Abstract
The coronavirus 2019 omicron variant has surged rapidly and raises concerns about immune evasion even in individuals with complete vaccination, because it harbors mutations. Here we examine the capability of booster vaccination following CoronaVac/AZD1222 prime to induce neutralizing antibodies (NAbs) against omicron (BA.1 and BA.2) and T‐cell responses. A total of 167 participants primed with heterologous CoronaVac/AZD1222 for 4–5 months were enrolled, to receive AZD1222, BNT162b2, or mRNA‐1273 as a third dose. Reactogenicity was recorded. Immunogenicity analyses of severe acute respiratory syndrome coronavirus 2‐binding antibodies were measured using enzyme‐linked immunosorbent assay. The NAb titers against omicron BA.1 and BA.2 were determined using the focus reduction neutralization test (FRNT50) and total interferon‐γ responses were measured to observe the T‐cell activation. A substantial loss in neutralizing potency to omicron variant was found at 4–5 months after receiving the heterologous CoronaVac/AZD1222. Following booster vaccination, a significant increase in binding antibodies and neutralizing activities toward delta and omicron variants was observed. Neutralization to omicron BA.1 and BA.2 were comparable, showing the highest titers after boosted mRNA‐1273 followed by BNT162b2 and AZD1222. In addition, individuals boosted with messenger RNA (mRNA) vaccines develop a T‐cell response to spike protein, whereas those boosted with AZD1222 did not. Reactogenicity was mild to moderate without serious adverse events. Our findings demonstrated that mRNA booster vaccination is able to overcome waning immunity to provide antibodies that neutralize omicron BA.1 and BA.2, as well as a T‐cell response.
Keywords: AZD1222, booster vaccine, COVID‐19, heterologous, mRNA vaccine, omicron
1. INTRODUCTION
As of November 2021, the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) omicron (B.1.1.529) variant quickly surged worldwide and raised concern about immune evasion. 1 Due to high transmissibility and the potential to evade immunity, the World Health Organization Technical Advisory Group on SARS‐CoV‐2 Virus Evolution declared the omicron as a variant of concern. 2 Omicron variant is now reported in more than 190 countries and causes the global case count of over 10 million weekly cases between December 2021 and March 2022. 3 During the omicron wave, the mortality rate was 0.27% over positive cases and 0.021% overpopulation as of February 2022. 4 The total number of estimated infections was about twofold higher for omicron and showed a twofold lower rate of mortality relative to delta. 5
The omicron variant is characterized by many mutations in the spike protein. Among these, 15 mutations located on the receptor‐binding domain (RBD), which are responsible for interactions with the angiotensin‐converting enzyme 2 (ACE2) receptor, and 8 amino acid changes are found at the N‐terminal domain (NTD). 6 In a study of mutated RBD profiles, various omicron mutations capable of escaping human neutralizing antibodies (NAbs) were found to contain epitopes overlapping the ACE2‐binding motif, indicating evasion of immunity and reduction in vaccine effectiveness. 7 Furthermore, mutations in NTD related to partial escape from the Nabs have been reported. 8 These findings are consistent with other clinical data demonstrating that the emergence of the omicron variant has led to an increase in the risk of reinfection. 9 During the ongoing viral evolution, the omicron variant has been divided into four subvariants, including BA.1, BA.1.1, BA.2, and BA.3. 10 Among them, BA.1 surged earlier and became the dominant subvariant circulating worldwide. However, the BA.2 subvariant has recently increased in multiple countries and appears to be more transmissible than BA.1. 11 Previous studies suggest that BA.1 and BA.2 are highly resistant to neutralization by monoclonal antibody therapy and vaccine‐induced immunity. 12 , 13
Another concern is the waning immunity that occurs over time. A previous study indicates that the IgG antibodies declined a consistent rate at 6 months after second dose of the messenger RNA (mRNA) vaccination, whereas NAbs declined rapidly over the first 3 months followed by a relatively slower decrease after that point. 14 A reduction in NAbs is related to an increased risk of symptomatic SARS‐CoV‐2 infection and reduced vaccine effectiveness. 15 Moreover, omicron variants were poorly or not at all neutralized in the sera sampled 5 months after completing the two‐dose BNT162b2 or AZD1222 vaccination courses. 16 Due to the emergence of omicron and waning immunity, a booster vaccination program has been implemented in many countries. 17 , 18 Thus, data on boosting immunity against omicron variant are needed.
Besides humoral immunity, cell‐mediated immunity also plays an essential role in limiting the SARS‐CoV‐2 infection 19 and reducing disease severity in acute coronavirus 2019 (COVID‐19) patients. 20 The SARS‐CoV‐2‐specific T cells persist at least 6 months after receiving the two‐dose regimen of either mRNA or adenoviral‐vectored vaccines, whereas the levels of NAb severely declined. 21 Furthermore, SARS‐CoV‐2‐specific T cells induced by vaccination or previous infection are highly cross‐reactive with the omicron variant and the omicron cross‐reactive T cells exhibiting the polyfunctional profiles were not significantly different compared with ancestral strain and other SARS‐CoV‐2 variants. 22 These findings indicate that a few mutations in the spike can minimally affect T‐cell recognition.
Seven vaccines have now been authorized in Thailand: (1) inactivated CoronaVac, (2) BBIBP‐CorV, (3) adenoviral‐vectored ChAdOx1‐S/AZD1222, (4) Ad26.COV2.S, (5) mRNA‐based BNT162b2, (6) mRNA‐1273, and (7) protein‐based NVX‐CoV2373 vaccines. 23 However, the vaccine effectiveness and capability of inducing immune responses differ with different types of vaccines and are affected by emerging variants. 24 Our previous study indicated that mRNA vaccine‐ or AZD1222‐boosted individuals after a two‐dose CoronaVac course elicited a higher immune response than in those receiving boosted inactivated vaccines. 25 In the COV‐BOOST trial, the heterologous boost after either two‐dose of AZD1222 or BNT162b2 prime showed an increased humoral and cell‐mediated immune response compared to homologous booster vaccination, although the reactogenicity was increasing in some heterologous boosted combination. 26 However, information about the effects of booster vaccinations on safety and immune responses against omicron variants following heterologous primed has been limited.
