Abstract
A multicenter retrospective study was designed to assess clinical outcome of COVID‐19 in patients with hematological malignancies (HM) following treatment with anti‐SARS‐CoV‐2 convalescent plasma (CP) or standard of care therapy. To this aim, a propensity score matching was used to assess the role of non‐randomized administration of CP in this high‐risk cohort of patients from the Italian Hematology Alliance on COVID‐19 (ITA‐HEMA‐COV) project, now including 2049 untreated control patients. We investigated 30‐ and 90‐day mortality, rate of admission to intensive care unit, proportion of patients requiring mechanical ventilatory support, hospitalization time, and SARS‐CoV‐2 clearance in 79 CP recipients and compared results with 158 propensity score‐matched controls. Results indicated a lack of efficacy of CP in the study group compared with the untreated group, thus confirming the negative results obtained from randomized studies in immunocompetent individuals with COVID‐19. In conclusion, this retrospective analysis did not meet the primary and secondary end points in any category of immunocompromized patients affected by HM.
Keywords: convalescent plasma, COVID‐19, disease severity, hematological malignancy, survival data
1. INTRODUCTION
The use of convalescent plasma (CP) from subjects recovered from Coronavirus 2 (SARS‐CoV‐2) infection has been considered a therapeutic modality for patients affected by mild or severe acute respiratory syndrome associated with Coronavirus disease 2019 (COVID‐19). 1 , 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 Three recent meta‐analyses of randomized clinical trials and matched‐control data showed that the use of CP was not associated with a lower mortality rate compared to patients treated with standard drugs. 11 , 12 , 13 Interestingly, a lower mortality was demonstrated in patients who were treated with high titer CP within 3 days of hospital admission, thus supporting a possible overall benefit of COVID‐19 CP in homecare patients as well as in non‐critical hospitalized patients. 14 , 15 , 16 , 17 , 18 , 19 As far the clinical implication of COVID‐19 infection in patients with concomitant hematological malignancies (HM) is concerned, several studies have reported a high mortality rate and the Italian Hematology Alliance on HM and COVID‐19 has found that HM patients with COVID‐19 have a more aggressive clinical course than patients with either HM or COVID‐19 alone. 20 , 21 , 22 , 23 In particular, the preexistence of COVID‐19 in patients with B‐cell lymphoma is associated with impaired generation of neutralizing antibody titers and reduced clearance of SARS‐CoV‐2. 24 A recent study has shown that CP treatment was able to neutralize antibody titers in this patient category and to improve clinical response in 80% of patients examined. 2 In a recent study from Visco et al, a high mortality rate in patients with lymphoma and COVID19 has been observed. 25 The clinical outcome may be easily predicted both in hospitalized and not hospitalized patients by demographics or hematological parameters. 25
In this paper we assessed the association of CP use with 30‐ and 90‐day mortality in COVID‐19 infected patients affected by HM. Secondary end points were represented by the rate of admission to intensive care unit, proportion of pts requiring mechanical ventilatory support, hospitalization time, and virus clearance. A propensity score matching analysis was performed using the Italian Hema‐COV data base including 2049 COVID‐19 patients with hematological malignancies.
1.1. Material and methods
This multicenter non‐interventional study, was based on a retrospective data analysis (previously published by Passamonti et al, 22 , 26 Visco et al, 25 and a second retrospective cohort study, aimed at evaluating the impact of the use of CP in reducing mortality in COVID‐19‐infected Italian patients. This sub‐analysis was performed in the same Italian Institutions reporting data over the last 18 months (April 2020‐ October 2021). The present study involved 64 hematology Institutions in Italy. The ITA‐HEMA‐COV (the ITAlian HEMatology Alliance on COVID‐19) worked on behalf of 5 Italian societies, namely SIE (Società Italiana di Ematologia), SIES (Società Italiana di Ematologia Sperimentale), GITMO (Gruppo Italiano Trapianto Midollo Osseo), SEIFEM (Sorveglianza Epidemiologica Infezioni nelle Emopatie), and FIL (Fondazione Italiana Linfomi). All patients (aged ≥18 years) with an established diagnosis of hematological malignancies and being treated with CP were registered by single centers between 25 Feb 2020 and 1 October 2020 (retrospective cohort), and then between April 2020 and October 2021 (retrospective/prospective cohort including the use of CP). Disease severity for COVID‐19 was classified according to the following categories; 1) Mild (non‐pneumonia and mild pneumonia); 2) Severe (dyspnea, respiratory frequency 30/min, SpO2 93%, PaO2/FiO2 <300 and/or lung infiltrates >50%); 3) Critical (respiratory failure, septic shock, and/or multiple organ dysfunction or failure).
