Abstract
The effectiveness of remdesivir on survival in coronavirus disease 2019 (COVID‐19), especially in cases treated in the intensive care unit (ICU), is controversial. We investigated the effectiveness of remdesivir with corticosteroids on the survival of COVID‐19 patients in a real ICU clinical practice. For laboratory‐confirmed COVID‐19 patients admitted to the ICU of a tertiary hospital in Tokyo (April 2020–November 2021) and who received corticosteroids, the effectiveness of remdesivir for survival, stratified by interval length (within 9 or 10+ days), was retrospectively analyzed using Cox regression model. A total of 168 patients were included: 35 with no remdesivir use (control), 96 with remdesivir use within 9 days, and 37 with remdesivir use with an interval of 10+ days. In‐hospital mortality was 45.7%, 10.4%, and 16.2%, respectively. After adjusting for possible covariates including comorbidities, laboratory data, oxygen demand, or level of pneumonia, remdesivir use within 9 days from symptom onset reduced mortality risk (hazard ratio [HR]: 0.10; 95% confidence interval (CI): 0.025–0.428) compared to the control group. However, remdesivir use with an interval of 10+ days showed no significant association with mortality (HR: 0.42; 95% CI: 0.117–1.524). Among COVID‐19 patients who received corticosteroids in ICU, remdesivir use within 9 days from symptom onset was associated with reduced in‐hospital mortality risk.
Keywords: COVID‐19, intensive care units, mortality, pneumonia, remdesivir
1. INTRODUCTION
The efficacy of remdesivir for coronavirus disease 2019 (COVID‐19) patients was investigated in several randomized controlled trials (RCTs), which reported a shortening of recovery time 1 , 2 ; however, a survival benefit has not been demonstrated. 1 , 2 , 3 An RCT from China 4 found that 28‐day mortality was lower among the group treated with remdesivir within 10 days from onset compared to placebo, although the finding was not significant. In addition, a retrospective study in India showed that patients with onset to remdesivir treatment interval within 9 days had a significantly lower mortality than those with intervals of over 10 days. 5 Therefore, in investigating the efficacy of remdesivir for mortality, the timing of treatment would be important.
Notably, previous trials did not focus on invasive mechanical ventilator (IMV) patients, and a subgroup analysis of one RCT did not show an effective recovery for those requiring IMV or extracorporeal membrane oxygenation (ECMO). 1 According to the CRoss Icu Searchable Information System database, information‐sharing system for intensive care unit (ICU) beds available across Japan, 6 as the survival rate of severe COVID‐19 patients requiring IMV or ECMO was better (71.4%–80.4%) 7 compared to other countries, 8 , 9 , 10 with little variation in each of the epidemic waves, we believed that effectiveness of remdesivir should be retrospectively evaluated in Japan by reviewing the actual course of treatment for COVID‐19 patients who required intensive care.
Although lung damage is a major factor for COVID‐19 mortality, few studies have considered the quantitative assessment of pneumonia to investigate the effectiveness of remdesivir. As the lung is the main target of remdesivir treatment, 11 and previous studies showed that total lung opacity volume of chest computed tomography (CT) scan could predict the adverse outcomes, 12 , 13 we investigated the effectiveness of remdesivir with the consideration of the severity of pneumonia from CT scan.
The aim of this study was to investigate the effectiveness of remdesivir with corticosteroids for COVID‐19 patients in Japanese ICU, stratified by remdesivir interval length, adjusted for possible covariates such as total lung opacity volume to assess the level of pneumonia.
2. METHODS
2.1. Data source and study design
COVID‐19 patients who were consecutively hospitalized at Tokyo Medical and Dental University (TMDU) Hospital, a tertiary care hospital located in Tokyo, between April 2020 and November 2021 and admitted to the ICU were included. Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) was detected by a real‐time reverse transcription‐PCR test. Exclusion criteria were: (i) those who stayed in ICU for less than 24 h, (ii) those who did not receive corticosteroids for COVID‐19 pneumonia treatment to collect patients with a similar condition, which was recommended for the treatment of patients with severe or critical COVID‐19 by the World Health Organization (WHO), 14 and (iii) those who did not have a CT scan available for analysis. A total of 665 laboratory‐confirmed COVID‐19 patients were consecutively hospitalized at TMDU Hospital, of which 225 COVID‐19 patients were admitted to the ICU. Patients who stayed in the ICU for less than 24h (n = 26), who did not receive corticosteroids (n = 26), and who did not have a CT scan available for analysis (n = 5) were excluded. Finally, 168 patients were analyzed (Supporting Information: Figure S1).
