Abstract
Purpose
The aim of the study was to compare the outcomes of patients with post‐COVID‐19 condition undergoing supervised therapeutic exercise intervention or following the self‐management WHO (World Health Organization) rehabilitation leaflet.
Methods
A randomized controlled trial was carried out that included 39 participants with post‐COVID‐19 condition who had a chronic symptomatic phase lasting >12 weeks. Comprehensive medical screening, patient‐reported symptoms, and cardiorespiratory fitness and muscular strength were assessed. Patients were randomly assigned to a tailored multicomponent exercise program based on concurrent training for 8 weeks (two supervised sessions per week comprised resistance training combined with aerobic training [moderate intensity variable training], plus a third day of monitored light intensity continuous training), or to a control group which followed the WHO guidelines for rehabilitation after COVID‐19.
Results
After follow‐up, there were changes in physical outcomes in both groups, however, the magnitude of the change pre–post intervention favored the exercise group in cardiovascular and strength markers: VO2max +5.7%, sit‐to‐stand −22.7% and load‐velocity profiles in bench press +6.3%, and half squat +16.9%, (p < 0.05). In addition, exercise intervention resulted in a significantly better quality of life, less fatigue, less depression, and improved functional status, as well as in superior cardiovascular fitness and muscle strength compared to controls (p < 0.05). No adverse events were observed during the training sessions.
Conclusion
Compared to current WHO recommendations, a supervised, tailored concurrent training at low and moderate intensity for both resistance and endurance training is a more effective, safe, and well‐tolerated intervention in post‐COVID‐19 conditions.
Keywords: fatigue, long COVID, physical activity, post‐COVID‐19 condition, post‐exercise malaise, quality of life
1. INTRODUCTION
A novel coronavirus, SARS‐CoV2 (severe acute respiratory syndrome coronavirus 2), was first described in Wuhan (China) in December 2019 as a new cause of respiratory illness, called Coronavirus disease 2019 (hereinafter, COVID‐19). Although recovery is the main prognostic feature, some people manifest a myriad of symptoms for a long period of time after the acute infection. The WHO (World Health Organization) recently named this as “post‐COVID‐19 condition.”. 1 It refers to a syndrome that can include a wide range of symptoms: of persistent or new onset, continuous or fluctuating in nature, lasting for more than 2 months after 12 weeks of a microbiologically confirmed or suspected SARS‐CoV2 infection, that cannot be explained by an alternative diagnosis. These post‐COVID‐19 conditions have also been named long COVID, post COVID‐19 syndrome, or chronic COVID‐19 since May 2020.
The large number of symptoms, lasting for months, the fluctuating nature, and relapsing pattern, 2 hugely impact the quality of life. The presence of fatigue, exhaustion post‐exercise, dyspnea, and neurocognitive derangements during the recovery period, even in mild cases, make daily life activities difficult. 2 Work and financial loss, difficulties to access an appropriate plan of rehabilitation, and mental health deterioration are all having a great impact on society. Estimates on the scope of this health problem are difficult since data on the prevalence of the disease vary depending on the diagnosis definition and patient characteristics. 3 In a systematic review with meta‐analysis that analyzed the prevalence of symptoms at 3 months, it was found that the overall prevalence of persistent symptoms was 45.9%. 4 Attending exclusively to mild, not hospitalized, cases in a longitudinal and prospective study, the prevalence of persistent symptoms after more than 6 months was 34.8%. 5 However, large‐scale population studies conducted in patients with mild disease show a significantly lower prevalence of ongoing symptoms after 3 months of evolution, estimated between 1% and 3%. 6
Even when vaccinations have been shown to have some impact on decreasing post‐COVID‐19 conditions, 7 and that preserving better cardiopulmonary health and physical condition was related to a lower intensity of symptoms, 8 up to now, no active strategies have been developed to improve symptomatology in these individuals. Preliminary data on some pharmacological treatments, such as a short course of corticosteroids, have not shown enough evidence so far. 9 The treatments offered for acute disease do not appear to have a prognostic effect on these persistent symptoms either. Guidelines on clinical management of patients with post‐COVID‐19 conditions indicate the need to address the control of symptoms and the quality of life of patients. 10 The proposals include rehabilitation plans and therapeutic exercise, indicating that the reintroduction of progressive exercise, according to the patient's tolerance, could be beneficial for the vast majority of them. 11
In the RECOVE trial (REhabilitation for post‐COVid‐19 condition through a supervised Exercise intervention), we evaluated non‐hospitalized people with the post‐COVID‐19 condition to identify the roll of a tailored exercise program, based on multicomponent exercise training, on the recovery of persistent or recurrent symptoms and functional limitation after COVID‐19 and compared this to the self‐management leaflet commonly used in outpatient scenarios. We hypothesized that the intervention would better improve physical and mental status after 8 weeks of physical training compared to the conventional self‐management recommendations.
2. MATERIAL AND METHODS
2.1. Study design, eligibility, and randomization
This study was registered at ClinicalTrials.gov (NCT04718506), approved by an ethical review board by Murcia University Ethics Committee (reference No. 3447/2021), and conducted according to the CONSORT statement. The required sample size was determined on the basis of the differences in aerobic exercise capacity in COVID‐19 patients’ post‐hospital discharge compared to comorbidity‐matched controls. 12 Effect sizes of d = 0.998 in aerobic exercise capacity can be identified with 16 participants per group, assuming an alpha error of α = 5% and power of 95%. Expecting a maximum loss of follow‐up of 20%, we recruited 19 participants per group (n = 38). Participants were recruited through advertisements on social media or via recommendations to general practitioners. Inclusion criteria were subjects aged over 18 who had a confirmed microbiological diagnosis of COVID‐19 by SARS‐CoV2 reverse transcription‐polymerase chain reaction on an oropharyngeal–nasopharyngeal swab or a positive rapid antigen test, who presented a chronic symptomatic phase, lasting >12 weeks from the onset of symptoms, and had not been hospitalized because of the acute COVID‐19 infection. All patients belonged to the mild acute infection category at the time of diagnosis, 13 characterized by the presence of typical symptoms, in the absence of shortness of breath, dyspnea, or abnormal chest imaging (SatO2 on room air ≥94%, breathing frequency <22 bpm [breaths per minute]). No one had received specific SARS‐CoV2 treatment. We excluded pregnant patients and those who had acute or unstable chronic diseases such as unstable myocardiopathy, ischemic heart disease, uncontrolled hypertension, uncontrolled chronic obstructive pulmonary disease (COPD), or major surgery in the past 3 months. After recruitment and baseline measurements, a VO2max‐stratified computer‐generated randomization sequence with 1:1 allocation into a control or exercise group was created.
