Abstract
Little information is available for antibody levels against SARS‐CoV‐2 variants of concern induced by Omicron breakthrough infection and a third booster with an inactivated vaccine (InV) or Ad5‐nCoV in people with completion of two InV doses. Plasma was collected from InV pre‐vaccinated Omicron‐infected patients (OIPs), unvaccinated OIPs between 0 and 22 days, and healthy donors (HDs) 14 days or 6 months after the second doses of an InV and 14 days after a homogenous booster or heterologous booster of Ad5‐nCoV. Anti‐Wuhan‐, Anti‐Delta‐, and Anti‐Omicron‐receptor binding domain (RBD)‐IgG titers were detected using enzyme‐linked immunosorbent assay. InV pre‐vaccinated OIPs had higher anti‐Wuhan‐, anti‐Delta‐, and anti‐Omicron‐RBD‐IgG titers compared to unvaccinated OIPs. Anti‐Wuhan‐RBD‐IgG titers sharply increased in InV pre‐vaccinated OIPs 0–5 days postinfection (DPI), while the geometric mean titers (GMTs) of anti‐Delta‐ and anti‐Omicron‐RBD‐IgG were 3.3‐fold and 12.0‐fold lower. Then, the GMT of anti‐Delta‐ and anti‐Omicron‐RBD‐IgG increased to 35 112 and 28 186 during 11–22 DPI, about 2.6‐fold and 3.2‐fold lower, respectively, than the anti‐Wuhan‐RBD‐IgG titer. The anti‐Wuhan‐, anti‐Delta‐, and anti‐Omicron‐RBD‐IgG titers declined over time in HDs after two doses of an InV, with 25.2‐fold, 5.6‐fold, and 4.5‐fold declination, respectively, at 6 months relative to the titers at 14 days after the second vaccination. Anti‐Wuhan‐, anti‐Delta‐, and anti‐Omicron‐RBD‐IgG titers elicited by a heterologous Ad5‐nCoV booster were significantly higher than those elicited by an InV booster, comparable to those in InV pre‐vaccinated OIPs. InV and Ad5‐nCoV boosters could improve humoral immunity against Omicron variants. Of these, the Ad5‐nCoV booster is a better alternative.
Keywords: Ad5‐nCoV booster, humoral immunity, inactivated vaccine booster, Omicron variants breakthrough infection
1. INTRODUCTION
Omicron variants obtain greater immune evasion and enhanced contagiousness due to more than 30 mutations in the S1 domain of the spike protein. 1 Accordingly, real‐world data analysis demonstrated that vaccine efficacy against Omicron variant infection declined with time after completion of the two‐dose mRNA vaccine schedule. 2 Omicron variants have since spread to become the predominant circulating strain worldwide following their first identification in South Africa in November 2021, 1 raising several concerns about the effectiveness of the antibody therapies and vaccination strategies conducted in different countries.
Various vaccines have been approved worldwide to build herd immunity, suppress SARS‐CoV‐2 transmission, and decrease severity after infection. 3 Although the clinical severity of infection in vaccinated individuals infected with Omicron variants was milder, the vaccine efficacy of the mRNA vaccine against Omicron variant breakthrough infection decreased over time. 2 Previous studies demonstrated that homogenous third inactivated vaccine (InV) boosters significantly improve spike binding and neutralizing antibody titers against Omicron variants compared with two doses of an InV. 4 , 5 However, the neutralizing antibody titers against Omicron variants elicited by homogenous third InV boosters were significantly lower than those of heterologous third vaccination with Ad5‐nCoV, a recombinant protein subunit vaccine and mRNA vaccine. 6 , 7
Accumulating studies have consistently shown that Omicron variant breakthrough infection could effectively induce robust protective humoral and cellular immunity against Omicron variants. 8 , 9 , 10 Therefore, boosting with a variants‐of‐concern‐matched vaccine is a potential strategy for providing robust protection immunity against Omicron variants. However, recent data have shown that mRNA‐1273 and mRNA‐Omicron elicit comparable neutralizing antibody titers against Omicron variants shortly after the boost and provide equivalent protection in the lungs of macaques against an Omicron challenge. 11 Additionally, studies have demonstrated that the Omicron variant spike‐based vaccine has lower immunogenicity compared with Delta variants and the Wuhan strain. 10 , 12 To date, no direct comparison analysis of humoral immunity induced by Omicron variant breakthrough infection and a third booster with an InV or Ad5‐nCoV in people who have completed the schedule of a fully InV has been reported. To address these knowledge gaps, we examined binding antibodies against Wuhan strain, Delta, and Omicron variants receptor binding domain (RBD) in individuals who had received either two doses of InV regimen, a standard regimen followed by a booster of InV or Ad5‐nCoV, or Omicron breakthrough infection following vaccination.
