Abstract
Maltose metabolism and the regulation of the glv operon of Bacillus subtilis, comprising three genes, glvA (6-phospho-α-glucosidase), yfiA (now designated glvR), and glvC (EIICB transport protein), were investigated. Maltose dissimilation was dependent primarily upon the glv operon, and insertional inactivation of either glvA, glvR, or glvC markedly inhibited growth on the disaccharide. A second system (MalL) contributed to a minor extent to maltose metabolism. Northern blotting revealed two transcripts corresponding to a monocistronic mRNA of glvA and a polycistronic mRNA of glvA-glvR-glvC. Primer extension analysis showed that both transcripts started at the same base (G) located 26 bp upstream of the 5′ end of glvA. When glvR was placed under control of the spac promoter, expression of the glv operon was dependent upon the presence of isopropyl-β-d-thiogalactopyranoside (IPTG). In regulatory studies, the promoter sequence of the glv operon was fused to lacZ and inserted into the amyE locus, and the resultant strain (AMGLV) was then transformed with a citrate-controlled glvR plasmid, pHYCM2VR. When cultured in Difco sporulation medium containing citrate, this transformant [AMGLV(pHYCM2VR)] expressed LacZ activity, but synthesis of LacZ was repressed by glucose. In an isogenic strain, [AMGLVCR(pHYCM2VR)], except for a mutation in the sequence of a catabolite-responsive element (cre), LacZ activity was expressed in the presence of citrate and glucose. Insertion of a citrate-controlled glvR plasmid at the amyE locus of ccpA+ and ccpA mutant organisms yielded strains AMCMVR and AMCMVRCC, respectively. In the presence of both glucose and citrate, AMCMVR failed to express the glv operon, whereas under the same conditions high-level expression of both mRNA transcripts was found in strain AMCMVRCC. Collectively, our findings suggest that GlvR (the product of the glvR gene) is a positive regulator of the glv operon and that glucose exerts its effect via catabolite repression requiring both CcpA and cre.
Bacteria have evolved a highly sophisticated multiprotein sugar transport and phosphorylation system, the phosphoenolpyruvate-sugar phosphotransferase system (PTS) (11, 14). Bacillus subtilis, whose whole genome sequence was established in 1997 (8), encodes 15 complete PTS permeases, of which only 7 have been characterized (15). We previously reported that the glv operon of B. subtilis encodes 6-phospho-α-glucosidase (GlvA), an unknown product (YfiA, now designated GlvR [regulatory protein of the glv operon]), and a PTS permease (GlvC), in that order, in the 76° region (36). GlvA is a polypeptide consisting of 449 amino acid residues, and this enzyme requires NAD(H) and divalent metal ions for activity (30). This glucosidase is assigned to the ∼20-member family 4 of the glycosylhydrolase superfamily (30, 32; SWISS-PROT protein sequence data bank [http://www.expasy.ch/cgi-bin/lists?glycosid.txt]). GlvR is a polypeptide consisting of 254 amino acid residues, and its amino acid sequence exhibits high similarity to those of RpiR/YebK/YfhH family members (SWISS-PROT). RpiR is involved in the regulation of rpiB expression in Escherichia coli, and its N-terminal region contains a helix-turn-helix DNA binding motif characteristic of many regulatory proteins (22). GlvC is a polypeptide consisting of 527 amino acid residues and is a PTS transport component with 12 transmembrane segments (36). Maltose is transported into the cytoplasm simultaneously with phosphorylation by GlvC, and maltose-6-phosphate is hydrolyzed intracellularly to glucose-6-phosphate and glucose by GlvA (30). Recent studies by Dahl and coworkers have identified a cluster of nine genes that promote the non-PTS-catalyzed transport and metabolism of maltose in B. subtilis (18, 19). Included in this putative operon is the gene malL. The gene encodes MalL (YvdL), a maltose-inducible α-glucosidase that exhibits high amino acid sequence similarity with several α-glucosidases and hydrolyzes various disaccharides such as maltose, sucrose, and isomaltose and longer maltodextrins (18). The malL gene is located in the deduced gene cluster (yvdE to yvdM) in the 302.8° to 303.9° region (18). Upstream of yvdE there is a ρ-independent terminator, and downstream of yvdM there is a clpA gene in the opposite orientation, followed by a ρ-independent terminator (BSORF database). In the cluster, yvdE is assumed to be a transcriptional regulatory gene belonging to the helix-turn-helix LacI family (BSORF database), and the yvdF and yvdG products are homologues of glucan 1,4-α-maltohydrolase and maltose- and maltodextrin-binding protein, respectively (SWISS-PROT). yvdH and yvdI are genes for maltodextrin transport system permeases, and yvdJ is a membrane protein with an ATP- and GTP-binding motif. The yvdK product has no identifiable motif, but yvdL (malL) and yvdM are the genes for sucrase-isomaltase-maltase and β-phosphoglucomutase, respectively (19; SWISS-PROT). Thus, the gene cluster may be a polycistronic operon associated with transport (as free sugar) and metabolism of maltose.
Catabolite repression is a global regulatory mechanism which, in B. subtilis and other gram-positive bacteria, comprises three major components (6, 23). First, the expression of many catabolic genes is repressed in the presence of a readily metabolizable carbon source such as glucose, fructose, or mannitol. Second, cis-acting sequences called catabolite responsive elements (cre) mediate catabolite repression of many genes (7, 12, 23). Mutations that result in release from cre-dependent catabolite repression occur in the gene encoding the catabolite control protein, CcpA, which belongs to the LacI family of transcriptional regulators (6, 23). The third important factor is HPr, an intermediate phosphoryl transfer protein in the PTS (4, 6, 23, 24). HPr phosphorylates not only EII for sugar transport but also certain catabolic enzymes such as glycerol kinase and transcriptional regulators for modulation of their activities (23).
In this report we show that regulation of the glv operon in B. subtilis requires a positive factor (GlvR), catabolite repression through CcpA and cre, and induction by maltose. We also discuss the function of malL in maltose metabolism.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
The bacterial strains used in this study are listed in Table 1. The bacterial strains were cultured in Luria-Bertani (LB) medium (5 g of yeast extract, 10 g of polypeptone, 5 g of NaCl per liter, pH 7.2) at 37°C. When required, ampicillin, erythromycin, tetracycline, kanamycin, chloramphenicol, or neomycin was added to a final concentration of 50, 0.3, 5, 5, 5, or 15 μg per ml, respectively. B. subtilis strains were grown in Difco sporulation medium (DSM) (17), Spizizen's minimal medium (SMM) (1), modified SMM (mSMM; glucose was replaced by maltose), or maltose minimal medium (MMM; C medium [13] supplemented with 50 mM l-glutamic acid and 10 mM maltose). When B. subtilis strains were grown in minimal medium, l-tryptophan was added to a final concentration of 50 μg per ml.
TABLE 1.
Bacterial strains used in this study
| Strain | Relevant genotype | Sourcea |
|---|---|---|
| B. subtilis | ||
| 168 | trpC2 | S. D. Ehrlich |
| GLVAd | trpC2 glvA::pMV1 | This study |
| GLVRd | trpC2 glvR::pMVR | This study |
| GLVCd | trpC2 glvC::pMV2 | This study |
| MALLdd | trpC2 malL::kan | This study |
| MLGLVAd | trpC2 malL::kan glvA::pMV1 | This study |
| GLVR-PSP | trpC2 glvR::pMVRSD (spac-glvR glvC) | This study |
| AMGLV | trpC2 amyE::(PglvA [−240 to +32]′-′lacZ cat)b | pDHΔglv→168 |
| AMGLVCR | trpC2 amyE::(PglvA [−240 to +32 region carrying a CG-to-AT dinucleotide change at positions +6 and +7 relative to the glvA start point]′-′lacZ cat)b | pDHΔglvCR→168 |
| AMCMVR | trpC2 amyE::(PcitM [−209 to +14]-glvR cat)c | pDAFBCMVR→168 |
| 1A1 | trpC2 ccpA::neo | Y. Fujita |
| AMCMVRCC | trpC2 amyE::(PcitM [−209 to +14]-glvR cat) ccpA::neoc | 1A1→AMCMVR |
| E. coli | ||
| JM109 | recA1 supE44 endA1 hsdR17 gyrA96 relA1 thi-1 Δ(lac-proAB)/F′ (traD36 proAB+laclqlacZ Δ M15) | Takara |
| C600 | supE44 hsdR17 thi-1 thr-1 leuB6 lacY1 tonA21 | Laboratory stock |
Arrows indicate construction by transformation.
