Severe acute respiratory syndrome (SARS) coronavirus (COV)‐2 causes flu‐like symptoms, including fever, cough, fatigue, and loss of sense of smell. Although many complications of this unprecedented virus have been specified, there is still a lack of information on neurologic post‐recovery complications. Studies suggest that this rapidly spreading virus can invade the nervous system and cause neurological problems even after recovery. 1 The most common neurological complications include headache, dizziness, myalgia, anosmia, gustatory, and olfactory dysfunctions. 2 Severe disorders such as encephalopathy, encephalitis, necrotizing hemorrhagic encephalopathy, stroke, epileptic seizures, rhabdomyolysis, and Guillain‐Barre syndrome have also been reported after coronavirus disease (COVID)‐19 infection. 3 There are just six case reports on post‐COVID‐19 chorea. As far as we know, the two patient cases noted below are the first to be reported with more than a 2‐week interval between post‐COVID‐19 encephalitis and initiation of chorea. 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11
A 67‐year‐old woman was referred to the movement disorder clinic because of acute onset of generalized choreiform movements. She stated an earlier admission because of coronavirus symptoms including nausea, loss of appetite, and high blood pressure 3 months prior. Moreover, she tested positive for coronavirus reverse‐transcription polymerase chain reaction (RT‐PCR) by nasopharyngeal swab. Additionally, 2 weeks after the beginning of her COVID‐19 infection, she developed confusion, illusion, aphasia, imbalance, and delirium. Consequently, she was diagnosed with post‐COVID‐encephalitis and received a tocilizumab injection. She was vaccinateded for COVID‐19 (AstraZeneca) 4 months previously. Her family history and previous history of any abnormal movements in any part of her body were negative.
She had normal neurological and systemic examinations. However, she was suffering from choreiform movements in her face and all four limbs, with right arm dominancy (Video 1). Brain magnetic resonance imaging (MRI) showed bilateral hyperintensity on fluid‐attenuated inversion recovery and T2 imaging on basal ganglia (Fig. 1). She was treated with tetrabenazine (12.5 mg twice daily). After a few days, her symptoms recovered rapidly, and she was discharged.
Video 1.
Shows the patient with choreiform movements in face and all four limbs, more prominently in the right upper extremity.
FIG 1.
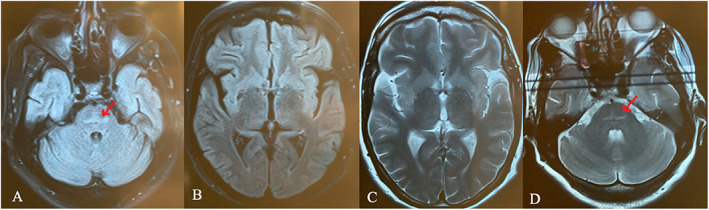
Axial fluid‐attenuated inversion recovery (FLAIR) and T2 brain magnetic resonance imaging (MRI) shows hyperintensity of basal ganglia and pons.
Another 62‐year‐old otherwise healthy female presented to the movement disorder clinic because she experienced sudden onset abnormal movements in her truncal and limbs. She had been admitted 15 days previously because of her COVID‐19 infection and treated with a remdesivir injection. Her past medical history, drug history, and family history were unremarkable. She had no pathological findings in examinations and brain MRI. Nonetheless, she had choreiform movements on her extremities, especially on the right side (Video 2). She was treated with tetrabenazine and chorea dramatically mended.
Video 2.
Shows the patient with chorea in all four limbs.
In both cases, laboratory tests including complete blood count, basic metabolite assessment (sodium, potassium, calcium, chloride, carbon dioxide, albumin, blood urea nitrogen, and fasting blood sugar), lipid panel, liver‐thyroid function tests, and paraneoplastic panels were found to be at normal ranges. However, there was an increase in erythrocyte sedimentation rate and C‐reactive protein levels in case 2. Accordingly, chorea was considered to be a complication of COVID‐19 infection.
Societies have been greatly affected by COVID‐19 consequences. These complications need to be elucidated. As mentioned earlier, neurologic complications after COVID‐19 infection have been stated before. Here, we report two women with right dominant choreiform movements that, unlike other reports, showed chorea after 2 weeks from COVID‐19 infection.