Seventeen Italian Institutions provided data on the use of CP in COVID‐19 patients with HM. CP was prepared according to national and international guidelines. Serum titer of specific neutralizing antibodies was >160 in all cases and >320 in 50% of preparation using the EIA method. Pathogen (viral) inactivation treatment of the plasma was performed in all CP preparations, in view of presence of viral DNA in donor population, according to national legislation. The plasma volume used was 200 mL of CP for two consecutive days (400 mL in total). CP was used in hospitalized patients. The levels of neutralizing antibody titers in the treated patients‐group were available in a small number of cases (no. 8).
Inclusion criteria were a diagnosis of hematological malignancy, according to standard WHO‐criteria and laboratory‐confirmed SARS‐CoV‐2 infection, tested by RT‐PCR on nasopharyngeal swabs following standardized national recommendations. The trial was approved by the institutional review board of Varese Hematology unit. Written informed consent was collected from all patients.
1.2. Statistical analysis
The baseline characteristics of the cohort of patients who received the CP were compared with those obtained from controls using a bivariate analysis and the Student's t‐test, Mann‐Whitney test, and Chi square test. Given the observational nature of the study, and considering that the treatment variables were not assigned in a randomized way, we created a propensity score in order to match controls with similar baseline characteristics of patients treated with CP.
The individual propensity score to receive plasma treatment was estimated through a multivariate logistic regression model using as covariates the baseline characteristics of the patients potentially influencing the outcomes: age, disease‐type, disease status, COVID severity. For the matching, the nearest‐neighbor method was used with a ratio of 1: 2 between treated and controls.
Survival was studied through Kaplan‐Meier curves and compared through log‐rank between CP and controls, considering both the overall cohort and the matched control sample. The significance threshold was 0.05 and the tests were all two‐sided.
Analyses were performed with the STATA software, version 14.2.
1.3. Results
This multicenter retrospective analysis has been conducted in 64 hematology Institutions in Italy, belonging to ITA‐HEMA‐COV (the ITAlian HEMatology Alliance on COVID‐19) network. The main aim of this study was to assess the clinical efficacy related to the use of convalescent plasma in the treatment of SARS‐CoV‐2 infected patients affected by concomitant HM. The main clinical and laboratory features of the treated and untreated patient subgroups are given in Table 1. Treated group was constituted by 79 patients, while the control group was initially composed by 2490 patients. In order to make a better comparison between treated and untreated patient groups, a propensity score matching analysis was performed. Based on this subanalysis, 79 CP recipients were compared with 158 matched untreated control patients (Table 2).
TABLE 1.