Nucleotide numbers correspond to the glvA promoter regions relative to the glvA start point.
Nucleotide numbers correspond to the citM promoter regions relative to the citM start point.
Construction of plasmids.
The plasmids and primers used in this study are listed in Tables 2 and 3, respectively. Derivatives of pMUTIN2 (33) and pGEM-3Zf(+) were used to construct mutants of B. subtilis 168 and to prepare gene-specific RNA probes, respectively. For the construction of gene-disrupted mutants, pMUTIN2 derivatives containing an internal region of each gene were used. For example, a glvA internal DNA fragment amplified with 168 DNA and primers V1-EF and V1-BR was digested with EcoRI and BamHI. Then the digested fragment was ligated to the EcoRI and BamHI sites of pMUTIN2, and E. coli JM109 was transformed with the mixture to obtain pMV1. In the same way, to obtain plasmids pMVR and pMV2, internal fragments of glvR and glvC were amplified with 168 DNA and primers VR-EF and VR-BR and V2-HF and V2-BR, respectively. E. coli C600 cells were transformed with these ligated DNAs. The nucleotide sequences of PCR products were always confirmed with a DNA sequencer (model 373A; Applied Biosystems). To prepare digoxygenin (DIG)-labeled RNA probes, HindIII- or EcoRI- and BamHI-digested PCR fragments were cloned into pGEM-3Zf(+) to generate plasmids pGV1, pGVR, and pGV2.
TABLE 2.
Plasmids used in this study
| Plasmid | Relevant genotype | Source or Reference |
|---|---|---|
| pMUTIN2 | bla erm lacZ lacI spac | 33 |
| pMUTIN4 | bla erm lacZ lacI spac oid | 33 |
| pBluescript II SK(+) | bla lacZ | Stratagene |
| pGEM-3Zf(+) | bla lacZ | Promega |
| pUC119 | bla lacZ | Takara |
| pDG782 | bla kan | BGSCa |
| pHY300PLK | bla tet | Takara |
| pMV1 | pMUTIN2::ΔglvA | This study |
| pMVR | pMUTIN2::ΔglvR | This study |
| pMV2 | pMUTIN2::ΔglvC | This study |
| pMVR-SD | pMUTIN4::ΔglvR (containing glvR SD sequence) | This study |
| pGV1 | pGEM-3Zf(+)::ΔglvA | This study |
| pGVR | pGEM-3Zf(+)::ΔglvR | This study |
| pGV2 | pGEM-3Zf(+)::ΔglvC | This study |
| pUCMALL | bla malL | This study |
| pUCMALLKm | bla malL::kan | This study |
| pBCM2 | pBluescript II SK(+) with a 242-bp citM promoter region | This study |
| pHYCM2 | pHY300PLK with a 223-bp citM promoter region | This study |
| pDHAFBLZ | bla amyE::(lacZ cat) | 34 |
| pHYCM2LZ | pHY300PLK with a 223-bp citM promoter region and lacZ | This study |
| pBVR-SD | bla glvR | This study |
| pHYCM2VR | pHY300PLK with a 223-bp citM promoter region and glvR | This study |
| pUCΔglv | pUC119 with a 272-bp glvA promoter region | This study |
| pUCΔglvCR | pUC119 with a 272-bp glvA promoter region (carrying a CG-to-AT dinucleotide change at positions +6 and +7 relative to the glvA start point) | This study |
| pDHΔglv | pDHAFBLZ with a 272-bp glvA promoter region fused to lacZ | This study |
| pDHΔglvCR | pDHAFBLZ with a 272-bp glvA promoter region fused to lacZ (carrying a CG-to-AT dinucleotide change at positions +6 and +7 relative to the glvA start point) | This study |
| pDHAFB | bla amyE::cat lacI | 34 |
| pDHAFB2 | bla amyE::cat | This study |
| pDAFBCMVR | pDHAFB2 with a 242-bp citM promoter region and glvR | This study |
BGSC, Bacillus Genetic Stock Center, The Ohio State University, Columbus.
TABLE 3.
Primers used in this study
| Primer | Sequence (5′→3′) or sourcea | Restriction site |
|---|---|---|
| V1-EF | gccgGAATTCCATTCTCAATCGTAATAGCG | EcoRI |
| V1-BR | gcgcGGATCCTTCCCTACTCTGATGTGC | BamHI |
| VR-EF | gccgGAATTCGAAGAACTGATCAATCAGC | EcoRI |
| VR-BR | gcgcGGATCCTCTTCCGGCTGATCTTCC | BamHI |
| V2-HF | gccgAAGCTTTCGTCGGTATCAGCACG | HindIII |
| V2-BR | gcgcGGATCCGACCTCTTGATTCATGTCG | BamHI |
| VR-PSPE1 | gccgGAATTCTATAATAGAAAGAAAATGGGG | EcoRI |
| VR-PSPB2 | cgcGGATCCACTGTAACCGCTGAAACC | BamHI |
| GLVR-SDB | gccgGGATCCAAATGGGGGGATCTGATAT | BamHI |
| GLVR-PROE2 | gcgcGAATTCGCTTCCAAAGCGCTGAAT | EcoRI |
| CMUD-F4 | gccgGAATTCTAAACGAACAGGACTGGG | EcoRI |
| CMUD-PH2B | gcgcGGATCCGTCTTGCCTTTTTGCCATC | BamHI |
| GLV-UPF | gccgGAATTCGGCATGTATCCGAATCG | EcoRI |
| GLV-UPR | gccgGTCGACCTTCATATGACGACCTCC | SalI |
| GLV-creF | ATAAATGGAATTGTAAAATTTATCAAGGAGGTCGTC | |
| GLV-creR | GACGACCTCCTTGATAAATTTTACAATTCCATTTAT | |
| Mal-EF | gccgGAATTCGTGGAAAGAAGCTGTCG | EcoRI |
| Mal-PB | gccgCTGCAGCTAATGCCCATCACAGC | PstI |
| CCPA-F1 | AGCGAGAGAAGCTAATGTAA | |
| CCPA-R2 | GTGCGGCAGTTCGACGA | |
| V1-PEX | AGAGCATGAGTACGATCC | |
| −21M13 | Universal primer (Takara) | |
| M13RV | Universal primer (Takara) | |
| PM-FK | cggGGTACCGTGTGGAATTGTGAGCG | KpnI |
| PM-T7 | TAATACGACTCACTATATAGTGTATCAACAAGCTGG |
The additional sequence (lowercase), restriction site (underlined), and the T7 promoter sequence (bold) are indicated. The PM-FK and PM-T7 primers were annealed to the outside of the multicloning site of pMUTIN derivatives.
Construction of glvARC and malL disruptants.
