Abstract
Emerging variants of SARS‐CoV‐2 and potential novel epidemic coronaviruses underline the importance of investigating various viral proteins as potential drug targets. The papain‐like protease of coronaviruses has been less explored than other viral proteins; however, its substantive role in viral replication and impact on the host immune response make it a suitable target to study. This review article focuses on the structure and function of the papain‐like protease (PLpro) of SARS‐CoV‐2, including variants of concern, and compares it to those of other coronaviruses, such as SARS‐CoV‐1 and MERS‐CoV. The protease's recognition motif is mirrored in ubiquitin and ISG15, which are involved in the antiviral immune response. Inhibitors, including GRL0617 derivatives, and their prospects as potential future antiviral agents are also discussed.
Keywords: antivirals, COVID-19, immune response, posttranslational modifications, protease inhibitors
Viral mutations highlight the importance of exploring a broad range of antiviral drug targets. The involvement of the SARS‐CoV‐2 papain‐like protease in the viral replication cycle and the host immune response offers a unique possibility for drug development. In our review, we focus on the structure and function of PLpro and discuss prospects of current inhibitors.
1. Introduction
The discovery of SARS‐CoV‐1 in 2003 induced a paradigm‐shift in the assessment of coronaviruses, as it related a human coronavirus to severe illness for the first time. [1] Since the emergence of the closely related SARS‐CoV‐2 and COVID‐19 in late 2019, [2] extraordinary efforts in pharmaceutical research have led to the rapid development of preventive vaccines (mRNA, vector, protein), [3] neutralizing monoclonal antibodies (mAbs), [4] and small molecule drugs. [5] Yet the advent of SARS‐CoV‐2 as the third severe coronavirus outbreak during the 21st century is a warning that future zoonotic transmissions of emerging coronaviruses are possible. [6]
SARS‐CoV‐1, MERS‐CoV, and SARS‐CoV‐2 are part of the Betacoronavirus genus, [7] and feature a large positive‐sense, single‐stranded RNA genome of circa 30 kb, which encodes for accessory factors, structural proteins, and non‐structural proteins. [8] The open reading frame ORF1ab spans more than two thirds of the total genome and encodes for two polyproteins (pp1a, pp1ab)[ 7 , 9 ] containing several non‐structural proteins, many of which are essential for viral replication (Figure 1a). [10] Proteolytical processing to liberate the individual non‐structural proteins is realized by the main protease (Mpro, 3CLpro, nsp5) and the papain‐like protease (PLpro, part of nsp3; Figure 1b).[ 7 , 9 , 11 ]
Figure 1.
Genome and polyprotein organization of SARS‐CoV‐2. (a) SARS‐CoV‐2 RNA genome with representations of the genes of ORF1a (yellow, orange), ORF1b (orange, red), and the spike (teal). (b) SARS‐CoV‐2 ORF1ab polyproteins pp1a and pp1ab. The arrows indicate a cleavage site recognized by either PLpro (green) or Mpro (brown). Individual sections of the polyproteins are not to scale.
Given that viral protease inhibitors are approved drugs against HIV and HCV, [12] both viral proteases of SARS‐CoV‐2 are prime drug targets. While the Mpro inhibitor nirmatrelvir is already approved and broadly used, [13] PLpro attracted considerably less attention. However, the release of nsp1–nsp4 is likewise important for the viral replication.[ 7 , 14 ] Inhibition of the proteolytic activity of PLpro may therefore stop the viral replication cycle.[ 7 , 14 ] Additional functions of PLpro were identified, such as interactions with the host immune system, e. g., via deubiquitination and de‐ISGylation. [15] In comparison to host deubiquitinases (DUBs), PLpro has a slightly different substrate scope, potentially permitting the design of specific antivirals. [16]
2. Structure and Function
2.1. SARS‐CoV‐2 PLpro is structurally conserved
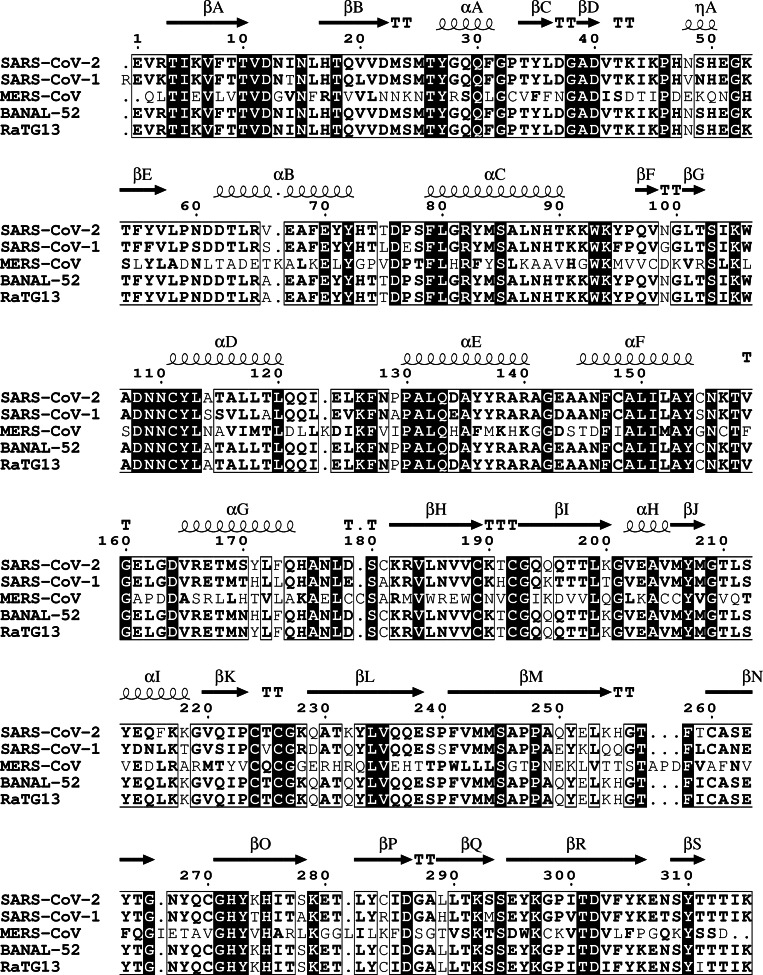
PLpro is one of the eight domains of the non‐structural protein 3 (nsp3),[ 10b , 14 ] which is the largest of the SARS‐CoV‐2 non‐structural proteins (212 kDa). [17] The functions of the nsp3 domains within the viral replication cycle are diverse [18] and have been reviewed for SARS‐CoV‐1. [14] PLpro is located on residues 746–1060 of nsp3 (1564–1878 of pp1ab[ 11b , 16 ]; SARS‐CoV‐2 reference sequence WIV04) [19] and consists of 315 amino acids (36 kDa).[ 11b , 16 ] PLpro is highly conserved among coronaviruses. The amino acid sequence of SARS‐CoV‐2 PLpro is 98 % identical to the related proteases from bat coronaviruses BANAL‐52 and RaTG13, [20] and 83 % identical to the related human coronavirus SARS‐CoV‐1. [21] The active sites of SARS‐CoV‐1 and SARS‐CoV‐2 PLpro are almost identical. [22] In contrast, the amino acid sequence of SARS‐CoV‐2 PLpro is only 29 % identical and only 51 % similar to MERS‐CoV PLpro (Figure 2). [23] However, superimposition of X‐ray crystal structures of PLpro from SARS‐CoV‐2, SARS‐CoV‐1 and MERS‐CoV demonstrate their structural similarity and high conservation (RMSD≤1.11 Å; Figure 3a). [24] Active‐site inhibitors designed for SARS‐CoV‐1 and SARS‐CoV‐2 PLpro appear to display lower activity against MERS‐CoV PLpro, potentially challenging the discovery of pan‐coronaviral PLpro inhibitors. [25]
Figure 2.
