Abstract
Background and purpose
Dizziness and vertigo are common symptoms after COVID‐19‐vaccination. We aimed to prospectively evaluate objective central or peripheral vestibular function in patients with dizziness, vertigo, and postural symptoms that started or worsened after COVID‐19‐vaccination.
Methods
Of 4137 patients who presented between January 2021 and April 2022 at the German Center for Vertigo and Balance Disorders, Ludwig Maximilian University of Munich, we identified 72 patients (mean age = 47 years) with enduring vestibular symptoms following COVID‐19 vaccination. All underwent medical history‐taking, and neurological and neuro‐otological workup with bithermal caloric test, video head‐impulse test, orthoptics, and audiometry. Diagnoses were based on international criteria. The distribution of diagnoses was compared to a cohort of 39,964 patients seen before the COVID‐19 pandemic.
Results
Symptom onset was within the first 4 weeks postvaccination. The most prevalent diagnoses were somatoform vestibular disorders (34.7%), vestibular migraine (19.4%), and overlap syndromes of both (18.1%). These disorders were significantly overrepresented compared to the prepandemic control cohort. Thirty‐six percent of patients with somatoform complaints reported a positive history of depressive or anxiety disorders. Nine patients presented with benign paroxysmal positional vertigo, three with acute unilateral vestibulopathy, and seven with different entities (vestibular paroxysmia, Ménière disease, polyneuropathy, ocular muscular paresis). Causally related central vestibular deficits were lacking. Novel peripheral vestibular deficits were found in four patients.
Conclusions
Newly induced persistent vestibular deficits following COVID‐19 vaccination were rare. The predominant causes of prolonged vestibular complaints were somatoform vestibular disorders and vestibular migraine, possibly triggered or aggravated by stress‐related circumstances due to the COVID‐19 pandemic or vaccination. An increase of other central or peripheral vestibular syndromes after COVID‐19 vaccination was not observed.
Keywords: COVID‐19, vaccination, vertigo, vestibular syndrome, vestibulopathy
INTRODUCTION
Dizziness, vertigo, and postural symptoms are common reasons for consulting a doctor, with a high lifetime prevalence of 17%–30% [1]. Dizziness is defined as disturbed spatial orientation without a false or distorted sense of motion and vertigo as a sensation of self‐motion without actual self‐motion [2]. They are thought to be generated by an acute sensorimotor conflict (mismatch) between the converging sensory inputs (vestibular, visual, somatosensory) and the expected sensory patterns or by an acute vestibular tone imbalance. Postural symptoms are balance symptoms related to maintenance of postural stability, occurring only while the subject is upright (seated, standing, or walking) [2]. Anatomically, vestibular syndromes can be caused by disturbances along the entire afferent peripheral and central vestibular pathways, starting from the vestibular end organ in the inner ear and the vestibular nerve. The central vestibular system further runs via the vestibular nuclei in the medullary brainstem, and brainstem–cerebellar circuits to cortical multisensory vestibular network areas in the temporoparietal cortex [3, 4]. Detailed history‐taking combined with clinical and instrument‐based (neuro‐ophthalmological and neuro‐otological) examination allows topographical assignment to specific vestibular structures and syndromes. Peripheral vestibular disorders are, for example, benign paroxysmal positional vertigo (BPPV), Ménière disease (MD), acute unilateral vestibulopathy (UVP), and bilateral vestibulopathy (BVP). Typical central vestibular disorders are, for example, vestibular migraine (VM) and brainstem–cerebellar syndromes induced by infarction (eg, Wallenberg syndrome) or degenerative disorders (eg, downbeat nystagmus syndrome). Furthermore, somatoform vestibular disorders, which are currently covered by the umbrella terms “persistent postural–perceptual dizziness” (PPPD) and “functional dizziness,” are frequent [5]. For most of the vestibular syndromes, clear diagnostic criteria according to international guidelines or consensus documents, for example, from the International Bárány Society and the International Headache Society, are available (Table S1).
In Germany, COVID‐19 vaccination began at the end of December 2020 with messenger RNA vaccines (Pfizer/BioNTech's Comirnaty® and later Moderna's Spikevax®) as well as vector‐based vaccines (AstraZeneca's Vaxzevria® and Johnson & Johnson's COVID‐19 Vaccine Janssen®). Vaccination side effects in Germany are collected and published by the Paul Ehrlich Institute (PEI), the Federal Agency for Vaccines and Biomedicines acting as a medical regulatory body and research institution. In line with international statistics, the PEI reports an overall low rate of serious adverse reactions and life‐threatening unsolicited events [6].