Main clinical characteristics of CP‐treated versus untreated control patients, obtained from ITA‐HEMA Covid registry (n 2490 patients with COVID‐19 and concomitant HM)
| Variable | Convalescent plasma recipient group (n 79) | Control group (n 2490) | p value |
|---|---|---|---|
| Median age | 62.1 years | 63.7 years | |
| Sex | Male 61.54% (n 48) | Male 59.46% (n 1477) | 0.713 |
| Female 38.46% (n 30) | Female 40.54% (n 1007) | ||
| Charlson index (m) | 4.53 | 4.24 | 0.190 |
| HM subtype: | |||
| AML/ALL | 16.88% (n 13) | 11.33% (n 282) | 0.000 |
| MPN/CML/MDS | 5.19% (n 4) | 21.49% (n 535) | |
| Aggressive NHL/HL | 42.86% (n 33) | 22.65% (n 564) | |
| Indolent NHL/CLL | 23.38% (n 18) | 23.78% (n 592) | |
| Plasma cell neoplasm | 11.69% (n 9) | 23.78% (n 517) | |
| HM status at the time of Covid‐19 infection | |||
| CR | 34.85% (n 23) | 41.62% (n 857) | 0.002 |
| PR | 34.85% (n 23) | 19.87% (n 396) | |
| PD | 19.07% (n 13) | 13.55% (n 270) | |
| SD | 10.14% (n 7) | 24.74% (n 493) | |
| Pneumonia | |||
| Yes 53.52% (n 38) | Yes 39.29% (n 855) | 0.016 | |
| No 46.48% (n 33) | No 60.71% (n 1321) | ||
| Severity of Covid‐19 | |||
| Mild | 32.00% (n 24) | 63.99% (n 1477) | 0.0001 |
| Severe | 53.33% (n 40) | 26.86% (n 620) | |
| Critical | 14.67% (n 11) | 9.14% (n 211) | |
| ICU admission | |||
| Yes 26.58% (n 21) | Yes 11.33% (n 282) | 0.0001 | |
| No 73.42% (n 58) | No 88.67% (n 2208) | ||
Abbreviations: ALL, Acute Lymphoblastic Leukemia; AML, Acute Myeloid Leukemia; CML, Chronic Myeloid Leukemia; CR, Complete Remission; HL, Hodgkin Lymphoma; HM, Hematological Malignancy; ICU, Intensive Care Unit; MDS, Myelodysplastic Syndrome; MPN, Chronic Myeloproliferative Neoplasm; NHL, Non Hodgkin Lymphoma; PD, Progressive Disease; PR, Partial Remission; SD, Stable Disease; WW, Watch and Wait.
TABLE 2.
Main clinical characteristics of CP‐treated versus untreated propensity score‐matched control patients
| Variable | Plasma convalescent‐treated group (n 79) | Control group (n 158) | p value |
|---|---|---|---|
| Age (median) | 62.1 years | 61.2 years | |
| Sex | Male 61.54% (n 48) | Male 59.87% (n 94) | 0.806 |
| Female 38.46% (n 30) | Female 40.13% (n 63) | ||
| Charlson index (m) | 4.53 | 3.86 | |
| HM subtype: | 16.88% (n 13) | 12.03% (n 19) | 0.222 |
| AML/ALL | 5.19% (n 4) | 12.66% (n 20) | |
| MPN/CML/MDS | 42.86% (n 33) | 35.44% (n 56) | |
| Aggressive/CLL | 23.38% (n 18) | 22.15% (n 35) | |
| Plasma cell neoplasia | 11.69% (n 9) | 17.72% (n 28) | |
| HM status at time of Covid infection | |||
| CR | 33.33% (n 23) | 31.48% (n 68) | 0.481 |
| PR | 33.33% (n 23) | 30.09% (n 65) | |
| PD | 18.84% (n 13) | 18.52% (n 40) | |
| SD | 10.14% (n 7) | 10.19% (n 22) | |
| WW | 4.35% (n 3) | 9.72% (n 21)) | |
| Pneumonia | 0.627 | ||
| Yes 53.52% (n 38) | Yes 39.29% (n 855) | ||
| No 46.48% (n 33) | No 60.71% (n 1321) | ||
| Severity of Covid‐19 | |||
| Mild | 32.00% (n 24) | 63.99% (n 1477) | 0.353 |
| Severe | 53.33% (n 40) | 26.86% (n 620) | |
| Critical | 14.67% (n 11) | 9.14% (n 211) | |
| ICU admission | |||
| Yes 26.58% (n 21) | Yes 25.95% (n 41) | 0.0917 | |
| No 73.42% (n 58) | No 74.05% (n 117) | ||
Abbreviations: ALL, Acute Lymphoblastic Leukemia; AML, Acute Myeloid Leukemia; CML, Chronic Myeloid Leukemia; CR, Complete Remission; HL, Hodgkin Lymphoma; HM, Hematological Malignancy; ICU, Intensive Care Unit; MDS, Myelodysplastic Syndrome; MPN, chronic myeloproliferative neoplasm; NHL, Non Hodgkin Lymphoma; PD, Progressive Disease; PR, Partial Remission; SD, Stable Disease; WW, Watch and Wait.
