Abstract
Background
Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection and the resulting coronavirus 2019 disease (COVID‐19) have spread all around the world since 2019 and have affected millions of people. The development of COVID‐19 vaccines helped to decelerate the spread of the virus. However, as in the case of vaccines against other infectious diseases, adverse events can also present with COVID‐19 vaccines.
Case Presentation
We report here a rare case of a 53‐year‐old man with knee‐joint synovitis, after the first dose of messenger RNA vaccine, with no fever and a negative COVID‐19 reverse transcription polymerase chain reaction test. During a clinical examination the suspicion of pyogenic arthritis was excluded by blood tests and by a complex joint effusion examination, including a microbiological and cytological‐energy analysis of the synovial fluid. The treatment received by our patient consisted of 3 doses of dexamethasone administered intravenously over a period of 3 days. All the symptoms improved after this therapy, and in the 3‐week follow‐up period we recorded full recovery with no consequences.
Conclusion
Case reports on patients undergoing COVID‐19 vaccination should be examined in order to detect rare and long‐term side‐effects. This is the first report to present the outcomes of an ultrastructural analysis of post‐vaccination synovitis.
Keywords: Covid‐19 vaccination, cytological‐energy analysis, reactive arthritis, synovial fluid
1. BACKGROUND
Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection and the resulting coronavirus 2019 disease (COVID‐19) have spread all around the world since 2019 and have affected millions of people. Although vaccines have helped to decelerate the spread of the disease, there have been adverse events associated with almost all types of COVID‐19 vaccines. These adverse events are mostly mild, and they range from injection‐side pain, muscle pain, fatigue and fever to autoimmune diseases such as reactive arthritis, systematic lupus erythematosus, vasculitis, and so on. 1 Severe symptoms are comparatively rare, and include severe pneumonia, acute respiratory distress syndrome, and even multiple organ failure. 2 Reactive arthritis is a sterile arthritis usually caused by an over‐stimulated autoimmune response by bacterial antigens deposited in the joints, typically followed by a gastrointestinal or urinary infection. 3 Although reactive arthritis is mostly associated with bacterial infection, viruses including SARS‐CoV‐2 have also been reported to trigger the condition. 4
Our case reports the development of acute reactive arthritis after a COVID‐19 vaccination. The primary suspicion of pyogenic arthritis was excluded by blood tests and by a complex joint effusion examination of the synovial fluid (SF), including a cytological‐energy analysis. Only 4 other cases of reactive arthritis after COVID‐19 vaccination have been published until now in the literature.
2. CASE PRESENTATION
Our patient is a 53‐year‐old Caucasian man with a medical history of arterial hypertension and diabetes mellitus type 2. He was admitted to the Internal Medicine Department in our small regional hospital with 4‐day persisting swelling, pain and monoarticular synovitis. According to the patient, all these symptoms started 3 days after the first dose of a messenger RNA (mRNA) vaccine (BNT162b2 Pfizer/BioNTech®). The whole time, the patient had no fever. He had no history of trauma, rheumatic disease, or arthrosis in the affected knee joint. There were no pathological findings in the X‐ray of the left knee (Figure 1). There were no abnormalities in the blood count, and C‐reactive protein (CRP) was elevated to 91.8 mg/L.
FIGURE 1.