Most often, the adverse events of COVID‐19 vaccination fall within general reactions such as fever, injection site pain, or asthenia usually subsiding after a few hours to days. An international meta‐analysis on nervous and muscular adverse events revealed that headache and myalgia account for the great majority (approximately 98%) of symptoms [7]. Other cerebrovascular or neuroimmunological disorders are very rare [8].
The data basis for the occurrence of vestibular symptoms is inconsistent. The above‐cited international meta‐analysis reported dizziness, drowsiness, and hypesthesia in approximately 2% [7]. In a British/American cross‐sectional vaccination study among health care workers, self‐reported “vertigo‐like symptoms” were found in 2.49% and “dizziness” in 8.34% of the participants [9, 10]. An Italian cross‐sectional study on 314,664 vaccinated subjects found “dizziness” in as many as 21% [11]. In Germany, the second most common vaccination side effect after headache was “Schwindelgefühl,” which is an unspecific umbrella term used for dizziness, vertigo, drowsiness, or balance disorders (PEI) [6]. It was self‐reported in approximately 0.1 of 1000 first or second doses of Comirnaty®, and approximately 0.2 of 1000 first or second doses of Spikevax®.
There are only case reports and one observational case series by Wichova et al. that focused on otological manifestation, for example, acute hearing loss and tinnitus after vaccination [12]. In the latter, no definite correlation between otological symptoms and COVID‐19 vaccination could be determined in 30 patients with otological symptoms, eight of whom also presented with dizziness and five with vertigo. To the best of our knowledge, there are only two case series exploring the source of vestibular side effects in more detail. In the first study [13], of 33 patients with “acute vertigo” after COVID‐19 vaccination, nine showed a “BPPV‐typical nystagmus”; in a further 17 patients, a peripheral vestibular dysfunction could be excluded. However, in the great majority of patients, the specific diagnoses and central nervous system involvement remained unclear due to the limited neuro‐ophthalmological and neurophysiological diagnostic setup. Another recent study described eight patients who had developed a UVP within 30 days after COVID‐19 vaccination [14]. However, it remained unclear whether UVP incidence after COVID‐19 vaccination was higher than in the prepandemic years. Up to now, a dedicated description of the spectrum and frequency of diagnoses based on comprehensive objective neuro‐otological measurements and established diagnostic criteria in patients with postvaccination vestibular symptoms is still lacking.
Thus, the aim of our study in patients with persistent complaints of vertigo, dizziness, and postural imbalance that started in temporal relation to their COVID‐19 vaccination was twofold: (i) to evaluate the central and peripheral vestibular function by detailed neuro‐otological and neuro‐ophthalmological testing and (ii) to determine the specific vestibular syndromes according to the current international diagnostic criteria. We therefore conducted a prospective cohort study at a tertiary referral center for patients with vertigo, dizziness, and balance disorders.
MATERIALS AND METHODS
Patient cohort
We prospectively screened 4137 patients at the interdisciplinary German Center for Vertigo and Balance Disorders (DSGZ) at the Ludwig Maximilian University of Munich Hospital between January 2021 and April 2022 for patients who reported vertigo, dizziness, or disorders of stance and gait following COVID‐19 vaccination as their main complaint. All patients underwent structured history‐taking (including vaccination date, type, temporal relation to symptom onset, direct adverse reactions) as well as a detailed clinical neurological and neuro‐otological examination, including video‐oculography during bithermal water caloric test, video head‐impulse test (vHIT), hearing tests, and a neuro‐orthoptic examination including vision testing, fundus photography, and assessment of perceptual vestibular deficits by measurements of the subjective visual vertical. Depending on the individual cases, cervical or ocular vestibular evoked myogenic potentials (VEMPs), posturography, gait analyses, or nerve conduction studies were also performed, if useful (for details, please see below). All patients were seen by senior specialists in neuro‐otology. Diagnoses were made according to international criteria established in consensus statements of the International Bárány Society for Neuro‐Otology (https://www.jvr‐web.org/ICVD.html) and the International Headache Society (https://www.ichd‐3.org).
Inclusion criteria for the subcohort of interest were as follows: age > 18 years and self‐reported new onset or noteworthy increase of pre‐existing vestibular symptoms in temporal relation to COVID‐19 vaccination. Patients who had had an additional SARS‐CoV‐2 infection were excluded to avoid false assignment.
As a control group, we analyzed the distribution of vestibular diagnosis in a cohort of 39,964 patients seen in the DSGZ before the start of the COVID‐19 pandemic. Furthermore, we additionally screened for patients who presented with vestibular symptoms starting in temporal relation to other vaccinations in the years before the COVID‐19 pandemic (2011–2020) from the DSGZ database and chart analysis.
