Sickle cell disease (SCD) is caused by homozygous or compound heterozygous inheritance of mutations in the beta‐globin chains. 1 , 2 Severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2), causing coronavirus disease 2019 (COVID‐19) may potentially lead to severe complications in patients with SCD due to immunocompromise. 3 , 4 The clinical course of COVID‐19 infection in SCD has been described in larger cohorts with COVID‐19 severity varying widely among patients. 5 , 6 , 7 , 8 The purpose of this study was to investigate COVID‐19 vaccination 9 , 10 , 11 patterns in SCD. We hypothesised that COVID‐19 vaccination did not cause significant side‐effects and was effective in lowering COVID‐19 infection in this patient population.
Adults aged ≥18 years (N = 535) with a diagnosis of SCD having encounters at the University of Illinois Hospital and Health Sciences System (UIHealth), a 485‐bed teaching hospital serving patients with various ethnicities, between 1 January and 31 December 2021 were included in this retrospective study. Demographic information, COVID‐19 vaccination status, COVID‐19 infection, healthcare encounters and influenza vaccination history were collected by the primary and co‐investigators. The COVID‐19 vaccination status for African‐American residents aged ≥18 years in Chicago was collected from the Chicago city official website (https://www.chicago.gov/city/en/sites/covid19‐vaccine/home/vaccination‐data‐at‐a‐glance.html). The national Area Deprivation Index (ADI) score was obtained from the Center of Health Disparities Research (https://www.neighborhoodatlas.medicine.wisc.edu/mapping). The severity of COVID‐19 was defined according to: https://www.covid19treatmentguidelines.nih.gov/overview/clinical‐spectrum/. Univariate analyses of patient characteristics were conducted using the Kruskal–Wallis test for continuous variables and the Chi‐square test for categorical variables. A p ≤ 0.05 with the Bonferroni correction was considered statistically significant. Stepwise logistic regression analysis was used to identify independent clinical correlates of COVID‐19 vaccination status and infection. Poisson regression was used to model the infection rate by vaccine status, focusing on events before the Omicron variant.
The median (interquartile range) age of this cohort was 34 (27–45) years, 38% were males and 66% had haemoglobin SS/Sβ0. By 31 December 2021, 52.3% of the patients had received at least one dose of COVID‐19 vaccine and 48.6% completed the primary series of vaccination (defined as two doses of the Pfizer/Moderna or one dose of the Janssen vaccine) (Figure 1A). Both rates were significantly lower than the vaccination rates among adult African‐American Chicago residents (52.3% vs. 64.7% and 48.6% vs. 59.1%, respectively, p < 0.001). In all, 81 (15.1%) of the patients with SCD had received a booster dose. Both uni‐ and multivariable analyses showed that receiving at least one dose of COVID‐19 vaccine was associated with older age, being employed or a student, and receiving influenza immunisation (Table 1; Table S1).
FIGURE 1.
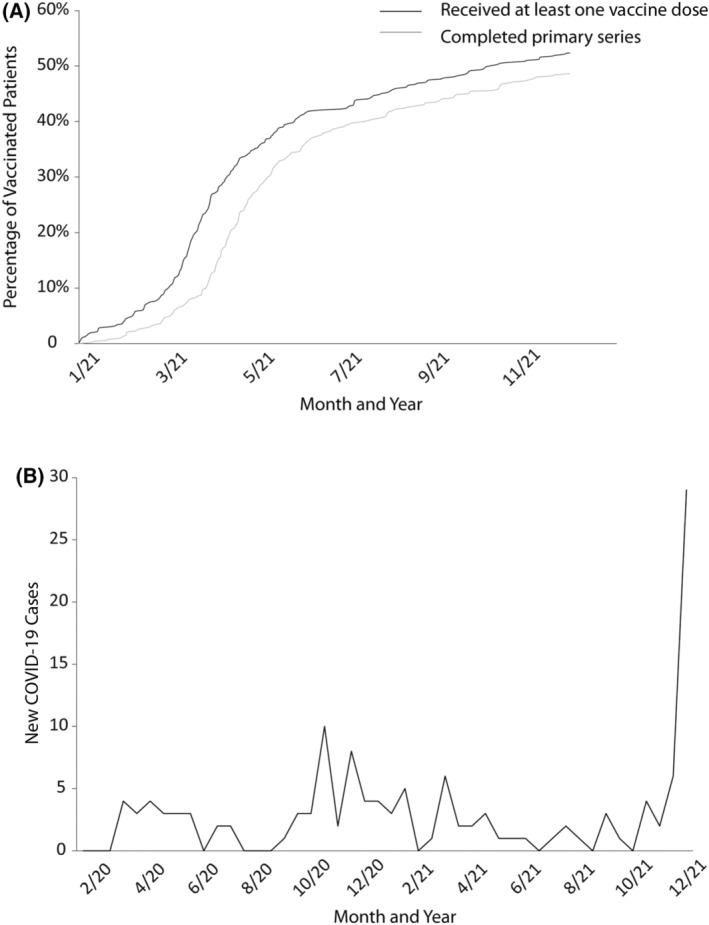
(A) Coronavirus disease 2019 (COVID‐19) vaccination rates in patients with sickle cell disease (SCD). (B) Incidence of COVID‐19 Infection in patients with SCD.
