Abstract
Streptomyces has been known to form two types of septa. The data in this research demonstrated that Streptomyces griseus forms another type of septum near the base of sporogenic hyphae (basal septum). To understand the regulation of the septation machinery in S. griseus, we investigated the expression of the ftsZ gene. S1 nuclease protection assays revealed that four ftsZ transcripts were differentially expressed during morphological differentiation. The vegetative transcript (emanating from Pveg) is present at a moderate level during vegetative growth, but is switched off within the first 2 h of sporulation. Two sporulation-specific transcripts predominantly accumulated, and the levels increased by approximately fivefold together shortly before sporulation septa begin to form. Consistently, the sporulation-specific transcripts were expressed much earlier and more abundantly in a group of nonsporulating mutants that form their sporulation septa prematurely. Promoter-probe studies with two different reporter systems confirmed the activities of the putative promoters identified from the 5′ end point of the transcripts. The levels and expression timing of promoter activities were consistent with the results of nuclease protection assays. The aseptate phenotype of the Pspo mutant indicated that the increased transcription from Pspo is required for sporulation septation, but not for vegetative or basal septum formation.
The early discernible event in bacterial cell division is the involvement of FtsZ, which governs the localization of septum formation. ftsZ is present in all bacteria so far examined, including wall-less bacteria (55), as well as in archaea (34, 56) and plastids of Arabidopsis (42). FtsZ forms the leading edge of the cell division septum by migrating from random cytoplasmic locations to the internal face of the cell membrane at the division plane, where FtsZ polymerizes into a ring structure (7). In Escherichia coli, the Min proteins appear to permit this ring structure to form at the center of the cell rather than at either pole (12). In Bacillus subtilis, ftsZ is required for both binary fission and asymmetric septation (6). Sharing some structural and biochemical properties with the eucaryotic cytoskeletal protein tubulin, FtsZ possesses GTPase activity (11, 40, 46). Interaction of FtsZ with GTP appears to be necessary for polymerization in vitro (9, 41). The inward growth of the division septum is presumably accompanied by the controlled depolymerization of the FtsZ ring, the appositional sliding of polymeric subunits (9), or a profound change in the ring architecture.
Although the role of FtsZ appears to be similar in procaryotes, the pattern of synthesis of FtsZ differs, apparently in compliance with the physiological demands of each species. The level of FtsZ from Caulobacter crescentus varies in concert with the division cycle; FtsZ reaches a maximal level in predivisional cells and is absent from swarmer cells, in which FtsZ appears to be specifically degraded (45). In E. coli, FtsZ is present at a constant number of molecules in each cell (1, 8). To guarantee this constancy independent of the physiological status of the cell, five promoters direct the expression of ftsZ; these promoters also govern the expression of adjacent genes in polycistronic transcription units (1, 47, 48). Recent evidence suggests that there may be rather small fluctuations in the expression of ftsZ that correspond to discrete stages in the E. coli cell cycle (16). Rothfield and colleagues have identified a gene (sdiA) the product of which enhances expression of ftsZ from the distal promoter primarily responsible for the transcription of ftsZ during steady-state growth (54); the synthesis of SdiA appears to be regulated in part by an extracellular, diffusible factor (17). Expression of ftsZ in B. subtilis appears to be less complicated: three promoters have been identified, two of which are recognized by the major vegetative form of RNA polymerase and the third of which is recognized by RNA polymerase containing the sporulation-specific sigma factor, ςH (18, 19). Elimination of the last promoter by mutation did not prevent sporulation, indicating that the sporulation-specific promoter is not necessary for the action of FtsZ during asymmetric septation.
Streptomyces is a gram-positive soil bacterium that is distinctive because of its morphological differentiation. Vegetative growth is characterized by production of branched, filamentous, multinucleoidal hyphae that contain relatively infrequent, thin, single-layer vegetative septa. The sporulation process includes the formation of specialized branches designated aerial (10) or sporogenic (29) hyphae and the synchronous septation events changing the sporogenic hyphae into chains of uninucleoidal compartments separated by double-layer, thick, lysozyme- and sonication-resistant sporulation septa (28, 29). At a later stage, chains of the uninucleoidal compartments are destined to become individual spores (28). The Streptomyces ftsZ gene has been studied to understand the unique septation machinery and regulation of the septum deposition during the reproductive cell division process (13, 36). The transient accumulation of FtsZ into ladder-like structures in sporogenic hyphae suggested that FtsZ assembles at sporulation septum sites and degrades after septum formation is complete (50). Like in other bacteria, the gene is also clustered in cell division and cell wall synthesis genes in Streptomyces, such as ftsQ, ftsW, and ftsI (36, 39). The viability of ftsZ and ftsQ null mutants of Streptomyces coelicolor (35, 36) indicates that two of the genes required for cell division in other bacteria are not essential for vegetative growth of Streptomyces.
Here we report by S1 nuclease protection and promoter probe studies that ftsZ expression is developmentally regulated at the transcription level in Streptomyces griseus. The wild-type strain accumulated sporulation-specific transcripts at high levels during submerged sporulation. A group of nonsporulating mutants, which are unimpaired in vegetative growth and vegetative septum formation but prematurely form sporulation septa, accumulated ftsZ transcripts much earlier and more abundantly than the wild-type strain. We also identified that S. griseus forms another kind of septa, designated basal septa, separating sporogenic from vegetative hyphae. Transmission electron microscopic study with the Pspo mutant showed that the upregulation of ftsZ transcription from the Pspo promoter is required for sporulation septation.
MATERIALS AND METHODS
Bacterial strains and plasmids.
S. griseus NRRL B-2682 obtained from the Northern Regional Research Laboratory (Peoria, Ill.) was used as the wild-type strain. Three nonsporulating mutants, SKK1015 (class IIIA), SKK1008 (class IIIB, bldA mutant), and SKK1003 (class IIIC), used in this research were described previously (29). SKK968 is an independent isolate containing the Pspo null allele in which 20 nucleotides (nt) between the −10 and −35 regions of the Pspo region have been deleted. S. lividans TK24 was used as the wild-type strain. E. coli DH5α was used for routine plasmid construction and preparation. E. coli ET12567 (dam dcm) (33) was used to demethylate plasmid DNA prior to its methylation in vitro and introduction into S. griseus. The characteristics and construction of plasmids used in this study are described in Table 1.
TABLE 1.
