Abstract
Background and Aim
To evaluate the demographic and prognostic significance of gastrointestinal (GI) symptoms in patients with coronavirus disease 2019 (COVID‐19).
Methods
A systematic search of electronic information sources was conducted. Combined overall effect sizes were calculated using random‐effects models for baseline demographic factors and outcomes including mortality, intensive care unit (ICU) admission, and length of hospital stay.
Results
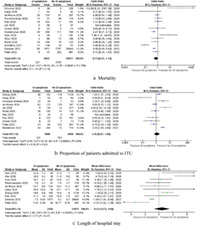
Twenty‐four comparative observational studies reporting a total of 51 522 COVID‐19 patients with (n = 6544) or without (n = 44 978) GI symptoms were identified. The patients with GI symptoms were of comparable age (mean difference [MD]: 0.25, 95% confidence interval [CI] −2.42 to 2.92, P = 0.86), rate of pre‐existing hypertension (odds ratio [OR]: 1.11, 95% CI 0.86–1.42, P = 0.42), diabetes mellitus (OR: 1.14, 95% CI 0.91–1.44, P = 0.26), and coronary artery disease (OR: 1.00, 95% CI 0.86–1.16, P = 0.98) compared with those without GI symptoms. However, there were significantly more male patients in the GI symptoms group (OR: 0.85, 95% CI 0.75–0.95, P = 0.005). The presence of GI symptoms was associated with similar risk of mortality (OR: 0.73; 95% CI 0.47–1.13, P = 0.16), ICU admission (OR: 1.15; 95% CI 0.67–1.96, P = 0.62), and length of hospital stay (MD: 0.43; 95% CI −0.73 to 1.60, P = 0.47) when compared with their absence.
Conclusion
Meta‐analysis of the best possible available evidence demonstrated that GI symptoms in COVID‐19 patients do not seem to affect patients with any specific demographic patterns and may not have any important prognostic significance. Although no randomized studies can be conducted on this topic, future high‐quality studies can provide stronger evidence to further understand the impact of GI symptoms on outcomes of COVID‐19 patients.
Keywords: coronavirus disease 2019, gastrointestinal symptoms, meta‐analysis
Meta‐analysis of the best possible available evidence demonstrated that gastrointestinal symptoms in COVID‐19 patients do not seem to affect patients with any specific demographic patterns and may not have any important prognostic significance.
Introduction
Coronavirus disease (COVID‐19) caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) was declared a Public Health Emergency of International Concern on 30 January 2020, 1 and a pandemic on 11 March 2020. 2
Clinical manifestations of COVID‐19 are variable from asymptomatic/mild cases to patients becoming critically ill with fulminant pneumonia and acute respiratory distress. All ages are susceptible with more severe disease generally seen in older males with underlying comorbidities. Common symptoms include fever, fatigue, and dry cough. 2 Other symptoms that have been reported include anosmia, ageusia, sputum production, chest discomfort, and gastrointestinal (GI) manifestations such as nausea, vomiting, abdominal discomfort, and diarrhea, 3 which can be chronic and disabling.
COVID‐19 can involve persistence (long COVID), sequelae, and other medical complications that last weeks to months after initial recovery. One study estimated a total of 55 long‐term effects associated with COVID‐19 including fatigue, headache, joint pain, and digestive tract symptoms. 4
Medical complications include the development of rare but severe disorders such as multisystem inflammatory syndrome in children (MIS‐C), associated with current or recent SARS‐CoV‐2 infection. 5 MIS‐C seems to show a male predilection with no significant racial predisposition. 6 Although this syndrome was first described in children, these sequalae may also develop in the adult population (MIS‐A). 7 GI signs and symptoms such as abdominal pain, nausea/vomiting, and diarrhea can appear prominently as presenting features of MIS‐C. 8 Prompt recognition and specialist treatment are required to prevent shock and multi‐organ failure.
The incidence of GI symptoms in the acute setting in patients with COVID‐19 varies and considering the existence of several confounding factors, estimation of the true incidence can be challenging. Although the respiratory tract appears to be the primary target of the novel coronavirus, the impact of GI symptoms on the severity of disease and outcomes remains undetermined.
We aimed to conduct a comprehensive systematic review and meta‐analysis of baseline characteristics and reported outcomes to evaluate the demographic and prognostic significance of GI symptoms in patients with COVID‐19.
Methods
The eligibility criteria, methodology, and investigated outcome parameters of this study were highlighted in a review protocol, which was registered at the International Prospective Register of Systematic Reviews (registration number: CRD42021283173). 9 Our methodology respected the Cochrane Handbook for Systematic Reviews of Interventions 10 and standards of Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) statement. 11
Study design: Considering that COVID‐19 with GI symptoms is a condition rather than an intervention, performing a randomized controlled trial (RCT) on the topic of the current study is not possible. Therefore, all comparative observational studies comparing the demographic factors and outcomes of COVID‐19 with and without associated GI symptoms were considered eligible for inclusion.
Population: Patients of any age or gender with a confirmed acute diagnosis of COVID‐19.
Exposure of interest: Presence of one or more GI symptoms at the time of presentation to a healthcare provider.
Comparison of interest: Absence of GI symptoms at the time of presentation.
The baseline demographic factors of interest: Age, gender, hypertension, diabetes mellitus, and coronary artery disease.
The outcome measures of interest: Mortality, intensive care unit (ICU) admission, and length of hospital stay.
Literature search strategy
A comprehensive search strategy was structured based on thesaurus headings, search operators, and limits in PubMed, Web of Science (WOS), and EMBASE. Two authors conducted the literature search via the above databases and searched World Health Organization International Clinical Trials Registry http://apps.who.int/trialsearch/, ClinicalTrials.gov http://clinicaltrials.gov/, and ISRCTN Register http://www.isrctn.com/ to identify ongoing and unpublished studies. Moreover, the same reviewers independently evaluated the reference lists of included studies and reviews to identify relevant trials. The last literature search was conducted on 08/05/2022. Appendix 1 presents the search strategy that was used to perform the literature search.
Selection of studies
The title and abstract of articles found as a result of the literature search were assessed by two authors. When deemed necessary, the full texts of relevant articles were retrieved and carefully assessed against the eligibility criteria of this review. Studies that met the inclusion criteria were considered for inclusion. Disagreements in this process were resolved by discussion between the authors. However, if the disagreement still existed, an independent author was consulted.