Literature that includes choreiform movements after coronavirus infection, published up to March 2022, was reviewed (Table 1). Subsequently, the relevant six case reports were extracted. We included our case report on hemichorea after the BBIBP‐CorV (Sinopharm) vaccine. 12 Although this is a rare condition, clinicians must be conscious of this possible outcome.
TABLE 1.
Presentation of available case reports
| Author | Patient | Clinical features | Symptoms onset day* | CSF | Lab tests | History | Brain CT/MRI | Treatment |
|---|---|---|---|---|---|---|---|---|
| Yüksel et al 9 | 14 yr F | Bilateral shoulder shrugging, choreiform movements in all four limbs and bilateral milkmaid's grip sign | 3 | NA | Iron deficiency anemia | Sydenham's chorea | Normal | Carbamazepine |
| Sawczyńska et al 5 | 77 yr F | Orofacial dyskinesia and involuntary chorea‐type movements of the trunk and all limbs | 11 | Normal | Elevated serum inflammation markers dyselectrolytemia | Arterial hypertension, diabetes mellitus, hypothyroidism, and urinary incontinence and three malignancies in remission | Marked features of cerebral small vessel disease. Diffuse white matter hyper intensities, cortical and subcortical atrophy | Steroids diazepam remdesivir, IVIG |
| Cotta Ramusino et al 6 | 62 yr M | Choreiform movements in all four limbs, head, and trunk. Mild encephalopathy (impulsivity, hyperactivity, and attention impairment) | Before | Mildly decreased glucose mildly increased albumin | Normal | Type 2 diabetes mellitus and arterial hypertension | SWI showed hypointense signal in the dorsolateral portion of both putamina | Tetrabenazine, haloperidol |
| Byrnes et al 7 | 36 yr M | Intermittent rapid, irregular, and no purposeful movements of the both upper extremities with mild encephalopathy | Before | Mildly elevated lymphocytes | Decreased lymphocytes, mildly increased ESR, CRP. | Drug abuser | Enchantment lesions affecting the bilateral medial putamen and left cerebellum. | Solu‐Medrol, IVIG, methylprednisolone |
| Ghosh et al 8 | 60 yr M | Right‐sided involuntary violent flinging movements in all limbs with semi‐purposeful dancing movement involving both right upper and lower limbs. | 2 | NA | Capillary blood glucose 540, mild neutrophilic leukocytosis, lymphopenia increased ESR CRP, metabolic acidosis, and ketonuria | None | Left striatal hyperintensity on T1‐weighted imaging | Insulin |
| Revert Barberà et al 11 | 69 yr F | Mixed aphasia, mild right hemiparesis, and choreic movements in all 4 limbs. Headache, focal neurological deficits, seizures, and diffuse encephalopathy. | Before | NA | Elevated d‐dimer levels (3160 μg/L), | Fatty liver, fibromyalgia | Capsuloganglionic and thalamic infarcts bilaterally, with thrombosis of the lateral veins, left lateral sinus, straight sinus, and vein of Galen | |
| Salari et al 12 | 13 yr M | Large‐amplitude choreic movements affecting the right side of his body that affected his gait | 7 after vaccination | 0 RBC, 0 WBC, protein 51 (g/L), glucose 56 (mg/dL) | Normal | None | Multiple white matter lesions, one of them enhanced with gadolinium | Intravenous methylprednisolone and tetrabenazine |
| Salari et al 12 | 18 yr M | Choreic movements that mainly affected the left upper limb, shoulder, and the left lower limb. | 7 after vaccination | 3 RBC, 4 WBC, protein 34 (g/L), glucose 64 (mg/dL) | Normal | None | Few nonspecific white matter lesions | Intravenous methylprednisolone and tetrabenazine |
| This paper case 1 | 67 yr F | Random, fast, irregular, and involuntary choreiform movements in her face and all four limbs, with right arm dominancy. | 180 | NA | Normal | None | Damaged bilateral basal ganglia | Tetrabenazine |
| This paper case 2 | 62 yr F | Choreiform movements on all limbs, especially on the right side | 15 | NA | Increased ESR, CRP | None | Normal | Tetrabenazine |
Day of choreiform movement onset since COVID‐19 first symptoms; CSF, cerebrospinal fluid; NA, no data available; IVIG, intravenous infusion of immunoglobulins; CT, computed tomography; MRI, magnetic resonance imaging.