Due to the limited vaccine supply, Thailand has administered the heterologous CoronaVac followed by AZD1222 vaccination as an alternative regimen for combatting the delta variant. This regimen could induce a higher immune response than the homologous CoronaVac regimen. 27 However, the antibodies wane over time, and the emergence of the omicron variant has raised concerns about booster vaccinations. In this study, the safety and capability of inducing NAbs against the omicron variant (BA.1 and BA.2 subvariants) and T‐cell responses after receiving AZD1222, BNT162b2, or mRNA‐1273 as a booster dose in individuals who were previously vaccinated with the heterologous CoronaVac/AZD1222 regimen were evaluated.
2. METHODS
2.1. Study design and ethical considerations
In a cohort study, 167 individuals aged 18 years and older, with received CoronaVac followed by AZD1222 as a primary series vaccine regimen at least 4–5 months earlier were eligible for recruitment. The exclusion criteria included the individuals with a previous laboratory‐confirmed SARS‐CoV‐2 infection, history of comorbidities that might potentially induce the reactogenicity after vaccination, history of anaphylaxis, pregnancy, and current use of anticoagulants. The eligible participants were offered immunization with one of three approved vaccines, including AZD1222, BNT162b2, or mRNA‐1273 vaccines. The cohort study started between November 30, 2021 and January 24, 2022. Blood samples were collected at baseline (Day 0) and on Days 14 and 28 after booster vaccination. The study protocol was reviewed and approved by the Institutional Review Board of the Faculty of Medicine, Chulalongkorn University (IRB numbers 871/64) and performed under the Declaration of Helsinki and Good Clinical Practice principles. This study was registered with the Thai Clinical Trials Registry (TCTR20211120002). All participants signed a written consent before being enrolled in this study.
2.2. Study vaccines
The study vaccines used as booster doses included AZD1222 (AstraZeneca), 28 BNT162b2 (Pfizer‐BioNTech Inc.), 29 and mRNA‐1273 (Moderna Inc.). 30 All vaccines were designed using the SARS‐CoV‐2 spike of ancestral strain as a template.
2.3. Reactogenicity assessment
Participants were observed adverse events (AEs) at least 30 min after vaccination. At the baseline visit, the participants were given the AEs report form to self‐record within 7 days after immunization. AEs report forms recorded the solicited local and systemic AEs, and unsolicited and AEs of special interests. The solicited local and systemic AEs were classified as mild (no limitation on normal activity), moderate (some limitation of daily activity), and severe (unable to perform or prevented daily activity).
2.4. Measurement binding antibody and neutralizing activity using enzyme‐linked immunosorbent assay (ELISA)‐based assay
All sera samples measured anti‐RBD IgG and anti‐nucleocapsid (N) IgG using ELISAs (Abbott Diagnostics). The anti‐RBD IgG was expressed as a binding antibody unit (BAU/ml) and a value ≥ 7.1 was considered positive. Anti‐N IgG reported as S/C ratio (optical density (OD) sample divided by calibrator) with a value ≥1.4 was considered positive. In addition, a subset of samples was randomly selected to perform surrogate virus neutralization assay (sVNT) against delta and omicron variants using a cPass™ SAR‐CoV‐2 NAb detection kit (GenScript Biotech) as previously described. 27 Neutralizing activity was reported as the percentage of inhibition (inhibition [%] = [1 − OD value of sample/average OD of negative control] × 100). A value ≥ 30% was defined as positive, indicating the presence of NAbs. The lower limit of detection was set as 0% inhibition.
2.5. Focus reduction neutralization test (FRNT50)
Live SARS‐CoV‐2 NAb titers in a subset of serum samples were determined using a 50% focus reduction neutralization test (FRNT50) against omicron BA.1 (accession number: EPI_ISL_8547017) and BA.2 (EPI_ISL_11698090) subvariants. Briefly, heat‐inactivated sera were used to prepare serial dilutions starting from 1:10 to 1:7290 and incubated with live virus for 1 h at 37°C. The virus–sera mixtures were transferred to monolayers of Vero cells in a 96‐well plate and incubated for 2 h. Foci development evaluation and infected cell counting were performed as previously described. 31 The focus reduction percentage for an individual sample was calculated and the half‐maximal inhibitory concentration was evaluated using PROBIT software. If no neutralization was observed, the FRNT50 was set as 10, which is one dilution step below the lower limit of detection (dilution 1:20).
2.6. Quantification of interferon‐γ (IFN‐γ) response
The SARS‐CoV‐2‐specific T‐cell response was evaluated by using a commercially available IFN‐γ release assay in whole blood according to the manufacturer's instructions (QuantiFERON, Qiagen). Heparinized whole blood was incubated with different antigens, including negative (Nil), positive (Mitogen), and two different SARS‐CoV‐2 antigens (Ag1 and Ag2). The Ag1 tube was coated with S1 subunit (RBD) peptides with CD4+ stimulation and Ag2 contained S1 + S2 peptides for CD4+ and CD8+ stimulation. After stimulation, total IFN‐γ production was measured as previously described. 27 The results were calculated from a standard curve and expressed as IU/ml after subtraction of the Nil control. The total IFN‐γ with a value ≥0.15 IU/ml and ≥25% of Nil were considered a positive response against SARS‐CoV‐2.
