Abstract
Background
Extracorporeal membrane oxygenation (ECMO) has shown variable results in COVID‐19 pneumonia however, some evidence supports benefit. Here we compare our institution's ECMO outcomes across multiple waves of the COVID‐19 pandemic.
Methods
All patients who received ECMO for COVID‐19 between March 1, 2020, and March 1, 2021, were reviewed. Patients received venovenous (VV) or right ventricular assist device (RVAD/ECMO) ECMO. Early (March 1–July 6, 2020, Era 1) and late (July 7, 2020–March 1, 2021, Era 2) pandemic RVAD/ECMO patients were compared.
Results
Fifty‐four patients received ECMO of which 16 (29.6%) patients received VV ECMO and 38 (70.4%) RVAD/ECMO. Median age was 53.0 years, body mass index 36.1 kg/m2, 41.2% female, and 49% Caucasian. The most common pre‐cannulation treatments included steroids (79.6%) and convalescent plasma (70.4%). Median time from admission to cannulation was 7.0 days. Median support time was 30.5 days (VV ECMO 35.0 days, RVAD/ECMO 26.0 days). In‐ hospital mortality was 42.6% (39.5% RVAD/ECMO, 50.0% VV ECMO). Significant morbidities included infection (80.8%), bleeding events (74.5%), and renal replacement therapy (30.8%). Cumulative mortality 120‐days post‐cannulation was 45.7% (VV ECMO 60.8%, RVAD/ECMO 40.0%). RVAD/ECMO Era 1 demonstrated a significantly lower cumulative mortality (16.2%) compared to Era 2 (60.4%). Competing risk analysis found age (HR 0.95, [95% CI 0.92, 0.98] p = 0.005) to be a protective factor for survival.
Conclusion
ECMO support for COVID‐19 is beneficial but carries significant morbidity. RVAD/ECMO support demonstrated consistent advantages in survival to VV‐ECMO, but with declining efficacy across time during the COVID‐19 pandemic.
Keywords: acute respiratory distress syndrome, COVID‐19, extracorporeal membrane oxygenation, mechanical circulatory support, outcomes
Outcomes for ECMO for COVID‐19 through multiple waves of the pandemic.
1. INTRODUCTION
The rapidity and magnitude of the SARS‐CoV‐2 (i.e., COVID‐19) pandemic has presented a unique challenge to the global medical community. Resources and health care systems have been stressed while the medical community has been pressured to devise effective therapies with limited, evolving evidence. Extracorporeal membrane oxygenation (ECMO) has reemerged as an important component of the support algorithm for acute respiratory distress syndrome (ARDS).
In response to early data regarding the unique pathophysiology of COVID‐19, our center adopted an early right ventricular assist device (RVAD)‐centric strategy (i.e., RVAD/ECMO) with favorable results. 1 This strategy was favored due to early evidence of myocardial involvement, particularly right ventricular dysfunction in COVID‐19 patients, as well as our robust institutional experience with the technique. 2 , 3 , 4 , 5 ECMO's use in COVID‐19 has come about with the same sense of urgency and time pressure as other therapies and similar to many centers, we have continually evaluated our outcomes with this approach in order to refine our technique.
Since our previous publication, the pandemic has progressed through multiple waves of disease, and we have diversified our approach to ECMO support in these patients to include both traditional venovenous ECMO (VV ECMO) and RVAD/ECMO. The present study summarizes our institutional experience with ECMO for COVID‐19 ARDS over one year of the pandemic.
2. METHODS
Approval for this study was obtain from the Medical College of Wisconsin Institutional Review Board on 4/10/2020 (PRO37778). The study population consisted of all patients admitted or transferred to our tertiary‐care center with COVID‐19 pneumonia who received ECMO between March 1, 2020, and March 1, 2021. All patients that received ECMO support for respiratory failure were evaluated by our multidisciplinary mechanical circulatory support team (“SHOCK” team). Eligible patients were those with severe ARDS, as defined by the Berlin criteria, refractory to conventional therapy meeting Extracorporeal Life Support Society (ELSO) indications for Adult Respiratory Failure. 6 Standard ELSO absolute contraindications were considered for all candidates, and additional institution‐specific criteria were also applied. Specifically, age greater than 70 years, body mass index (BMI) greater than 50 kg/m2, and KDIGO stage 3 acute kidney injury were all considered contraindications for ECMO support.