Data extraction and management
An electronic data extraction spreadsheet according to the Cochrane's recommendations for intervention reviews was created and was pilot tested in randomly selected articles and adjusted accordingly. The following information was extracted from each of the included studies by two independent reviewers:
study‐related data;
baseline demographic and clinical information of the study populations;
primary and secondary outcome data.
Discrepancies in this stage were resolved following consultation with an additional author.
Assessment of risk of bias
As all the included studies were observational, assessment of their methodological quality and risk of bias were carried out by two authors using the Newcastle–Ottawa scale (NOS). 12 The NOS is a star‐based scoring system (maximum score: 9), which enables review authors to evaluate an observational study in the following aspects: the selection of the study groups, the comparability of the groups, and the ascertainment of outcome of interest. Studies with score of nine stars were deemed to be at low risk of bias, studies with score of seven or eight stars were deemed to be at medium risk of bias, and those that scored six or less were judged to be at high risk of bias. Disagreements in this stage were resolved by discussion between the assessing authors. A third reviewer was consulted if the discrepancies remained unresolved.
Summary measures and synthesis
Analyses were conducted using Review Manager 5.4 software. Mean difference (MD) was computed for continuous outcome variables and odds ratio (OR) was calculated for dichotomous outcome variables. The I 2 using Cochran Q test (χ 2) was used to quantify heterogeneity. When mean values were not available for continuous outcomes, data on median and interquartile range (IQR) were extracted and subsequently converted to mean and SD using the well‐practiced equation described by Hozo et al. 13 Random‐effects modeling was used for analysis. We reported the results of our analysis for each outcome parameter in a forest plot with 95% confidence intervals (CIs).
The unit of analysis regarding all evaluated outcomes was an individual participant. Where possible, data regarding dropouts, withdrawals, and other missing information were recorded. We planned to contact authors of the included studies where information about our outcome of interest was not reported.
Heterogeneity among the studies was assessed using the Cochran Q test (χ 2). We quantified inconsistency by calculating I 2 and interpreted it using the following guide: 0–50% might not be important; 50–75% may represent moderate heterogeneity; 75–100% may represent substantial heterogeneity. Moreover, where more than 10 studies were available for analysis of an outcome parameter, funnel plots were constructed to assess their symmetry to visually evaluate publication bias.
We conducted sensitivity analyses to explore potential sources of heterogeneity and assess the robustness of our results. We evaluated the effect of each study on the overall effect size and heterogeneity by repeating the analysis after excluding one study at a time (leave‐one‐out sensitivity analysis).
Meta‐regression analysis of effect estimates were modeled to assess whether the difference in age, gender, hypertension, coronary artery disease, and diabetes between the two groups affected the effect estimates.
Results
The literature search resulted in 9609 articles. Of those, 102 studies were shortlisted for potential inclusion following assessment of their titles, abstracts, or full texts. Further, 78 studies were excluded as 61 were single‐arm studies and 17 did not provide sufficient data. Therefore, 24 comparative observational studies 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 were deemed appropriate for inclusion (Fig. 1). The total number of included patients was 51 522 patients of whom 6544 patients had COVID‐19 with GI symptoms and 44 978 patients had COVID‐19 without GI symptoms.
Figure 1.
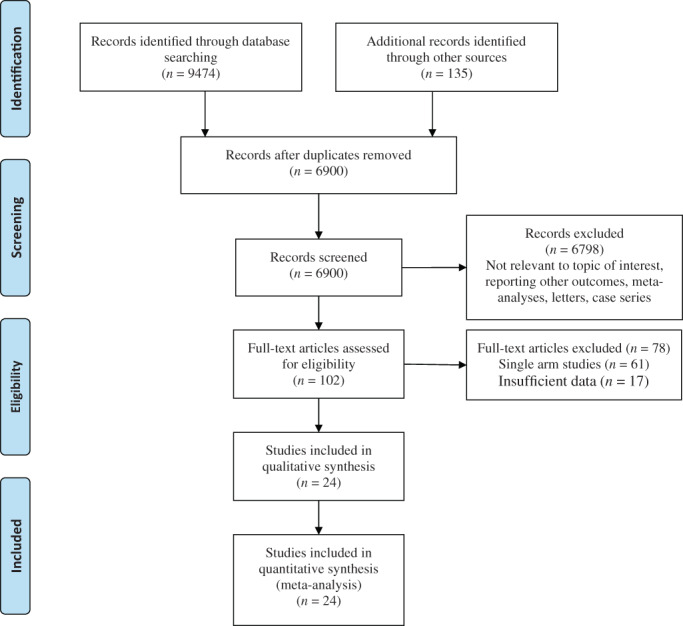
PRISMA flow diagram
Of the 24 studies included in this review, 20 reported on hospitalized patients only. 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 34 , 36 The remainder 14 , 33 , 35 , 37 provided data on a mixed cohort of patients including those hospitalized (in‐patients) and those not admitted (outpatients/ambulatory).
Moreover, 18 of our included studies 15 , 16 , 17 , 18 , 22 , 24 , 25 , 26 , 27 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 assessed GI symptoms only once at the time of presentation to a healthcare facility. One study assessed GI symptoms both on presentation and during hospital admission. 21
For the remaining five studies, 14 , 19 , 20 , 23 , 28 timing of assessment of GI symptoms was not explicitly stated. Although one can reasonably infer that this was once only at the point of admission/presentation. Tables 1 and 2 present the date of publication and country of origin, study design, and sample size of the included studies along with the definition of GI symptoms.
Table 1.