2.7. Statistical analysis
Baseline characteristics were expressed as number or percentage and median with interquartile ranges (IQRs). The AEs were presented as the frequency of each solicited AEs and determined the risk difference between vaccine groups. The levels of binding antibody and NAbs were presented as geometric mean titers. A comparison of log‐transformed data was determined using one‐way analysis of variance with Bonferroni adjustment. The Kruskal–Wallis test with Dunn's posthoc correction and Mann–Whitney test were used for unpaired samples, whereas the Friedman and Wilcoxon signed‐rank tests were used for paired samples in cases in which data were not normally distributed. Spearman's R was used to determine the correlation. A p < 0.05 was considered statistically significant. All statistical analyses were performed using SPSS v23.0 (IBM Corp.). Figures were generated using GraphPad Prism v9.0 (GraphPad) and R version 3.6.0 (R Foundation). Details of statistical analysis for each experiment are described in the figure legends.
3. RESULTS
3.1. Study participants
A total of 167 vaccinated individuals who received heterologous Coronavac/AZD1222 were enrolled to receive AZD1222 (n = 60), BNT162b2 (n = 55), and mRNA‐1273 (n = 52) with a booster interval ranging from 4 to 5 months post second dose (Figure 1A). Overall, participants included 83 (49.7%) women and 84 (50.3%) men with ages ranging from 19 to 64 years. The mean age of participants who received AZD1222 was 41.2 years (IQR: 38.5–43.9), BNT162b2 39.0 years (IQR: 36.4–41.6), and mRNA‐1273 43.9 years (IQR:40.9–46.8), as booster doses were not significantly different (Table 1). Additionally, no statistically significant differences were observed in the time intervals between the first and the second doses for any group. However, the median interval between the second and third dose in AZD1222 (125 days, IQR: 118–134.5) was slightly shorter (but not statistically significant) than BNT162b2 (130 days, IQR: 110–141) and mRNA‐1273 (131 days, IQR: 102.3–133).
Figure 1.
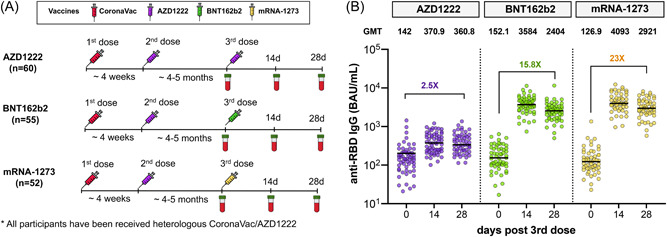
Study design and measurement of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2)‐specific receptor‐binding domain (RBD)‐binding antibody responses. Schematic depicting a total of 167 vaccinated individuals with heterologous CoronaVac/AZD1222 enrolled in cohort study. They were assigned to receive booster vaccines, either AZD1222 (n = 60), BNT162b2 (n = 55), or mRNA‐1273 (n = 52), and blood samples were collected on Days 0, 14, and 28 after booster vaccination (A). The anti‐RBD IgG (BAU/ml) in sera from boosted individuals with different vaccines, AZD1222 (purple), BNT162b2 (green), and mRNA‐1273 (yellow), were compared (B). Error bars in B indicate the geometric mean titers (GMTs).
Table 1.
Characteristics of participants in the study.
| Characteristics | Total (n = 167) | AZD1222 (n = 60) | BNT162b2 (n = 55) | mRNA‐1273 (n = 52) |
|---|---|---|---|---|
| Sex (n, %) | ||||
| Female | 83 (49.7%) | 37 (61.7%) | 26 (47.3%) | 20 (38.5%) |
| Male | 84 (50.3%) | 23 (38.3%) | 29 (52.7%) | 32 (61.5%) |
| Age in years (mean, range) | 41.3 (35–48) | 41.2 (38.5–43.9) | 39.0 (36.4–41.6) | 43.9 (40.9–46.8) |
| Interval between 1st and 2nd dose (median, IQR) | 27 (21–28) | 24 (21–28) | 27 (21–28) | 27 (24.3–28) |
| Interval between 2nd and 3rd dose (median, IQR) | 130 (110–135) | 125 (118–134.5) | 130 (110–141) | 131 (102.3–133) |
Abbreviation: IQR, interquartile range.
3.2. Increase binding antibodies level after boost
Waning immunity against SARS‐CoV‐2 in heterologous CoronaVac/AZD1222 primed individuals was determined. The anti‐RBD IgG at 1 month (n = 35) as reported in a previous study 27 was individually compared with 4–5 months after the second dose (baseline in this cohort) as shown in Supporting Information: Figure 1. As expected, a significant drop (5.9‐fold) in anti‐RBD IgG within 4–5 months (109.6 BAU/ml) occurred compared with 1 month post second dose (652.1 BAU/ml; p < 0.001). This result indicates a decline in immunity over time in vaccinated individuals who received heterologous CoronaVac/AZD1222. Following booster vaccination, the anti‐RBD IgG significantly increased and peaked at Day 14 for all vaccines (p < 0.001) as shown in Figure 1B. Comparing pre‐ and post‐boost, mRNA‐1273‐boosted individuals achieved an anti‐RBD IgG with a 23‐fold increase (126.9 vs. 2921 BAU/ml) and showed a higher level than the other vaccine groups, whereas BNT162b2 and AZD1222 groups were induced by 15.8‐fold (152.1 vs. 2404 BAU/ml) and 2.5‐fold (142 vs. 360.8 BAU/ml) higher than baseline. Most boosted individuals were seronegative for anti‐N IgG, indicating no SARS‐CoV‐2 exposure during the study period (Supporting Information: Figure 2). Although one participant had an anti‐N IgG level above the cutoff value, the anti‐RBD IgG level was comparable with other participants at baseline, suggesting the anti‐N IgG may be induced by CoronaVac prime vaccination.
