Abstract
We aimed to evaluate the seroconversion rates after two doses of inactive COVID‐19 vaccine (CoronaVac) and the benefit of a third dose mRNA vaccine booster in patients with cancer receiving active treatment. Patients with solid tumors receiving active treatment (n = 101) and patients with no‐cancer (n = 48) as the control group were included in the study. All the patients and controls had received two doses of CoronaVac and a third booster dose of the mRNA vaccine (Bnt162b2). Anti‐SARS‐CoV‐2 Spike Receptor Binding Domain IgG antibody levels after the second and third dose were measured with quantitative ELISA. The median age of the patients was 66 (IQR 60‐71). 79% of the patients were receiving chemotherapy, and 21% were receiving immunotherapy at the time of vaccination. Antibody levels measured after two doses of CoronaVac were significantly lower in patients with cancer than in the control group (median 0 μg/ml [IQR 0‐1.17 μg/ml] vs median 0.91 μg/ml [IQR 0‐2.24 μg/ml], respectively, P = .002). Seropositivity rates were 46.5% in patients with cancer and 72.9% in the control group (P = .002). Antibody measurement was performed in 26 patients after the third dose. Seroconversion rate increased from 46.5% to 88.5% (P < .001), and the antibody titers significantly increased with the third‐dose booster (median 0 μg/ml [IQR 0‐1.17 μg/ml] after two doses vs 12.6 μg/ml [IQR 1.8‐69.1 μg/ml] after third booster dose, P < .001). Immunogenicity of CoronaVac is low in patients with cancer receiving active treatment, and administering a third dose of an mRNA vaccine is effective in terms of improving seroconversion rates.
Keywords: cancer, chemotherapy, COVID‐19, immunotherapy, vaccine response
What's new?
The existing data on the efficacy of inactive COVID‐19 vaccines and the benefit of heterologous boosters remains limited in patients with cancer, who may be immunocompromised by cancer itself or anti‐cancer treatments. This study shows that the immunogenicity of two doses of inactive COVID‐19 vaccine is lower in patients with cancer receiving active treatment compared with controls. Administering a third booster dose of an mRNA vaccine significantly improves seroconversion rates in these patients. The results could be important for clinical practice considering the limited access to mRNA vaccines in several parts of the world.

Abbreviations
- COVID‐19
Coronavirus Disease 19
- FDA
Food and Drug Administration
- IQR
Interquartile Range
- mRNA
messenger RNA
- RBD
receptor binding domain
- WHO
World Health Organization
1. INTRODUCTION
The COVID‐19 pandemic caused more than 500 million infections and 6 million deaths worldwide as of April 22, 2022. 1 , 2 While the disease affected people of all ages, older people and patients with chronic comorbidities were affected most. 3 , 4 Patients with cancer, particularly those under active treatment and those with hematological malignancies, were among the most significantly affected populations from the pandemic with significantly increased morbidity and mortality. 5 In addition, the effects of cancer care disruptions during the pandemic are currently better appraised and will most likely have short‐ and long‐term untoward consequences. 6
Vaccination is one of the most essential strategies against the SARS‐CoV‐2 pandemic. As of April 22, 2022, there are currently over 20 vaccines approved after phase III clinical trials, and more than five billion people worldwide have been vaccinated so far. 2 , 7 While mRNA vaccines have been commonly used in Europe and North America, the inactivated SARS‐CoV‐2 vaccine (CoronaVac) has been widely used in Turkey, where the first phase III trial of CoronaVac was also conducted and showed a high level of activity after two doses (83.5% effectiveness). 8 However, data on vaccine efficacy is limited in cancer patients due to the exclusion of these patients from the phase III vaccine clinical trials. Various observational studies have shown lower seroconversion rates with two‐dose vaccination in patients with cancer, particularly in those under active treatment. 9 , 10 , 11 Both cancer itself and the immunosuppression created by the anti‐cancer treatments were factored in the reduced vaccine efficacy in patients with cancer, probably with a more important role of the anti‐cancer treatments. 12 However, these reports mostly included patients vaccinated with mRNA vaccines and data with other vaccines were scarce. Two noncomparative studies showed low efficacy of CoronaVac in cancer. 13 , 14
Third dose booster vaccination is currently recommended in these patients based on lower efficacy of the vaccines in these patients and waning of immunity over time. Booster vaccination may be with the same vaccine (homologous) or with a different type of vaccine (heterologous). Heterologous vaccination elicited a higher immune response in randomized trials in healthy individuals. 15 , 16 , 17 Among patients who received two doses of inactivated vaccine (CoronaVac), Clemens et al. showed that the magnitude of the immune boost was greater with third dose adenoviral vaccines or mRNA vaccine compared with the homologous vaccine. 17
In this study, we assessed the immunogenicity of two doses of CoronaVac in patients with solid tumors receiving active systemic treatment in comparison with patients with no‐cancer and evaluated the activity of a third heterologous booster with an mRNA vaccine. To our knowledge, this is the first study showing the efficacy of two‐dose vaccination with CoronaVac followed by a third dose booster with mRNA vaccine (Bnt162b2) in this high‐risk cancer patient population.