Vestibular testing
Neuro‐orthoptic assessment included testing for nystagmus with Frenzel goggles (spontaneous and head‐shaking nystagmus), ocular motor examination (smooth pursuit, saccades, optokinetic reflex, etc.), fundus photography using a scanning laser ophthalmoscope (Rodenstock, Munich, Germany), and adjustments of the subjective visual vertical (SVV) to rule out central vestibular deficits and acute vestibular tone imbalance [3]. Visual acuity was assessed using a Snellen chart. The SVV as a sensitive sign of a graviceptive vestibular tone imbalance in the roll plane [15] was assessed with the subject sitting upright in front of a half‐spherical dome with the head fixed on a chin rest. Caloric testing to measure the function of the horizontal semicircular canals in the low‐frequency range of the vestibulo‐ocular reflex was done using 30°C cool and 44°C warm water irrigation measuring peak slow‐phase velocity of caloric nystagmus by video‐oculography (EyeSeeCam; Interacoustics, Middelfart, Denmark). A pathological result was defined as >25% asymmetry between the right‐ and left‐sided responses [16]. Standardized vHIT measurements of the semicircular function in the high‐frequency range were obtained in a bright room with a red target affixed at eye level at a distance of 1.8 m using the EyeSeeCamHIT system (Interacoustics, Middelfart, Denmark), with the procedure as described in Heuberger et al. [17]. A median gain < 0.8 at 60 ms (eye velocity in °/s divided by head velocity in °/s) during head impulses was considered pathological. Posturographic measurements were performed using a stabilometer platform (Kistler 9261A; Kistler Group, Winterthur, Switzerland) in an upright standing position. Displacement of center of gravity was assessed by the total sway path for x, y, and z directions (for x‐ and y‐axes: m/min and for z‐axis: kN/min) for 10 different standing conditions of increasing difficulty. In addition to the regular analysis, sway was analyzed over all conditions by an artificial neuronal network and categorized as normal or with functionally phobic, cerebellar, orthostatic, or vestibular patterns [18]. VEMPs as short‐latency, mainly otolith‐driven vestibular reflexes elicited by air‐conducted sound or bone‐conducted vibration, were recorded in a standardized manner [19]. Standardized routine pure tone audiometry as a fast clinical assessment of hearing acuity was conducted in soundproof cabins at 0.5, 1, 2, 4, 8, and 10 kHz.
Statistical analyses
After data collection, all data were irreversibly anonymized for data analyses and processed using Microsoft Excel (v2021) and SPSS (v27). For data description, we used mean values and SD for continuous variables and absolute and relative frequencies for categorical variables. The observed distribution of diagnoses in the post COVID‐19 vaccination subgroup was statistically compared to expected distribution in the 39,964 patients of the prepandemic control group by Fisher exact test. We qualitatively compared the number of visits to the total number of COVID‐19 vaccinations (derived from official data from the German Ministry of Health at www.impfdashboard.de). To ensure high data quality, the RECORD (Reporting of Studies Conducted Using Observational Routinely Collected Data) guidelines [20] were followed.
The study was approved by the data protection officer and the institutional review board of Ludwig Maximilian University of Munich (standardized database Dizziness Register, no. 414–15), and all patients gave informed consent. The study was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
RESULTS
In total, 72 of 4137 patients (mean age = 47.2 ± 15.7 years, 46 female) fulfilled the inclusion criteria of onset or worsening of vestibular symptoms after COVID‐19 vaccination (Figure 1). The administered vaccines were Comirnaty (67%), Vaxzevria (15%), Vaxzevria plus Comirnaty (5%), Spikevax (11%), and Spikevax plus Comirnaty (2%).
FIGURE 1.
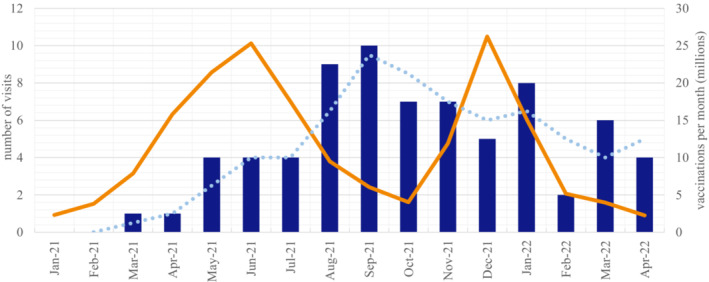
Chart depicting the number of patients presenting at the German Center for Vertigo and Balance Disorders with vestibular symptoms following a COVID‐19 vaccination from January 2021 and April 2022 (blue bars; dotted blue line = trend line). This is compared with the total number of COVID‐19 vaccinations in Germany in the respective time period (orange line). COVID‐19 vaccination data were extracted from the national database of the German Ministry of Health and the vaccination dashboard of the Robert Koch Institute
Vestibular symptom onset occurred in 22 patients <24 h after vaccination, and in 23 patients within 1 week, whereas the remaining 27 patients described an onset within between 2 and 4 weeks. The mean time interval between onset and presentation in our outpatient clinic was 144 ± 83.8 days (minimum = 19, maximum = 366 days). The frequency and time course of patient visits paralleled the total number of COVID‐19 vaccinations in Germany, with a time delay of a mean of 2 months (Figure 1).