Study characteristics
| Author | Year | Country | Study design | Characteristics of included studies | ||||
|---|---|---|---|---|---|---|---|---|
| Study population | Total SARS‐CoV‐2 positive (n) | Gastrointestinal symptoms (n) | Timing of GI symptoms assessment | |||||
| Present | Absent | |||||||
| Ghoshal et al. | 2020 | India | Observational (prospective) | Hospitalized/ambulatory | 252 | 26 | 226 | NR |
| Kang et al. | 2020 | Korea | Observational (retrospective) | Hospitalized | 118 | 54 | 64 | Initial presentation |
| Jin et al. | 2020 | China | Observational (retrospective) | Hospitalized | 651 | 74 | 577 | Initial presentation |
| de Moura et al. | 2020 | Brazil | Single‐center cohort study (prospective) | Hospitalized | 400 | 133 | 267 | Initial presentation |
| Ramachandran et al. | 2020 | United States | Single‐center cohort study (retrospective) | Hospitalized | 150 | 31 | 119 | Initial presentation |
| Zhang et al. | 2020 | China | Observational (retrospective) | Hospitalized | 505 | 164 | 341 | NR |
| Cao et al. | 2020 | China | Observational | Hospitalized | 157 | 63 | 94 | NR |
| Lin et al. | 2020 | China | Single‐center cohort study (retrospective) | Hospitalized | 95 | 58 | 37 | Initial presentation and during hospital admission |
| Wan et al. | 2020 | China | Multi‐center observational study (retrospective) | Hospitalized | 230 | 49 | 181 | Initial presentation |
| Wei et al. | 2020 | China | Single‐center cohort study (retrospective) | Hospitalized | 84 | 26 | 58 | NR |
| Han et al. | 2020 | China | Single‐center cohort study (retrospective) | Hospitalized | 206 | 117 | 89 | Initial presentation |
| Pan et al. | 2020 | China | Descriptive, cross‐sectional, multi‐center study | Hospitalized | 204 | 103 | 101 | Initial presentation |
| Grover et al. | 2021 | United States | Multi‐center cohort study (prospective) | Hospitalized | 395 | 23 | 13 | Initial presentation |
| Zheng et al. | 2020 | China | Observational (retrospective) | Hospitalized | 1320 | 192 | 1128 | Initial presentation |
| Zhou et al. | 2020 | China | Single‐center cohort study | Hospitalized | 254 | 66 | 188 | NR |
| Xiong et al. | 2021 | China | Observational (prospective) | Hospitalized | 244 | 34 | 210 | Initial presentation |
| Gonzalez Jimenez et al. | 2020 | Spain | Multi‐center, descriptive, observational study | Hospitalized | 101 | 58 | 43 | Initial presentation |
| Redd et al. | 2020 | United States | Multi‐center cohort study (prospective) | Hospitalized | 318 | 195 | 123 | Initial presentation |
| Schettino et al. | 2021 | Italy | Single‐center cohort study (prospective) | Hospitalized | 190 | 138 | 52 | Initial presentation |
| Hajifathalian et al. | 2020 | United States | Observational (retrospective) | Hospitalized/outpatients | 1059 | 349 | 710 | Initial presentation |
| Bishehsari et al. | 2022 | United States | Observational (retrospective) | Hospitalized/outpatients | 921 | 206 | 715 | Initial presentation |
| Delavari et al. | 2022 | Iran | Observational | Hospitalized/outpatients | 42 964 | 4187 | 38 777 | Initial presentation |
| Fallouh et al. | 2022 | United States | Single‐center cohort study (retrospective) | Hospitalized | 382 | 154 | 228 | Initial presentation |
| Patel et al. | 2022 | United States | Observational (retrospective) | Hospitalized | 1672 | 44 | 637 | Initial presentation |
GI, gastrointestinal; NR, not recorded.
Table 2.
Study characteristics
| Author | Year | Country | Definition of GI symptoms |
|---|---|---|---|
| Ghoshal et al. | 2020 | India | Anorexia, nausea, vomiting, diarrhea, abdominal pain/discomfort |
| Kang et al. | 2020 | Korea | Diarrhea |
| Jin et al. | 2020 | China | At least one of: nausea, vomiting, diarrhea |
| de Moura et al. | 2020 | Brazil | Diarrhea, nausea, anorexia, vomiting, abdominal pain, dysphagia, weight loss, GI bleed, constipation |
| Ramachandran et al. | 2020 | United States | Nausea, vomiting, diarrhea, or abdominal pain |
| Zhang et al. | 2020 | China | Not specified |
| Cao et al. | 2020 | China | One or more of: anorexia, nausea, diarrhea |
| Lin et al. | 2020 | China | Not specified |
| Wan et al. | 2020 | China | Diarrhea |
| Wei et al. | 2020 | China | Not specified but divided into diarrhea versus non‐diarrhea group |
| Han et al. | 2020 | China | One or more including: anorexia, vomiting, diarrhea, abdominal pain |
| Pan et al. | 2020 | China | Lack of appetite, diarrhea, vomiting, abdominal pain |
| Grover et al. | 2021 | United States | Not specified |
| Zheng et al. | 2020 | China | Diarrhea, abdominal pain, nausea & vomiting, anorexia |
| Zhou et al. | 2020 | China | Not specified |
| Xiong et al. | 2021 | China | At least one of: diarrhea, nausea & vomiting, abdominal pain, decreased feeding |
| Gonzalez Jimenez et al. | 2020 | Spain | Not specified |
| Redd et al. | 2020 | United States | Not specified |
| Schettino et al. | 2021 | Italy | Abdominal pain, diarrhea, nausea, vomiting, hyporexia/anorexia |
| Hajifathalian et al. | 2020 | United States | Nausea, vomiting, diarrhea, or abdominal pain |
| Bishehsari et al. | 2022 | United States | Diarrhea, nausea/vomiting, abdominal pain |
| Delavari et al. | 2022 | Iran | Any self‐reported stomach pain, nausea, vomiting, diarrhea, anorexia, and fever |
| Fallouh et al. | 2022 | United States | Abdominal pain, nausea, vomiting, or diarrhea |
| Patel et al. | 2022 | United States | Diarrhea, nausea, vomiting, abdominal pain |
GI, gastrointestinal.
Risk of bias in included studies
Table 3 highlights the outcomes of methodological quality assessment based on the NOS.
Table 3.