2. PATIENTS AND METHODS
2.1. Study cohort
Patients with solid tumors receiving active cancer treatment in Hacettepe University Cancer Center, Turkey, between February 2021 and May 2021 were included in this prospective study. The study institution is an ESMO‐designated cancer center accepting patients from all parts of Turkey as a reference center. All patients treated with chemotherapy or immunotherapy in the day chemotherapy unit were invited to the study during the study period. Patients with various comorbidities (other than cancer) but not receiving immunosuppressive treatment were included from the general internal medicine outpatient clinic as the control group. Patients and controls with a clinical history of Covid‐19 infection and patients who received only one dose of the CoronaVac vaccine were excluded. All the patients and the controls were vaccinated with the CoronaVac vaccine for the initial two vaccine doses due to the earlier availability of this vaccine in our country. Patient demographics, tumor types, active anti‐cancer treatment, vaccination dates and systemic treatment dates were collected from the patient files and individual interviews with patients by the investigators on the day of serum sample collection.
The primary aim of the study was to compare seropositivity rates after two doses of CoronaVac between patients with cancer and controls. Due to a lack of available data on the seroconversion rates with CoronaVac vaccine in patients with cancer during the planning of the study, a target population of 100 patients was selected as the sample size considering the continuous nature of target variables as previously suggested. 18 The control group target was 50 to ensure a 2:1 patient to control ratio. The secondary aim was to describe the possibly increased immune response after the third dose booster with the mRNA vaccine in patients with cancer.
2.2. Antibody measurement
Serum samples were obtained from all participants at least 14 days after the second dose. In addition, a serum sample was drawn after the third‐dose vaccine in patients with cancer when available. The serum samples were stored at −20°C until testing. Antibody measurement was performed by Coronahunter Anti‐SARS‐CoV‐2 Spike Receptor Binding Domain (RBD) ELISA kit (Matriks Biotechnology Co. Ltd, Turkey), which is based on the sandwich principle and has been developed for the quantitative analysis of Spike RBD antibodies in serum and plasma samples. The National Institute for Biological Standards and Control (NIBSC) sera with the catalog numbers NIBSC 20/B764, NIBSC 20/130, NIBSC 20/162 and NIBSC 20/B770 were used as reference. The results were evaluated in accordance with WHO cut‐off values. Anti‐SARS‐CoV‐2 antibody titers from NIBSC were compared with the study calibrated against the WHO International anti‐SARS‐CoV‐2 Immunoglobulin Standards (NIBSC code 20/136). In the results obtained, the value given by NIBSC in binding antibody units per milliliter (BAU/ml) was calculated in ng/ml in the study. As a result of the verification studies, one BAU/ml value corresponded to 10 ng/ml. 19
2.3. Statistical analyses
We presented the descriptive characteristics by the median and interquartile range (IQR) for continuous variables and percentages for categorical variables. We compared patient demographics, factors affecting seroconversion rates, and antibody titers in the patient and control groups with the Mann‐Whitney U, Chi‐square and Fischer exact tests. Additional adjustments for the clinical factors affecting the seroconversion rates in patients and controls were conducted with binary logistic regression. Seroconversion rates between the second and third‐dose vaccination were compared with the McNemar test and antibody titers with the Wilcoxon test. We used Statistical Package for Social Sciences version (SPSS) version 25.0 (IBM Inc., Armonk, NY) in the analyses and considered P values below .05 statistically significant.
