Abstract
SARS‐CoV‐2 infection causes syncytial pneumocyte in patients and this has been considered as a defining feature of severe COVID‐19 cases. Traditional methods of syncytia quantification require the morphology characterization of fused cells either with light microscope or fluorescent microscope, which is time‐consuming and not accurate. Here we developed a rapid and sensitive coculture system measuring spike‐induced syncytia by using NanoLuc complementation system. We found the formation of syncytia occurred rapidly after ACE2‐expressing cells exposure to spike protein. In addition, we found furin cleavage as well as the cell surface protease TMPRSS2 enhanced syncytia formation. Finally, we showed that this coculture system can be used to test the ability of different compound to inhibit syncytia formation, thus providing a useful tool to screen anti‐syncytial drugs.
Keywords: luciferase assay, SARS‐CoV‐2, spike, syncytia
1. INTRODUCTION
Syncytia formation is a common cytopathic effect caused by virus infection, which is thought to facilitate viral dissemination and evasion of host immune responses. 1 SARS‐CoV‐2 infection also causes multinucleated lung epithelial syncytia, which is a commonly observed pathological phenomenon of severe COVID‐19 cases. 2 , 3 Patients died as a direct consequence of SARS‐CoV‐2 infection had higher rate of syncytia formation than those who died of other causes. 2 The molecular mechanism underlying the induction of pathogen‐induced syncytia is not fully understood. During SARS‐CoV‐2 infection, spike protein expressed on the surface of infected cells is believed to interact and fuse with ACE2‐expressing neighboring cells, leading to the formation of syncytia.
Syncytia is usually assessed by visualization of multinucleated cells with light microscopy, which does not allow accurate quantification and could introduce false positive results due to cell clustering. To address these issues, dual‐color fluorescent protein labeling or split GFP complementation system 2 , 3 , 4 , 5 has been employed for syncytia assays. For example, Buchrieser et al. reported to use split GFP system to measure SARS‐CoV‐2 induced syncytia. 4 While this method gives better visualized cell morphology changes, it takes long time before syncytia could be measured (after 6 h). We also tried to use this method to measure spike‐induced syncytia and found this assay time‐consuming and was not very sensitive (Supporting Information: Figure S1A). More importantly, the quantification of fluorescent syncytia requires high‐content microscopy, which limited its use in high‐throughput screening. Here taking advantage of the split NanoLuc system, we developed a more rapid and quantitative luminescent assay to measure spike‐induced syncytia and employed this system to evaluate the role of host factors and small molecular weight chemicals in the formation of syncytia.
2. MATERIALS AND METHODS
2.1. Syncytia coculture system and luminescence measurement
For coculture optimization, 293T cells were first seeded in 12‐well plate and cells were transfected with HiBiT‐, LgBiT‐, spike‐ or ACE2‐expressing plasmid 24 h later (0.4 μg each except for Figure 1G,H). Cells were cultured for 16 h before they were split and cocultured at 1:1 ratio for indicated times before luminescence measurement. For other assays, 293T‐ACE2/LgBiT and 293T‐HiBiT stable cell lines were used and 293T‐HiBiT was first transfected to express spike or spike mutant. Cells were then cocultured at 1:1 ratio and incubated for indicated times before syncytia or cell viability testing. For TMPRSS2 related assays, TMPRSS2‐expressing plasmid was transfected into 293T‐ACE2/LgBiT cells and cocultured as described above.
Figure 1.