Alignment of the amino acid sequences of PLpro. Sequences originate from SARS‐CoV‐2 (WIV04) [19] and related coronaviruses (% identity, % similarity): SARS‐CoV‐1 (83 %, 88 %), [21] MERS‐CoV (29 %, 51 %), [23] BANAL‐52 (98 %, 98 %), [20a] RaTG13 (98 %, 98 %). [20b] Secondary structural elements of crystallized SARS‐CoV‐2 PLpro are indicated (PDB: 6WZU). [24a] The alignment was generated with Clustal Omega, [26] calculations were performed in Sequence Manipulation Suite 2, [27] and the illustration created in ESPript 3. [28]
Figure 3.
X‐ray crystal structure representations of PLpro. Zinc ions are indicated in the Fingers domain as gray spheres. (a) Superimposition of PLpro of SARS‐CoV‐2 (PDB: 6WZU), [24a] SARS‐CoV‐1 (RMSD: 0.95 Å; PDB: 2FE8), [24b] and MERS‐CoV (RMSD: 1.11 Å; PDB: 4RNA). [24c] (b) Domains of SARS‐CoV‐2 PLpro: Ub‐like (1‐61), thumb (62‐178), fingers (179‐240), palm (241‐315). (c) Location of the catalytic triad of SARS‐CoV‐2 PLpro: Cys111 (yellow), His272 (blue), Asp286 (red). (d) Location of mutations of PLpro in SARS‐CoV‐2 variants of concern (VOC): Lys92Asn, Beta (blue), Ala145Asp, Alpha (red), Lys232Gln, Gamma (yellow). The graphics were generated with UCSF Chimera. [31]
SARS‐CoV‐2 PLpro is a cysteine protease consisting of four domains, three of which form the catalytically relevant right‐handed thumb‐palm‐fingers architecture (Figure 3b). The catalytic triad is located between the thumb (62–178) and palm (241–315) domains, and consists of Cys111, His272, and Asp286 (Figure 3c). The fingers domain (179–240) contains a zinc binding site made up of four cysteine residues (Cys189, Cys192, Cys224, Cys226). Mutagenesis studies of these cysteine residues in SARS‐CoV‐1 PLpro demonstrated that coordination of zinc is required for catalytic activity. [29] The fourth domain of SARS‐CoV‐2 PLpro is a ubiquitin‐like (Ub‐like) domain (1–61); however, its specific function is not yet fully understood (Figure 3b).[ 22 , 30 ]
The general architecture of PLpro resembles[ 14 , 16 ] that of human deubiquitinating enzymes (DUB) (e. g., ubiquitin‐specific proteases; USP). [32] However, their sequences and structures appear to be dissimilar enough to allow for the development of specific PLpro inhibitors, despite concerns about potential off‐target effects of PLpro inhibitors.[ 25 , 33 ] It is good practice to counter‐screen PLpro inhibitors against a set of human DUBs, and in case of covalent inhibitors additionally against other human cysteine proteases. [25] A factor associated with selective binding to PLpro over DUBs is the highly mobile β‐loop (BL2, 266–271),[ 14 , 24a , 25 , 33 ] which is identical in sequence for SARS‐CoV‐1 and SARS‐CoV‐2 (Figure 2).
Since SARS‐CoV‐2 continues to evolve, several variants of the virus have been identified, with some classified by health authorities as variants of concern (VOC; Alpha, Beta, Gamma, Delta, Omicron). [34] These variants show pronounced genetic variability in comparison to the ancestral SARS‐CoV‐2 strain. [35] Mutations have accumulated particularly in the spike protein, [36] which governs host cell recognition and infectivity.[ 35b , 37 ] Other viral proteins are also affected by mutations of the genome, as exemplified by the presence of certain Mpro mutants in SARS‐CoV‐2 variants (e. g., Omicron Mpro P132H). [38] An analysis of the SARS‐CoV‐2 VOC genomes with the Outbreak.info database [39] revealed the presence of three abundant (>20 %) point mutations in PLpro. While the Delta and Omicron variants do not display a change in the amino acid sequence of PLpro, the Alpha (Ala145Asp, >95 %), Beta (Lys92Asn, >90 %) and Gamma (Lys232Gln, >95 %) VOCs contain single amino acid mutations in PLpro (Figure 3d). The Lys232Gln mutation present in SARS‐CoV‐2 Gamma is located in the fingers domain of PLpro and was associated with enhanced catalytic activity in vitro. [40] Notably, the mutations Tyr268Thr (as found in MERS‐CoV PLpro) and Tyr268Gly impact negatively on the binding of naphthalene‐based inhibitors such as GRL0617. [17]
2.2. SARS‐CoV‐2 PLpro processes the viral polyproteins
One function of PLpro is the liberation of the first non‐structural proteins (nsp1–nsp4), which are essential for viral replication (Figure 1b). [7] By binding to the human ribosome, nsp1 inhibits host cell translation, [41] and nsp2 is thought to disrupt the cell cycle progression and apoptosis. [42] Apart from PLpro, nsp3 contains seven other domains and is among other functions involved in facilitating the assembly of the replication and transcription complex (RTC),[ 10b , 14 , 42 ] as is nsp4. [42] Accordingly, SARS‐CoV‐2 PLpro is involved in its autocleavage, [43] equivalent to the second viral protease Mpro. [11a] It may be worth noting that nsp3 of certain coronaviruses contain an additional PLpro, as is the case for the human coronaviruses NL63, OC43, HKU1, and 229E. [44]
PLpro recognizes Leu‐Xaa‐Gly‐Gly↓Xaa substrate motifs (P4–P1′, Schechter‐Berger nomenclature) [45] in the viral polyprotein (pp1a, pp1ab). [46] The PLpro cleavage sites (P5–P5′) within the viral polyprotein are identical between SARS‐CoV‐2 and its closest known bat coronavirus relatives RaTG13 and BANAL‐52 (Table 1). Similarly, the PLpro cleavage sites (P5–P5′) of SARS‐CoV‐2, SARS‐CoV‐1 and MERS‐CoV polyproteins share a high degree of identity (Table 1, Figure 4). The requirement for Gly in P1 and P2 was confirmed by a combinatorial substrate library study of SARS‐CoV‐1 and SARS‐CoV‐2 PLpro. [46] The protease's recognition is less specific in P3 and P4, as demonstrated by substrates containing homotyrosine (hTyr; P3) and diaminopropionic acid (Dap; P4). [46]
Table 1.