Plasmids constructed in this study
| Plasmid | Vector (reference) | Contents |
|---|---|---|
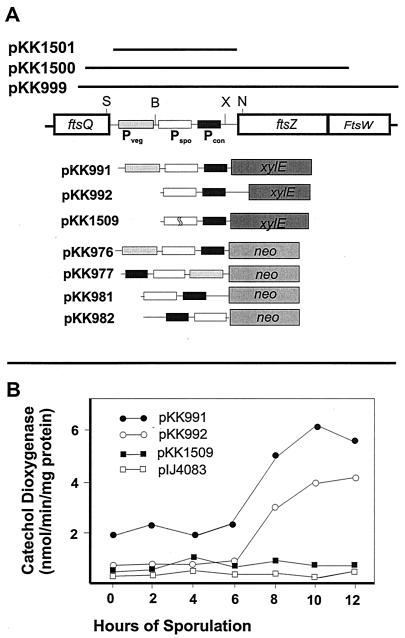
| pKK991 | pIJ4083 (43) | 400-bp SmaI-XhoI fragment containing Pveg and Pspo from pKK970 as an EcoRI-BglII fragment into EcoRI and BamHI sites |
| pKK992 | pIJ4083 | 340-bp BclI-Nrul fragment containing Pspo from pKK975 as an EcoRI-HindIII fragment |
| pKK1509 | pIJ4083 | 200-bp MscI-StuI fragment containing Pveg and Pspo from pKK967 as an EcoRI-BglII fragment into EcoRI and BamHI sites |
| pKK976 | pIJ486 (57) | 400-bp SmaI-XhoI fragment containing Pveg and Pspo from pKK970 as an EcoRI-HindIII fragment |
| pKK977 | pIJ487 (57) | 400-bp SmaI-XhoI fragment containing Pveg and Pspo from pKK970 as an EcoRI-HindIII fragment |
| pKK981 | pIJ486 | 340-bp BclI-NruI fragment containing Pspo from pKK975 as an EcoRI-HindIII fragment |
| pKK982 | pIJ487 | 340-bp BclI-NruI fragment containing Pspo from pKK975 as an EcoRI-HindIII fragment |
| pKK999 | pXE4 (23) | 3.0-kb BglII fragment containing ftsQ, ftsZ, and ftsW from pKK920 (A. J. Dharmatilake and K. E. Kendrick) |
| pKK1500 | pXE4 | 2.2-kb BglII fragment containing ftsZ and ftsW from pKK932 (Dharmatilake and Kendrick) |
| pKK1501 | pXE4 | 400-bp SmaI-XhoI fragment containing Pveg and Pspo from pKK970 as a BglII fragment into BamHI site |
| pKK2000 | pIJ2925 (24) | 1.4-kb apramycin resistance cassette BamHI site |
| pKK984 | pKK2000 | 3.0-kb BamHI fragment containing Pspo mutation from pKK967 (200-bp BstXI-Xhol fragment of pKK920 replaced by same fragment from pKK967) |
| pKK970 | pIJ2925(24) | 400-bp SmaI-XhoI fragment containing Pveg and Pspo from pKK920 (Dharmatilake and Kendrick) |
| pKK975 | pIJ2925 | 340-bp BclI-NruI fragment containing Pspo from pKK920 at BamHI and SmaI sites |
| pKK966 | pIJ2925 | 200-bp MscI-StuI fragment from pKK920 at the HincII site |
| pKK967 | pIJ2925 | SalI site of 200-bp MscI-StuI fragment from pKK966 digested with SalI and treated with S1 nuclease; missing 20 nt in Pspo region |
| pKK968 | pIJ2925 | SalI site of 200-bp MscI-StuI fragment from pKK966 digested with SalI and filled in with Klenow fragment in Pspo region |
Growth and induction of sporulation.
E. coli cultures were grown in Luria-Bertani medium (LB) (2) supplemented with ampicillin (100 μg/ml) or apramycin (100 μg/ml) as needed. Starter cultures of S. griseus were grown in SpM (28) for 2 to 5 days to generate spore suspensions that were then used as inoculums for induction of sporulation. SpM agar, supplemented as needed with apramycin (20 μg/ml) or thiostrepton (5 μg/ml), was used for maintenance of S. griseus strains. Two or 15 μg of thiostrepton per ml was used for maintaining pIJ486 and pIJ4083 in S. griseus or S. lividans, respectively. SpMR was used for protoplast transformation (3). Trypticase soy broth (BBL), 2XYT (49), or LB was used for isolation of genomic and plasmid DNA from S. griseus (21). R2YE medium was used for S. lividans transformation (21). Sporulation was induced by phosphate starvation or nutritional downshift (31).
DNA manipulation and analysis.
Standard (2, 21, 49) or previously published (29) methods were used for analysis and manipulation of DNA fragments for colony hybridization, plasmid DNA minipreparations, and transformations. For Southern analysis, we used the Genius II system after transfer of the DNA to a positively charged membrane (Boehringer).
RNA studies.
RNA was purified from vegetative and sporulating cultures of S. griseus NRRL B-2682, SKK1003, SKK1008, and SKK1015 by a standard method (21) with modifications (31). The 1.45-kb BamHI-MluI DNA fragment from pKK920 was used as a probe for low-resolution S1 nuclease protection studies. A double-stranded DNA probe for high-resolution S1 nuclease protection experiments was produced by PCR amplification with Deep Vent polymerase (NEB) with the upstream primer 158 (5′-GCAGGTGTGGCAGGGGACAC-3′, corresponding to nt 418 to 437) and the downstream primer 162 (5′-ATTCGGTTGATGGCATTGACA-3′, complementary to nt 983 to 963). The reaction conditions were the same as those recommended by the manufacturer, except that 2% dimethyl sulfoxide and 3 mM MgCl2 were combined in reaction buffer. The fragments were purified from an agarose gel by the phenol freeze-fracture method (22). We used the S1 nuclease protection assay (13, 21) by combining 50 μg of RNA with 100,000 cpm of end-labeled DNA probes. The sequencing reactions were prepared with the same primer used to make the probe. E. coli tRNA was served as the negative control. A 1.0% agarose gel was used for low-resolution S1 nuclease protection experiments. A 6% polyacrylamide gel was used for fractionation of the protected fragments for high resolution (31). The relative intensity of transcripts was compared by densitometry (ChemiImager; Alpha Innotech Corporation) with developed films.
Catechol dioxygenase assay.
Crude cell extracts were prepared according to the method of Ingram et al. (23), except that the mycelia were disrupted by high pressure with a French press. Catechol dioxygenase activity was determined spectrophotometrically by monitoring the rate of appearance of 2-hydroxymuconic acid semialdehyde (59). The absorption coefficient is 33 × 103 mol−1 Cm−1 (5).
Gene disruption.
To generate disruption in the −10 and −35 regions of Pspo, pKK984 was passed through E. coli ET12467 and subsequently methylated with HpaII methyltransferase. After incubation for 1 h at 30°C, the methylated DNA was introduced into protoplasts of S. griseus NRRL B-2682 suspended in P-buffer (21). Apramycin-resistant transformants were selected, and the single-crossover event was confirmed by Southern hybridization. The single-crossover transformants were incubated for 4 days in an apramycin-free SpM liquid medium and plated on apramycin-negative SpM plates. The apramycin-sensitive strains, indicative of a double-crossover event, were isolated by replica plating. The Pspo mutants were isolated by Southern hybridization and confirmed by nucleotide sequencing, after PCR amplification of the genomic DNA. Seven such strains, SKK968-1 through SKK968-7, were identified.
Microscopy.
Phase-contrast microscopy and transmission electron microscopy were performed according to methods used in the previous experiment (31). TMax 400 film was used for the photographs. Either the prints or the negatives were scanned and imported as TIFF files into Corel PhotoPaint 8 for cropping, and then CorelDraw 8 was used for labeling and assembly of composite photographs. The appearance of each TIFF file was adjusted with Corel PhotoPaint 8 to resemble the microscopic view as closely as possible.
Sonication resistance.
Sonication resistance units (SRU) were measured according to methods used in previous experiments (29).
RESULTS
Identification of the basal septum.