The final diagnoses included PPPD/functional dizziness (n = 25, 34.7%), VM (n = 14, 19.4%), and a combination of both (n = 13, 18.1%). BPPV was found in nine patients (12.5%), UVP and MD in three patients each (4.2%), and vestibular paroxysmia (VP) in two patients. Furthermore, there was gait instability due to diabetic polyneuropathy in one patient, oscillopsia due to an eye muscle paresis in another patient, and subjective coordination deficits possibly due to suspected mild cerebellar dysfunction in the remaining patient. A detailed overview of the audiovestibular testing results can be seen in Table 1. Statistical comparison of diagnostic distribution in the COVID‐19 vaccination subgroup to a cohort of patients with vertigo, dizziness, and imbalance (n = 39,964) diagnosed before the pandemic started showed a significantly increased rate for PPPD/functional dizziness, VM, and overlap syndrome in the COVID‐19 vaccination cohort by 2.4‐fold (Fisher exact test, p = 0.03), whereas other peripheral vestibular disorders (BPPV, UVP, MD, VP) were similarly frequent (p = 0.49).
TABLE 1.
Overview of patient characteristics, history, vaccination‐related symptoms, and results of audiovestibular testing in the subgroup of COVID‐19‐related vestibular syndromes (n = 72)
| PPPD/FD | VM | PPPD/FD + VM | BPPV | UVP | MD | VP | Other | |
|---|---|---|---|---|---|---|---|---|
| Patient characteristics | ||||||||
| n (%) | 25 (34.7%) | 14 (19.4%) | 13 (18.1%) | 9 (12.5%) | 3 (4.2%) | 3 (4.2%) | 2 (2.8%) | 3 (4.2%) |
| Female/male, n | 10/15 | 12/2 | 12/1 | 7/2 | 2/1 | 2/1 | 0/2 | 0/3 |
| Age, years | 44.4 ± 13.9 | 42.1 ± 13.4 | 39.1 ± 11.6 | 66.3 ± 14.2 | 45.4 ± 5.9 | 52.7 ± 5.8 | 55.4 ± 5.6 | 63.1 ± 11.4 |
| Patient history, n | ||||||||
| Preceding psychiatric disease | 9 | 1 | 5 | – | 2 | 1 | – | – |
| Headache | 5 | 9 | 12 | 1 | 2 | – | – | – |
| Vaccination‐related symptoms, n | ||||||||
| General reaction | 9 | 1 | 2 | 1 | 3 | 1 | 1 | – |
| Migrainelike symptoms | – | 9 | 6 | 2 | – | – | – | – |
| Onset of vestibular symptoms (postvaccination) | ||||||||
| 0–24 h | 1 | 7 | 7 | 4 | 1 | 1 | 1 | – |
| 1–7 days | 10 | 4 | 2 | 5 | – | – | – | 2 |
| 2–4 weeks | 14 | 3 | 4 | – | 2 | 2 | 1 | 1 |
| Audiovestibular testing, n | ||||||||
| Peripheral vestibular function | ||||||||
| Normal | 23 | 12 | 13 | 6 | – | 1 | 2 | 2 |
| Old unilateral deficit | 2 | 1 | – | 2 | – | 1 | – | 1 |
| Old bilateral deficit | – | 1 | – | 1 | – | – | – | – |
| New unilateral deficit | – | – | – | – | 3 | 1 | – | – |
| New bilateral deficit | – | – | – | – | – | – | – | – |
| Central vestibular function | ||||||||
| Central vestibular/ocular motor signs | – | – | – | 1 | – | – | – | – |
| Acute vestibular tone imbalance | – | – | – | 2 | 1 | – | – | – |
| Posturography | ||||||||
| PPPD/FD sway | 11 | – | 1 | – | – | – | – | – |
| Normal | 12 | 4 | 10 | – | 1 | 1 | 1 | 1 |
| Hearing function | ||||||||
| Normal | 23 | 14 | 12 | 6 | 3 | – | 2 | 3 |
| New or worsening of hearing dysfunction | – | – | – | – | – | 3 | – | – |
Abbreviations: BPPV, benign peripheral paroxysmal vertigo; MD, Ménière disease; PPPD/FD, persistent postural perceptual dizziness/functional dizziness; UVP, unilateral vestibulopathy; VM, vestibular migraine; VP, vestibular paroxysmia.