3.3. Neutralizing activity to delta and omicron measured using sVNT
Neutralizing activity to the delta and omicron variants was measured in sera at baseline and 28 days post‐boost using the surrogate virus neutralization test. All boosted individuals could restore neutralizing activity to delta by more than 90% (Figure 2A). Although baseline neutralizing activity to omicron declined at 4–5 months after the second dose, a 20% (6/30), 83% (25/30), and 90% (27/30) of individuals boosted with AZD1222, BNT162b2, and mRNA‐1273, respectively, were detected to possess omicron variant neutralizing potential (Figure 2B). By comparison, it was noted that individuals boosted with mRNA vaccines demonstrated a higher level of neutralizing activity than those boosted with AZD1222. The median of neutralizing activity to omicron was 10.1% for AZD1222, 55.9% for BNT162b2, and 78.2% for mRNA‐1273 after booster vaccination. Although neutralizing activity against omicron was significantly lower than that against the delta variant, most individuals have detected the neutralizing activity against omicron after receiving booster mRNA vaccines (Figure 2C).
Figure 2.
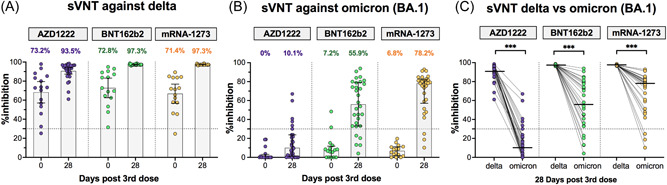
Neutralizing activities measured using surrogate virus neutralization test against delta and omicron (BA.1). A subset of samples from boosted individuals with AZD1222 (purple), BNT162b2 (green), and mRNA‐1273 (yellow), which was randomly selected to test the surrogate virus neutralization test (sVNT) that included sera collected at baseline (n = 15/group) and sera collected at 28 days post‐boost (n = 30/group). Neutralizing activities against delta (A) and omicron (BA.1) (B) were compared between pre‐ and post‐booster vaccination. Numbers above the bar graph indicate the percentage of inhibition between human angiotensin converting enzyme 2 and receptor binding domain (ACE‐2 and RBD, respectively) proteins. A comparison of the neutralizing activity between delta and omicron variants at 28 days after booster vaccination is shown in (C). Median values are shown as horizontal bars. Dotted lines indicate cut‐off values (30%). The comparison was perform using Wilcoxon signed‐rank test (two‐tailed). ***p < 0.001.
3.4. Comparison of NAb titers to omicron BA.1 and BA.2
The functional NAb titers against omicron BA.1 and BA.2 were quantified using a live virus neutralization test (FRNT50). At baseline, 80% (24/30) and 43% (13/30) of vaccinated individuals with heterologous CoronaVac/AZD1222 had NAbs to omicron BA.1 and BA.2, respectively, which dropped below detectable levels (Figure 3). After 28 days post‐boost, NAb titers against omicron BA.1 were 40.3, 171.0, and 271.6 after AZD1222, BNT162b2, and mRNA‐1273 boosters, respectively, reflecting a 3.16‐, 9.91‐, and 24.78‐fold increase compared with baseline, respectively (Figure 3A). The NAb titers against omicron BA.2 in AZD1222, BNT162b2, and mRNA‐1273 groups were 59.3, 130.7, and 235.3, which reached a 2.43‐, 4.63‐, and 19.67‐fold induction relative to baseline, respectively (Figure 3B). This finding indicates that the omicron variant is more susceptible to neutralization by sera from individuals boosted with mRNA vaccines (BNT162b2 and mRNA‐1273) than those boosted with AZD1222. Overall, the NAb titers to BA.1 and BA.2 were comparable. By comparison, the NAb titer to BA.2 was 1.47‐fold higher in the AZD1222 group and 1.31‐ and 1.15‐fold lower in the BNT162b2 and mRNA‐1273 groups, respectively, compared with BA.1 (Figure 3C). Moreover, it was shown that anti‐RBD IgG and sVNT to omicron correlated well with the NAb titers against omicron BA.1. and BA.2 as measured using the live virus neutralization test (FRNT50), as shown in Supporting Information: Figure 3.
Figure 3.
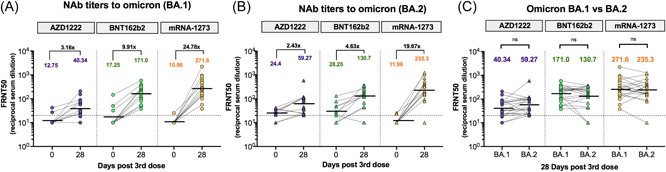
Neutralizing antibody (NAb) titers against omicron BA.1 and BA.2 measured using focus reduction neutralization test (FRNT50). A subset of samples from boosted individuals with AZD1222 (purple), BNT162b2 (green), and mRNA‐1273 (yellow), which was randomly selected to test the FRNT50 included sera collected at baseline (n = 10/group) and sera collected at 28 days post‐boost (n = 30/group). NAb titers against omicron BA.1 (A) and omicron BA.2 (B) were compared between baseline (Day 0) and 28 days post‐booster vaccination with different vaccines. Numbers above the plot indicate the geometric mean titers (GMTs). Fold increases for each comparison are denoted. NAb titers against BA.1 and BA.2 at 28 days after booster vaccination were compared (C). Statistical analysis was done using Wilcoxon signed‐rank test (two‐tailed). The horizontal dotted line indicates the limit of detectable value of FRNT50. Values below the limit of detection (<20) were set at a titer of 10 before statistical analysis. ns, no significant difference.