TABLE 1.
Independent clinical correlates of receiving coronavirus disease 2019 (COVID‐19) vaccines
| Odds ratio | 95% CI | p | |
|---|---|---|---|
| Age (10 years) | 1.533 | 1.253–1.876 | <0.001 |
| Employment/student | 3.574 | 2.077–6.149 | <0.001 |
| Received flu vaccination in either 2020 or 2021 | 7.334 | 4.437–12.122 | <0.001 |
Note: A stepwise ordinal logistic regression analysis was performed of categories of receiving COVID‐19 vaccine or not. The covariates originally placed into the analysis were age, gender, national Area Deprivation Index score, distance to the closet pharmacy, being employed or a student, and receiving flu vaccination. Age, being employed or a student, and receiving flu vaccination remained in the regression model after covariates with p > 0.20 were eliminated from the model in a stepwise manner. N = 535.
Abbreviation: CI, confidence interval.
In the SCD cohort, 133 COVID‐19 infections occurred in 121 (22.6%) of the patients (Figure 1B), with 8% non‐symptomatic, 55% mild, 20% moderate, 10% severe and 7% critical cases. Those with a COVID‐19 infection had a significantly higher number of acute care centre/emergency department/hospitalisation encounters in 2021, whereas other factors including the haemoglobin genotype had no effect (Table 2; Table S2). Completion of the primary series of vaccination was associated with a significantly lower infection risk compared to unvaccinated patients (odds ratio 0.311, 95% confidence interval 0.127–0.766; p = 0.01). There was no significant difference in COVID‐19 infection severity or incidence of side‐effects in patients with versus without COVID‐19 vaccination.
TABLE 2.
Clinical correlates of contracting coronavirus disease 2019 (COVID‐19)
| Odds ratio | 95% CI | p | |
|---|---|---|---|
| Total number of admission/ER/ACC visits in 2021 | 1.042 | 1.025–1.059 | <0.001 |
| Received COVID‐19 vaccine booster | 0.502 | 0.240–1.050 | 0.067 |
Note: A stepwise ordinal logistic regression analysis was performed of categories of contracting the COVID‐19 infection or not. The patients who contracted COVID‐19 infection were compared to those who did not. The covariates originally placed into the analysis were age, gender, national Area Deprivation Index score, received COVID‐19 booster, and number of ACC/ED/hospitalisation. Total number of ACC/ED/hospitalisation and receiving COVID‐19 vaccine booster remained in the regression model after covariates with p > 0.20 were eliminated from the model in a stepwise manner. N = 535.
Abbreviations: ACC, acute care centre; CI, confidence interval; ED, emergency department.
The COVID‐19 vaccination completion rate in SCD was lower than the general African‐American population. Older age, being employed or a student, and previous influenza immunization history were significant predictors of COVID‐19 vaccination completion. COVID‐19 vaccination decreased the infection risk by 70% and did not cause significant side‐effects. The relatively young age of our cohort (34 years) and low employment/student status (32.3%) may contribute to the lower vaccination rates, as both were important predictors for receiving COVID‐19 vaccination in our cohort. The association of older age with higher vaccination rate in SCD is consistent with the trend in the general population. The association with employment/student status may reflect the mandatory vaccination requirement at school and work settings. The significant correlation between COVID‐19 and influenza vaccination suggests that immunisation hesitancy is not specific to the newly developed COVID‐19 vaccine. Many reasons, including socioeconomic and medical factors, may contribute to the observed vaccination hesitancy, which needs to be addressed to improve the vaccination rate in this patient population.
The COVID‐19 vaccines appear to not cause significant side‐effects in this patient population, and completion of the primary series of COVID‐19 vaccinations decreased the infection risk by 70% before the Omicron surge. No significant difference was observed in the severity of the COVID‐19 infection after vaccination. The Omicron variant surge by the end of December 2021 accounted for a large portion of the infection cases in our cohort. The Omicron variant tends to cause less serious complications 12 and this may mask the potential benefits of COVID‐19 vaccination on infection severity. There was no COVID‐19‐related deaths in our cohort, but SCD was associated with increased mortality even after vaccination in another cohort. 13
Study limitations include that the majority of vaccinated patients in this cohort had <1 year of follow‐up after vaccination and an even shorter time frame after receiving the booster, which makes interpretation of the COVID‐19 vaccination efficacy difficult. Selection bias is also a possibility, as only patients with SCD with encounters at UIHealth in 2021 were included in the study. Additionally, when assessing healthcare encounters as a risk factor for infection, the information is not restricted to the time before contracting COVID‐19, and it is unclear how COVID‐19 infection impacts acute care use after infection. Lastly, lack of community level factors in this study is also a limitation to understand the risk of contracting COVID‐19. In conclusion, we found that COVID‐19 vaccination appeared to be safe in patients with SCD and to decrease the infection risk by 70%. Older age, being employed or a student, and previous immunisation acceptance are strong predictors for receiving COVID‐19 vaccination despite a relatively low acceptance rate, which emphasises the importance of developing strategies to address immunisation hesitance in this vulnerable population. This study contributes to the scarce existing literature 14 on COVID‐19 vaccination in SCD.