None of the patients with PPPD/functional dizziness showed new peripheral or central vestibular deficits (Table 1); there were two patients with a known old, compensated unilateral peripheral vestibular partial deficit and only one patient with preexisting congenital mild ocular motor dysfunction. All of the PPPD/functional dizziness patients described typical ongoing symptoms exacerbated by certain external triggers (e.g., busy environments) and mostly improved when distracted (e.g., during sports). The clinical finding was further technically confirmed by posturography in 11 patients (44%) with a typical psychosomatic sway pattern with improvement during more demanding tasks. A history of psychiatric diseases, such as depression or anxiety disorder, was reported by nine of the 24 patients (36%), and acute general side effects of vaccination (e.g., myalgia, general weakness, orthostatic dysregulation, or anaphylaxis) by eight patients (36%).
All patients of the VM group showed unremarkable results in their audiovestibular tests, except for two with a known stable partial unilateral or bilateral peripheral deficit. All VM patients described typical episodic vertigo/dizziness attacks with migrainelike symptoms (e.g., phono‐/photophobia, headache, aura) and often additional mild signs of vestibular hypersensitivity (e.g., kinetosis or strong vegetative reaction after caloric test). Transient migrainelike side effects in the acute postvaccination phase (e.g., headache, phono‐/photophobia) were reported by nine patients (64%).
In the overlap group (i.e., PPPD/functional dizziness + VM) that fulfilled the diagnostic criteria of both entities, five patients had suffered from psychiatric diseases in the past (38%). Testing excluded relevant peripheral or central vestibular deficits in all patients. Acute postvaccination migrainelike headache or symptoms were reported by four patients (46%).
Of the BPPV group, one patient showed an isolated mild bilateral high‐frequency vestibular hypofunction (vHIT) in terms of presbyvestibulopathy, two an old centrally compensated unilateral deficit, and one a cerebellar ocular motor dysfunction with downbeat nystagmus, all of which have been documented earlier. Signs of discrete acute vestibular tone imbalance measured by mild SVV deviation were evident in two patients, as often found in BPPV. Beyond that finding, no other audiovestibular deficits were present.
There were three patients with acute UVP, one of whom developed typical symptoms <24 h postvaccination, whereas the other two did in the third and fourth week, respectively, postvaccination. All three reported acute general vaccine reactions with myalgia, fatigue, and unspecific discomfort. Hearing was unaffected in all three patients. The patient with early onset had been suffering from fever and general malaise in the days before vaccination and still had an elevated body temperature on the day of vaccination; nevertheless, the vaccine was administered. At the time of presentation, in one patient central compensation was still incomplete 8 weeks after symptom onset (residual SVV deviation and head‐shaking nystagmus), but compensation was complete at the follow‐up 4 months later. The other two presented 4 and 6 months, respectively, after system onset in an already centrally compensated state. One of these patients was taking antiretroviral drugs due to a human immunodeficiency virus infection at the time of vaccination, whereas the other did not suffer from chronic diseases or take immunosuppressive drugs.
There were three patients with MD and two with VP, with only one of each showing a new onset of MD and VP. The remaining patients reported a worsening of attack frequency or intensity. In two further patients, the final diagnoses were diabetic axonal polyneuropathy and eye muscle paresis.
Retrospective collection of cases with vaccination‐related vestibular symptoms from the prepandemic years (2011–2020) revealed only five patients (three females; one seasonal influenza, one seasonal influenza/diphtheria, one tuberculosis, one diphtheria/tetanus/polio/pertussis, one unclear). Three of them were finally diagnosed as PPPD/functional dizziness without central or peripheral vestibular deficits, one as VM, and one as UVP. Although the number of patients presenting in the DSGZ in the prepandemic years was comparable to the years 2021/2022, the estimated number of total vaccinations in Germany was approximately 30–40 million doses per year compared to 151.7 million COVID‐19 vaccinations in 2021 and 26.7 million in 2022.
DISCUSSION
Our prospective cohort study of 4137 patients revealed 72 patients with enduring vertigo or dizziness after COVID‐19 vaccination that led to presentation in our referral center. The main findings in this cohort were the following:
Overall, newly induced central vestibular or ocular motor dysfunctions were not present at all, and peripheral vestibular dysfunction was rare. In nine patients, only preexisting unilateral or bilateral deficits were confirmed in the audiovestibular diagnostics.