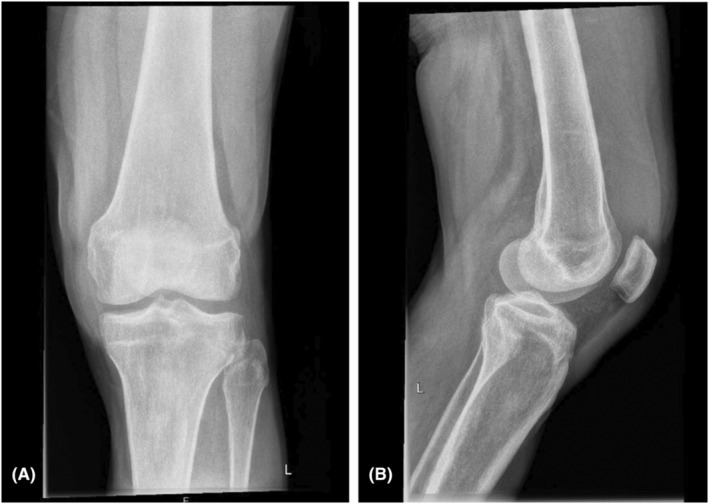
X‐ray of the left knee: anterior‐posterior (A), lateral (B) views
The Orthopedic Department of our hospital was consulted to exclude the suspicion of pyogenic arthritis. During a physical examination, slightly limited flection due to the pain showed up. An ultrasound examination detected an effusion in the left knee joint. A puncture from the suprapatellar recess of the left knee guided by ultrasound was performed, and approximately 50 mL of serous SF sample were aspired; 20 mL of the SF sample were sent for a microbiological analysis, and the remaining 30 mL were sent for a cytological‐energy analysis. The results from the cytological‐energy analysis were known within hours and excluded a pyogenic inflammation process in the affected knee joint, since the metabolic activity was normal. In addition, elevated levels of CRP (22.0 mg/L) were revealed in the SF, which correlated with the levels in the blood sample. The normal level of aspartate aminotransferase (AST) in the SF (17.4 IU/L) excluded local tissue damage in the knee joint. The normal uric acid concentration (237.0 mmol/L) showed no gouty impairment in this location. The treatment that we established was 3 doses of dexamethasone administered intravenously over a period of 3 days. In the 3‐day follow‐up, it was observed that all the symptoms had improved, and that the CRP levels had decreased to 8.75 mg/L. According to the ultrasound a small amount of joint effusion persisted in the left knee. The effusion was punctured and was sent once more for a microbiological and cytological‐energy analysis. The results from the microbiological analysis finally returned with no detection of microbes in either of the SF samples of the left knee. The results of the cytological‐energy analysis confirmed again that there was no presence of an ongoing inflammatory process in the affected knee joint. The levels of CRP decreased to normal levels. The patient was sent home from the inpatient unit, and in the 3‐week follow‐up we recorded a full recovery of our patient with no consequences.
In the analysis of the first SF sample, pleocytosis of neutrophils was found (number of nucleated cells = 4680/1 μL; percentage of neutrophils = 89.0%; percentage of lymphocytes = 8.0%; percentage of monocytes = 3.0%) (Figure 2). The aerobic metabolism in the SF is determined by the coefficient of energy balance (KEB). In the first SF sample area the aerobic metabolism with KEB = 34.1 reliably excluded the purulent type of inflammation induced by extracellular bacteria. 5 , 6 The analysis of the second SF sample manifested a decrease in the cell count (360/1 μL) and a significant decrease in neutrophils (percentage of neutrophils = 6.0%; percentage of lymphocytes = 71.0%; percentage of monocytes = 23.0%) (Figure 3). The persisting aerobic metabolism in the SF sample area (KEB = 33.5) again excluded a significant inflammatory reaction in this site. 5 , 6
FIGURE 2.
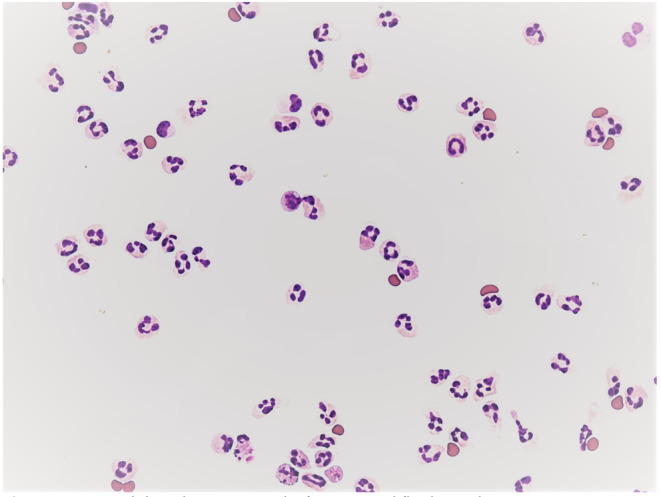
Neutrophil predominance in the first synovial fluid sample
FIGURE 3.
