Abstract
Liver fibrosis (LF) is the wound healing response to chronic liver injury. LF is the endpoint of chronic liver disease (CLD) regardless of etiology and the single most important determinant of long-term liver-related clinical outcomes. Quantification of LF is important for staging, to evaluate response to treatment and to predict outcomes. LF is traditionally staged by liver biopsy. However, liver biopsy is invasive and suffers from sampling errors when biopsy size is inadequate; therefore, non-invasive tests (NITs) have found important roles in clinical care. NITs include simple laboratory-based serum tests, panels of serum tests, and imaging biomarkers. NITs are validated against the liver biopsy and will be used in the future for evaluation of nearly all CLDs with invasive liver biopsy reserved for some cases. Both serum tests and some imaging biomarkers such as elastography are currently used clinically as surrogate markers for LF. Several other imaging biomarkers are still considered research and awaiting clinical application in the future. As the evaluation of imaging biomarkers will likely become the norm in the future, understanding pathogenesis of LF is important. Knowledge of properties measured by imaging biomarkers and its correlation with LF is important to understand the application of NITs by abdominal radiologists. In this review, we present a brief overview of pathogenesis of LF, spatiotemporal evolution of LF in different CLD, and severity assessment with liver biopsy. This will be followed by a brief discussion on properties measured by imaging biomarkers and their relationship to the LF.
Keywords: Hepatic fibrosis, Fibrosis burden, Non-invasive tests, Imaging biomarkers, Elastography, Diffusion, T1-mapping, Susceptibility, Hepatobiliary uptake, Surface nodularity, Volumetry
Introduction
Liver fibrosis (LF) is a wound healing response to chronic liver parenchymal injury with extracellular matrix (ECM) deposition [1]. Common causes of chronic liver disease (CLD) include chronic hepatitis B, (CHB), chronic hepatitis C (CHC), alcoholic liver disease (ALD), non-alcoholic fatty liver disease (NAFLD), primary biliary cholangitis (PBC), primary sclerosing cholangitis (PSC), and autoimmune hepatitis (AIH). When the injury to liver parenchyma is persistent due to a CLD, excessive and abnormal ECM deposition leads to progressive replacement of liver parenchyma by LF [2]. Untreated and progressive LF leads to cirrhosis and its associated complications, including hepatic insufficiency, portal hypertension, and the development of hepatocellular carcinoma, which often has poor outcomes and high mortality. LF is the single most important factor associated with increased likelihood of liver-related complications and increased overall and liver-related mortality in CLD [3, 4]. Fortunately, LF is reversible and near complete resolution is possible when the active injury can be treated, especially in early fibrosis stages. Therefore, the detection and determination of severity of LF has important implications in the management of CLD.
The historical standard for assessment of LF is histological evaluation by liver biopsy. Although liver biopsy is the only method that provides direct visualization of liver fibrosis, etiologic diagnosis, and assessment of inflammation, the fibrosis burden evaluation is semi-quantitative [5]. Furthermore, liver biopsy evaluation is a single timepoint assessment and repeat biopsies are generally avoided due to its invasiveness and associated non-negligible risk of complications. For these reasons, non-invasive tests (NITs) including serum tests, liver test panels, and imaging biomarkers have emerged as important biomarkers in the assessment of LF for both baseline assessment and in the serial evaluation of LF [6–8].
Therefore, the understanding of pathogenesis of LF in CLD, relationship of fibrosis burden with outcome, and regression with successful treatment would be useful for practising abdominal radiologists. Nearly all the imaging biomarkers are quantitative as compared to semi-quantitative histologic staging of LF [5]. Knowledge of physical properties measured by imaging biomarkers and their correlation with severity of fibrosis is important in the interpretation of imaging biomarkers [9–11]. Although excellent reviews on different imaging techniques for evaluation of liver fibrosis exist, a description of pathogenesis of fibrosis, regression of fibrosis, subclassification of cirrhosis, an overview of blood-based and imaging biomarkers and their correlation of fibrosis has been lacking, particularly for abdominal radiologists [5, 9, 12, 13]. In this review, we will first provide an overview of pathogenesis of LF, spatiotemporal evolution of LF in different CLD, and severity assessment with liver biopsy. This will be followed by discussion on blood-based tests and imaging biomarkers and their relationship to the LF.
Pathology of liver fibrosis
In the normal liver parenchyma, the ECM provides the architectural support in the form of Glisson capsule, as the interstitial matrix around the vessels and portal tracts, and as low-density basement membrane within the space of Disse around the sinusoids. The ECM comprises less than 3% of normal liver tissue cross-section at histology and approximately 0.5% of wet weight [14] and is composed mainly of proteins such as collagen, glycoproteins, and proteoglycans. Collagen is the major protein and bulk component and normal ECM mostly contains type I and type III collagen and basement membrane components. The normal liver parenchyma collagen content is about 2 to 8 mg/g of wet tissue [15], but in LF, two to tenfold or more increase in total collagen content occurs [16, 17]. The factors determining the degree of collagen deposition (fibrosis burden) in LF is not well known and different degrees of collagen deposition may occur in patients with the same CLD, and between patients with different CLDs at similar stages of LF [15, 18].
LF is the result of dynamic and complex interactions between fibrinogenesis and fibrinolysis that occur simultaneously in CLD. When fibrinogenesis dominates and exceeds fibrinolysis, progressive accumulation of ECM occurs, and conversely fibrinolysis dominates over fibrinogenesis during regression of LF. Although several cellular processes are identified, the basic mechanisms of fibrinogenesis and fibrinolysis, and the factors controlling the two interactive processes remains incompletely understood.
Fibrinogenesis
Fibrogenesis is the process of deposition of abnormal ECM which is quantitatively (total content and collagen type) and qualitatively (disorganized, fibrillar collagen) different from the normal liver ECM. The source of ECM are myofibroblasts which are not normally present in healthy liver but are either activated and/or recruited in response to chronic liver injury [16, 17]. Myofibroblasts are stellate or spindle shaped cells with contractile properties and have abundant intracellular proteins including actin and myosin and are predominantly derived from transdifferentiated (activated) hepatic stellate cells (HSC) [17]. The other possible sources of myofibroblasts include peribiliary fibroblasts and myofibroblasts in the portal tract, myofibroblasts around the centrolobular vein, smooth muscle cells localized in the vessel walls, myofibroblasts recruited from bone marrow and from epithelial-mesenchymal transition [17, 18]. The recruitment of myofibroblasts occurs in response to increased tension in the ECM and release of proinflammatory cytokines. Injury to liver parenchyma including hepatocytes, endothelial cells, cholangiocytes, and Kupffer cells result in release and/or secretion of proinflammatory cytokines. These cytokines can activate both HSCs and Kupffer cells. Kupffer cells also stimulate matrix synthesis, cell proliferation, and release of retinoids by HSCs through cytokines [18]. The activated HSCs acquire contractile, proinflammatory and fibrogenic properties and become myofibroblasts [19, 20].
Collagen that is deposited for a long duration becomes stiffer due to non-reducible crosslinking by the action of several matrix enzymes which makes them resistant to protease digestion or removal during fibrosis regression [19, 20]. Other proteins that are found in the abnormal ECM include proteoglycans, glycosaminoglycans, matricellular proteins, matrix-bound growth factors, fibronectin, and elastin. Elastin typically accumulates in greater quantities in advanced stages when there is less degradation [21]
Fibrinolysis
Fibrinolysis involves active degradation of abnormal ECM, a task mainly performed by matrix degrading proteases also know matrix-metalloproteinases (MMPs). There are several families of MMPs found in liver. HSCs are the principal source of MMP-2 and the sources of other MMPs are not well established [18]. The MMPs may be regulated by the tissue inhibitors of metalloproteinases (TIMPs) [21]. HSCs are also source of some of these TIMPs, making HSCs the center of both fibrogenesis and fibrinolysis.
Spontaneous resolution of LF can occur after successful treatment or removal of underlying disease/injury, and the duration to achieve complete resolution is variable. During fibrosis resolution, MMP activity increases due to rapid decrease in expression of TIMP-2. Degradation of fibrillar collagen and altered interaction between activated HSCs and ECM favors apoptosis [22] which leads to fibrosis resolution [20]. Several ongoing research studies are focused on identifying key factors in the fibrinolysis and fibrinogenesis to modulate the LF process and delay progression of disease.
Hepatic fibrosis progression
ECM deposition typically occurs in the regions of chronic inflammation/injury. LF develops with different spatial and temporal patterns that is directly related to initial injury site within the liver, and influenced by the relative concentration of proinflammatory and pro-fibrinogenic factors and the prevalent mechanisms depending on the cause of parenchymal damage [22].
LF progresses through several steps (Fig. 1): (1) portal fibrosis (a synonym is periportal fibrosis): collagen deposition that expands the portal tracts; (2) bridging fibrosis: this is characterized by portal-central or portal to portal bridging fibrosis; (3) cirrhosis: this is defined as a diffuse process with bands of fibrosis surrounding regenerative nodules. In addition, pericellular and central vein fibrosis can be seen prior to or alongside of portal fibrosis in cases of steatohepatitis and chronic venous outflow obstruction (Fig. 1).
Fig. 1.
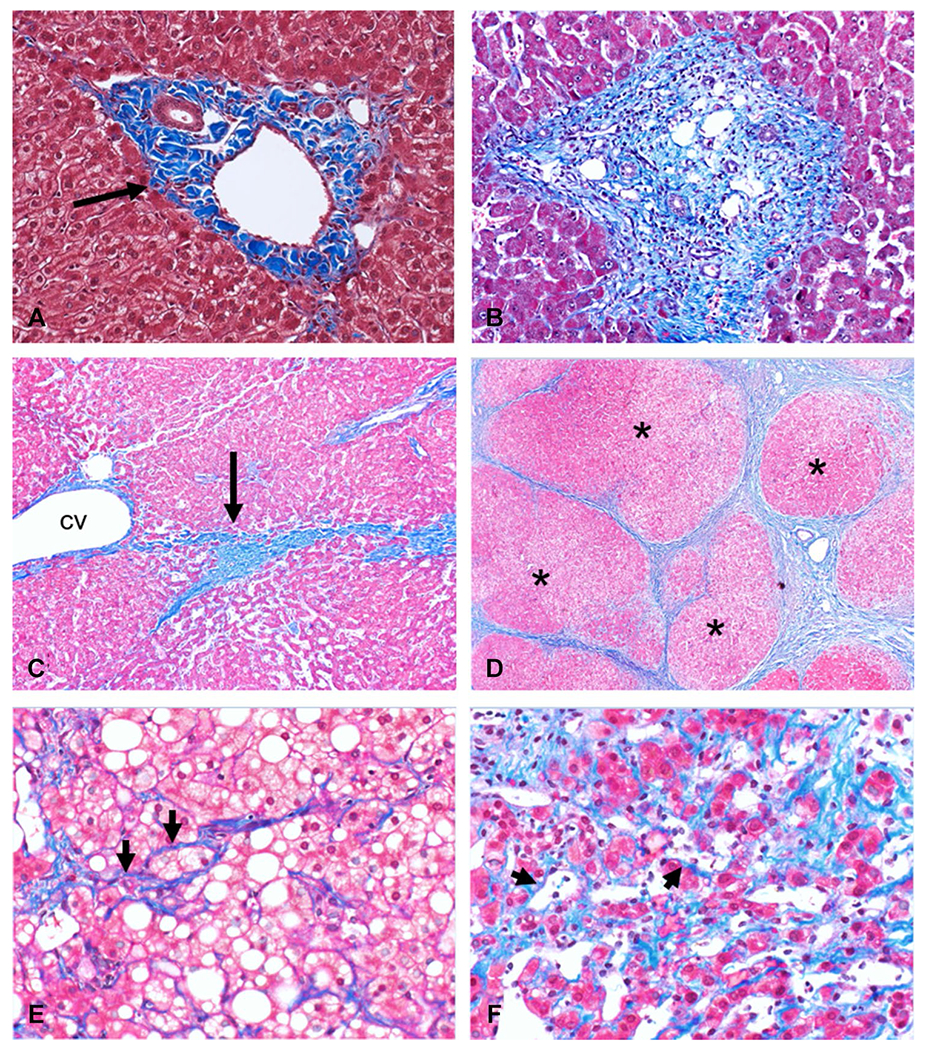
Histological images (Masson-Trichrome stain) showing a normal portal tract with normal amount of collagen (black arrow, A) within the portal tract. In portal fibrosis or periportal fibrosis (B) there is increased collagen deposition that expands the portal tract. The next stage of bridging fibrosis (C) is characterized by fibrotic bands (black arrow, C) that connect portal tract to portal tract or portal tract to central vein (cv). At the cirrhosis stage (D), fibrosis bands surround regenerative nodules (asterisk). Pericellular fibrosis where collagen fibrils surround hepatocytes (arrowheads, E and F) is typically seen in steatohepatitis (E) and chronic venous outflow obstruction (F), respectively
The onset of LF is usually insidious, and progression depending on the inciting etiology is typically slow, occurring over years or decades. Symptoms typically occur late in CLD with few exceptions. The severity of the inflammation or active liver injury correlates with the rate of progression of LF, albeit imperfectly. LF progresses at variable rates in different CLD depending on the etiology, environmental factors, and the host factors. Concurrent injury by more than one agent is synergistic for the progression of LF. Some factors are known to be associated with faster progression to advanced stages such as age at infection, obesity, diabetes mellitus, male gender, daily alcohol intake, and hepatic iron content [22, 23]. LF can rapidly progress when patients are immunocompromised as in post-transplant status or hepatitis C and human immunodeficiency co-infection [24, 25].