Three quarters of patients showed somatoform vestibular disorders or vestibular migraine or a combination of both.
A clear increase of other vestibular syndromes was not observed.
In our cohort, the most frequent cause of vertigo, dizziness, and imbalance was somatoform vestibular disorders in approximately 50%, as the only diagnosis or in combination with VM. These disorders were significantly more prevalent than in our cohort of patients from before the pandemic, as well as in reported cohorts from referral centers for vertigo and dizziness, where somatoform vestibular disorders comprise up to approximately 20% [21]. In our COVID‐19 vaccination‐related PPPD/functional subgroup, mild general adverse reactions were not more prevalent than in the general population (Table 1). However, one may speculate that these patients subjectively perceived higher impairment, which triggered secondary functional progression of vertigo and dizziness symptoms, especially because almost 40% of them had a prior history of psychiatric illness as a predisposing factor. It is well accepted that a preexisting anxiety diathesis and a highly anxious response to acute physical symptoms increase the likelihood of persistently changing the postural control toward a more conscious and inappropriate strategy with increased perception of body sway [22].
VM was found significantly more often (37%) in patients after COVID‐19 vaccination compared to the prevalence in our and other cohorts (12%–15%). This is in line with findings in migraine headache that showed ongoing modulation of attack frequency after COVID‐19 vaccination [23, 24]. Whether this is a direct immunological reaction to vaccination, for example, by cytokine release [25], or due to vaccine‐related stress, for example, caused by direct or accompanying psychological factors, remains unclear [26]. Moreover, patients with VM often suffer from psychiatric comorbidities that can predispose to somatoform reactions after stressors [27].
BPPV was found to be the next most frequent entity (13%). However, its proportion corresponds to the usual frequency at our center and was not increased. Although transient immobility (i.e., by a postvaccine prolonged resting period) could theoretically predispose for BPPV, we did not find an accumulation of this diagnosis. Due to the suspected immunological cause, with reactivation of herpes simplex virus (HSV) type II from the vestibular ganglion [28], acute UVP following vaccination appears to be an interesting subgroup. A recent study by Schmid et al. found eight patients in a 6‐month period who had developed a UVP in timely conjunction with COVID‐19 vaccination [14]; in our patient subcohort, only three patients suffered from a UVP after their vaccination (within a 4‐week interval), which was less frequent than in the prepandemic control cohort. Although we cannot exclude that the inflammatory response to COVID‐19 vaccination may contribute to the evolution of a vestibular neuritis by reactivation of HSV II in single cases, a specific clustering above likelihood of spontaneous incidence was not observed. Interestingly, bilateral vestibulopathy, which is expected to be the most prevalent phenotype of a systemic autoimmune process, was not seen at all. Because the time span between vaccination and presentation in our department was up to 1 year, it appears unlikely that we completely dismissed slowly progressive autoimmune syndromes.
Our respective analysis of patients presenting with vestibular symptoms after immunization with other vaccines indicated that somatoform vestibular disorders also were the leading diagnosis. However, the number of patients who presented to our tertiary dizziness center with COVID‐19 vaccination‐related symptoms of somatoform origin was disproportionately higher (by an estimated factor of 70). This might be explained by the mass media and misinformation, such as claims of late occurring vaccination effects, reaching wider audiences than ever before during this pandemic [29]. These factors paired with public discussion of possible side effects, political interests in reaching high vaccination rates, and a general pandemic‐related psychosocial burden [30] might add further psychological stressors or foster introspective behavior after vaccination. Similar mechanisms have been proposed regarding the high prevalence of unspecific vaccine reactions (such as fatigue or headache) in the placebo arms of the COVID‐19 vaccine trials, in which almost one of three placebo recipients reported one or more systemic side effects [31].
The main limitations of the present study are a potential selection bias because of the patients' admission to an interdisciplinary tertiary university hospital center. Thus, the results cannot be transferred to the general population, and do not allow assessment or comparison of vestibular syndrome prevalences. However, one would expect that in the case of significantly increased frequency of organic vestibular dysfunction in temporal relation to vaccination, more patients would present at a specialized center like ours. Because we did not see the patients in the acute phase, we are not able to exclude transient vestibular syndromes. However, the finding that newly acquired persistent audiovestibular deficits were very rare and central vestibular dysfunction was completely lacking after a mean time span of 4 months makes relevant structural affection of the vestibular system very unlikely. Organic vestibular disorders (e.g., UVP, BVP, central ischemia or inflammation) typically leave neuro‐otological and/or neuro‐ophthalmological signs that are detectable even in the asymptomatic follow‐up.