3. RESULTS
3.1. Baseline characteristics
A total of 101 patients and 48 controls were included in the study (Table 1). The median age of the patients was 66 (IQR 60‐71), and 57% of the patients were male. The age distribution of the patients and controls was similar (P = .480), while the control group had female predominance (43% vs 71% in patients and controls, respectively, P = .001). Lung cancer was the most common diagnosis (24%), followed by colorectal cancer in 21% of the patients. Eighty patients (79.2%) were treated with chemotherapy, while 22 patients received immunotherapy (21 as monotherapy and one combined with chemotherapy) (Table 1). Twenty‐six patients vaccinated with a third‐dose booster mRNA vaccine (Bnt162b2) were eligible and included in the analyses.
TABLE 1.
Baseline clinical features of the patient (n = 101) and control (n = 48) cohorts
| Clinical feature | Patient Cohort n (%) | Control Cohort n (%) | P value |
|---|---|---|---|
| Age (median, IQR) | 66 (60–71) | 64 (59‐71) | .480 |
| Sex | |||
| Male | 58 (57) | 14 (29) | .001 |
| Female | 43 (43) | 34 (71) | |
| Any comorbidity | 51 (50) | 43 (90) | <.001 |
| Hypertension | 36 (36) | 32 (67) | <.001 |
| Diabetes mellitus | 18 (18) | 21 (44) | .001 |
| Coronary heart disease | 10 (10) | 8 (17) | .236 |
| Hypothyroidism | 8 (8) | 5 (10.4) | .757 |
| Primary tumor | |||
| Lung | 24 (24) | ||
| Colorectal | 21 (21) | ||
| Breast | 9 (9) | ||
| Hepatobiliary | 9 (9) | ||
| Gynecological | 8 (8) | ||
| Genitourinary | 7 (7) | ||
| Gastric | 7 (7) | ||
| Other | 16 (15) | ||
| Disease stage | |||
| Localized | 23 (23) | ||
| Advanced | 78 (77) | ||
| Treatment type | |||
| Chemotherapy | 79 (79) | ||
| Immunotherapy | 21 (20) | ||
| Immunotherapy + chemotherapy | 1 (1) | ||
Abbreviation: IQR, interquartile range.
3.2. Antibody results after two‐dose vaccination
The median time between the second dose of vaccination and antibody testing was 42 days (min‐max: 14‐110 days). The COVID‐19 Spike RBD IgG antibody titers after two‐dose vaccination were significantly lower in patients with cancer compared with the control group (median 0 μg/ml [IQR 0‐1.17 μg/ml] vs median 0.91 μg/ml [IQR 0‐2.24 μg/ml], respectively, P = .002) (Figure 1). Seroconversion rates were 46.5% (47 of 101 patients) among the patients with cancer and 72.9% (35 of 48 patients) among the controls after two‐dose vaccination (P = .002). Seropositivity rates were higher in female patients (68% vs 42% in male patients, P = .002), in patients <70 years of age (61% vs 38% in patients ≥70 years of age, P = .009), a trend toward a higher seropositivity rate was observed in patients with hypertension (63% vs 48% in those without hypertension, P = .065). Seroconversion rates did not have a statistically significant association with tumor type (P = .350) or tumor stage (localized vs advanced, P = .890). Multivariate analysis was made to refine the confounding effect of these factors. The seroconversion rates after two‐dose vaccination remained significantly lower in patients compared with controls after additional adjustments according to sex, age and presence of hypertension (Table 2).
FIGURE 1.