PLpro cleavage sites of different coronaviruses.[a]
|
|
nsp1↓nsp2 |
nsp2↓nsp3 |
nsp3↓nsp4 |
|---|---|---|---|
|
SARS‐CoV‐2 |
ELNGG↓AYTRY |
TLKGG↓APTKV |
ALKGG↓KIVNN |
|
SARS‐CoV |
ELNGG↓AVTRY |
RLKGG↓APIKG |
SLKGG↓KIVST |
|
MERS‐CoV |
KLIGG↓DVTPV |
RLKGG↓APVKK |
KIVGG↓APTWF |
|
BANAL‐52 |
ELNGG↓AYTRY |
TLKGG↓APTKV |
ALKGG↓KIVNN |
|
RaTG13 |
ELNGG↓AYTRY |
TLKGG↓APTKV |
ALKGG↓KIVNN |
[a] Substrate recognition sequence is shown from P5–P5′ according to the Schechter‐Berger nomenclature [45] within the viral polyprotein (pp1a, pp1ab) between nsp1, nsp2, nsp3, and nsp4 of SARS‐CoV‐2, [19] SARS‐CoV‐1, [21] MERS‐CoV, [23] BANAL‐52, [20a] and RaTG13. [20b] The arrow (↓) indicates the position of proteolysis within the sequence. The consensus sequence over the three cleavage sites is illustrated in Figure 4.
Figure 4.
Viral polyprotein cleavage sites recognized by PLpro. Represented are the recognition motifs of SARS‐CoV‐2, [19] SARS‐CoV‐1, [21] and MERS‐CoV, [23] covering residues P5–P5′ (Schechter‐Berger nomenclature) [45] within pp1a and pp1ab. Note that the recognized polyprotein sequences of SARS‐CoV‐2, [19] BANAL‐52, [20a] and RaTG13 [20b] are identical from P5–P5′ (Table 1). The consensus sequence over the three cleavage sites (nsp1↓nsp2, nsp2↓nsp3, nsp3↓nsp4) was plotted using WebLogo 3. [47]
2.3. SARS‐CoV‐2 PLpro acts as deubiquitinase
SARS‐CoV‐2 PLpro participates in the virus's suppression of the host immune system. [11b] Its effect on specific immunological pathways was comprehensively reviewed by Mahmoudvand et al. [11b] Importantly, PLpro removes post‐translational ubiquitination and ISG‐ylation, [43] which are central protein modifications of the antiviral immune response. [48] In vitro studies showed that SARS‐CoV‐2 PLpro catalyzes the hydrolysis of fluorogenic Ub and ISG15 (interferon‐stimulated gene 15) substrates. [46] PLpro recognizes the C‐terminus of ubiquitin (Leu73‐Arg74‐Gly75‐Gly76). [46] Similarly, it was shown that the six C‐terminal residues of ISG15 (Leu152‐Arg153‐Leu154‐Arg155‐Gly156‐Gly157) are essential for its interaction with PLpro. [49] Evidently, the C‐terminal amino acid sequences of Ub and ISG15 are related to the protease's viral substrate recognition sequences (Table 1).[ 46 , 49b ]
2.4. SARS‐CoV‐2 PLpro interacts with ubiquitin‐like proteins
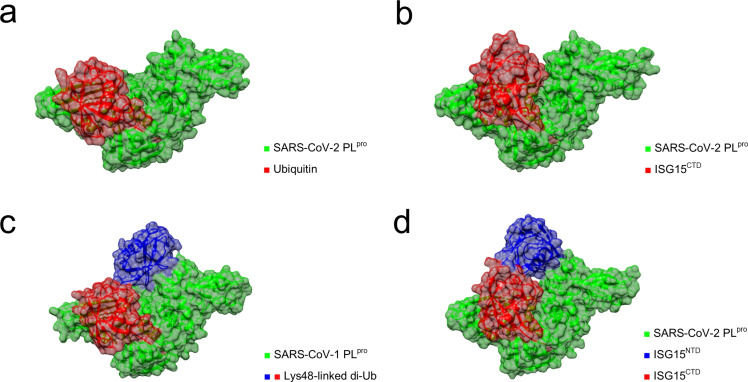
Ub and ISG15 interact with two binding sites (S1, S2) on the surface of SARS‐CoV‐2 PLpro (Figure 5).[ 16 , 50 ] Mono‐Ub binds to the S1 site of the enzyme (Figure 5a), while Lys48‐linked poly‐Ub interacts with both the S1 and S2 sites (Figure 5c). [16] While SARS‐CoV‐2 PLpro can bind mono‐Ub, poly‐Ub is preferentially processed. [16] Notably, Lys48‐linked poly‐Ub substrates are cleaved less efficiently by SARS‐CoV‐2 PLpro than by SARS‐CoV‐1 PLpro, which may be due to the cumulative effect of amino acid substitutions in the S2 binding site.[ 40 , 46 , 50 ] ISG15 consists of two Ub‐like domains linked by a short hinge region. [48c] When interacting with PLpro, ISG15 binds simultaneously to S1 and S2. The C‐terminal domain (ISG15CTD) binds to S1 while the N‐terminal domain (ISGNTD) binds to S2 (Figure 5b, Figure 5d). While ISG15 and Ub interact with PLpro at the same surfaces, there are specific dissimilarities in their binding geometry (see Figure 5a vs. Figure 5b), which were thoroughly reviewed by Olsen and co‐workers. [50] As most host DUBs preferentially bind mono‐Ub, the slightly different substrate scope of PLpro may be beneficial for the development of less cross‐reactive, specific inhibitors. [16]
Figure 5.
X‐ray crystal structures of PLpro in complex with Ub‐like proteins. (a) SARS‐CoV‐2 PLpro in complex with ubiquitin bound to the S1 binding site (PDB: 6XAA) [16] (b) SARS‐CoV‐2 PLpro in complex with ISG15CTD (PDB: 6XA9). [16] (c) SARS‐CoV‐1 PLpro in complex with a Lys48‐linked di‐ubiquitin probe bound to the S1 and S2 binding sites (PBD: 5E6J). [51] (d) SARS‐CoV‐2 PLpro in complex with ISG15 (PDB: 7RBS). [24a] The graphics were generated with UCSF Chimera. [31]
3. PLpro Inhibitors
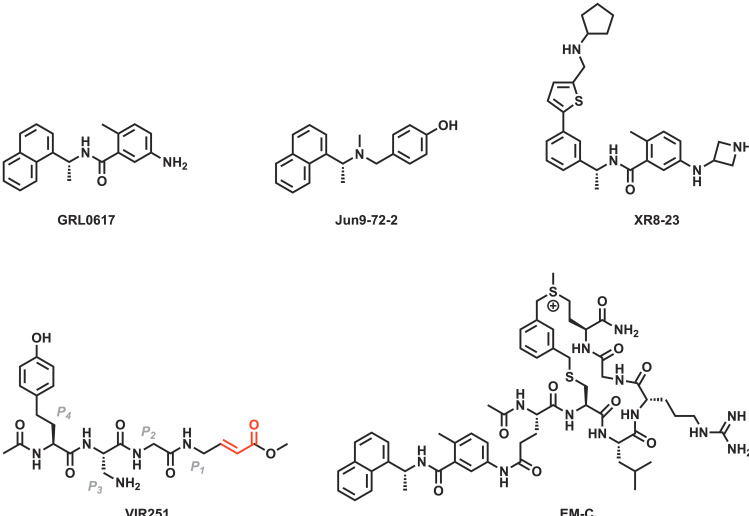
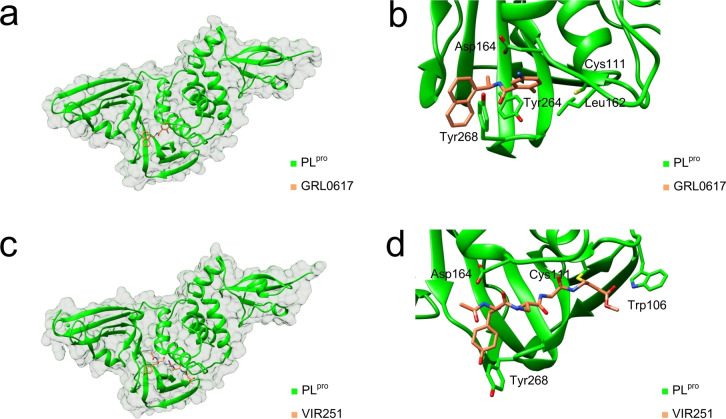
Several inhibitors of SARS‐CoV‐2 PLpro were originally identified as inhibitors of SARS‐CoV‐1 PLpro. Among those is GRL0617, which was discovered by Ratia et al., [52a] and has since become one of the most frequently studied inhibitors of SARS‐CoV‐2 PLpro (Figure 6).[ 16 , 24a , 49b , 52b , 53 ] This small molecule is a reversible competitive inhibitor of PLpro (Figure 7a, Figure 7b). [52a] Half‐maximal inhibitory concentrations (IC50) of GRL0617 against SARS‐CoV‐2 PLpro have been determined in various biochemical in vitro assays and range from 0.88 μM to 2.6 μM.[ 52b , 52c ] Evaluations of the compound in a cell culture‐based assay indicated micromolar half‐maximal effective concentrations (EC50=20 μM–33 μM).[ 52b , 53c ] GRL0617 is ineffective against MERS‐CoV PLpro due to a single amino acid substitution – an effect which could similarly be observed in recombinant SARS‐CoV‐2 PLpro mutants (Tyr268Thr, Tyr268Gly). [17]
Figure 6.