2.2. Interventions
The training sessions were carried out in the medical center and the physiology laboratory at the Faculty of Sports Sciences of the University of Murcia. Participants from the experimental group completed 8 weeks of a tailored and supervised multicomponent exercise program adapted from the ACSM guidelines for chronic obstructive pulmonary disease and cardiovascular disease. 14 Participants completed a 3 days‐a‐week concurrent training routine: 2 days of resistance training (50% 1RM [one‐repetition maximum], 3 sets, 8 repetitions, 4 exercises [squat, bench press, deadlift, and bench pull]) combined with moderate intensity variable training (MIVT: 4–6 × 3–5 min at 70%–80% heart rate reserve [HRR]/2–3 min at 55%–65% HRR), and 1 day of light intensity continuous training (LICT: 30–60 min, 65%–70% HRR). During the resistance training, a constant programming model (intensity and intra‐set volume kept constant throughout the training plan) was carried out. Training loads were individually determined for each session following the velocity‐based training. 15 On the other hand, a weekly linear programming model (volume was varied in session or set of sessions) was conducted for endurance sessions. All participants in the training group used a heart rate chest band for monitoring the intensity of endurance exercise in real‐time through the Polar Beat application on their mobile phones.
Progressions in endurance sessions were individualized and consistent with patient tolerance, according to the abovementioned range. In addition, the subjective rate of perceived exertion (RPE, according to the modified Borg scale) was continuously assessed for all participants with a visual scale in all training sessions reaching a score between 11 and 12 in LICT and not exceeding a score of 16 in MIVT. This RPE monitoring 16 allowed us to control the exertion intensity of patients with the impossibility of reaching the estimated heart rate due to severe dyspnea or serious fatigue. Sessions were directed by certified strength and conditioning coaches, graduated in Sports Sciences, and conducted under medical supervision. Attendance ≥85% (at least 20 of the 24 scheduled sessions) was an essential requirement; if any participant did not show this adherence, he or she was withdrawn from the study.
Participants from the control group were informed (non‐supervised) to follow the WHO guidelines: Support for Rehabilitation: Self‐Management after COVID‐19 Related Illness. 17 In summary, aerobic exercise for 20–30 min was recommended, 5 days a week at an intensity that allows breathless speech plus strength exercises in 3 weekly sessions (3 × 10 repetitions of the seven recommended exercises).
3. MEASUREMENTS
3.1. Baseline characteristics
Participants initially completed a clinical evaluation that included an interview, physical examination, and standardized questionnaire on medical history, conducted by an internal medicine physician (infectious diseases consultant) and a cardiology team. Body composition and body mass index (BMI) were measured by a multifrequency segmental body composition analyzer (Tanita MC‐780U, Tokyo, Japan). Blood tests (cardiac and muscle injury markers, coagulation, and inflammatory markers), spirometry, resting electrocardiogram, and echocardiography were also performed to rule out any potential major cardiopulmonary issues.
3.2. Severity of symptoms
Patient‐reported outcomes (PROs) included health‐related quality of life by the 12‐item Short Form Survey (SF‐12), 18 calculating the mental component (MH) and physical component scores (PA). Anxiety and depression symptoms were calculated using the General Anxiety Disorder Questionnaire‐7 (GAD‐7) 19 and the Patient Health Questionnaire‐9 (PHQ‐9). 20 A cut‐off score for moderate–severe depression and anxiety ≥10 points was considered for secondary analyses. Perception of dyspnea and the disability produced by this was estimated using the Modified Medical Research Council Dyspnea l Scale (mMRC). 21 A cut‐off score for severe breathlessness ≥2 was considered for secondary analyses. Fatigue intensity was determined using the Chalder Fatigue Scale (CFQ‐11) with the Likert scoring system 22 and the average score on Fatigue Severity Scale (FSS). 23 Scores of ≥18 and ≥4, respectively, indicate severe fatigue. 24 A bimodal CFQ‐11 bimodal score was used to measure the frequency of symptoms related to tiredness in a syndrome characterized by fatigue. The DePaul Symptom Questionnaire Short Form (DSQ‐14 short form) 25 was used to screen myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) symptoms by measuring its frequency and severity over the past 6 months. Functional limitations after COVID‐19 were calculated using the Post‐COVID‐19 Functional Status (PCFS) scale. 26 All the indicated scales are validated in their respective versions in Spanish.
3.3. Physical fitness
Participants completed a submaximal multistage and individualized cardiopulmonary exercise test on a cycle ergometer (Ergoline, Ergoselect 200) while wearing heart rate (HR) monitors (Polar V800, Kempele, Finland) and reporting their rate of perceived exertion (RPE 6‐20) according to the Ekblom‐Bak protocol. 27 Mean heart rate during the last minute at the higher work rate was recorded. Maximal oxygen consumption (VO2max) was estimated by sex‐specific equations further adjusted by sex and age. 28
Muscular strength measurements included a handgrip (HG) test using a calibrated digital dynamometer (Takei 5401‐C, Shinagawa‐Ku, Tokyo), the 5‐time sit‐to‐stand test, 29 a 3‐second isometric knee extension test at 110° of knee flexion angle using a force sensor (Chronojump, BoscoSystem, Barcelona) recording in Newtons (N), 30 and a progressive submaximal loading test using a Smith machine for the bench press (BP) and half squat (HSQ) exercises, using a linear velocity transducer (T‐Force, Ergotech Consulting, Murcia, Spain). 31 The progressive loading tests were performed from a starting load of 5 kg, increasing to reach a target mean propulsive velocity corresponding to ~50% of the 1‐repetition maximum effort (1RM): 0.89–0.93 m s−1 for the BP 32 and 0.66–0.70 m s−1 for the HSQ. 32
WHO Global Physical Activity Questionnaire (GPAQ) for physical activity surveillance was used to quantify the number of minutes of physical activity per week. 33 Intensity, duration, and frequency of physical activity (PA) were assessed.
3.4. Cardiopulmonary function
A resting ECG and an echocardiogram were performed following standard procedures 34 by a team of cardiologists. The ultrasound system (Philips CX50; Guilford, England), methodology, and interpretation were the same for all patients. Participants completed a forced spirometry test (MetaLyzer 3B‐R3, Cortex Biophysik GmbH, Leipzig, Germany) following standardized procedures. 35 Data from the forced vital capacity (FVC), forced expiratory volume at the end of the first second (FEV1), forced expiratory flow rate at the mid‐portion of FVC (FEV 25%–75%), and maximum voluntary ventilation (MMV) were collected.