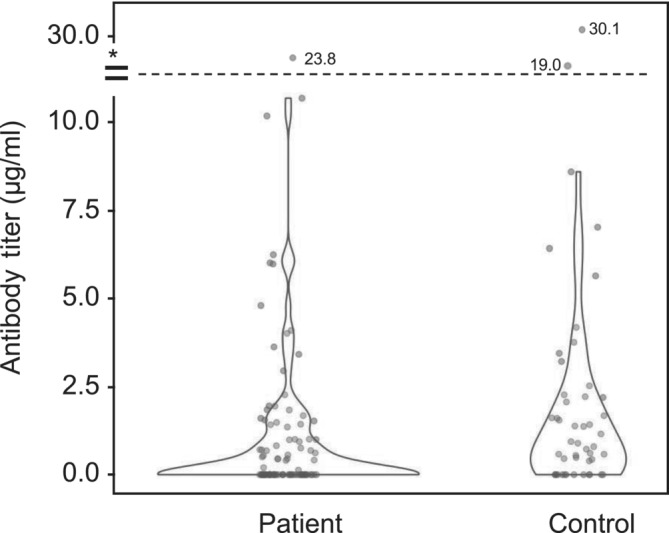
Antibody titers in patient and control groups after two‐dose vaccination
TABLE 2.
Multivariate analysis of factors associated with seropositivity after two‐dose vaccination
| Univariate HR | 95% CI | P | Multivariate HR | 95% CI | P value | |
|---|---|---|---|---|---|---|
| Patient group | ||||||
| Cancer (ref) | 1 | |||||
| Control | 3.09 | 1.46‐6.53 | .003 | 2.73 | 1.23‐6.07 | .014 |
| Gender | ||||||
| Male (ref) | 1 | |||||
| Female | 2.91 | 1.49‐5.68 | .002 | 2.25 | 1.11‐4.58 | .025 |
| Age | ||||||
| ≥70 (ref) | 1 | |||||
| <70 | 2.15 | 1.11‐4.15 | .023 | 2.68 | 1.22‐5.89 | .014 |
| Hypertension | 1.85 | 0.96‐3.58 | .066 | 1.69 | 0.79‐3.60 | .175 |
| Diabetes mellitus | 0.82 | 0.39‐1.69 | .584 | — | — | — |
Abbreviations: CI, confidence interval; HR, hazard ratio.
3.2.1. Antibody results after third booster dose
A total of 70 patients with cancer received a booster dose, and 26 of these patients had serum samples available for antibody measurements. The median time between the second and third dose was 111 (min‐max: 82‐174) days. The seroconversion rate was increased from 46.5% (47 of 101 patients) to 88.5% (23 of 26 patients) after the third‐dose booster (P < .001). Additionally, the antibody titers were significantly increased with the third‐dose (median 0 μg/ml (IQR 0‐1.17 μg/ml) after second dose vs 12.6 μg/ml (IQR 1.8‐69.1 μg/ml) after third dose, P < .001) (Figure 2). Among patients receiving chemotherapy, the seroconversion rate was increased from 50% (40 of 80 patients) to 94% (16 of 17 patients) (P < .001), and among those receiving immunotherapy, the seroconversion rate was increased from 33% (seven of 21 patients) to 78% (seven of nine patients) after the third‐dose booster (P = .001). Seropositivity rates after third dose were higher in patients <70 years of age (100% vs 50% in patients ≥70 years of age, P = .008), and a trend toward a higher rate was observed in female patients (100% vs 81% in male patients, P = .260).
FIGURE 2.
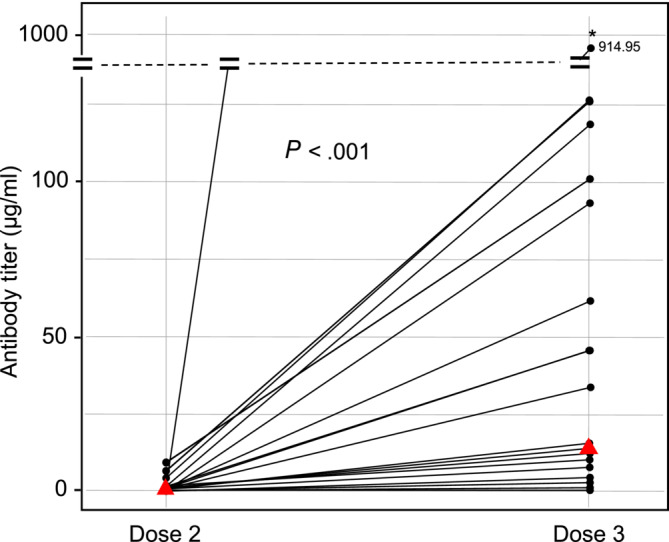
Antibody titers (μg/ml) after two (left) and three (right) vaccine doses. Red triangles denote median values [Color figure can be viewed at wileyonlinelibrary.com]
4. DISCUSSION
In this study, we have shown that immunogenicity of CoronaVac was lower in patients with cancer receiving active treatment compared with controls and administering a third booster dose of an mRNA vaccine significantly improved seroconversion rates in these patients. Older age and male gender were also associated with lower seroconversion rates.