Chemical structures of selected inhibitors of PLpro.[ 33 , 46 , 52 ] The α,β‐unsaturated carbonyl of VIR251 is relevant for the compound's Michael reactivity with the catalytic cysteine (Cys111) and is highlighted in red. Binding subsites of VIR251 are indicated.
Figure 7.
X‐ray co‐crystal structures of inhibitor‐bound SARS‐CoV‐2 PLpro. (a) PLpro in complex with bound GRL0617 (PDB: 7JRN). [52b] (b) Close‐up view of GRL0617 (Figure 6) in the active site with key residues [49b] highlighted (PDB: 7JRN). [52b] (c) PLpro in complex with covalently‐bound VIR251 (PDB: 6WX4). [46] (d) Close‐up view of VIR251 (Figure 6) in the active site with key residues [40] highlighted (PDB: 6WX4). [46] The graphics were generated with UCSF Chimera. [31]
Two small molecules which resulted from the same screening campaign against SARS‐CoV‐1 conducted by Ratia et al. [52a] were also tested against SARS‐CoV‐2 PLpro and showed high micromolar inhibition (7724772, IC50=24 μM; 6577871, IC50=101 μM). [53a] Three further SARS‐CoV‐1 PLpro inhibitors were assessed against SARS‐CoV‐2 PLpro by Klemm et al. [16] The naphthalene‐containing small molecules rac5c, rac3j, and rac3k – originally developed by Mesecar and co‐workers [33] – inhibit SARS‐CoV‐2 PLpro as strongly as GRL0617 (rac5c, IC50=0.81 μM; rac3j, IC50=1.4 μM; rac3k, IC50=1.2 μM). [16]
The majority of reported SARS‐CoV‐2 PLpro inhibitors are repurposed small molecules identified from high‐throughput screenings. A study by Lim et al. identified five small molecules with sub‐micromolar in vitro activity against SARS‐CoV‐2 PLpro: GNF‐Pf‐3800 (IC50=0.26 μM), ryuvidine (IC50=0.39 μM), Ro 08–2750 (IC50=0.53 μM), dihydrotanshinone I (IC50=0.59 μM), and beta‐lapachone (IC50=0.61 μM). [53c] The same study also found tanshinone IIA (IC50=1.6 μM) and cryptotanshinone (IC50=1.3 μM) to be single‐digit micromolar inhibitors of SARS‐CoV‐2 PLpro, [53c] and the latter was also identified in a high‐throughput screening performed by Zhao et al. (IC50=5.6 μM). [53d] This screening also identified tanshinone I (IC50=2.2 μM) and YM155 (IC50=2.5 μM) as SARS‐CoV‐2 PLpro inhibitors. [53d] Lim et al. also reported that Ro 08–2750 (EC50=20 μM) and dihydrotanshinone I (EC50=8.1 μM) were active against SARS‐CoV‐2 in a cell culture‐based assay. [53c] On the contrary, Wang and Ma reported that tanshinone I, tanshinone IIa, cryptotanshinone, and dihydrotanshinone I were inactive in both thermal‐shift binding SARS‐CoV‐2 PLpro and cell culture‐based anti‐coronaviral assays, suggesting nonspecific interactions. [54]
Additional SARS‐CoV‐2 PLpro inhibitors were discovered in another high‐throughput screening by Wang and co‐workers. [52b] Extensive structure‐activity‐relationship (SAR) studies on the basis of two micromolar hits led to the discovery of 30 related small molecule inhibitors of SARS‐CoV‐2 PLpro (e. g., Jun9‐75‐5, IC50=0.56 μM). [52b] Multiple compounds were also evaluated in cell‐based antiviral assays, in which Jun9‐72‐2 (Figure 6) displayed the strongest anti‐coronaviral activity (EC50=6.6–7.9 μM). [52b]
Structure‐based efforts to improve activity led to the discovery of several sub‐micromolar SARS‐CoV‐2 PLpro inhibitors. [55] Central to the study was the replacement of the common naphthalene motif by a 2‐phenylthiophene moiety. [55] Inhibitors like XR8‐23 (IC50=0.39 μM, EC50=1.4 μM, Figure 6) interact with the protease through a novel binding mode by associating with BL2 close to the active site, and appear to have promising in vivo pharmacokinetics. [55]
By featuring peptide substrates of SARS‐CoV‐2 PLpro with C‐terminal electrophilic vinylmethyl ester warheads, Rut et al. developed covalently binding peptidomimetics. [46] The inhibitors VIR250 and VIR251 show IC50 values in the two‐digit micromolar range against both SARS‐CoV‐1 and SARS‐CoV‐2 PLpro (Figure 6). [46] Patchett et al. further characterized the interactions of VIR250 and VIR251 with the active site of PLpro by X‐ray crystallography (Figure 7c, Figure 7d). [40] Recently, several antiviral peptide‐drug conjugates targeting SARS‐CoV‐2 PLpro were also reported. [52c] The peptide‐drug conjugate EM‐C consists of a sulfonium‐tethered peptide substrate linked to GRL0617 and was reported to have an IC50 of 7.4 μM against SARS‐CoV‐2 PLpro (Figure 6). [52c]
It was also suggested that ebselen, disulfiram and their analogues are SARS‐CoV‐2 PLpro inhibitors. [56] However, it is noted that these compounds are non‐specific cysteine modifiers.[ 54 , 57 ]
At the time of writing, six clinical studies registered on ClinicalTrials.gov were associated with PLpro, [58] evaluating isotretinoin‐containing treatments and ebselen. The use of isotretinoin as PLpro inhibitor appears to be mainly supported by published in silico data. [59] Ebselen was also investigated as SARS‐CoV‐2 Mpro inhibitor, but – as discussed previously – has been found to be an unspecific modifier of cysteine proteases.[ 54 , 57 ] All six studies were initially uploaded on ClinicalTrials.gov in early to mid‐2020. [58] As of July 2022, the four studies involving isotretinoin‐derived treatments were not yet recruiting and the two studies on ebselen were enrolling by invitation. [58]
4. Conclusion and Outlook
PLpro is a promising drug target for coronavirus infections. The cysteine protease is arranged in the characteristic thumb‐palm‐fingers architecture and hydrolyses the viral polyproteins at three defined positions, always after Gly‐Gly motifs. PLpro also targets posttranslational ubiquitin and ISG15 modifications, which are part of the antiviral immune response. The association of PLpro with both the viral replication cycle and the modulation of the host immune response is a unique opportunity for drug development.