2. MATERIALS AND METHODS
2.1. Study cohort
This study was conducted in line with the Declaration of Helsinki and was approved by the Ethics Committee of the Second Affiliated Hospital of Guangzhou Medical University (approval no. 2021‐hs‐43) and Dongguan Ninth People's Hospital (approval no. 2022‐8). Omicron variant‐infected patients (OIPs) were enrolled in Dongguan Ninth People's Hospital from February 25, 2022, to March 21, 2022, and the vaccinated healthy donors (HDs) were from the Second Affiliated Hospital of Guangzhou Medical University. A confirmed OIP was defined as having a positive result through a real‐time reverse‐transcriptase‐polymerase‐chain‐reaction (RT‐PCR) assay of nasopharyngeal swab specimens. The clinical parameters, including sex, age, vaccination history, and Cycle threshold (Ct) value of the nucleic acid test, were extracted from the medical records. The OIPs were classified as symptomless or having mild or moderate symptoms based on previous guidelines. 13
2.2. Sample collection
A total of 80 serum samples from OIPs (n = 77) were retrieved after completion of routine clinical laboratory testing, then stored at −80°C after inactivation at 56°C for 30 min (Figure 1). Of them, 9 serum samples were from 9 unvaccinated OIPs, who did not receive any SARS‐CoV‐2 vaccine, between 0 and 16 days; and 71 serum samples were from 68 InV pre‐vaccinated OIPs, who completed a two‐dose InV schedule before infection, between 0 and 22 days (Figure 1). Eighty‐one serum samples were also collected from HDs 14 days after the second dose of an InV (n = 19), 6 months after the second dose of an InV (n = 21), 14 days after the third homogenous InVk booster (n = 23), and 14 days after a heterologous Ad‐5‐nCOV vaccine booster (n = 18; Figure 1).
Figure 1.
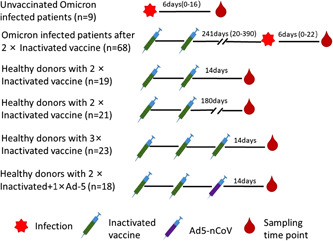
Overview of serum sampling from Omicron variant‐infected patients at different times after infection and healthy donors at different times after vaccination
2.3. Enzyme‐linked immunosorbent assay
Antibody titers were determined based on a previous study. 14 Briefly, 96‐well plates were coated with Wuhan‐RBD (Dongkang; 120 ng/well), Delta‐RBD (Fapon; 120 ng/well), and Omicron‐RBD (Fapon; 120 ng/well) overnight. The sequences of these RBDs are shown in Supporting Information: Figure S1. After washing with a phosphate‐buffered solution containing 0.1% Tween‐20 (PBST), 100‐μl 5% milk blocking solution was added to each well plate and incubated for 2 h. Afterward, threefold serially diluted serum samples starting from 1:20 were added to well plates and incubated at 37°C for 1 h. After washing, each well plate was coated with 100‐μl diluted HRP labeled anti‐human‐IgG antibody (1:10 000; Southern Biotech) and incubated for 1h. Subsequently, 50‐μl TMB substrate (Neobioscience) was added to well plates after washing (five times) and incubated at 37°C for 5 min. Then, the chromogenic reaction was terminated by adding 50 μl 1 M H2SO4 solution. The optical density (OD) value at 450 nm was measured using a microplate absorbance reader (Tecan Sunrise). The antibody's endpoint titer was determined by the highest dilution giving an OD value higher than the mean + 3 SD OD values of 3 serum pools from 45 archived sera collected from healthy individuals in 2016 at the same dilution.
2.4. Statistical analysis
Binding antibody titers were expressed as geometric mean titers (GMTs) and 95% confidence interval (CI). The median (interquartile range [IQR]) was used to present continuous variables in this study. Categorical variables were described as the count and percentage. All statistical analyses were performed using GraphPad Prism 7.0. All the binding antibody titers were firstly processed by log transformation, then independent group t‐test (normal distribution) and the Mann–Whitney U test (non‐normal distribution) were used to determine the difference between groups, and paired analysis was performed using the Wilcoxon test. Other parameters, including age and Ct value, were analyzed using a Mann–Whitney U test. The Pearson chi‐square was used to determine the differences in proportions between the two groups. Two‐sided p value ˂ 0.05 was considered statistically significant.