The total amount of fibrosis, i.e., fibrosis burden, is different in different patients and may represent the influence of several host factors and disease mechanisms. The same disease process at the same stage may result in different fibrosis burdens in different individual patients (Fig. 2), and fibrosis burden is different at same stage of disease from different etiologies (Fig. 3). Progression of LF leads to cirrhosis, which is characterized by conversion of normal liver architecture into regenerative nodules that are separated and encapsulated by fibrous tissue (fibrous septa) and accompanied by major vascular remodeling [1].
Fig. 2.
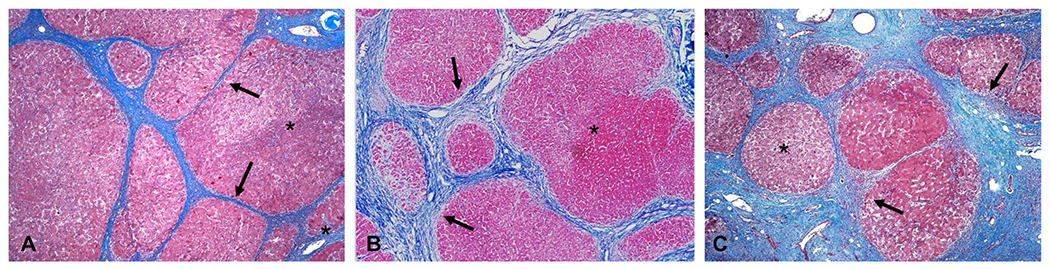
Three examples of hepatitis C cirrhosis showing different degrees of fibrosis deposition. The regenerative nodules (asterisk) are larger in examples (A) and (B) compared to (C). The fibrous bands (black arrows) in (B) and (C) are thicker than that in (A)
Fig. 3.
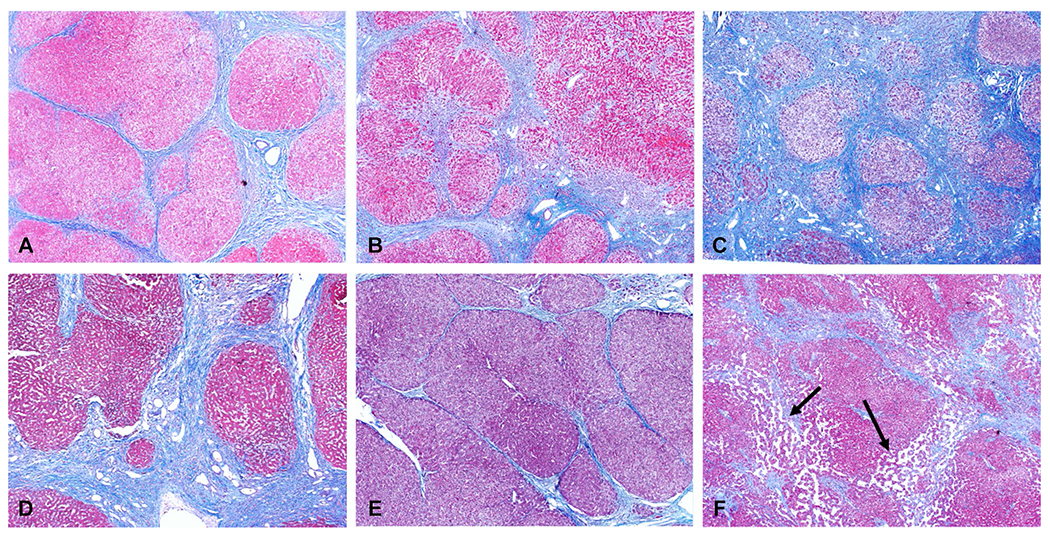
Examples of cirrhosis from chronic hepatitis C (A), NASH-non-alcoholic steatohepatitis (B), ASH-alcoholic steatohepatitis (C), primary sclerosing cholangitis (D), auto immune hepatitis (E) and cardiac cirrhosis (F). Note the different degrees of fibrosis deposition. No hepatic steatosis is seen in both NASH and ASH examples which is common at this stage of chronic liver disease. Dilated sinusoidal spaces (arrows, F) are seen secondary to chronic venous outflow obstruction in this case of cardiac cirrhosis
Vascular remodeling
In addition to the ECM deposition, LF causes changes in endothelial cell porosity, vascular thrombosis, increased sinusoidal resistance, and vascular flow reorganization [26–28]. In normal liver, sinusoidal endothelial cells lack a basement membrane and exhibit fenestrations through which exchange of substances between hepatocytes and blood in the sinusoid occurs across the space of Disse. With increased ECM in the space of Disse, the fenestrations reduce or disappear and the microvilli of hepatocytes on the sinusoidal aspect reduce leading to impairment of exchange of substances between hepatocytes and sinusoidal blood [26, 27]. These changes in the structure of sinusoids is termed capillarization of sinusoids [28, 29].
Increased ECM in the space of Disse reduces the sinusoidal lumen leading to presinusoidal vascular resistance [28]. Activated HSC with contractile properties also cause constriction of sinusoids contributing to increased resistance. Fibrosis around the perivenular region causes increased post-sinusoidal resistance, and along with increased presinusoidal resistance forms the basis for portal hypertension. In addition, vascular thrombosis mainly affecting portal vein branches occur and propagate the effects of LF on portal flow. Bridging fibrous septa between portal veins and hepatic veins provide avenues for portovenous and arteriovenous shunting to occur. Direct interconnections between arteriolar branches and portal venules may develop [30, 31]. As fibrosis progresses around the perivenular region, more blood gets shunted through the arteriovenous shunts which also contributes to the development of portal hypertension.
Regression of liver fibrosis
Removal of etiology is the most effective method in the treatment of liver fibrosis [8]. Once the causative agent of CLD is removed, regression of LF occurs through decreased production of pro-inflammatory and fibrogenic cytokines, increased fibrinolysis, suppression of ECM production, suppression of hepatocyte apoptosis, inhibition of hepatic inflammation, inhibition of myofibroblast activation, modulation and suppression of ECM deposition, and finally removal/disappearance of hepatic myofibroblasts [32]. All these processes lead to dissolution of fibrous scar [16]. However, note that term regression is used instead of reversal or resolution of LF. As mentioned earlier, longstanding collagen fibrils in LF may form non-reducible links which prevents their complete removal, and vascular remodeling that occurs during advanced stages of LF do not completely disappear. Therefore, the architectural changes and residual collagen fibrils may persist even after near complete removal of ECM, particularly in cirrhosis, hence the term regression is preferred as it indicates improvement in fibrosis but does not imply return to normal histology or normal parenchyma architecture [33].
Regression of fibrosis has been demonstrated in nearly all CLDs including hepatitis B, hepatitis C, secondary biliary fibrosis, autoimmune hepatitis, alcoholic liver disease, and NASH [34–44]. Histologically, regression of LF is seen as broken or thinned out fibrous septa with residual lobulations, and islands of hepatocytes within the fibrous septations suggesting regrowth of hepatocytes (Fig. 4).
Fig. 4.
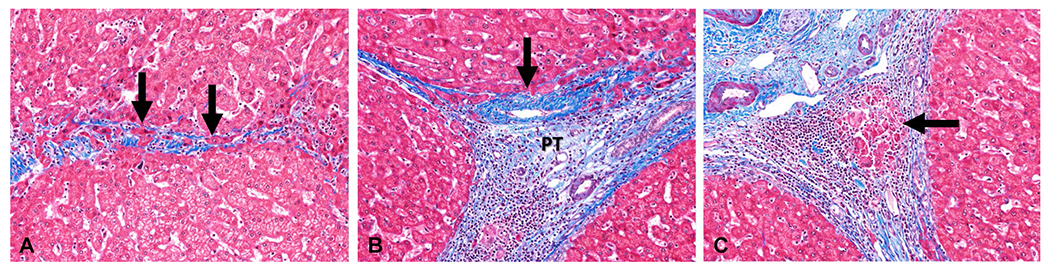
Histologic changes associated with the regression of liver fibrosis. The specimens are Masson-Trichrome stained, with collagen fibers staining blue and the hepatocytes staining red. (A) Resorbing bridging fibrosis (arrows). (B) Adhered central vein (arrow) to the portal tract (PT). (C) Regression of cirrhosis with an early bud of hepatocytes (arrow) in a fibrous band
Histological evaluation of liver fibrosis
Histological staging with liver biopsy is considered the historical standard and is the only method that allows direct observation of LF. LF can be seen on routine histological sections stained with hematoxylin and eosin but is formally assessed with special histochemical stains such as Masson’s trichrome (Fig. 1, 2, 3, and 4) and Sirius red. Masson’s trichrome stain imparts a blue color to collagen against a background of red hepatocytes and other structures. The trichrome stain typically stains type I collagen which is also present in normal portal tracts and vessel walls [18].
The first proposal of a formal grading and staging system for chronic viral hepatitis was by Knodell et al. [45] who combined scores for fibrosis stage and inflammation grade into a single numerical value. Subsequently, other scoring methods separated the inflammation grade and fibrosis stages. These methods include the Ishak system [46], Scheuer system [47], Batt-Ludwig system [48], and recently the METAVIR system [49]. These systems were mainly used in the evaluation of predominant CLD, which at that time was primarily chronic hepatitis C (CHC), chronic hepatitis B (CHB), and autoimmune hepatitis. Non-alcoholic fatty liver disease (NAFLD) uses a new system for staging—NASH clinical research network (NASH CRN) scoring system [50]. In this system, stage 1 fibrosis is divided into 1A and 1B, which shows pericellular fibrosis alone, and 1C which describes portal fibrosis only, with stage 2 indicating portal and pericellular fibrosis, stage 3 bridging fibrosis, and stage 4 cirrhosis. A congestive hepatic fibrosis score has also been proposed, in which stage 1 describes central zone fibrosis only and stage 2 describes central and periportal fibrosis with subdivisions into 2A and 2B depending on central or periportal predominance of fibrosis [51]. In most staging systems, the final stage is 4, or cirrhosis, with the exception of the Ishak system, where stage 6 is cirrhosis.
Histological evaluation provides semi-quantitative descriptors of architectural changes [52–54]. In order to provide fully quantitative data, morphometric evaluation was introduced using computer aided digital image analysis of histological sections. Collagen proportionate area (CPA) is the ratio of collagen area to hepatic tissue area in microscopy using digital image analysis [18]. Several approaches have been described including manual thresholding morphometry [55], second-harmonic generation/two photon excitation fluorescence (SHG/TPEF) microscopy [56, 57], and recently employment of artificial intelligence [58, 59]. However, these morphometric analyses require operator experience in the imaging software used [55] and are not routinely available [60].
Extending cirrhosis subclassification?
Traditionally cirrhosis was considered as the irreversible end stage of LF as there was no definitive treatment available. However, with recent availability of treatment for chronic viral hepatitis and interventions in NAFLD to prevent progression, LF can be reversed and therefore cirrhosis is no longer consider a permanent disease pattern [32, 61]. In addition, it is also recognized that cirrhosis is not a single stage but a spectrum with variable outcomes which are not fully predicted by either histologic staging systems or standard clinical parameters in patients with either a low model for end-stage liver disease (MELD) or Child–Pugh score [62–64]. As mentioned earlier, the fibrosis burden of patients are different in different diseases and therefore further staging of cirrhosis is important for predicting outcomes and individualize treatment [62].
Histologically, cirrhosis has been subclassified using the Laennec system. In the Laennec system, stage 0 indicates no definite fibrosis; stage 1, minimal fibrosis (no septa or rare thin septum and/or /portal expansion or mild sinusoidal fibrosis), stage 2, mild fibrosis (occasional thin septa); stage 3, moderate fibrosis (moderate thin septa; up to incomplete cirrhosis); stage 4A, mild cirrhosis, definite or probable; 4B, moderate cirrhosis (at least 2 broad septa or less than half of biopsy length composed of minute nodules); 4C, severe cirrhosis (at least one very broad septum or more than half of biopsy length composed of minute nodules) [65]. Other methods of classification of cirrhosis have focused on semiquantitatively assessing fibrosis burden either by evaluating septal thickness and nodule size [63, 64] or by measuring fibrosis area [66] at histological evaluation. All the cirrhosis subclassification methods have shown correlation with clinical stage (compensated versus decompensated cirrhosis) and grade of portal hypertension (hepatic venous wedge pressure, HVPG) [62–65]. In summary, methods of subclassification of cirrhosis are based on semiquantitatively evaluating fibrosis burden, and the estimated fibrosis burden correlates with clinical stage of disease.
Non-invasive tests (NITs)
Histological staging of LF is an excellent method for understanding the pathogenesis and identifying coexistent pathological processes in the CLD. However, it does have limitations, as it is invasive and associated with rare but definite complications, sampling errors, and subjectivity in interpretation [8, 52, 67–71]. As more treatment options emerge, it will become increasingly important to evaluate the response to the treatment and perform serial follow-up of CLD for assessment, and therefore a non-invasive test (NIT) is desirable for fibrosis burden quantification. Liver biopsy still has the role of the final arbiter in cases of inconclusive NIT or when NIT results do not correlate with clinical findings, and when there is concern for additional or multiple active disease processes. NITs are now increasingly used clinically to improve diagnosis of LF and assessing prognosis in CLD from various etiologies. However, some of the NITs are not widely available, are expensive, and not validated for all etiologies or not validated in different clinical scenarios. Careful selection of NIT is therefore required depending on its availability, expertise to interpret, cost and appropriateness for the clinical situation.
An ideal NIT should be reliable and reproducible, easy to perform and accurate for diagnosis of LF and quantification of degree of fibrosis burden. Recent European Association for the Study of the Liver guidelines recommend that a candidate NIT should be able to correctly classify at least 80% of patients with cut-offs with high sensitivity and specificity chosen for a particular scenario (i.e., rule out fibrosis or rule in cirrhosis) to be of value in clinical practice [72].
NITs for LF can be broadly classified into (1) Blood-based tests that includes serum tests or panel of serum tests for fibrosis and other variables; and (2) imaging-based methods which can be further classified into: (a) methods assessing liver morphology and other organs and (b) methods assessing physical properties of liver parenchyma [72].