Various SARS‐CoV‐2 PLpro inhibitors have been explored. Repurposed molecules exhibited SARS‐CoV‐2 PLpro inhibition,[ 53c , 53d ] and peptide‐based compounds were also explored.[ 46 , 52c ] Notably, many promising inhibitors are derived from the small molecule GRL0617, which was first developed as a SARS‐CoV‐1 PLpro inhibitor. [52a] Encouraging advancements of GRL0617 include the compounds designed by Wang and co‐workers, [52b] as well as the 2‐phenylthiophene compounds described by Shen et al., [55] which exhibit low micromolar activity in cell culture‐based assays. [52b] However, further improvement of the pharmacokinetic and pharmacodynamic properties might be necessary to advance to clinical trials. [60]
In the case of PLpro, small molecules with reversible binding modes were more explored than covalent peptide‐based inhibitors. This is a notable difference to Mpro, where the latter class dominates the drug discovery landscape. [61] An obvious disadvantage of covalent inhibitors is their potential proneness to off‐target effects, [62] while the additional covalent bond to the catalytically active residue boosts affinity and may offer better protection against resistance. The development of substrate‐derived peptidomimetics may be challenged due to the Gly‐Gly (P2–P1) recognition motif of PLpro, as compared to the unique Gln (P1) specificity of Mpro. [11a] In contrast to the rapid success with Mpro inhibitors like nirmatrelvir,[ 5b , 13 ] the pharmacological profiles of PLpro inhibitors might so far have prevented an approved drug candidate.
The COVID‐19 pandemic continues to have a devastating impact on societies worldwide. [63] While the first anti‐coronaviral drugs are available, the possibility of emerging resistance should not be disregarded. [64] To enhance the toolbox available in the fight against COVID‐19, SARS‐CoV‐2 PLpro appears to be a suitable target for the creation of new antivirals.
Conflict of interest
The authors declare no conflict of interest.
5.
Biographical Information
Sven Ullrich is a PhD candidate at the Australian National University, where he works at the Research School of Chemistry on viral proteases and modified peptides as their inhibitors. He studied pharmacy at Heidelberg University, Germany, with a placement in Nancy, France. He relocated to the Australian National University supported by the German Academic Exchange Service PROMOS programme and was a visiting PhD student at the University of Alberta, Canada.

Biographical Information
Christoph Nitsche completed his PhD at Heidelberg University in 2014 supported by the German Academic Scholarship Foundation. He was awarded a Feodor Lynen Fellowship (Alexander von Humboldt Foundation) to work at the Australian National University from 2015 to 2018, followed by a period as Rising Star Fellow at the Free University of Berlin. In 2019, he returned to the ANU as an ARC DECRA Fellow and was appointed Senior Lecturer in 2020. His research program focuses on drug discovery against infectious diseases, biocompatible chemistry, and peptide and protein modification.

Acknowledgements
CN gratefully acknowledges funding by the Australian Research Council (DECRA: DE190100015; Discovery Project: DP200100348). The authors gratefully acknowledge Junming He (Australian National University) who designed and created the artwork for the frontispiece. Open Access publishing facilitated by Australian National University, as part of the Wiley ‐ Australian National University agreement via the Council of Australian University Librarians.
S. Ullrich, C. Nitsche, ChemBioChem 2022, e202200327.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
References
- 1. Drosten C., Günther S., Preiser W., van der Werf S., Brodt H.-R., Becker S., Rabenau H., Panning M., Kolesnikova L., Fouchier R. A. M., Berger A., Burguière A.-M., Cinatl J., Eickmann M., Escriou N., Grywna K., Kramme S., Manuguerra J.-C., Müller S., Rickerts V., Stürmer M., Vieth S., Klenk H.-D., Osterhaus A. D. M. E., Schmitz H., Doerr H. W., N. Engl. J. Med. 2003, 348, 1967–1976. [DOI] [PubMed] [Google Scholar]
- 2.
- 2a. Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., Zhao X., Huang B., Shi W., Lu R., Niu P., Zhan F., Ma X., Wang D., Xu W., Wu G., Gao G. F., Tan W., et al., N. Engl. J. Med. 2020, 382, 727–733; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2b. Lu R., Zhao X., Li J., Niu P., Yang B., Wu H., Wang W., Song H., Huang B., Zhu N., Bi Y., Ma X., Zhan F., Wang L., Hu T., Zhou H., Hu Z., Zhou W., Zhao L., Chen J., Meng Y., Wang J., Lin Y., Yuan J., Xie Z., Ma J., Liu W. J., Wang D., Xu W., Holmes E. C., Gao G. F., Wu G., Chen W., Shi W., Tan W., Lancet 2020, 395, 565–574; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2c. Ciotti M., Ciccozzi M., Terrinoni A., Jiang W.-C., Wang C.-B., Bernardini S., Crit. Rev. Clin. Lab. Sci. 2020, 57, 365–388. [DOI] [PubMed] [Google Scholar]
- 3. Kudlay D., Svistunov A., BioEngineering 2022, 9, 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.
- 4a. Corti D., Purcell L. A., Snell G., Veesler D., Cell 2021, 184, 3086–3108; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4b. Taylor P. C., Adams A. C., Hufford M. M., de la Torre I., Winthrop K., Gottlieb R. L., Nat. Rev. Immunol. 2021, 21, 382–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.
- 5a. Painter G. R., Natchus M. G., Cohen O., Holman W., Painter W. P., Curr. Opin. Virol. 2021, 50, 17–22; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5b. Owen D. R., Allerton C. M. N., Anderson A. S., Aschenbrenner L., Avery M., Berritt S., Boras B., Cardin R. D., Carlo A., Coffman K. J., Dantonio A., Di L., Eng H., Ferre R., Gajiwala K. S., Gibson S. A., Greasley S. E., Hurst B. L., Kadar E. P., Kalgutkar A. S., Lee J. C., Lee J., Liu W., Mason S. W., Noell S., Novak J. J., Obach R. S., Ogilvie K., Patel N. C., Pettersson M., Rai D. K., Reese M. R., Sammons M. F., Sathish J. G., Singh R. S. P., Steppan C. M., Stewart A. E., Tuttle J. B., Updyke L., Verhoest P. R., Wei L., Yang Q., Zhu Y., Science 2021, 374, 1586–1593. [DOI] [PubMed] [Google Scholar]
- 6.