Our approach to ECMO for COVID‐19, technical details, and post‐cannulation care have been described in our institution's previous publication. 1 In brief, early in the pandemic, we instituted an empiric RVAD‐centric support strategy in conjunction with venovenous (VV) ECMO (RVAD/ECMO) utilizing a dual‐lumen, percutaneous RVAD cannula (TandemLife Protek Duo™, LivaNova, UK). This was done for several reasons: (1) early evidence suggesting myocardial involvement of COVID‐19, (2) a high prevalence of right ventricular (RV) dysfunction in severe disease, (3) observation of a unique clinical pathophysiology of COVID‐19 with acute myocardial injury being a critical component preceding death in non‐survivors, and (4) our extensive experience with this support modality for respiratory failure with favorable outcomes. 2 , 3 , 4 , 5 , 7 , 8 As our experience grew, and in order to more effectively use limited mechanical circulatory support resources given rising demand for ECMO, concomitant RV support was prioritized to candidates with RV dysfunction at the time of cannulation as assessed by mandatory pre‐cannulation transthoracic or transesophageal echocardiography. Patients with RV dysfunction or failure were selected for RVAD/ECMO support while those with preserved RV function were supported with conventional dual‐lumen cannulation (Crescent™, Medtronic, Minneapolis, MN), most often via right internal jugular vein cannulation. Echocardiographic features suggestive of RV dysfunction or failure included ventricular systolic or diastolic wall motion abnormalities, new or worsening tricuspid regurgitation, elevated central venous or pulmonary artery pressures, and right atrial dilatation. Anticoagulation for COVID ECMO patients was achieved with a low‐intensity heparin protocol with a goal unfractionated heparin level of 0.2–0.35 U/ml, which remained consistent through this time period of the pandemic.
Disease characteristics were summarized using descriptive statistics. ECMO patients were separated according to support modality with main groups being VV ECMO and RVAD/ECMO with RVAD/ECMO patients subdivided into early pandemic (i.e., Era 1, March 1–July 6, 2020, previously published cohort) and late pandemic (i.e., Era 2, July 7, 2020–March 1, 2021). Our institutions outcomes for RVAD/ECMO Era 1 patients have been previously published by our group representing our early experience with ECMO for COVID‐19. 1 Patients in Era 2 represent our most recent cohort of patients treated with RVAD/ECMO. Comparative statistics were performed only between RVAD/ECMO Era 1 and Era 2 groups and not between VV ECMO and RVAD/ECMO groups given the inherent selection bias these patients experienced when being selected for their given ECMO support modality (i.e., concomitant requirement for RV support or not). Medians with interquartile ranges were used to summarize continuous variables while counts and percentages are used for categorical variables. Fine‐Gray model was used to evaluate the effect of specific independent patient variables on weaning without death (i.e., survival). Variables of interest included age, BMI, sex, race, transfer from a referral center, days from symptoms to admission, days from admission to cannulation, comorbidities (e.g., diabetes, hypertension, hyperlipidemia, chronic lung disease, history of transplant), and ECMO support type (RVAD/ECMO, VV ECMO). We prescreened by running univariate analysis for each of variable with a p‐value of <0.2 as the inclusion criteria. Significant variables were included in the finalized Fine‐Gray model. Stacked cumulative incidence plots were used to demonstrate mortality and survival to wean for COVID‐19 ECMO through 120 days post‐cannulation. Analyses were performed using SAS 9.4 (SAS Institute, Cary, NC).
3. RESULTS
Between March 1, 2020, and March 1, 2021, 54 patients received ECMO for severe ARDS secondary to COVID‐19 pneumonia (Figure 1). Thirty‐eight (70.4%) patients received RVAD/ECMO and 16 (29.6%) patients received VV ECMO. During the early pandemic (i.e., Era 1), 18 patients (47.4%) received RVAD/ECMO, while 20 patients (52.6%) received RVAD/ECMO during the latter pandemic (i.e., Era 2).
FIGURE 1.
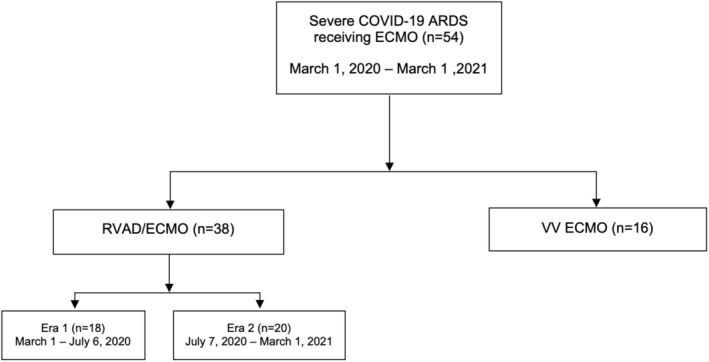
Consort diagram for COVID‐19 ECMO. ARDS, acute respiratory distress syndrome; ECMO, extracorporeal membrane oxygenation; RVAD, right ventricular assist device; VV, venovenous.