3.5. Statistical analysis
Descriptive statistics were used to summarize the characteristics of the sample by the treatment group. The independence of groups at baseline was verified using the t test. Analysis of covariance (ANCOVA) was conducted to determine whether the post‐test values in health markers and symptoms were different between the two groups after controlling for age, sex, duration of symptoms, BMI, and baseline scores. Binomial variables were studied by chi‐square analyses. Effects size was interpreted by means of partial eta square (η p 2), interpreted as small (0.01), medium (0.06), and large (0.14), and Cramer's V, interpreted as low (0.10), medium (0.30), and large (0.50). A 2 (group: RECOVE vs. CONTROL) × 2 (time: pre [T0] vs. post [T1]) factorial analysis of variance (ANOVA) with Bonferroni adjustment was used to analyze the differences between groups for physical fitness markers. The significance level was set at p < 0.05. Calculations and plots were performed with JASP v.0.15 for windows.
4. RESULTS
Thirty‐nine participants met the inclusion/exclusion criteria and were randomly assigned into control (n = 20) and exercise (n = 19) groups. Overall, patients were 45.2 years old (SD 9.5), and 74.4% female sex (n = 29). The mean time from diagnosis to study entry was 33 weeks (SD 20.5). All the participants were mild cases in their acute phase of the infection, without evidence of pneumonia, who did not require admission or specific treatment for SARS‐CoV2 infection. There were no differences between groups at baseline in any of the symptoms referred (Table 1, p from 0.106 to 0.935) or in any of the studied variables (Table 2, p from 0.093 to 0.970). No abnormalities were found in cardiac or pulmonary evaluations by ECG, echocardiogram, or spirometry. There were no adverse events during the training sessions. Among participants in the intervention group, one abandoned the training program because of commitment problems.
TABLE 1.
Baseline characteristics of the sample
| Control | Exercise | Control | Exercise | ||
|---|---|---|---|---|---|
| Age (years) | 46.0 ± 9.5 | 44.6 ± 9.9 | Number of symptoms | 8.7 ± 4.4 | 7.9 ± 3.6 |
| Female sex | 16 (80) | 13 (68) | Weeks of symptoms | 36.7 ± 23.4 | 29.3 ± 16.8 |
| Symptoms | Evolution of symptoms | ||||
| Low‐grade fever | 3 (15.0) | 4 (21.1) | Fluctuating course | 11 (55.0) | 9 (47.4) |
| Fatigue | 16 (80.0) | 16 (84.2) | Progressive improvement | 17 (85.0) | 12 (66.7) |
| Dyspnea | 12 (60.0) | 11 (57.9) | Intensity of symptoms | ||
| Myalgia | 10 (50.0) | 8 (42.1) | Mild | 7 (35.0) | 10 (52.6) |
| Headache | 9 (45.0) | 8 (42.1) | Moderate | 11 (55.0) | 6 (31.6) |
| Loss appetite | 6 (30.0) | 2 (10.5) | Severe | 2 (10.0) | 3 (15.8) |
| Weight loss | 1 (5.0) | 2 (10.5) | Medication | ||
| Chest pain | 8 (40.0) | 2 (10.5) | Taking any medication | 12 (60.0) | 14 (73.7) |
| Cough | 3 (15.0) | 3 (15.8) | Antidepressants | 7 (35.0) | 7 (36.8) |
| Loss of smell/taste | 9 (45.0) | 5 (26.3) | Benzodiazepines | 6 (30.0) | 7 (36.8) |
| Low mood | 11 (55.0) | 7 (38.9) | Bronchodilators | 7 (35.0) | 3 (15.8) |
| Anxiety | 6 (30.0) | 6 (31.6) | Toxic habits | ||
| Lack concentration | 10 (50.0) | 12 (63.2) | Alcohol | 0 (0.0) | 2 (10.5) |
| Brain fog | 10 (50.0) | 11 (61.1) | Active smoker | 1 (5.0) | 2 (11.1) |
| Memory problems | 10 (50.0) | 11 (61.1) | Former smoker | 6 (30.0) | 6 (33.3) |
| Sleep disturbances | 11 (55.5) | 9 (47.4) | Comorbidity | ||
| Dizziness | 3 (15.0) | 4 (21.1) | Hypertension | 0 (0.0) | 1 (5.3) |
| Palpitations | 5 (25.0) | 4 (21.1) | Diabetes | 0 (0.0) | 1 (5.3) |
| Hair loss | 5 (25.0) | 5 (26.3) | Asthma | 3 (15.0) | 2 (10.5) |
| Diarrhea | 5 (25.0) | 0 (0.0) | Structural heart disease | 2 (10.0) | 1 (5.3) |
| Nausea/vomiting | 1 (5.0) | 0 (0.0) | Cerebrovascular disease | 0 (0.0) | 1 (5.3) |
| Abdominal pain | 4 (20.0) | 3 (15.8) | Psychiatric conditions | 4 (20.0) | 7 (36.8) |
Note: Data are frequencies and percentages (n [%]) or means and standard deviation (M ± SD).
TABLE 2.