- 6a. da Costa V. G., Moreli M. L., Saivish M. V., Arch. Virol. 2020, 165, 1517–1526; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6b. Grange Z. L., Goldstein T., Johnson C. K., Anthony S., Gilardi K., Daszak P., Olival K. J., O'Rourke T., Murray S., Olson S. H., Togami E., Vidal G., Mazet J. A. K., Anderson K., Auewarakul P., Coffey L., Corley R., Dauphin G., Epstein J., Fukuda K., Goodman S., Han B., Hughes J., Jeggo M., Karesh W., Kazwala R., Kelly T., Keusch G., Kurilla M., Mackenzie J., Markotter W., Monagin C., Morens D., Munster V., Muhlberger E., Pandit P., Peel A., Pfeiffer D., Restif O., Tomori O., Towner J., Van Der Werf S., VonDobschetz S., Wacharapluesadee S., Ward M., Weirsma L., Wilson M., Wolking D., Wongsathapornchai K., Brierley L., Tambrana-Torellio C., Islam A., Islam S., Raman Z., Hul V., Duong V., Mouiche M., Nwobegahay J., Coulibaly K., Kumakamba C., Syaluha E. K., Lukusa J.-P., Belay D., Kebede N., Ampofo W., Bel-Nono S., Suu-Ire R., Douokoro K., Dursman H., Pamungkas I., Rachmitasari N., Saputro S., Damanik W., Kusumaningrum T., Rambitan M., Rey B., Safari D., Soebandrio A., Triastuti J., Abu-Basha E., Allan K., Joseph K., Samson M., Khamphaphonphane B., Theppanga W., Desmond J., Samules S., Lee M. H., Lee J., Damdinjav B., Shiilegdamba E., Aung O., Bista M., Karmacharya D., Shrestha R., Nziza J., Tumushime J.-C., Lo M. M., Ndiaye A., Seck M. C., et al., Proc. Natl. Acad. Sci. USA 2021, 118, e2002324118.33822740 [Google Scholar]
- 7. Hartenian E., Nandakumar D., Lari A., Ly M., Tucker J. M., Glaunsinger B. A., J. Biol. Chem. 2020, 295, 12910–12934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.
- 8a. Gordon D. E., Jang G. M., Bouhaddou M., Xu J., Obernier K., White K. M., O'Meara M. J., Rezelj V. V., Guo J. Z., Swaney D. L., Tummino T. A., Hüttenhain R., Kaake R. M., Richards A. L., Tutuncuoglu B., Foussard H., Batra J., Haas K., Modak M., Kim M., Haas P., Polacco B. J., Braberg H., Fabius J. M., Eckhardt M., Soucheray M., Bennett M. J., Cakir M., McGregor M. J., Li Q., Meyer B., Roesch F., Vallet T., Mac Kain A., Miorin L., Moreno E., Naing Z. Z. C., Zhou Y., Peng S., Shi Y., Zhang Z., Shen W., Kirby I. T., Melnyk J. E., Chorba J. S., Lou K., Dai S. A., Barrio-Hernandez I., Memon D., Hernandez-Armenta C., Lyu J., Mathy C. J. P., Perica T., Pilla K. B., Ganesan S. J., Saltzberg D. J., Rakesh R., Liu X., Rosenthal S. B., Calviello L., Venkataramanan S., Liboy-Lugo J., Lin Y., Huang X.-P., Liu Y., Wankowicz S. A., Bohn M., Safari M., Ugur F. S., Koh C., Savar N. S., Tran Q. D., Shengjuler D., Fletcher S. J., O'Neal M. C., Cai Y., Chang J. C. J., Broadhurst D. J., Klippsten S., Sharp P. P., Wenzell N. A., Kuzuoglu-Ozturk D., Wang H.-Y., Trenker R., Young J. M., Cavero D. A., Hiatt J., Roth T. L., Rathore U., Subramanian A., Noack J., Hubert M., Stroud R. M., Frankel A. D., Rosenberg O. S., Verba K. A., Agard D. A., Ott M., Emerman M., Jura N., et al., Nature 2020, 583, 459–468; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8b. Wu F., Zhao S., Yu B., Chen Y.-M., Wang W., Song Z.-G., Hu Y., Tao Z.-W., Tian J.-H., Pei Y.-Y., Yuan M.-L., Zhang Y.-L., Dai F.-H., Liu Y., Wang Q.-M., Zheng J.-J., Xu L., Holmes E. C., Zhang Y.-Z., Nature 2020, 579, 265–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kim D., Lee J.-Y., Yang J.-S., Kim J. W., Kim V. N., Chang H., Cell 2020, 181, 914–921.e910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.
- 10a. Yadav R., Chaudhary J. K., Jain N., Chaudhary P. K., Khanra S., Dhamija P., Sharma A., Kumar A., Handu S., Cells 2021, 10, 821; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10b. Arya R., Kumari S., Pandey B., Mistry H., Bihani S. C., Das A., Prashar V., Gupta G. D., Panicker L., Kumar M., J. Mol. Biol. 2021, 433, 166725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.
- 11a. Ullrich S., Nitsche C., Bioorg. Med. Chem. Lett. 2020, 30, 127377; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11b. Mahmoudvand S., Shokri S., Scand. J. Immunol. 2021, 94, e13044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Agbowuro A. A., Huston W. M., Gamble A. B., Tyndall J. D. A., Med. Res. Rev. 2018, 38, 1295–1331. [DOI] [PubMed] [Google Scholar]
- 13. Lamb Y. N., Drugs 2022, 82, 585–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lei J., Kusov Y., Hilgenfeld R., Antivir. Res. 2018, 149, 58–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Báez-Santos Y. M., St. John S. E., Mesecar A. D., Antivir. Res. 2015, 115, 21–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Klemm T., Ebert G., Calleja D. J., Allison C. C., Richardson L. W., Bernardini J. P., Lu B. G. C., Kuchel N. W., Grohmann C., Shibata Y., Gan Z. Y., Cooney J. P., Doerflinger M., Au A. E., Blackmore T. R., Heden van Noort G. J., Geurink P. P., Ovaa H., Newman J., Riboldi-Tunnicliffe A., Czabotar P. E., Mitchell J. P., Feltham R., Lechtenberg B. C., Lowes K. N., Dewson G., Pellegrini M., Lessene G., Komander D., EMBO J. 2020, 39, e106275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shin D., Mukherjee R., Grewe D., Bojkova D., Baek K., Bhattacharya A., Schulz L., Widera M., Mehdipour A. R., Tascher G., Geurink P. P., Wilhelm A., van der Heden van Noort G. J., Ovaa H., Müller S., Knobeloch K.-P., Rajalingam K., Schulman B. A., Cinatl J., Hummer G., Ciesek S., Dikic I., Nature 2020, 587, 657–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lou Z., Rao Z., Annu. Rev. Biochem. 2022, 91, 381–401. [DOI] [PubMed] [Google Scholar]
- 19. Zhukova A., Blassel L., Lemoine F., Morel M., Voznica J., Gascuel O., C. R. Biol. 2021, 344, 57–75. [Google Scholar]
- 20.