In the CP‐treated group, no adverse events were detected following the administration of convalescent plasma therapy.
The statistical analysis performed on the comparative assessment of the whole Ita‐Hema‐COV series (n 2490) and CP ‐treated cases (n 79) has shown that CP was mainly used in patients having a more severe COVID‐19 infection (advanced pulmonary infection 53.52% vs. 39.29% ‐p 0.016‐ as well as those hospitalized with several complications 53.33% vs. 26.86% ‐p 0.001) compared with the untreated group. Furthermore, CP was mainly used in patients having a more aggressive stage of hematological disease (i.e., aggressive LNH 42.86% vs. 22.65%; p 0.0001), who were treated with more aggressive chemotherapy courses and not yet achieving a remission phase of the disease (partial response: 34.85% vs. 19.87%; p 0.002).
Based on this preliminary statistical analysis, a propensity score‐matched analysis was performed. The Propensity score‐matched control group was composed by 156 cases fully matched for all clinical and laboratory variables (including type of HM). Individual propensities considered several variables, including sex, age, race, ECOG performance status, obesity, hypertension, renal comorbidities, presence of type 2 diabetes, pulmonary impairment, hematologic cancer type, clinical stage and histologic variant, type of treatment, remission phase achieved after treatment, receipt of cytotoxic chemotherapy within 3 months of COVID‐19 diagnosis.
In brief, no statistical difference was seen in the two groups (treated vs. untreated group) in terms of 30‐ and 90‐day survival, severity of COVID‐19 disease.
Survival data were comparable in the two groups (treated vs. untreated ‐ Figure 1, p, not significant values).
FIGURE 1.
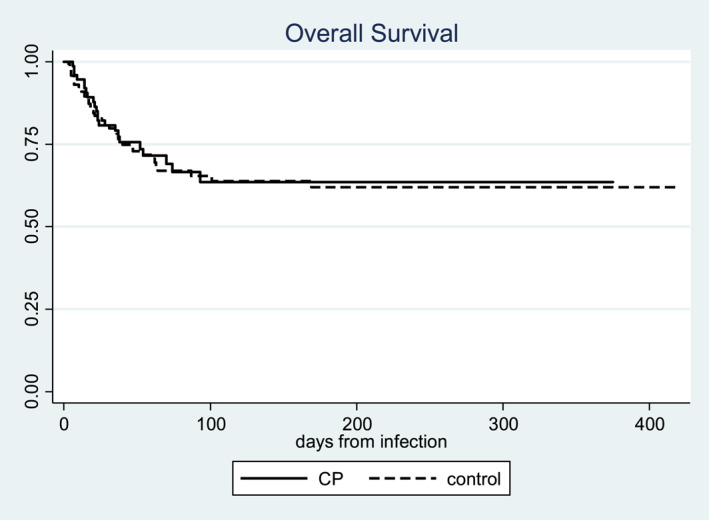
Survival analysis curves obtained from CP‐treated patient group (n 79) versus untreated matched patient group (n 158). No differences were seen. CP was used in hospitalized patients
Reasons for death and follow‐up data were comparable in the two groups and are given in Table 3.
TABLE 3.