Post‐test results and ANCOVA showing the effect of the 8‐week supervised exercise intervention (exercise) compared with no intervention (controls) in people with post‐COVID‐19 condition
| Variable | Control | Exercise | Group effect | |||
|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | p | ηp 2 | |
| Number of symptoms (n) | ||||||
| Number of symptoms | 8.7 ± 4.4 | 4.9 ± 3.5 | 7.9 ± 3.6 | 4.0 ± 3.0 | 0.443 | 0.01 |
| Pulmonary function | ||||||
| FVC (L) | 3.6 ± 1.0 | 3.6 ± 1.1 | 3.9 ± 1.1 | 3.8 ± 1.1 | 0.141 | 0.71 |
| %FVC | 94.1 ± 13.8 | 93.6 ± 14.0 | 97.2 ± 13.7 | 98.3 ± 10.4 | 0.186 | 0.06 |
| FEV‐1 (L) | 3.1 ± 0.9 | 3.0 ± 1.0 | 3.3 ± 1.0 | 3.3 ± 1.0 | 0.574 | 0.01 |
| %FEV‐1 | 102.4 ± 17.2 | 103.3 ± 18.9 | 110.7 ± 13.3 | 108.5 ± 16.8 | 0.100 | 0.09 |
| FEV‐1/FVC | 83.6 ± 5.3 | 84.6 ± 6.0 | 87.3 ± 3.1 | 85.4 ± 3.6 | 0.093 | 0.09 |
| FEV25‐75%(L·s−1) | 3.5 ± 1.4 | 3.5 ± 1.3 | 4.2 ± 1.2 | 4.1 ± 1.3 | 0.605 | 0.10 |
| MVV (L) | 97.5 ± 44.0 | 111.1 ± 45.6 | 101.8 ± 38.1 | 120.3 ± 39.4 | 0.945 | <0.01 |
| %MVV | 82.7 ± 23.0 | 90.7 ± 21.4 | 83.9 ± 16.2 | 102.1 ± 15.3 | 0.115 | 0.09 |
| Body composition | ||||||
| Body mass (kg) | 72.0 ± 12.9 | 72.4 ± 12.9 | 72.7 ± 13.6 | 73.7 ± 13.7 | 0.287 | 0.04 |
| Fat mass (%) | 30.4 ± 8.9 | 29.8 ± 8.7 | 31.1 ± 7.8 | 30.1 ± 8.1 | 0.605 | 0.01 |
| Lean body mass (%) | 49.5 ± 10.5 | 49.7 ± 11.7 | 50.3 ± 11.9 | 51.5 ± 11.7 | 0.251 | 0.04 |
| Quality of life and fatigue | ||||||
| SF‐12 (PA) | 37.2 ± 11.0 | 41.2 ± 11.2 | 35.7 ± 11.6 | 47.8 ± 10.6 | 0.024 a | 0.170 |
| SF‐12 (MH) | 39.6 ± 11.5 | 43.5 ± 10.9 | 46.1 ± 12.2 | 49.3 ± 9.7 | 0.444 | 0.02 |
| mMRC | 1.5 ± 1.0 | 0.94 ± 0.93 | 1.3 ± 1.1 | 0.42 ± 0.77 | 0.090 | 0.10 |
| CFQ‐11 (bimodal) | 8.1 ± 2.9 | 6.9 ± 3.8 | 8.1 ± 2.8 | 3.5 ± 3.7 | 0.007 a | 0.23 |
| CFQ‐11 (Likert) | 21.0 ± 7.2 | 18.2 ± 7.3 | 22.8 ± 6.0 | 11.4 ± 8.6 | 0.018 a | 0.15 |
| FSS | 5.2 ± 1.4 | 4.7 ± 1.5 | 5.0 ± 1.4 | 3.4 ± 1.7 | 0.024 a | 0.17 |
| DSQ‐14 | 54.9 ± 20.4 | 44.8 ± 19.6 | 53.1 ± 16.7 | 33.6 ± 13.2 | 0.094 | 0.10 |
| PCSF | 2.5 ± 1.0 | 1.8 ± 1.1 | 2.6 ± 1.1 | 1.1 ± 1.2 | 0.033 a | 0.15 |
| Anxiety and depression | ||||||
| GAD‐7 | 10.2 ± 5.3 | 7.3 ± 4.7 | 7.3 ± 4.2 | 4.7 ± 3.8 | 0.556 | 0.01 |
| PHQ‐9 | 12.7 ± 6.4 | 8.4 ± 4.9 | 10.7 ± 4.9 | 5.0 ± 4.0 | 0.021 a | 0.18 |
| Cardiovascular fitness | ||||||
| VO2max (ml/kg/min) | 36.4 ± 10.1 | 36.1 ± 9.5 | 36.8 ± 10.2 | 38.9 ± 10.8 | 0.035 a | 0.14 |
| Final RPE 6–20 | 15.4 ± 1.0 | 14.7 ± 1.5 | 14.7 ± 1.5 | 11.8 ± 2.5 | 0.003 a | 0.27 |
| Final HR (b·m−1) | 142 ± 17 | 140.3 ± 19.1 | 146 ± 13 | 136.0 ± 12.8 | 0.045 a | 0.13 |
| Muscular strength | ||||||
| Sit‐to‐stand (s) | 8.3 ± 3.5 | 6.6 ± 1.5 | 6.6 ± 2.5 | 5.1 ± 1.2 | 0.009 a | 0.21 |
| Handgrip (kg) | 34.5 ± 9.9 | 34.5 ± 9.9 | 35.7 ± 9.7 | 36.0 ± 9.8 | 0.123 | 0.08 |
| BP‐50% 1RM (m·s−1) | 0.91 ± 0.08 | 0.90 ± 0.10 | 0.94 ± 0.05 | 1.00 ± 0.09 | 0.012 a | 0.20 |
| HSQ‐50% 1RM (m·s−1) | 0.70 ± 0.06 | 0.74 ± 0.09 | 0.71 ± 0.05 | 0.83 ± 0.10 | 0.032 a | 0.17 |
| Leg extension (N) | 401 ± 151 | 421.6 ± 153.6 | 472 ± 183 | 485.3 ± 173.2 | 0.706 | 0.01 |
Note: Data are frequencies and percentages, n (%), or means and standard deviation, M ± SD.
Abbreviations: η p 2 , partial eta squared effect size; FVC, forced ventilatory capacity; FEV, forced expiratory volume; MVV, maximum voluntary ventilation; SF‐12, Short Form Survey; PA, physical activity; MH, mental health; mMRC, Modified Medical Research Council Dyspnea scale; CFQ‐11, Chalder Fatigue Questionnaire; FSS, Fatigue Severity Scale; DSQ‐14, The DePaul Symptom Questionnaire Short Form; PCFS, Post‐COVID‐19 Functional Status scale; GAD‐7, Generalized Anxiety Disorder scale; PHQ‐9, Patient Health Questionnaire; RPE, rate of perceived exertion; HR, heart rate; BP, bench press; HSQ, half squat.
Significant group effect at post‐test (ANCOVA p < 0.05 adjusted for age, sex, duration of symptoms, body mass index [BMI], and baseline scores).
Overall, the exercise group described improvements in all the physical variables examined. Figure 1 shows the comparison of physical condition (cardiovascular fitness and strength) within and between groups after 8 weeks of follow‐up. STS test and HSQ 50% 1RM improved significantly over time, both in the control and in the exercise group, in addition to estimated VO2max and BP 50% 1RM, which also did so in the exercise group. Significant differences (p ≤ 0.005) were found in the four designated variables when compared by intervention allocation. No differences were found in HG and leg extension (dominant) within groups by time or intervention. On the other hand, patient‐reported outcomes (PROs) on fatigue (CFQ‐11 bimodal and Likert and FSS) and quality of life (SF‐12, both dimensions) scales did not show any improvement over time in the control group, even though, partial amelioration in perception of dyspnea (mMRC) and functionality (PCFS) were detected (p = 0.02 and p = 0.009, respectively) after 8 weeks.
FIGURE 1.