Patients with cancer are among the most vulnerable groups affected by COVID‐19. Mortality and morbidity of COVID‐19 are high secondary to immunocompromise by cancer itself and anti‐cancer treatments. 6 , 20 , 21 , 22 This immunocompromise also affects antibody responses to vaccination. Furthermore, two recent meta‐analyses demonstrated significantly diminished antibody responses in patients receiving active treatment, especially in patients treated with chemotherapy, pointing out the importance of iatrogenic immunosuppression by anti‐cancer treatments and rendering the patients under active anti‐cancer treatment a specific high‐risk group for reduced antibody response to vaccination. 12 , 23 It was previously demonstrated that patients with cancer had decreased antibody responses to seasonal influenza and hepatitis B vaccines. 24 , 25 A similar pattern emerged during the COVID‐19 vaccination period, and studies demonstrated significantly lower seroconversion rates and antibody titers after the first and second doses of vaccination. 10 , 11 Guven et al. recently reported 19% lower seroconversion rates in patients with cancer compared with controls with two‐dose COVID‐19 vaccination in the pooled analysis of 10 studies encompassing 1448 patients. The seroconversion rate was lower in patients with hematological malignancies (72.6 vs 99.4% in patients and controls, respectively), while 91.6% of the patients with solid tumors achieved seroconversion. 26 This is considerably higher than the seroconversion rate in our study after two‐dose vaccination (48.1%). Patient characteristics in our study seem to be similar to previous studies, but our patients were mainly vaccinated with two doses of the inactivated vaccine (CoronaVac), in contrast to mRNA vaccines in the previous studies. This may be the main reason for the discrepant results. Likewise, Karacin et al. reported a 63.8% seroconversion rate in 47 patients with cancer vaccinated with two doses of CoronaVac. 13 These findings emphasize the importance of additional boosters to high‐risk populations vaccinated with two doses of the CoronaVac vaccine.
While the patients under active treatment have a significantly worse prognosis with COVID‐19 disease, patients treated with immune checkpoint inhibitors had similar outcomes to the general population, 27 possibly due to the relatively intact immune system and activated T‐lymphocyte machinery in immunotherapy‐treated patients. 28 Based on a similar mechanism, the immunotherapy‐treated patients could have more robust responses to vaccination, although the data is unequivocal. Massarweh et al. showed similar antibody titers in patients treated with immunotherapy or chemotherapy, while the patients treated with combined chemo‐immunotherapy had the lowest antibody titers. 10 Addeo et al. observed similar seroconversion rates in patients treated with immunotherapy or chemotherapy (93% for both groups), while the median antibody titers were higher in immunotherapy‐treated patients (1116 vs 611 U/ml). 11 The very recent VOICE study reported numerically higher seroconversion rates in immunotherapy‐treated patients compared with patients treated with chemotherapy (93.1 vs 83.8%). 29 Recently, Tang et al. demonstrated similar seroconversion rates in patients treated with immunotherapy compared with those without active treatment in their pooled analysis of 17 study cohorts, including patients with both hematologic and solid tumors. However, in the subgroup analyses, the patients with solid tumors treated with immunotherapy had a significantly increased risk of seronegativity (odds ratio: 1.71, 95% CI: 1.03‐2.84) 23 than patients without active treatment, similar to our results. We observed similar seroconversion rates and antibody titers in patients treated with chemotherapy and immunotherapy but lower seroconversion rates compared with previous studies. Both the use of inactivated CoronaVac vaccine in contrast to mRNA vaccines and the delayed use of immunotherapy in later lines of treatment (after chemotherapy) in our country could be the reasons for relatively low seroconversion rates in immunotherapy‐treated patients in our study.