Syncytia assay system based on split NanoLuc. (A) Diagram of 293T coculture system for syncytia detection. Fused cells of 293T‐ACE2/LgBiT and 293T‐spike/HiBiT lead to complementation of NanoLuc, which can be detected with Nano‐Glo live cell reagent. (B) Optimization of protein combination for efficient syncytia detection. 293T cells transfected to express the indicated proteins were cocultured and luminescence was measured 12 h later. (C) Luminescences of coculture cells expressing ACE2/LgBiT and spike/HiBiT (S + ACE2), ACE2/LgBiT and HiBiT (No spike) or LgBiT and spike/HiBiT(No ACE2) were determined at indicated time points. (D) Real‐time measurement of luminescences of coculture cells as described in (C) with extended Nano‐Glo substrate (Endurazine). Inset, enlargement of the first 2 h. (E) Represented images of syncytia formed at 8 h (upper panel) or 24 h (lower panel) of coculture cells as described in (C). Bar, 20 μm. (F) Cell viability of cocultured cells as described above were measured at the indicated time points with Cell‐Titer Glo. (G) Cells transfected with increasing amount of ACE2 and 0.4 μg of LgBiT plasmid were cocultured with fixed amount of spike/HiBiT cells and luminescences were measured at 8 h post‐coculture. ACE2 expression was immunoblotted. (H) Cells transfected with increasing amount of spike and 0.4 μg of HiBiT plasmid were cocultured with fixed amount of ACE2/LgBiT cells and luminescences were measured at 8 h post‐coculture. Spike expression was immunoblotted.
To test complemented NanoLuc luciferase, cells were first washed once with PBS and then treated with Nano‐Glo live cell reagent according to manufacturer's instruction (Promega Corporation). For real‐time luminescence monitoring, cells were first transfected and cocultured as described above. Cocultured cells were then plated in DMEM buffered with HEPES to maintain pH. Cells were incubated at 37°C for 30 min for cell adherence before luminescence was measured every 15 min for 10 h with the lid on using a BioTek Neo 2 microplate reader at 37°C with settings to prevent lid condensation.
2.2. Syncytia inhibition assay
Cells were first transfected and cocultured as described above. Indicated drugs or inhibitors were administrated when cells were mixed for coculture. Cells were incubated at 37°C for 8 h before luciferase was measured as described above.
3. RESULTS AND DISCUSSION
Similar to split GFP, NanoLuc can be split into a large subunit(LgBiT) and a small subunit (HiBiT). 6 Luminescence is produced when these two subunits are complemented. We established coculture system where spike‐ACE2 interaction led to cell fusion and NanoLuc complementation. Luciferase can then be detected by the Live‐Cell NanoGlo substrate without cell lysis, and thus avoiding any nonspecific signals from unfused cells (Figure 1A). We first optimized the combination of protein expression in 293T coculture system and found that cells expressing ACE2/LgBiT and spike/HiBiT fusion generated best luminescence signal (Figure 1B, Supporting Information: Figure S1B). Luciferase can only be detected when both ACE2 and spike were expressed (Figure 1C, Supporting Information: Figure S1C), suggesting the formation of syncytia is mediated by spike interacting with receptor ACE2. More importantly, we found that syncytia luciferase is significantly increased at 4 h post‐coculture and continued to increase during 24 h of testing. To more accurately monitor the syncytia formation kinetics, we used the extended NanoLuc live cell substrate (Endurazine). Differences in luciferase signal were detected at the first 15‐min time point post‐coculture and continued to expand for the duration of the testing (Figure 1D). In contrast, complemented GFP signals did not increase significantly before 8 h post‐coculture (Supporting Information: Figure S1D), suggesting the split NanoLuc system assay is very rapid and sensitive. Similarly, syncytia can hardly be visualized under light microscope before 4 h of coculture and by 8 h they begin to be visualized (Figure 1E, upper panel). By 24 h post‐coculture, even bigger syncytia were visualized with cytoplasmic vacuolization in fused cells (Figure 1E, lower panel), suggesting cytotoxicity began to appear. Consistent with this observation, we found syncytia cell viability was significantly lower than that of normal cells (Figure 1F). Previous studies have also shown that other viral‐induced syncytia lead to apoptosis or cell death. 7 , 8
We next tested how the expression level of ACE2/spike protein affects syncytia formation. We found syncytia formation correlated with ACE2 expression (Figure 1G). In contrast, moderate expression of spike was required for efficient syncytia formation and further increase in spike expression did not lead to more syncytia (Figure 1H), suggesting minimum spike is sufficient to induce syncytia during infection.