Pre‐cannulation characteristics and demographics are presented in Table 1. Patients were median age 53.0 years, predominantly male (62.1%) and Caucasian (51.9%) with a median body mass index (BMI) in the morbidly obese category (36.1 kg/m2). The most common comorbidities included diabetes (42.6%), hypertension (64.8%), and chronic lung disease (46.3%). The most common pre‐cannulation therapies were steroids (79.6%), remdesivir (55.6%), convalescent plasma (70.4%), and antibiotics (63.0%). VV ECMO and RVAD/ECMO patients were similar with the exception of a greater incidence of a prior solid organ transplantation (31.3 vs. 5.3%), chronic kidney disease (18.8 vs. 2.6%) and hyperlipidemia (56.3 vs. 28.9%) in VV ECMO patients. Patients presented median of 7.0 days after symptoms and were cannulated 6.0 days after admission and within 4.5 days of intubation. Twenty‐four patients (44.4%) were transfers from referral centers. The majority of VV ECMO patients (56.3%) were transferred compared to a minority (39.5%) of RVAD/ECMO patients. Cannulation was often performed less than 24 h after intubation (median 0.0 days).
TABLE 1.
Baseline characteristics, demographics, and pre‐cannulation treatment
| Total (n = 54) | VV ECMO (n = 16) | RVAD/ECMO (n = 38) | |
|---|---|---|---|
| Age (years) | 53.0 (27.0–69.0) | 53.5 (29.0–69.0) | 53.0 (27.0–69.0) |
| Female | 21 (38.9) | 7 (43.8) | 14 (36.8) |
| Race | |||
| Caucasian | 28 (51.9) | 9 (56.3) | 19 (50.0) |
| Black | 13 (24.1) | 5 (31.3) | 8 (21.1) |
| Asian | 3 (5.6) | 0 (0.0) | 3 (7.9) |
| American Indian | 2 (3.7) | 1 (6.3) | 1 (2.6) |
| Hispanic | 7 (13.0) | 1 (6.3) | 6 (15.8) |
| Other | 1 (1.9) | 0 (0.0) | 1 (2.6) |
| Body mass index (kg/m2) | 36.1 (30.0–40.9) | 38.9 (28.7–40.7) | 35.3 (30.3–41.4) |
| Body surface area (m2) | 2.3 (2.0–2.5) | 2.3 (2.0–2.5) | 2.2 (2.0–2.4) |
| Comorbidities | |||
| Diabetes | 23 (42.6) | 7 (43.8) | 16 (42.1) |
| Chronic kidney disease | 4 (7.4) | 3 (18.8) | 1 (2.6) |
| Hypertension | 35 (64.8) | 12 (75.0) | 23 (60.5) |
| Coronary artery disease | 1 (1.9) | 0 (0.0) | 1 (2.6) |
| Hyperlipidemia | 20 (37.0) | 9 (56.3) | 11 (28.9) |
| Chronic lung disease | 25 (46.3) | 7 (43.8) | 18 (47.4) |
| Tobacco use | 18 (33.3) | 4 (25.0) | 14 (36.8) |
| Prior solid organ transplantation | 7 (13.0) | 5 (31.3) | 2 (5.3) |
| PaO2/FiO2 | 69.0 (37.0–98.0) | 73.0 (37.0–98.0) | 68.5 (39.0–97.0) |
| Pre‐cannulation treatment | |||
| Remdesivir | 30 (55.6) | 11 (68.8) | 19 (50.0) |
| Steroids | 43 (79.6) | 14 (87.5) | 29 (76.3) |
| Antibiotics | 34 (63.0) | 7 (43.8) | 26 (68.4) |
| Tocilizumab | 18 (33.3) | 2 (12.5) | 16 (42.1) |
| Proning | 50 (92.6) | 13 (81.3) | 37 (97.4) |
| Paralytics | 21 (38.9) | 8 (50.0) | 13 (34.2) |
| Convalescent plasma | 38 (70.4) | 12 (75.0) | 26 (68.4) |
| Hydroxychloroquine | 10 (18.5) | 2 (12.5) | 8 (21.1) |
| Pulmonary vasodilators | 19 (35.2) | 6 (37.5) | 13 (34.2) |
| Transfer from referral center | 24 (44.4) | 9 (56.3) | 15 (39.5) |
| Days symptoms to admission | 7.0 (1.0–14.0) | 7.0 (3.0–14.0) | 7.0 (1.0–13.0) |
| Days admission to intubation | 4.5 (0.0–29.0) | 8.0 (0.0–29.0) | 3.0 (0.0–16.0) |
| Days admission to cannulation | 6.0 (0.0–20.0) | 7.5 (1.0–20.0) | 5.5 (0.0–19.0) |