CONCLUSIONS
There was no evidence that COVID‐19 vaccination induces enduring peripheral or central vestibular deficits more frequently than expected from spontaneous incidence. The most common origin of postvaccination dizziness or vertigo in our patient cohort was somatoform vestibular disorders and/or VM. Without clear clustering or evidence for a causal context, we saw few cases of acute UVP and BPPV that are both well treatable and have very good prognosis. We recommend a clinical neurological examination and in justified cases a neuro‐otological workup to rule out coincidental onset of vestibular diseases. Furthermore, the management of patients should include education about the unimpaired vestibular functioning in the great majority of patients and the overall favorable prognosis, especially to prevent chronification and long‐term sick leave with a socioeconomic burden.
AUTHOR CONTRIBUTIONS
Johannes Gerb, Sandra Becker‐Bense, and Doreen Huppert conceived and conducted the study. Johannes Gerb collected the data, did the statistical analyses, and designed the tables. Johannes Gerb, Sandra Becker‐Bense, and Doreen Huppert drafted the manuscript. All authors interpreted the data. Andreas Zwergal revised the manuscript and designed the figure. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
FUNDING INFORMATION
German Federal Ministry of Education and Research (grant code 01‐EO‐1401), Deutsche Stiftung Neurologie.
CONFLICT OF INTEREST
The authors declare that they have no competing financial interests.
Supporting information
Table S1
ACKNOWLEDGMENTS
We would like to thank the DSGZ staff and the orthoptic and medical technical assistants. Furthermore, we thank Katie Göttlinger for copyediting and Ralf Strobl for statistical support. Open Access funding enabled and organized by Projekt DEAL.
Gerb J, Becker‐Bense S, Zwergal A, Huppert D. Vestibular syndromes after COVID‐19 vaccination: A prospective cohort study. Eur J Neurol. 2022;00:1‐8. doi: 10.1111/ene.15546
Johannes Gerb and Sandra Becker‐Bense contributed equally to this study.
DATA AVAILABILITY STATEMENT
Individual‐level data from all patients are anonymously given in the article. Any data are available from the corresponding author on reasonable request (Johannes.Gerb@med.uni‐muenchen.de).
REFERENCES
- 1. Murdin L, Schilder AGM. Epidemiology of balance symptoms and disorders in the community: a systematic review. Otol Neurotol. 2015;36:387‐392. [DOI] [PubMed] [Google Scholar]
- 2. Bisdorff A, von Brevern M, Lempert T, Newman‐Toker DE. Classification of vestibular symptoms: towards an international classification of vestibular disorders. J Vestib Res. 2009;19:1‐13. [DOI] [PubMed] [Google Scholar]
- 3. Brandt T, Dieterich M. The dizzy patient: don't forget disorders of the central vestibular system. Nat Rev Neurol. 2017;13:352‐362. [DOI] [PubMed] [Google Scholar]
- 4. Kirsch V, Keeser D, Hergenroeder T, et al. Structural and functional connectivity mapping of the vestibular circuitry from human brainstem to cortex. Brain Struct Funct. 2016;221:1291‐1308. [DOI] [PubMed] [Google Scholar]
- 5. Dieterich M, Staab JP. Functional dizziness: from phobic postural vertigo and chronic subjective dizziness to persistent postural‐perceptual dizziness. Curr Opin Neurol. 2017;30:107‐113. [DOI] [PubMed] [Google Scholar]
- 6. Coronavirus und COVID‐19 ‐ Sicherheit von COVID‐19‐Impfstoffen. https://www.pei.de/DE/newsroom/dossier/coronavirus/arzneimittelsicherheit.html. Accessed January 9, 2022.
