrG2) containing plasmids pBAD18-Kan (vector; lanes 3 and 4), pAraG (+LcrG; lanes 5 to 8), and pJM89 (+LcrG A16R; lanes 9 to 12) were grown in TMH with or without calcium. Arabinose was added at 0.2% (wt/vol) to the cultures prior to temperature shift to 37°C to induce the expression of LcrG or LcrG A16R from the plasmids. Cultures were harvested after 4 h of growth at 37°C, and samples were fractionated into whole-cell and cell-free culture supernatants. Whole-cell samples were separated by SDS-PAGE in a 12.5% polyacrylamide gel and analyzed by immunoblotting with an antibody cocktail containing α-LcrV, α-YopN, and α-YopE. Duplicate immunoblots were probed with α-YopM. Samples were separated by SDS-PAGE in a 15% polyacrylamide gel and analyzed by immunoblotting with α-LcrQ and α-LcrG on separate immunoblots. Proteins were visualized by probing with alkaline phospatase-conjugated secondary antibodies and developing with NBT-BCIP.
The expression profiles were also examined in Y. pestis strain KIM8-3002.7 (ΔlcrG3) to determine if the LcrG A16R mutant had a different effect on Yops expression in the presence of wild-type levels of LcrV. In agreement with Fields et al. (7), we observed elevated Yops and LcrV expression in the ΔlcrG3 strain whether calcium was present or not (Fig. 3B, lanes 3 and 4). Transcomplementation of the ΔlcrG3 strain with LcrG restored calcium regulation of the LCR (Fig. 3B, lanes 7 and 8). Yops and LcrV levels were reduced to levels similar to that of the wild-type strain in the presence of calcium (Fig. 3B, compare lane 7 to lane 1). When the ΔlcrG3 strain was transcomplemented with LcrG A16R, Yops and LcrV levels were reduced in both the presence and absence of calcium (Fig. 3B, lanes 11 and 12), whereas only the presence of calcium lowered expression levels when LcrG was overexpressed (Fig. 3B, lanes 7 and 8).
LcrG A16R blocks translocation of Yops into HeLa cells.
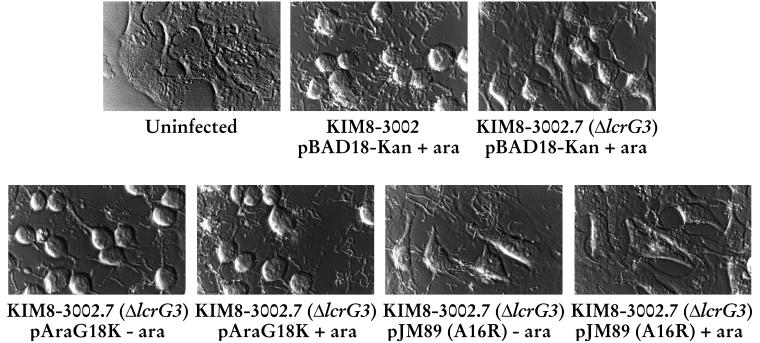
Due to the blockage of secretion observed for the ΔlcrG3 strain transcomplemented with LcrG A16R in our defined medium, we tested the ability of this strain to induce cytotoxicity in infected HeLa cells. The cytotoxicity assay has been shown to be a reliable and sensitive indicator of Yops targeting into eukaryotic cells (6). After 4 h of infection with the parent Y. pestis strain (KIM8-3002), the HeLa cells showed strong cytotoxicity, manifested as “rounding up,” compared with uninfected cells (Fig. 4). Strain ΔlcrG3 showed an intermediate phenotype 4 h postinfection as previously reported (7), indicating that LcrG has a facilitative, but not essential, role in Yops targeting. When LcrG was supplied in trans in the ΔlcrG3 strain, full cytotoxicity was restored. Cytoxicity was observed irrespective of arabinose induction of the lcrG copy carried on the complementing plasmid. This result is consistent with previously reported results (7) and is due to the very small amount of LcrG that is sufficient to correct the defect. However, when the ΔlcrG3 strain was transcomplemented with LcrG A16R, there was no visible cytotoxicity when the plasmid was induced with arabinose (Fig. 4). This result indicates that Yops were not being targeted into the HeLa cell cytoplasm when the LcrG-LcrV interaction was disrupted. These data are in agreement with the lack of secretion of LcrV and Yops observed in vitro. They further suggest that the role of the LcrG-LcrV interaction is at the level of controlling the activity of the Ysc translocation apparatus.