AUTHOR CONTRIBUTIONS
Jin Han, Santosh L. Saraf and Victor R. Gordeuk designed and performed research, analysed the data and wrote the paper. Xu Zhang analysed the data. Robert E. Molokie, Faiz Hussain, Franklin Njoku, Marwah Farooqui, Insia Rizvi designed and performed research and wrote the paper.
CONFLICT OF INTEREST
Victor R. Gordeuk receives research support and provides consulting for Global Blood Therapeutics. Santosh L. Saraf receives research support, serves on the speakers bureau, and provides consulting for Global Blood Therapeutics.
Supporting information
Tables S1‐S2 Supporting Information
ACKNOWLEDGEMENTS
Santosh L. Saraf was supported by the National Institutes of Health (NIH) through grant R01 HL‐153161. Victor R. Gordeuk was supported by the NIH through grant U01HL‐134042. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
DATA AVAILABILITY STATEMENT
Data are available upon requests to the corresponding author.
REFERENCES
- 1. Steinberg MH. Management of sickle cell disease. N Engl J Med. 1999;340:1021–30. [DOI] [PubMed] [Google Scholar]
- 2. Modell B, Darlison M. Global epidemiology of haemoglobin disorders and derived service indicators. Bull World Health Organ. 2008;86:480–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Booth C, Inusa B, Obaro SK. Infection in sickle cell disease: a review. Int J Infect Dis. 2010;14:e2–e12. [DOI] [PubMed] [Google Scholar]
- 4. Battersby AJ, Knox‐Macaulay HH, Carrol ED. Susceptibility to invasive bacterial infections in children with sickle cell disease. Pediatr Blood Cancer. 2010;55:401–6. [DOI] [PubMed] [Google Scholar]
- 5. Arlet JB, Lionnet F, Khimoud D, Joseph L, de Montalembert M, Morisset S, et al. Risk factors for severe COVID‐19 in hospitalized sickle cell disease patients: A study of 319 patients in France. Am J Hematol. 2022;97:E86–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Telfer P, de la Fuente J, Sohal M, Brown R, Eleftheriou P, Roy N, et al. Real‐time national survey of COVID‐19 in hemoglobinopathy and rare inherited anemia patients. Haematologica. 2020;105:2651–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Minniti CP, Zaidi AU, Nouraie M, Manwani D, Crouch GD, Crouch AS, et al. Clinical predictors of poor outcomes in patients with sickle cell disease and COVID‐19 infection. Blood Adv. 2021;5:207–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mucalo L, Brandow AM, Dasgupta M, Mason SF, Simpson PM, Singh A, et al. Comorbidities are risk factors for hospitalization and serious COVID‐19 illness in children and adults with sickle cell disease. Blood Adv. 2021;5:2717–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the BNT162b2 mRNA Covid‐19 vaccine. N Engl J Med. 2020;383:2603–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Baden LR, el Sahly HM, Essink B, Kotloff K, Frey S, Novak R, et al. Efficacy and safety of the mRNA‐1273 SARS‐CoV‐2 vaccine. N Engl J Med. 2021;384:403–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sadoff J, Gray G, Vandebosch A, Cárdenas V, Shukarev G, Grinsztejn B, et al. Safety and efficacy of single‐dose Ad26.COV2.S vaccine against Covid‐19. N Engl J Med. 2021;384:2187–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Madhi SA, Kwatra G, Myers JE, Jassat W, Dhar N, Mukendi CK, et al. Population immunity and Covid‐19 severity with omicron variant in South Africa. N Engl J Med. 2022;386:1314–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hippisley‐Cox J, Coupland CAC, Mehta N, Diaz‐Ordaz K, Lyons RA, Sheikh A, et al. Risk prediction of covid‐19 related death and hospital admission in adults after covid‐19 vaccination: national prospective cohort study. BMJ. 2021;374:n2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Varelas C, Gavriilaki E, Sakellari I, Klonizakis P, Koravou EE, Christodoulou I, et al. Immune response of adult sickle cell disease patients after COVID‐19 vaccination: the experience of a Greek center. J Clin Med. 2022;11:937. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1‐S2 Supporting Information
Data Availability Statement
Data are available upon requests to the corresponding author.