Spike proteins from different variants have been reported to have different fusogenicity, among which, spike protein of Delta variant is more syncytiogenic. 1 , 5 , 9 We then tested syncytia formation of different spike sequences with our system. Mutation in the furin cleavage site (fur/mut) of spike compromised syncytia formation compared to the ancestral Wuhan spike (WH), while spike bearing the unique set of Delta mutations (Delta), which showed enhanced spike cleavage, induced highest syncytia (Figure 2A,B, upper panel, Supporting Information: Figure S2A). These results indicated that spike cleavage between S1 and S2 could enhance syncytia formation. To test the role of furin in syncytia formation, we treated the coculture system with furin inhibitor, naphthofluorescein, which efficiently blocked spike protein cleavage (Figure 2B, lower panel), and found that it also inhibited syncytia in a dose‐dependent manner without showing cytotoxicity (Figure 2C).
Figure 2.
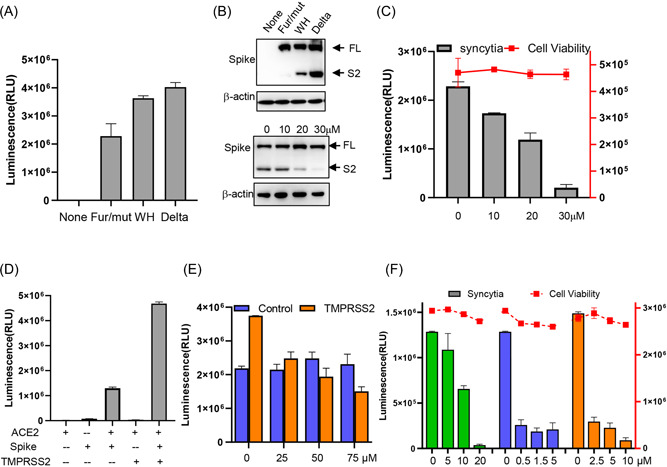
Viral and host factors affecting syncytia formation. (A) 293T‐HiBiT cells transfected with the indicated spike variant were cocultured with 293T‐ACE2/LgBiT cells and luminescences were determined 8 h later. Fur/mut, furin mutation spike; WH, spike form Wuhan strain; Delta, spike bearing delta mutations. (B) upper panel, cells were transfected as described in (A) and spike expression was immunoblotted. lower panel, coculture cells treated with indicated amount of naphthofluorescein were immunoblotted for spike cleavage. (C) Coculture cells were treated with indicated amount of naphthofluorescein and luminescences were determined 8 h later. (D) Coculture cells with or without TMPRSS2 overexpression were assayed for syncytia. (E) Coculture cells with or without TMPRSS2 overexpression were treated with indicated amount of camostat and luminescences were determined 8 h later. (F) Coculture cells were treated with indicated amount of Nelfinavir(green), Niclosamide(blue), or Salinomycin(orange) and luminescences were determined 8 h later. Cell viability was shown as curves.
Cell surface serine protease TMPRSS2, which primes spike for efficient viral entry, has been reported to increase syncytia formation. 4 Here we also found that overexpressing TMPRSS2 enhanced syncytia (Figure 2D). When TMPRSS2 inhibitor camostat was administrated to the coculture system, we found it did not inhibit syncytia (Figure 2E, blue, Supporting Information: Figure S2B, upper panel), and this result was in agreement with a previous report. 10 However, camostat did inhibit syncytia when the ACE2/LgBiT cells were overexpressed with TMPRSS2 (Figure 2E, orange, Supporting Information: Figure S2B, lower panel, Supporting Information: Figure S2C,D). These results suggested that camostat only targets TMPRSS2‐high cells, and explained why 293T and Vero E6 cell syncytia could not be inhibited.
We next tested our system with several known syncytia inhibitors. Anti‐HIV drug Nelfinavir mesylate, 11 antihelminthic drug niclosamide, and antibacterial drug salinomycin 3 have been reported to inhibit spike‐induced syncytia formation. When these drugs were administrated to our coculture system, all three drugs exhibited potent inhibition against syncytia while showing little cytotoxicity (Figure 2F), suggesting this model is suitable to test or screen drugs with anti‐syncytia activity.