Risk of bias assessment
| Study | Representativeness of the exposed cohort | Selection of the non‐exposed cohort | Ascertainment of exposure | Demonstration that outcome of interest was not present at start of study | Comparability of cohorts based on the design or analysis controlled for confounders | Assessment of outcome | Was follow‐up long enough for outcomes to occur? | Adequacy of follow‐up of cohorts |
|---|---|---|---|---|---|---|---|---|
| Ghoshal et al. (2020) | * | * | * | * | ** | * | * | |
| Kang et al. (2020) | * | * | * | * | * | * | * | |
| De Moura et al. (2020) | * | * | * | * | * | * | * | |
| Ramachandran et al. (2020) | * | * | * | * | * | * | ||
| Zhang et al. (2020) | * | * | * | * | * | * | ||
| Cao et al. (2020) | * | * | * | * | * | * | ||
| Lin et al. (2020) | * | * | * | * | * | |||
| Jin et al. (2020) | * | * | * | * | ** | * | * | |
| Wan et al. (2020) | * | * | * | * | * | * | ||
| Wei et al. (2020) | * | * | * | * | * | |||
| Han et al. (2020) | * | * | * | ** | * | * | * | |
| Pan et al. (2020) | * | * | * | * | * | * | * | |
| Grover et al. (2021) | * | * | * | ** | * | * | ||
| Zheng et al. (2020) | * | * | * | * | * | * | * | |
| Zhou et al. (2020) | * | * | * | * | * | * | * | |
| Xiong et al. (2021) | * | * | * | * | * | |||
| Gonzalez Jimenez et al. (2020) | * | * | * | * | * | |||
| Redd et al. (2020) | * | * | * | * | * | * | ||
| Schettino et al. (2021) | * | * | * | * | * | * | * | |
| Hajifathalian et al. (2020) | * | * | * | * | * | * | ||
| Bishehsari et al. (2022) | * | * | * | * | * | * | ||
| Delavari et al. (2022) | * | * | * | ** | * | * | * | |
| Fallouh et al. (2022) | * | * | * | * | * | * | ||
| Patel et al. (2022) | * | * | * | * | * |
* = 1 point.
** = 2 points.
Demographic factors
Demographic factors are summarized in Figure 2.
Figure 2.
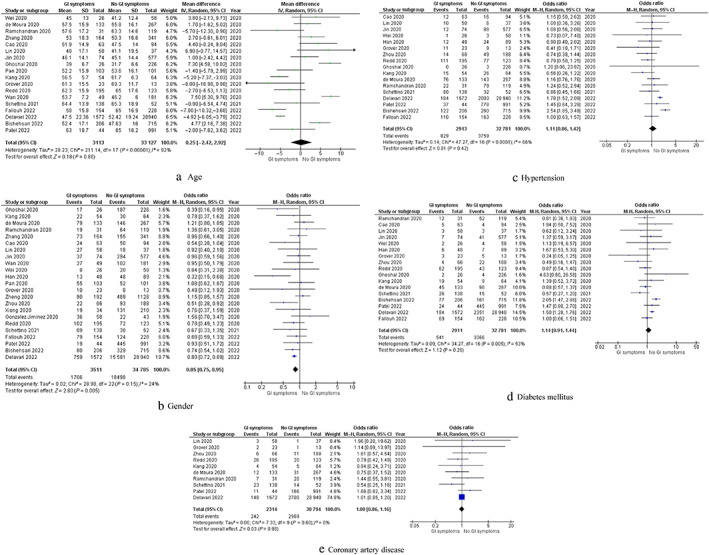
Forest plots of comparison of (a) age, (b) gender (c) hypertension, (d) diabetes mellitus, and (e) coronary artery disease. The solid squares denote the odds ratio, the horizontal lines represent the 95% confidence intervals (CIs), and the diamond denotes the pooled effect size. GI, gastrointestinal; M–H, Mantel–Haenszel test
Age: Eighteen studies reported data on age of the included populations. Pooled analysis of 36 240 patients demonstrated no significant difference in age between GI symptoms and no GI symptoms groups (MD: 0.25, 95% CI −2.42 to 2.92, P = 0.86). Cochran Q test revealed a significant level of between‐study heterogeneity (I 2 = 92%, P < 0.00001).
Male gender: Twenty‐three studies provided data on gender of the included populations. Pooled analysis of 38 296 patients showed significantly higher number of males in patients with GI symptoms compared with those without GI symptoms (OR: 0.85, 95% CI 0.75–0.95, P = 0.005). Cochran Q test revealed a low level of between‐study heterogeneity (I 2 = 24%, P = 0.15).
Hypertension: Seventeen studies provided data on the number of hypertensive patients in their study groups. Pooled analysis of 35 694 patients did not show any significant difference in the number of hypertensive patients between the two groups (OR: 1.11, 95% CI 0.86–1.42, P = 0.42). Cochran Q test revealed a moderate level of between‐study heterogeneity (I 2 = 66%, P < 0.0001).
Diabetes mellitus: Seventeen studies provided data on the number of diabetic patients in their study groups. Pooled analysis of 35 692 patients did not show any significant difference in the number of diabetic patients between the two groups (OR: 1.14, 95% CI 0.91–1.44, P = 0.26). Cochran Q test revealed a moderate level of between‐study heterogeneity (I 2 = 53%, P = 0.005).
Coronary artery disease: Ten studies provided data on the number of patients with coronary artery disease in their study groups. Pooled analysis of 33 108 patients did not show any significant difference in the number of patients with coronary artery disease between the GI symptoms and no GI symptoms groups (OR: 1.00, 95% CI 0.86–1.16, P = 0.98). Cochran Q test revealed a low level of between‐study heterogeneity (I 2 = 0%, P = 0.60).
Outcome synthesis
Outcomes are summarized in Figures 3 and 4.
Figure 3.
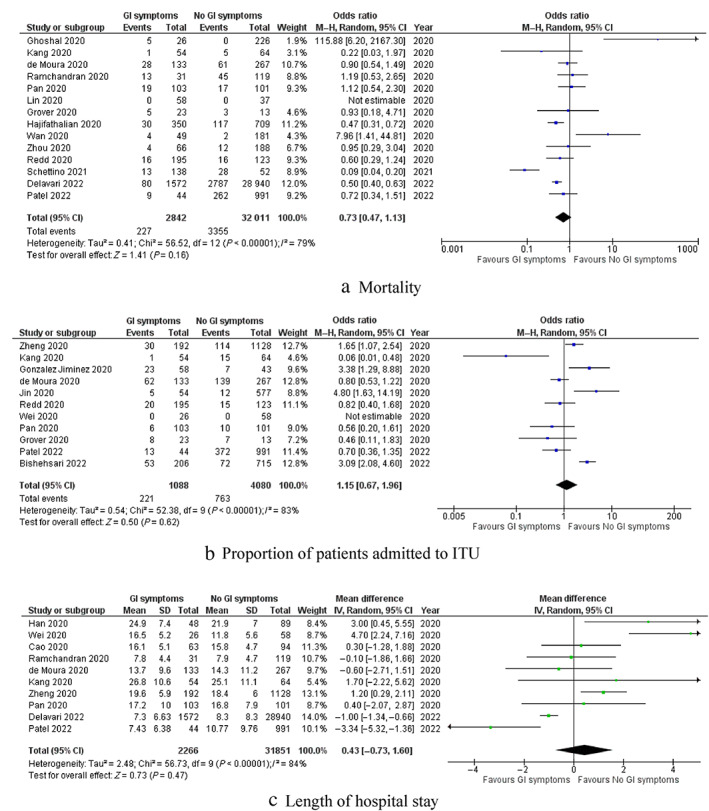
Forest plots of comparison of (a) mortality, (b) proportion of patients admitted to intensive therapy unit, and (c) length of hospital stay. The solid squares denote the odds ratio or mean difference. The horizontal lines represent the 95% confidence intervals (CIs), and the diamond denotes the pooled effect size. GI, gastrointestinal; M–H, Mantel–Haenszel test
Figure 4.