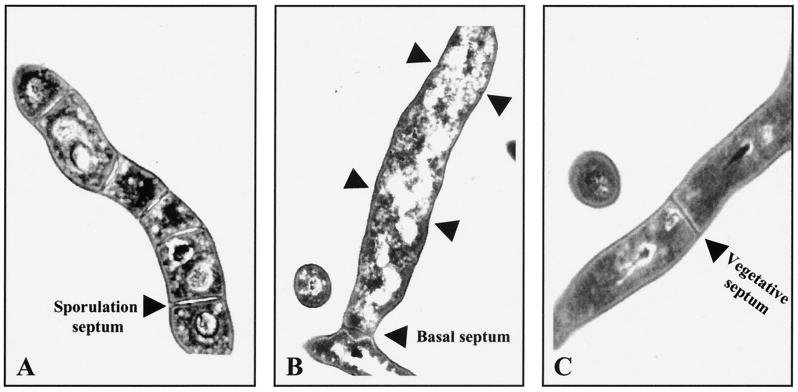
Cefoxitin inhibits sporulation septation, but not vegetative septation, by its binding to 85-kDa sporulation-specific penicillin-binding protein (20). Transmission electron microscopy showed that in the presence of 50 μg of cefoxitin per ml, septum materials accumulated to form inward growths at the sporulation septum sites of sporogenic hyphae, but failed to centripetally grow to become mature sporulation septa (Fig. 1A). However, another type of septum was observed at the bases of all sporogenic hyphae after 12 h of phosphate starvation, when treated with the same concentration of cefoxitin (Fig. 1B). The septa differ from vegetative septa, since vegetative septa are much thinner and are found in the middle of vegetative hyphae but not at branch points (Fig. 1C). Phase-contrast microscopy showed that the aseptate sporogenic hyphae were detached from vegetative hyphae at the branch septum sites between 12 and 16 h of phosphate starvation (data not shown). We measured sonication resistance of the cultures treated with 50 μg of cefoxitin per ml. Thick walls of sporogenic hyphae and spores are resistant to ultrasonication, so that the culture sporulated in liquid culture shows approximately 6 × 109 viable counts after sonication (SRU per milliliter), whereas the vegetatively growing culture has approximately 3 × 104 SRU/ml (29). After 12 h of phosphate starvation, the cefoxitin-treated cultures showed approximately 104 times higher sonication resistance than vegetative cultures and approximately 10- to 20-fold less sonication resistance than the sporulated cultures without treatment (Table 2). These observations indicate that the basal septum is resistant to ultrasonication, and each sporogenic hypha treated with cefoxitin contains 1 SRU.
FIG. 1.
Ultrastructure of three different types of septa in S. griseus. (A) Sporogenic hypha in untreated culture after 12 h of phosphate starvation. (B) Sporogenic hypha in 50-μg/ml cefoxitin-treated culture after 12 h of phosphate starvation. Arrowheads mark the initiated sporulation septa. (C) Vegetative septum.
TABLE 2.
Sonication resistance of the Pspo mutant and cefoxitin-treated cultures
| Culture | Sonication resistance (SRU/ml)
|
||
|---|---|---|---|
| Wild type | Pspo mutant | Cefoxitin treated | |
| Vegetative | (2.2 ± 0.4) × 104 | (1.1 ± 0.8) × 104 | (3.8 ± 1.9) × 104 |
| Sporulated for 12 h | (6.7 ± 1.6) × 109 | (4.2 ± 1.0) × 108 | (3.1 ± 1.3) × 108 |
Transcription of ftsZ in the wild-type strain of S. griseus.
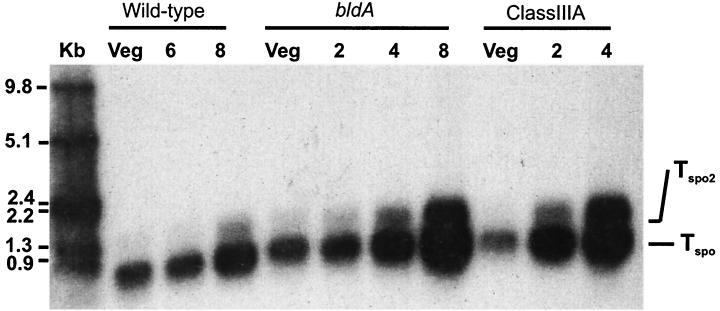
To determine whether ftsZ expression is regulated during growth of S. griseus, we investigated the transcription of ftsZ in the wild-type strain during vegetative growth and sporulation. Low-resolution S1 nuclease protection assays with a 1.44-kb DNA probe (extending 902 bp upstream and 538 downstream of the translation start site) showed that a ftsZ transcript was present at substantial levels during vegetative growth (Fig. 2). However, from the RNA isolated from cultures induced to sporulate by phosphate starvation, two protected fragments, one major and the other minor, accumulated at much higher levels than from the vegetative growth (Fig. 2).
FIG. 2.
Analysis of the ftsZ transcript by low-resolution S1 nuclease protection studies. A double-stranded 1,440-bp DNA probe (including 903 bp upstream and 437 bp downstream of the translation start site) was generated by restriction digestion of the ftsZ structural gene with BamHI and MluI. Kb indicates the molecular size standard (in kilobases). The numbers at the top (2, 4, 6, and 8) indicate the number of hours of culture after the shift to sporulation induction medium. Veg, vegetatively growing cells.
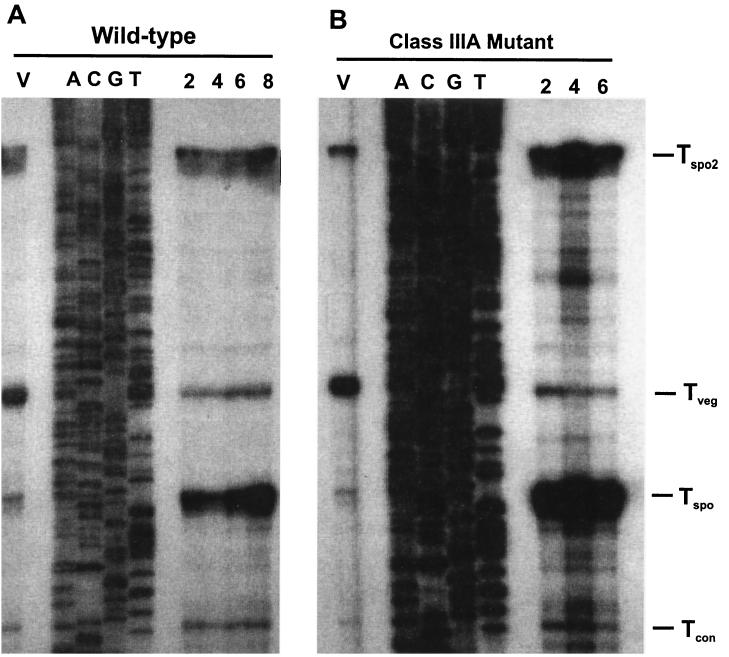
To more precisely characterize the transcripts, we performed high-resolution S1 nuclease protection assays with the same RNA preparations. The probe was designed to extend 486 bp upstream and 80 bp downstream of the translation start codon. The experiments revealed that there were at least four protected fragments upstream of the translation start site (Fig. 3). A transcript was present at a high level only during vegetative growth. The 5′ end point of the vegetative transcript (Tveg) mapped 207 nt upstream of the translation start codon. After 8 h of phosphate starvation, at least two transcripts (a major transcript and a readthrough transcript) accumulating at much higher levels than other transcripts were detected (Fig. 3). The major sporulation-specific transcript (Tspo) mapped at 151 nt upstream transcript. The minor sporulation-specific transcript (readthrough; Tspo2) mapped more than 500 bp upstream of ftsZ inside of the ftsQ open reading frame, since Tspo2 was approximately 0.4 kb bigger than Tspo, as shown in the low-resolution S1 protection assay (Fig. 2). We also compared the amounts of transcripts at different time points during sporulation in the wild-type strain (Fig. 3A). Within the first 2 h of sporulation, the level of Tspo appeared equal to that during vegetative growth and started to increase about 6 h after phosphate starvation. The transcript reached a maximum at 8 h of phosphate starvation, at which point the level of this transcript was approximately four- to fivefold higher than that of the vegetative transcript (Fig. 3A). The minor sporulation-specific transcript (Tspo2) reached its maximum levels after 8 h at which time point the level of Tspo2 was slightly higher than that of the vegetative transcript. Another transcript, Tcon, which mapped 101 bp upstream of the translation start codon, accumulated at a relatively constant level throughout growth (Fig. 2 and 3A). The same pattern of transcription was observed in the same experiments after nutritional downshift, an alternative method for inducing sporulation (data not shown).