| Days intubation to cannulation | 0.0 (0.0–8.0) | 0.5 (0.0–5.0) | 0.0 (0.0–8.0) |
Comparisons of Era 1 and Era 2 RVAD/ECMO pre‐cannulation characteristics and demographics are presented in Table S1. Comparing Era 1 and Era 2 RVAD/ECMO cohorts, patients in the Era 2 were more often Caucasian (33.3 vs. 65.0%, p = 0.013), had significantly higher BMIs (32.6 vs. 39.7 kg/m2, p = 0.014) and body surface areas (2.1 vs. 2.3 m2, p = 0.012), and had a history of tobacco use (22.2 vs. 50.0%, p = 0.003). Changes in empiric medical therapies were observed with fewer patients receiving steroids in Era 1 than Era 2 (50.0 vs. 100.0%, p < 0.001), a significant decline in tocilizumab (66.7 vs. 20.0%, p = 0.004), hydroxychloroquine (44.4 vs. 0.0%, p < 0.001), and empiric antibiotics (77.8 vs. 40.0%, p = 0.019) paralleling trends in emerging evidence. 9 , 10 , 11 As the pandemic progressed, a greater portion of patients were transferred from outside facilities, although this difference did not reach statistical significance (27.8 vs. 50.0%, p = 0.162). Times from admission to both intubation and cannulation were similar, although the time from intubation to cannulation increased from a median of 0.0 to 1.0 days between the eras (p = 0.077), which was not statistically significant.
Post‐cannulation outcomes are in Table 2. The median duration of ECMO support was 30.5 days among survivors with VV ECMO patients requiring a longer support interval than RVAD/ECMO patients (35.0 vs. 26.0 days). Overall in‐hospital mortality was 42.6% with VV ECMO patients having a higher incidence than RVAD/ECMO (50.0 vs. 39.5%). Four patients (7.4%) were still on support at the time of this analysis. The most frequent morbidities included renal failure requiring renal replacement therapy (RRT) (30.8% overall, VV ECMO 42.6%, RVAD/ECMO 26.3%), concomitant infection (80.8%), bleeding (70.4%), and stroke (16.7%). Bleeding events were exceedingly common in our cohort despite low‐intensity heparin anticoagulation. Bleeding severity definitions can be found in the Supporting Information (“COVID ECMO bleeding severity definitions”). “Major” bleeding events were the most commonly seen with 42.6% of patients experiencing at least one event, occurring with similar frequency in VV ECMO and RVAD/ECMO groups (43.8 vs. 42.1%). VV ECMO patients more often experienced “minor” events (42.9%) while RVAD/ECMO patients experienced more “moderate” events (42.1%). Patients were intubated a median 21.0 days after cannulation with 53.7% requiring reintubation and 50.0% needing tracheostomy, all of which were numerically higher for VV ECMO patients than RVAD/ECMO patients. Two VV ECMO patients (12.5%) developed RV failure requiring conversion to RVAD/ECMO. Several cannula‐related complications occurred at similar frequency in both VV ECMO and RVAD/ECMO groups (15.4 vs. 13.2%). These one peri‐cannula thrombus and one upper extremity limb ischemia in VV ECMO patients and one peri‐cannula thrombus, one canula fracture, and three instances of RV perforation in RVAD/ECMO patients (details below). Multivariate (Fine‐Gray) competing‐risk analysis for survival demonstrated age to be the only significant factor impacting survival (HR 0.95, [95% CI 0.92, 0.98] p = 0.005) (Table 3).
TABLE 2.