3.5. Total IFN‐γ response
Besides the NAbs, the T‐cell response were assessed by measuring total IFN‐γ responses in whole blood from AZD1222‐, BNT162b2‐, and mRNA‐1273‐boosted individuals after S1 (RBD) peptides for CD4+ stimulation or Ag1 (Figure 4A) and S1+ S2 peptides for CD4+ and CD8+ stimulation or Ag2 (Figure 4B). At baseline, 47% (43/90) and 57.7% (52/90) of participants could elicit IFN‐γ responses after Ag1 and Ag2 stimulation after 4–5 months post second dose. Following booster vaccination, IFN‐γ levels significantly increased at 14 days after receiving BNT162b2 and mRNA‐1273 as booster doses. Notably, 90%–97% of individuals boosted with BNT162b2 and mRNA‐1273 could induce IFN‐γ responses after stimulation with the spike protein of the ancestral strain. On the contrary, no difference in IFN‐γ response in those boosted with AZD1222 compared with baseline was found. This result indicates that individuals boosted with BNT162b2 and mRNA‐1273 vaccines could induce a T‐cell response, which elicits a higher level of IFN‐γ response, but this process was not observed in those boosted with the AZD1222 vaccine.
Figure 4.
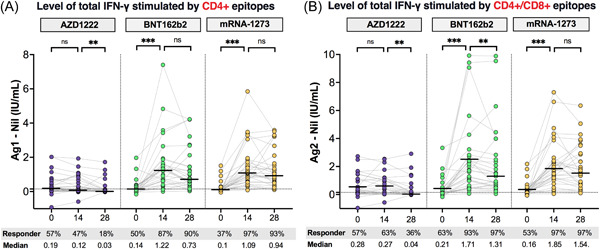
Comparison of total interferon‐γ (IFN‐γ)‐releasing T‐cell responses to severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) antigens. Heparinized whole blood samples from vaccinated individuals receiving heterologous CoronaVac/AZD1222 followed by a third booster with AZD1222 (n = 30, purple), BNT162b2 (n = 30, green), or mRNA‐1273 (n = 30, yellow) were stimulated by Ag1, which is a CD4+ epitope derived from receptor‐binding domain (RBD), minus negative control (Nil) (A), and Ag2 which is CD4+ and CD8+ epitopes derived from S1 and S2 subunits, minus negative control (Nil) (B). Horizontal bars indicate the median. The cut‐off values were represented by horizontal dotted line. Levels of IFN‐γ above cut‐off values (0.15 IU/ml and ≥25% of Nil) indicate a reactive response. Statistical analysis was done using Wilcoxon signed‐rank test (two‐tailed). ns, no significant difference; **p < 0.01; ***p < 0.001.
3.6. Reactogenicity after booster vaccination
Local and systemic reactogenicity were self‐reported within 7 days after booster vaccination. A high frequency of AEs was observed within 2–3 days following the booster dose and were predominantly mild to moderate (Supporting Information: Figure 6). Boosted individuals with BNT162b2 and mRNA‐1273 vaccines reported greater local and systemic reactogenicity than those receiving the AZD1222 vaccine. Overall, the most common AEs observed in boosted individuals were injection site pain, redness, and swelling indicating local AEs, whereas myalgia headache and chills were frequently reported as systemic AEs (Figure 5). However, no serious AEs were reported.
Figure 5.
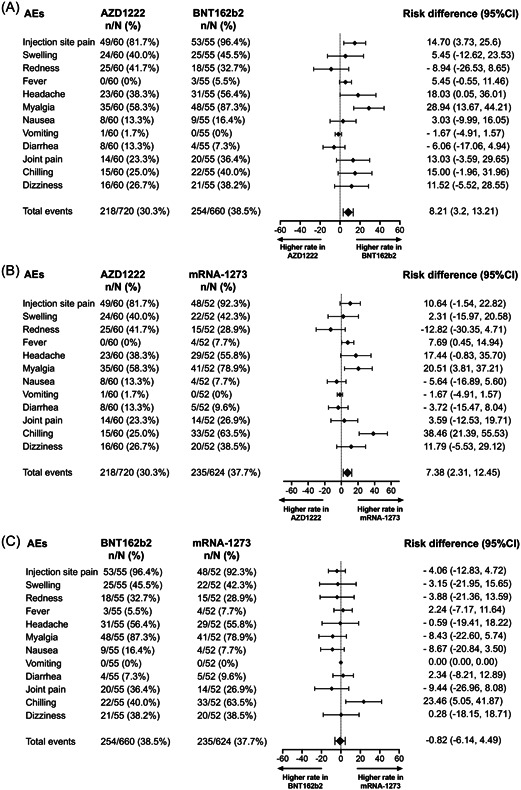
Forest plot showed the risk difference with 95% confidence intervals of adverse events (AEs) after booster vaccination. The proportion of participants with any grade of solicited AEs after receipt the third dose were compared between AZD1222 versus BNT162b2 vaccine (A), AZD1222 versus mRNA‐1273 (B), and BNT162b2 versus mRNA‐1273 (C).
4. DISCUSSION
This study demonstrated the NAb response against omicron BA.1 and BA.2 subvariants and T‐cell responses after boosting with AZD1222, BNT162b2, and mRNA‐1273 in vaccinated individuals who had received heterologous CoronaVac/AZD1222 prime. It was found that a booster vaccination could restore the binding antibody response and induce NAb titers against omicron BA.1 and BA.2. Of note, our findings indicate that individuals boosted with mRNA‐1273 and BNT162b2 vaccines could induce humoral and T‐cell responses higher than those boosted with AZD1222. Although mRNA vaccines showed a higher frequency of AEs than AZD1222 vaccine, the reactogenicity was in acceptable ranges, indicating a good safety profiles after booster vaccination.