Reason for death and length of Covid‐19 in the various patient groups
| CP‐treated n. (%) | CP‐untreated n. (%) | Total n. (%) | p value (chi2 test) | ||
|---|---|---|---|---|---|
| Reason of death | HM | 2/19 (10.5) | 5/46 (10.9) | 7/65 (10.8) | 0.806 |
| Covid‐19 | 16/19 (84.2) | 40/46 (87.0) | 56/65 (86.2) | ||
| Other | 1/19 (5.3) | 1/46 (2.2) | 2/65 (3.1) | ||
| Recovery_from Covid‐19 (follow‐up) | Yes | 34/72 (47.2) | 81/149 (54.4) | 115/221 (52.0) | 0.319 |
Furthermore, the statistical analysis failed to show meaningful differences between the two groups (treated vs. untreated) in terms of the rate of admission to intensive care unit (26.58% vs. 25.95%; p 0.0001), proportion of patients requiring mechanical ventilatory support 47,2% versus. 54,4%; p 0.319), hospitalization time (71,2 days vs. 38,8 days; p 0.4), and timing of virus clearance (p 0.91) (Tables 1 and 3).
Furthermore, there was no meaningful difference in the mortality in patients receiving early versus late administration of convalescent plasma therapy.
2. DISCUSSION
The administration of convalescent plasma (CP) has been recently proposed in severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2)‐infected individuals characterized by severe acute respiratory syndrome. 1 , 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 However, three recent meta‐analyses of randomized clinical trials and matched‐control data clearly showed that the use of CP was not associated with a lower mortality rate compared to patients treated with standard drugs. 11 , 12 , 13 However, a lower mortality was documented in patients who were treated with high titer CP within 3 days of hospital admission, thus supporting a possible overall benefit of COVID‐19 CP in homecare patients as well as in non‐critical hospitalized patients. 14 , 15 , 16 , 17 , 18 , 19
As far the clinical implication of COVID‐19 in patients with concomitant hematological malignancies (HM) is concerned, several studies have reported a high mortality rate and the Italian Hematology Alliance on HM and COVID‐19 has demonstrated that this patient category is characterized by an aggressive clinical course in most of the examined cases. 20 , 21 , 22 , 23 In particular, the preexistence of COVID‐19 in patients with B‐cell lymphoma is associated with an impaired production of neutralizing antibody titers and reduced clearance of SARS‐CoV‐2. 24 , 25 , 26 , 27 , 28 Recent studies have shown that CP treatment was able to neutralize antibody titers in this patient category and to improve clinical response in a significant number of patients examined. 27 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 Furthermore, a recent study indicates an impaired production of SARS‐CoV‐2‐ neutralizing antibodies in an immunosuppressed individual treated with CP, possibly supporting the notion that virus escape, particularly in immunocompromized individuals where prolonged viral replication occurs, and this may limit the efficacy of CP treatment in at least some HM patients. 40
Although most randomized controlled trials have shown negative results on the use of CP for the treatment of COVID‐19, we thought to be wise to conduct a retrospective analysis aimed at evaluating the role played by CP for adults with COVID‐19 and concomitant HM. 11 , 12 , 13 , 20 , 21 , 22 , 23 , 24
In our series, a propensity score matching was used to better assess the role played by the non‐randomized administration of CP in SARS‐CoV‐2 infected patients affected by concomitant HM. Individual propensities took into account clinical characteristics such as sex, age, race, ECOG performance status, obesity, hypertension, renal comorbidities, presence of type 2 diabetes, pulmonary impairment, hematologic cancer type, clinical stage and histologic variant, type of treatment, receipt of cytotoxic chemotherapy within 3 months of COVID‐19 diagnosis.
The association of CP use with 30‐ and 90‐day mortality, rate of admission to intensive care unit, proportion of patients requiring mechanical ventilatory support, hospitalization time, and SARS‐CoV‐2 clearance was investigated in a series of Italian patients affected by COVID‐19 and HM. 79 convalescent plasma recipients were firstly compared with 2049 untreated control patients affected by COVID‐19 and concomitant HM, obtained from the Italian Hematology Alliance on HM and COVID‐19. A second analysis was performed using a propensity score matching to better assess the role played by the non‐randomized administration of CP in SARS‐CoV‐2 infected patients with concomitant hematological malignancies. Results indicate a lack of efficacy of CP in the study group compared with a fully matched untreated group (n. 158 cases). Unfortunately, the levels of SARS‐CoV‐2 antibodies on patients examined were available in a few cases, thus not allowing us to correlate the efficacy of CP with antibody response to COVID‐19 infection and/or previous vaccination. Furthermore, in our series, early administration of CP was not associated with an improvement in survival rates and did not meet secondary end points. These data failed to show an improvement in patients' clinical status and did not result in faster clearance of the virus.