GLVAd, GLVRd, and GLVCd were constructed by means of Campbell-type integration with plasmids prepared from E. coli C600 cells harboring pMV1, pMVR, and pMV2, respectively. To construct the B. subtilis MALLdd (malL::kan) strain, a fragment containing malL was amplified with primers Mal-EF and Mal-PB and then digested with EcoRI and PstI, followed by ligation to the corresponding sites of pUC119. The resultant plasmid, pUCMALL, was digested at the HincII site in the malL gene and then ligated to a StuI-SmaI fragment containing the kanamycin-resistant cassette from pDG782, followed by transformation of E. coli. Plasmids were isolated from the Kmr transformants, and a plasmid (pUCMALLKm) containing the Kmr cassette in the reverse direction with respect to the malL gene was selected. The pUCMALLKm plasmid was linearized with AatII and used for transformation of B. subtilis 168. A malL null mutant, MALLdd, was selected on agar medium containing kanamycin. To construct B. subtilis MLGLVAd, B. subtilis GLVAd was transformed with chromosomal DNA prepared from the MALLdd strain, and kanamycin-resistant transformants were selected.
Construction of a strain containing isopropyl-β-d-thiogalactopyranoside (IPTG)-inducible glvRC.
A fragment containing the Shine-Dalgarno sequence and the 5′ end of glvR was amplified with primers VR-PSPE1 and VR-PSPB2, followed by digestion with EcoRI and BamHI. The digested fragment was ligated to the corresponding sites of pMUTIN4, resulting in pMVR-SD. The plasmid from E. coli C600(pMVR-SD) was used for transformation of B. subtilis 168. The resultant Emr transformant (GLVR-PSP) contains glvRC which is regulated by the spac promoter. Proper integration of the plasmids was confirmed by Southern hybridization.
Construction of plasmids integrated into the amyE locus.
A 272-bp fragment containing the glvA promoter region was amplified with primers GLV-UPF and GLV-UPR, followed by digestion with EcoRI and SalI. The digested fragment was ligated to the corresponding sites of pUC119, resulting in pUCΔglv. An EcoRI-SalI fragment of pUCΔglv was cloned into the corresponding site of an integration vector, pDHAFBLZ, resulting in pDHΔglv. Then B. subtilis 168 was transformed with the linear fragment of pDHΔglv obtained with PstI, and a Cmr strain (AMGLV) was selected. Proper integration was confirmed by PCR, and moreover, the amylase deficiency due to integration into the amyE locus was confirmed by plating on LB agar medium with starch, followed by the addition of the I2-KI solution (20).
Construction of strains containing citrate-controlled genes.
The citM gene is regulated by the two-component system, CitS (sensor) and CitT (positive regulator), and the target site of phosphorylated CitT is located in the upstream region of the 5′ end of citM (35). citM is repressed by glucose via a cre sequence just upstream of the ribosome-binding site of citM (35). A 242-bp fragment containing the citM promoter region without the cre sequence, but with the phosphorylated CitT target site (35), was amplified with 168 DNA and primers CMUD-F4 and CMUD-PH2B, followed by digestion with EcoRI and BamHI. The digested fragment was ligated to the corresponding sites of pBluescriptII SK(+). The resultant plasmid, pBCM2, was digested with HindIII and BamHI and the resultant 223-bp fragment was cloned into the HindIII and BglII sites of pHY300PLK, resulting in pHYCM2. To construct a strain containing a PcitM-lacZ gene, pHYCM2 was digested with SalI, followed by a fill-in reaction with T4 DNA polymerase (DNA blunting kit; Takara). After digestion with BamHI, the plasmid DNA fragment was ligated to the SmaI-BamHI fragment containing lacZ of pDHAFBLZ, resulting in pHYCM2LZ. After transformation of B. subtilis 168 with pHYCM2LZ, transformants expressed β-galactosidase activity only on the addition of 2 mM citrate (data not shown).
To construct strains containing a citrate-controlled glvR gene in the amyE locus, the BamHI-BglII fragment of pDHAFB was self-ligated to produce pDHAFB2. Then the 242-bp EcoRI-BamHI fragment containing the citM promoter region from pBCM2 was mixed with the BamHI-EcoRV fragment (containing glvR) from pBVR-SD and the EcoRI-SmaI fragment from pDHAFB2, followed by ligation. The resulting plasmid, pDAFBCMVR, was digested with PstI, followed by transformation of B. subtilis 168. The resultant Cmr strain, AMCMVR, was plated on LB agar medium with starch for the amylase assay, and proper recombination at the amyE locus was confirmed by PCR. AMCMVR was transformed with B. subtilis 1A1 DNA and then selected on LB agar medium containing neomycin and chloramphenicol. The resultant transformant, AMCMVRCC, was a ccpA-deficient strain and contained the citrate-controlled glvR gene. Correct recombination was confirmed by PCR with primers CCPA-F1 and CCPA-R2.
Construction of a plasmid to produce GlvR in B. subtilis cells.
A glvR-containing fragment was amplified with B. subtilis 168 DNA and primers GLVR-SDB and GLVR-PROE2 and then digested with BamHI and EcoRI. The digested fragment was cloned into the corresponding sites of pBluescriptII SK(+), resulting in pBVR-SD. After digestion of pBVR-SD with BamHI and EcoRI, the digested fragment was ligated to the corresponding sites of pHYCM2, resulting in pHYCM2VR.
Site-directed mutagenesis.
Two-base replacement in the center of the consensus cre sequence from CG to AT at positions 6 and 7 upstream of the translational start point was performed with a Quick Change site-directed mutagenesis kit (Stratagene) according to the manufacturer's instructions. pUCΔglvCR was constructed by PCR using plasmid pUCΔglv DNA and primers GLV-creF and GLV-creR. After confirmation of the two-base replacement by sequencing, the EcoRI-SalI fragment from pUCΔglvCR was ligated to the corresponding sites of pDHAFBLZ, resulting in pDHΔglvCR. This plasmid was linearized with PstI and then used for transformation of B. subtilis 168. Transformants (AMGLVCR) were selected on LB medium containing chloramphenicol, and proper recombination was confirmed by PCR and amylase assaying.
Transformation of E. coli and B. subtilis.
Transformation of E. coli cells was performed as described by Sambrook et al. (16). Conventional transformation of B. subtilis cells was performed according to the procedure of Anagnostopoulos and Spizizen (1).
Northern blot and primer extension analyses.
RNA preparation and probe labeling were performed as described previously (34). Northern blot analysis of RNAs fractionated by electrophoresis in agarose-formaldehyde gels was performed as described by Sambrook et al. (16). Probe labeling was performed with a DIG RNA labeling kit (Roche Diagnostics) according to the manufacturer's instructions, with some minor modifications. Briefly, the internal regions inserted into pGEM-3Zf(+) derivatives (pGV1, pGVR, and pGV2) were amplified by PCR with −21M13 and M13RV as primers. The amplified fragments were digested with HindIII or EcoRI and then used as templates for in vitro runoff transcription with T7 or SP6 RNA polymerase, yielding probes A, R, and C, respectively. The internal region inserted into a pMUTIN derivative (pMVR-SD) was amplified by PCR with PM-FK and PM-T7 as primers. The amplified fragments were digested with EcoRI and then used as templates for in vitro runoff transcription with T7 RNA polymerase. Hybridization and detection were performed with a DIG luminescent detection kit (Roche Diagnostics) according to the manufacturer's instructions.
Primer extension analysis was performed as described previously (9) with an end-labeled V1-PEX primer.
β-Galactosidase assay.
After shaking at 37°C, samples were withdrawn at various times to assay β-galactosidase activity. Measurement and calculation of β-galactosidase activity (expressed as units per milligram of protein or optical density at 600 nm) were carried out as described by Shimotsu and Henner (21). One unit of β-galactosidase activity was defined as the amount of enzyme necessary to release 1 nmol of 2-nitrophenol from O-nitrophenyl-β-d-galactopyranoside (ONPG) in 1 min at 28°C.
RESULTS
Transcriptional analysis of the glvARC genes of B. subtilis.