Post‐cannulation outcomes
| Total (n = 54) | VV ECMO (n = 16) | RVAD/ECMO (n = 38) | |
|---|---|---|---|
| Days intubated after cannulation | 21.0 (0.0–80.0) | 30.5 (0.0–43.0) | 16.0 (1.0–80.0) |
| Reintubation | 29 (53.7) | 12 (75.0) | 17 (44.7) |
| Tracheostomy | 27 (50.0) | 11 (68.8) | 16 (42.1) |
| Cannulation days | 30.5 (3.0–85.0) | 35.0 (3.0–78.0) | 26.0 (4.0–85.0) |
| Length of stay (days) | 53.0 (13.0–104.0) | 75.0 (57.0–102.0) | 48.0 (13.0–104.0) |
| ICU length of stay (days) | 39.0 (6.0–102.0) | 47.0 (7.0–102.0) | 37.0 (6.0–91.0) |
| In‐hospital mortality | 23 (42.6) | 8 (50.0) | 15 (39.5) |
| Renal failure requiring RRT | 16 (30.8) | 6 (42.9) | 10 (26.3) |
| Infection | 42 (80.8) | 12 (85.7) | 30 (78.9) |
| Pneumonia | 32 (61.5) | 11 (78.6) | 21 (55.3) |
| Bacteremia | 19 (36.5) | 6 (42.9) | 13 (34.2) |
| Bleeding event | 38 (70.4) | 12 (75.0) | 26 (68.4) |
| Minor | 17 (31.5) | 6 (42.9) | 11 (28.9) |
| Moderate | 21 (38.9) | 5 (31.3) | 16 (42.1) |
| Major | 23 (42.6) | 7 (43.8) | 16 (42.1) |
| Bleeding requiring reoperation | 8 (14.8) | 4 (25.0) | 4 (10.5) |
| Intracranial hemorrhage | 8 (14.8) | 2 (12.5) | 6 (15.8) |
| Stroke | 10 (18.5) | 3 (18.8) | 7 (18.4) |
| Hemorrhagic stroke | 8 (14.8) | 2 (12.5) | 6 (15.8) |
| Conversion to RVAD/ECMO | 2 (3.7) | 2 (12.5) | N/A |
| Cannula‐associated complications | 7 (13.0) | 2 (15.4) | 5 (13.2) |
| Cytokine filter a | 13 (24.1) | 3 (18.8) | 10 (26.3) |
| Ongoing support | 4 (7.4) | 3 (18.8) | 1 (2.6) |
| Discharge disposition | |||
| Home | 15 (30.6) | 1 (7.7) | 14 (38.9) |
| Long term care facility | 4 (8.2) | 1 (7.7) | 3 (8.3) |
| Inpatient rehabilitation | 4 (8.2) | 2 (15.4) | 2 (5.6) |
| Subacute rehabilitation | 3 (6.1) | 1 (7.7) | 2 (5.6) |
Abbreviations: ECMO, extracorporeal membrane oxygenation; ICU, intensive care unit; RRT, renal replacement therapy; RVAD, right ventricular assist device.
Cytokine filter refers to the use of the CytoSorb® extracorporeal cytokine absorber (Cytosorbents Medical, Inc., Monmouth Junction, NJ) authorized under an FDA Emergency Use Authorization for the management of cytokine storm in extracorporeal circulation in COVID‐19 pneumonia. Patients were selected for cytokine filter therapy according to serum levels c‐reactive protein, ferritin, and plasma free hemoglobin.
TABLE 3.
Fine‐Gray competing‐risk model for survival
| Variable | Hazard ratio (95% CI) | p‐value |
|---|---|---|
| Age (years) | 0.95 (0.92, 0.98) | 0.005 |
| Body mass index (kg/m2) | 0.98 (0.95, 1.01) | 0.114 |
| Transfer from referral center | 1.00 (0.37, 2.74) | 0.999 |
| Duration symptoms to admission | 1.01 (0.89, 1.14) | 0.907 |
| Cytokine filter a | 0.88 (0.34, 2.29) | 0.800 |
Cytokine filter refers to the use of the CytoSorb® extracorporeal cytokine absorber (Cytosorbents Medical, Inc., Monmouth Junction, NJ) authorized under an FDA Emergency Use Authorization for the management of cytokine storm in extracorporeal circulation in COVID‐19 pneumonia.
Bolid/italic value indicates p‐values that reached statistical significance (i.e., < 0.05).