It was found that individuals who received the heterologous CoronaVac/AZD1222 vaccination exhibited a 5.9‐fold reduction in binding antibody and less detectable NAbs to omicron variants after 4–5 months post second dose, indicating waning of vaccine‐induced immunity. 14 It has been well established that booster vaccinations could overcome the waning immunity. 18 , 25 , 26 , 32 Rapid elevation in binding antibodies after boosting with mRNA and adenoviral‐vectored vaccines were found. Similar results have been reported after receiving either a viral‐vectored or an mRNA vaccine as a third dose, indicating that it was sufficient to recall the memory B cells. 32
NAb titers is a highly predict the immune protection against SARS‐CoV‐2 infection. Higher levels of NAbs correlated with a reduced risk of infection and severe disease. 15 Our study found a substantial loss of NAb titers against omicron variants at pre‐booster vaccination. This effect might be related to harboring of mutations concentrated around the RBD. 7 However, the NAb titers against omicron were retained upon booster vaccination even though the current vaccines composition was based on the ancestral strain. Consistent results were observed after receiving mRNA vaccine following either homologous inactivated, adenoviral‐vectored, or mRNA‐based vaccines prime. 18 , 25 , 26 , 33 Although the low number of omicron‐neutralizing memory B cells produced after two‐doses of mRNA vaccination has previously been reported, their coverages were improved through affinity maturation over time and might be sufficient to expand the coverage of neutralization against SARS‐CoV‐2 variants after receiving a booster dose. 34 Furthermore, a reduced neutralizing activity against omicron compared with delta variant was found. Similar results were found concerning neutralizing activity to omicron, which was reduced by 6‐ to 23‐fold lower than delta after booster vaccination. 16
With the surge of omicron BA.1 and BA.2 subvariants, a substantial loss of NAbs to omicron BA.1 and BA.2 subvariants was observed in vaccinated individuals with two doses of mRNA vaccine and patients who previously were infected with wild‐type SARS‐CoV‐2. 35 Furthermore, a study of the NAb of omicron subvariants indicated that a 23‐fold reduction for BA.1 and a 27‐fold reduction for BA.2 was observed compared with wild type in vaccinated individuals who received the two doses of BNT162b2 vaccine. 33 Our findings indicate that booster vaccination with mRNA and adenoviral‐vectored vaccines could increase the NAb titers and coverage against omicron BA.1 and BA.2. Consistent results showed an improvement in neutralization sensitivity against BA.1 and BA.2 after individuals were boosted with the mRNA vaccine. 33 , 36 Although both subvariants shared several common amino acid changes, the unique mutations found in each subvariant might affect the differences in neutralization potency. 35 However, it was found that the NAb titer of BA.1 and BA.2 were comparable and trended higher in the adenoviral‐vectored booster and lower in mRNA vaccines. A recent study showed a 1.4‐fold lower NAb titer to BA.2 compared with BA.1. 33 This result indicates that booster vaccination is a useful strategy for controlling the omicron BA.1 and BA.2 pandemic.
A study of T‐cell responses targeting the omicron spike protein suggested that no differences in T‐cell profiles and cytokine production between omicron and wild type were found. 21 This finding indicates cross‐recognition of T‐cells was minimally affected by mutations in the omicron spike protein. 22 , 37 A current study showed that individuals boosted with the mRNA vaccine could induce T‐cell activity in whole blood. Cross‐recognition of different SARS‐CoV‐2 variants by T cells was maintained after being boosted with mRNA vaccine has been reported. 21 , 38 On the contrary, our result showed that individuals boosted with AZD1222 following heterologous CoronaVac/AZD1222 appeared to abolish T‐cell responses.
Study limitations included the small sample size and the detection limits of the sVNT assay. Most boosted individuals elicited an elevation in antibody level that was higher than the upper limit of detection; thus, this method may not have provided the actual NAb level in case of a robust immune response. Furthermore, the effect of omicron peptide stimulation on T‐cell response was not examined. However, van Kessel et al. 21 showed that no difference in cytokine production was observed upon stimulation with S‐peptide pools derived from the ancestral strain and omicron variants. Further studies are required to define long‐term immunity and durability of immune responses against SARS‐CoV‐2 variants, particularly omicron subvariants, after administration of booster vaccinations.
In conclusion, these findings indicate that booster vaccination could retain the level of anti‐RBD IgG and improved the neutralizing activity against delta and omicron variants. Of note, a booster dose could induce NAb titers to omicron BA.1 comparable to BA.2. Furthermore, individuals boosted with mRNA vaccines could induce IFN‐γ responses higher than those boosted with AZD1222. Hence, giving mRNA vaccines as the booster dose could improve humoral and T‐cell responses and induce neutralization coverage against omicron subvariants. These findings support the policymakers' choices about which booster vaccines to use in the population to overcome waning immunity and prevent breakthrough infections during the recent emergence of the SAR‐CoV‐2 variants.
AUTHOR CONTRIBUTIONS
Conceptualization: Nungruthai Suntronwong, Pornjarim Nilyanimit, Nasamon Wanlapakorn, and Yong Poovorawan. Data collection: Jira Chansaenroj, Donchida Srimuan, Thaksaporn Thatsanatorn, and Natthinee Sudhinaraset. Formal analysis: Nungruthai Suntronwong, Sitthichai Kanokudom, Suvichada Assawakosri, and Yong Poovorawan. Methodology: Nungruthai Suntronwong, Chompoonut Auphimai, Thanunrat Thongmee, Preeyaporn Vichaiwattana, Thaneeya Duangchinda, Warangkana Chantima, Pattarakul Pakchotanon, Juthathip Mongkolsapaya, Sitthichai Kanokudom, Suvichada Assawakosri, and Jiratchaya Puenpa. Project administration: Yong Poovorawan. Writing—original draft: Nungruthai Suntronwong. Writing—review and editing: Nungruthai Suntronwong, Nasamon Wanlapakorn, and Yong Poovorawan. All authors have read and agreed to the published version of the manuscript.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ETHICS STATEMENT
This study was performed following the Declaration of Helsinki and Good Clinical Practice principles. The study protocol was reviewed and approved by the Institutional Review Board of the Faculty of Medicine of Chulalongkorn University (IRB number 871/64). This study was registered with the Thai Clinical Trials Registry (TCTR20211120002). All participants signed a written consent before being enrolled.