FIG. 4.
LcrG A16R blocks Y. pestis-induced cytotoxicity of HeLa cells. Y. pestis KIM8-3002 containing pBAD18-Kan, and KIM8-3002.7 (ΔlcrG3) containing pBAD18-Kan, pAraG, and pJM89 were used to infect HeLa cells at a multiplicity of infection of 5 to 10. Arabinose (ara) was added at 0.2% (wt/vol) to induce the expression of LcrG or LcrG A16R from the plasmids. After 4 h of expression, the cultures were viewed with Hoffman modulation optics to evaluate cytotoxicity and photographed with a green filter.
Phenotype of a strain deleted for lcrG and lcrV.
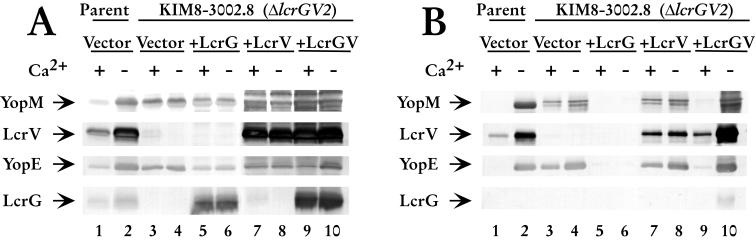
A postulate of the LcrG titration hypothesis is that a Yersinia strain with a deletion of lcrG should demonstrate the same phenotype as a strain with deletions of lcrG and lcrV. Previously, Skrzypek and Straley demonstrated that a Y. pestis strain with partial deletions in lcrG and lcrV was Ca2+ blind and constitutively secreted Yops at 37°C (32). To confirm their result and to create a strain with a clean deletion of lcrGV, we constructed a deletion that removed nearly all of lcrG (only codons 1 to 5 remained) and extended to codon 268 of lcrV. To ensure that no portion of LcrV was produced, codon 6 of lcrG was changed to a stop codon. This newly constructed ΔlcrGV strain (KIM8-3002.8 ΔlcrGV2) exhibited a Ca2+-blind phenotype with respect to growth at 37°C (data not shown). As anticipated from the growth phenotype, the strain has increased levels of LCR-regulated proteins, e.g., YopM, YopE, and LcrV (Fig. 5A, lanes 3 and 4). Additionally, it secreted Yops irrespective of the presence of Ca2+ (Fig. 5B, lanes 3 and 4). This result confirmed the previous observation of Skrzypek and Straley (32) and supports our model of LCR induction. To further characterize this strain, we conducted transcomplementation studies with plasmids expressing LcrG, LcrV, or LcrG and LcrV. As expected, transcomplementation with LcrG resulted in a Ca2+-independent phenotype with an accompanying loss of Yops secretion (Fig. 5B, lanes 5 and 6). Expression of LcrV in ΔlcrGV2 maintained the Ca2+-blind phenotype and increased the levels of other LCR-regulated proteins, suggesting an activating role for LcrV independent of that obtained by neutralizing LcrG function. Introduction of LcrG and LcrV into the ΔlcrGV2 strain restored a Ca2+-dependent phenotype with accompanying control of Yops secretion (Fig 5B, lanes 9 and 10). These results demonstrate that a Y. pestis strain with clean deletions of lcrG and lcrV is Ca2+ blind and constitutively secretes Yops. This strain can be complemented with lcrG and lcrV on plasmids demonstrating that the phenotype we obtained is indeed the result of the lcrGV deletion.
FIG. 5.
Phenotype of lcrGV. Cells of Y. pestis KIM8-3002 containing pBAD18-Kan (vector; lanes 1 and 2) and Y. pestis KIM8-3002.8 (ΔlcrGV2) containing plasmids pBAD18-Kan (vector; lanes 3 and 4), pAraG (+LcrG; lanes 5 and 6), pAraV (+LcrV; lanes 7 and 8), and pAraGV (+LcrGV; lanes 9 and 10) were grown in TMH with or without calcium. Arabinose was added at 0.2% (wt/vol) to each of the cultures immediately prior to the temperature shift to 37°C to induce the expression of LcrG, LcrV, or LcrG and LcrV from the plasmids. Cultures were harvested after 4 h of growth at 37°C, and samples were fractionated into whole-cell (A) and cell-free culture supernatants (B). Samples were separated by SDS-PAGE in a 12.5% polyacrylamide gel and analyzed by immunoblotting with an antibody cocktail containing α-LcrV, α-YopE, and α-LcrG. Duplicate immunoblots were probed with α-YopM. Proteins were visualized by probing with alkaline phospatase-conjugated secondary antibodies and developing with NBT-BCIP.