Here in this study, we established a syncytia assay based on split NanoLuc luciferase. Compared to the commonly used split GFP system, this system is more rapid and sensitive. In addition, when this coculture system was recapitulated with A549 or Huh 7 cells, these cells also produced luminescence when both ACE2 and spike present (Supporting Information: Figure S3A,B), suggesting this in vitro syncytia assay is not cell line specific and thus could be adapted to test syncytia from different tissue origins. With this system, we showed that minimal spike is needed for the formation of syncytia, this may explain why syncytia is a common observed feature in SARS‐CoV‐2 infected tissue. We also showed that protease TMPRSS2 enhanced syncytia formation and furin cleavage was important for spike‐induced syncytia formation. Finally, we used our system for several known syncytia inhibitors and showed this system is very rapid and simple to use. Since luciferase has been proved advantageous in screenings, our system could be easily scaled down to 384‐ or 1536‐well format, and thus very useful for high‐throughput screening of anti‐syncytia inhibitors.
AUTHOR CONTRIBUTIONS
Conceived and planned the overall structure of the article; performed the experiments; wrote and edited the manuscript: Hongliang Wang. Development of methodology and interpretation of data; prepared the figures: Shangrui Guo and Hang Yang. All authors contributed to the article and approved the submitted version.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Supporting information
Additional supplementary information can be found online in the Supporting information section at the end of this article.
ACKNOWLEDGMENTS
This study was supported by the National Natural Science Foundation of China (81871662, 82150201) and Fundamental Research Funds for the Central Universities (xzy012019066 and xzy032020037).
Wang H, Guo S, Yang H. Rapid quantitative monitoring of SARS‐CoV‐2 spike protein mediated syncytia formation using split NanoLuc. J Med Virol. 2022;1‐5. 10.1002/jmv.28053
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Rajah MM, Bernier A, Buchrieser J, Schwartz O. The mechanism and consequences of SARS‐CoV‐2 spike‐mediated fusion and syncytia formation. J Mol Biol. 2021;434:167280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sanders DW, Jumper CC, Ackerman PJ, et al. SARS‐CoV‐2 requires cholesterol for viral entry and pathological syncytia formation. eLife. 2021;10:e65962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Braga L, Ali H, Secco I, et al. Drugs that inhibit TMEM16 proteins block SARS‐CoV‐2 spike‐induced syncytia. Nature. 2021;594(7861):88‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Buchrieser J, Dufloo J, Hubert M, et al. Syncytia formation by SARS‐CoV‐2‐infected cells. EMBO J. 2021;39(23):e106267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rajah MM, Hubert M, Bishop E, et al. SARS‐CoV‐2 Alpha, Beta, and Delta variants display enhanced Spike‐mediated syncytia formation. EMBO J. 2021;40(24):e108944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dixon AS, Schwinn MK, Hall MP, et al. NanoLuc complementation reporter optimized for accurate measurement of protein interactions in cells. ACS Chem Biol. 2016;11(2):400‐408. [DOI] [PubMed] [Google Scholar]
- 7. Ferri KF, Jacotot E, Geuskens M, Kroemer G. Apoptosis and karyogamy in syncytia induced by the HIV‐1‐envelope glycoprotein complex. Cell Death Differ. 2000;7(11):1137‐1139. [DOI] [PubMed] [Google Scholar]
- 8. Higuchi H, Bronk SF, Bateman A, Harrington K, Vile RG, Gores GJ. Viral fusogenic membrane glycoprotein expression causes syncytia formation with bioenergetic cell death: implications for gene therapy. Cancer Res. 2000;60(22):6396‐6402. [PubMed] [Google Scholar]
- 9. Yang H, Yuan H, Zhao X, et al. Cytoplasmic domain and enzymatic activity of ACE2 are not required for PI4KB dependent endocytosis entry of SARS‐CoV‐2 into host cells. Virol Sin. 2022;37:380‐389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cheng YW, Chao TL, Li CL, et al. Furin inhibitors block SARS‐CoV‐2 spike protein cleavage to suppress virus production and cytopathic effects. Cell Rep. 2020;33(2):108254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Musarrat F, Chouljenko V, Dahal A, et al. The anti‐HIV drug nelfinavir mesylate (Viracept) is a potent inhibitor of cell fusion caused by the SARS‐CoV‐2 spike (S) glycoprotein warranting further evaluation as an antiviral against COVID‐19 infections. J Med Virol. 2020;92(10):2087‐2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional supplementary information can be found online in the Supporting information section at the end of this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