In conclusion, this retrospective analysis conducted in a large series of COVID‐19 patients with hematological malignancies seem to confirm the negative results obtained from randomized studies in SARS‐CoV‐2‐infected individuals. Although this survey has some limitations, due to its retrospective nature, the lack of antibody titers against Sars‐CoV‐2, and avoidance of a standardized use of CP in combination with other treatment measures, we do believe that this study may be informative for the medical community. Prospective randomized clinical trials may provide further insights in this high‐risk population.
CONFLICT OF INTEREST
The author declares that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1002/hon.3060.
Lanza F, Monaco F, Ciceri F, et al. Lack of efficacy of convalescent plasma in COVID‐19 patients with concomitant hematological malignancies: an Italian retrospective study. Hematol Oncol. 2022;1‐7. 10.1002/hon.3060
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are openly available in SIE ‐ Società Italiana Ematologia at https://www.siematologia.it/.
REFERENCES
- 1. Shen C, Wang Z, Zhao F, et al. Treatment of 5 critically ill patients with COVID‐19 with convalescent plasma. JAMA. 2020;323(16):1582‐1589. 10.1001/jama.2020.4783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Casadevall A, Pirofski LA. The convalescent sera option for containing COVID‐19. J Clin Invest. 2020;130(4):1545‐1548. 10.1172/JCI138003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Seghatchian J, Lanza F. Convalescent plasma, an apheresis research project targeting and motivating the fully recovered COVID‐19 patients: a rousing message of clinical benefit to both donors and recipients alike. Transfus Apher Sci. 2020;59(3):102794. 10.1016/j.transci.2020.102794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Joyner JM, Carter RE, Senefeld JW, et al. Convalescent plasma antibody levels and the risk of death from COVID‐19. N Engl J Med. 2021;384(11):1015‐1027. 10.1056/NEJMoa2031893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lanza F, Seghatchian J. Reflection on passive immunotherapy in those who need most: some novel strategic arguments for obtaining safer therapeutic plasma or autologous antibodies from recovered COVID‐19 infected patients. Br J Haematol. 2020;190(1):e27‐9. 10.1111/bjh.16814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Duan K, Liu B, Li C, et al. Effectiveness of convalescent plasma therapy in severe COVID‐19 patients. Proc Natl Acad Sci U. S. A. 2020;117(17):9490‐9496. 10.1073/pnas.2004168117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Li L, Zhang W, Hu Y, et al. Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life‐threatening COVID‐19: a randomized clinical trial. JAMA. 2020;324(5):460‐470. 10.1001/jama.2020.10044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Liu STH, Lin HM, Baine I, et al. Convalescent plasma treatment of severe COVID‐19: a propensity score–matched control study. Nat Med. 2020;26(11):1708‐1713. 10.1038/s41591-020-1088-9 [DOI] [PubMed] [Google Scholar]
- 9. Ortigoza M, Yoon H, Goldfeld K, et al. Efficacy and safety of COVID‐19 convalescent plasma in hospitalized patients. JAMA Intern Med. 2022;182(2):115‐126. 10.1001/jamainternmed.2021.6850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bégin P, Callum J, Jamula E, et al. Convalescent plasma for hospitalized patients with COVID‐19: an open‐label, randomized controlled trial. Nat Med. 2021;27(11):2012‐2024. Epub. 9 Sep 2021. 10.1038/s41591-021-01488-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Janiaud P, Axfors C, Schmitt AM, et al. Association of convalescent plasma treatment with clinical outcomes in patients with COVID‐19: a systematic review and meta‐analysis. JAMA. 2021;325(12):1185‐1195. 