DISCUSSION
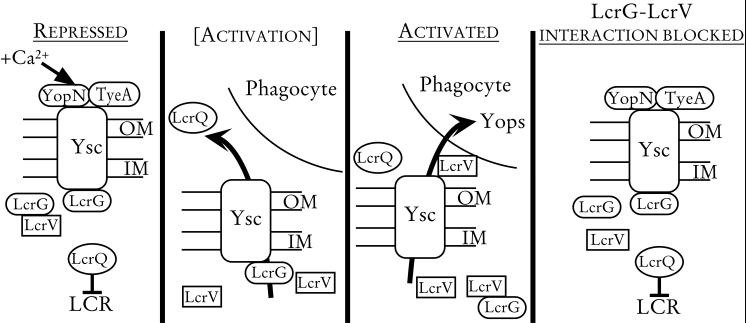
The present work expands the LcrG titration model (Fig. 6) for the regulation of Yops secretion that was first proposed by Nilles et al. (22). This model states that LcrG may directly or indirectly block the secretion machinery, acting from within the bacterial cytoplasm. YopN and TyeA also act in a similar manner to block Yops secretion (5, 9, 13); however, YopN may exert its activity at the bacterial cell surface. In the absence of inducing conditions, LcrG, YopN, and TyeA likely work together as blocks for secretion of Yops and the negative transcriptional regulator, LcrQ. Removal of calcium from the growth medium or contact with a eukaryotic cell may cause the release of YopN from the surface of the bacterium (9). The loss of YopN could enable LcrQ secretion, thereby allowing increased expression of LCR-regulated genes, including the positive regulator, lcrV. It is hypothesized that increased levels of LcrV remove LcrG from its secretion-blocking function through the stable interaction between the two proteins (22). The subsequent loss of both the LcrG and YopN secretion blocks results in the secretion of Yops and LcrQ and in full induction of the LCR.
FIG. 6.
Model for LCR regulation in Y. pestis. In the presence of calcium, LcrG, YopN, and TyeA block the Ysc. LcrG is hypothesized to exert its blocking activity from the cytoplasm while YopN may block secretion at the cell surface. The combined secretion block retains LcrQ in the cell, resulting in repression of LCR-regulated genes (Repressed). In the absence of calcium or in the presence of eukaryotic cell contact, a block (possibly YopN) is released, allowing secretion of LcrQ ([Activation]). Secretion of LcrQ is believed to allow induction of LCR-regulated genes, including lcrV. Increased LcrV levels in the cytoplasm titrate LcrG away from the Ysc by forming a stable LcrG-LcrV complex. The removal of LcrG results in Yops and LcrV secretion and full induction of the LCR (Activated). If LcrG and LcrV are incapable of interacting, LcrG cannot be titrated away from the Ysc. This results in a constitutive blockage of Yops secretion regardless of calcium concentration or eukaryotic cell contact (LcrG-LcrV interaction blocked).
To date, a convincing body of evidence exists to support this model. It has been shown that LcrG is found primarily in the cytoplasm and that the LcrG-LcrV complex is also found in the bacterial cytoplasm (22). YopN has been shown to be surface localized and is thought to act as a sensor of calcium concentration and cell contact (3, 9). YopN, LcrG, and LcrV have been shown to function in the same pathway, and the positive regulatory function of LcrV is actually a result of counteracting negative regulation in this system (32). The relative levels of LcrG and LcrV in the bacterium are important in regulating secretion as overexpression of LcrG in the presence of low LcrV levels can block secretion, even under secretion-inducing conditions (21). Finally, we have shown in this study that LcrG blocks secretion of Yops in the presence of wild-type levels of LcrV, even under secretion-inducing conditions, when the two proteins are incapable of interacting. This finding indicates that the interaction of LcrG and LcrV is necessary for controlling Yops secretion by the Ysc.