FIG. 3.
Analysis of the ftsZ transcripts by high-resolution S1 nuclease protection. A double-stranded 567-bp DNA probe (including 487 bp upstream and 80 bp downstream of the translation start site) was synthesized by PCR with oligonucleotides 158 and 162. In each case, the sequence ladder (A, C, G, and T) was extended from the same primer and used as a size marker. (A) Time course of ftsZ transcription in the wild-type strain during phosphate starvation. Total RNA was prepared from vegetative cells (V) and phosphate-starved cells for 2, 4, 6, and 8 h. E. coli tRNA served as the negative control. (B) ftsZ transcripts in the class III mutants during phosphate starvation. Total RNA was prepared from class III developmental mutant SKK1015 (class IIIA) during vegetative growth (V) and after phosphate starvation for 2, 4, and 6 h. Tveg, Tspo, Tspo2, and Tcon indicate the vegetative, major sporulation-specific, minor sporulation-specific, and constant transcripts, respectively.
Transcription of ftsZ in the class III nonsporulating mutants.
Among the developmental mutants of S. griseus that we have isolated is one phenotypic class, class III. All three subclasses of class III nonsporulating mutants are characterized by their premature and ectopic sporulation septum formation (29). To investigate whether the premature septation of the mutants is caused by a premature increase in ftsZ expression, the ftsZ transcripts were analyzed by S1 nuclease protection assays in the class IIIA (SKK1015), class IIIB (bldA, SKK1008), and class IIIC (SKK1003) mutants. Low- and high-resolution S1 nuclease protection assays showed that transcripts of the class III nonsporulating mutants accumulated at higher levels than the wild-type strain, whereas the same amounts of Tveg were detected during vegetative growth (Fig. 2 and 3B). Tspo accumulated at levels approximately 4 times higher than that of Tveg after 2 h in SKK1015 and after 4 h in SKK1008 (Fig. 2 and 3B). After 4 h of phosphate starvation, the level of Tspo was approximately 10 times higher than that of the vegetative transcript in SKK1015 (Fig. 2 and 3B). In SKK1008, the level of Tspo reached a maximum after 6 h of sporulation, at which time point Tspo accumulated at levels approximately 8 times higher than that of Tveg. In both mutants, the minor sporulation-specific transcript (Tspo2) was also evident after 2 to 4 h of phosphate starvation and showed maximum expression at 4 to 6 h of phosphate starvation, at which time the level of this transcript was slightly higher than that of the vegetative transcript (Fig. 3B). Like the wild-type strain, the level of Tcon was unchanged during sporulation. The transcription pattern of SKK1003 was the same as that of SKK1015 (data not shown).
Promoter activity.
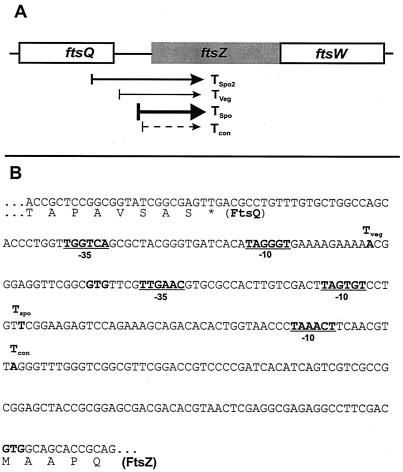
The S1 nuclease protection assays revealed that there were three putative promoters assigned from the 5′ end points of these transcripts. The putative vegetative promoter (Pveg) included −10 (TAGGGT) and −35 (TTGGTC) regions, and the putative sporulation-specific promoter (Pspo) contained −10 (TAGTGT) and −35 (TTGAAC) regions at appropriate distances upstream from the 5′ end points (Fig. 4). The putative constitutive promoter (Pcon) has a −10 sequence motif (TAAACT), but not an obvious −35 region upstream region, so that further confirmation is required (Fig. 4B). Both putative promoters resembled the consensus sequence for the Eς70-like promoters in Streptomyces (25, 51). The nucleotide sequences in the −10 and −35 regions of Pspo were identical in 16 of 20 nt to those of the promoter of eshA in S. griseus, which encodes a sporulation-specific factor required for sporogenic hypha formation (31). To confirm the location of the vegetative promoter (Pveg) and the major sporulation-specific promoter (Pspo) lying immediately upstream of ftsZ, we tested promoter activities by using the neo reporter system that contains a promoterless neomycin phosphotransferase gene (pIJ486 and -487) (57), conferring resistance to kanamycin in both S. griseus and S. lividans TK24. Insertion of a 400-bp DNA fragment containing both Pveg and Pspo of ftsZ into the promoter-probe vector pIJ486 (pKK976) led to expression of the neo reporter in S. lividans and S. griseus, whereas the same fragment in the opposite orientation in pIJ487 (pKK977) did not confer kanamycin resistance to either strain. A 340-bp BclI-NruI fragment containing only Pspo in pIJ486 and pIJ487 (pKK981 and pKK982) did not confer kanamycin resistance to S. lividans and S. griseus.
FIG. 4.
Transcription map of ftsZ from S. griseus. (A) Diagram of four transcripts of ftsZ. The open reading frames at the ftsZ locus are indicated (boxes), together with approximate 5′ ends of transcripts (arrows) as identified by high- and low-resolution S1 nuclease protection studies. The thickness of each arrow indicates the relative amount of each transcript. Tspo, Tspo2, Tveg, and Tcon represent the major sporulation-specific, minor sporulation-specific, vegetative, and constant transcripts, respectively. (B) Locations of the 5′ ends of the two major ftsZ transcripts as determined by high-resolution nuclease protection studies. The 5′ ends of transcripts are marked in boldface. The −10 and −35 regions of promoters are underlined. The C-terminal region of FtsQ and the N-terminal region of FtsZ are shown.
The promoter activity was also determined by using another promoter-probe vector, pIJ4083, which contains the xylE gene encoding catechol dioxygenase from Pseudomonas putida (23), since the antibiotic resistance can be detected only during vegetative growth. S. lividans strains containing plasmid pKK991 (400-bp SmaI-XhoI fragment containing Pveg and Pspo in pIJ4083) showed substantially high levels of xylE expression during vegetative growth (until 2 days on plates) and early stages of sporulation between 2 and 3 days on plates). A more intense expression was observed in the plates incubated for more than 5 days when colonies were in a more advanced stage of sporulation. S. lividans TK24 containing pKK992 (340-bp BclI-NruI fragment containing only Pspo in pIJ4083 in the proper orientation) did not develop a yellow color until 2 days, when colonies were in the vegetative growth stage. As expected, the strain carrying pKK1509, in which 20 nt had been deleted from the Pspo region, did not develop a yellow color after prolonged incubation.