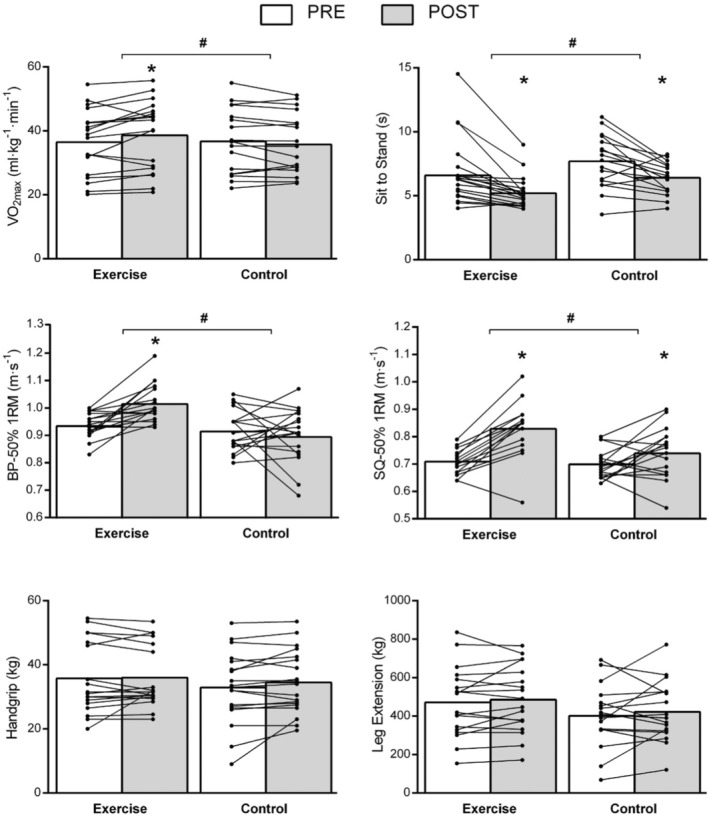
Intra‐ and intergroup effects of the 8‐week supervised exercise intervention (exercise) compared with no intervention (controls) in people with post‐COVID‐19 condition in physical fitness markers
When adjusted for age, sex, duration of symptoms, BMI, and baseline scores in ANCOVA models (Table 2), we identified significant changes in the exercise group at post‐test compared to controls in health markers for quality of life and fatigue (SF‐12, bimodal and Likert CFQ‐11, FSS, and PCSF), depression symptomatology (PHQ‐9), cardiovascular fitness (VO2max, final RPE, and HR) and muscular strength (5 reps sit‐to‐stand test [STS], BP, and HSQ 50% 1RM).
Both exercise and control groups experienced a similar reduction in the total number of symptoms after the 8‐week period. However, some symptoms disappeared in a more pronounced way after the exercise intervention, especially in reported dyspnea (controls vs. exercise: 83.3% vs. 5.4%, p = 0.003; V = 0.48) and fatigue (61.1% vs. 34.6%, p = 0.072; V = 0.30). People from the exercise group reported a better progressive improvement of symptoms after the intervention (94.7% vs. 72.2%, p = 0.063; V = 0.31) and were more likely to become asymptomatic (42.1% vs. 16.7%, p = 0.091; V = 0.28) than controls. PROs also revealed a significant change in the intervention group: SF‐12 (physical activity domain): 41.5% versus 6.5%, p = 0.003; bimodal CFQ‐11: −58.0% versus −16.5%, p = 0.01; Likert CFQ‐11: −50.0% versus −13.3, p = 0.008; FSS: −31.2% versus −1.1%, p = 0.02; and PCSF: −64.3% versus −29.1%, p = 0.007. In cardiovascular parameters, a loss in the main determinant of fitness was observed in the control group (VO2max, 5.7% vs. −0.8%, p = 0.01) and in final HR (−50.0% vs. −13.3%, p = 0.01). Meanwhile, the strength of the lower limbs was recovered in a similar way in both groups when measuring STS test (−22.7% vs. −20.7%) and leg extension (−5.0% vs. −2.8%), regardless of the assigned group, but more efficiently on HSQ in the exercise intervention (17.1% vs. 7.0%, p = 0.02).
5. DISCUSSION
The main finding of the present study indicates that 8 weeks of a supervised, tailored exercise program based on multicomponent exercise training significantly improve health markers for the quality of life and fatigue (SF‐12, bimodal CFQ‐11, FSS, and PCSF), depression perceived symptoms (PHQ‐9), cardiovascular fitness (VO2max, final RPE, and HR) and muscular strength (sit‐to‐stand, BP, and HSQ), more so than self‐management rehabilitation recommendations.
This intervention cohort is characterized by patients who suffered from mild COVID‐19, not requiring hospitalization, which represents the most frequent form of presentation of the disease. These patients present substantial limitations caused by persistent symptoms, such as fatigue (82%), breathlessness (59%), and neurocognitive impairment (lack of concentration [56.4%], brain fog [55.5%], memory problems [53.8%], and sleep disturbances [51.3%]), and secondary functional deterioration that prevents the return to their usual state of health and the return to normal work, as others have shown. 36 , 37
Despite fair VO2max values for a non‐athletic population, 38 the intervention group significantly improved their VO2max by a mean of 2.1 ml kg min−1 (5.9%, SD 9.2). Increases >1 ml kg min−1 are usually considered clinically relevant in the population with cardiopulmonary disease and are related to “hard” clinical outcomes, such as mortality, readmissions, or quality of life. 39 The number needed to treat (NNT) to improve VO2max by this minimal clinical important difference of 1 ml kg min−1 was 1.82, meaning that physical exercise is highly effective in these patients to increase VO2max. The association with the decrease in the final recordings of heart rate (HR) and perception of exertion (RPE), also significant, corroborate this finding. We additionally show how there is an improvement in the parameters of functional estimation of strength by performing the 5‐time STS test and the speed of load execution on BP 50% 1RM (m·s‐1) and HSQ 50% 1RM (m·s‐1).
Given the recent findings in other studies that indicate that peripheral limitation in O2 extraction is the main determinant of VO2peak along with lower VO2 at anaerobic threshold and greater ventilatory inefficiency in patients with long COVID‐19, 40 , 41 the significant improvement in VO2max in our population is possibly the main mechanism that explains symptomatic improvement in the number of patients who reported dyspnea (controls vs. exercise: 83.3% vs. 5.4%, p = 0.003; V = 0.48) and fatigue (61.1% vs. 34.6%, p = 0.072; V = 0.30) at the end of study. This phenomenon is also observed when evaluating the differences in the scores that quantify quality of life (SF‐12 [physical activity domain] 41.5% vs. 6.5%, p = 0.003); fatigue intensity (bimodal CFQ‐11 score, −58% vs. –16.5%, p = 0.01, and FSS mean score, −31.2% vs. −1.1%, p = 0.02), Likert CFQ‐11 score (−48.2% vs. −11.7%, p = 0.02), and functional status (PCSF, −64.3% vs. −29.1%, p = 0.007).