Due to concerns with the efficacy of two‐dose vaccination in high‐risk populations and the rise of variants of concern, the FDA recently recommended a third‐dose booster for immunocompromised adults with a high risk of severe COVID‐19. 30 Afterward, two community‐level studies from Israel reported over 90% protection rates from severe COVID‐19 protection with a third‐dose booster. 31 , 32 Similarly, in a study from France including patients with cancer under active treatment and vaccinated with two doses of mRNA vaccines, a third‐dose booster created antibody responses in 75% (27/36) of the seronegative patients after two‐dose vaccination. 33 Recently, Clemens et al. reported the efficacy of booster vaccination in adults who received two doses of the CoronaVac vaccine in Brazil. In this study, booster vaccination with a different type of vaccine (an mRNA vaccine or a recombinant adenoviral‐vectored vaccine) resulted in substantially higher anti‐Spike IgG and neutralizing antibody responses compared with homologous booster vaccination with CoronaVac. 17 These studies support using third‐dose boosters for the general population; however, specific data on heterologous boosters for patients with cancer was lacking. Our study showed a significant increase in antibody titers and a nearly 2‐fold increase in seroconversion rate from 46.5 to 88.5% after heterologous third‐dose booster with Bnt162b2 vaccine. Given the correlation between antibody levels and vaccine efficacy, such a robust enhancement in antibody titers may imply stronger protection against COVID‐19 in cancer patients with this vaccination strategy, which needs to be verified in clinical studies. In a recent population‐level study from Israel, the effectiveness of two doses of the mRNA BNT162b2 vaccine was evaluated. In this study, patients with hematological malignancies still had an increased risk of severe COVID‐19 (OR 2.27) and COVID‐19 related death (OR 1.66) compared with healthy controls. 34 Booster doses with either homologous or heterologous vaccines may augment the protection level against the virus in this high‐risk population.
Limitations of our study include a limited sample size precluding additional analyses according to tumor types and treatment lines. Second, baseline COVID‐19 antibody testing was unavailable for the patients and controls. Therefore, some patients may have baseline antibody positivity because of previous asymptomatic infection, resulting in an overestimation of seroconversion rates. On the contrary, the interval between vaccination and antibody testing was not uniform, extending up to 110 days after the second or the third dose. This may have affected antibody titers, which is known to wane over time. Lastly, the control group had a higher frequency of comorbidities, although this difference would have driven the results toward the null hypothesis rather than a false positive result.
In conclusion, our study provides important data regarding the lower efficacy of CoronaVac in patients with cancer compared with controls. Older age and male gender are other risk factors that may further compromise vaccine immunogenicity. Additionally, we demonstrated significantly increased seroconversion rates with a third‐dose booster mRNA vaccine after two doses of CoronaVac in patients with cancer for the first time in literature. Further research is needed to define the optimal vaccination schedule in patients with cancer.
AUTHOR CONTRIBUTIONS
The work reported in the paper has been performed by the authors, unless clearly specified in the text. CRediT author statement is listed below. Deniz Can Guven: Conceptualization, Data Curation, Methodology, Writing Original Draft. Fatma Gul Gulbahce Incesu: Investigation, Data Curation. Hasan Cagri Yildirim: Investigation, Data Curation. Enes Erul: Investigation, Data Curation. Elvin Chalabiyev: Investigation, Data Curation. Burak Yasin Aktas: Investigation, Data Curation. Deniz Yuce: Data Curation, Methodology, Visualization. Zafer Arik: Data Curation, Methodology, Writing Original Draft. Saadettin Kilickap: Data Curation, Methodology, Writing Original Draft. Sercan Aksoy: Data Curation, Methodology, Writing Original Draft. Mustafa Erman: Data Curation, Methodology, Writing Original Draft. Kadir Mutlu Hayran: Data Curation, Methodology, Visualization. Serhat Unal: Data Curation, Methodology, Supervision. Alpaslan Alp: Data Curation, Methodology, Writing‐Review&Editing, Supervision, Omer Dizdar: Conceptualization, Writing‐Review&Editing, Supervision, Project Administration.