3. RESULTS
3.1. Demographic and clinical characteristics of the Omicron variant‐infected patients and the vaccinated HDs in our cohort
A total of 60 vaccinated HDs and 77 OIPs were enrolled in our study (Table 1). The median age of the vaccinated HDs was 38 (IQR: 29–50) years, not different than that of the OIPs (median = 39, IQR = 29–53, p = 0.668; Table 1). The sex proportion in OIPs and vaccinated HDs was comparable (Table 1). Among the OIPs, 9 patients did not receive any SARS‐CoV‐2 vaccine (unvaccinated OIPs) and 68 patients completed a two‐dose InV schedule before infection (InV pre‐vaccinated OIPs), with a median interval between the second vaccination and the Omicron variant infection of 241 (IQR: 20–390) days (Figure 1). The age and sex rate of unvaccinated and InV pre‐vaccinated OIPs were not different (Table 1). The Ct value of nasopharyngeal samples from unvaccinated and InV pre‐vaccinated OIPs was not different (Table 1). The asymptomatic infection rate in InV pre‐vaccinated OIPs was 36.76% (25/68), higher than that in unvaccinated OIPs (0% (0/9), p = 0.027; Table 1). Then, we explored the difference RT‐PCR Ct values of Open reading frames 1 a/b (orf1ab) and nucleocapsid protein (NP) genes among OIPs with different clinical severities. The results indicated the Ct value of NP and orf1ab genes were not significantly different among asymptomatic, mildly symptomatic, and moderately symptomatic OIPs (Supporting Information: Figure S2).
Table 1.
Demographic and epidemiologic characteristics of the enrolled Omicron variant‐infected patients and healthy donors receiving the SARS‐CoV‐2 vaccine
| Variables (n [%] or median [IQR]) | Vaccinated HDs (n = 60) | OIPs (n = 77) | p Value | Pre‐vaccinated OIPs (n = 68) | Unvaccinated OIPs (n = 9) | p Value |
|---|---|---|---|---|---|---|
| Age (years) | 38 (29–50) | 39 (29–53) | 0.668 | 40 (30.25–53) | 30 (12–54.5) | 0.086 |
| Sex | ||||||
| Female | 28 (46.6%) | 38 (49.3%) | 0.755 | 33 (48.5%) | 5 (55.5%) | 0.692 |
| Male | 32 (53.3%) | 39 (50.6%) | 35 (51.4%) | 4 (44.4%) | ||
| Ct value of RT‐PCR | 29.5 (24.25–32) | 29.5 (24.25–32) | 29 (23.5–39.42) | 0.898 | ||
| Severity | ||||||
| Asymptomatic | 25 (32.47%) | 25 (36.76%) | 0 | 0.027 | ||
| Symptomatic | 52 (67.53%) | 43 (63.24%) | 9 (100%) | |||
| Mild | 48 (62.34%) | 40 (58.82 | 8 (88.89%) | |||
| Moderate | 4 (5.19%) | 3 (4.41%) | 1 (11.11%) |
Abbreviations: Ct, cycle threshold; HD, healthy donor; IQR, interquartile range; OIP, Omicron variants‐infected patient; RT‐PCR, reverse transcription‐polymerase chain reaction.
3.2. Wuhan, Delta, and Omicron variant RBD‐specific antibody titers in Omicron‐infected patients
The anti‐Wuhan‐RBD‐, anti‐Delta‐RBD‐, and anti‐Omicron‐RBD‐IgG titers in OIPs were explored over time using enzyme‐linked immunosorbent assay. The experimental data showed that InV pre‐vaccinated OIPs had higher anti‐Wuhan‐RBD‐, anti‐Delta‐RBD‐, and anti‐Omicron‐RBD‐IgG titers relative to unvaccinated OIPs (Figure 2A–C). Regarding the InV pre‐vaccinated OIPs, the anti‐Wuhan‐RBD‐IgG titer sharply increased early, with a GMT of 1067 during 0–5 days postinfection (DPI; Figure 2D). During the same period, the anti‐Delta‐RBD‐ and anti‐Omicron‐RBD‐IgG titers were 4.3‐fold and 12.9‐fold lower than the anti‐Wuhan‐RBD‐IgG titers, respectively (Supporting Information: Figure S3). The anti‐Wuhan‐RBD‐, anti‐Delta‐RBD‐, and anti‐Omicron‐RBD‐IgG titers increased over time in InV pre‐vaccinated OIPs and peaked during 11–22 DPI (Figure 2D–F), whereas anti‐Delta‐RBD‐ and anti‐Omicron‐RBD‐IgG titers were 2.6‐fold and 3.2‐fold lower than anti‐Wuhan‐RBD‐IgG titers, respectively (Supporting Information: Figure S3). The dynamics of anti‐Wuhan‐RBD‐, anti‐Delta‐RBD‐, and anti‐Omicron‐RBD‐IgG titers were further analyzed according to the stratification of clinical severity, which was previously positively associated with SARS‐CoV‐2 specific antibody titers in COVID‐19 patients. 15 The anti‐Wuhan‐RBD‐, anti‐Delta‐RBD‐, and anti‐Omicron‐RBD‐IgG titers in asymptomatic InV pre‐vaccinated OIPs increased over time, with GMTs of 764, 242, and 59, respectively, during 0–5 DPI, and GMTs of 55 835, 34 265, and 26 843, respectively, during 6–22 DPI (Figure 2G–I). Comparable anti‐Wuhan‐RBD‐, Delta‐RBD‐, and anti‐Omicron‐RBD‐IgG titers were observed in mild InV pre‐vaccinated OIPs during the same period (Figure 2G–I). However, the patients with moderate symptoms had higher anti‐Wuhan‐RBD‐, anti‐Delta‐RBD‐, and anti‐Omicron‐RBD‐IgG titers than those with mild symptoms during 6–22 DPI, but there was no difference in comparison with asymptomatic patients (Figure 2G–I). The RT‐PCR Ct value of respiratory samples is an indicator of viral load in nasopharyngeal samples from pre‐vaccinated OIPs. 16 Further analysis was conducted to explore the correlation between the Ct values of NP and orf1ab genes and anti‐RBD‐IgG level during different periods after infection. However, we did not find an association between the Ct value and the anti‐RBD‐IgG level in InV pre‐vaccinated OIPs (Supporting Information: Figure S4).