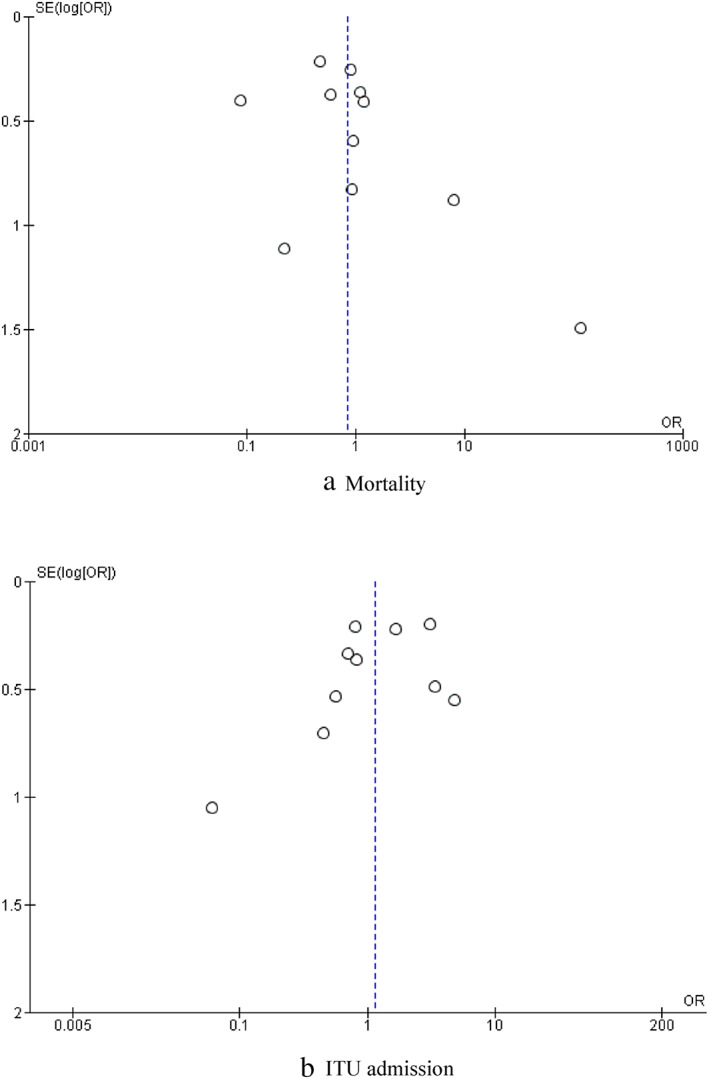
Funnel plot of comparison of (a) mortality and (b) intensive therapy unit (ITU) admission. OR, odds ratio
Mortality: Fourteen studies reported mortality of their patients as an outcome. The mortality rate in patients with GI symptoms was 8.0%, while it was 10.5% in patients without GI symptoms. Pooled analysis of 34 853 patients demonstrated no significant difference in mortality rate between the two groups (OR: 0.73; 95% CI 0.47–1.13, P = 0.16). Cochran Q test revealed a significant level of between‐study heterogeneity (I 2 = 79%, P < 0.00001).
ICU admission: Eleven studies reported rate of ICU admission as an outcome. The rate of ICU admission in patients with and without GI symptoms were 20.3 and 18.7%, respectively. Pooled analysis of 5168 patients demonstrated no significant difference in ICU admission rate between the two groups (OR: 1.15; 95% CI 0.67–1.96, P = 0.62). Cochran Q test revealed a significant level of between‐study heterogeneity (I 2 = 83%, P < 0.00001).
Length of hospital stay: Ten studies reported length of hospital stay of the patients as an outcome. The mean length of stay in patients with and without GI symptoms were 15.7 ± 6.7 days and 15.1 ± 5.4 days, respectively. Pooled analysis of 34 117 patients demonstrated no significant difference in length of hospital stay between the two groups (MD: 0.43; 95% CI −0.73 to 1.60, P = 0.47). Cochran Q test revealed a significant level of between‐study heterogeneity (I 2 = 84%, P < 0.00001).
Sensitivity analysis
The direction of pooled effect size remained unchanged when the OR, risk ratio (RR), or risk difference (RD) was calculated or during leave‐one‐out sensitivity analysis.
Meta‐regression analysis
Meta‐regression analyses suggested that the baseline difference in age (P = 0.046) and diabetes (P = 0.003) between the two groups affected the effect estimate for mortality, but the effect estimate for mortality was not affected by baseline difference in gender (P = 0.904), hypertension (P = 0.200), or coronary artery disease (P = 0.139).
Discussion
In view of unknown prognostic significance of GI symptoms associated with COVID‐19, we conducted a comprehensive literature search and identified 24 comparative observational studies 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 reporting a total of 51 522 COVID‐19 patients of whom 6544 patients had GI symptoms and 44 978 patients did not have any GI symptoms. The subsequent meta‐analysis of outcomes demonstrated that the presence of GI symptoms was associated with similar risk of mortality, ICU admission, and length of hospital stay when compared with their absence. The between‐study heterogeneity was significant in the analysis of all the evaluated outcomes.
Furthermore, alongside the outcome parameters, we objectively evaluated the baseline characteristics of the study populations and demonstrated that the patients with GI symptoms were of comparable age, rate of pre‐existing hypertension, coronary artery disease, and diabetes mellitus compared with those without GI symptoms although there were more male patients in the GI symptoms group.
Moreover, we conducted meta‐regression analysis, which indicated that the baseline difference in age and diabetes between the two groups affected the effect estimate for mortality, but the effect estimate for mortality was not affected by baseline difference in gender, hypertension, or coronary artery disease.
The reported incidence of GI symptoms in COVID‐19 patients varies with estimates ranging between 3 and 39%. 38 Moreover, the literature on the association between these symptoms and COVID‐19 disease severity is also conflicting. Some studies have suggested a “protective” effect with patients experiencing a mild/moderate illness, 39 while others have found more severe symptoms of pneumonia. 22 This may in part reflect on the quality and type of studies commenting on this association (largely observational retrospective data).