Blood-based tests
Blood-based or serum tests for LF can be either tests for direct biomarkers or indirect biomarkers of LF. The direct biomarkers of fibrosis are molecules which are generated during fibrinogenesis and fibrinolysis, and their serum levels correlate with turnover of the ECM. The indirect tests evaluate the changes in liver function by assessing molecules released by the hepatocytes (secondary to direct or indirect injury by the etiologic agent of the chronic liver disease) into the blood stream or commonly measured for liver functions (e.g., bilirubin level) and are not directly related to ECM metabolism [73, 74]. The direct biomarkers can be further classified into those associated with ECM deposition, ECM degradation, and cytokines associated with the process of fibrinogenesis. Note that direct biomarkers also include the matrix degradation markers as fibrinolysis occurs simultaneously with fibrinogenesis as discussed in the section on pathogenesis of liver fibrosis. The markers of ECM turnover are not specific to liver and can be affected by inflammation and fibrosis occurring simultaneously elsewhere in the body. Furthermore, most of the markers only reflect the rate of ECM turnover and not deposition and therefore, do not actually assess the LF burden. Finally, the biomarker levels are affected by their clearance rate from the circulation which can be impaired due to alterations in renal function, liver function, and/or impaired biliary excretion. Biomarkers have been combined into a panel with other patient specific factors to improve sensitivity and specificity. These panels are formed from various combinations of direct markers, indirect markers, or both. Many such combinations as well as combination of the panels exists. A list of blood-based markers and panels of tests are presented in Table 1.
Table 1.
Blood-based tests for assessment of liver fibrosis
| Direct markers | Indirect markers |
|---|---|
| Extracellular matrix deposition | Liver enzymes |
| Hyaluronic acid | Serum alanine aminotransferase (ALT) |
| Laminin | Serum aspartate aminotransferase (AST) |
| Procollagen I peptide | The AST/ALT ratio (AAR) |
| Procollagen III peptide | AST to platelet ratio index (APRI) |
| Type I collagen | |
| Type IV collagen | Indirect biomarkers used in panels |
| YXL-40 (chondrex) | Gamma glutamyl transferase (GGT) |
| Extracellular matrix degradation | Serum haptoglobin |
| Matrix metalloproteinase (MMP) -1,-2 | Alpha2-macroglobulin |
| Tissue inhibitor of metalloproteinase (TIMP) | Apolipoprotein A1 |
| Cytokines | Serum bilirubin |
| Transforming growth factor-alpha (TGF-α) | Serum cholesterol |
| Transforming growth factor-beta (TGF-β) | Platelet count |
| Platelet derived growth factor (PDGF) | |
| Panels of serum tests (with or without other clinical parameters) | |
| Forns index—age, platelet count, cholesterol level, gamma glutamyl transferase (GGT) | |
| FibroTest® or FibroSure®—age, gender, serum haptoglobin, alpha2-macroglobulin, apolipoprotein A1, GGT, and bilirubin | |
| Hepascore—age, gender, bilirubin, GGT, Hyaluronic acid and alpha2-macroglobulin | |
| PGA and PGAA index—GGT, the prothrombin index, apolipoprotein A and alpha2-macroglobulin | |
| ACTI test—Fibrotest+ALT | |
| Fibro-index—platelet count, AST, and gamma globulin | |
| FIB-4 score—age, platelet count, AST, gamma globulin | |
| NAFLD fibrosis score (NFS)—age, body mass index (BMI), blood glucose levels, ALT, AST, platelet count and albumin | |
| Fibrometer™—age, platelet count, prothrombin index, AST, alpha2-macroglobulin, hyaluronic acid, BUN | |
| BARD score—BMI, AAR, and Diabetes (presence/absence) | |
| FibroSpect-II—Hyaluronic acid, TIMP-1 and alpha2-macroglobulin | |
| Enhanced liver fibrosis (ELF™) panel—Hyaluronic acid level, amino-terminal propeptide of type III collagen level, and TIMP-1 | |
Although blood-based tests are easy to perform and repeatable, several of them still require validation in different etiologies of CLD. Some of the commonly used blood-based tests include APRI in chronic hepatitis, and FIB-4 score and Fibrotest, in NAFLD. APRI (AST to platelet ratio index), a simple test has an accuracy of 0.80 with 84% sensitivity and 41% specificity for significant fibrosis (stage 2 or higher) in CHB [75]. A meta-analysis showed that APRI had accuracies of 0.77, 0.80, and 0.83 for detecting significant fibrosis, advanced fibrosis, and cirrhosis in CHC patients, respectively [76]. Fibrosure and Fibrotest are identical proprietary panels of tests used in USA and Europe, respectively, and the panel is composed of indirect markers. A meta-analysis showed that Fibrosure/Fibrotest has an accuracy of 0.84 for significant fibrosis and 0.87 for cirrhosis in CHB [77]. A large metaanalyses of 30 studies and pooled 6378 subjects including CHB, CHC, NAFLD, ALD, and mixed etiologies showed good performance of Fibrosure/Fibrotest with mean observed accuracy of 0.80 [78]. Interestingly, in the same study, analysis of individual data from about 3300 subjects showed the accuracy for distinguishing adjacent stages of fibrosis (Stage 1 vs 2, stage 2 vs stage 3, etc.) ranging from 0.62 to 0.69. The authors concluded that Fibrotest could be used as an alternative to liver biopsy for the four common CLDs.
FIB-4 test was found to perform better than APRI and VCTE for identification of advanced fibrosis in chronic viral hepatitis [79]. However, the metaanalyses had a limitation of variable cut-offs used in the individual studies. Recently, enhanced liver fibrosis (ELF) received Food and Drug Administration (FDA) approval for use in chronic liver disease. A metaanalysis of 9 studies comprised of subjects with CHB, CHC, NAFLD,ALD, PBC, and others showed that the accuracy of ELF for assessment of significant fibrosis, advanced fibrosis, and cirrhosis ranged from 0.82 to 0.98, 0.70 to 0.99, and 0.68 to 0.92, respectively, suggesting a good diagnostic value for prediction of fibrosis stages [80].
Currently there is no individual marker or panel that is considered standard of care and the use of a particular blood-based test or panel depends on their availability, and some are proprietary panels that may require mailing of blood samples for analysis. Overall, the blood tests perform well for detection of advanced fibrosis but may not be reliable for differentiating adjacent and intermediate stages of fibrosis [81–84], and their role in assessing complications, and categorizing patients with CLD into compensated and decompensated advanced liver disease is not well known or evaluated. However, these blood tests may be useful in monitoring once fibrosis stage is determined by other NITs or liver biopsy.
Studies have compared blood-based tests and imaging methods for non-invasive assessment of liver fibrosis and found variable results with some clearly favoring blood-based tests while others showing superior performance of imaging methods [79, 80]. Since blood tests and imaging methods are not widely available and not validated in all causes of CLDs, various society guidelines, and expert panels have advocated use of both blood-based tests and imaging methods in combination or in stepwise fashion depending on the etiology of the CLD for screening, to improve risk stratification and staging [72, 85–88]. They also recommend the use of blood-based tests only in resource limited settings [85]. Discussion of various society recommendations is out of scope of this review article and readers are encouraged to review these published guidelines [72, 85–88].
Imaging methods
Imaging methods are rapidly increasing in popularity and in their acceptance for use in clinical practice. Conventional methods such as ultrasound, computed tomography (CT), and magnetic resonance imaging (MRI) are useful in evaluating the morphologic changes that occur in liver in advanced fibrosis and cirrhosis stages [89]. These changes in liver anatomy include right lobe atrophy, caudate lobe hypertrophy, left lobe hypertrophy, nodular outline of the liver, enlarged periportal space, enlarged gall bladder fossa sign, and posterior hepatic notch sign [5, 90–92] (Fig. 5). Other signs of portal hypertension such as presence of portosystemic collaterals and splenomegaly may also be present in advanced fibrosis and cirrhosis. Although these signs can be specific for diagnosis of cirrhosis, they are not sensitive signs for detection of early fibrosis stages particularly in CLD such as NAFLD. The presence or absence of these imaging signs are therefore not useful for assessing early fibrosis and their utility in the assessment of changes during follow-up after treatment or intervention remains unknown.
Fig. 5.
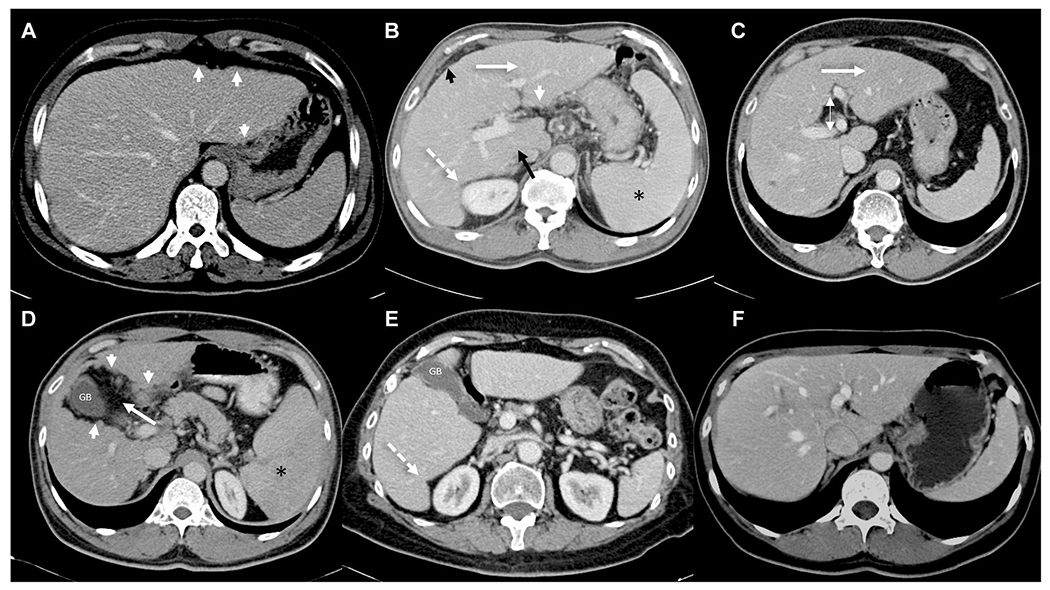
Contrast enhanced CT images from different patients illustrating the morphological changes in liver in chronic liver disease. Nodular outline of the liver (short arrows in A, B, and D), atrophic right lobe with enlarged caudate lobe (black arrow in B), enlarged left lobe (white arrow in B and C), increased abdominal fat or creeping fat sign (short black arrow, B), increased periportal space (double headed arrow, C), enlarged gall bladder fossa sign (white arrow, D), posterior hepatic notch sign (broken white arrow, B and E). Note splenomegaly (asterisk, B and D) consistent with portal hypertension. Compare with normal appearance of liver in a chronic hepatitis B patient (F) with biopsy proven stage 2 fibrosis. Morphological changes are usually seen in advanced fibrosis or cirrhosis and mostly absent in early fibrosis stages. GB = gall bladder
Quantitative imaging methods have evolved in the past few decades particularly using MRI. Some of the quantitative methods, the parameter assessed, their advantages and limitations are summarized in Table 2. Detailed description of the methods and their clinical application are described elsewhere in other articles in this special issue. Among the currently available methods, elastography methods such as vibration controlled transient elastography (VCTE), acoustic radiation force impulse (ARFI) based methods including both point and 2D shear wave elastography (SWE) (Fig. 6) and MR elastography (MRE) (Fig. 7) are widely available and validated in several studies and in multiple etiologies [81, 93–96]. Several metaanalysis results have established elastography techniques as very accurate for prediction of significant fibrosis, advanced fibrosis, and cirrhosis [97–102]. Diffusion weighted imaging (DWI) and intravoxel incoherent imaging (IVIM) are very attractive non-invasive options [99, 103, 104]. However, the reproducibility and repeatability across scanners of different magnetic strengths and vendors is poor to moderate and therefore have not found wide clinical application in the evaluation of LF. Studies have shown elastography techniques performing significantly better than DWI [99] [105].
Table 2.