- 20a. Temmam S., Vongphayloth K., Baquero E., Munier S., Bonomi M., Regnault B., Douangboubpha B., Karami Y., Chrétien D., Sanamxay D., Xayaphet V., Paphaphanh P., Lacoste V., Somlor S., Lakeomany K., Phommavanh N., Pérot P., Dehan O., Amara F., Donati F., Bigot T., Nilges M., Rey F. A., van der Werf S., Brey P. T., Eloit M., Nature 2022, 604, 330–336; [DOI] [PubMed] [Google Scholar]
- 20b. Zhou P., Yang X.-L., Wang X.-G., Hu B., Zhang L., Zhang W., Si H.-R., Zhu Y., Li B., Huang C.-L., Chen H.-D., Chen J., Luo Y., Guo H., Jiang R.-D., Liu M.-Q., Chen Y., Shen X.-R., Wang X., Zheng X.-S., Zhao K., Chen Q.-J., Deng F., Liu L.-L., Yan B., Zhan F.-X., Wang Y.-Y., Xiao G.-F., Shi Z.-L., Nature 2020, 579, 270–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rota P. A., Oberste M. S., Monroe S. S., Nix W. A., Campagnoli R., Icenogle J. P., Peñaranda S., Bankamp B., Maher K., Chen M.-H., Tong S., Tamin A., Lowe L., Frace M., DeRisi J. L., Chen Q., Wang D., Erdman D. D., Peret T. C. T., Burns C., Ksiazek T. G., Rollin P. E., Sanchez A., Liffick S., Holloway B., Limor J., McCaustland K., Olsen-Rasmussen M., Fouchier R., Günther S., Osterhaus A. D. M. E., Drosten C., Pallansch M. A., Anderson L. J., Bellini W. J., Science 2003, 300, 1394–1399. [DOI] [PubMed] [Google Scholar]
- 22. Bosken Y. K., Cholko T., Lou Y.-C., Wu K.-P., Chang C.-E. A., Front. Mol. Biosci. 2020, 7, 174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. van Boheemen S., de Graaf M., Lauber C., Bestebroer T. M., Raj V. S., Zaki A. M., Osterhaus A. D. M. E., Haagmans B. L., Gorbalenya A. E., Snijder E. J., Fouchier R. A. M., Buchmeier M. J., mBio 2012, 3, e00473–00412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.
- 24a. Osipiuk J., Azizi S.-A., Dvorkin S., Endres M., Jedrzejczak R., Jones K. A., Kang S., Kathayat R. S., Kim Y., Lisnyak V. G., Maki S. L., Nicolaescu V., Taylor C. A., Tesar C., Zhang Y.-A., Zhou Z., Randall G., Michalska K., Snyder S. A., Dickinson B. C., Joachimiak A., Nat. Commun. 2021, 12, 743; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24b. Ratia K., Saikatendu K. S., Santarsiero B. D., Barretto N., Baker S. C., Stevens R. C., Mesecar A. D., Proc. Natl. Acad. Sci. USA 2006, 103, 5717–5722; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24c. Lee H., Lei H., Santarsiero B. D., Gatuz J. L., Cao S., Rice A. J., Patel K., Szypulinski M. Z., Ojeda I., Ghosh A. K., Johnson M. E., ACS Chem. Biol. 2015, 10, 1456–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tan H., Hu Y., Jadhav P., Tan B., Wang J., J. Med. Chem. 2022, 65, 7561–7580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Madeira F., Pearce M., Tivey A. R. N., Basutkar P., Lee J., Edbali O., Madhusoodanan N., Kolesnikov A., Lopez R., Nucleic Acids Res. 2022, 50, W276-W279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Stothard P., BioTechniques 2000, 28, 1102–1104. [DOI] [PubMed] [Google Scholar]
- 28. Robert X., Gouet P., Nucleic Acids Res. 2014, 42, W320-W324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Barretto N., Jukneliene D., Ratia K., Chen Z., Mesecar A. D., Baker S. C., J. Virol. 2005, 79, 15189–15198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Narayanan A., Toner S. A., Jose J., Biochem. Soc. Trans. 2022, 50, 151–165. [DOI] [PubMed] [Google Scholar]
- 31. Pettersen E. F., Goddard T. D., Huang C. C., Couch G. S., Greenblatt D. M., Meng E. C., Ferrin T. E., J. Comp. Chem. 2004, 25, 1605–1612. [DOI] [PubMed] [Google Scholar]
- 32. Mevissen T. E. T., Komander D., Annu. Rev. Biochem. 2017, 86, 159–192. [DOI] [PubMed] [Google Scholar]
- 33. Báez-Santos Y. M., Barraza S. J., Wilson M. W., Agius M. P., Mielech A. M., Davis N. M., Baker S. C., Larsen S. D., Mesecar A. D., J. Med. Chem. 2014, 57, 2393–2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.
- 34a.World Health Organization, Tracking SARS-CoV-2 variants, 2022. https://www.who.int/en/activities/tracking-sars-cov-2-variants. Accessed 13 May 2022;
- 34b.Centers for Disease Control and Prevention, SARS-CoV-2 variant classifications and definitions, 2022; https://www.cdc.gov/coronavirus/2019-ncov/variants/variant-classifications.html, Accessed 13 May 2022.
- 35.
- 35a. Tao K., Tzou P. L., Nouhin J., Gupta R. K., de Oliveira T., Kosakovsky Pond S. L., Fera D., Shafer R. W., Nat. Rev. Genet. 2021, 22, 757–773; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35b. Harvey W. T., Carabelli A. M., Jackson B., Gupta R. K., Thomson E. C., Harrison E. M., Ludden C., Reeve R., Rambaut A., Peacock S. J., Robertson D. L., Nat. Rev. Microbiol. 2021, 19, 409–424; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35c. de Souza A. S., de Freitas Amorim V. M., Guardia G. D. A., dos Santos F. F., Ulrich H., Galante P. A. F., de Souza R. F., Guzzo C. R., Viruses 2022, 14, 827; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35d. Dumache R., Enache A., Macasoi I., Dehelean C. A., Dumitrascu V., Mihailescu A., Popescu R., Vlad D., Vlad C. S., Muresan C., Pathogens 2022, 11, 516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.
- 36a. Stapleford K., Mittal A., Khattri A., Verma V., PLOS Pathog. 2022, 18, e1010260; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36b. Magazine N., Zhang T., Wu Y., McGee M. C., Veggiani G., Huang W., Viruses 2022, 14, 640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.
- 37a. Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., Schiergens T. S., Herrler G., Wu N.-H., Nitsche A., Müller M. A., Drosten C., Pöhlmann S., Cell 2020, 181, 271–280.e278; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37b. Walls A. C., Park Y.-J., Tortorici M. A., Wall A., McGuire A. T., Veesler D., Cell 2020, 181, 281–292.e286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.
- 38a. Ullrich S., Ekanayake K. B., Otting G., Nitsche C., Bioorg. Med. Chem. Lett. 2022, 62, 128629; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38b. Greasley S. E., Noell S., Plotnikova O., Ferre R., Liu W., Bolanos B., Fennell K., Nicki J., Craig T., Zhu Y., Stewart A. E., Steppan C. M., J. Biol. Chem. 2022, 298, 101972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gangavarapu K., Abdel Latif A., Mullen J. L., Alkuzweny M., Hufbauer E., Tsueng G., Haag E., Zeller M., Aceves C. M., Zaiets K., Cano M., Zhou J., Qian Z., Sattler R., Matteson N. L., Levy J. I., Suchard M. A., Wu C., Su A. I., Andersen K. G., Hughes L. D., medRxiv 2022, 10.1101/2022.01.27.22269965.. [DOI] [Google Scholar]
- 40. Patchett S., Lv Z., Rut W., Békés M., Drag M., Olsen S. K., Huang T. T., Cell Rep. 2021, 36, 109754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Simeoni M., Cavinato T., Rodriguez D., Gatfield D., Commun. Biol. 2021, 4, 715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Suryawanshi R. K., Koganti R., Agelidis A., Patil C. D., Shukla D., Trends Microbiol. 2021, 29, 224–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wong N. A., Saier M. H., Int. J. Mol. Sci. 2021, 22, 1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Woo P. C. Y., Huang Y., Lau S. K. P., Yuen K.-Y., Viruses 2010, 2, 1804–1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Schechter I., Berger A., Biochem. Biophys. Res. Commun. 1967, 27, 157–162. [DOI] [PubMed] [Google Scholar]
- 46. Rut W., Lv Z., Zmudzinski M., Patchett S., Nayak D., Snipas S. J., El Oualid F., Huang T. T., Bekes M., Drag M., Olsen S. K., Sci. Adv. 2020, 6, eabd4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Crooks G. E., Hon G., Chandonia J.-M., Brenner S. E., Genome Res. 2004, 14, 1188–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.