Regarding post‐cannulation outcomes for RVAD/ECMO patients (Table S1), Era 2 patients experienced a longer intubation duration (3.0 vs. 24.0 days, p = 0.026), higher incidences of reintubation (27.8 vs. 60.0%, p = 0.046), in‐hospital mortality (16.7 vs. 60.0%, p = 0.006), RRT (0.0 vs. 50.0%, p < 0.001), and infection (61.1 vs. 95.0%, p = 0.016) driven by a significant increase in the rate of secondary bacterial pneumonia (22.2 vs. 85.0%, p < 0.001). Era 2 patients were significantly less likely to be discharged home (72.2 vs. 5.6%, p < 0.001). Era 2 patients suffered significantly more cannula‐associated complications (25.0 vs. 0.0%, p = 0.048) including three instances of RV perforation, one cannula tip thrombus, and one cannula fracture. Two occurrences of RV perforation occurred at the time of cannula placement believed to be due to improper guidewire selection while the third occurred followed cardiopulmonary resuscitation for cardiac arrest in a patient who had been on RVAD/ECMO support for 60 days. Regarding bleeding complications, Era 2 patients experienced significantly more “major” bleeding events (22.2 vs. 60.0%, p = 0.025).
The cumulative incidence of mortality at 120 days post‐cannulation for all patients was 45.7% with RVAD/ECMO patients demonstrating a lower incidence (40.0%) than VV ECMO patients (60.8%) (Figure 2A–C). Era 2 patients had a much higher cumulative incidence of mortality (60.4%) compared to Era 1 patients (16.2%) reported in our original publication (Figure 2D,E). 1
FIGURE 2.
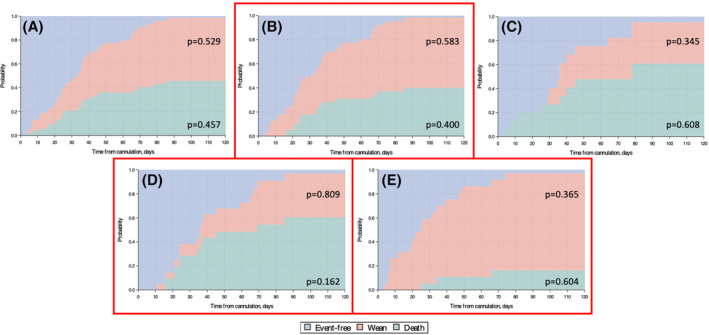
Stacked cumulative incidence plots for mortality and survival to wean for COVID‐19 ECMO. (A) All COVID ECMO patients, (B) RVAD/ECMO, (C) VV ECMO, (D) Era 1 (March 1–July 6, 2020), (E) Era 2 (July 7, 2020–March 1, 2021). p‐values represent the cumulative probability at 120 days post‐cannulation for the variable corresponding to the shaded area in which they lie.
4. DISCUSSION
Here we present an updated analysis of our institution's unique approach to ECMO for COVID‐19 ARDS. Since our previous publication, much has changed in the pandemic and reassessment of our institutional outcomes with this support strategy remains important in informing critical care practices. Regarding mortality, the present study is congruent with other reports demonstrating an overall mortality of 30%–40% in contemporary series. 12 , 13 , 14 , 15 , 16 Most interesting, however, is the varying mortality between early and late eras. One would think that increased experience with treatment of an emerging disease would improve survival, however, the increased mortality noted in this study has been redemonstrated by others as well. 14 , 15 The causes for this are undoubtedly multifaceted, and may include significant changes in adjunct therapies, patient referral patterns, specific disease variants, and/or ECMO support strategies, choices, and implementation decisions. We recognize that our earlier successes with ECMO may have liberalized our later patient selection, potentially making us more willing to use ECMO in higher risk patients, and thus negatively affecting statistical survival rates.
Similar to others' results, this study demonstrates a longitudinal picture of declining benefit of ECMO in COVID‐19 in later pandemic waves. Our institution's initial published experience with COVID‐19 utilizing an RVAD‐centric approached proved promising demonstrating a favorable mortality rate of 11.1% (i.e., Era 1, final mortality 16.7%). 1 These results were echoed by others from a similar time period of the pandemic. 17 Following this early pandemic experience, we have noted a significant increase in late era mortality in excess of 60%. The reasons for this declining efficacy are not entirely clear given the multiple factors at play, however, this trend has been observed by others as well. 14 , 15 , 18 Multivariate analysis revealed age was a significant factor impacting mortality, with older patients demonstrating a worse prognosis across both eras. This is consistent with previous studies. 14 , 16
Although not the focus of the present study, a notable observation was the difference in mortality between ECMO support modality. In‐hospital mortality for conventional VV ECMO was notably higher at 50.0% compared to 39.5% for RVAD/ECMO over the study period, raising the question of if RV support is beneficial in COVID‐19. A unique feature of COVID‐19 is the myocardial involvement, particularly the RV dysfunction present in a significant number of patients. 2 , 7 , 8 Our early experience utilizing an RVAD‐centric approach largely consisted of patients without evidence of overt RV dysfunction. 1 However, in an attempt to better allocate resources, we modified our approach to prioritize concomitant RV support to those with objective evidence of RV dysfunction and subsequently noticed, as demonstrated in this data, a significant increase in mortality. In fact, latter RVAD‐supported patients had near equivalent mortality to VV ECMO patients. Thus, we hypothesize that early RV support may be protective in the COVID‐19 ECMO population and may improve outcomes. This may be because RV failure can be insidious and precipitate worsening organ dysfunction, particularly renal failure. Thus, when the RV goes unsupported or is supported later when RV function has already deteriorated, patients fare worse. These observations combined with the growing evidence of a high frequency of RV dysfunction in COVID‐19 and the probability of developing RV dysfunction during prolonged (and getting longer) ECMO support periods, empiric or early RV support may be beneficial. The above point, however, is purely speculative given the limitations and design of the current study but is a hypothesis that warrants future investigation in an appropriate fashion.