However, a faint yellow color was observed on plates of S. griseus strains containing pKK991 or pKK992 only in heavily inoculated areas during late sporulation, presumably due to the low copy number of pIJ4083. The vector, a derivative of pIJ486, exists in multiple copies in S. lividans, but less than a single copy in S. griseus, for unknown reasons (A. J. Dharmatilake, unpublished observations). To more quantitatively estimate the xylE expressions in S. griseus, we used spectrophotometry after inducing sporulation in liquid culture by phosphate starvation. The S. griseus strain carrying pKK991 and -992 showed a relatively high level of xylE activity during vegetative growth, compared to the S. griseus strain carrying pIJ4083 and pKK1509 (Fig. 5). The levels of expression substantially increased after 10 and 12 h of phosphate starvation (Fig. 5). Pspo::xylE (pKK992) was active only during sporulation (Fig. 5). After 10 and 12 h of phosphate starvation, the xylE activity was approximately 3 times higher than that of vegetative growth, respectively (Fig. 5). These observations were in agreement with the previous results obtained from the S1 nuclease protection experiments and with the neomycin phosphotransferase reporter system regarding the location of Pveg and Pspo and the lack of expression from Pspo during vegetative growth.
FIG. 5.
Promoter-probe studies of the vegetative and sporulation-specific promoters. (A) Diagram of transcription fusions of each promoter region with xylE (pIJ4083) and neo (pIJ486 and 487). The open reading frames are indicated by open bars. Pspo, Pveg, and Tcon below the boxes indicate the sporulation-specific, vegetative, and constant promoters, respectively. The restriction enzymes used for the fusion construction are BclI (B), NruI (N), SmaI (S), and XhoI (X). Further details for cloning plasmids are given in Table 1. (B) Catechol dioxygenase activities of S. griseus strains transformed with pIJ4083 derivatives containing transcription fusions during sporulation induced by phosphate starvation.
Characterization of a Pspo mutant.
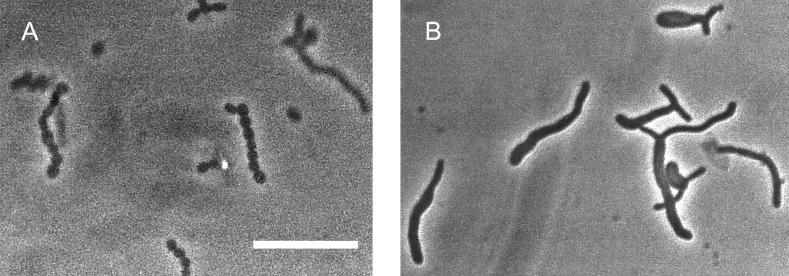
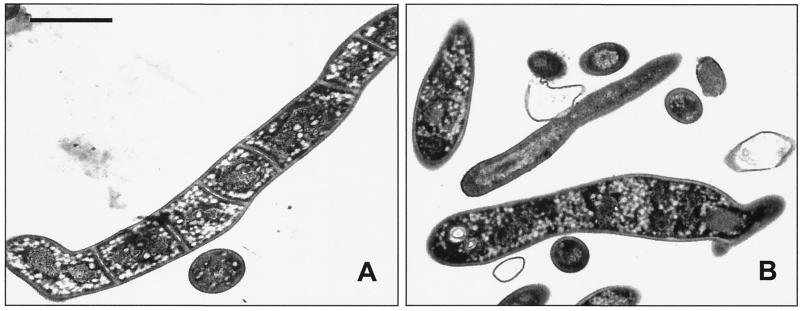
To study the role of the major sporulation-specific promoter (Pspo) in sporulation septum formation, we constructed a Pspo mutant (SKK968) in which 20 nt, including the 18-nt spacer region, was deleted between the −10 and −35 regions of Pspo. The proper construction of the mutated gene was confirmed by Southern hybridization and restriction digestion of the genome, and the nucleotide sequence of the PCR amplified genomic DNA from the mutant. The mutant did not accumulate Tspo, but did accumulate Tspo2, Tveg, and Tcon to the same levels after 8 h of phosphate starvation (data not shown). SKK968 had no obvious phenotypic defect during vegetative growth. The mutant grew at the same rate as the wild-type strain in complex medium and had a hairy colony morphology indistinguishable from that of the wild-type strain, indicating that the mutant produces sporogenic hyphae on plate growth. Phase-contrast microscopy revealed that sporogenic hyphae formed in the mutant after growth on agar and induction of submerged sporulation in complex medium and by phosphate starvation. However, the mutant sporogenic hyphae of the length of approximately 10 to 15 spores did not develop into spore chains but did develop thick walls and appear bright under the phase-contrast microscope, which is characteristic of mature spores (Fig. 6). Electron micrographs of strain SKK968 cultures phosphate starved for 12 h showed that each sporogenic hypha contained no sporulation septa (Fig. 7B). Like in the cefoxitin-treated cells, one basal septum was present in all of the aseptate sporogenic hyphae. The mutant strain starved for 12 h had more than 104 times the number of SRU of the vegetative cells and 10 to 20 times less SRU than the wild-type strain induced to sporulate for 12 h (Table 2).
FIG. 6.
Phase-contrast microscopy of the Pspo mutant (SKK968) and the wild-type strain of S. griseus after 16 h of sporulation induced by phosphate starvation. (A) Spore chains were evident in the sporogenic hyphae of the wild-type strain. (B) No sporulation septation was found from the sporogenic hyphae of the mutant. The bar represents 10 μm. No differences in vegetative cultures of the mutant and wild-type strains were apparent.
FIG. 7.
Transmission electron microscopy of the Pspo mutant (SKK968) and the mutant complemented with pKK999 after 12 h of sporulation induced by phosphate starvation. (A) The sporogenic hypha was divided by sporulation septa in the complemented mutant. (B) No sporulation septum was formed in the sporogenic hypha of the Pspo mutant. Bar, 1 μm.
Complementation of the phenotype of SKK968.
A potential open reading frame encoding a 24-amino-acid polypeptide was found in the intergenic region between ftsQ and ftsZ. orf24 lacks the GC bias at the third position of the codons, which is characteristic of streptomycete open reading frames. However, the deduced amino acid sequence and the rare TTA codon are highly conserved in S. coelicolor (90% amino acid identity) (15). Therefore, the 20-nt deletion mutation in strain SKK968 has the potential to affect the expression of orf24 and ftsW located downstream of the ftsZ gene. To distinguish between these various possibilities, we complemented mutant SKK968 with the following fragments. As described in Table 1, pKK1501 contained the 400-bp intergenic region (orf24); pKK1500 contained the intergenic region (orf24) and the whole ftsZ open reading frame; and pKK999 contained orf24, ftsZ, and ftsW. Transmission electron microscopy showed that the septum formation in sporogenic hyphae was restored, when the mutant was transformed with only pKK999 or pKK1501 (Fig. 7B).