A study on the significance of GI symptoms in COVID‐19 patients found 22.4% of their cohort reporting at least one symptom at the onset of infection, with diarrhea being the most common complaint (70%). This group (compared to absence of GI symptoms) were older with higher BMI and more prevalent rates of diabetes and hypertension. 35
Even after adjustment for demographics, comorbidities, and other clinical symptoms, the presence of GI symptoms and in particular diarrhea and/or abdominal pain were independently linked to poor outcomes including hospitalization, intensive therapy unit (ITU) admission, acute respiratory distress syndrome (ARDS), and increased rate of intubation. 35
A meta‐analysis of 21 articles 38 (mainly from China) suggested regional differences in the relationship between diarrhea and COVID‐19 severity. Overall analysis showed a severe rate of COVID‐19 in patients with diarrhea of 41.1% with a pooled OR reaching statistical significance. However, further subgroup and sensitivity analysis failed to show a significant association.
The authors did demonstrate a significant correlation between abdominal pain and disease severity. A 2.8‐fold increased risk of severe COVID‐19 was estimated in patients with abdominal pain. However, due to the variable reporting a significant association between nausea and vomiting, disease severity was not established. 38
A further review showed a similar significant association between abdominal pain and severe COVID‐19. The incidence of other common GI symptoms showed no difference between severe and mild–moderate cases. 40
In terms of GI symptom frequency in patients infected with SARS‐CoV‐2, a large systematic review (158 studies, n = 78 798) reported as follows: diarrhea (16.5%), nausea (9.7%), anorexia/loss of appetite (1.6%), vomiting (1.5%), and abdominal pain (4.5%). They also failed to demonstrate a significant association between the presence of GI symptoms and indicators of severe disease such as admission to ITU and increased mortality rate. 41
Cumulative incidence of GI symptoms occurring in COVID‐19 patients was estimated as follows by Wang et al. 42 : diarrhea (25%), nausea (16%), vomiting (7.5%), and abdominal pain (3.6%). In keeping with previous conclusions, no significant correlation between the presence of GI symptoms and mortality was demonstrated.
Finally, Mao et al. 43 reported a pooled prevalence of GI symptoms of 15% in patients with COVID‐19 at diagnosis and these were associated with a significantly increased risk of developing ARDS. However, pooled rates of hospital discharge, length of stay, and mortality rates were unaffected by the presence of digestive symptoms. In keeping with the above findings, abdominal pain seemed to have a higher prevalence in patients with severe disease compared with non‐severe COVID‐19.
In summary, from the published literature, it is difficult to determine disease severity and risk stratify COVID‐19 patients based on the presence or absence of GI symptoms. Some evidence suggests a possible link between abdominal pain and increased COVID‐19 severity. This link remains unclear, and the “gut–lung” axis has been proposed as a potential mechanism. Altered gut flora secondary to viral invasion may lead to changes in immune regulation and effects on the respiratory system, but this warrants further investigation.
Overall, the analysis of best available evidence does not seem to demonstrate a significant correlation between GI symptoms and disease severity in patients with COVID‐19. Our results agree with previous studies and show no real prognostic significance of GI symptoms in patients with COVID‐19.
We did find a higher proportion of males in the GI symptoms group. A large‐scale global analysis 44 showed that both sexes were at equivalent risk of infection, but male sex was associated with the development of severe disease as measured by ITU admission and death. Sex differences have previously been reported in both the innate and adaptive immune systems. 44 Robust antiviral interferon response and increased adaptive immunity toward viral antigens in females may help to contain and localize the infection and may account for the differences seen in risk of developing GI symptoms.
Diarrhea, whether on presentation or developing during hospital admission, is an important consideration for healthcare professionals. Viral colonization of the gut and viral shedding in the stool (even after it becomes undetectable in the upper respiratory tract) has clinical implications for possible fecal–oral mode of transmission.
Infection prevention and control principles therefore become necessary to prevent further viral propagation and include screening, triaging, and regular testing of both patients and healthcare providers. Correct personal protective equipment, compliance with good hand hygiene, use of single rooms (where possible), regular decontamination of the care environment including toilets/commodes and frequently touched surfaces should all help in minimizing the risk of spread.
Therefore, GI symptoms and in particular diarrhea in addition to the commonly reported respiratory complaints should be monitored as a sign of an actively infectious state.
COVID‐19 has had (and continues to have) an enormous impact on healthcare resources worldwide, including critical care facilities. The need to risk stratify patients early and identify those potentially becoming critically ill for closer monitoring and early intervention is vital to reduce morbidity and mortality.
The coronavirus virion is made up of several structural proteins, including the nucleocapsid (N), membrane (M), envelope (E), and spike (S) proteins. 45 Angiotensin‐converting enzyme 2 (ACE2) is the obligate receptor for SARS‐CoV‐2 entry into host cells. ACE2 was originally identified in 2003 and acts as the receptor for other alpha‐ and beta‐coronaviruses. Viral entry including attachment to host cell membrane and fusion are mediated by the S glycoprotein. This process is also dependent on transmembrane protease, serine 2 (TMPRSS2). 45 Receptor expression in the GI tract and the demonstration in human small intestinal organoid models producing infectious virions is thought to be the mechanism responsible for GI symptoms. Moreover, viral RNA and proteins have been observed in biopsies from different parts of the GI tract and show infiltration of plasma cells and lymphocytes in the lamina propria. 46
It remains unclear whether the GI tract is affected primarily by the virus, or the dysfunction occurs secondary to critical illness, and its associated systemic inflammation [systemic inflammatory response syndrome (SIRS) response], hypoperfusion, coagulopathy, and treatments. 46
Changes in the gut microbiota may also play a role. COVID‐19 patients have significantly reduced bacterial diversity and in particular a reduction in species with immunomodulatory potential and relative increases in opportunistic pathogens. Data suggesting altered microbiome may be related to COVID‐19 severity and biochemical markers of inflammation. 46 However, future well‐designed studies are needed to explore this further.
The current study has some limitations that should be considered when interpreting its findings. The best available evidence comes mainly from retrospective observational studies that are subject to selection bias. Other inherent problems with this study design include recall bias and missing data. They are often subject to confounding factors and unable to determine causation being limited to association. Moreover, temporal relationships are difficult to assess.
