Dear Editor,
Given the widespread vaccination of the population with the novel mRNA vaccines against COVID‐19, it is of utmost importance to identify any possible adverse effects they may have. In clinical trials with the BNT162b2 COVID‐19 mRNA vaccine (Pfizer‐ΒioNTech), adverse effects included mostly injection‐site reactions, fatigue and headache. 1
Herein, we report a case of vitiligo following vaccination with the BNT162b2 COVID‐19 mRNA vaccine (Pfizer/BioNTech).
A 69‐year‐old male presented with white macules on his face, abdomen, back, and upper and lower limbs that covered approximately 20% of his total body surface area (Figure 1). The lesions had appeared several months before his presentation, 3 days after his second dose of the BNT162b2 COVID‐19 mRNA vaccine (Pfizer/BioNTech). The patient reported that the lesions appeared initially on the dorsal area of his hands, progressed to the rest of his body the following month and since then they had remained stable. There was no progression of the lesions after the third dose of the vaccine. The patient recalled that a few days after the first dose of the vaccine he noticed a few hypomelanotic lesions in his forearms which were very subtle and for which he did not seek medical advice. His medical history was unremarkable and he reported no family history of vitiligo. A biopsy and immunohistochemical analysis were performed which showed reduction/absence of melanocytes and absence of melanin in the basal layer of the epidermis. The patient was diagnosed with vitiligo and the diagnosis was supported with examination under Wood's lamp. The patient was started on narrowband UVB phototherapy. Some improvement was observed after 2 months of treatment, especially on the face.
FIGURE 1.
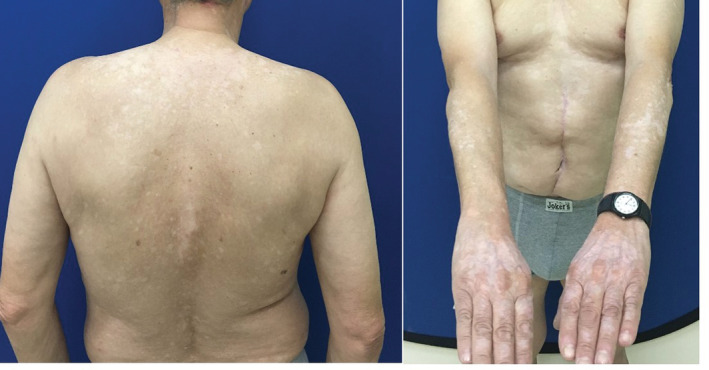
Vitiligo lesions on our patient.
To our knowledge, six other mRNA vaccine related vitiligo cases have been reported so far with four of them being related to the BNT162b2 COVID‐19 mRNA vaccine (Pfizer/BioNTech) 2 , 3 , 4 , 5 and two of them to the Moderna (mRNA‐1273) COVID‐19 vaccine. 6 , 7 All of them were adults except for one 13‐year‐old girl. 5 All patients reported that their lesions appeared approximately 1–2 weeks after the first dose of the vaccine, with the exception of one patient, who developed only very faint hypopigmented macules after the first dose, which progressed to overt vitiligo after the second dose, as was the case in our patient. 6 In another patient, no deterioration was reported after the second dose of the vaccine. 2 Two patients reported a personal history of other autoimmune diseases [ulcerative colitis 3 and ankylosing spondylitis 4 ], which may have contributed to the appearance of vitiligo. 3 A positive family history of vitiligo was reported by two patients. 2 , 5
Vaccination in general has already been associated with autoimmunity, 8 but, to our knowledge, not with vitiligo. The temporal association between anti‐COVID 19 vaccination and vitiligo in our patient may suggest a possible contribution of the vaccine to the development of the disease. The exact pathophysiological mechanism linking the vaccination and the disease remains unclear. A proposed mechanism could be the production of type Ι interferons (IFN‐Ι) through stimulation of plasmacytoid dendritic cells, a process that can be induced by coronaviruses 9 and is also associated with the pathogenesis of vitiligo. 10
The benefits of anti‐COVID 19 vaccination far outweigh the suspected adverse effects and all eligible candidates should be encouraged to receive the vaccines. At the same time, clinicians should also be encouraged to report possible adverse effects of vaccination in an effort to contribute to the best possible counselling of patients. Since a very high percentage of the population in most European countries have already been vaccinated, it would be of great interest to search for a possible post‐vaccination increase in vitiligo incidence in these countries.
FUNDING INFORMATION
The authors have received no funding for this work.
CONFLICT OF INTEREST
None.
ACKNOWLEDGEMENTS
The patient in this manuscript has given written informed consent to the publication of his case details.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid‐19 vaccine. N Engl J Med. 2020;383:2603–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ciccarese G, Drago F, Boldrin S, Pattaro M, Parodi A. Sudden onset of vitiligo after COVID‐19 vaccine. Dermatol Ther. 2022;35:e15196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Aktas H, Ertuğrul G. Vitiligo in a COVID‐19‐vaccinated patient with ulcerative colitis: coincidence? Clin Exp Dermatol. 2022;47:143–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Uğurer E, Sivaz O, Kıvanç Altunay İ. Newly‐developed vitiligo following COVID‐19 mRNA vaccine. J Cosmet Dermatol. 2022;21:1350–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bukhari AE. New‐onset of vitiligo in a child following COVID‐19 vaccination. JAAD Case Rep. 2022;22:68–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kaminetsky J, Rudikoff D. New‐onset vitiligo following mRNA‐1273 (Moderna) COVID‐19 vaccination. Clin Case Rep. 2021;9:e04865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Militello M, Ambur AB, Steffes W. Vitiligo possibly triggered by COVID‐19 vaccination. Cureus. 2022;14:e20902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Salemi S, D'Amelio R. Could autoimmunity be induced by vaccination? Int Rev Immunol. 2010;29:247–69. [DOI] [PubMed] [Google Scholar]
- 9. Onodi F, Bonnet‐Madin L, Meertens L, et al. SARS‐CoV‐2 induces human plasmacytoid predendritic cell diversification via UNC93B and IRAK4. J Exp Med. 2021;218:e20201387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bertolotti A, Boniface K, Vergier B, et al. Type I interferon signature in the initiation of the immune response in vitiligo. Pigment Cell Melanoma Res. 2014;27:398–407. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