- 48a. Ben-Neriah Y., Nat. Immunol. 2002, 3, 20–26; [DOI] [PubMed] [Google Scholar]
- 48b. Davis M. E., Gack M. U., Virology 2015, 479–480, 52–65; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48c. Perng Y.-C., Lenschow D. J., Nat. Rev. Microbiol. 2018, 16, 423–439; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48d. Nakashima H., Nguyen T., Goins W. F., Chiocca E. A., J. Biol. Chem. 2015, 290, 1485–1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.
- 49a. Narasimhan J., Wang M., Fu Z., Klein J. M., Haas A. L., Kim J.-J. P., J. Biol. Chem. 2005, 280, 27356–27365; [DOI] [PubMed] [Google Scholar]
- 49b. Fu Z., Huang B., Tang J., Liu S., Liu M., Ye Y., Liu Z., Xiong Y., Zhu W., Cao D., Li J., Niu X., Zhou H., Zhao Y. J., Zhang G., Huang H., Nat. Commun. 2021, 12, 488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lv Z., Cano K. E., Jia L., Drag M., Huang T. T., Olsen S. K., Front. Chem. 2022, 9, 819165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Békés M., van der Heden van Noort G. J., Ekkebus R., Ovaa H., Huang T. T., Lima C. D., Mol. Cell 2016, 62, 572–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.
- 52a. Ratia K., Pegan S., Takayama J., Sleeman K., Coughlin M., Baliji S., Chaudhuri R., Fu W., Prabhakar B. S., Johnson M. E., Baker S. C., Ghosh A. K., Mesecar A. D., Proc. Natl. Acad. Sci. USA 2008, 105, 16119–16124; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52b. Ma C., Sacco M. D., Xia Z., Lambrinidis G., Townsend J. A., Hu Y., Meng X., Szeto T., Ba M., Zhang X., Gongora M., Zhang F., Marty M. T., Xiang Y., Kolocouris A., Chen Y., Wang J., ACS Cent. Sci. 2021, 7, 1245–1260; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52c. Liu N., Zhang Y., Lei Y., Wang R., Zhan M., Liu J., An Y., Zhou Y., Zhan J., Yin F., Li Z., J. Med. Chem. 2022, 65, 876–884. [DOI] [PubMed] [Google Scholar]
- 53.
- 53a. Freitas B. T., Durie I. A., Murray J., Longo J. E., Miller H. C., Crich D., Hogan R. J., Tripp R. A., Pegan S. D., ACS Infect. Dis. 2020, 6, 2099–2109; [DOI] [PubMed] [Google Scholar]
- 53b. Gao X., Qin B., Chen P., Zhu K., Hou P., Wojdyla J. A., Wang M., Cui S., Acta Pharm. Sin. B 2021, 11, 237–245; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53c. Lim C. T., Tan K. W., Wu M., Ulferts R., Armstrong L. A., Ozono E., Drury L. S., Milligan J. C., Zeisner T. U., Zeng J., Weissmann F., Canal B., Bineva-Todd G., Howell M., O′Reilly N., Beale R., Kulathu Y., Labib K., Diffley J. F. X., Biochem. J. 2021, 478, 2517–2531; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53d. Zhao Y., Du X., Duan Y., Pan X., Sun Y., You T., Han L., Jin Z., Shang W., Yu J., Guo H., Liu Q., Wu Y., Peng C., Wang J., Zhu C., Yang X., Yang K., Lei Y., Guddat L. W., Xu W., Xiao G., Sun L., Zhang L., Rao Z., Yang H., Protein Cell 2021, 12, 877–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ma C., Wang J., ACS Pharmacol. Transl. Sci. 2022, 5, 102–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Shen Z., Ratia K., Cooper L., Kong D., Lee H., Kwon Y., Li Y., Alqarni S., Huang F., Dubrovskyi O., Rong L., Thatcher G. R. J., Xiong R., J. Med. Chem. 2021, 65, 2940–2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.
- 56a. Sargsyan K., Lin C.-C., Chen T., Grauffel C., Chen Y.-P., Yang W.-Z., Yuan H. S., Lim C., Chem. Sci. 2020, 11, 9904–9909; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56b. Weglarz-Tomczak E., Tomczak J. M., Talma M., Burda-Grabowska M., Giurg M., Brul S., Sci. Rep. 2021, 11, 3640; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56c. Chen T., Fei C.-Y., Chen Y.-P., Sargsyan K., Chang C.-P., Yuan H. S., Lim C., ACS Pharmacol. Transl. Sci. 2021, 4, 898–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.
- 57a. Ma C., Hu Y., Townsend J. A., Lagarias P. I., Marty M. T., Kolocouris A., Wang J., ACS Pharmacol. Transl. Sci. 2020, 3, 1265–1277; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57b. Ma C., Tan H., Choza J., Wang Y., Wang J., Acta Pharm. Sin. B 2022, 12, 1636–1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.National Institutes of Health United States National Library of Medicine, ClinicalTrials.gov: search results for ′papain-like protease′, 2022. http://www.clinicaltrials.gov/search?term=%22papain-like+protease%22. Accessed 23 May 2022.
- 59. Wu C., Liu Y., Yang Y., Zhang P., Zhong W., Wang Y., Wang Q., Xu Y., Li M., Li X., Zheng M., Chen L., Li H., Acta Pharm. Sin. B 2020, 10, 766–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Hefti F. F., BMC Neurosci. 2008, 9, S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.
- 61a. Pillaiyar T., Manickam M., Namasivayam V., Hayashi Y., Jung S.-H., J. Med. Chem. 2016, 59, 6595–6628; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61b. Ullrich S., Sasi V. M., Mahawaththa M. C., Ekanayake K. B., Morewood R., George J., Shuttleworth L., Zhang X., Whitefield C., Otting G., Jackson C., Nitsche C., Bioorg. Med. Chem. Lett. 2021, 50, 128333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.
- 62a. Baillie T. A., Angew. Chem. Int. Ed. 2016, 55, 13408–13421; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2016, 128, 13606–13619; [Google Scholar]
- 62b. Ray S., Murkin A. S., Biochemistry 2019, 58, 5234–5244. [DOI] [PubMed] [Google Scholar]
- 63. Hiscott J., Alexandridi M., Muscolini M., Tassone E., Palermo E., Soultsioti M., Zevini A., Cytokine Growth Factor Rev. 2020, 53, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Irwin K. K., Renzette N., Kowalik T. F., Jensen J. D., Virus Evol. 2016, 2, vew014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.