A certain degree of spontaneous improvement can be expected over time in a number of symptoms or some functional tests. 42 In our control group, the STS test and the HSQ 50% 1RM (Figure 1) along with the mMRC, PCFS, PHQ‐9, and GAD‐7 scales had significantly improved over the 8‐week period. This may be due to time or non‐specific interventions such as rehabilitation recommendations and, perhaps, to the recovery of leisure time, to less restrictive policies around COVID‐19, or to the recovery of work activity. This change, however, is not expected in cardiovascular fitness (VO2max) or its surrogate parameters (RPE or HR), nor in dynamic limb strength (HSQ and BP 50%1RM), if there are no active interventions, including physical exercise, that allow better physical recovery. However, it is clear that “one‐fits all” recommendations do not seem to be sufficiently effective to guarantee recovery. In fact, although there were no differences between vigorous or total minutes of PA declared by the participants between groups (46 min/week vs. 88 min/week, p = 0.35 and 294 min/week vs. 506 min/week, p = 0.225, respectively) at the beginning of the study, the participants in the control group, despite the recommendations, did not significantly increase their PA after follow‐up.
Patients undergoing exercise training exhibited better results in depression symptoms, with a remarkable effect size (p = 0.021, ηp 2 = 0.18). On the other hand, no detectable changes in anxiety symptoms (GAD‐7, p = 0.556, ηp 2 = 0.01) and general mental well‐being (SF‐12 [MH], p = 0.444, ηp 2 = 0.02) have been found. Recent data from a meta‐analysis 43 indicate that exercise is a viable treatment option for the treatment of anxiety, however, high‐intensity training was found to be more effective than low‐intensity regimen. It is therefore possible that the exercise modality considered in this study is not the best for patients with a post‐COVID‐19 condition who show signs of anxiety.
A recent review of the literature of the effectiveness of multidisciplinary pulmonary rehabilitation (PR) for post‐COVID symptoms 44 includes nine studies, most of them small, quasi‐experimental, and of low quality. Hospitalized patients (many had required intensive care) with a maximum duration of symptoms of 125 days (4.5 months) were analyzed. All these studies reported improvements in exercise capacity, in lung function measured by spirometry (we did not find this change in pulmonary volumes; Table 2), and/or improvements in the quality of life. To our knowledge, the RECOVE study is the first randomized clinical trial to show similar results with concurrent training in non‐hospitalized patients with a mean evolutionary symptom for more than 6 months. Nopp and colleges 45 in a prospective observational cohort study on 58 patients, 4.4 months after testing positive for SARS‐CoV‐2, observed significant improvements following Austrian PR guidelines in exercise capacity (6MWT), functional status (PCFS), dyspnea, fatigue (FAS), and quality of life. Since PR was hospital based, it presents a clear practical limitation when it comes to its implementation in large populations. However, a structured and secure program such as the one we propose, supervised by certified strength and conditioning coaches, easily implemented in other facilities outside the hospital setting, would cover a significantly higher number of patients.
5.1. Safety considerations
As patients with a post‐COVID condition may share some of the symptoms that occur in patients experiencing myalgic encephalomyelitis/chronic fatigue syndrome, management of post‐exertional malaise and individualization should be one of the main goals of exercise programs in this population. Thanks to the fact that all of them were treated individually, adjusting the intensity of intra‐session training (always completing the pre‐established volume), no patient dropped out due to tolerance issues, though it is a highly demanding population due to the large symptom density.
An appropriate medical screening should be guided by the patient history, physical examination, clinical findings, and results of previous test. A basic laboratory test (e.g., complete blood count, basic metabolic panel, cardiac markers, C‐reactive protein included) and considerations of ECG, echocardiogram, chest imaging (X‐ray and/or CT), and/or pulmonary function tests are recommended when patients have impediments on returning to amateur exercise practice. However, in patients with post‐COVID‐19 conditions after mild forms of the disease, it is common to find a decreased exercise capacity even in the presence of a completely normal cardiac workout. 46
5.2. Limitations
As this is a study representing an outpatient population of limited size, the results may not be directly applicable to other cohorts with post‐COVID‐19 conditions or post‐COVID‐19 sequelae, especially when the severity of acute SARS‐CoV2 infection was moderate or severe, requiring admission. Furthermore, participants did not undergo an exercise stress test before infection, so changes from their normal baseline values are difficult to assess. The Ekblom–Bak test could fail to estimate VO2max in subjects with extreme values of physical condition or with HR limitations (such as chronotropic incompetence, CI). For these reasons, participants with extreme VO2max values (<19 or >62 ml min kg−1 for women and <24 or >76 ml min kg−1 for men) and those with suspected CI were excluded from the selection process.
A relevant number of participants were receiving treatment for mood disorders and significant reductions in physical activity have been found in the population before limiting the effectiveness of the results in exercise projects. 47 It is possible that cognitive behavior therapy, with or without psychiatric intervention, may improve the response to exercise and vice versa. The results of this exercise program cannot be extrapolated to other combinations or training strategies (e.g., inspiratory muscle training or high‐intensity interval training [HIIT]), which must be studied and could result in different health outcomes.
5.3. Perspective
The beneficial physiological adaptations to cardiopulmonary and skeletal muscle associated to a tailored concurrent training may be an effective, safe, and well‐tolerated intervention in post‐COVID‐19 conditions. Improvements in the quality of life, mood disorder symptoms, and cardiovascular and strength fitness suggest that exercise could have a main roll in recovering active life when suffering long‐term disability because of post‐COVID‐19 condition. There is an urgent need to explore other exercise‐based treatment strategies that could, together with neurocognitive and behavioral strategies, provide greater benefits for these patients.
FUNDING INFORMATION
The authors thank the volunteers for their dedication to the training. This work was partially funded by the Spanish Ministry of Science and Innovation (grant no. PID2019‐108202RA‐I00) and by the Centro Médico Virgen de la Caridad (agreement no. 35110).
Jimeno‐Almazán A, Franco‐López F, Buendía‐Romero Á, et al. Rehabilitation for post‐COVID‐19 condition through a supervised exercise intervention: A randomized controlled trial. Scand J Med Sci Sports. 2022;00:1‐11. doi: 10.1111/sms.14240
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are openly available in [RECOVE dataset] at [http://www.hpsportsscience.com/recove/]. We do not have reference number.