Supporting information
Supplementary information.
ACKNOWLEDGMENTS
We would like to thank all the staffs of the Center of Excellence in Clinical Virology and all the participants for helping and supporting in this project. We also appreciate the Ministry of Public Health, Chulabhorn Royal Academy, and Zullig pharma for providing the vaccines for this study. This study was supported by the National Research Council of Thailand, the Health Systems Research Institute, the Center of Excellence in Clinical Virology of Chulalongkorn University, and King Chulalongkorn Memorial Hospital, National Center for Genetic Engineering and Biotechnology (BIOTEC Platform No. P2051613). Nungruthai Suntronwong reports that financial support was also provided by the Second Century Fund Fellowship of Chulalongkorn University.
Suntronwong N, Kanokudom S, Auphimai C, et al. Effects of boosted mRNA and adenoviral‐vectored vaccines on immune responses to omicron BA.1 and BA.2 following the heterologous CoronaVac/AZD1222 vaccination. J Med Virol. 2022;1‐10. 10.1002/jmv.28044
DATA AVAILABILITY STATEMENT
All data generated during this study are contained within this manuscript and its Supporting Information files.
REFERENCES
- 1. Viana R, Moyo S, Amoako DG, et al. Rapid epidemic expansion of the SARS‐CoV‐2 omicron variant in Southern Africa. Nature. 2022;603:679‐686. 10.1038/s41586-022-04411-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. World Health Organization . 2022. Classification of Omicron (B.1.1.529): SARS‐CoV‐2 Variant of Concern. Accessed June 30, https://www.who.int/news/item/26-11-2021-classification-of-omicron-(b.1.1.529)-sars-cov-2-variant-of-concern
- 3. World Health Organization . 2022. Accessed June 30, 2022. https://covid19.who.int
- 4. Larrauri BJ, Malbrán A, Larrauri JA. Omicron and vaccines: an analysis on the decline in COVID‐19 mortality. medRxiv . 2022. 10.1101/2022.05.20.22275396 [DOI]
- 5. Sigal A, Milo R, Jassat W. Estimating disease severity of omicron and delta SARS‐CoV‐2 infections. Nat Rev Immunol. 2022;22(5):267‐269. 10.1038/s41577-022-00720-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Harvey WT, Carabelli AM, Jackson B, et al. SARS‐CoV‐2 variants, spike mutations and immune escape. Nat Rev Microbiol. 2021;19:409‐424. 10.1038/s41579-021-00573-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cao Y, Wang J, Jian F, et al. Omicron escapes the majority of existing SARS‐CoV‐2 neutralizing antibodies. Nature. 2022;602:657‐663. 10.1038/s41586-021-04385-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Liu L, Iketani S, Guo Y, et al. Striking antibody evasion manifested by the omicron variant of SARS‐CoV‐2. Nature. 2022;602:676‐681. 10.1038/s41586-021-04388-0 [DOI] [PubMed] [Google Scholar]
- 9. Pulliam J, van Schalkwyk C, Govender N, et al. Increased risk of SARS‐CoV‐2 reinfection associated with emergence of Omicron in South Africa. Science. 2022;376:eabn4947. 10.1126/science.abn4947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kumar S, Karuppanan K, Subramaniam G. Omicron (BA. 1) and Sub‐Variants (BA. 1, BA. 2 and BA. 3) of SARS‐CoV‐2 spike infectivity and pathogenicity: a comparative sequence and structural‐based computational assessment. J Med Virol . 2022. 10.1002/jmv.27927 [DOI] [PMC free article] [PubMed]
- 11. GISAID . 2022. Overview of variants/mutations. Accessed April 16, 2022. https://covariants.org/per-variant
- 12. Takashita E, Kinoshita N, Yamayoshi S, et al. Efficacy of antibodies and antiviral drugs against Covid‐19 omicron variant. N Engl J Med. 2022;386:995‐998. 10.1056/NEJMc2119407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Takashita E, Kinoshita N, Yamayoshi S, et al. Efficacy of antiviral agents against the SARS‐CoV‐2 omicron subvariant BA. 2. N Engl J Med. 2022;386:1475‐1477. 10.1056/NEJMc2201933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Levin EG, Lustig Y, Cohen C, et al. Waning immune humoral response to BNT162b2 Covid‐19 vaccine over 6 months. N Engl J Med. 2021;385:e84. 10.1056/NEJMoa2114583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Khoury DS, Cromer D, Reynaldi A, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS‐CoV‐2 infection. Nat Med. 2021;27:1205‐1211. 10.1038/s41591-021-01377-8 [DOI] [PubMed] [Google Scholar]
- 16. Planas D, Saunders N, Maes P, et al. Considerable escape of SARS‐CoV‐2 omicron to antibody neutralization. Nature. 2022;602:671‐675. 10.1038/s41586-021-04389-z [DOI] [PubMed] [Google Scholar]