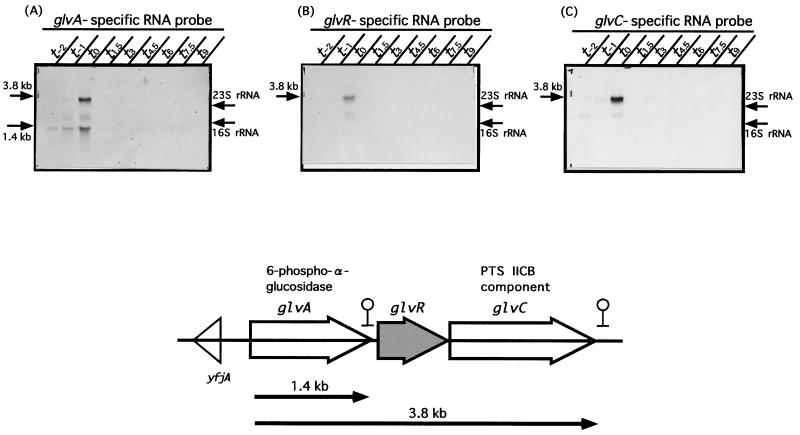
Analysis of the B. subtilis genome sequence shows that glvA, glvR, and glvC are transcribed in the same direction. In addition, deduced ρ-independent terminators are located between glvA and glvR and downstream of glvC. yfjA that is transcribed in the opposite direction is located upstream of glvA (Fig. 1). To determine whether or not these three genes are transcribed as a polycistronic mRNA, we performed Northern blot analysis using specific probes A, R, and C for glvA, glvR, and glvC, respectively. Figure 1 shows that all three probes hybridized to a 3.8-kb mRNA at t0 (time [0 h] after onset of sporulation) and that the glvA-specific probe hybridized only to a 1.4-kb mRNA throughout all growth phases (Fig. 1A). These results indicate that the 1.4-kb mRNA is a glvA transcript and the 3.8-kb one is a polycistronic (glvARC) mRNA.
FIG. 1.
Transcriptional analysis of the glvA, glvR, and glvC genes of B. subtilis, B. subtilis 168 cells were cultured in a rich sporulation medium (DSM) at 37°C for various periods (t0 means the time of onset of sporulation, and t−x and tx mean x hours before and after t0, respectively). mRNAs were prepared (see Materials and Methods) and subjected to Northern blot analysis. Ten micrograms of each RNA was separated on a 1% formaldehyde–agarose gel. Signals were detected with DIG-labeled RNA probes (panel A, probe A; panel B, probe R; panel C, probe C) specific to the glvA, glvR, and glvC mRNAs, respectively. The positions of mRNA signals and rRNAs are indicated by arrows on the left and right, respectively. A map of the three genes encoded by the glv operon is shown below the panels.
Cell growth of mutants deficient in the glv operon.
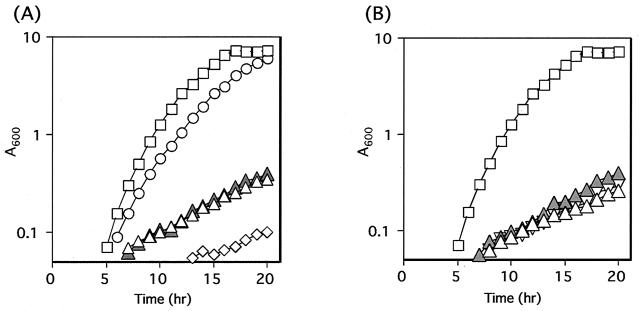
Previous results suggested that maltose is transported into cells and then metabolized through at least two systems; the PTS system related to the glv operon and the ABC transporter related to the malL gene cluster (18). Therefore, we examined the growth of organisms disrupted with respect to glvA, glvR, glvC, malL, and glvA malL in MMM (Fig. 2). The glvA, glvR, and glvC genes were disrupted by a single crossover of pMUTIN2 derivative plasmids (pMV1, pMVR, and pMV2), with the GLVAd, GLVRd, and GLVCd strains being obtained, respectively. In these mutants, the downstream genes can be expressed in the presence of IPTG. The GLVAd strain grew very poorly in MMM with or without IPTG. The MALLdd strain lacking malL, due to a double crossover as described in Materials and Methods, grew quite well in MMM (Fig. 2A). The GLVRd and GLVCd strains also grew very poorly in MMM with or without IPTG (Fig. 2B). Therefore, all the genes in the glv operon are required for growth in MMM, but malL is not required, although some growth inhibition was observed (Fig. 2).
FIG. 2.
Growth of various mutants in MMM. (A) Open squares, 168 (wild type); open triangles, GLVAd (glvA::pMV1); filled triangles, GLVAd with 1 mM IPTG; open circles, MALLdd (malL::kan); open diamonds, MLGLVAd (glvA::pMV1 malL::kan). (B) Open squares, 168 (wild type); open triangles, GLVRd (glvR::pMVR); filled triangles, GLVRd with IPTG; inverted triangles, GLVCd (glvC::pMV2).
Maltose induction and glucose repression of expression of the glv operon.
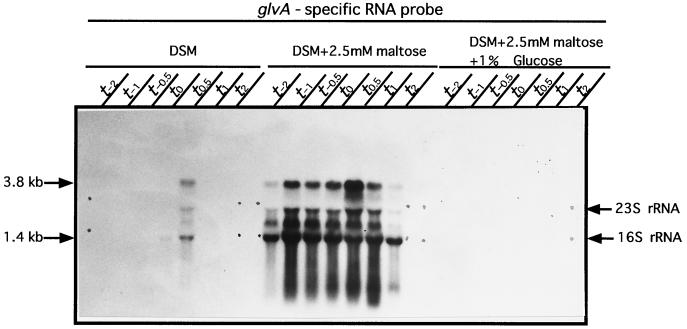
The effects of maltose and glucose on glv expression in the wild-type strain are shown in Fig. 3. Transcripts (1.4 and 3.8 kb) were produced in DSM at t0.5, but much stronger expression was observed for both transcripts in DSM supplemented with 2.5 mM maltose throughout the growth phase from t−2 to t2. But the addition of 1% glucose completely repressed glv expression (Fig. 3). These results indicate that maltose is an inducer and glucose is a strong repressor of expression of the glv operon.
FIG. 3.
Effects of maltose and glucose on transcription of the glv operon. Maltose (2.5 mM) and/or 1% glucose was added to B. subtilis 168 cells at the beginning of growth in DSM. Northern blot analysis was carried out with probe A.
Primer extension analysis of the glv operon.
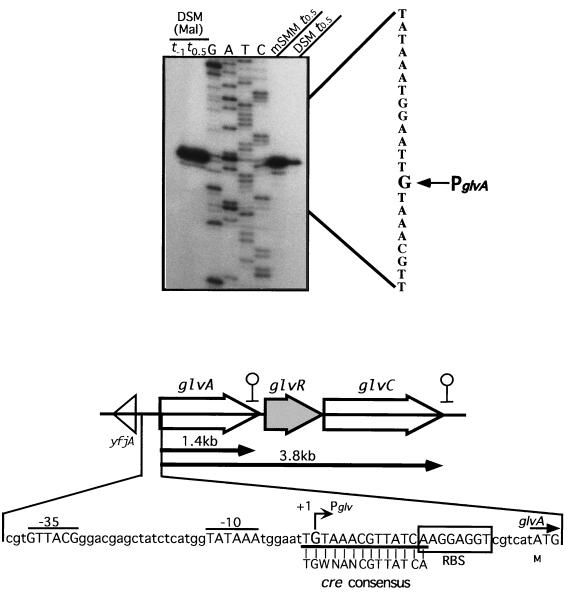
The transcriptional start point of the glv operon of the wild-type strain was determined as described in Materials and Methods. The primer was designed for the sequence from +85 to +102 with respect to the 5′ end of glvA. The transcriptional start point was G for RNA transcripts from cells cultured in DSM supplemented with maltose at t−1 and t0.5, mSMM at t0.5, and DSM at t0.5 (Fig. 4). We could not detect any other significant transcripts within a region 150-bp upstream of the 5′ end of glvA. These results suggest that the 1.4- and 3.8-kb transcripts start at the same position. The −35 region (GTTACG) and the −10 region (TATAAA), with a spacing of 18 bp, were similar to those of the ςA consensus sequence (TTGACA for the −35 region and TATAAT for the −10 region, with a spacing of 17 bp) (Fig. 4) (5). Between the −10 region and the ribosome binding site, there is a sequence (TGTAAACGTTATCA) identical to the cre consensus sequence (TGWNANCGTTATCA) (Fig. 4) (7). This sequence suggests that the glv operon is regulated by carbon catabolite repression through the ccpA gene and the cre sequence.