LcrG is proposed to have several functions in the yersiniae. Because an LcrG null mutant constitutively expresses and secretes Yops regardless of calcium concentration, it is apparent that LcrG is a negative regulator in the LCR (33). Specifically, LcrG has been proposed to block the secretion of Yops and LcrV along with other proteins, thus preventing full induction of the LCR in the presence of calcium or in the absence of eukaryotic cell contact. Additionally, LcrG may have a chaperone-like activity in its role of facilitating Yops translocation (4, 8, 15). Another proposed function of LcrG is that of a cell surface receptor that senses eukaryotic cell contact by interacting with heparin sulfate ligands (4). Finally, LcrG has a role in the translocation of LcrV and the Yops into target cells (7, 31).
The role of LcrG in blocking Yops secretion was first demonstrated by Skrzypek and Straley (33), who constructed a nonpolar LcrG mutant that failed to downregulate the expression and secretion of Yops and LcrV, whether calcium was present in the growth medium or not. This calcium-blind growth phenotype is observed when negative regulators (such as LcrG, YopN, TyeA, or LcrQ) of the LCR are absent. Further evidence that LcrG has a secretion-blocking function was provided by Nilles et al. (21) by using a strain of Y. pestis that had a deletion of lcrG and produced reduced amounts of LcrV. When LcrG was overexpressed in the presence of low LcrV levels, secretion was blocked regardless of the presence of calcium, indicating that LcrG has a primary function of blocking secretion (21). However, that effect can be observed only under conditions where the LcrV concentration in the bacterial cytoplasm is significantly reduced. LcrG mutant strains that produce wild-type levels of LcrV cannot have secretion blocked by overexpression of LcrG (7). We have provided additional evidence for the secretion-blocking function of LcrG in this work. We demonstrated that when wild-type LcrV levels are present, secretion was constitutively blocked by LcrG if LcrG and LcrV could not physically interact. These results contribute additional evidence that one component of the control of Yops secretion by the Ysc is the stable interaction of LcrG with LcrV.
LcrG has a demonstrated role in the translocation of LcrV and Yops into eukaryotic cells. Sarker et al. showed that LcrG is required for efficient translocation of Yops, because after 2 h of infection, no cytotoxicity was observed when HeLa cells were infected with an lcrG mutant (31). Nilles et al. also observed a lack of cytotoxicity at 4 h postinfection with an lcrG mutant that produces small amounts of LcrV (21). However, strong cytotoxicity could be restored in this strain by overexpressing LcrV, but not by overexpressing LcrG (21). These results indicate that LcrV is required for HeLa cell cytotoxicity and that LcrG is not directly required but has a facilitative role. Fields et al. demonstrated that an lcrG deletion strain producing wild-type LcrV levels is delayed in cytotoxicity, as the rounding up phenotype is only partial at 4 h postinfection and strong cytotoxicity is observed at 6 h (7). This is consistent with the observation that while LcrG is not strictly required for LcrV secretion, it may be required for maximal or efficient secretion of LcrV (7). These results indicate that LcrG has a facilitative role in Yops targeting by promoting LcrV secretion, whereas LcrV is essential for translocation (7, 21, 25).
LcrG has been proposed to be a chaperone for LcrV (15). This model is based on the observation that LcrG has been demonstrated to improve the secretion of LcrV and to make Yops translocation more efficient (4, 7). While this is an attractive idea since the two proteins have been shown to form a stable interaction and LcrG remains primarily in the cytoplasm, there is significant evidence suggesting other roles for LcrG. LcrG has a small size (95 amino acids) like other known chaperones, but unlike the other known chaperones, LcrG has a basic pI (8.64) (27), not an acidic pI. None of the other identified Yop chaperones have described functions other than binding to a Yop and preventing its degradation in the cytoplasm before the protein is secreted or assisting in translocation of the Yop (36). Additionally, LcrV can be secreted (although possibly less efficiently) and targeted into eukaryotic cells in lcrG deletion strains, demonstrating that LcrG is not required for either secretion or translocation of LcrV (8, 21). Possibly the most compelling evidence against LcrG functioning simply as a chaperone for LcrV is that LcrG has a function in the absence of LcrV: it blocks secretion. Therefore, while LcrG may have some chaperone-like characteristics, it clearly has regulatory functions in the type III secretion system of Y. pestis.