FUNDING INFORMATION
This study was funded by Hacettepe University Scientific Research Projects Coordination Unit. Project number: THD‐2021‐19 639.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ETHICS STATEMENT
This study was performed after the approval of the Ethics Committee of Hacettepe University and with specific permission from the Turkish Ministry of Health.
Guven DC, Incesu FGG, Yildirim HC, et al. Immunogenicity of two doses of inactive COVID‐19 vaccine and third booster dose mRNA vaccine in patients with cancer receiving active systemic therapy. Int J Cancer. 2022;1‐7. doi: 10.1002/ijc.34280
Funding information Hacettepe University Scientific Research Projects Coordination Unit, Grant/Award Numbers: THD‐2021‐19639, THD‐2021‐19 639
DATA AVAILABILITY STATEMENT
The study data is available upon reasonable request to the authors.
REFERENCES
- 1. Hopkins J. 2020. COVID‐19 map‐Johns Hopkins coronavirus resource center. Johns Hopkins Coronavirus Resource Center 2020;1.
- 2. Roser M, Ritchie H, Ortiz‐Ospina E, Hasell J 2020. Coronavirus disease (COVID‐19)–statistics and research. Our World in Data 2020;4.
- 3. Gold MS, Sehayek D, Gabrielli S, Zhang X, McCusker C, Ben‐Shoshan M. COVID‐19 and comorbidities: a systematic review and meta‐analysis. Postgrad Med. 2020;132:749‐755. [DOI] [PubMed] [Google Scholar]
- 4. Dessie ZG, Zewotir T. Mortality‐related risk factors of COVID‐19: a systematic review and meta‐analysis of 42 studies and 423,117 patients. BMC Infect Dis. 2021;21:855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lee LYW, Cazier JB, Starkey T, et al. COVID‐19 prevalence and mortality in patients with cancer and the effect of primary tumour subtype and patient demographics: a prospective cohort study. Lancet Oncol. 2020;21:1309‐1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Guven DC, Sahin TK, Yildirim HC, et al. Newly diagnosed cancer and the COVID‐19 pandemic: tumour stage migration and higher early mortality. BMJ Support Palliat Care. 2021;bmjspcare‐2021‐003301. [DOI] [PubMed] [Google Scholar]
- 7. Organization WH . COVID‐19 vaccine tracker and landscape, 2022.
- 8. Tanriover MD, Doganay HL, Akova M, et al. Efficacy and safety of an inactivated whole‐virion SARS‐CoV‐2 vaccine (CoronaVac): interim results of a double‐blind, randomised, placebo‐controlled, phase 3 trial in Turkey. Lancet. 2021;398:213‐222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Herishanu Y, Avivi I, Aharon A, et al. Efficacy of the BNT162b2 mRNA COVID‐19 vaccine in patients with chronic lymphocytic leukemia. Blood. 2021;137:3165‐3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Massarweh A, Eliakim‐Raz N, Stemmer A, et al. Evaluation of Seropositivity following BNT162b2 messenger RNA vaccination for SARS‐CoV‐2 in patients undergoing treatment for cancer. JAMA Oncol. 2021;7:1133‐1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Addeo A, Shah PK, Bordry N, et al. Immunogenicity of SARS‐CoV‐2 messenger RNA vaccines in patients with cancer. Cancer Cell. 2021;39:1091‐1098. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gagelmann N, Passamonti F, Wolschke C, et al. Antibody response after vaccination against SARS‐CoV‐2 in adults with hematological malignancies: a systematic review and meta‐analysis. Haematologica. 2021;107:1840‐1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Karacin C, Eren T, Zeynelgil E, et al. Immunogenicity and safety of the CoronaVac vaccine in patients with cancer receiving active systemic therapy. Future Oncol. 2021;17:4447‐4456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yasin AI, Aydin SG, Sümbül B, et al. Efficacy and safety profile of COVID‐19 vaccine in cancer patients: a prospective, multicenter cohort study. Future Oncol. 2022;18:1235‐1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Li J, Hou L, Guo X, et al. Heterologous prime‐boost immunization with CoronaVac and Convidecia. medRxiv. 2021;1‐37. doi: 10.1101/2021.09.03.21263062 [DOI] [Google Scholar]
- 16. Atmar RL, Lyke KE, Deming ME, et al. Homologous and heterologous Covid‐19 booster vaccinations. N Engl J Med. 2022;386:1046‐1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Clemens SAC, Weckx L, Clemens R, et al. Heterologous versus homologous COVID‐19 booster vaccination in previous recipients of two doses of CoronaVac COVID‐19 vaccine in Brazil (RHH‐001): a phase 4, non‐inferiority, single blind, randomised study. Lancet. 2022;399:521‐529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Johnston KM, Lakzadeh P, Donato BMK, Szabo SM. Methods of sample size calculation in descriptive retrospective burden of illness studies. BMC Med Res Methodol. 2019;19:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Coronahunter Anti‐SARS‐CoV‐2 Spike Receptor Binding Domain ELISA, 2022.