- 17. Bar‐On YM, Goldberg Y, Mandel M, et al. Protection of BNT162b2 vaccine booster against Covid‐19 in Israel. N Engl J Med. 2021;385:1393‐1400. 10.1056/NEJMoa2114255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Munro APS, Janani L, Cornelius V, et al. Safety and immunogenicity of seven COVID‐19 vaccines as a third dose (booster) following two doses of ChAdOx1 nCov‐19 or BNT162b2 in the UK (COV‐BOOST): a blinded, multicentre, randomised, controlled, phase 2 trial. Lancet. 2021;398:2258‐2276. 10.1016/S0140-6736(21)02717-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. McMahan K, Yu J, Mercado NB, et al. Correlates of protection against SARS‐CoV‐2 in rhesus macaques. Nature. 2021;590:630‐634. 10.1038/s41586-020-03041-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Moderbacher CR, Ramirez SI, Dan JM, et al. Antigen‐specific adaptive immunity to SARS‐CoV‐2 in acute COVID‐19 and associations with age and disease severity. Cell. 2020;183:996‐1012. 10.1016/j.cell.2020.09.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. van Kessel CH, Geers D, Schmitz KS, et al. Divergent SARS CoV‐2 omicron‐reactive T‐ and B cell responses in COVID‐19 vaccine recipients. Sci Immunol. 2022;7:eabo2202. 10.1126/sciimmunol.abo2202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Keeton R, Tincho MB, Ngomti A, et al. T cell responses to SARS‐CoV‐2 spike cross‐recognize omicron. Nature. 2022;603:488‐492. 10.1038/s41586-022-04460-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Snyder J, Root‐Wiley M. 2022. 7 Vaccines approved for use in Thailand [Internet]. Accessed 16 April, 2022. https://covid19.trackvaccines.org/country/thailand/
- 24. Andrews N, Stowe J, Kirsebom F, et al. Covid‐19 vaccine effectiveness against the omicron (B. 1.1. 529) variant. N Engl J Med. 2022;386:1532‐1546. 10.1056/NEJMoa2119451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Assawakosri S, Kanokudom S, Suntronwong N, et al. Neutralizing activities against the omicron variant after a heterologous booster in healthy adults receiving two doses of CoronaVac vaccination. J Infect Dis . 2022:jiac092. 10.1093/infdis/jiac092 [DOI] [PubMed]
- 26. Liu X, Munro AP, Feng S, et al. Persistence of immunogenicity after seven COVID‐19 vaccines given as third dose boosters following two doses of ChAdOx1 nCov‐19 or BNT162b2 in the UK: three month analyses of the COV‐BOOST trial. J Infect. 2022;S0163‐4453(22):00200‐00206. 10.1016/j.jinf.2022.04.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wanlapakorn N, Suntronwong N, Phowatthanasathian H, et al. Safety and immunogenicity of heterologous and homologous inactivated and adenoviral‐vectored COVID‐19 vaccines in healthy adults. Hum Vaccin Immunother. 2022;18:2029111. 10.1080/21645515.2022.2029111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Folegatti PM, Ewer KJ, Aley PK, et al. Safety and immunogenicity of the ChAdOx1 nCoV‐19 vaccine against SARS‐CoV‐2: a preliminary report of a phase 1/2, single‐blind, randomised controlled trial. Lancet. 2020;396:467‐478. 10.1016/S0140-6736(20)31604-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. US Food and Drug Administration . 2022. Package Insert–Comrinaty [Internet]. Accessed April 16, 2022. https://www.fda.gov/media/151707/download
- 30. US Food and Drug Administration . 2022. Fact sheet for healthcare providers administering vaccine‐the moderna covid‐19 vaccine [Internet]. Accessed 16 April 16, 2022. https://www.fda.gov/media/144637/download
- 31. Supasa P, Zhou D, Dejnirattisai W, et al. Reduced neutralization of SARS‐CoV‐2 B. 1.1. 7 variant by convalescent and vaccine sera. Cell. 2021;184:2201‐2211. 10.1016/j.cell.2021.02.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Liu Y, Zeng Q, Deng C, et al. Robust induction of B cell and T cell responses by a third dose of inactivated SARS‐CoV‐2 vaccine. Cell Discov. 2022;8:10. 10.1038/s41421-022-00373-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yu J, Collier AY, Rowe M, et al. Neutralization of the SARS‐CoV‐2 omicron BA.1 and BA.2 variants. N Engl J Med. 2022;386:1579‐1580. 10.1056/NEJMc2201849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kotaki R, Adachi Y, Moriyama S, et al. SARS‐CoV‐2 omicron‐neutralizing memory B‐cells are elicited by two doses of BNT162b2 mRNA vaccine. Sci Immunol. 2022;7:eabn8590. 10.1126/sciimmunol.abn8590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Iketani S, Liu L, Guo Y, et al. Antibody evasion properties of SARS‐CoV‐2 omicron sublineages. Nature. 2022;604:553‐556. 10.1038/s41586-022-04594-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bowen JE, Sprouse KR, Walls AC, et al. Omicron BA. 1 and BA. 2 neutralizing activity elicited by a comprehensive panel of human vaccines. bioRxiv . 2022. 10.1101/2022.03.15.484542 [DOI] [PMC free article] [PubMed]
- 37. Gao Y, Cai C, Grifoni A, et al. Ancestral SARS‐CoV‐2‐specific T cells cross‐recognize the omicron variant. Nat Med. 2022;28:472‐476. 10.1038/s41591-022-01700-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Naranbhai V, Nathan A, Kaseke C, et al. T cell reactivity to the SARS‐CoV‐2 omicron variant is preserved in most but not all prior infected and vaccinated individuals. medRxiv . 2022. 10.1101/2022.01.04.21268586 [DOI]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information.
Data Availability Statement
All data generated during this study are contained within this manuscript and its Supporting Information files.