Imaging based quantitative methods for evaluation of liver fibrosis
| Method | Parameter (s) evaluated | Confounding factors | Advantages | Limitations |
|---|---|---|---|---|
| Ultrasound based | ||||
| Vibration controlled transient elastography (VCTE or Fibroscan®) [81, 82, 95, 96] | Bulk modulus @50Hz | Fatty liver Inflammation Biliary obstruction Venous congestion Diffuse infiltrative disorders Post prandial state Excessive alcohol |
Rapid, easy to perform Repeatable Widely available Validated in all common CLDs Excellent for distinguishing advanced fibrosis from mild or no fibrosis Shown to be useful for predicting outcome and complications |
Dependent on operator’s experience Limited depth of penetration Patient factors-small intercostal space, excessive visceral fat, obesity, and ascites Blinded technique and region of interest cannot be chosen |
| Point shear wave elastography (pSWE) and two-dimensional shear wave elastography (2D-SWE) [72, 83, 84, 95, 96, 112] | Shear stiffness at variable frequencies | Fatty liver Inflammation Biliary obstruction Venous congestion Diffuse infiltrative disorders Post prandial state Excessive alcohol |
Can be performed with routine ultrasound of liver Region of interest (ROI) can be chosen Measures stiffness in real time High performance for diagnosis of significant fibrosis and cirrhosis |
Operator dependent Patient factors-obesity and ascites |
| CT based | ||||
| Dual energy CT | Extracellular volume (ECV) Iodine washout rate (IWR) Parenchymal iodine density |
Portal flow Inflammation Congestion |
Can be performed easily on dual energy CT scanners | Prospective evaluation results are lacking Intravenous contrast Exposure to radiation No standardized acquisition Unknown reproducibility across vendors Availability of dual energy CT technique is limited |
| MRI based | ||||
| Magnetic resonance elastography (MRE) [81–83, 87, 89, 94, 95, 97, 112] | Shear stiffness @60 Hz | Inflammation Biliary obstruction Venous congestion Diffuse infiltrative disorders |
High repeatability and reproducibility Whole liver evaluation possible High diagnostic performance for early stages of fibrosis Not affected by obesity or fatty change in the liver Validated in all common CLDs Useful for predicting outcome and complications |
Needs dedicated hardware and software Not widely available Moderate to severe iron deposition |
| Diffusion weighted imaging (DWI) and Intravoxel incoherent imaging (IVIM) [89, 99] | Apparent diffusion coefficient (ADC) Diffusion coefficient (D) Pseudo-diffusion coefficient (D*) Perfusion fraction (f) |
Fatty liver Iron deposition Inflammation |
DWI widely available in all clinical MR scanners No intravenous contrast required High performance differentiating advanced fibrosis/cirrhosis from normal liver |
Motion sensitive Lack of standardized acquisition parameters Poor repeatability and reproducibility Low to moderate performance for intermediate stages IVIM not widely available |
| T1-mapping [89, 104, 105] | T1-relaxation time | Iron Fatty change Acute liver disease |
Can be performed with or without intravenous contrast | No standardization of acquisition parameters and analysis Repeatability and reproducibility across scanners not widely established |
| T1ρ [103, 105] | Spin–lattice relaxation time | Magnetic field inhomogeneities | No additional hardware required No contrast required |
Sensitive to B0 and B1 field inhomogeneities High specific absorption rate Research studies and not validated for clinical use |
| Hepatobiliary contrast uptake on MRI | Gadoxetate uptake ratio | Genetic variability of expression of proteins Competing drugs for uptake |
Can be performed on any available clinical scanner Short post processing calculation |
Specific use of hepatobiliary contrast agent Needs longer imaging time for the hepatobiliary phase Decreased enhancement in fatty or iron loaded livers |
| CT or MRI | ||||
| Surface nodularity score (CT or MRI) [106, 107, 110, 111] | Nodularity of the surface | Disease that can mimic cirrhosis such as nodular regenerative hyperplasia and treated multifocal metastases | Semiautomatic Performed on existing or previously acquired CT/MRI Relatively less affected by inflammation, acute biliary obstruction, and hepatic congestion |
Computation time Not validated in all etiologies May be less accurate in NAFLD May be difficult to evaluate in patients with ascites and very thin patients |
| CT volumetric assessment [89] | Caudate to right lobe ratio (CRL) Liver segment volume ratio (LSVR) Splenic volume (SV) | Volumetric changes without fibrosis can occur. For example, from portal vein occlusion in perihilar cholangiocarcinoma or severe biliary strictures in PSC | No special technique required Performed on existing CT studies Can be performed on MRI as well |
Computation of volumes take time Ionizing radiation with CT Low sensitivity for early fibrosis Other causes of organomegaly may confound volumetry |
| Fractional extracellular space (CT or MRI) | Extracellular space which becomes wider or larger in LF due to deposition of fibrosis in extracellular space | Edema Congestion |
Can be performed with CT or MRI | Need prospective evaluation Specific phases of contrast need to be obtained such as equilibrium phases Motion artifacts Not validated in all etiologies Poor discrimination between intermediate stages of fibrosis |
| Perfusion CT/MRI | Mean transit time (MTT) Portal venous perfusion Hepatic arterial perfusion Arterial perfusion fraction |
Inflammation may increase blood flow Passive venous congestion may reduce portal flow |
Applicable to any contrast enhanced studies Can be incorporate into any existing CT or MRI scanners |
Need prospective evaluation Fasting is essential Prone for motion artifacts Acquisition protocols and reconstruction methods are not standardized Additional computation and complex modeling required Software not available widely |
Fig. 6.
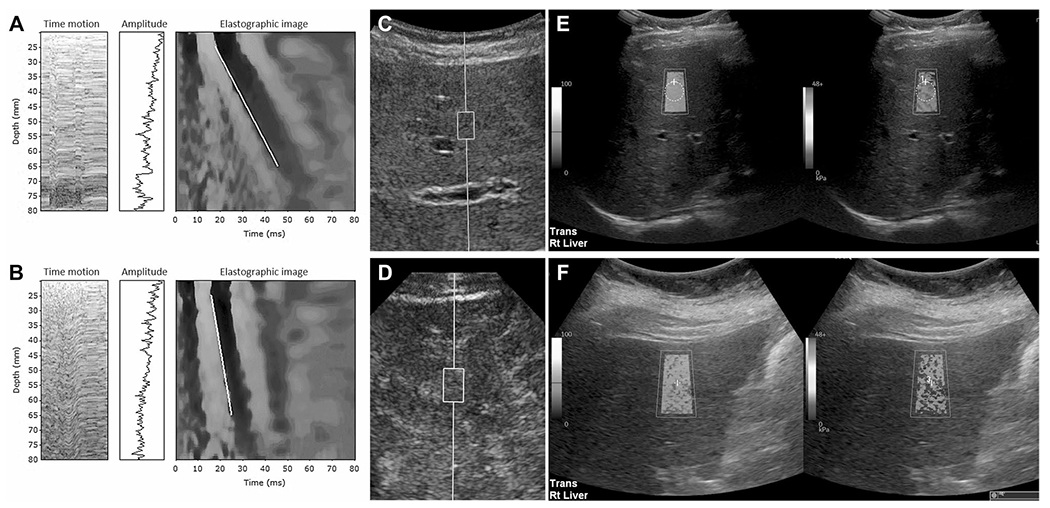
Examples of vibration controlled transient elastography (VCTE) (A, B), point shear wave elastography (pSWE) (C, D) and two-dimensional shear wave elastography (2D SWE) (E, F). A VCTE image in a 70-year-old woman with primary biliary cholangitis. The bulk modulus (E) is 5.1 kPa and within normal range. B VCTE image in a 49-year-old male with chronic hepatitis C with E value of 46.4 kPa consistent with cirrhosis. C pSWE image in a 31-year-old female with congestive hepatopathy with measured median velocity of 2.1 m/s corresponding to calculated value of 13.1 kPa consistent with advanced fibrosis and confirmed with biopsy. D pSWE image in a 36-year-old female with post Fontan congestive hepatopathy showing a median velocity of 4.25 m/s corresponding with 54 kPa consistent with biopsy confirmed cirrhosis. E 2D SWE image in a 50-year-old male with known hepatitis C. The median liver stiffness is 5.25 kPa which is within normal limits. F 2D SWE image in 64-year-old female with chronic liver disease of unknown etiology. The median stiffness is 16.7 kPa consistent with advanced fibrosis
Fig. 7.
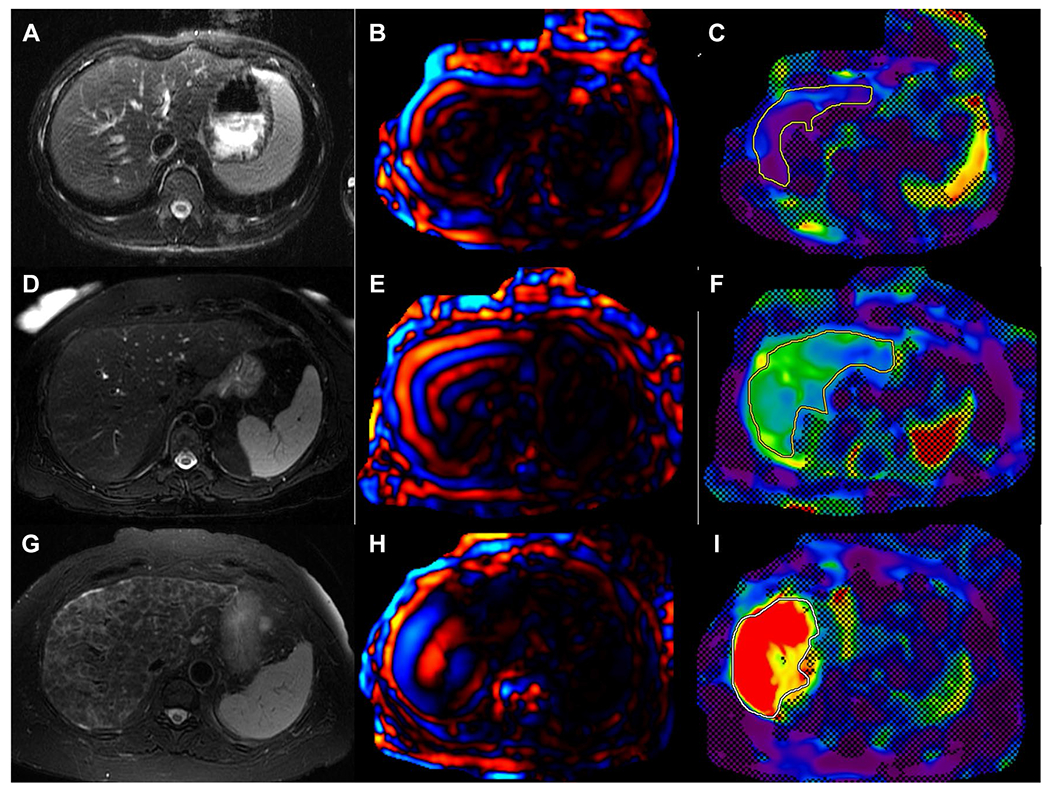
Examples of magnetic resonance elastography (MRE). Axial T2W images (A, D, G), with corresponding level color wave images (D, E, H) and color stiffness maps with confidence map overlay (C, F, I). Regions of interest (solid lines) drawn within the confidence map which provides mean stiffness of liver parenchyma at that slice level. Top row: A 18-year-old male with primary sclerosing cholangitis (PSC) with mean liver stiffness of 1.8 kPa which is within normal limits and excluding significant fibrosis. Middle row: A 62-year-old male with PSC and mean liver stiffness of 3.3 kPa suggestive of mild fibrosis. Bottom row: A 70-year-old female with primary biliary cholangitis and non-alcoholic steatohepatitis overlap with a mean liver stiffness of 7.7 kPa consistent with stage 4 fibrosis or cirrhosis
Another non-invasive parameter of interest is T1-mapping of liver parenchyma (Fig. 8) [103] and has been shown to be of value in evaluation of inflammation and fibrosis particularly in nonalcoholic steatohepatitis [104, 105]. However, the technique is not widely available or routinely used in clinical practice. Morphological signs as discussed above are not useful for differentiating early stages of fibrosis; however, methods that quantify the surface nodularity have been found useful in the diagnosis of fibrosis. Liver surface nodularity (Fig. 9) is accurate for diagnosis of cirrhosis in CLD from chronic viral hepatitis and may be combined with other volumetric indices [106–109]. The technique has the advantage that it can be performed on both CT and MRI without need for intravenous contrast and with routinely obtained images. However, the performance of LSN in NAFLD is somewhat lesser compared to that in chronic viral hepatitis and therefore needs additional studies for further validation and in large cohorts [110, 111]. Several emerging techniques include T1p-mapping, T1-mapping with hepatobiliary contrast agents, dual energy CT, perfusion CT, and fractional extracellular space quantification. These techniques are promising and the subject of research projects, and may be of value for clinical practice in the future.
Fig. 8.
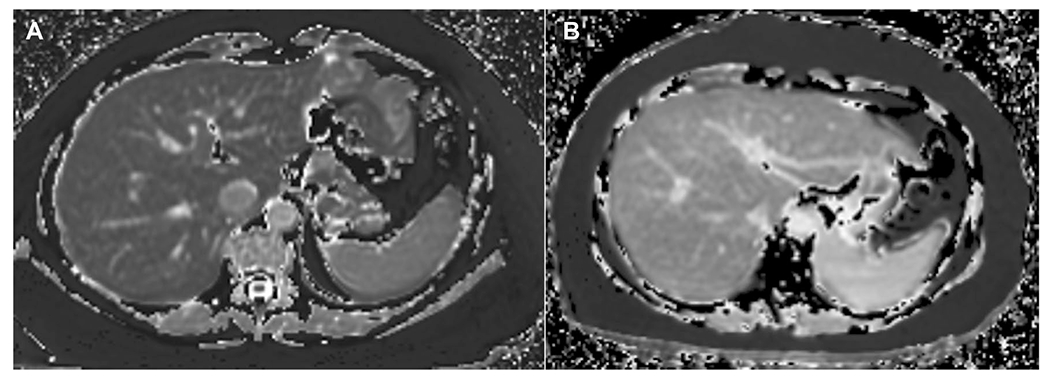
Examples of T1-maps of liver. A A 50-year-old female with obesity and mean liver T1 of 740 ms. Liver biopsy confirmed a normal liver. B A 65-year-old female with obesity and mean liver T1 of 901 ms. Liver biopsy showed grade 1 steatosis and fatty liver, mild lobular inflammation, ballooning of hepatocytes, and stage 4 fibrosis consistent with non-alcoholic steatohepatitis. (Image courtesy, Drs. Jiahui Li and Meng Yin, Radiology, Mayo clinic, Rochester, MN, USA)
Fig. 9.