- 20. Garassino MC, Whisenant JG, Huang LC, et al. COVID‐19 in patients with thoracic malignancies (TERAVOLT): first results of an international, registry‐based, cohort study. Lancet Oncol. 2020;21:914‐922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lai AG, Pasea L, Banerjee A, et al. Estimated impact of the COVID‐19 pandemic on cancer services and excess 1‐year mortality in people with cancer and multimorbidity: near real‐time data on cancer care, cancer deaths and a population‐based cohort study. BMJ Open. 2020;10:e043828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ozdemir N, Dizdar O, Yazici O, et al. Clinical features and outcomes of COVID‐19 in patients with solid tumors: Turkish National Registry Data. Int J Cancer. 2020. [DOI] [PubMed] [Google Scholar]
- 23. Tang K, Wei Z, Wu X. Impaired serological response to COVID‐19 vaccination following anticancer therapy: a systematic review and meta‐analysis. J Med Virol. 2022;94:4860‐4868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pleyer C, Ali MA, Cohen JI, et al. Effect of Bruton tyrosine kinase inhibitor on efficacy of adjuvanted recombinant hepatitis B and zoster vaccines. Blood. 2021;137:185‐189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shehata MA, Karim NA. Influenza vaccination in cancer patients undergoing systemic therapy. Clin Med Insights Oncol. 2014;8:57‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Guven DC, Sahin TK, Kilickap S, Uckun FM. Antibody responses to COVID‐19 vaccination in cancer: a systematic review. Front Oncol. 2021;11:759108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yekeduz E, Utkan G, Urun Y. A systematic review and meta‐analysis: the effect of active cancer treatment on severity of COVID‐19. Eur J Cancer. 2020;141:92‐104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Vivarelli S, Falzone L, Grillo CM, Scandurra G, Torino F, Libra M. Cancer management during COVID‐19 pandemic: is immune checkpoint inhibitors‐based immunotherapy harmful or beneficial? Cancers (Basel). 2020;12:2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Oosting SF, van der Veldt AAM, GeurtsvanKessel CH, et al. mRNA‐1273 COVID‐19 vaccination in patients receiving chemotherapy, immunotherapy, or chemoimmunotherapy for solid tumours: a prospective, multicentre, non‐inferiority trial. Lancet Oncol. 2021;22:1681‐1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. In brief: third dose of mRNA‐based COVID‐19 vaccines for immunocompromised persons. Med Lett Drugs Ther. 2021;63:145‐146. [PubMed] [Google Scholar]
- 31. Barda N, Dagan N, Cohen C, et al. Effectiveness of a third dose of the BNT162b2 mRNA COVID‐19 vaccine for preventing severe outcomes in Israel: an observational study. Lancet. 2021;398:2093‐2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bar‐On YM, Goldberg Y, Mandel M, et al. Protection of BNT162b2 vaccine booster against Covid‐19 in Israel. N Engl J Med. 2021;385:1393‐1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fenioux C, Teixeira L, Fourati S, et al. SARS‐CoV‐2 antibody response to 2 or 3 doses of the BNT162b2 vaccine in patients treated with anticancer agents. JAMA Oncol. 2022;8:612‐617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mittelman M, Magen O, Barda N, et al. Effectiveness of the BNT162b2mRNA Covid‐19 vaccine in patients with hematological neoplasms. Blood. 2022;139(10):1439‐1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The study data is available upon reasonable request to the authors.