FIG. 4.
Determination of the transcriptional start site by primer extension analysis. Total RNAs (40, 10, 10, and 10 μg) from B. subtilis 168 cells cultured in DSM at t0.5, DSM with 2.5 mM maltose [DSM (Mal)] at t−1 and t0.5, and mSMM at t0.5, respectively, were used as RNA samples. Signals were detected with 32P-labeled primer V1-PEX. Dideoxy DNA sequencing reaction mixtures with the same primer were electrophoresed in parallel (lanes G, A, T, and C). The nucleotide sequence of the transcribed strand is given beside the sequence ladder and the arrow indicates the nucleotide at the transcriptional start site. A map of the glv operon and the nucleotide sequence of the upstream region of glv are shown below the primer extension analysis results.
Effect of a glvR mutation on expression of the glv operon.
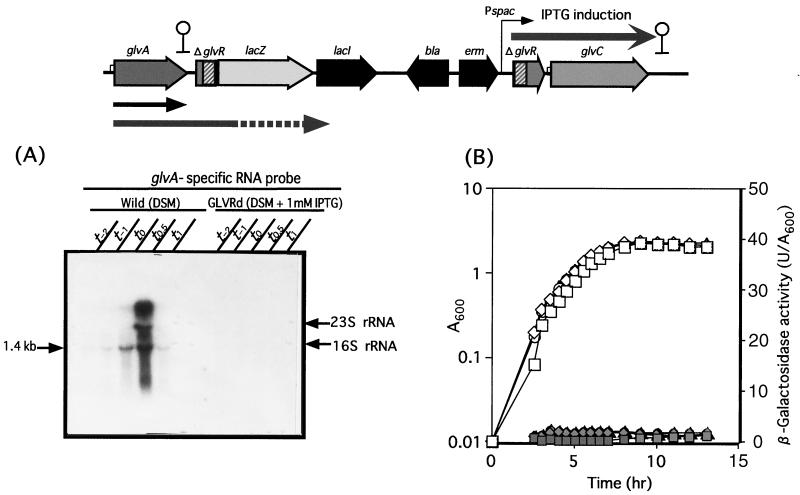
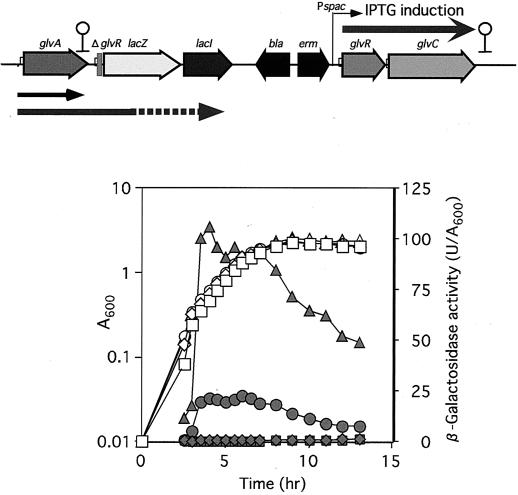
The glvR-deficient disruptant (GLVRd) containing a glvR-lacZ transcriptional fusion was cultured in DSM with or without IPTG. A glvA-specific probe hybridized to the 1.4 and 3.8 kb transcripts for the wild-type strain cultured in DSM (Fig. 5A). In contrast, no significant hybridization with transcript was observed for the GLVRd culture in DSM plus 1 mM IPTG (Fig. 5A). These results indicated that GlvR is essential for transcription of the glv operon. The LacZ assay results for GLVRd with or without IPTG supported the above finding that there is no transcription of the glvA gene even with no polarity effect (Fig. 5B). Maltose failed to induce expression of the glv operon in the GLVRd strain. To further confirm the positive effect of GlvR on glv expression, a GLVR-PSP strain containing the intact glvR gene controlled by the spac promoter and a glvR-lacZ transcriptional fusion was constructed (Fig. 6). LacZ activity was not observed for GLVR-PSP without IPTG, but there was a significant level of LacZ activity with IPTG, and the activity considerably increased in the presence of both IPTG and maltose. These results indicated that GlvR is a positive regulator and that maltose is also required for induction of the glv operon.
FIG. 5.
Northern blot analysis (A) and β-galactosidase activity (B) of the glvR-lacZ transcriptional fusion strain constructed in the B. subtilis chromosome. (A) Wild and GLVRd strains were grown at 37°C in DSM without and with 1 mM IPTG, respectively. RNAs prepared from cells were separated on a gel, and signals were detected with a DIG-labeled specific RNA probe (probe A). (B) Cell growth (A600) and β-galactosidase activity (units per A600) of the glvR-lacZ transcriptional fusion strain (GLVRd) are shown by open and filled symbols, respectively. Squares, B. subtilis 168 (wild type); diamonds, GLVRd; circles, GLVRd with IPTG; triangles, GLVRd with IPTG plus maltose. A map of the insertionally inactivated glv operon of GLVRd is shown at the top.
FIG. 6.
β-Galactosidase activity of the glvR-lacZ transcriptional fusion strain (GLVR-PSP) with the intact glvR gene. Strain GLVR-PSP was grown in DSM with or without 1 mM IPTG and 2.5 mM maltose at 37°C. Growth (A600) and β-galactosidase activity (units per A600) are shown by open and filled symbols, respectively. Squares, B. subtilis 168 (wild type); diamonds, GLVR-PSP; circles, GLVR-PSP with IPTG; triangles, GLVR-PSP with IPTG plus maltose. A map of the glv operon of GLVR-PSP is shown at the top.
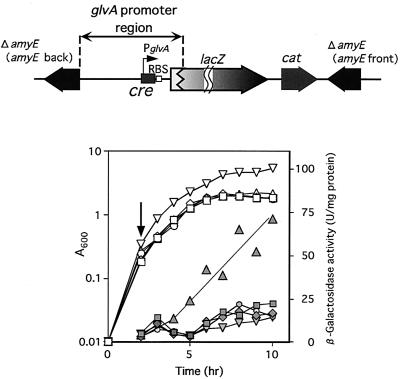
To further investigate the glucose repression, we constructed an AMGLV strain containing the glvA promoter region fused with lacZ in the amyE locus (Fig. 7). Additionally, we constructed a GlvR-producing plasmid controlled by the citM promoter (pHYCM2VR). The glvR gene of pHYCM2VR was only expressed in the presence of citrate (data not shown). The AMGLV strain harboring the pHYCM2VR plasmid was cultured in DSM, followed by the addition of 2 mM citrate at t−2 (Fig. 7). GlvA-LacZ activity was observed on glvR expression due to citrate. If we added glucose to the citrate-containing AMGLV(pHYCM2VR) culture, the LacZ activity was completely repressed. This indicates that glucose represses the glvA operon and that the target site for the sugar is located between −240 and +32 with respect to the transcriptional start point of the glv operon (covering the −35 and −10 promoter regions and the translational start codon of glvA).