10.1001/jama.2021.2747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Klassen SA, Senefeld JW, Johnson PW, et al. The effect of convalescent plasma therapy on mortality among patients with COVID‐19: systematic review and meta‐analysis. Mayo Clin Pro. 2021;96(5):1262‐1275. Epub 2021 Feb 17. 10.1016/j.mayocp.2021.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Troxel AB, Petkova E, Goldfeld K, et Al. Association of convalescent plasma treatment with clinical status in patients hospitalized with COVID‐19: a meta‐analysis. JAMA. 2022;5(1):e2147331. 10.1001/jamanetworkopen.2021.47331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shenoy AG, Hettinger AZ, Fernandez SJ, Blumenthal J, Baez V. Early mortality benefit with COVID‐19 convalescent plasma: a matched control study. Br J Haematol. 2021;192(4):706‐713. 10.1111/bjh.17272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Katz LM. (A little) clarity on convalescent plasma for COVID‐19. N Engl J Med. 2021;384(7):666‐668. Epub. 13 Jan 2021. 10.1056/NEJMe2035678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tobian AAR, Shaz BH. Earlier the better: convalescent plasma. Blood. 2020;136(6):652‐654. 10.1182/blood.2020007638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Korley FK, Durkalski‐Mauldin V, Yeatts SD, et Al. Early convalescent plasma for high‐risk outpatients with COVID‐19. N Engl J Med. 2021;385(21):1951‐1960. 10.1056/NEJMoa2103784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Libster R, Pérez Marc G, Wappner D, et al. Early high‐titer plasma therapy to prevent severe COVID‐19 in older adults. N Engl J Med. 2021;384(7):610‐618. 10.1056/NEJMoa2033700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Joyner MJ, Scott Wright R, Fairweather D, et al. Early safety indicators of COVID‐19 convalescent plasma in 5000 patients. J Clin Invest. 2020;130(9):4791‐4797. 10.1172/JCI140200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hu Y, Wang L, Cui Q, et al. COVID‐19 in hematologic malignancies: big challenges. Clinical Hematology International. 2020;2(4):173‐175. 10.2991/chi.k.200919.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Brissot E, Labopin M, Baron F, et al. Management of patients with acute leukemia during the COVID‐19 outbreak: practical guidelines from the acute leukemia working party of the European Society for Blood and Marrow Transplantation. Bone Marrow Transpl. 2021;56(3):532‐535. 10.1038/s41409-020-0970-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Passamonti F, Cattaneo C, Arcaini L, et al. ITA‐HEMA‐COV Investigators. Clinical characteristics and risk factors associated with COVID‐19 severity in patients with haematological malignancies in Italy: a retrospective, multicentre, cohort study. Lancet Haematol 2020;7(10):e737‐e745. 10.1016/S2352-3026(20)30251-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mileham KF, Bruinooge SS, Aggarwal C, et al. Changes over time in COVID‐19 severity and mortality in patients undergoing cancer treatment in the United States: initial report from the ASCO registry. JCO Oncol Pract. 2022;18(4):e426‐e441. Epub 2021 Oct 25. 10.1200/OP.21.00394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Karataş A, Çağkanİnkaya A, Demiroğlu H, et al. Prolonged viral shedding in a lymphoma patient with COVID‐19 infection receiving convalescent plasma. Transfus Apher Sci. 2020;59(5):102871. Epub 2020 Jul 3. 10.1016/j.transci.2020.102871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Visco C, Marcheselli L, Mina R, et al. A prognostic model for patients with lymphoma and COVID‐19: a multicentre cohort study. Blood Adv. 2022;6(1):327‐338. 10.1182/bloodadvances.2021005691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Passamonti F, Romano A, Salvini M, et al. COVID‐19 elicits an impaired antibody response against SARS‐CoV‐2 in patients with hematological malignancies. Br J Haematol. 2021;195(3):371‐377. Epub 2021 Jul 16. 