DISCUSSION
Key events in Streptomyces differentiation include the extensive growth of multinucleoidal vegetative cells and massive and relatively synchronous septation necessary to form the compartments destined to become the spores. So far only two septa, vegetative and sporulation, have been found in research with the wild-type strain. However, in this report, by using the Pspo mutant and cefoxitin-treated culture in which sporulation septum formation is arrested, we could identify another type of septum during growth of S. griseus, named basal septum, which differs from vegetative or sporulation septa. Unlike vegetative septa, basal septa are located near the bases of sporogenic hyphae and are resistant to ultrasonication, so that multinucleoidal structures of the aseptate sporogenic hyphae from the Pspo mutant and cefoxitin-treated cultures are resistant to sonication. This leads to viable counts approximately 104 times higher than those of vegetative cells, but approximately 10 to 20 times lower than that of the sporulated culture of the wild-type strain. However, the lack of sporulation septa does not prevent certain subsequent developmental steps such as wall thickening and acquisition of phase brightness in the sporogenic hyphae, as observed in the sporogenic hyphae of the Pspo mutant and the cefoxitin-treated cells. We speculate that the basal septum formation is complete between 10 and 12 h of submerged sporulation, based on the timing of sonication resistance and the timing of the basal septum detection.
Increased ftsZ transcription from sporulation-specific promoters is required for sporulation septation, but not for vegetative or basal septum formation. This was true in the sporulation processes of the wild-type strain, the Pspo mutant, and the class III nonsporulating mutants. The very low level of ftsZ expression from Pveg may not yield enough FtsZ for massive septation during sporulation. Sporulation septation also seems to require ftsZ expression localized to sporogenic hyphae. Transcription fusion study of ftsZ in S. coelicolor showed that the sporulation-specific promoter is only transcribed in aerial hyphae (15). The ftsZ expression in sporogenic hyphae may be caused by differential expression or activity of the trans-active factors, including RNA polymerase sigma factors and activators suggested by recent experiments with S. coelicolor (15). In compliance with the localized expression, the basal septum may function as a physical barrier for dividing two different cell types: vegetative and sporogenic hyphae.
The sporulation septation occurs in a highly coordinated manner in S. griseus. When S. griseus is induced to sporulate in liquid culture, it begins to deposit sporulation septa from the sporogenic hyphae after 10 h of phosphate starvation and completed its septum formation by 12 h of phosphate starvation (29). Results from nuclease protection studies clearly demonstrated that the ftsZ expression is tightly regulated at the transcription level during the life cycle of s. griseus in such a manner that the accumulation timing of the ftsZ transcripts coincides with the timing of sporulation septum deposition. The regulation of ftsZ transcription appeared to be exerted at two discrete stages: the switch from the vegetative to the sporulation-specific transcript and the subsequent increase in the level of sporulation-specific transcript.
The appearance of the sporulation-specific transcript is the consequence of a developmentally regulated promoter activity rather than transcript processing. Based on the extensive similarity in nucleotide sequence and expression timing of eshA and Pspo transcripts (31), we speculate that Pspo and the promoter of the eshA gene may be recognized by the same RNA polymerase holoenzyme and may be subject to the same temporal regulation. The nucleotide sequences of Pveg and Pspo resemble those recognized by the housekeeping sigma factor in Streptomyces (51), although the spacing is not optimal. Still it is possible that Pspo is recognized by the sporulation-specific sigma factor WhiG (26, 38), although the nucleotide sequence of Pspo does not align extensively with the WhiG consensus sequence (52). However, we do not think that Pspo is recognized by RNA polymerase containing the sigma factor, SigF, which is required in S. coelicolor for sporulation events that occur after septation (26, 44). Recent studies of ftsZ transcription in S. coelicolor also demonstrated that ftsZ expression is controlled at the transcription level through modification of transcription activities from several different promoters (15). Four ftsZ transcripts have almost the same 5′ end points as those of S. griseus, and the same promoters have been suggested (15).
Expression of ftsZ from Pspo may require not only RNA polymerase holoenzyme but also a trans-active transcription factor. Circumstantial evidence suggests that ftsZ transcription is activated by a positive regulatory factor during sporulation rather than being repressed by a negative factor during vegetative growth. The presence of the entire region upstream of ftsZ on a high-copy plasmid did not impart a phenotype to the transformant, suggesting that this region could not titrate out a negative regulatory factor (A. J. Dharmatilake, unpublished observations). In S. coelicolor, ftsZ transcription from the Pspo counterpart is greatly reduced or eliminated by null mutations in several regulatory genes, including whiG, whiH, and whiI, which may encode families of transcription activators (15).
Our previous results also support the presence of other regulatory factors that govern ftsZ expression. We have identified three genetic complementation groups among the class III mutants that do form their sporulation septa prematurely from the preexisting vegetative hyphae (30–32, 37). Our results to date suggest that the class III mutants have not undergone extensive rearrangements or multiple mutations, but rather are the consequence of point mutations (30; J. Kwak, unpublished observations). The earlier and more abundant accumulation of the sporulation-specific transcripts of ftsZ is consistent with the precocious and abundant sporulation septation in these mutants. This defect appears to be due to the enhanced production of the sporulation-specific transcripts from Pspo and Pspo2. The straightforward interpretation of these results is that the class III genes are required for the regulation of ftsZ transcription from the promoters. Because septation is not the only defect in these mutants, however, the products of these loci probably indirectly influence expression of ftsZ.
Despite of its functional similarity, regulatory mechanisms of ftsZ expression in other bacteria differ from that of Streptomyces. This seems to be due to the fact that bacteria have different life cycles, so that they need to respond to different developmental or environmental signals. In E. coli, transcription of ftsZ oscillates during the cell division cycle: ftsZ transcription increases when DNA replication is initiated (16), and ftsZ expression is maximal in the middle of the cell cycle and minimal at the end of cell division (58). ftsZ is transcribed from at least five promoters that are recognized by different sigma factors (1, 4, 14, 53). Unlike the case of E. coli, Caulobacter crescentus ftsZ is transcribed by a single promoter, and the transcription is negatively regulated by the global cell cycle regulator CtrA, presumably by binding to a site that overlaps the transcription start site (27). The complexity of ftsZ expression shows that ftsZ is regulated at many different regulatory factors responding to many signals during the cell cycle.
ACKNOWLEDGMENTS
We thank M. Bibb for providing pIJ4083, R. Baltz and P. Solenberg for the apramycin resistance cassette, and D. MacNeil for E. coli strain ET12567. We are also grateful for the assistance of Kathy Wolken of the Campus Microscopy and Imaging Facility (School of Medicine).
This work was supported by grant MCB-9724038 from The National Science Foundation.