- 7. Chen J, Cai Y, Chen Y, Williams AP, Gao Y, Zeng J. Nervous and muscular adverse events after COVID‐19 vaccination: a systematic review and meta‐analysis of clinical trials. Vaccines (Basel). 2021;9(8):939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Finsterer J. Neurological side effects of SARS‐CoV‐2 vaccinations. Acta Neurol Scand. 2022;145:5‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kadali RAK, Janagama R, Peruru S, Malayala SV. Side effects of BNT162b2 mRNA COVID‐19 vaccine: a randomized, cross‐sectional study with detailed self‐reported symptoms from healthcare workers. Int J Infect Dis. 2021;106:376‐381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kadali RAK, Janagama R, Yedlapati SH, et al. Side effects of messenger RNA vaccines and prior history of COVID‐19, a cross‐sectional study. Am J Infect Control. 2022;50:8‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gianfredi V, Minerva M, Casu G, et al. Immediate adverse events following COVID‐19 immunization. A cross‐sectional study of 314,664 Italian subjects. Acta Biomed. 2021;92:e2021487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wichova H, Miller ME, Derebery MJ. Otologic manifestations after COVID‐19 vaccination: the house ear clinic experience. Otol Neurotol. 2021;42:e1213‐e1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Di Mauro P, La Mantia I, Cocuzza S, et al. Acute vertigo after COVID‐19 vaccination: case series and literature review. Front Med (Lausanne). 2021;8:790931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schmid MB, Bächinger D, Pangalu A, Straumann D, Dlugaiczyk J. Acute unilateral peripheral vestibulopathy after COVID‐19 vaccination: initial experience in a tertiary neurotology center. Front Neurol. 2022;13:1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dieterich M, Brandt T. Ocular torsion and tilt of subjective visual vertical are sensitive brainstem signs. Ann Neurol. 1993;33:292‐299. [DOI] [PubMed] [Google Scholar]
- 16. Jongkees LB, Maas JP, Philipszoon AJ. Clinical nystagmography. A detailed study of electro‐nystagmography in 341 patients with vertigo. Pract Otorhinolaryngol (Basel). 1962;24:65‐93. [PubMed] [Google Scholar]
- 17. Heuberger M, Sağlam M, Todd NS, Jahn K, Schneider E, Lehnen N. Covert anti‐compensatory quick eye movements during head impulses. PLoS One. 2014;9:e93086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Krafczyk S, Tietze S, Swoboda W, Valkovic P, Brandt T. Artificial neural network: a new diagnostic posturographic tool for disorders of stance. Clin Neurophysiol. 2006;117:1692‐1698. [DOI] [PubMed] [Google Scholar]
- 19. Dlugaiczyk J. Evidenzbasierte VEMP‐Diagnostik: Von den neurophysiologischen Grundlagen zur klinischen Anwendung. HNO. 2020;68:69‐78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Benchimol EI, Smeeth L, Guttmann A, et al. The REporting of studies conducted using observational routinely‐collected health data (RECORD) statement. PLoS Med. 2015;12:e1001885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Brandt T, Huppert D, Strupp M, Dieterich M. Functional dizziness: diagnostic keys and differential diagnosis. J Neurol. 2015;262:1977‐1980. [DOI] [PubMed] [Google Scholar]
- 22. Dieterich M, Staab JP, Brandt T. Functional (psychogenic) dizziness. Handb Clin Neurol. 2016;139:447‐468. [DOI] [PubMed] [Google Scholar]
- 23. Sekiguchi K, Watanabe N, Miyazaki N, et al. Incidence of headache after COVID‐19 vaccination in patients with history of headache: a cross‐sectional study. Cephalalgia. 2022;42(3):266‐272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Göbel CH, Heinze A, Karstedt S, et al. Clinical characteristics of headache after vaccination against COVID‐19 (coronavirus SARS‐CoV‐2) with the BNT162b2 mRNA vaccine: a multicentre observational cohort study. Brain Commun. 2021;3:fcab169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kelman L. The triggers or precipitants of the acute migraine attack. Cephalalgia. 2007;27:394‐402. [DOI] [PubMed] [Google Scholar]
- 26. Hervé C, Laupèze B, Del Giudice G, Didierlaurent AM, Da Tavares SF. The how's and what's of vaccine reactogenicity. NPJ Vaccines. 2019;4:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Eggers SDZ, Neff BA, Shepard NT, Staab JP. Comorbidities in vestibular migraine. J Vestib Res. 2014;24:387‐395. [DOI] [PubMed] [Google Scholar]
- 28. Himmelein S, Lindemann A, Sinicina I, et al. Differential involvement during latent herpes simplex virus 1 infection of the superior and inferior divisions of the vestibular ganglia: implications for vestibular neuritis. J Virol. 2017;91(14):e00331‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Puri N, Coomes EA, Haghbayan H, Gunaratne K. Social media and vaccine hesitancy: new updates for the era of COVID‐19 and globalized infectious diseases. Hum Vaccin Immunother. 2020;16:2586‐2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Salari N, Hosseinian‐Far A, Jalali R, et al. Prevalence of stress, anxiety, depression among the general population during the COVID‐19 pandemic: a systematic review and meta‐analysis. Global Health. 2020;16:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Haas JW, Bender FL, Ballou S, et al. Frequency of adverse events in the placebo arms of COVID‐19 vaccine trials: a systematic review and meta‐analysis. JAMA Netw Open. 2022;5:e2143955. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Data Availability Statement
Individual‐level data from all patients are anonymously given in the article. Any data are available from the corresponding author on reasonable request (Johannes.Gerb@med.uni‐muenchen.de).