Another possible function of LcrG is that of a surface receptor that senses eukaryotic cell contact by binding heparin sulfate (4). The significance of the observation that LcrG can bind heparin sulfate is not known at this time, as LcrG has not been demonstrated on the bacterial surface or demonstrated to be surface accessible in yersinae. While some LcrG can be detected in culture supernatants (33), we typically find LcrG secretion only when LcrG is overexpressed in the presence of LcrV. The LcrV-noninteracting LcrG variant described in this study was not detected in culture supernatants even when overexpressed (Fig. 2B), and we speculate that LcrG secretion occurs due to the interaction with LcrV. Failure to detect LcrG A16R in culture supernatants may be due to the A16R mutation itself. If LcrG is a substrate for the Ysc, then the N-terminal change could effect the Ysc secretion signal.
To further expand our model for the role of the LcrG-LcrV interaction in controlling Yops secretion, we constructed a new lcrGV deletion strain (ΔlcrGV2) that produces no LcrG and no LcrV. Consistent with a previously described lcrGV strain (32), this strain was Ca2+ blind for growth at 37°C and secreted Yops irrespective of Ca2+ concentration (Fig. 5B). This new strain exhibited one surprising characteristic: it had decreased levels of LcrG-regulated proteins. Expression was restored by introduction of LcrV into the strain (Fig. 5A). This result is similar to a report from Pettersson et al., where a yopN/lcrV strain of Yersinia pseudotuberculosis was analyzed (25). Pettersson et al. showed that a yopN/lcrV strain produces lower levels of Yops than does the isogenic yopN strain (25). The result of Pettersson et al. combined with analysis of our lcrGV strain may suggest an independent role for LcrV in LCR induction independent of the LcrG interaction. Pettersson et al. constructed a strain with a specific deletion of lcrV and characterized its secretion and expression of Yops. They found that their strain was Ca2+ independent for growth but demonstrated Ca2+-dependent Yops secretion albeit at lower levels. This result is somewhat at odds with our observation that the ΔlcrGV2 strain overexpressing LcrG is blocked for Yops secretion regardless of the presence of Ca2+. However, our ΔlcrGV2 Y. pestis strain overexpressing LcrG and their lcrV Y. pseudotuberculosis strain likely express very different LcrG levels, which could account for the differences in Yops secretion. Indeed, we find that in situations where LcrG or LcrG A16R overexpression is blocking secretion under normally permissive conditions that the LcrG block becomes “leaky” with increased time at 37°C (J. S. Matson and M. L. Nilles, unpublished data). This leaky secretion block may be due to effects from other control proteins, such as YopN or TyeA.
In summary, we further characterized the role of LcrG in the LCR by testing the hypothesis that LcrG can be relieved from its secretion-blocking function through its interaction with LcrV. An LcrG mutant that could no longer interact with LcrV was able to block the Ysc constitutively, regardless of LCR-inducing conditions. This provides further evidence in support of the proposed LcrG titration model and demonstrates the importance of the LcrG-LcrV interaction in regulating Yops secretion.
ACKNOWLEDGMENTS
We thank Susan Straley (University of Kentucky) for strains and antibodies and Kevin Young and Thomas Hill (University of North Dakota) for critical reading of the manuscript.