Figure 2.
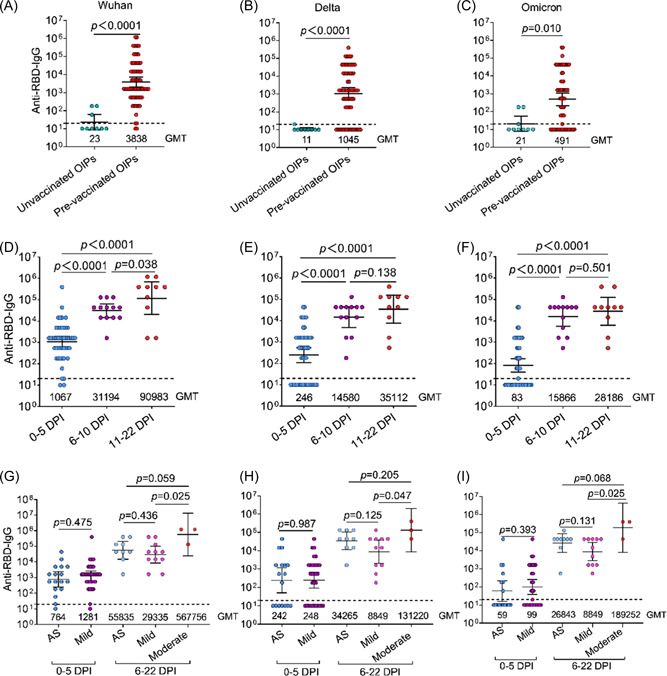
Comparative analysis of anti‐Wuhan, Delta, and Omicron variant receptor binding domain (RBD) antibody IgG levels in Omicron variant‐infected patients (OIPs) with different clinical parameters. (A–C) The anti‐Wuhan (A), Delta (B), and Omicron variant (C) RBD antibody IgG titers in unvaccinated OIPs and OIPs pre‐vaccination with an inactivated vaccine (pre‐vaccinated OIPs). (D–F) The anti‐Wuhan (D), Delta (E), and Omicron variant (F) RBD antibody IgG titers in pre‐vaccinated OIPs during 0–5, 6–10, and 11–22 days after infection. (G–I) The anti‐Wuhan (G), Delta (H), and Omicron variants (I) RBD antibody IgG titers in pre‐vaccinated OIPs who were asymptomatic and those with mild and moderate infection during 0–5, 6–10, and 11–22 days after infection. AS, asymptomatic; DPI, days postinfection, GMT, geometric mean titer. Dotted line represents the detection of limits.
3.3. Comparative analysis of the Wuhan, Delta, and Omicron variant RBD‐specific antibody IgG titers in vaccinated HDs and patients with Omicron variant breakthrough infections
Vaccination is an optimal countermeasure for conferring protective immunity against SARS‐CoV‐2 variants. Homogenous InV and heterologous Ad5‐nCoV boosters following a two‐dose regimen of InV are being implemented in China. Therefore, the anti‐Wuhan‐RBD‐, anti‐Delta‐RBD‐, and anti‐Omicron‐RBD‐IgG titers in HDs after completing two doses of InV, homogenous InV, and a heterologous Ad5‐nCoV booster were explored. As shown in Figure 3A, high anti‐Wuhan‐RBD‐IgG titers were induced in HDs at 14 days after two doses of InV, and there was a marked decline at 6 months. Compared with anti‐Wuhan‐RBD‐IgG titers, there were 8.4‐fold and 36.7‐fold declines in anti‐Delta‐RBD‐IgG and anti‐Omicron‐RBD‐IgG titers, respectively, in HDs 14 days after two doses of InV (Figure 3B–D), then a continuous declined at 6 months, with GMTs of 46 and 13, respectively (Figure 3B,D). As expected, a homogenous InV booster induced 12.7‐fold, 42.6‐fold, and 7.2‐fold enhancements of anti‐Wuhan‐RBD‐, anti‐Delta‐RBD‐, and anti‐Omicron‐RBD‐IgG titers at 14 days after the booster compared with two doses of InV (Figure 3A–C). In particular, the heterologous Ad5‐nCoV booster induced 5.4‐fold, 2.9‐fold, and 67.2‐fold higher anti‐Wuhan‐RBD‐, anti‐Delta‐RBD‐, and anti‐Omicron‐RBD‐IgG titers compared with the homogenous InV