REFERENCES
- 1.Aldea M, Garrido T, Pla J, Vicente M. Division genes in Escherichia coli are expressed coordinately to cell septum requirements by gearbox promoters. EMBO J. 1990;9:3787–3794. doi: 10.1002/j.1460-2075.1990.tb07592.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ausubel F M, Brent A, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K. Current protocols in molecular biology. New York, N.Y: Wiley Interscience; 1990. [Google Scholar]
- 3.Babcock M J, Kendrick K E. Cloning of DNA involved in sporulation of Streptomyces griseus. J Bacteriol. 1988;170:2802–2808. doi: 10.1128/jb.170.6.2802-2808.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ballesteros M, Kusano S, Ishihama A, Vicente M. The ftsQ1p gearbox promoter of Escherichia coli is a major sigma S-dependent promoter in the ddlB-ftsA region. Mol Microbiol. 1998;30:419–430. doi: 10.1046/j.1365-2958.1998.01077.x. [DOI] [PubMed] [Google Scholar]
- 5.Bayly R C, Dagley S, Gibson D T. The metabolism of cresols by species of Pseudomonas. Biochem J. 1966;101:293–301. doi: 10.1042/bj1010293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beall B, Lutkenhaus J. FtsZ in Bacillus subtilis is required for vegetative septation and for asymmetric septation during sporulation. Genes Dev. 1991;5:447–455. doi: 10.1101/gad.5.3.447. [DOI] [PubMed] [Google Scholar]
- 7.Bi E, Lutkenhaus J. FtsZ ring structure associated with division in Escherichia coli. Nature. 1991;354:161–164. doi: 10.1038/354161a0. [DOI] [PubMed] [Google Scholar]
- 8.Bi E, Lutkenhaus J. Genetics of bacterial cell division. In: Mohan S, Dow C, Coles J A, editors. Prokaryotic structure and function: a new perspective. Cambridge, United Kingdom: Cambridge University Press; 1991. pp. 123–152. [Google Scholar]
- 9.Bramhill D, Thompson C M. GTP-dependent polymerization of Escherichia coli FtsZ protein to form tubules. Proc Natl Acad Sci USA. 1994;91:5813–5817. doi: 10.1073/pnas.91.13.5813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chater K F. Developmental decisions during sporulation in the aerial mycelium in Streptomyces. In: Brun Y V, Shimkets L J, editors. Prokaryotic development. Washington, D.C.: American Society for Microbiology; 2000. pp. 33–48. [Google Scholar]
- 11.Dai K, Lutkenhaus J. ftsZ is an essential cell division gene in Escherichia coli. J Bacteriol. 1991;173:3500–3506. doi: 10.1128/jb.173.11.3500-3506.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Boer P, Crossley R, Rothfield L. The essential bacterial cell-division protein FtsZ is a GTPase. Nature. 1992;359:254–256. doi: 10.1038/359254a0. [DOI] [PubMed] [Google Scholar]
- 13.Dharmatilake A J, Kendrick K E. Expression of the division-controlling gene, ftsZ, during growth and sporulation of the filamentous bacterium, Streptomyces griseus. Gene. 1994;147:21–28. doi: 10.1016/0378-1119(94)90034-5. [DOI] [PubMed] [Google Scholar]
- 14.Donachie W D. The cell cycle of Escherichia coli. Annu Rev Microbiol. 1993;47:199–230. doi: 10.1146/annurev.mi.47.100193.001215. [DOI] [PubMed] [Google Scholar]
- 15.Flardh K, Leibovitz E, Buttner M J, Chater K F. Generation of a non-sporulating strain of Streptomyces coelicolor A3(2) by the manipulation of a developmentally controlled ftsZ promoter. Mol Microbiol. 2000;38:737–749. doi: 10.1046/j.1365-2958.2000.02177.x. [DOI] [PubMed] [Google Scholar]
- 16.Garcia-Lara J, Shang L H, Rothfield L I. An extracellular factor regulates expression of sdiA, a transcriptional activator of cell division genes in Escherichia coli. J Bacteriol. 1996;178:2742–2748. doi: 10.1128/jb.178.10.2742-2748.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garrido T, Sánchez M, Palacios P, Aldea M, Vicente M. Transcription of ftsZ oscillates during the cell cycle of Escherichia coli. EMBO J. 1993;12:3957–3965. doi: 10.1002/j.1460-2075.1993.tb06073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gholamhoseinian A, Shen Z, Wu J-J, Piggot P. Regulation of transcription of the cell division gene ftsA during sporulation of Bacillus subtilis. J Bacteriol. 1992;174:4647–4656. doi: 10.1128/jb.174.14.4647-4656.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gonzy-Tréboul G, Karmazyn-Campelli C, Stragier P. Developmental regulation of transcription of the Bacillus subtilis ftsAZ operon. J Mol Biol. 1992;224:967–979. doi: 10.1016/0022-2836(92)90463-t. [DOI] [PubMed] [Google Scholar]
- 20.Hao J, Kendrick K E. Visualization of penicillin-binding proteins during sporulation of Streptomyces griseus. J Bacteriol. 1998;180:2125–2132. doi: 10.1128/jb.180.8.2125-2132.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hopwood D A, Bibb M J, Chater K F, Kieser T, Bruton C J, Kieser H M, Lydiate D J, Smith C P, Ward J M, Schrempf H. Genetic manipulation of Streptomyces. A laboratory manual. Norwich, Great Britain: The John Innes Foundation; 1985. [Google Scholar]
- 22.Huff J P. Rapid isolation and purification of DNA from agarose gels: the phenol-freeze-fracture method. Bio/Technology. 1991;10:724. [PubMed] [Google Scholar]
- 23.Ingram C, Brawner M, Youngman P, Westpheling J. xylE functions as an efficient reporter gene in Streptomyces spp.: use for the study of galP1, a catabolite-controlled promoter. J Bacteriol. 1989;171:6617–6624. doi: 10.1128/jb.171.12.6617-6624.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Janssen G R, Bibb M J. Derivatives of pUC18 that have BglII sites flanking a modified multiple cloning site and that retain the ability to identify recombinant clones by visual screening of Escherichia coli colonies. Gene. 1993;124:133–134. doi: 10.1016/0378-1119(93)90774-w. [DOI] [PubMed] [Google Scholar]
- 25.Kang J G, Hahn M Y, Ishihama A, Roe J H. Identification of sigma factors for growth phase-related promoter selectivity of RNA polymerases from Streptomyces coelicolor A3(2) Nucleic Acids Res. 1997;25:2566–2573. doi: 10.1093/nar/25.13.2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kelemen G H, Brown G L, Kormanec J, Potúcková L, Chater K F, Buttner M J. The positions of the sigma-factor genes, whiG and sigF, in the hierarchy controlling the development of spore chains in the aerial hyphae of Streptomyces coelicolor A3(2) Mol Microbiol. 1996;21:593–603. doi: 10.1111/j.1365-2958.1996.tb02567.x. [DOI] [PubMed] [Google Scholar]
- 27.Kelly A J, Sackett M J, Din N, Quardokus E, Brun Y V. Cell cycle-dependent transcriptional and proteolytic regulation of FtsZ in Caulobacter. Genes Dev. 1998;12:880–893. doi: 10.1101/gad.12.6.880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kendrick K E, Ensign J C. Sporulation of Streptomyces griseus in submerged culture. J Bacteriol. 1983;155:357–366. doi: 10.1128/jb.155.1.357-366.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kwak J, Kendrick K E. Bald mutants of Streptomyces griseus that prematurely undergo key events of sporulation. J Bacteriol. 1996;178:4643–4650. doi: 10.1128/jb.178.15.4643-4650.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kwak J, McCue L A, Kendrick K E. Identification of bldA mutants of Streptomyces griseus. Gene. 1996;171:75–78. doi: 10.1016/0378-1119(96)00066-2. [DOI] [PubMed] [Google Scholar]