REFERENCES
- 1.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons; 1998. [Google Scholar]
- 2.Bergman T, Häkansson S, Forsberg Å, Norlander L, Macellaro A, Bäckman A, Bölin I, Wolf-Watz H. Analysis of the V antigen lcrGVH-yopBD operon of Yersinia pseudotuberculosis: evidence for a regulatory role of LcrH and LcrV. J Bacteriol. 1991;173:1607–1616. doi: 10.1128/jb.173.5.1607-1616.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boland A, Sory M-P, Iriarte M, Kerbourch C, Wattiau P, Cornelis G R. Status of YopM and YopN in the Yersinia Yop virulon: YopM of Y. enterocolitica is internalized inside the cytosol of PU5–1.8 macrophages by the YopB, D, N delivery apparatus. EMBO J. 1996;15:5191–5201. [PMC free article] [PubMed] [Google Scholar]
- 4.Boyd A P, Sory M-P, Iriarte M, Cornelis G R. Heparin interferes with translocation of Yop proteins into HeLa cells and binds to LcrG, a regulatory component of the Yersinia Yop apparatus. Mol Microbiol. 1998;27:425–436. doi: 10.1046/j.1365-2958.1998.00691.x. [DOI] [PubMed] [Google Scholar]
- 5.Cheng L W, Schneewind O. Yersinia enterocolitica TyeA, an intracellular regulator of the type III macinery, is required for specific targeting of YopE, YopH, YopM, and YopN into the cytosol of eukaryotic cells. J Bacteriol. 2000;182:3183–3190. doi: 10.1128/jb.182.11.3183-3190.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cornelis G R, Wolf-Watz H. The Yersinia Yop virulon: a bacterial system for subverting eukaryotic cells. Mol Microbiol. 1997;23:861–867. doi: 10.1046/j.1365-2958.1997.2731623.x. [DOI] [PubMed] [Google Scholar]
- 7.Fields K A, Nilles M L, Cowan C, Straley S C. Virulence role of V antigen of Yersinia pestis at the bacterial surface. Infect Immun. 1999;67:5395–5408. doi: 10.1128/iai.67.10.5395-5408.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fields K A, Straley S C. LerV of Yersinia pestis enters infected eukaryotic cells by a virulence plasmid-independent mechanism. Infect Immun. 1999;67:4801–4813. doi: 10.1128/iai.67.9.4801-4813.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Forsberg Å, Viitanen A-M, Skurnik M, Wolf-Watz H. The surface-located YopN protein is involved in calcium signal transduction in Yersinia pseudotuberculosis. Mol Microbiol. 1991;5:977–986. doi: 10.1111/j.1365-2958.1991.tb00773.x. [DOI] [PubMed] [Google Scholar]
- 10.Guzman L-M, Belin D, Carson M J, Beckwith J. Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J Bacteriol. 1995;177:4121–4130. doi: 10.1128/jb.177.14.4121-4130.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hanahan D, Jessee J, Bloom F R. Plasmid transformation of Escherichia coli and other bacteria. Methods Enzymol. 1991;204:63–113. doi: 10.1016/0076-6879(91)04006-a. [DOI] [PubMed] [Google Scholar]
- 12.Holmström A, Olsson J, Cherepanov P, Maier E, Nordfelth R, Pettersson J, Benz R, Wolf-Watz H, Forsberg A A. LerV is a channel size-determining component of the Yop effector translocon of Yersinia. Mol Microbiol. 2001;39:620–632. doi: 10.1046/j.1365-2958.2001.02259.x. [DOI] [PubMed] [Google Scholar]
- 13.Iriarte M, Sory M-P, Boland A, Boyd A P, Mills S D, Lambermont I, Cornelis G R. TyeA, a protein involved in control of Yop release and in translocation of Yersinia Yop effectors. EMBO J. 1998;17:1907–1918. doi: 10.1093/emboj/17.7.1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 15.Lee V T, Tam C, Schneewind O. Yersinia enterocolitia type III secretion LcrV, a substrate for type III secretion, is required for toxin-targeting into the cytosol of HeLa cells. J Biol Chem. 2000;275:36869–36875. doi: 10.1074/jbc.M002467200. [DOI] [PubMed] [Google Scholar]
- 16.Metcalf W W, Jiang W, Daniels L L, Kim S-K, Haldimann A, Wanner B L. Conditionally replicative and conjugative plasmids carrying lacZα for cloning, mutagenesis, and allele replacement in bacteria. Plasmid. 1996;35:1–13. doi: 10.1006/plas.1996.0001. [DOI] [PubMed] [Google Scholar]
- 17.Miller J H. A short course in bacterial genetics: a laboratory manual and handbook for Escherichia coli and related bacteria. Plainview, NY: Cold Spring Harbor Laboratory Press; 1992. [Google Scholar]