booster (Figure 3A–C). Moreover, the InV pre‐vaccinated OIPs had 33.9‐fold, 94.8‐fold, and 471.6‐fold higher anti‐Wuhan‐RBD‐, anti‐Delta‐RBD‐, and anti‐Omicron‐RBD‐IgG titers, respectively, than HDs 14 days after two doses of InV, comparable to anti‐Wuhan‐RBD‐ and anti‐Delta‐RBD‐IgG titers, but 65.6‐fold higher anti‐Omicron‐RBD‐IgG titers relative to those of HDs after a homogenous InV booster (Figure 3A–C). Interestingly, the anti‐Wuhan‐RBD‐, anti‐Delta‐RBD‐, and anti‐Omicron‐RBD‐IgG titers in HDs 14 days after heterologous Ad5‐nCoV booster were equal to those in InV pre‐vaccinated OIPs (Figure 3A–C). Compared with anti‐Wuhan‐RBD‐IgG titers, there were 1.5‐fold and 63.6‐fold declines in anti‐Delta‐RBD‐ and anti‐Omicron‐RBD‐IgG titers in HDs 14 days after the homogenous InV booster, and 3.6‐fold and 4.2‐fold declines in anti‐Delta‐RBD‐ and anti‐Omicron‐RBD‐IgG titers in HDs 14 days after a heterologous Ad5‐nCoV booster; 2.3‐fold and 1.6‐fold declines were observed in anti‐Delta‐RBD‐IgG and anti‐Omicron‐RBD‐IgG titers in InV pre‐vaccinated OIPs as well (Figure 3D).
Figure 3.
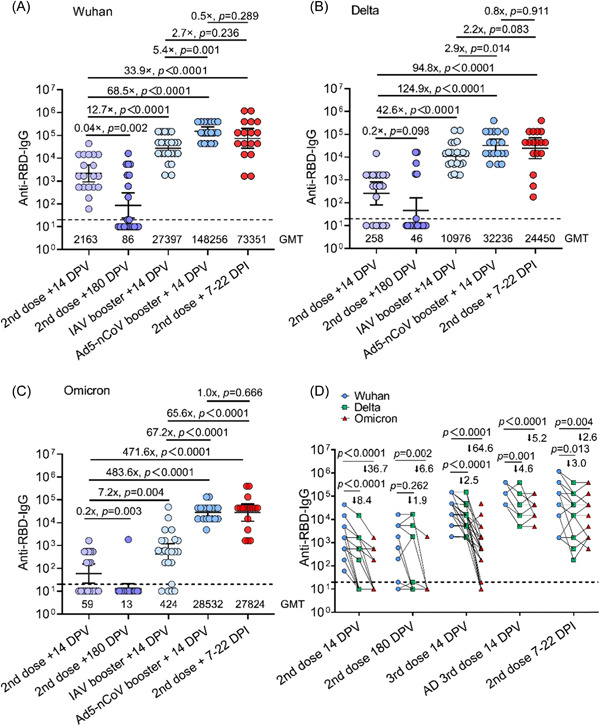
Comparative analysis of anti‐Wuhan, Delta, and Omicron variant receptor binding domain (RBD) antibody IgG levels in vaccinated healthy donors (HDs) and pre‐vaccinated Omicron variant‐infected patients (OIPs). (A) The anti‐Wuhan RBD antibody IgG titers in HDs at 14 days and 6 months after the second vaccine dose, 14 days after an inactivated vaccine or Ad5‐nCoV booster, and in pre‐vaccinated OIPs 7–21 days after infection. (B) The anti‐Delta RBD antibody IgG titers in HDs at 14 days and 6 months after the second vaccine dose, 14 days after an inactivated vaccine or Ad5‐nCoV booster, and in pre‐vaccinated OIPs 7–21 days after infection. (C) The anti‐Omicron RBD antibody IgG titers in HDs at 14 days and 6 months after the second vaccine dose, 14 days after an inactivated vaccine or Ad5‐nCoV booster, and in pre‐vaccinated OIPs 7–21 days after infection. (D) Paired analysis of anti‐Wuhan, Delta, and Omicron variants RBD antibody IgG titers in HDs at 14 days and 6 months after the second vaccine dose, 14 days after an inactivated vaccine or Ad5‐nCoV booster, and in pre‐vaccinated OIPs 7–21 days after infection. DPI, days postinfection; DPV, days postvaccination; GMT, geometric mean titer; IAV, inactivated vaccine. Dotted line represents the detection limits.