The nature of emerging variants and their unique pathophysiology could also play an important role in the evolving outcomes observed here and elsewhere. Each subsequent variant since the original wild‐type strain has demonstrated differences in risk for hospitalization and mortality. 19 This may, in and of itself, impact patient prognosis. The impact of health care resource shortages, including staff and supplies, is not yet clear but is potentially a contributing factor in our results. Later waves have certainly resulted in the presentation of sicker patients with more comorbidities as seen here and elsewhere. 14 As more patients require advanced therapies, triaging of care and rationing of resources may be a factor as such systems and practices have had to be implemented, which has been the case at our institution with the waitlisting and prioritization of ECMO candidates.
Aside from declining survival benefit, ECMO is not without significant morbidity. While our initial success with RVAD/ECMO was remarkable for no instances of renal failure requiring RRT, we have subsequently observed a significant increase with an overall rate of 30.8%. By support type, 42.9% of VV ECMO patients required RRT compared with 26.3% of RVAD/ECMO patients. This is more in line with other reports (34%–46%), but worrisome for an increasing incidence overall. 12 , 13 , 14 , 16 Infection is another notable morbidity observed in our patients with 80.8% of patients developing at least one secondary infection while on support, most commonly bacteremia (36.5%) or bacterial pneumonia (61.5%). This high infection rate has been observed in other series 13 , 14 and is likely related to cannulation duration which, especially in our series, is increasingly prolonged. Compared to other series, our duration of support is generally longer, and reflects more recent pandemic data showing an overall increasing support duration. 14 , 16 Finally, bleeding events continue to be a complication of these patient as experienced in our original series and others with a somewhat unique propensity for nasal and oropharyngeal bleeding. 1 , 13 , 14
Our study has several limitations. Its retrospective design is a limitation in the context of a pandemic with many evolving factors. Additionally, these results represent the experience of a single, high‐volume center in a relatively small cohort and, therefore, differences in referral patterns, ECMO management, and eligibility criteria are not generalizable and must be considered. Emerging variants with differing pathogenicity, the availability and impact of immunizations and novel therapies, and the everchanging socioeconomic impact of this disease are not controlled for in this analysis but are very much encompassed by the time frame of this study. Thus, while the contributions of these various factors are not accounted for and may not be until future analyses, they must also be considered when interpreting these results.
AUTHOR CONTRIBUTIONS
Nathan J. Smith: Study design, data collection, analysis, manuscript preparation and editing; Sarah Park: Data collection, manuscript preparation and editing; M. Tracy Zundel: Study design, analysis, manuscript preparation and editing; Huaying Dong and Aniko Szabo: Data analysis, manuscript preparation and editing; Michael T. Cain: Data collection, manuscript preparation and editing; Lucian A. Durham: Principle investigator, study design, manuscript preparation and editing.
CONFLICT OF INTEREST
None of the authors listed have any conflicts of interest to disclose in the submission of this manuscript.
Supporting information
Appendix S1
ACKNOWLEDGMENTS
A special thank you to Emily Neumann, RN for which this project and many others would not be possible. A debt of gratitude is extended to the health care professionals who took care of the patients included in this study and have borne the stress and burden of practicing in the midst of a once in a generation global crisis.
Smith NJ, Park S, Zundel MT, Dong H, Szabo A, Cain MT, et al. Extracorporeal membrane oxygenation for COVID‐19: An evolving experience through multiple waves. Artif Organs. 2022;00:1–9. 10.1111/aor.14381
Meeting presentation: 68th annual Southern Thoracic Surgical Association (STSA) meeting, November 5, 2021, Atlanta, GA.