- 18.Nakajima R, Brubaker R R. Association between virulence of Yersinia pestis and suppression of gamma interferon and tumor necrosis factor alpha. Infect Immun. 1993;61:23–31. doi: 10.1128/iai.61.1.23-31.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakajima R, Motin V L, Brubaker R R. Suppression of cytokines in mice by protein A-V antigen fusion peptide and restoration of synthesis by active immunization. Infect Immun. 1995;63:3021–3029. doi: 10.1128/iai.63.8.3021-3029.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nemeth J, Straley S C. Effect of Yersinia pestis YopM on experimental plague. Infect Immun. 1997;65:924–930. doi: 10.1128/iai.65.3.924-930.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nilles M L, Fields K A, Straley S C. The V antigen of Yersinia pestis regulates Yop vectorial targeting as well as Yop secretion through effects on YopB and LcrG. J Bacteriol. 1998;180:3410–3420. doi: 10.1128/jb.180.13.3410-3420.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nilles M L, Williams A W, Skrzypek E, Straley S C. Yersinia pestis LcrV forms a stable complex with LcrG and may have a secretion-related regulatory role in the low-Ca2+ response. J Bacteriol. 1997;179:1307–1316. doi: 10.1128/jb.179.4.1307-1316.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perry R D, Fetherson J D. Yersinia pestis—etiologic agent of plague. Clin Microbiol Rev. 1997;10:35–66. doi: 10.1128/cmr.10.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perry R D, Pendrak M, Schuetze P. Identification and cloning of a hemin storage locus involved in the pigmentation phenotype of Yersinia pestis. J Bacteriol. 1990;172:5929–5937. doi: 10.1128/jb.172.10.5929-5937.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pettersson J, Holmström A, Hill J, Leary S, Frithz-Lindsten E, von Euler-Matell A, Carlsson E, Titball R, Forsberg Å, Wolf-Watz H. The V-antigen of Yersinia is surface-exposed before targent cell contact and involved in virulence protein translocation. Mol Microbiol. 1999;32:961–976. doi: 10.1046/j.1365-2958.1999.01408.x. [DOI] [PubMed] [Google Scholar]
- 26.Pettersson J, Nordfelth R, Dubinina E, Bergman T, Gustafsson M, Magnusson K E, Wolf-Watz H. Modulation of virulence factor expression by pathogen target cell contact. Science. 1996;273:1231–1233. doi: 10.1126/science.273.5279.1231. [DOI] [PubMed] [Google Scholar]
- 27.Price S B, Leung K Y, Barve S S, Straley S C. Molecular analysis of lcrGVH, the V antigen operon of Yersinia pestis. J Bacteriol. 1989;171:5646–5653. doi: 10.1128/jb.171.10.5646-5653.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rimpiläinen M, Forsberg Å, Wolf-Watz H. A novel protein, LcrQ, involved in the low-calcium response of Yersinia pseudotuberculosis, shows extensive homology to YopH. J Bacteriol. 1992;174:3355–3363. doi: 10.1128/jb.174.10.3355-3363.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 30.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sarker M R, Sory M-P, Boyd A P, Iriarte M, Cornelis G R. LcrG is required for efficient translocation of Yersinia Yop effector proteins into eukaryotic cells. Infect Immun. 1998;66:2976–2979. doi: 10.1128/iai.66.6.2976-2979.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Skrzypek E, Straley S C. Differential effects of deletions in lcrV on secretion of V antigen, regulation of the low-Ca2+ response, and virulence of Yersinia pestis. J Bacteriol. 1995;177:2530–2542. doi: 10.1128/jb.177.9.2530-2542.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Skrzypek E, Straley S C. LcrG, a secreted protein involved in negative regulation of the low-calcium response in Yersinia pestis. J Bacteriol. 1993;175:3520–3528. doi: 10.1128/jb.175.11.3520-3528.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Straley S C, Bowmer W S. Virulence genes regulated at the transcriptional level by Ca2+ in Yersinia pestis include structural genes for outer membrane proteins. Infect Immun. 1986;51:445–454. doi: 10.1128/iai.51.2.445-454.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Straley S C, Plano G V, Skrzypek E, Haddix P L, Fields K A. Regulation by Ca2+ in the Yersinia low-Ca2+ response. Mol Microbiol. 1993;8:1005–1010. doi: 10.1111/j.1365-2958.1993.tb01644.x. [DOI] [PubMed] [Google Scholar]
- 36.Wattiau P, Woestyn S, Cornelis G R. Customized secretion chaperones in pathogenic bacteria. Mol Microbiol. 1996;20:255–262. doi: 10.1111/j.1365-2958.1996.tb02614.x. [DOI] [PubMed] [Google Scholar]
- 37.Williams A W, Straley S C. YopD of Yersinia pestis plays a role in the negative regulation of the low-calcium response in addition to its role in the translocation of Yops. J Bacteriol. 1998;180:350–358. doi: 10.1128/jb.180.2.350-358.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]