4. DISCUSSION
Herein, the comprehensive analysis demonstrated that the fraction of asymptomatic infections in vaccinated OIPs was higher than that in unvaccinated OIPs. InV pre‐vaccinated OIPs had higher anti‐Wuhan‐RBD‐, anti‐Delta‐RBD‐, and anti‐Omicron‐RBD‐IgG titers compared with unvaccinated OIPs. Among the InV pre‐vaccinated OIPs, COVID‐19 patients with moderate symptoms obtained higher anti‐Wuhan‐RBD‐, anti‐Delta‐RBD‐, and anti‐Omicron‐RBD‐IgG levels compared with mildly symptomatic COVID‐19 patients at 6–22 DPI. Promisingly, a third dose homogenous InV or heterologous Ad5‐nCoV booster following completion of two doses InV regimen significantly improved the anti‐Wuhan‐RBD‐, anti‐Delta‐RBD‐, and anti‐Omicron‐RBD‐IgG titers. Especially, the Ad5‐nCoV booster elicited higher anti‐Wuhan‐RBD‐, anti‐Delta‐RBD‐, and anti‐Omicron‐RBD‐IgG titers than did the homogenous InV booster, comparable anti‐Wuhan‐RBD‐, anti‐Delta‐RBD‐, and anti‐Omicron‐RBD‐IgG titers to InV pre‐vaccinated OIPs.
In line with previous studies, our study showed that individuals who have completed the schedule of InV have a higher fraction of asymptomatic Omicron variant infections compared to unvaccinated patients, supporting the protection efficiency of InV against Omicron variants. 17 The breakthrough infections of all the enrolled InV pre‐vaccinated OIPs occurred 6 months after two doses of InV when anti‐Omicron‐RBD‐IgG levels had significantly declined and the patients were therefore prone to infection by Omicron variants. However, a majority of InV pre‐vaccinated OIPs presented with asymptomatic infection or mild symptoms, the underlying mechanism of which might be related to the durable memory of B and T cells, which provided cross‐reactive protection against Omicron variants. 5 , 18 Consistent with previous studies on COVID‐19 patients with other SARS‐CoV‐2 variants, our study showed that InV pre‐vaccinated OIPs with moderate symptoms had higher antibody levels against Wuhan and Delta variants, as well as Omicron variant RBD, relative to patients with mild symptoms; this may be linked to the high viral loads in the patients with moderate symptoms. 15 Of note, unvaccinated OIPs had lower antibody levels against Omicron variants and had a risk of re‐infection with SARS‐CoV‐2 variants. 13 , 19 Therefore, unvaccinated OIPs need to be further vaccinated to provide better protective immunity against emergent SARS‐CoV‐2 variants. 19
It should be mentioned that anti‐Wuhan‐RBD‐IgG and anti‐Delta‐RBD‐IgG titers sharply increased during the early stage of infection in pre‐vaccinated OIPs and were higher than the anti‐Omicron‐RBD‐IgG titer. The phenomenon was also reported in previous studies and is called immune imprinting. 20 , 21 These enhanced antibodies could provide protection via a viral neutralizing function, antibody‐dependent cellular phagocytosis, and anti‐body‐dependent cellular cytotoxicity. 21 In addition, the cross‐reactive protective T cell immunity against the conserved sites between Omicron variants and Wuhan virus isolates could contribute to the recovery of pre‐vaccinated OIPs. 22 Finally, the coordinated protection of conserved T cells immunity and the early induced humoral immune response contribute to rapid virus clearance and recovery of pre‐vaccinated OIPs. 13 , 23
Accumulating data provided evidence that enhanced cross‐reactive protective immunity could be significantly improved by continuous exposure to spike antigens. 8 , 24 Therefore, a third and even fourth booster were implemented worldwide. 25 Consistent with a previous study, our study demonstrated that homologous InV and heterologous Ad5‐nCoV boosters significantly enhanced antibody titers against the Wuhan and Delta variants, as well as the Omicron variant RBD. 7 Inspiritingly, the antibody titer against Omicron variant RBD elicited by the Ad5‐nCoV booster was markedly higher than that elicited by a homologous InV, and comparable with that in InV pre‐vaccinated OIPs. Previous data demonstrated that older people, immunocompromised patients, and blood cancer patients have diminished immune responses after vaccination. 17 , 26 , 27 Our results support using Ad5‐nCoV to boost the immunity of individuals who have completed two doses of the InV, especially these individuals with diminished immunogenicity after two doses of the InV. Another issue of concern is the durability of the immunity induced by the Ad5‐nCoV booster. Therefore, the key indicators of immunity durability, including memory B cell and T cell immunity, should be assessed in Ad5‐nCoV boosted individuals and pre‐vaccinated OIPs in 6 months.