FIG. 7.
β-Galactosidase activity of a PglvA-lacZ translational fusion localized at the amyE locus. The AMGLV strain having the PglvA-lacZ fusion at the amyE locus was transformed with a citrate-regulated glvR plasmid, pHYCM2VR, and then β-galactosidase activity of the transformant cultured in DSM with or without 1% glucose and 2 mM citrate was measured. Glucose was added at 0 h and citrate was added at the time indicated by an arrow. Growth (A600) and β-galactosidase activity (units per A600) are shown by open and filled symbols, respectively. pHYCM2 is a control plasmid without the glvR gene. Squares, B. subtilis 168(pHYCM2); diamonds, AMGLV(pHYCM2); circles, AMGLV(pHYCM2VR); triangles, AMGLV(pHYCM2VR) with citrate; inverted triangles, AMGLV(pHYCM2VR) with citrate and glucose. A map of the amyE locus of AMGLV is shown at the top.
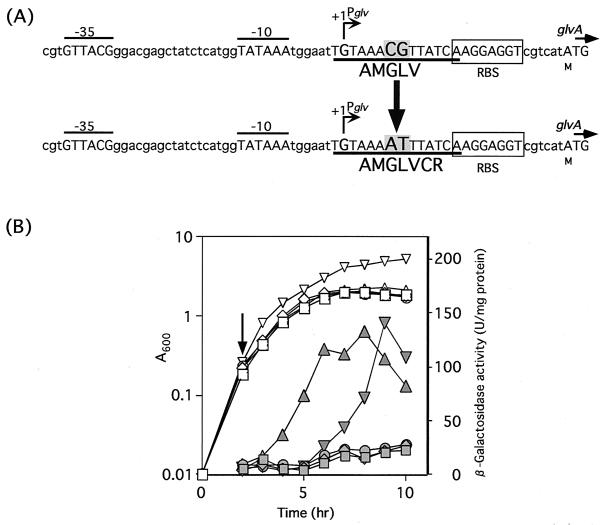
Effect of a cre mutation on expression of the glv operon.
Since the target region contains the deduced cre sequence, we changed the cre sequence of the wild type to a mutated cre sequence (with a CG-to-AT change in the center of cre) (Fig. 8). The mutant strain AMGLVCR harboring pHYCM2VR expressed LacZ activity after citrate addition, and this activity was not repressed by glucose (Fig. 8). The maximal expression level in a medium containing citrate and glucose was very similar to that in a medium containing citrate, and thus the cre sequence was essential for glucose repression of the glv operon. There was a 3-h delay of glvA expression in the medium containing citrate and glucose (Fig. 8). Although the reason for this phenomenon has not been determined, the mutated cre sequence of AMGLVCR might retain weak binding for CcpA.
FIG. 8.
The deduced cre and mutated cre sequences of AMGLV and AMGLVCR, respectively (A), and β-galactosidase activity of AMGLVCR(pHYCM2VR) (B). AMGLVCR was constructed by changing the cre sequence to a mutated cre sequence upstream of the glvA-lacZ fusion at the amyE locus. β-Galactosidase activity of AMGLVCR(pHYCM2VR) cultured in DSM with or without 1% glucose and 2 mM citrate was measured. Glucose was added at 0 h and citrate was added at the time indicated by an arrow. Growth (A600) and β-galactosidase activity (units per A600) are shown by open and filled symbols, respectively. Squares, B. subtilis 168(pHYCM2); diamonds, AMGLVCR(pHYCM2); circles, AMGLVCR(pHYCM2VR); triangles, AMGLVCR(pHYCM2VR) with citrate; inverted triangles, AMGLVCR(pHYCM2VR) with citrate and glucose.
Effect of a ccpA mutation on expression of the glv operon.
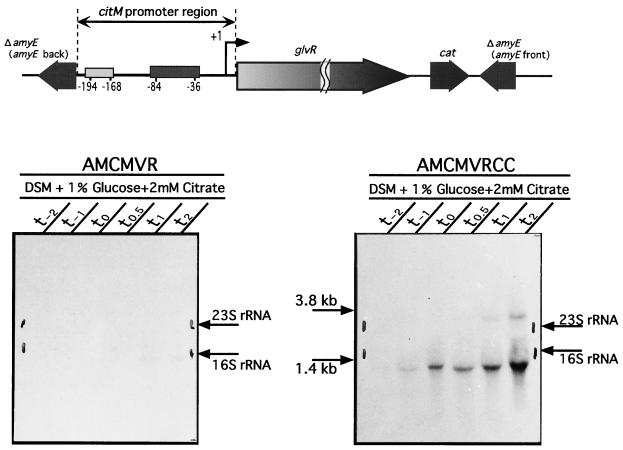
Since the cre sequence is known to be a target of CcpA for glucose repression (6), a ccpA mutation was introduced into AMCMVR containing the citrate-controlled glvR gene in the amyE locus of the chromosome. The resultant ccpA mutant strain, AMCMVRCC, was cultured in DSM containing 2 mM citrate and 1% glucose, and then transcripts were submitted to Northern blot analysis (Fig. 9). AMCMVR failed to generate the 1.4- and 3.8-kb transcripts of glv, whereas AMCMVRCC clearly produced both transcripts. These results indicate that glucose repression of the glv operon requires both CcpA and the cre sequence.
FIG. 9.
Northern blot analysis of the ccpA+ strain, AMCMVR, and the ccpA mutant, AMCMVRCC, in DSM with 2 mM citrate and 1% glucose. AMCMVR and AMCMVRCC contain PcitM-glvR at the amyE locus (top). Glucose was added at t−4 and citrate was added at t−2. Probe A was used for Northern blotting.
DISCUSSION
Many Bacillus strains utilize maltose as sole carbon and energy source, but whether a maltose-specific phosphoenolpyruvate-PTS was present in members of this genus has long been unclear. Indeed, early studies with Bacillus popilliae (28) and later investigations with Bacillus licheniformis (27) and B. subtilis (26) had failed to detect maltose-PTS activity in these species. However, discovery of the three-gene glv operon in 1996 (36) during sequencing of the B. subtilis genome led to suggestions that this operon might participate in the PTS-mediated dissimilation of α-glucosides (including maltose) in B. subtilis. Two significant findings provided experimental support for this proposal. First, the gene glvA was shown to encode a unique NAD+- and metal ion-dependent phospho-α-glucosidase that catalyzes the hydrolysis of maltose-6-phosphate (30). Second, the gene glvC encodes an EII(CB)mal component of the PTS, and mutation of this membrane protein (designated MalP by Reizer et al. [15]) severely curtailed the growth of the organism on maltose. In the present communication we have defined the regulatory function of the protein GlvR encoded by the second gene (glvR) in the glv operon. Additionally, we demonstrate that mutational inactivation of any of these genes almost abolishes growth of B. subtilis in the minimal medium containing maltose. A second route for transport (via an ATP-binding cassette) and metabolism of maltose is also present in B. subtilis, and Dahl and his colleagues have described a maltose-inducible α-glucosidase, MalL (19), whose gene is located in a large operon that also encompasses genes yvdE to yvdM (8, 19). Cells defective in malL grew poorly in MMM, but growth was not inhibited to the degree noted in the glvA-deficient organism (Fig. 2). The PTS–phospho-α-glucosidase would appear to be the more significant of the two metabolic routes for disaccharide metabolism. Inspection of the glv operon (Fig. 1) reveals the absence of a gene whose sugar-specific product (EIIA) is required for functional operation of all sugar-phosphotransferase systems. Interestingly, operons for the PTS-mediated translocation of both sucrose and trehalose by B. subtilis also lack the corresponding (and expected) disaccharide-specific EIIA genes. Evidence presented by Sutrina et al. (25) and Dahl (3) indicates that EIIAglc can serve as a substitute for these disaccharide-specific PTSs. A similar cross-complementation may also occur between EIIAglc and EII(CB)mal components to yield an operational maltose-PTS in B. subtilis.