REFERENCES
- 1. Cain MT, Smith NJ, Barash M, Simpson P, Durham LA III, Makker H, et al. Extracorporeal membrane oxygenation with right ventricular assist device for COVID‐19 ARDS. J Surg Res. 2021;264:81–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Inciardi RM, Lupi L, Zaccone G, Italia L, Raffo M, Tomasoni D, et al. Cardiac involvement in a patient with coronavirus disease 2019 (COVID‐19). JAMA Cardiol. 2020;5(7):819–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shi S, Qin M, Shen B, Cai Y, Liu T, Yang F, et al. Association of cardiac injury with mortality in hospitalized patients with COVID‐19 in Wuhan, China. JAMA Cardiol. 2020;5(7):802–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Badu B, Cain MT, Durham LA 3rd, Joyce LD, Sundararajan S, Gaglianello N, et al. A dual‐lumen percutaneous cannula for managing refractory right ventricular failure. ASAIO J. 2020;66(8):915–21. [DOI] [PubMed] [Google Scholar]
- 6. ELSO . Extracorporeal Life Support Organization (ELSO) guidelines for adult respiratory failure, v1.4; Extracorporeal Life Support Organization (ELSO); 2017. https://elso.org/ecmo‐resources/elso‐ecmo‐guidelines.aspx [Google Scholar]
- 7. Argulian E, Sud K, Vogel B, Bohra C, Garg VP, Talebi S, et al. Right ventricular dilation in hospitalized patients with COVID‐19 infection. JACC Cardiovasc Imaging. 2020;13(11):2459–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Li Y, Li H, Zhu S, Xie Y, Wang B, He L, et al. Prognostic value of right ventricular longitudinal strain in patients with COVID‐19. JACC Cardiovasc Imaging. 2020;13(11):2287–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, et al. Dexamethasone in hospitalized patients with COVID‐19. N Engl J Med. 2021;384(8):693–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Boulware DR, Pullen MF, Bangdiwala AS, Pastick KA, Lofgren SM, Okafor EC, et al. A randomized trial of hydroxychloroquine as postexposure prophylaxis for COVID‐19. N Engl J Med. 2020;383(6):517–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ginsburg AS, Klugman KP. COVID‐19 pneumonia and the appropriate use of antibiotics. Lancet Glob Health. 2020;8(12):e1453–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ramanathan K, Shekar K, Ling RR, Barbaro RP, Wong SN, Tan CS, et al. Extracorporeal membrane oxygenation for COVID‐19: a systematic review and meta‐analysis. Crit Care. 2021;25(1):211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schmidt M, Hajage D, Lebreton G, Monsel A, Voiriot G, Levy D, et al. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome associated with COVID‐19: a retrospective cohort study. Lancet Respir Med. 2020;8(11):1121–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schmidt M, Langouet E, Hajage D, James SA, Chommeloux J, Bréchot N, et al. Evolving outcomes of extracorporeal membrane oxygenation support for severe COVID‐19 ARDS in Sorbonne hospitals, Paris. Crit Care. 2021;25(1):355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Barbaro RP, MacLaren G, Boonstra PS, Combes A, Agerstrand C, Annich G, et al. Extracorporeal membrane oxygenation for COVID‐19: evolving outcomes from the international Extracorporeal Life Support Organization Registry. Lancet. 2021;398(10307):1230–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Barbaro RP, MacLaren G, Boonstra PS, Iwashyna TJ, Slutsky AS, Fan E, et al. Extracorporeal membrane oxygenation support in COVID‐19: an international cohort study of the Extracorporeal Life Support Organization registry. Lancet. 2020;396(10257):1071–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mustafa AK, Alexander PJ, Joshi DJ, Tabachnick DR, Cross CA, Pappas PS, et al. Extracorporeal membrane oxygenation for patients with COVID‐19 in severe respiratory failure. JAMA Surg. 2020;155(10):990–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jacobs JP, Stammers AH, Louis JS, Hayanga JWA, Firstenberg MS, Mongero LB, et al. Multi‐institutional analysis of 100 consecutive patients with COVID‐19 and severe pulmonary compromise treated with extracorporeal membrane oxygenation: outcomes and trends over time. ASAIO J. 2021;67(5):496–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lin L, Liu Y, Tang X, He D. The disease severity and clinical outcomes of the SARS‐CoV‐2 variants of concern. Front Public Health. 2021;9:775224. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1