Another strategy to confer better protection against Omicron variants is the development of their matched vaccine. To this end, a study on Omicron‐matched mRNA vaccine booster in rhesus macaques was conducted and demonstrated that an Omicron‐matched mRNA vaccine booster led to comparable neutralizing titers and protection against Omicron variants with the approved Wuhan spike‐based mRNA vaccine mRNA‐1273, which may be related to the marked increase in cross‐reactive memory B cell numbers after both boosters. 11 However, a recent experiment in mice showed that an Omicron‐matched mRNA booster after two doses of mRNA‐1273 primary series induced higher neutralizing antibody levels against Omicron variants than an mRNA‐1273 booster. 28 Boosting with an Omicron‐matched mRNA vaccine elicits slightly greater protection against lung infection, and inflammation was slightly greater in animals following a low‐dose primary vaccination series compared with those boosted with an mRNA‐1273mRNA vaccine, but not in animals with a high‐dose primary vaccination, 28 indicating that individuals with lower immunity response elicited by primary immunity could obtain more benefit from Omicron‐matched mRNA vaccine boosters than those who receive an mRNA‐1273mRNA booster. Our study, along with others, indicated that the adenovirus vector vaccine booster is a promising strategy to confer better humoral and cellular immunity against Omicron variants. 7 Therefore, the immunogenicity and vaccine efficiency of adenovirus vector‐based Omicron variant vaccines need to be further explored in the future.
5. CONCLUSIONS
In summary, the early elicitation of humoral immunity response upon Omicron variant breakthrough infection contributes to milder symptoms. Unvaccinated OIPs have lower antibody levels against Omicron variants and need to be further vaccinated. The Ad5‐nCoV booster is a better alternative to confer better protective immunity against Omicron variants than the homogenous InV.
AUTHOR CONTRIBUTIONS
Conceptualization: Tianxing Ji. Clinical sample and data collection: Jianfeng Ruan, Jun Wang, An Kang, Qingyang Zhong, Ruhong Xu, Yuemei Liang, Lei Zhang, Changchun Lai, Li Wan, Qiulan Huang, and Shilong Xiong. Methodology devising and performing experiments and data analysis: Weiya Kong, Mingxiao Chen, Pei Yu, Minhong Wang, Min Deng, Meifang Lin, Weikang Mai, Lu Chen,Yuemei Liang, Nan Qin, and Jianqiang Zhu. Writing – original draft: Tianxing Ji, Weiya Kong, Mingxiao Chen, and Pei Yu. Funding acquisition: Tianxing Ji, Lei Zhang, and Qingyang Zhong. Supervision: Tianxing Ji. All authors contributed to data analysis and interpretation and edited the manuscript.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Supporting information
Supplementary information.
ACKNOWLEDGMENTS
The authors thank all participants in this study. This study received the following funding: Guangzhou Health Science and Technology Project (20201A011078), Guangzhou Science and Technology Project (202102010094), Guangdong Basic and Applied Basic Research Foundation (2021A1515012550), Clinical research project of Guangzhou Medical University Second Affiliated Hospital (2021‐LCYJ‐05), Guangdong Medical Research Fund (A2022255), Key Clinical Specialty of Guangzhou Medical University (0F03031), and Guangzhou Key Discipline of Urology.
Kong W, Zhong Q, Chen M, et al. Ad5‐nCoV booster and omicron variant breakthrough infection following two doses of inactivated vaccine elicit comparable antibody levels against Omicron variants. J Med Virol. 2022;1‐9. 10.1002/jmv.28163
Weiya Kong, Qingyang Zhong, Mingxiao Chen, Pei Yu, and Ruhong Xu contributed equally to this study.
Contributor Information
An Kang, Email: kangan1@qq.com.
Jun Wang, Email: 1046594653@qq.com.
Wenrui Li, Email: jiujiangliwenrui@163.com.
Tianxing Ji, Email: jitianxing7021@163.com.
DATA AVAILABILITY STATEMENT
Data are available on request from the authors.