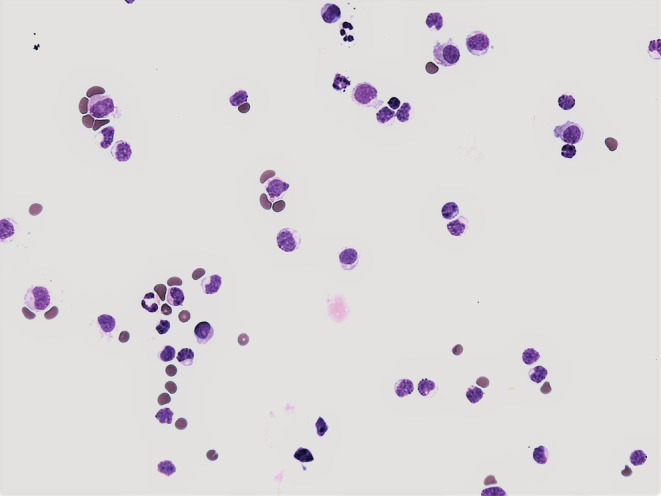
Lymphocyte predominance in the second synovial fluid sample
An evaluation of these findings led us to the conclusion that there had been significant reactive changes in the knee joint, followed by regression.
3. DISCUSSION
Like COVID‐19 vaccines, vaccines against other infectious diseases have helped to decelerate the spread of diseases. Although vaccination is a very beneficial option, adverse events and effects may also be present. Most of the reported adverse events after the Pfizer‐BioNTech vaccine, which was the first mRNA vaccine, are mild and self‐limited. They include injection‐site pain, fatigue, muscle pain and fever. Severe reactions, for example autoimmune diseases such as reactive arthritis, systematic lupus erythematosus, vasculitis or anaphylactic shock are rare. 7 The mechanism of the development of an autoimmune disease after vaccination is not fully known. It may be caused by the inactivated virus or by the adjuvant found in the vaccines. 8
Only 4 other cases of reactive arthritis after COVID‐19 vaccination have been published in the literature, but none of them developed after the Pfizer‐BioNTech vaccine. In 2 of these cases, reactive arthritis presented in small joints of the hand 9 and in 1 case in the elbow joint. 10 The last case report describes reactive arthritis in the knee joint, as in our case. 1 In all these cases, the onset of the symptoms of reactive arthritis appeared several days after inoculation. The treatment in all of the cases reported until now has included the application of a corticosteroid. However, the method of application has varied from oral administration to an intra‐articular injection into the affected joint. In the treatment of arthritis of small hand joints, oral corticoid tablets were prescribed for 1 week. 9 The arthritis in the elbow joint and in the knee joint were treated with a single intra‐articular injection of corticoids. 1 , 10 In our case, we used 3 doses applied intravenously for a period of 3 days. In all of the reported cases, the patients recovered fully without any recurrences or consequences.
In our case report, we applied a cytological‐energy analysis of the knee joint effusion as a part of our diagnostic process. This is an accessible, rapid and affordable method which facilitates the clarification if there is an ongoing local inflammatory process in the joint effusion. The results of a cytological‐energy analysis are available within a few hours after sampling. This is far earlier than the results of a microbiological analysis, which are available only after several days. If it is necessary to determine whether or not the inflammation is purulent, a cytological‐energy analysis can promptly exclude pyogenic complication. This exclusion of an ongoing inflammatory process in the SF also clarifies that there is no ongoing inflammatory process in the extravascular body fluids. 11
This is the first use of a cytological‐energy analysis for analyzing joint effusion after a COVID‐19 vaccination. This method reveals immunocompetent cells, and also their metabolic activity. In the first SF sample, before our treatment, there were predominantly neutrophils with a segmented nucleus. In the SF sample after the corticosteroid therapy the neutrophils were less numerous. We obtained information about the concentration of CRP. The levels of CRP were elevated in the first SF sample, which correlates with levels of CRP in the blood sample. In the second SF sample, the levels of CRP were substantially decreased as in the blood sample.
In both SF samples, before and after the treatment, there was normal metabolic activity. This excluded the presence of an ongoing inflammatory process with an oxidative burst. This analysis also reveals the levels of AST and uric acid in the SF sample. In our case report, there was a normal level of AST in the joint effusion, indicating there was no destruction of the tissue in the located area. In addition, the normal level of uric acid indicated that there were no gout alterations. A cytological‐energy analysis of the SF can also be used in the diagnostic process, for typing the tumor and for accelerating the start of treatment. 6 There are also case reports in the literature of reactive arthritis developing during or after COVID‐19 infection. 12 , 13 , 14
4. CONCLUSION
Although reactive synovitis after COVID‐19 vaccination is rare, it should be taken into consideration in patients presenting with joint pain and swelling after inoculation, in order to exclude pyogenic complications. As we have presented in this case report, the cytological‐energy analysis can promptly exclude pyogenic inflammation. In the basis of this finding, effective treatment can be started without delay.