Primer extension analysis indicated that the two transcripts (1.4 and 3.8 kb) start at the same point in the glv operon (Fig. 4). Only the ςA consensus sequence was found in the −10 and −35 promoter regions, the former being highly conserved. Since the major transcript was the glvA transcript, it is considered that transcription mainly stopped between glvA and glvR. That the glvARC operon has a strong stem-loop structure (ΔG = −30.1 kcal/mol) at 59 to 6 bp upstream from the glvR translational start point and a weaker stem-loop structure (ΔG = −18.6 kcal/mol) downstream of glvC may be reflected in the relative amounts of the two transcripts.
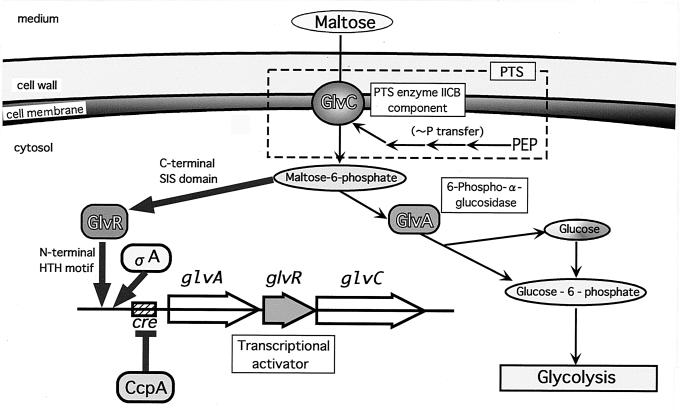
Our findings indicate that the glv operon is autoregulated by the positive regulator GlvR, which is a potential helix-turn-helix DNA-binding protein (N-terminal amino acid residues 1 to 106; Pfam software; Sanger Centre). GlvR has the sugar isomerase (SIS) domain in the C-terminal region (residues 107 to 243; Pfam software). The SIS domain is a phosphosugar-binding domain found in many phosphosugar isomerases and phosphosugar binding proteins (22). SIS domains are also found in proteins that regulate the expression of genes involved in the synthesis of phosphosugars. It is therefore likely that maltose-6-phosphate binds to GlvR to exert a positive effect on glvARC transcription. The upstream region (−240 to +32 with respect to the transcriptional start point) of the glvA gene seems to be the target for GlvR, because the β-galactosidase activity of PglvA-lacZ of AMGLV(pHYCM2VR) was completely dependent on the citrate-induced expression of GlvR (Fig. 7). Maltose is an inducer of the glv operon, and this induction may be caused by GlvR being strongly activated by the higher accumulation of maltose-6-phosphate. Actually, induction of glvA on the plasmid yielding the decrease of maltose-6-phosphate led to repression of the glv operon (data not shown). These proposed mechanisms for regulation of the glv operon are illustrated in Fig. 10. The role of the malL operon for maltose metabolism is not presented in Fig. 10, because the precise function(s) and contributions of this system in B. subtilis have yet to be resolved.
FIG. 10.
Illustration of proposed mechanisms of PTS-dependent maltose transport and metabolism. Maltose is also incorporated into cells via an ABC transporter, whose system is explained with the PTS system in Discussion. Thin arrows indicate the metabolic pathway, and thick arrows and perpendicular ones indicate positive and negative controls, respectively. PEP, phosphoenolpyruvate; HTH, helix-turn-helix.
The cre sequence (TGTAAACGTTATCA), which is completely identical to the consensus sequence, located between the −10 sequence and a ribosome-binding site of the glv operon was important for catabolite repression by glucose (Fig. 8). A CG-to-AT change in the center of cre was made in the AMGLVCR strain. Expression of the glv operon in the mutated cre strain was not severely affected by glucose (Fig. 8). The lack of CcpA also led to expression of the glv operon even in the presence of glucose (Fig. 9). Therefore, glucose repression of the glv operon is mediated by CcpA and cre. Recently, Marino et al. (10) reported the two-dimensional gel electrophoretic patterns of proteins formed during adaptation of B. subtilis under the shift from aerobic to anaerobic conditions. Together with proteins of inositol and melibiose operons, GlvA was induced during this transition. The glv operon is also regulated through anaerobic stress, but the molecular basis for this response has yet to be defined.
Our descriptions of the genetic, biochemical, and regulatory components of the glv operon in B. subtilis provide the first unequivocal evidence for the PTS-catalyzed metabolism of maltose in any bacterial species. However, the recent discovery of homologous genes for both PTS proteins and NAD+- and metal-dependent phospho-α-glucosidase in such diverse organisms as Fusobacterium mortiferum (2, 29), Klebsiella pneumoniae (31), and Clostridium acetobutylicum (Thompson et al., unpublished data) suggests that the maltose (α-glucoside)-PTS may be considerably more widespread than is presently envisaged.
ACKNOWLEDGMENT
This work was supported by a Grant-in-Aid for Scientific Research on Priority Areas (C), “Genome Biology,” from the Ministry of Education, Science, Sports, and Culture of Japan.
REFERENCES
- 1.Anagnostopoulos C, Spizizen J. Requirements for transformation in Bacillus subtilis. J Bacteriol. 1961;81:741–746. doi: 10.1128/jb.81.5.741-746.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bouma C L, Reizer J, Reizer A, Robrish S A, Thompson J. 6-Phospho-α-glucosidase from Fusobacterium mortiferum: cloning, expression and assignment to family 4 of the glycosylhydrolases. J Bacteriol. 1997;179:4129–4137. doi: 10.1128/jb.179.13.4129-4137.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dahl M K. Enzyme IIGlc contributes to trehalose metabolism in Bacillus subtilis. FEMS Microbiol Lett. 1997;148:233–238. [Google Scholar]
- 4.Deutscher J, Reizer J, Fischer C, Galinier A, Saier M H, Jr, Steinmetz M. Loss of protein kinase-catalyzed phosphorylation of HPr, a phosphocarrier protein of the phosphotransferase system, by mutation of the ptsH gene confers catabolite repression resistance to several catabolic genes of Bacillus subtilis. J Bacteriol. 1994;176:3336–3344. doi: 10.1128/jb.176.11.3336-3344.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haldenwang W G. The sigma factors of Bacillus subtilis. Microbiol Rev. 1995;59:1–30. doi: 10.1128/mr.59.1.1-30.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hueck C J, Hillen W. Catabolite repression in Bacillus subtilis: a global regulatory mechanism for the gram-positive bacteria? Mol Microbiol. 1995;15:395–401. doi: 10.1111/j.1365-2958.1995.tb02252.x. [DOI] [PubMed] [Google Scholar]
- 7.Hueck C J, Hillen W, Saier M H., Jr Analysis of a cis-active sequence mediating catabolite repression in gram-positive bacteria. Res Microbiol. 1994;145:503–518. doi: 10.1016/0923-2508(94)90028-0. [DOI] [PubMed] [Google Scholar]
- 8.Kunst F, Ogasawara N, Moszer I, Albertini A M, Alloni G, Azeveno V, et al. The complete genome sequence of the gram-positive bacterium Bacillus subtilis. Nature. 1997;390:249–256. doi: 10.1038/36786. [DOI] [PubMed] [Google Scholar]
- 9.Kuroda A, Sekiguchi J. High-level transcription of the major Bacillus subtilis autolysin operon depends on expression of the sigma D gene and is affected by a sin(flaD) mutation. J Bacteriol. 1993;175:795–801. doi: 10.1128/jb.175.3.795-801.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marino M, Hoffmann T, Schmid R, Möbitz H, Jahn D. Changes in protein synthesis during the adaptation of Bacillus subtilis to anaerobic growth conditions. Microbiology. 2000;146:97–105. doi: 10.1099/00221287-146-1-97. [DOI] [PubMed] [Google Scholar]