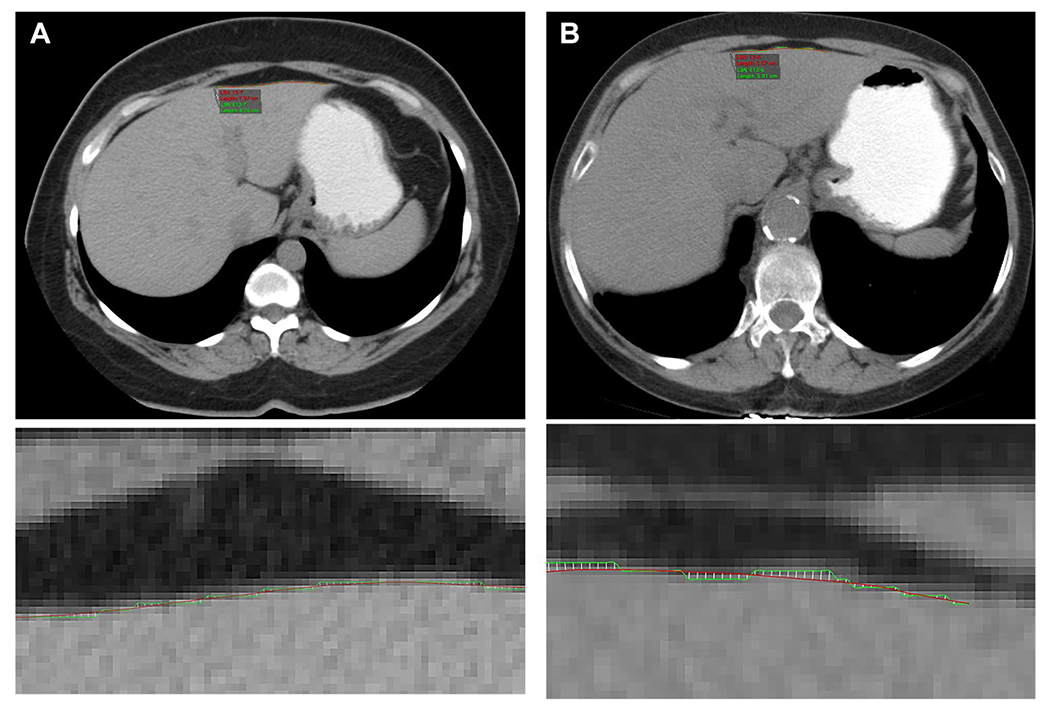
Examples of liver surface nodularity (LSN) scores derived from routine abdominal CT images. (A, B wide FOV-top row images; small FOV-bottom images) A A 46-year-old male with abdominal pain undergoes abdominal CT. The liver has normal morphological appearance, and the LSN score was normal at 1.59. B A 55-year-old male with abdominal distension undergoes nonenhanced abdominal CT. The patient had a history of hepatitis C chronic liver disease. The liver has liver surface nodularity and the LSN score was abnormal at 2.98 indicating cirrhosis (Image courtesy: Dr. Andrew Smith, University of Alabama Medical Center, UABC, Birmingham, AL)
The imaging biomarkers are only surrogate markers and have confounding factors or processes as outlined in Table 2. Some of the imaging biomarkers have technical limitations or are not fully validated across imaging systems or not validated in all types of CLD or for multiple clinical scenarios. Currently there is no perfect imaging biomarker for LF, but elastography based biomarkers are widely used in clinical practice due to their reliability and accuracy.
A summary of sensitivity, specificity, and area under ROC of several NITs for distinguishing any fibrosis (≥ stage 1), significant fibrosis (≥ stage 2), advanced fibrosis (≥ stage), and cirrhosis (stage 4) is presented in Table 3. The numbers in the table are derived from several metaanalysis studies and some individual studies. Several of these studies have limitations in collection of data, selection bias, and are not applicable to all CLD. Note that most NITs have excellent performance for distinguishing cirrhosis from lesser stages of fibrosis or normal liver.
Table 3.
Accuracies of non-invasive tests (NITs) for diagnosis of fibrosis, significant fibrosis, advanced fibrosis, and cirrhosis
| NIT | Fibrosis (≥ stage 1) |
Significant fibrosis (≥ stage 2) |
Advanced fibrosis (≥ stage 3) |
Cirrhosis (= stage 4) |
Ref | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AUROC | Sensitivity (%) | Specificity (%) | AUROC | Sensitivity (%) | Specificity (%) | AUROC | Sensitivity (%) | Specificity (%) | AUROC | Sensitivity (%) | Specificity (%) | ||
| APRI | 0.83 | 79–82 | 73–83 | 0.54–0.87 | 60–69 | 69–77 | 070–0.78 | 33–73 | 67–91 | 0.75–0.77 | 56–62 | 72–86 | [81–84] |
| FIB-4 | 0.76 | 79–83 | 50–73 | 0.56–0.85 | 62–65 | 70–75 | 0.77–0.80 | 32–78 | 70–96 | 0.82–0.85 | 65–76 | 80–83 | [81–83, 85] |
| VCTE | 0.78–0.85 | 64–82 | 65–88 | 0.83–0.86 | 65–94 | 58–80 | 0.85–0.91 | 70–88 | 66–90 | 0.89–0.94 | 70–100 | 77–91 | [81, 93–96] |
| pSWE | 0.77–0.88 | 64–79 | 76–86 | 0.85–0.86 | 69–75 | 83–85 | 0.89–0.94 | 80–87 | 86–87 | 0.90–0.93 | 76–87 | 87–88 | [95, 100, 101] |
| SWE | 0.65–0.92 | 53–83 | 90–100 | 0.72–0.91 | 71–87 | 67–95 | 0.72–0.95 | 72–90 | 72–92 | 0.88–0.97 | 78–100 | 84–86 | [81, 83, 93, 95, 96, 102] |
| MRE | 0.80–0.97 | 60–83 | 73–98 | 0.88–0.97 | 73.2–94 | 85–95 | 0.92–0.98 | 83–92 | 83–96 | 0.90–0.99 | 81–98 | 81–94 | [81, 83, 93–95, 97–100, 102] |
| DWI/IVIM | 0.83–0.89 | 72–87 | 67–91 | 0.79–0.87 | 71–82 | 69–84 | 0.83–0.89 | 60–82 | 77–89 | 0.86–0.88 | 80–87 | 73–77 | [99, 103–105] |
| LSN | 0.85–0.90 | 65–77 | 88–100 | 0.80–0.93 | 68–80 | 62–97 | 0.84–0.98 | 67–89 | 84–89 | 0.83–0.96 | 90–98 | 79–85 | [110–114] |
The numbers are from single reference or multiple references when the values are single or in range, respectively
The values may not be applicable to chronic liver diseases not evaluated
AUROC Area under receiver operating curve (0 to 1), APRI aspartate to platelet ratio index, FIB-4 Fibrosis-4, VCTE vibration controlled transient elastography, pSWE point shear wave elastography, 2D SWE two-dimensional shear wave elastography, MRE magnetic resonance elastography, DWI diffusion weighted imaging, IVIM Intravoxel incoherent imaging, LSN liver surface nodularity
NITs, particularly elastography techniques have been demonstrated to be useful in the assessment of response to intervention. Interval improvement in stiffness may represent improvement in inflammation, fibrosis or both depending on the clinical interval and expected time course for response to treatment. The interval change in stiffness (delta change) is more useful than absolute values [112]. The same technique, and if possible same equipment, should ideally be used for follow-up assessment. For example, if the baseline evaluation was performed with SWE, the follow-up should also be performed with SWE for meaningful assessment and not be done with VCTE or MRE because of its availability or superior accuracy, respectively.
When regression of fibrosis occurs in cirrhotic liver with treatment such as in chronic hepatitis C, improvement in fibrosis burden occurs gradually and the remodeling can take years to occur. At follow-up, the liver may still have a cirrhotic appearance, and the liver stiffness may be normal or only mildly elevated and should not be interpreted as failure of technique. However, a combination of blood markers and liver stiffness measurement would be useful in determining the response. Morphology based tests including LSN, volumetric changes and possibly textural analysis may fail to show the changes in the short term and their utility in the assessment of changes in fibrosis burden is not known.
Textural analysis
Liver parenchyma becomes coarser and appears heterogeneous in LF on ultrasound, CT, and MRI images. Computer based textural analysis may be able to detect subtle differences between fibrotic livers and normal livers which are not visible to the human eye. Textural analysis can be carried out on any imaging modality and does not require prospective acquisition. In one study using texture analysis of noncontrast enhanced T1W and T2W images found comparable performance with MRE [113] Although it can be performed on non-contrast enhanced images, contrast enhanced images appear to produce better results [114] and this can be a limiting factor in wide application for the purpose of LF quantification. However, the analysis is not standardized, and requires specialized software for analysis and the need for additional computing time. The utility of textural analysis in determining the long term outcome in CLD and response assessment to treatment is not yet determined.
Artificial intelligence and deep learning algorithms
Given the great interest in application of artificial intelligence (AI) and deep learning (DL), many research studies have been performed for LF staging using US, CT, or MRI images [115–119]. The specific advantage of this approach is that available images can be used for training. High performance ranging from 0.74 to 0.97 have been demonstrated for distinguishing LF stages using deep convolutional neural network (DCNN) using non-contrast enhanced and contrast enhanced CT and MRI images including hepatobiliary phases [115–118]. The field of AI and DL is exciting with a lot of promising applications; however, there is no standardized approach and therefore clinical applications are lacking. However, in the future one may expect to see AI and DL methods integrated with NITs.
Correlation of quantitative NITs with semiquantitative histology staging
Currently several research and clinical studies perform validation of quantitative imaging biomarkers against semiquantitative histologic staging; however, this is an imperfect approach. There are several limitations with this approach. First, the histologic stages are not equidistant but at key points in the continuum between normal and stages of fibrosis. For example, stage 2 LF does not have double the amount of fibrosis burden as that in stage 1 LF. Furthermore, morphometric analyses have shown that the increase in fibrosis burden through successive stages of LF is not linear [18, 120, 121]. There is a small incremental increase in fibrosis burden in early stages and the increase in LF burden is exponential in advanced fibrosis and cirrhosis stages. Second, studies have spectrum bias toward advanced fibrosis, which is unavoidable as there are rarely clinical indications for liver biopsy in early LF. As a consequence, there is less representation of early and intermediate stages of LF. Third, sampling error of liver biopsy due to heterogeneity of the disease process introduces variability in the assessment of biomarkers. In addition, liver biopsy analysis has a low level of diagnostic performance in intermediate stages of fibrosis compared to advanced stages of fibrosis due to multiple factors including less sampling errors in advanced stages of fibrosis [122]. In general, NITs tend to have lower accuracy for intermediate stages of LF and high accuracy for advanced stage fibrosis. The reasons for these are multifactorial as described above and not necessarily due to low performance of the biomarker.
Even in the best possible scenario of high accuracy of liver biopsy, the “perfect surrogate biomarker” may only reach accuracy of 0.90 [123]. There may be a perfect surrogate biomarker existing but it may not be recognized. Also, a biomarker may have a correlated error with liver biopsy, therefore the same false positive and false negative results as liver biopsy, and this could be falsely interpreted as high performance.
As the performance of any surrogate biomarker is limited by the presence of confounders, careful interpretation of the NITs should be performed by considering the clinical presentation and results from other liver function tests. For example, in acute flare of viral hepatitis, interpretation of increased stiffness as advanced fibrosis or cirrhosis should be avoided by correlating with clinical presentation, increased serum transaminase levels, particularly serum ALT and other inflammatory markers if available.
As mentioned before, the stage of cirrhosis can be further classified for prognostication and surveillance [62–64]. Subclassification of cirrhosis is an emerging concept and likely to become integrated into clinical practice in the future. Severe fibrosis and compensated cirrhosis are difficult to differentiate but it is important to identify subjects at risk for screening and surveillance of hepatocellular carcinoma and varices. Therefore, the concept compensated advanced chronic liver disease (cACLD) has been introduced [72, 115]. cACLD includes patients at risk of developing portal hypertension and clinical decompensation. Several NITs have proven to be accurate for distinguishing advanced fibrosis and cirrhosis, and are likely to play role in identifying cACLD patients for clinical practice. VCTE is the most widely studied NIT and nearly all types of CLD and has shown accuracy > 0.9 for ruling out advanced liver disease and has been included under Baveno VI recommendations [72]. MRE is accurate for diagnosing advanced fibrosis in NAFLD [98]. Recently Gidener et al. [116] showed that MRE can predict future cirrhosis in patients with NAFLD with 2.93 HR per 1 kPa increment from baseline liver stiffness measurement (LSM), predict decompensation or death in patients with compensated cirrhosis with 1.32 HR per 1 kPa increment. Another study with > 1200 subjects showed that MRE can predict long-term progression from baseline LSM. In this study, baseline LSM predicted development of cirrhosis in non-cirrhotics, future decompensation in cirrhotic patients, and death or transplant with HR of 2.38, 1.22, and 1.11, respectively [117]. In this study, the non-decompensated cirrhosis group showed increasing risk of decompensation as the baseline liver stiffness increased. The cumulative decompensation at 10 years for LSM < 4 kPa, 4 to 5 kPa, 5 to 6 kPa, 6 to 7 kPa, 7 to 8 kPa, and > 8 kPa were 13.4%, 21.6%, 55%, 46%, 76%, and 78%, respectively. These results are encouraging that MRE could be used for stratifying the cirrhotic subjects for prognostication. Other NITs may also play role in the subclassification; however, studies with long term outcomes are not yet available.
Similarly, NITs can be useful for predicting clinically significant portal hypertension or in the assessment of severity of portal hypertension, defining high risk gastroesophageal varices. Spleen stiffness may be another useful parameter in such assessment. Combination of NITs may be advantageous for such purposes [72]
NITs can also be useful in the evaluation of patients with non-cirrhotic portal hypertension (NCPH) as the distinction from cirrhosis is important for management. Nodular regenerative hyperplasia, a common cause for NCPH often appears similar to cirrhotic liver with portal hypertension; however, the liver functions are usually normal and liver stiffness is only mild to moderately elevated [118, 119]. The combination of LSM and spleen stiffness can reliably distinguish NCPH from cirrhotic livers [119].
In conclusion, with the introduction of NITs into clinical practice, liver biopsies are going to be fewer and less often performed, and there will be a lack of reference standard for validation of NITs, particularly newly discovered biomarkers. Non-invasive tests alone or in combination with clinical and other parameters will play an important role in the diagnosis and management of chronic liver disease. A new reference standard in the form of quantified fibrosis burden that correlates with patient outcomes and able to predict complications related to CLD will be needed.