In several of our included studies GI symptoms were not clearly defined and, in the majority, they were assessed once only at the time of initial presentation and not repeatedly throughout the study period. The definition of what constitutes severe COVID‐19 compared with mild/moderate cases also varied between studies introducing heterogeneity. Additionally, due to lack of data, subgroup analysis based on severity of illness in patients with and without GI symptoms was not feasible.
Furthermore, there is heterogeneity in the cohort of patients included in this review. Most of the included data were from hospitalized patients but a small number of studies also included ambulatory/outpatients. Due to a lack of data, it was not feasible to perform separate subgroup analysis for these groups.
Following hospital admission, extraneous factors other than COVID‐19 such as antibiotic or antiviral treatments, associated infections can potentially result in the development of GI symptoms. Future analyses in carefully selected studies considering GI symptoms at presentation and subsequent development during admission and their impact on outcomes and prognosis would be interesting. Similarly, association with disease severity in matched cohorts would provide for further valuable insight into this topic.
Finally missing data may result in less precise and possibly biased effect estimates in single studies. This bias arising from individual studies with incomplete outcome datasets can then be propagated into subsequent meta‐analyses. To address this, we attempted to contact corresponding authors of the included studies where information about our outcome of interest was not reported.
Some studies reported their continuous parameters as median and IQR. We have calculated the mean and SD from median and IQR applying a widely acceptable equation described by Hozo et al. 13 This might have introduced some bias to our findings.
In conclusion, GI symptoms are an important clinical feature of COVID‐19 and in some patients can be particularly debilitating leading to a prolonged recovery. However, our meta‐analysis of the best possible available evidence (level 2) demonstrated that GI symptoms in COVID‐19 do not seem to affect patients with any specific demographic pattern and may not have any important prognostic significance. However, in hospitalized patients, especially in the presence of diarrhea, the necessary precautions need to be taken to prevent further disease transmission and disruption of healthcare provision.
Although no randomized studies can be conducted on this topic, future high‐quality studies can provide stronger evidence to further understand the impact of GI symptoms on outcomes of COVID‐19 patients.
Supporting information
Appendix S1. Search strategy.
Declaration of conflict of interest: None.
Author contribution: Shafquat Zaman, Shahin Hajibandeh, Shahab Hajibandeh, and Andrew D Beggs were involved in study conception and design. Shafquat Zaman, Shahin Hajibandeh, and Ali Yasen Y Mohamedahmed were involved in acquisition of data. Shafquat Zaman, Shahin Hajibandeh, Shahab Hajibandeh, Mohammed E El‐Asrag were involved in analysis and interpretation of data. All authors were involved in drafting the manuscript, critical revision of the manuscript, and final approval of the manuscript.
References
- 1. Guan WJ, Ni ZY, Hu Y et al. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020; 382: 1708–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hu B, Guo H, Zhou P, Shi ZL. Characteristics of SARS‐CoV‐2 and COVID‐19. Nat. Rev. Microbiol. 2021; 19: 141–54. Erratum in: Nat. Rev. Microbiol. 2022;20 (5):315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cheung KS, Hung IFN, Chan PPY et al. Gastrointestinal manifestations of SARS‐CoV‐2 infection and virus load in fecal samples from a Hong Kong cohort: systematic review and meta‐analysis. Gastroenterology. 2020; 159: 81–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lopez‐Leon S, Wegman‐Ostrosky T, Perelman C et al. More than 50 long‐term effects of COVID‐19: a systematic review and meta‐analysis. Sci. Rep. 2021; 11: 16144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Payne AB, Gilani Z, Godfred‐Cato S et al. Incidence of multisystem inflammatory syndrome in children among US persons infected with SARS‐CoV‐2. JAMA Netw. Open. 2021; 4: e2116420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dhar D, Dey T, Samim MM et al. Systemic inflammatory syndrome in COVID‐19‐SISCoV study: systematic review and meta‐analysis. Pediatr. Res. 2022; 91: 1334–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Joshee S, Vatti N, Chang C. Long‐term effects of COVID‐19. Mayo Clin. Proc. 2022; 97: 579–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Miller J, Cantor A, Zachariah P, Ahn D, Martinez M, Margolis KG. Gastrointestinal symptoms as a major presentation component of a novel multisystem inflammatory syndrome in children that is related to coronavirus disease 2019: a single center experience of 44 cases. Gastroenterology. 2020; 159: 1571–1574.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zaman S, Hajibandeh S, Hajibandeh S et al. Meta‐analysis of the demographic and prognostic significance of gastrointestinal symptoms in COVID‐19 patients . PROSPERO 2021 CRD42021283173. [DOI] [PMC free article] [PubMed]
- 10. Higgins JPT, Thomas J, Chandler J et al. Cochrane handbook for systematic reviews of interventions version 6.1 (updated September 2020). Cochrane, 2020. Cited 15 Oct 2021.
- 11. Liberati A, Altman DG, Tetzlaff J et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009; 339: b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wells GA, Shea B, O'Connell D et al. The Newcastle‐Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta‐analyses . Cited 28 Nov 2018.