- 11.Meadow N D, Fox D K, Roseman S. The bacterial phosphoenolpyruvate: glycose phosphotransferase system. Annu Rev Biochem. 1990;59:497–542. doi: 10.1146/annurev.bi.59.070190.002433. [DOI] [PubMed] [Google Scholar]
- 12.Miwa Y, Nakata A, Ogiwara A, Yamamoto M, Fujita Y. Evaluation and characterization of catabolite-responsive elements (cre) of Bacillus subtilis. Nucleic Acids Res. 2000;28:1206–1210. doi: 10.1093/nar/28.5.1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Msadek T, Kunst F, Henner D, Klier A, Rapoport G, Dedonder R. Signal transduction pathway controlling synthesis of a class of degradative enzymes in Bacillus subtilis: expression of the regulatory genes and analysis of mutations in degS and degU. J Bacteriol. 1990;172:824–834. doi: 10.1128/jb.172.2.824-834.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Postma P W, Lengeler J W, Jacobson G R. Phosphoenolpyruvate: carbohydrate phosphotransferase systems of bacteria. Microbiol Rev. 1993;57:543–594. doi: 10.1128/mr.57.3.543-594.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reizer J, Bachem S, Reizer A, Arnaud M, Saier M H, Jr, Stülke J. Novel phosphotransferase system genes revealed by genome analysis—the complete complement of PTS proteins encoded within the genome of Bacillus subtilis. Microbiology. 1999;145:3419–3429. doi: 10.1099/00221287-145-12-3419. [DOI] [PubMed] [Google Scholar]
- 16.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 17.Schaeffer P, Millet J, Aubert J P. Catabolite repression of bacterial sporulation. Proc Natl Acad Sci USA. 1965;54:704–711. doi: 10.1073/pnas.54.3.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schönert S, Buder T, Dahl M K. Identification and enzymatic characterization of the maltose-inducible α-glucosidase MalL (sucrase-isomaltase-maltase) of Bacillus subtilis. J Bacteriol. 1998;180:2574–2578. doi: 10.1128/jb.180.9.2574-2578.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schönert S, Buder T, Dahl M K. Properties of maltose-inducible α-glucosidase MalL (sucrase-isomaltase-maltase) in Bacillus subtilis: evidence for its contribution to maltodextrin utilization. Res Microbiol. 1999;150:167–177. doi: 10.1016/s0923-2508(99)80033-3. [DOI] [PubMed] [Google Scholar]
- 20.Sekiguchi J, Takada N, Okada H. Genes affecting the productivity of α-amylase in Bacillus subtilis Marburg. J Bacteriol. 1975;121:688–694. doi: 10.1128/jb.121.2.688-694.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shimotsu H, Henner D J. Modulation in Bacillus subtilis levansucrase gene expression by sucrose, and regulation of the steady-state mRNA level by sacU and sacQ genes. J Bacteriol. 1986;168:380–388. doi: 10.1128/jb.168.1.380-388.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sorensen K I, Hove-Jensen B. Ribose catabolism of Escherichia coli: characterization of the rpiB gene encoding ribose phosphate isomerase B and of the rpiR gene, which is involved in regulation of rpiB expression. J Bacteriol. 1996;178:1003–1011. doi: 10.1128/jb.178.4.1003-1011.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stülke J, Hillen W. Regulation of carbon catabolism in Bacillus species. Annu Rev Microbiol. 2000;54:849–880. doi: 10.1146/annurev.micro.54.1.849. [DOI] [PubMed] [Google Scholar]
- 24.Stülke J, Martin-Verstraete I, Charrier V, Klier A, Deutscher J, Rapoport G. The Hpr protein of the phosphotransferase system links induction and catabolite repression of the Bacillus subtilis levanase operon. J Bacteriol. 1995;177:6928–6936. doi: 10.1128/jb.177.23.6928-6936.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sutrina S L, Reddy P, Saier M H, Jr, Reizer J. The glucose permease of Bacillus subtilis is a single polypeptide chain that functions to energize the sucrose permease. J Biol Chem. 1990;265:18581–18589. [PubMed] [Google Scholar]
- 26.Tangney M, Buchanan C J, Priest F G, Mitchell W J. Maltose uptake and its regulation in Bacillus subtilis. FEMS Microbiol Lett. 1992;97:191–196. doi: 10.1016/0378-1097(92)90385-2. [DOI] [PubMed] [Google Scholar]
- 27.Tangney M, Smith P, Priest F G, Mitchell W J. Maltose transport in Bacillus licheniformis NCIB 6346. J Gen Microbiol. 1992;138:1821–1827. [Google Scholar]
- 28.Taylor D C, Costilow R N. Uptake of glucose and maltose by Bacillus popilliae. Appl Environ Microbiol. 1977;34:102–104. doi: 10.1128/aem.34.1.102-104.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thompson J, Gentry-Weeks C R, Nguyen N Y, Folk J E, Robrish S A. Purification from Fusobacterium mortiferum ATCC 25557 of a 6-phosphoryl-O-α-d-glucopyranosyl: 6-phosphoglucohydrolase that hydrolyzes maltose 6-phosphate and related phospho-α-d-glucosides. J Bacteriol. 1995;177:2505–2512. doi: 10.1128/jb.177.9.2505-2512.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thompson J, Pikis A, Ruvinov S B, Henrissat B, Yamamoto H, Sekiguchi J. The gene glvA of Bacillus subtilis 168 encodes a metal-requiring, NAD(H)-dependent 6-phospho-α-glucosidase. Assignment to family 4 of the glycosylhydrolase superfamily. J Biol Chem. 1998;273:27347–27356. doi: 10.1074/jbc.273.42.27347. [DOI] [PubMed] [Google Scholar]
- 31.Thompson J, Robrish S A, Pikis A, Brust A, Lichtenthaler F W. Phosphorylation and metabolism of sucrose and its five linkage-isomeric α-d-glucosyl-d-fructoses by Klebsiella pneumoniae. Carbohydr Res. 2001;331:149–161. doi: 10.1016/s0008-6215(01)00028-3. [DOI] [PubMed] [Google Scholar]
- 32.Thompson J, Ruvinov S B, Freedberg D I, Hall B G. Cellobiose-6-phosphate hydrolase (CelF) of Escherichia coli: characterization and assignment to the unusual family 4 of glycosylhydrolases. J Bacteriol. 1999;181:7339–7345. doi: 10.1128/jb.181.23.7339-7345.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vagner V, Dervyn E, Ehrlich S D. A vector for systematic gene inactivation in Bacillus subtilis. Microbiology. 1998;144:3097–3104. doi: 10.1099/00221287-144-11-3097. [DOI] [PubMed] [Google Scholar]
- 34.Yamamoto H, Mori M, Sekiguchi J. Transcription of genes near the sspE locus of the Bacillus subtilis genome. Microbiology. 1999;145:2171–2180. doi: 10.1099/13500872-145-8-2171. [DOI] [PubMed] [Google Scholar]
- 35.Yamamoto H, Murata M, Sekiguchi J. The CitST two-component system regulates the expression of the Mg-citrate transporter in Bacillus subtilis. Mol Microbiol. 2000;37:898–912. doi: 10.1046/j.1365-2958.2000.02055.x. [DOI] [PubMed] [Google Scholar]
- 36.Yamamoto H, Uchiyama S, Fajar A N, Ogasawara N, Sekiguchi J. Determination of a 12 kb nucleotide sequence around the 76° region of the Bacillus subtilis chromosome. Microbiology. 1996;142:1417–1421. doi: 10.1099/13500872-142-6-1417. [DOI] [PubMed] [Google Scholar]