REFERENCES
- 1. Viana R, Moyo S, Amoako DG, et al. Rapid epidemic expansion of the SARS‐CoV‐2 Omicron variant in Southern Africa. Nature. 2022;603(7902):679‐686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tseng HF, Ackerson BK, Luo Y, et al. Effectiveness of mRNA‐1273 against SARS‐CoV‐2 Omicron and Delta variants. Nature Med. 2022;28:1063‐1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tregoning JS, Flight KE, Higham SL, Wang Z, Pierce BF. Progress of the COVID‐19 vaccine effort: viruses, vaccines and variants versus efficacy, effectiveness and escape. Nat Rev Immunol. 2021;21(10):626‐636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Xue JB, Lai DY, Jiang HW, et al. Landscape of the RBD‐specific IgG, IgM, and IgA responses triggered by the inactivated virus vaccine against the Omicron variant. Cell Discov. 2022;8(1):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wang K, Jia Z, Bao L, et al. Memory B cell repertoire from triple vaccinees against diverse SARS‐CoV‐2 variants. Nature. 2022;603(7903):919‐925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cheng SMS, Mok CKP, Leung YWY, et al. Neutralizing antibodies against the SARS‐CoV‐2 Omicron variant BA.1 following homologous and heterologous CoronaVac or BNT162b2 vaccination. Nature Med. 2022;28(3):486‐489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhang Z, Wu S, Liu Y, et al. Aerosolized Ad5‐nCoV booster vaccination elicited potent immune response against the SARS‐CoV‐2 Omicron variant after inactivated COVID‐19 vaccine priming. medRxiv. 2022. 10.1101/2022.03.08.22271816 [DOI] [Google Scholar]
- 8. Walls AC, Sprouse KR, Bowen JE, et al. SARS‐CoV‐2 breakthrough infections elicit potent, broad, and durable neutralizing antibody responses. Cell. 2022;185(5):872‐880.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Evans JP, Zeng C, Carlin C, et al. Neutralizing antibody responses elicited by SARS‐CoV‐2 mRNA vaccination wane over time and are boosted by breakthrough infection. Sci Transl Med. 2022;14(637):eabn8057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Servellita V, Syed AM, Morris MK, et al. Neutralizing immunity in vaccine breakthrough infections from the SARS‐CoV‐2 Omicron and Delta variants. Cell. 2022;185:1539‐1548.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gagne M, Moliva JI, Foulds KE, et al. mRNA‐1273 or mRNA‐Omicron boost in vaccinated macaques elicits similar B cell expansion, neutralizing responses, and protection from Omicron. Cell. 2022;185:1556‐1571.e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. He C, He X, Yang J, et al. Spike protein of SARS‐CoV‐2 Omicron (B.1.1.529) variant have a reduced ability to induce the immune response. Signal Transduct Target Ther. 2022;7(1):119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lai C, Liu X, Yan Q, et al. Low innate immunity and lagged adaptive immune response in the re‐tested viral RNA positivity of a COVID‐19 patient. Front Immunol. 2021;12:664619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chen Y, Yin S, Tong X, et al. Dynamic SARS‐CoV‐2‐specific B‐cell and T‐cell responses following immunization with an inactivated COVID‐19 vaccine. Clin Microbiol Infect Official Publ Eur Soc Clin Microbiol Infect Dis. 2022;28(3):410‐418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sette A, Crotty S. Adaptive immunity to SARS‐CoV‐2 and COVID‐19. Cell. 2021;184(4):861‐880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bergwerk M, Gonen T, Lustig Y, et al. Covid‐19 breakthrough infections in vaccinated health care workers. N Engl J Med. 2021;385(16):1474‐1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Smith DJ, Hakim AJ, Leung GM, et al. COVID‐19 mortality and vaccine coverage – Hong Kong Special Administrative Region, China, January 6, 2022‐March 21, 2022. Morb Mortal Wkly Rep. 2022;71(15):545‐548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Keeton R, Tincho MB, Ngomti A, et al. T cell responses to SARS‐CoV‐2 spike cross‐recognize Omicron. Nature. 2022;603(7901):488‐492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lu L, Chen LL, Zhang RRQ, et al. Boosting of serum neutralizing activity against the Omicron variant among recovered COVID‐19 patients by BNT162b2 and CoronaVac vaccines. EBioMedicine. 2022;79:103986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Röltgen K, Nielsen SCA, Silva O, et al. Immune imprinting, breadth of variant recognition, and germinal center response in human SARS‐CoV‐2 infection and vaccination. Cell. 2022;185(6):1025‐1040.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nichols AL, Sonnappa‐Naik M, Gardner L, et al. SARS‐CoV‐2 Omicron triggers cross‐reactive neutralization and Fc effector functions in previously vaccinated, but not unvaccinated, individuals. Cell Host Microbe. 2022;107:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Choi SJ, Kim DU, Noh JY, et al. T cell epitopes in SARS‐CoV‐2 proteins are substantially conserved in the Omicron variant. Cell Mol Immunol. 2022;19(3):447‐448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gao Y, Cai C, Grifoni A, et al. Ancestral SARS‐CoV‐2‐specific T cells cross‐recognize the Omicron variant. Nature Med. 2022;28(3):472‐476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gruell H, Vanshylla K, Tober‐Lau P, et al. mRNA booster immunization elicits potent neutralizing serum activity against the SARS‐CoV‐2 Omicron variant. Nature Med. 2022;28(3):477‐480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Magen O, Waxman JG, Makov‐Assif M, et al. Fourth dose of BNT162b2 mRNA Covid‐19 vaccine in a nationwide setting. N Engl J Med. 2022;386(17):1603‐1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Neurath MF. COVID‐19: biologic and immunosuppressive therapy in gastroenterology and hepatology. Nat Rev Gastroenterol Hepatol. 2021;18(10):705‐715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mair MJ, Mitterer M, Gattinger P, et al. Enhanced SARS‐CoV‐2 breakthrough infections in patients with hematologic and solid cancers due to Omicron. Cancer Cell. 2022;40:444‐446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ying B, Scheaffer SM, Whitener B, et al. Boosting with variant‐matched or historical mRNA vaccines protects against Omicron infection in mice. Cell. 2022;185(9):1572‐1587.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information.
Data Availability Statement
Data are available on request from the authors.