Acknowledgements
Dr. Venkatesh acknowledges support from National Institute of Health Grant (EB001981) and U.S. Department of Defense Grant (W81XWH-19-1-0583-01).
Funding
The authors did not receive support from any organization for the submitted work.
Footnotes
Conflict of interest Financial interests: The author declares no financial interests. Non-financial interests: The author declares no non-financial interests.
Research involving human participants and/or animals This review article did not involve any research involving human participants and/or animals. No ethics approval was needed.
Data availability
No data available.
References
- 1.Anthony PP, Ishak KG, Nayak NC, Poulsen HE, Scheuer PJ, Sobin LH (1977) The morphology of cirrhosis: definition, nomenclature, and classification. Bull World Health Organ 55 (4):521–540 [PMC free article] [PubMed] [Google Scholar]
- 2.Hernandez-Gea V, Friedman SL (2011) Pathogenesis of liver fibrosis. Annu Rev Pathol 6:425–456. 10.1146/annurev-pathol-011110-130246 [DOI] [PubMed] [Google Scholar]
- 3.Rosenberg WM (2003) Rating fibrosis progression in chronic liver diseases. J Hepatol 38 (3):357–360. 10.1016/s0168-8278(03)00010-2 [DOI] [PubMed] [Google Scholar]
- 4.Angulo P, Kleiner DE, Dam-Larsen S, Adams LA, Bjornsson ES, Charatcharoenwitthaya P, Mills PR, Keach JC, Lafferty HD, Stahler A, Haflidadottir S, Bendtsen F (2015) Liver Fibrosis, but No Other Histologic Features, Is Associated With Long-term Outcomes of Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology 149 (2):389–397.e310. 10.1053/j.gastro.2015.04.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Horowitz JM, Venkatesh SK, Ehman RL, Jhaveri K, Kamath P, Ohliger MA, Samir AE, Silva AC, Taouli B, Torbenson MS, Wells ML, Yeh B, Miller FH (2017) Evaluation of hepatic fibrosis: a review from the society of abdominal radiology disease focus panel. Abdom Radiol (NY) 42 (8):2037–2053. 10.1007/s00261-017-1211-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rockey DC, Bissell DM (2006) Noninvasive measures of liver fibrosis. Hepatology (Baltimore, Md) 43 (2 Suppl 1):S113–120. 10.1002/hep.21046 [DOI] [PubMed] [Google Scholar]
- 7.Rockey DC (2008) Noninvasive assessment of liver fibrosis and portal hypertension with transient elastography. Gastroenterology 134 (1):8–14. 10.1053/j.gastro.2007.11.053 [DOI] [PubMed] [Google Scholar]
- 8.Rockey DC, Caldwell SH, Goodman ZD, Nelson RC, Smith AD, Diseases AAftSoL (2009) Liver biopsy. Hepatology (Baltimore, Md) 49 (3):1017–1044. 10.1002/hep.22742 [DOI] [PubMed] [Google Scholar]
- 9.Petitclerc L, Gilbert G, Nguyen BN, Tang A (2017) Liver Fibrosis Quantification by Magnetic Resonance Imaging. Top Magn Reson Imaging 26 (6):229–241. 10.1097/rmr.0000000000000149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yin M, Venkatesh SK (2018) Ultrasound or MR elastography of liver: which one shall I use? Abdom Radiol (NY) 43 (7):1546–1551. 10.1007/s00261-017-1340-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tang A, Cloutier G, Szeverenyi NM, Sirlin CB (2015) Ultrasound Elastography and MR Elastography for Assessing Liver Fibrosis: Part 2, Diagnostic Performance, Confounders, and Future Directions. AJR Am J Roentgenol 205 (1):33–40. 10.2214/AJR.15.14553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith AD, Porter KK, Elkassem AA, Sanyal R, Lockhart ME (2019) Current Imaging Techniques for Noninvasive Staging of Hepatic Fibrosis. AJR Am J Roentgenol 213 (1):77–89. 10.2214/AJR.19.21144 [DOI] [PubMed] [Google Scholar]
- 13.Sun M, Kisseleva T (2015) Reversibility of liver fibrosis. Clin Res Hepatol Gastroenterol 39 Suppl 1:S60–63. 10.1016/j.clinre.2015.06.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Geerts A (2001) History, heterogeneity, developmental biology, and functions of quiescent hepatic stellate cells. Semin Liver Dis 21 (3):311–335. 10.1055/s-2001-17550 [DOI] [PubMed] [Google Scholar]
- 15.Poynard T, Mathurin P, Lai CL, Guyader D, Poupon R, Tainturier MH, Myers RP, Muntenau M, Ratziu V, Manns M, Vogel A, Capron F, Chedid A, Bedossa P (2003) A comparison of fibrosis progression in chronic liver diseases. J Hepatol 38 (3):257–265 [DOI] [PubMed] [Google Scholar]
- 16.Bataller R, Brenner DA (2005) Liver fibrosis. J Clin Invest 115 (2):209–218. 10.1172/JCI24282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kisseleva T, Brenner DA (2008) Mechanisms of fibrogenesis. Exp Biol Med (Maywood) 233 (2):109–122. 10.3181/0707-MR-190 [DOI] [PubMed] [Google Scholar]
- 18.Standish RA, Cholongitas E, Dhillon A, Burroughs AK, Dhillon AP (2006) An appraisal of the histopathological assessment of liver fibrosis. Gut 55 (4):569–578. 10.1136/gut.2005.084475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brenner DA, Waterboer T, Choi SK, Lindquist JN, Stefanovic B, Burchardt E, Yamauchi M, Gillan A, Rippe RA (2000) New aspects of hepatic fibrosis. J Hepatol 32 (1 Suppl):32–38. 10.1016/s0168-8278(00)80413-4 [DOI] [PubMed] [Google Scholar]
- 20.Issa R, Zhou X, Constandinou CM, Fallowfield J, Millward-Sadler H, Gaca MD, Sands E, Suliman I, Trim N, Knorr A, Arthur MJ, Benyon RC, Iredale JP (2004) Spontaneous recovery from micronodular cirrhosis: evidence for incomplete resolution associated with matrix cross-linking. Gastroenterology 126 (7):1795–1808. 10.1053/j.gastro.2004.03.009 [DOI] [PubMed] [Google Scholar]
- 21.Pellicoro A, Aucott RL, Ramachandran P, Robson AJ, Fallow-field JA, Snowdon VK, Hartland SN, Vernon M, Duffield JS, Benyon RC, Forbes SJ, Iredale JP (2012) Elastin accumulation is regulated at the level of degradation by macrophage metalloelastase (MMP-12) during experimental liver fibrosis. Hepatology (Baltimore, Md) 55 (6):1965–1975. 10.1002/hep.25567 [DOI] [PubMed] [Google Scholar]
- 22.Pinzani M, Rombouts K (2004) Liver fibrosis: from the bench to clinical targets. Dig Liver Dis 36 (4):231–242. 10.1016/j.dld.2004.01.003 [DOI] [PubMed] [Google Scholar]
- 23.Safdar K, Schiff ER (2004) Alcohol and hepatitis C. Semin Liver Dis 24 (3):305–315. 10.1055/s-2004-832942 [DOI] [PubMed] [Google Scholar]
- 24.Shiffman ML, Stravitz RT, Contos MJ, Mills AS, Sterling RK, Luketic VA, Sanyal AJ, Cotterell A, Maluf D, Posner MP, Fisher RA (2004) Histologic recurrence of chronic hepatitis C virus in patients after living donor and deceased donor liver transplantation. Liver Transpl 10 (10):1248–1255. 10.1002/lt.20232 [DOI] [PubMed] [Google Scholar]
- 25.Benhamou Y, Bochet M, Di Martino V, Charlotte F, Azria F, Coutellier A, Vidaud M, Bricaire F, Opolon P, Katlama C, Poynard T (1999) Liver fibrosis progression in human immunodeficiency virus and hepatitis C virus coinfected patients. The Multivirc Group. Hepatology (Baltimore, Md) 30 (4):1054–1058. 10.1002/hep.510300409 [DOI] [PubMed] [Google Scholar]
- 26.McGuire RF, Bissell DM, Boyles J, Roll FJ (1992) Role of extracellular matrix in regulating fenestrations of sinusoidal endothelial cells isolated from normal rat liver. Hepatology 15 (6):989–997. 10.1002/hep.1840150603 [DOI] [PubMed] [Google Scholar]
- 27.Hammoutene A, Rautou PE (2019) Role of liver sinusoidal endothelial cells in non-alcoholic fatty liver disease. J Hepatol 70 (6):1278–1291. 10.1016/j.jhep.2019.02.012 [DOI] [PubMed] [Google Scholar]
- 28.Lee JS, Semela D, Iredale J, Shah VH (2007) Sinusoidal remodeling and angiogenesis: a new function for the liver-specific pericyte? Hepatology 45 (3):817–825. 10.1002/hep.21564 [DOI] [PubMed] [Google Scholar]
- 29.Parola M, Pinzani M (2019) Liver fibrosis: Pathophysiology, pathogenetic targets and clinical issues. Mol Aspects Med 65:37–55. 10.1016/j.mam.2018.09.002 [DOI] [PubMed] [Google Scholar]
- 30.POPPER H, ELIAS H, PETTY DE (1952) Vascular pattern of the cirrhotic liver. Am J Clin Pathol 22 (8):717–729. 10.1093/ajcp/22.8.717 [DOI] [PubMed] [Google Scholar]
- 31.HALES MR, ALLAN JS, HALL EM (1959) Injection-corrosion studies of normal and cirrhotic livers. Am J Pathol 35:909–941 [PMC free article] [PubMed] [Google Scholar]
- 32.Kisseleva T, Brenner D (2021) Molecular and cellular mechanisms of liver fibrosis and its regression. Nat Rev Gastroenterol Hepatol 18 (3):151–166. 10.1038/s41575-020-00372-7 [DOI] [PubMed] [Google Scholar]
- 33.Zois CD, Baltayiannis GH, Karayiannis P, Tsianos EV (2008) Systematic review: hepatic fibrosis - regression with therapy. Aliment Pharmacol Ther 28 (10):1175–1187. 10.1111/j.1365-2036.2008.03840.x [DOI] [PubMed] [Google Scholar]
- 34.Serpaggi J, Carnot F, Nalpas B, Canioni D, Guechot J, Lebray P, Vallet-Pichard A, Fontaine H, Bedossa P, Pol S (2006) Direct and indirect evidence for the reversibility of cirrhosis. Hum Pathol 37 (12):1519–1526. 10.1016/j.humpath.2006.07.007 [DOI] [PubMed] [Google Scholar]
- 35.Abdalla AF, Zalata KR, Ismail AF, Shiha G, Attiya M, Abo-Alyazeed A (2009) Regression of fibrosis in paediatric autoimmune hepatitis: morphometric assessment of fibrosis versus semiquantiatative methods. Fibrogenesis Tissue Repair 2 (1):2. 10.1186/1755-1536-2-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ellis EL, Mann DA (2012) Clinical evidence for the regression of liver fibrosis. J Hepatol 56 (5):1171–1180. 10.1016/j.jhep.2011.09.024 [DOI] [PubMed] [Google Scholar]
- 37.Vukobrat-Bijedic Z, Husic-Selimovic A, Mehinovic L, Mehmedovic A, Junuzovic D, Bjelogrlic I, Sofic A, Djurovic A (2014) Analysis of effect of antiviral therapy on regression of liver fibrosis in patient with HCV infection. Mater Sociomed 26 (3):172–176. 10.5455/msm.2014.26.172-176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hammel P, Couvelard A, O’Toole D, Ratouis A, Sauvanet A, Fléjou JF, Degott C, Belghiti J, Bernades P, Valla D, Ruszniewski P, Lévy P (2001) Regression of liver fibrosis after biliary drainage in patients with chronic pancreatitis and stenosis of the common bile duct. N Engl J Med 344 (6):418–423. 10.1056/NEJM200102083440604 [DOI] [PubMed] [Google Scholar]
- 39.Arthur MJ (2002) Reversibility of liver fibrosis and cirrhosis following treatment for hepatitis C. Gastroenterology 122 (5):1525–1528. 10.1053/gast.2002.33367 [DOI] [PubMed] [Google Scholar]
- 40.Kweon YO, Goodman ZD, Dienstag JL, Schiff ER, Brown NA, Burchardt E, Schoonhoven R, Brenner DA, Fried MW, Burkhardt E (2001) Decreasing fibrogenesis: an immunohistochemical study of paired liver biopsies following lamivudine therapy for chronic hepatitis B. J Hepatol 35 (6):749–755. 10.1016/s0168-8278(01)00218-5 [DOI] [PubMed] [Google Scholar]
- 41.Dixon JB, Bhathal PS, Hughes NR, O’Brien PE (2004) Non-alcoholic fatty liver disease: Improvement in liver histological analysis with weight loss. Hepatology (Baltimore, Md) 39 (6):1647–1654. 10.1002/hep.20251 [DOI] [PubMed] [Google Scholar]
- 42.Czaja AJ, Carpenter HA (2004) Decreased fibrosis during corticosteroid therapy of autoimmune hepatitis. J Hepatol 40 (4):646–652. 10.1016/j.jhep.2004.01.009 [DOI] [PubMed] [Google Scholar]
- 43.Parés A, Caballería J, Bruguera M, Torres M, Rodés J (1986) Histological course of alcoholic hepatitis. Influence of abstinence, sex and extent of hepatic damage. J Hepatol 2 (1):33–42. 10.1016/s0168-8278(86)80006-x [DOI] [PubMed] [Google Scholar]