- 31.Kwak J, McCue L A, Trczianka K, Kendrick K E. Identification and characterization of a developmentally regulated protein, EshA, required for sporogenic hyphal branches in Streptomyces griseus. J Bacterol. 2001;183:3004–3015. doi: 10.1128/JB.183.10.3004-3015.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lawlor E J, Baylis H A, Chater K F. Pleiotropic morphological and antibiotic deficiencies result from mutations in a gene encoding a tRNA-like product in Streptomyces coelicolor A3(2) Genes Dev. 1987;1:1305–1310. doi: 10.1101/gad.1.10.1305. [DOI] [PubMed] [Google Scholar]
- 33.MacNeil D J, Gewain K M, Ruby C L, Dezeny G, Gibbons P H, MacNeil T. Analysis of Streptomyces avermitilis genes required for avermectin biosynthesis utilizing a novel integration vector. Gene. 1992;111:61–68. doi: 10.1016/0378-1119(92)90603-m. [DOI] [PubMed] [Google Scholar]
- 34.Margolin W, Wang R, Kumar M. Isolation of an ftsZ homolog from the archaebacterium Halobacterium salinarium: implications for the evolution of FtsZ and tubulin. J Bacteriol. 1996;178:1320–1327. doi: 10.1128/jb.178.5.1320-1327.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McCormick J R, Losick R. Cell division gene ftsQ is required for efficient sporulation but not growth and viability in Streptomyces coelicolor A3(2) J Bacteriol. 1996;178:5295–5301. doi: 10.1128/jb.178.17.5295-5301.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McCormick J R, Su E P, Driks A, Losick R. Growth and viability of Streptomyces coelicolor mutant for the cell division gene ftsZ. Mol Microbiol. 1994;14:243–254. doi: 10.1111/j.1365-2958.1994.tb01285.x. [DOI] [PubMed] [Google Scholar]
- 37.McCue L A, Kwak J, Wang J, Kendrick K E. Analysis of a gene that suppresses the morphological defect of bald mutants of Streptomyces griseus. J Bacteriol. 1996;178:2867–2875. doi: 10.1128/jb.178.10.2867-2875.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mendez C, Chater K F. Cloning of whiG, a gene critical for sporulation of Streptomyces coelicolor A3(2) J Bacteriol. 1987;169:5715–5720. doi: 10.1128/jb.169.12.5715-5720.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mikulik K, Zhulanova E, Kratky M, Kofronova O, Benada O. Isolation and characterization of dcw cluster from Streptomyces collinus producing kirromycin. Biochem Biophys Res Commun. 2000;268:282–288. doi: 10.1006/bbrc.2000.2127. [DOI] [PubMed] [Google Scholar]
- 40.Mukherjee A, Dai K, Lutkenhaus J. Escherichia coli cell division protein FtsZ is a guanine nucleotide binding protein. Proc Natl Acad Sci USA. 1993;90:1053–1057. doi: 10.1073/pnas.90.3.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mukherjee A, Lutkenhaus J. Guanine nucleotide-dependent assembly of FtsZ into filaments. J Bacteriol. 1994;176:2754–2758. doi: 10.1128/jb.176.9.2754-2758.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Osteryoung K W, Vierling E. Conserved cell and organelle division. Nature. 1995;376:473–474. doi: 10.1038/376473b0. [DOI] [PubMed] [Google Scholar]
- 43.Paradkar A S, Petrich A K, Leskiw B K, Aidoo K A, Jensen S E. Transcriptional analysis and heterologous expression of the gene encoding beta-lactamase inhibitor protein (BLIP) from Streptomyces clavuligerus. Gene. 1994;144:31–36. doi: 10.1016/0378-1119(94)90199-6. [DOI] [PubMed] [Google Scholar]
- 44.Potúcková L, Keleman G H, Findlay K C, Lonetto M A, Buttner M J, Kormanec J. A new RNA polymerase sigma factor, ςF, is required for the late stages of morphological differentiation in Streptomyces spp. Mol Microbiol. 1995;17:37–48. doi: 10.1111/j.1365-2958.1995.mmi_17010037.x. [DOI] [PubMed] [Google Scholar]
- 45.Quardokus E, Din N, Brun Y V. Cell cycle regulation and cell type-specific localization of the FtsZ division initiation protein in Caulobacter crescentus. Proc Natl Acad Sci USA. 1996;93:6314–6319. doi: 10.1073/pnas.93.13.6314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.RayChaudhuri D, Park J T. Escherichia coli cell-division gene ftsZ encodes a novel GTP-binding protein. Nature. 1992;359:251–254. doi: 10.1038/359251a0. [DOI] [PubMed] [Google Scholar]
- 47.Robin A, Joseleau-Petit D, D'Ari R. Transcription of the ftsZ gene and cell division in Escherichia coli. J Bacteriol. 1990;172:1392–1399. doi: 10.1128/jb.172.3.1392-1399.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Robinson A C, Kenan D J, Hatfull G F, Sullivan N F, Spiegelberg R, Donachie W D. DNA sequence and transcriptional organization of essential cell division genes ftsQ and ftsA of Escherichia coli: evidence for overlapping transcriptional units. J Bacteriol. 1984;160:546–555. doi: 10.1128/jb.160.2.546-555.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sambrook J, Maniatis T, Fritsch E F. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 50.Schwedock J, McCormick J R, Angert E R, Nodwell J R, Losick R. Assembly of the cell division protein FtsZ into ladder-like structure in the aerial hyphae of Streptomyces coelicolor. Mol Microbiol. 1997;25:847–858. doi: 10.1111/j.1365-2958.1997.mmi507.x. [DOI] [PubMed] [Google Scholar]
- 51.Strohl W R. Compilation and analysis of DNA sequences associated with apparent streptomycete promoters. Nucleic Acids Res. 1992;20:961–974. doi: 10.1093/nar/20.5.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tan H, Chater K F. Two developmentally controlled promoters of Streptomyces coelicolor A3(2) that resemble the major class of motility-related promoters in other bacteria. J Bacteriol. 1993;175:933–940. doi: 10.1128/jb.175.4.933-940.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vicente M, Errington J. Structure, function and controls in microbial division. Mol Microbiol. 1996;20:1–7. doi: 10.1111/j.1365-2958.1996.tb02482.x. [DOI] [PubMed] [Google Scholar]
- 54.Wang X, de Boer P A J, Rothfield L I. A factor that positively regulates cell division by activating transcription of the major cluster of essential cell division genes of Escherichia coli. EMBO J. 1991;10:3363–3372. doi: 10.1002/j.1460-2075.1991.tb04900.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang X, Lutkenhaus J. Characterization of the ftsZ gene from Mycoplasma pulmonis, an organism lacking a cell wall. J Bacteriol. 1996;178:2314–2319. doi: 10.1128/jb.178.8.2314-2319.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang X, Lutkenhaus J. FtsZ ring: the eubacterial division apparatus conserved in archaebacteria. Mol Microbiol. 1996;21:313–319. doi: 10.1046/j.1365-2958.1996.6421360.x. [DOI] [PubMed] [Google Scholar]
- 57.Ward J M, Janssen G R, Kieser T, Bibb M J, Buttner M J, Bibb M J. Construction and characterisation of a series of multi-copy promoter-probe plasmid vectors for Streptomyces using the aminoglycoside phosphotransferase gene from Tn5 as indicator. Mol Gen Genet. 1986;203:468–478. doi: 10.1007/BF00422072. [DOI] [PubMed] [Google Scholar]
- 58.Zhou P, Heimstetter C E. Relationship between ftsZ gene expression and chromosome replication in Escherichia coli. J Bacteriol. 1994;176:6100–6106. doi: 10.1128/jb.176.19.6100-6106.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zukowski M M, Gaffney D F, Speck D, Kauffman M, Findeli A, Wisecup A, Lecocq J P. Chromogenic identification of genetic regulatory signals in Bacillus subtilis based on expression of a cloned Pseudomonas gene. Proc Natl Acad Sci USA. 1983;80:1101–1105. doi: 10.1073/pnas.80.4.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]