AUTHOR CONTRIBUTIONS
Writing ‐ original draft preparation and conceptualization, E.V., P.K. and T.N. Supervision, T.N.; funding acquisition, T.N. All authors have read and agreed to the published version of the manuscript.
FUNDING INFORMATION
This work was supported by Krajska zdravotni a.s., Usti nad Labem, Czech Republic (No. IGA‐KZ‐217116003) and by Faculty of Health Studies, University J.E. Purkinje, Usti nad Labem, Czech Republic.
INSTITUTIONAL REVIEW BOARD STATEMENT
This was a purely observational case study which did not alter the patient's management and clinical outcomes. Thus, ethics approval was not required for this case report.
INFORMED CONSENT STATEMENT
Informed consent was obtained from the patient involved in the study. Written informed consent has been obtained from the patient to publish this paper.
Vanaskova E, Kelbich P, Novotny T. Reactive synovitis of the knee joint after COVID‐19 vaccination: The first ultrastructural analysis of synovial fluid. Int J Rheum Dis. 2022;00:1‐4. doi: 10.1111/1756-185X.14411
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. An QJ, Qin DA, Pei JX. Reactive arthritis after COVID‐19 vaccination. Hum Vaccin Immunother. 2021;17(9):2954‐2956. doi: 10.1080/21645515.2021.1920274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Won JH, Lee H. The current status of drug repositioning and vaccine developments for the COVID‐19 pandemic. Int J Mol Sci. 2020;21(24):9775. doi: 10.3390/ijms21249775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Selmi C, Gershwin ME. Diagnosis and classification of reactive arthritis. Autoimmun Rev. 2014;13(4–5):546‐549. doi: 10.1016/j.autrev.2014.01.005 [DOI] [PubMed] [Google Scholar]
- 4. Wendling D, Verhoeven F, Chouk M, Prati C. Can SARS‐CoV‐2 trigger reactive arthritis? Joint Bone Spine. 2021;88(1):105086. doi: 10.1016/j.jbspin.2020.105086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kelbich P, Vachata P, Maly V, et al. Neutrophils in extravascular body fluids: cytological‐energy analysis enables rapid, reliable and inexpensive detection of purulent inflammation and tissue damage. Life. 2022;12:160. 10.3390/life12020160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vanaskova E, Kelbich P, Cegan M, Novotny T. Malignant knee joint effusion—a new dimension of laboratory diagnostics. Applied Sciences. 2022;12:994. 10.3390/app12030994 [DOI] [Google Scholar]
- 7. Dighriri IM, Alhusayni KM, Mobarki AY, et al. Pfizer‐BioNTech COVID‐19 vaccine (BNT162b2) side effects: a systematic review. Cureus. 2022;14(3):e23526. doi: 10.7759/cureus.23526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shoenfeld Y, Aron‐Maor A. Vaccination and autoimmunity‐'vaccinosis': a dangerous liaison? J Autoimmun. 2000;14(1):1‐10. doi: 10.1006/jaut.1999.0346 [DOI] [PubMed] [Google Scholar]
- 9. Enginar AU. Arthritis following COVID‐19 vaccination: report of two cases. Int Immunopharmacol. 2021;101(Pt B):108256. doi: 10.1016/j.intimp.2021.108256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Baimukhamedov C. Arthritis of the left elbow joint after vaccination against SARS‐CoV‐2 infection. Int J Rheum Dis. 2021;24(9):1218‐1220. doi: 10.1111/1756-185X.14202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kelbich P, Hejcl A, Stanek I, Svitilova E, Hanuljakova E, Sames M. Principles of the cytological‐energy analysis of the extravascular body fluids. Biochem Mol Biol J. 2017;3:6. [Google Scholar]
- 12. Ono K, Kishimoto M, Shimasaki T, et al. Reactive arthritis after COVID‐19 infection. RMD Open. 2020;6(2):e001350. doi: 10.1136/rmdopen-2020-001350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. López‐González MD, Peral‐Garrido ML, Calabuig I, et al. Case series of acute arthritis during COVID‐19 admission. Ann Rheum Dis Apr. 2020;80(4):e58. doi: 10.1136/annrheumdis-2020-217914 [DOI] [PubMed] [Google Scholar]
- 14. Alkindi F, Al‐Nokhatha S, Alseiari K, Alnaqb KA. Reactive hip arthritis and avascular necrosis after severe Covid‐19 infection: a case report and comprehensive review of literature. EMJ. 2022;7(1):48‐55. doi: 10.33590/emj/21-00261 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