- 44.Hafeez S, Ahmed MH (2013) Bariatric surgery as potential treatment for nonalcoholic fatty liver disease: a future treatment by choice or by chance? J Obes 2013:839275. 10.1155/2013/839275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Knodell RG, Ishak KG, Black WC, Chen TS, Craig R, Kaplowitz N, Kiernan TW, Wollman J (1981) Formulation and application of a numerical scoring system for assessing histological activity in asymptomatic chronic active hepatitis. Hepatology (Baltimore, Md) 1 (5):431–435. 10.1002/hep.1840010511 [DOI] [PubMed] [Google Scholar]
- 46.Ishak K, Baptista A, Bianchi L, Callea F, De Groote J, Gudat F, Denk H, Desmet V, Korb G, MacSween RN (1995) Histological grading and staging of chronic hepatitis. J Hepatol 22 (6):696–699. 10.1016/0168-8278(95)80226-6 [DOI] [PubMed] [Google Scholar]
- 47.Scheuer PJ (1991) Classification of chronic viral hepatitis: a need for reassessment. J Hepatol 13 (3):372–374. 10.1016/0168-8278(91)90084-o [DOI] [PubMed] [Google Scholar]
- 48.Batts KP, Ludwig J (1995) Chronic hepatitis. An update on terminology and reporting. Am J Surg Pathol 19 (12):1409–1417. 10.1097/00000478-199512000-00007 [DOI] [PubMed] [Google Scholar]
- 49.Bedossa P, Poynard T (1996) An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology (Baltimore, Md) 24 (2):289–293. 10.1002/hep.510240201 [DOI] [PubMed] [Google Scholar]
- 50.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A, Yeh M, McCullough AJ, Sanyal AJ, Network NSCR (2005) Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology (Baltimore, Md) 41 (6):1313–1321. 10.1002/hep.20701 [DOI] [PubMed] [Google Scholar]
- 51.Dai DF, Swanson PE, Krieger EV, Liou IW, Carithers RL, Yeh MM (2014) Congestive hepatic fibrosis score: a novel histologic assessment of clinical severity. Mod Pathol 27 (12):1552–1558. 10.1038/modpathol.2014.79 [DOI] [PubMed] [Google Scholar]
- 52.Bedossa P, Dargere D, Paradis V (2003) Sampling variability of liver fibrosis in chronic hepatitis C. Hepatology (Baltimore, Md) 38 (6):1449–1457. 10.1016/j.hep.2003.09.022 [DOI] [PubMed] [Google Scholar]
- 53.Poynard T, Ratziu V, Bedossa P (2000) Appropriateness of liver biopsy. Canadian journal of gastroenterology = Journal canadien de gastroenterologie 14 (6):543–548 [DOI] [PubMed] [Google Scholar]
- 54.Rousselet MC, Michalak S, Dupre F, Croue A, Bedossa P, Saint-Andre JP, Cales P (2005) Sources of variability in histological scoring of chronic viral hepatitis. Hepatology (Baltimore, Md) 41 (2):257–264. 10.1002/hep.20535 [DOI] [PubMed] [Google Scholar]
- 55.Goodman ZD, Becker RL, Pockros PJ, Afdhal NH (2007) Progression of fibrosis in advanced chronic hepatitis C: evaluation by morphometric image analysis. Hepatology (Baltimore, Md) 45 (4):886–894. 10.1002/hep.21595 [DOI] [PubMed] [Google Scholar]
- 56.Sun W, Chang S, Tai DC, Tan N, Xiao G, Tang H, Yu H (2008) Nonlinear optical microscopy: use of second harmonic generation and two-photon microscopy for automated quantitative liver fibrosis studies. J Biomed Opt 13 (6):064010. 10.1117/1.3041159 [DOI] [PubMed] [Google Scholar]
- 57.Tai DC, Tan N, Xu S, Kang CH, Chia SM, Cheng CL, Wee A, Wei CL, Raja AM, Xiao G, Chang S, Rajapakse JC, So PT, Tang HH, Chen CS, Yu H (2009) Fibro-C-Index: comprehensive, morphology-based quantification of liver fibrosis using second harmonic generation and two-photon microscopy. J Biomed Opt 14 (4):044013. 10.1117/1.3183811 [DOI] [PubMed] [Google Scholar]
- 58.Gawrieh S, Sethunath D, Cummings OW, Kleiner DE, Vuppalanchi R, Chalasani N, Tuceryan M (2020) Automated quantification and architectural pattern detection of hepatic fibrosis in NAFLD. Ann Diagn Pathol 47:151518. 10.1016/j.anndiagpath.2020.151518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yu Y, Wang J, Ng CW, Ma Y, Mo S, Fong ELS, Xing J, Song Z, Xie Y, Si K, Wee A, Welsch RE, So PTC, Yu H (2018) Deep learning enables automated scoring of liver fibrosis stages. Sci Rep 8 (1):16016. 10.1038/s41598-018-34300-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bedossa P (2010) Harmony in liver fibrosis.. J Hepatol 52 (3):313–314. 10.1016/j.jhep.2009.11.020 [DOI] [PubMed] [Google Scholar]
- 61.Friedman SL (2008) Hepatic fibrosis -- overview. Toxicology 254 (3):120–129. 10.1016/j.tox.2008.06.013 [DOI] [PubMed] [Google Scholar]
- 62.Garcia-Tsao G, Friedman S, Iredale J, Pinzani M (2010) Now there are many (stages) where before there was one: In search of a pathophysiological classification of cirrhosis. Hepatology 51 (4):1445–1449. 10.1002/hep.23478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nagula S, Jain D, Groszmann RJ, Garcia-Tsao G (2006) Histological-hemodynamic correlation in cirrhosis-a histological classification of the severity of cirrhosis. J Hepatol 44 (1):111–117. 10.1016/j.jhep.2005.07.036 [DOI] [PubMed] [Google Scholar]
- 64.Kumar M, Sakhuja P, Kumar A, Manglik N, Choudhury A, Hissar S, Rastogi A, Sarin SK (2008) Histological subclassification of cirrhosis based on histological-haemodynamic correlation. Aliment Pharmacol Ther 27 (9):771–779. 10.1111/j.1365-2036.2008.03653.x [DOI] [PubMed] [Google Scholar]
- 65.Kim MY, Cho MY, Baik SK, Park HJ, Jeon HK, Im CK, Won CS, Kim JW, Kim HS, Kwon SO, Eom MS, Cha SH, Kim YJ, Chang SJ, Lee SS (2011) Histological subclassification of cirrhosis using the Laennec fibrosis scoring system correlates with clinical stage and grade of portal hypertension. J Hepatol 55 (5):1004–1009. 10.1016/j.jhep.2011.02.012 [DOI] [PubMed] [Google Scholar]
- 66.Sethasine S, Jain D, Groszmann RJ, Garcia-Tsao G (2012) Quantitative histological-hemodynamic correlations in cirrhosis. Hepatology (Baltimore, Md) 55 (4):1146–1153. 10.1002/hep.24805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Khalifa A, Rockey DC (2020) The utility of liver biopsy in 2020. Curr Opin Gastroenterol 36 (3):184–191. 10.1097/MOG.0000000000000621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bravo AA, Sheth SG, Chopra S (2001) Liver biopsy. N Engl J Med 344 (7):495–500. 10.1056/nejm200102153440706 [DOI] [PubMed] [Google Scholar]
- 69.Ratziu V, Charlotte F, Heurtier A, Gombert S, Giral P, Bruckert E, Grimaldi A, Capron F, Poynard T, Group LS (2005) Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterology 128 (7):1898–1906. 10.1053/j.gastro.2005.03.084 [DOI] [PubMed] [Google Scholar]
- 70.Maharaj B, Maharaj RJ, Leary WP, Cooppan RM, Naran AD, Pirie D, Pudifin DJ (1986) Sampling variability and its influence on the diagnostic yield of percutaneous needle biopsy of the liver. Lancet (London, England) 1 (8480):523–525 [DOI] [PubMed] [Google Scholar]
- 71.Abdi W, Millan JC, Mezey E (1979) Sampling variability on percutaneous liver biopsy. Arch Intern Med 139 (6):667–669 [PubMed] [Google Scholar]
- 72.Berzigotti A, Boursier J, Castera L, Cazzagon N, Friedrich-Rust M, Petta S, Thiele M, Tsochatzis E, Liver EAftSot, order) Lopma (2021) Easl Clinical Practice Guidelines (Cpgs) On Non-Invasive Tests For Evaluation Of Liver Disease Severity And Prognosis-2020 Update. J Hepatol 10.1016/j.jhep.2021.05.025 [DOI] [Google Scholar]
- 73.Baranova A, Lal P, Birerdinc A, Younossi ZM (2011) Non-invasive markers for hepatic fibrosis. BMC Gastroenterol 11:91. 10.1186/1471-230X-11-91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pinzani M, Rombouts K, Colagrande S (2005) Fibrosis in chronic liver diseases: diagnosis and management. J Hepatol 42 Suppl (1):S22–36. 10.1016/j.jhep.2004.12.008 [DOI] [PubMed] [Google Scholar]
- 75.Parikh P, Ryan JD, Tsochatzis EA (2017) Fibrosis assessment in patients with chronic hepatitis B virus (HBV) infection. Ann Transl Med 5 (3):40. 10.21037/atm.2017.01.28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lin ZH, Xin YN, Dong QJ, Wang Q, Jiang XJ, Zhan SH, Sun Y, Xuan SY (2011) Performance of the aspartate aminotransferase-to-platelet ratio index for the staging of hepatitis C-related fibrosis: an updated meta-analysis. Hepatology (Baltimore, Md) 53 (3):726–736. 10.1002/hep.24105 [DOI] [PubMed] [Google Scholar]
- 77.Salkic NN, Jovanovic P, Hauser G, Brcic M (2014) FibroTest/Fibrosure for significant liver fibrosis and cirrhosis in chronic hepatitis B: a meta-analysis. Am J Gastroenterol 109 (6):796–809. 10.1038/ajg.2014.21 [DOI] [PubMed] [Google Scholar]
- 78.Poynard T, Morra R, Halfon P, Castera L, Ratziu V, Imbert-Bismut F, Naveau S, Thabut D, Lebrec D, Zoulim F, Bourliere M, Cacoub P, Messous D, Munteanu M, de Ledinghen V (2007) Meta-analyses of FibroTest diagnostic value in chronic liver disease. BMC Gastroenterol 7:40. 10.1186/1471-230X-7-40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Houot M, Ngo Y, Munteanu M, Marque S, Poynard T (2016) Systematic review with meta-analysis: direct comparisons of biomarkers for the diagnosis of fibrosis in chronic hepatitis C and B. Aliment Pharmacol Ther 43 (1):16–29. 10.1111/apt.13446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Xie Q, Zhou X, Huang P, Wei J, Wang W, Zheng S (2014) The performance of enhanced liver fibrosis (ELF) test for the staging of liver fibrosis: a meta-analysis. PLoS One 9 (4):e92772. 10.1371/journal.pone.0092772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Xiao G, Zhu S, Xiao X, Yan L, Yang J, Wu G (2017) Comparison of laboratory tests, ultrasound, or magnetic resonance elastography to detect fibrosis in patients with nonalcoholic fatty liver disease: A meta-analysis. Hepatology (Baltimore, Md) 66 (5):1486–1501. 10.1002/hep.29302 [DOI] [PubMed] [Google Scholar]
- 82.Xu XY, Wang WS, Zhang QM, Li JL, Sun JB, Qin TT, Liu HB (2019) Performance of common imaging techniques vs serum biomarkers in assessing fibrosis in patients with chronic hepatitis B: A systematic review and meta-analysis. World J Clin Cases 7 (15):2022–2037. 10.12998/wjcc.v7.i15.2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dong B, Lyu G, Chen Y, Lin G, Wang H, Qin R, Gu J (2021) Comparison of two-dimensional shear wave elastography, magnetic resonance elastography, and three serum markers for diagnosing fibrosis in patients with chronic hepatitis B: a meta-analysis. Expert Rev Gastroenterol Hepatol 15 (9):1077–1089. 10.1080/17474124.2021.1880894 [DOI] [PubMed] [Google Scholar]
- 84.Lu Q, Lu C, Li J, Ling W, Qi X, He D, Liu J, Wen T, Wu H, Zhu H, Luo Y (2016) Stiffness Value and Serum Biomarkers in Liver Fibrosis Staging: Study in Large Surgical Specimens in Patients with Chronic Hepatitis B. Radiology 280 (1):290–299. 10.1148/radiol.2016151229 [DOI] [PubMed] [Google Scholar]
- 85.Shiha G, Ibrahim A, Helmy A, Sarin SK, Omata M, Kumar A, Bernstien D, Maruyama H, Saraswat V, Chawla Y, Hamid S, Abbas Z, Bedossa P, Sakhuja P, Elmahatab M, Lim SG, Lesmana L, Sollano J, Jia JD, Abbas B, Omar A, Sharma B, Payawal D, Abdallah A, Serwah A, Hamed A, Elsayed A, AbdelMaqsod A, Hassanein T, Ihab A, GHaziuan H, Zein N, Kumar M (2017) Asian-Pacific Association for the Study of the Liver (APASL) consensus guidelines on invasive and non-invasive assessment of hepatic fibrosis: a 2016 update. Hepatol Int 11 (1):1–30. 10.1007/s12072-016-9760-3 [DOI] [PubMed] [Google Scholar]
- 86.Singh S, Muir AJ, Dieterich DT, Falck-Ytter YT (2017) American Gastroenterological Association Institute Technical Review on the Role of Elastography in Chronic Liver Diseases. Gastroenterology 152 (6):1544–1577. 10.1053/j.gastro.2017.03.016 [DOI] [PubMed] [Google Scholar]
- 87.Lim JK, Flamm SL, Singh S, Falck-Ytter YT, Association CGCotAG (2017) American Gastroenterological Association Institute Guideline on the Role of Elastography in the Evaluation of Liver Fibrosis. Gastroenterology 152 (6):1536–1543. 10.1053/j.gastro.2017.03.017 [DOI] [PubMed] [Google Scholar]