- 13. Hozo S, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med. Res. Methodol. 2005; 5: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ghoshal UC, Ghoshal U, Mathur A et al. The spectrum of gastrointestinal symptoms in patients with coronavirus disease‐19: predictors, relationship with disease severity, and outcome. Clin. Transl. Gastroenterol. 2020; 11: e00259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kang MK, Kim KO, Kim MC et al. Clinical characteristics of coronavirus disease 2019 patients with diarrhea in Daegu. Korean J. Intern. Med. 2020; 35: 1261–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jin X, Lian JS, Hu JH et al. Epidemiological, clinical and virological characteristics of 74 cases of coronavirus‐infected disease 2019 (COVID‐19) with gastrointestinal symptoms. Gut. 2020; 69: 1002–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. de Moura DTH, Proença IM, McCarty TR et al. Gastrointestinal manifestations and associated health outcomes of COVID‐19: a Brazilian experience from the largest South American public hospital. Clinics. 2020; 75: e2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ramachandran P, Onukogu I, Ghanta S et al. Gastrointestinal symptoms and outcomes in hospitalized coronavirus disease 2019 patients. Dig. Dis. 2020; 38: 373–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhang H, Liao YS, Gong J, Liu J, Xia X, Zhang H. Clinical characteristics of coronavirus disease (COVID‐19) patients with gastrointestinal symptoms: a report of 164 cases. Dig. Liver Dis. 2020; 52: 1076–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cao C, Chen M, He L, Xie J, Chen X. Clinical features and outcomes of COVID‐19 patients with gastrointestinal symptoms. Crit. Care. 2020; 24: 340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lin L, Jiang X, Zhang Z et al. Gastrointestinal symptoms of 95 cases with SARS‐CoV‐2 infection. Gut. 2020; 69: 997–1001. [DOI] [PubMed] [Google Scholar]
- 22. Wan Y, Li J, Shen L et al. Enteric involvement in hospitalised patients with COVID‐19 outside Wuhan. Lancet Gastroenterol. Hepatol. 2020; 5: 534–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wei XS, Wang X, Niu YR et al. Diarrhea is associated with prolonged symptoms and viral carriage in corona virus disease 2019. Clin. Gastroenterol. Hepatol. 2020; 18: 1753–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Han C, Duan C, Zhang S et al. Digestive symptoms in COVID‐19 patients with mild disease severity: clinical presentation, stool viral RNA testing, and outcomes. Am. J. Gastroenterol. 2020; 115: 916–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pan L, Mu M, Yang P et al. Clinical characteristics of COVID‐19 patients with digestive symptoms in Hubei, China: a descriptive, cross‐sectional, multicenter study. Am. J. Gastroenterol. 2020; 115: 766–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Grover S, Redd WD, Zhou JC et al. High prevalence of gastrointestinal manifestations of COVID‐19 infection in hospitalized patients with cancer. J. Clin. Gastroenterol. 2021; 55: 84–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zheng T, Yang C, Wang HY et al. Clinical characteristics and outcomes of COVID‐19 patients with gastrointestinal symptoms admitted to Jianghan Fangcang Shelter Hospital in Wuhan, China. J. Med. Virol. 2020; 92: 2735–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhou Z, Zhao N, Shu Y, Han S, Chen B, Shu X. Effect of gastrointestinal symptoms in patients with COVID‐19. Gastroenterology. 2020; 158: 2294–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Xiong XL, Wong KKY, Chi SQ et al. Comparative study of the clinical characteristics and epidemiological trend of 244 COVID‐19 infected children with or without GI symptoms. Gut. 2021; 70: 436–8. [DOI] [PubMed] [Google Scholar]
- 30. Gonzalez Jimenez D, Velasco Rodríguez‐Belvís M, Ferrer Gonzalez P et al. COVID‐19 gastrointestinal manifestations are independent predictors of PICU admission in hospitalized pediatric patients. Pediatr. Infect. Dis. J. 2020; 39: e459–62. [DOI] [PubMed] [Google Scholar]
- 31. Redd WD, Zhou JC, Hathorn KE et al. Prevalence and characteristics of gastrointestinal symptoms in patients with severe acute respiratory syndrome coronavirus 2 infection in the United States: a multicenter cohort study. Gastroenterology. 2020; 159: 765–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Schettino M, Pellegrini L, Picascia D et al. Clinical characteristics of COVID‐19 patients with gastrointestinal symptoms in Northern Italy: a single‐center cohort Study. Am. J. Gastroenterol. 2021; 116: 306–10. [DOI] [PubMed] [Google Scholar]
- 33. Hajifathalian K, Krisko T, Mehta A et al. Gastrointestinal and hepatic manifestations of 2019 novel coronavirus disease in a large cohort of infected patients from New York: clinical Implications. Gastroenterology. 2020; 159: 1137–1140.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Patel HK, Kovacic R, Chandrasekar VT et al. Correlation of gastrointestinal symptoms at initial presentation with clinical outcomes in hospitalized COVID‐19 patients: results from a large health system in the southern USA. Dig. Dis. Sci. 2022: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bishehsari F, Adnan D, Deshmukh A et al. Gastrointestinal Symptoms Predict the Outcomes From COVID‐19 Infection. J. Clin. Gastroenterol. 2022; 56: e145–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fallouh NA, Naik KH, Udochi CO et al. Better clinical outcomes in hospitalized COVID‐19 minority patients with accompanying gastrointestinal symptoms. J. Natl. Med. Assoc. 2022; 113: 626–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Delavari A, Asgari S, Alimohamadi Y et al. xsGastrointestinal symptoms are associated with a lower risk of hospitalization and mortality and Outcomes in COVID‐19. BMC Gastroenterol. 2022; 22: 119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zeng W, Qi K, Ye M et al. Gastrointestinal symptoms are associated with severity of coronavirus disease 2019: a systematic review and meta‐analysis. Eur. J. Gastroenterol. Hepatol. 2022; 34: 168–76. [DOI] [PubMed] [Google Scholar]
- 39. Nobel YR, Phipps M, Zucker J et al. Gastrointestinal symptoms and coronavirus disease 2019: a case‐control study from the United States. Gastroenterology. 2020; 159: 373–375.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hayashi Y, Wagatsuma K, Nojima M et al. The characteristics of gastrointestinal symptoms in patients with severe COVID‐19: a systematic review and meta‐analysis. J. Gastroenterol. 2021; 23: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Shehab M, Alrashed F, Shuaibi S, Alajmi D, Barkun A. Gastroenterological and hepatic manifestations of patients with COVID‐19, prevalence, mortality by country, and intensive care admission rate: systematic review and meta‐analysis. BMJ Open Gastroenterol. 2021; 8: e000571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wang Y, Li Y, Zhang Y, Liu Y, Liu Y. Are gastrointestinal symptoms associated with higher risk of Mortality in COVID‐19 patients? A systematic review and meta‐analysis. BMC Gastroenterol. 2022; 22: 106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mao R, Qiu Y, He JS et al. Manifestations and prognosis of gastrointestinal and liver involvement in patients with COVID‐19: a systematic review and meta‐analysis. Lancet Gastroenterol. Hepatol. 2020; 5: 667–78. Erratum in: Lancet Gastroenterol. Hepatol. 2020; 5: e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Peckham H, de Gruijter NM, Raine C et al. Male sex identified by global COVID‐19 meta‐analysis as a risk factor for death and ITU admission. Nat. Commun. 2020; 11: 6317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jackson CB, Farzan M, Chen B, Choe H. Mechanisms of SARS‐CoV‐2 entry into cells. Nat. Rev. Mol. Cell Biol. 2022; 23: 3–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Reintam Blaser A, Gunst J, Arabi YM. The gut in COVID‐19. Intensive Care Med. 2021; 47: 1024–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Search strategy.