REFERENCES
- 1. A clinical case definition of post COVID‐19 condition by a Delphi consensus, 6 October 2021. https://www.who.int/publications/i/item/WHO‐2019‐nCoV‐Post_COVID‐19_condition‐Clinical_case_definition‐2021.1. Accessed March 19, 2022. [DOI] [PMC free article] [PubMed]
- 2. Fernández‐de‐las‐Peñas C, Palacios‐Ceña D, Gómez‐Mayordomo V, et al. Prevalence of post‐COVID‐19 symptoms in hospitalized and non‐hospitalized COVID‐19 survivors: A systematic review and meta‐analysis. Eur J Intern Med. 2021;92:55‐70. doi: 10.1016/J.EJIM.2021.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Han Q, Zheng B, Daines L, Sheikh A. Long‐term sequelae of COVID‐19: A systematic review and meta‐analysis of one‐year follow‐up studies on post‐COVID symptoms. Pathogens. 2022;11(2):269. doi: 10.3390/PATHOGENS11020269/S1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fernández‐de‐las‐Peñas C, Palacios‐Ceña D, Gómez‐Mayordomo V, et al. Prevalence of post‐COVID‐19 symptoms in hospitalized and non‐hospitalized COVID‐19 survivors: A systematic review and meta‐analysis. Eur J Intern Med. 2021;92:55‐70. doi: 10.1016/J.EJIM.2021.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Augustin M, Schommers P, Stecher M, et al. Post‐COVID syndrome in non‐hospitalised patients with COVID‐19: a longitudinal prospective cohort study. Lancet Reg Heal Eur. 2021;6:100122. doi: 10.1016/J.LANEPE.2021.100122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Prevalence of ongoing symptoms following coronavirus (COVID‐19) infection in the UK ‐ Office for National Statistics. https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/bulletins/prevalenceofongoingsymptomsfollowingcoronaviruscovid19infectionintheuk/6may2022. Accessed June 12, 2022.
- 7. Kuodi P, Gorelik Y, Zayyad H, et al. Association between vaccination status and reported incidence of post‐acute COVID‐19 symptoms in Israel: a cross‐sectional study of patients tested between March 2020 and November 2021. medRxiv. January 2022:2022.01.05.22268800. doi: 10.1101/2022.01.05.22268800 [DOI]
- 8. Jimeno‐Almazán A, Martínez‐Cava A, Buendía‐Romero Á, et al. Relationship between the severity of persistent symptoms, physical fitness, and cardiopulmonary function in post‐COVID‐19 condition. A population‐based analysis. Intern Emerg Med. 2022. doi: 10.1007/s11739-022-03039-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Utrero‐Rico A, Ruiz‐Ruigómez M, Laguna‐Goya R, et al. A short corticosteroid course reduces symptoms and immunological alterations underlying long‐COVID. Biomedica. 2021;9(11):1540. doi: 10.3390/BIOMEDICINES9111540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Management of Post‐COVID Conditions . Evaluating and caring for patients with post‐COVID conditions: interim guidance. https://www.cdc.gov/coronavirus/2019‐ncov/hcp/clinical‐care/post‐covid‐management.html#print. Accessed March 19, 2022.
- 11. Daynes E, Gerlis C, Chaplin E, Gardiner N, Singh SJ. Early experiences of rehabilitation for individuals post‐COVID to improve fatigue, breathlessness exercise capacity and cognition ‐ A cohort study. Chron Respir Dis. 2021;18:147997312110156. doi: 10.1177/14799731211015691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Raman B, Cassar MP, Tunnicliffe EM, et al. Medium‐term effects of SARS‐CoV‐2 infection on multiple vital organs, exercise capacity, cognition, quality of life and mental health, post‐hospital discharge. EClinicalMedicine. 2021;31:100683. doi: 10.1016/J.ECLINM.2020.100683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. National Institute of Health (NIH) . COVID‐19 Treatment Guidelines. Clinical spectrum of SARS‐CoV2 infection. https://www.covid19treatmentguidelines.nih.gov/overview/clinical‐spectrum/. Accessed June 13, 2022.
- 14. Riebe D, Ehrman JK, Liguori G, Magal M. ACSM's Guidelines for Exercise Testing and Prescription. 10th ed. American College of Sports Medicine; 2018. [Google Scholar]
- 15. Hernández‐Belmonte A, Martínez‐Cava A, Morán‐Navarro R, Courel‐Ibáñez J, Pallarés JG. A comprehensive analysis of the velocity‐based method in the shoulder press exercise: stability of the load‐velocity relationship and sticking region parameters. Biol Sport. 2021;38(2):235‐243. doi: 10.5114/BIOLSPORT.2020.98453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Scherr J, Wolfarth B, Christle JW, Pressler A, Wagenpfeil S, Halle M. Associations between Borg's rating of perceived exertion and physiological measures of exercise intensity. Eur J Appl Physiol. 2013;113(1):147‐155. doi: 10.1007/S00421-012-2421-X/TABLES/2 [DOI] [PubMed] [Google Scholar]
- 17. Support for rehabilitation: self‐management after COVID‐19‐related illness, 2nd ed. December 2021. https://www.euro.who.int/en/health‐topics/Life‐stages/disability‐and‐rehabilitation/publications/support‐for‐rehabilitation‐self‐management‐after‐covid‐19‐related‐illness,‐2nd‐ed. Accessed April 10, 2022.