Author Roles
(1) Research project: A. Conception, B. Organization, C. Execution. 2 Manuscript: A. Writing of the First Draft, B. Review and Critique.
F.A.: 1A, 1B, 1C.
M.S.:1A, 1B, 1C, 2A.
F.H.P.: 2A.
Disclosures
Ethical Compliance Statement: This study was reviewed by Shahid Beheshti University of medical sciences. The patients have given written and informed consent for online publication of their videos. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this work is consistent with those guidelines.
Funding Sources and Conflicts of Interest: The authors declare that there are no conflicts of interest relevant to this work.
Financial Disclosures for the Previous 12 Months: The authors declare that there are no additional disclosures to report.
Relevant disclosures and conflict of interest are listed at the end of this article.
References
- 1. Lopez‐Leon S, Wegman‐Ostrosky T, Perelman C, Sepulveda R, Rebolledo PA, Cuapio A, Villapol S. More than 50 long‐term effects of COVID‐19: A systematic review and meta‐analysis. Sci Rep 2021;11(1):16144. 10.1038/s41598-021-95565-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sharifian‐Dorche M, Huot P, Osherov M, et al. Neurological complications of coronavirus infection; a comparative review and lessons learned during the COVID‐19 pandemic. J Neurol Sci 2020;417:117085. 10.1016/j.jns.2020.117085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Harapan BN, Yoo HJ. Neurological symptoms, manifestations, and complications associated with severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) and coronavirus disease 19 (COVID‐19). J Neurol 2021;268(9):3059–3071. 10.1007/s00415-021-10406-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hassan M, Syed F, Ali L, Rajput HM, Faisal F, Shahzad W, Badshah M. Chorea as a presentation of SARS‐CoV‐2 encephalitis: A clinical case report. JMD 2021;14(3):245–247. 10.14802/jmd.20098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sawczyńska K, Wężyk K, Bosak M, et al. Acute‐onset chorea and confusional state in 77‐year‐old COVID‐19 patient: A case report. Neurol Neurochir Pol 2022;56(1):106–110. 10.5603/PJNNS.a2022.0003. [DOI] [PubMed] [Google Scholar]
- 6. Cotta Ramusino M, Perini G, Corrao G, Farina L, Berzero G, Ceroni M, Costa A. sars‐cov −2 in a patient with acute chorea: Innocent bystander or unexpected actor? Mov Disord Clin Pract 2021;8(6):950–953. 10.1002/mdc3.13274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Byrnes S, Bisen M, Syed B, et al. COVID‐19 encephalopathy masquerading as substance withdrawal. J Med Virol 2020;92(11):2376–2378. 10.1002/jmv.26065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ghosh R, Dubey S, Roy D, Ray A, Pandit A, Ray BK, Benito‐León J. Choreo‐ballistic movements heralding COVID‐19 induced diabetic ketoacidosis. Diabetes Metab Syndr Clin Res Rev 2021;15(3):913–917. 10.1016/j.dsx.2021.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yüksel MF, Yıldırım M, Bektaş Ö, Şahin S, Teber S. A sydenham chorea attack associated with COVID‐19 infection. Brain Behav Immun Health 2021;13:100222. 10.1016/j.bbih.2021.100222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Aggarwal A, Adukia S, Bhatt M. Video anthology of movement disorders due to infections in South Asia. Mov Disord Clin Pract. 2021;8(6):843–858. 10.1002/mdc3.13275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Revert Barberà A, Estraguès Gazquez I, Beltrán Mármol MB, Rodríguez Campello A. Corea bilateral como forma de presentación de trombosis venosa cerebral asociada a COVID‐19. Neurologia 2021;37:507–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Salari M, Etemadifar M. Two cousins with acute Hemichorea after bbibp‐corv (Sinopharm) covid ‐19 vaccine. Mov Disord 2022;37:1101–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]