10.1111/bjh.17704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lanza F, Agostini V, Monaco F, Passamonti F, Seghatchian J. Therapeutic use of convalescent plasma in COVID‐19 infected patients with concomitant hematological disorders. Clin Hematol Int. 2021;3(3):77‐82. 10.2991/chi.k.210403.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gagelmann N, Passamonti F, Wolschke C, et al. Antibody response after vaccination against SARS‐CoV‐2 in adults with haematological malignancies: a systematic review and meta‐analysis. Haematologica. [Epub ahead of print]. 16 Dec 2021. 10.3324/haematol.2021.280163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Thompson MA, Henderson JP, Shah PK, et al. Convalescent Plasma and Improved Survival in Patients with Hematologic Malignancies and COVID‐19. JAMA Oncology. 2021. [Epub ahead of print]. 10.1101/2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ferrari S, Caprioli C, Weber A, Rambaldi A, Lussana F. Convalescent hyperimmune plasma for chemo‐immunotherapy induced immunodeficiency in COVID‐19 patients with hematological malignancies. Leuk Lymphoma. 2021;62(6):1490‐1496. 10.1080/10428194.2021.1872070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jeyaraman P, Agrawal N, Bhargava R, et al. Convalescent plasma therapy for severe COVID‐19 in patients with hematological malignancies. Transfus Apher Sci. 2021;60(3):103075. Epub 3 Feb 2021. 10.1016/j.transci.2021.103075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Biernat MM, Kolasińska A, Kwiatkowski J, et al. Early administration of convalescent plasma improves survival in patients with hematological malignancies and COVID‐19 viruses. Viruses. 2021;13(3):436. 10.3390/v13030436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Senefeld JW, Klassen SA, Ford SK, et al. Use of convalescent plasma in COVID‐19 patients with immunosuppression. Transfusion. 2021;61(8):2503‐2511. Epub 2021 Jun 1. 10.1111/trf.16525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mamez A.‐C, Pradier A, Giannotti F, et al. Antibody responses to SARS‐CoV2 vaccination in allogeneic hematopoietic stem cell transplant recipients. Bone Marrow Transpl. 2021;56(12):3094‐3096. 10.1038/s41409-021-01466-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Betrains A, Godinas L, Sherida FJ, et al. Convalescent plasma treatment of persistent severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2) infection in patients with lymphoma with impaired humoral immunity and lack of neutralizing antibodies. Br J Haematol. 2021;192(6):1100‐1105. 10.1111/bjh.17266 [DOI] [PubMed] [Google Scholar]
- 36. Hueso T, Pouderoux C, Péré H, et al. Convalescent plasma therapy for B‐cell‐depleted patients with protracted COVID‐19. Blood. 2020;136(20):2290‐2295. 10.1182/blood.2020008423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kemp SA, Collier DA, Datir R, et al. Neutralising antibodies in Spike mediated SARS‐CoV‐2 adaptation. medRxiv:20241927. [Epub ahead of print] Dec 2020. 10.1101/2020.12.05 [DOI] [Google Scholar]
- 38. Abu Jabal K, Ben‐Amram H, Beiruti K, et al. Impact of age, ethnicity, sex and prior infection status on immunogenicity following a single dose of the BNT162b2 mRNA COVID‐19 vaccine: real‐world evidence from healthcare workers, Israel, December 2020 to January 2021. Euro Surveill. 2021;26(6):2100096. 10.2807/1560-7917.ES.2021.26.6.2100096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Vasireddy D, Vanaparthy R, Mohan G, Malayala SV, Atluri P. Review of COVID‐19 variants and COVID‐19 vaccine efficacy: what the clinician should know? J Clin Med Res. 2021;13(6):317‐325. 10.14740/jocmr4518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Uriu K, Kimura I, Shirakawa K, et al. Ineffective neutralization of the SARS‐CoV‐2 Mu variant by convalescent and vaccine sera. bioRxiv. 2021. [Epub ahead of print]. 10.1101/2021.09.06.459005 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are openly available in SIE ‐ Società Italiana Ematologia at https://www.siematologia.it/.