- 18. Gandek B, Ware JE, Aaronson NK, et al. Cross‐validation of item selection and scoring for the SF‐12 Health Survey in nine countries: Results from the IQOLA Project. J Clin Epidemiol. 1998;51(11):1171‐1178. doi: 10.1016/S0895-4356(98)00109-7 [DOI] [PubMed] [Google Scholar]
- 19. Spitzer RL, Kroenke K, Williams JBW, Löwe B. A brief measure for assessing generalized anxiety disorder: The GAD‐7. Arch Intern Med. 2006;166(10):1092‐1097. doi: 10.1001/archinte.166.10.1092 [DOI] [PubMed] [Google Scholar]
- 20. Kroenke K, Spitzer RL, Williams JBW. The PHQ‐9: Validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606‐613. doi: 10.1046/j.1525-1497.2001.016009606.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mahler DA, Wells CK. Evaluation of clinical methods for rating dyspnea. Chest. 1988;93(3):580‐586. doi: 10.1378/chest.93.3.580 [DOI] [PubMed] [Google Scholar]
- 22. Jackson C. The Chalder Fatigue Scale (CFQ 11). Occup Med (Chic Ill). 2015;65(1):86. doi: 10.1093/occmed/kqu168 [DOI] [PubMed] [Google Scholar]
- 23. Krupp LB, Larocca NG, Muir Nash J, Steinberg AD. The fatigue severity scale: Application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol. 1989;46(10):1121‐1123. doi: 10.1001/archneur.1989.00520460115022 [DOI] [PubMed] [Google Scholar]
- 24. Cella M, Chalder T. Measuring fatigue in clinical and community settings. J Psychosom Res. 2010;69(1):17‐22. doi: 10.1016/J.JPSYCHORES.2009.10.007 [DOI] [PubMed] [Google Scholar]
- 25. Sunnquist M, Lazarus S, Jason LA. The development of a short form of the DePaul Symptom Questionnaire. Rehabil Psychol. 2019;64(4):453‐462. doi: 10.1037/REP0000285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Klok FA, Boon GJAM, Barco S, et al. The post‐COVID‐19 functional status scale: A tool to measure functional status over time after COVID‐19. Eur Respir J. 2020;56(1):2001494. doi: 10.1183/13993003.01494-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ekblom‐Bak E, Björkman F, Hellenius ML, Ekblom B. A new submaximal cycle ergometer test for prediction of VO2max. Scand J Med Sci Sports. 2014;24(2):319‐326. doi: 10.1111/SMS.12014 [DOI] [PubMed] [Google Scholar]
- 28. Björkman F, Ekblom‐Bak E, Ekblom Ö, Ekblom B. Validity of the revised Ekblom Bak cycle ergometer test in adults. Eur J Appl Physiol. 2016;116(9):1627‐1638. doi: 10.1007/S00421-016-3412-0/FIGURES/2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Courel‐Ibáñez J, Buendía‐Romero Á, Pallarés JG, García‐Conesa S, Martínez‐Cava A, Izquierdo M. Impact of tailored multicomponent exercise for prevent weakness and falls on nursing home residents' functional capacity. J Am Med Dir Assoc. 2021;23:98‐104. doi: 10.1016/j.jamda.2021.05.037 e3. [DOI] [PubMed] [Google Scholar]
- 30. Buendía‐Romero Á, Hernández‐Belmonte A, Martínez‐Cava A, et al. Isometric knee extension test: A practical, repeatable, and suitable tool for lower‐limb screening among institutionalized older adults. Exp Gerontol. 2021;155:111575. doi: 10.1016/j.exger.2021.111575 [DOI] [PubMed] [Google Scholar]
- 31. Courel‐Ibáñez J, Martínez‐Cava A, Morán‐Navarro R, et al. Reproducibility and repeatability of five different technologies for bar velocity measurement in resistance training. Ann Biomed Eng. 2019;47(7):1523‐1538. doi: 10.1007/s10439-019-02265-6 [DOI] [PubMed] [Google Scholar]
- 32. Martínez‐Cava A, Morán‐Navarro R, Hernández‐Belmonte A, et al. Range of motion and sticking region effects on the bench press load‐velocity relationship. J Sports Sci Med. 2019;18(4):645‐652. [PMC free article] [PubMed] [Google Scholar]
- 33. Noncommunicable Disease Surveillance, Monitoring and Reporting . Physical activity surveillance.Global physical activity questionnaire (GPAQ). https://www.who.int/teams/noncommunicable‐diseases/surveillance/systems‐tools/physical‐activity‐surveillance. Accessed June 21, 2022.
- 34. Mitchell C, Rahko PS, Blauwet LA, et al. Guidelines for performing a comprehensive transthoracic echocardiographic examination in adults: recommendations from the american society of echocardiography. J Am Soc Echocardiogr. 2019;32(1):1‐64. doi: 10.1016/j.echo.2018.06.004 [DOI] [PubMed] [Google Scholar]
- 35. Graham BL, Steenbruggen I, Barjaktarevic IZ, et al. Standardization of spirometry 2019 update an official American Thoracic Society and European Respiratory Society technical statement. Am J Respir Crit Care Med. 2019;200(8):E70‐E88. doi: 10.1164/rccm.201908-1590ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tabacof L, Tosto‐Mancuso J, Wood J, et al. Post‐acute COVID‐19 syndrome negatively impacts physical function, cognitive function, health‐related quality of life, and participation. Am J Phys Med Rehabil. 2022;101(1):48‐52. doi: 10.1097/PHM.0000000000001910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tenforde MW, Kim SS, Lindsell CJ, et al. Symptom duration and risk factors for delayed return to usual health among outpatients with COVID‐19 in a multistate health care systems network — United States, March–June 2020. MMWR Morb Mortal Wkly Rep. 2022;69(30):993‐998. doi: 10.15585/MMWR.MM6930E1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kaminsky LA, Arena R, Myers J, et al. Updated reference standards for cardiorespiratory fitness measured with cardiopulmonary exercise testing: data from the fitness registry and the importance of exercise national database (FRIEND). Mayo Clin Proc. 2022;97(2):285‐293. doi: 10.1016/J.MAYOCP.2021.08.020 [DOI] [PubMed] [Google Scholar]
- 39. Taylor JL, Bonikowske AR, Olson TP. Optimizing outcomes in cardiac rehabilitation: the importance of exercise intensity. Front Cardiovasc Med. 2021;8:734278. doi: 10.3389/FCVM.2021.734278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Singh I, Joseph P, Heerdt PM, et al. Persistent exertional intolerance after COVID‐19: insights from invasive cardiopulmonary exercise testing. Chest. 2022;161(1):54‐63. doi: 10.1016/j.chest.2021.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Baratto C, Caravita S, Faini A, et al. Impact of COVID‐19 on exercise pathophysiology: A combined cardiopulmonary and echocardiographic exercise study. J Appl Physiol. 2021;130(5):1470‐1478. doi: 10.1152/japplphysiol.00710.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Xiong L, Li Q, Cao X, et al. Dynamic changes of functional fitness, antibodies to SARS‐CoV‐2 and immunological indicators within 1 year after discharge in Chinese health care workers with severe COVID‐19: a cohort study. BMC Med. 2021;19(1):1‐11. doi: 10.1186/s12916-021-02042-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Aylett E, Small N, Bower P. Exercise in the treatment of clinical anxiety in general practice – a systematic review and meta‐analysis. BMC Health Serv Res. 2018;18(1):559. doi: 10.1186/S12913-018-3313-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Soril LJJ, Damant RW, Lam GY, et al. The effectiveness of pulmonary rehabilitation for Post‐COVID symptoms: A rapid review of the literature. Respir Med. 2022;195:106782. doi: 10.1016/J.RMED.2022.106782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Nopp S, Moik F, Klok FA, et al. Outpatient pulmonary rehabilitation in patients with long COVID improves exercise capacity, functional status, dyspnea, fatigue, and quality of life. Respiration. 2022;101:1‐9. doi: 10.1159/000522118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hughes DC, Orchard JW, Partridge EM, La GA, Broderick C. Return to exercise post‐COVID‐19 infection: a pragmatic approach in Mid‐2022. J Sci Med Sport. 2022;25(7):544‐547. doi: 10.1016/J.JSAMS.2022.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zschucke E, Gaudlitz K, Ströhle A. Exercise and physical activity in mental disorders: clinical and experimental evidence. J Prev Med Public Health. 2013;46(Suppl 1):S12‐S21. doi: 10.3961/JPMPH.2013.46.S.S12 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are openly available in [RECOVE dataset] at [http://www.hpsportsscience.com/recove/]. We do not have reference number.