- 88.Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, Harrison SA, Brunt EM, Sanyal AJ (2018) The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology (Baltimore, Md) 67 (1):328–357. 10.1002/hep.29367 [DOI] [PubMed] [Google Scholar]
- 89.Huber A, Ebner L, Heverhagen JT, Christe A (2015) State-of-the-art imaging of liver fibrosis and cirrhosis: A comprehensive review of current applications and future perspectives. European Journal of Radiology Open 2:90–100. 10.1016/j.ejro.2015.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Brown JJ, Naylor MJ, Yagan N (1997) Imaging of hepatic cirrhosis. Radiology 202 (1):1–16. 10.1148/radiology.202.1.8988182 [DOI] [PubMed] [Google Scholar]
- 91.Brancatelli G, Federle MP, Ambrosini R, Lagalla R, Carriero A, Midiri M, Vilgrain V (2007) Cirrhosis: CT and MR imaging evaluation. Eur J Radiol 61 (1):57–69. 10.1016/j.ejrad.2006.11.003 [DOI] [PubMed] [Google Scholar]
- 92.Dodd GD, Baron RL, Oliver JH, Federle MP (1999) Spectrum of imaging findings of the liver in end-stage cirrhosis: part I, gross morphology and diffuse abnormalities. AJR Am J Roentgenol 173 (4):1031–1036. 10.2214/ajr.173.4.10511173 [DOI] [PubMed] [Google Scholar]
- 93.Zhang YN, Fowler KJ, Boehringer AS, Montes V, Schlein AN, Covarrubias Y, Wolfson T, Hong CW, Valasek MA, Andre MP, Loomba R, Sirlin CB (2021) Comparative diagnostic performance of ultrasound shear wave elastography and magnetic resonance elastography for classifying fibrosis stage in adults with biopsy-proven nonalcoholic fatty liver disease. Eur Radiol. 10.1007/s00330-021-08369-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hsu C, Caussy C, Imajo K, Chen J, Singh S, Kaulback K, Le MD, Hooker J, Tu X, Bettencourt R, Yin M, Sirlin CB, Ehman RL, Nakajima A, Loomba R (2019) Magnetic Resonance vs Transient Elastography Analysis of Patients With Nonalcoholic Fatty Liver Disease: A Systematic Review and Pooled Analysis of Individual Participants. Clin Gastroenterol Hepatol 17 (4):630–637.e638. 10.1016/jxgh.2018.05.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Selvaraj EA, Mózes FE, Jayaswal ANA, Zafarmand MH, Vali Y, Lee JA, Levick CK, Young LAJ, Palaniyappan N, Liu CH, Aithal GP, Romero-Gómez M, Brosnan MJ, Tuthill TA, Anstee QM, Neubauer S, Harrison SA, Bossuyt PM, Pavlides M, Investigators L (2021) Diagnostic accuracy of elastography and magnetic resonance imaging in patients with NAFLD: A systematic review and meta-analysis. J Hepatol 75 (4):770–785. 10.1016/j.jhep.2021.04.044 [DOI] [PubMed] [Google Scholar]
- 96.Sharpton SR, Tamaki N, Bettencourt R, Madamba E, Jung J, Liu A, Behling C, Valasek MA, Loomba R (2021) Diagnostic accuracy of two-dimensional shear wave elastography and transient elastography in nonalcoholic fatty liver disease. Therap Adv Gastroenterol 14:17562848211050436. 10.1177/17562848211050436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Singh S, Venkatesh SK, Wang Z, Miller FH, Motosugi U, Low RN, Hassanein T, Asbach P, Godfrey EM, Yin M, Chen J, Keaveny AP, Bridges M, Bohte A, Murad MH, Lomas DJ, Talwalkar JA, Ehman RL (2015) Diagnostic performance of magnetic resonance elastography in staging liver fibrosis: a systematic review and meta-analysis of individual participant data. Clin Gastroenterol Hepatol 13 (3):440–451.e446. 10.1016/jxgh.2014.09.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Singh S, Venkatesh SK, Loomba R, Wang Z, Sirlin C, Chen J, Yin M, Miller FH, Low RN, Hassanein T, Godfrey EM, Asbach P, Murad MH, Lomas DJ, Talwalkar JA, Ehman RL (2016) Magnetic resonance elastography for staging liver fibrosis in non-alcoholic fatty liver disease: a diagnostic accuracy systematic review and individual participant data pooled analysis. Eur Radiol 26 (5):1431–1440. 10.1007/s00330-015-3949-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wang QB, Zhu H, Liu HL, Zhang B (2012) Performance of magnetic resonance elastography and diffusion-weighted imaging for the staging of hepatic fibrosis: A meta-analysis. Hepatology (Baltimore, Md) 56 (1):239–247. 10.1002/hep.25610 [DOI] [PubMed] [Google Scholar]
- 100.Bota S, Herkner H, Sporea I, Salzl P, Sirli R, Neghina AM, Peck-Radosavljevic M (2013) Meta-analysis: ARFI elastography versus transient elastography for the evaluation of liver fibrosis. Liver International 33 (8):1138–1147. 10.1111/liv.12240 [DOI] [PubMed] [Google Scholar]
- 101.Hu X, Qiu L, Liu D, Qian L (2017) Acoustic Radiation Force Impulse (ARFI) Elastography for non-invasive evaluation of hepatic fibrosis in chronic hepatitis B and C patients: a systematic review and meta-analysis. Med Ultrason 19 (1):23–31. 10.11152/mu-942 [DOI] [PubMed] [Google Scholar]
- 102.Imajo K, Honda Y, Kobayashi T, Nagai K, Ozaki A, Iwaki M, Kessoku T, Ogawa Y, Takahashi H, Saigusa Y, Yoneda M, Kirikoshi H, Utsunomiya D, Aishima S, Saito S, Nakajima A (2020) Direct Comparison of US and MR Elastography for Staging Liver Fibrosis in Patients With Nonalcoholic Fatty Liver Disease. Clin Gastroenterol Hepatol. 10.1016/j.cgh.2020.12.016 [DOI] [PubMed] [Google Scholar]
- 103.Takayama Y, Nishie A, Asayama Y, Ushijima Y, Okamoto D, Fujita N, Morita K, Shirabe K, Kotoh K, Kubo Y, Okuaki T, Honda H (2015) T1 ρ Relaxation of the liver: A potential biomarker of liver function. J Magn Reson Imaging 42 (1):188–195. 10.1002/jmri.24739 [DOI] [PubMed] [Google Scholar]
- 104.Banerjee R, Pavlides M, Tunnicliffe EM, Piechnik SK, Sarania N, Philips R, Collier JD, Booth JC, Schneider JE, Wang LM, Delaney DW, Fleming KA, Robson MD, Barnes E, Neubauer S (2014) Multiparametric magnetic resonance for the non-invasive diagnosis of liver disease. J Hepatol 60 (1):69–77. 10.1016/j.jhep.2013.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hoffman DH, Ayoola A, Nickel D, Han F, Chandarana H, Shanbhogue KP (2020) T1 mapping, T2 mapping and MR elastography of the liver for detection and staging of liver fibrosis. Abdom Radiol (NY) 45 (3):692–700. 10.1007/s00261-019-02382-9 [DOI] [PubMed] [Google Scholar]
- 106.Smith AD, Branch CR, Zand K, Subramony C, Zhang H, Thaggard K, Hosch R, Bryan J, Vasanji A, Griswold M, Zhang X (2016) Liver Surface Nodularity Quantification from Routine CT Images as a Biomarker for Detection and Evaluation of Cirrhosis. Radiology 280 (3):771–781. 10.1148/radiol.2016151542 [DOI] [PubMed] [Google Scholar]
- 107.Lubner MG, Jones D, Said A, Kloke J, Lee S, Pickhardt PJ (2018) Accuracy of liver surface nodularity quantification on MDCT for staging hepatic fibrosis in patients with hepatitis C virus. Abdom Radiol (NY). 10.1007/s00261-018-1572-6 [DOI] [PubMed] [Google Scholar]
- 108.Pickhardt PJ, Malecki K, Kloke J, Lubner MG (2016) Accuracy of Liver Surface Nodularity Quantification on MDCT as a Noninvasive Biomarker for Staging Hepatic Fibrosis. AJR Am J Roentgenol 207 (6):1194–1199. 10.2214/AJR.16.16514 [DOI] [PubMed] [Google Scholar]
- 109.Kim SW, Kim YR, Choi KH, Cho EY, Song JS, Kim JE, Kim TH, Lee YH, Yoon KH (2020) Staging of Liver Fibrosis by Means of Semiautomatic Measurement of Liver Surface Nodularity in MRI. AJR Am J Roentgenol 215 (3):624–630. 10.2214/AJR.19.22041 [DOI] [PubMed] [Google Scholar]
- 110.Catania R, Furlan A, Smith AD, Behari J, Tublin ME, Borhani AA (2021) Diagnostic value of MRI-derived liver surface nodularity score for the non-invasive quantification of hepatic fibrosis in non-alcoholic fatty liver disease. Eur Radiol 31 (1):256–263. 10.1007/s00330-020-07114-y [DOI] [PubMed] [Google Scholar]
- 111.Dioguardi Burgio M, Sartoris R, Beaufrere A, Grégory J, Guiu B, Guillot C, Rautou PE, Castera L, Bouattour M, Paradis V, Vilgrain V, Ronot M (2021) Liver surface nodularity on non-contrast MRI identifies advanced fibrosis in patients with NAFLD. Eur Radiol. 10.1007/s00330-021-08261-6 [DOI] [PubMed] [Google Scholar]
- 112.Barr RG, Wilson SR, Rubens D, Garcia-Tsao G, Ferraioli G (2020) Update to the Society of Radiologists in Ultrasound Liver Elastography Consensus Statement. Radiology 296 (2):263–274. 10.1148/radiol.2020192437 [DOI] [PubMed] [Google Scholar]
- 113.Schawkat K, Ciritsis A, von Ulmenstein S, Honcharova-Biletska H, Jüngst C, Weber A, Gubler C, Mertens J, Reiner CS (2020) Diagnostic accuracy of texture analysis and machine learning for quantification of liver fibrosis in MRI: correlation with MR elastography and histopathology. Eur Radiol 30 (8):4675–4685. 10.1007/s00330-020-06831-8 [DOI] [PubMed] [Google Scholar]
- 114.Lubner MG, Jones D, Kloke J, Said A, Pickhardt PJ (2019) CT texture analysis of the liver for assessing hepatic fibrosis in patients with hepatitis C virus. Br J Radiol 92 (1093):20180153. 10.1259/bjr.20180153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Choi KJ, Jang JK, Lee SS, Sung YS, Shim WH, Kim HS, Yun J, Choi JY, Lee Y, Kang BK, Kim JH, Kim SY, Yu ES (2018) Development and Validation of a Deep Learning System for Staging Liver Fibrosis by Using Contrast Agent-enhanced CT Images in the Liver. Radiology 289 (3):688–697. 10.1148/radiol.2018180763 [DOI] [PubMed] [Google Scholar]
- 116.Yasaka K, Akai H, Kunimatsu A, Abe O, Kiryu S (2018) Deep learning for staging liver fibrosis on CT: a pilot study. Eur Radiol 28 (11):4578–4585. 10.1007/s00330-018-5499-7 [DOI] [PubMed] [Google Scholar]
- 117.Yasaka K, Akai H, Kunimatsu A, Abe O, Kiryu S (2018) Liver Fibrosis: Deep Convolutional Neural Network for Staging by Using Gadoxetic Acid-enhanced Hepatobiliary Phase MR Images. Radiology 287 (1):146–155. 10.1148/radiol.2017171928 [DOI] [PubMed] [Google Scholar]
- 118.Hectors SJ, Kennedy P, Huang KH, Stocker D, Carbonell G, Greenspan H, Friedman S, Taouli B (2021) Fully automated prediction of liver fibrosis using deep learning analysis of gadoxetic acid-enhanced MRI. Eur Radiol 31 (6):3805–3814. 10.1007/s00330-020-07475-4 [DOI] [PubMed] [Google Scholar]
- 119.Phelps A (2019) Liver Ultrasound Texture Analysis: The Computer Finds More to Quantify Than Meets the Eye. Acad Radiol 26 (8):1008–1009. 10.1016/j.acra.2019.03.013 [DOI] [PubMed] [Google Scholar]
- 120.Lazzarini AL, Levine RA, Ploutz-Snyder RJ, Sanderson SO (2005) Advances in digital quantification technique enhance discrimination between mild and advanced liver fibrosis in chronic hepatitis C. Liver Int 25 (6):1142–1149. 10.1111/j.1478-3231.2005.01155.x [DOI] [PubMed] [Google Scholar]
- 121.Masugi Y, Abe T, Tsujikawa H, Effendi K, Hashiguchi A, Abe M, Imai Y, Hino K, Hige S, Kawanaka M, Yamada G, Kage M, Korenaga M, Hiasa Y, Mizokami M, Sakamoto M (2018) Quantitative assessment of liver fibrosis reveals a nonlinear association with fibrosis stage in nonalcoholic fatty liver disease. Hepatol Commun 2 (1):58–68. 10.1002/hep4.1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Poynard T, Lenaour G, Vaillant JC, Capron F, Munteanu M, Eyraud D, Ngo Y, M’Kada H, Ratziu V, Hannoun L, Charlotte F (2012) Liver biopsy analysis has a low level of performance for diagnosis of intermediate stages of fibrosis. Clin Gastroenterol Hepatol 10 (6):657–663.e657. 10.1016/j.cgh.2012.01.023 [DOI] [PubMed] [Google Scholar]
- 123.Mehta SH, Lau B, Afdhal NH, Thomas DL (2009) Exceeding the limits of liver histology markers. J Hepatol 50 (1):36–41. 10.1016/j.jhep.2008.07.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data available.