As a previous study showed the association of timing of remdesivir initiation with in‐hospital mortality, 5 remdesivir‐treated patients were stratified into two groups by the interval length from symptom onset to remdesivir initiation: remdesivir use within 9 days and with an interval of 10 days or more. The primary outcome was all‐cause in‐hospital mortality. All patients were followed to hospital discharge, death, or back transfer. We censored patients who were discharged alive to home or back transferred. In addition, adverse events of remdesivir were reported. According to the Kidney Disease: Improving Global Outcomes criteria, 15 acute kidney injury (AKI) was defined as 1.5 times increase in creatinine from baseline or ≥0.3 mg/dl within 2 days of remdesivir treatment. Liver injury was defined as alanine aminotransferase (ALT) of more than five times the upper normal limit (23 U/L).
Considering the retrospective study design and complete anonymization, we applied opt‐out method. Ethical approval was obtained from Ethics Committee at TMDU (M2021‐167) and conforms to the provisions of the Declaration of Helsinki.
2.2. Data collection
Demographic and clinical data were retrospectively collected from medical records. Demographic information included age, sex, height, weight, comorbidities, and smoking status. Clinical data included severity scores, oxygen demand, radiological findings on CT scan, laboratory results, complications, prognosis and the date of onset, admission and discharge, and duration of ICU stay, treatments, and medication. The observation period was from the baseline date to discharge. In patients who developed COVID‐19 after admission to TMDU Hospital, the baseline date was set as the date of symptom onset; otherwise, the baseline date was the date of admission. The laboratory results were taken from the baseline date or the most recent data within 2 days of the date. Before anonymization, patient names were categorized into Japanese and non‐Japanese according to a previous study, 16 and we checked the origin country.
2.3. Quantitative method of pneumonia
The lung percentage of opacity volume, which was measured automatically using a deep‐learning‐based image analysis, was used to assess the level of pneumonia. Chest CT scans taken at the time of admission to TMDU Hospital or at a previous hospital before transfer were analyzed according to published protocol. 12 In patients who developed COVID‐19 after admission to TMDU Hospital, CT scan on the day of symptom onset was used.
2.4. Statistical analysis
The baseline characteristics of the three groups were described as proportions (percent), means (standard deviation [SD]) or median (interquartile range [IQR]) as appropriate. To evaluate the differences, Pearson's χ 2 test was used for categorical variables. For continuous variables, analysis of variance (ANOVA) followed by Dunnett's test or Kruskal–Wallis test followed by Dunn's test (with reference to the control group), respectively, we used. p < 0.05 were considered statically significant.
Cumulative survival probabilities were described using the Kaplan–Meier method, followed by Bonferroni‐adjusted log‐rank tests. To estimate the relative risks between remdesivir use (stratified by interval length) and in‐hospital mortality, Cox proportional hazards regression models were applied. In full multivariable models, the following variables, which were associated with both remdesivir use and mortality, were adjusted as confounders: age, sex, comorbidities with risk of mortality (i.e., cardiovascular disease, cerebrovascular disease, chronic respiratory disease, cancer and Type 2 diabetes), 17 the date of admission and baseline severity (i.e., renal dysfunction, liver dysfunction, oxygen demands, and chest CT opacity volume classified in tertiles).
As a sensitivity analysis, we performed the analysis with a Cox‐proportional hazard model, excluding 39 patients who had started remdesivir before admission to TMDU Hospital and two patients whose CT scan for pneumonia evaluation was taken after the start of remdesivir, as for these patients, covariates such as laboratory data or total lung opacity volume became mediators rather than confounders. All statistical analyses were carried out using Stata version 16 (Stata Corp. College Station).
3. RESULTS
3.1. Demographic and clinical characteristics
One hundred and sixty‐eight patients (control: 36 cases; remdesivir use within 9 days: 96 cases, remdesivir use with interval 10+: 37 cases) were included in the study. The mean (SD) age was 60.9 (±13.5) years, range was 26–94 years, and 81% of patients were male (Table 1). The percentage of non‐Japanese was 4.8%, and all were from East or Southeast Asia. Sixty percent of the patients were transferred from other hospitals due to the need for advanced care. The median (IQR) length of stay in the previous hospital was 4 (2–7) days. The control group had a high prevalence of Type 2 diabetes (54.3%) and cardiovascular disease (28.6%). The mean (SD) interval of onset to admission was 8.3 (±4.2) days, and it was the longest in the group of remdesivir use with an interval of 10+ days. The number of newly COVID‐19‐confirmed cases in Tokyo and COVID‐19‐hospitalized patients at TMDU Hospital is shown in Supporting Information: Figure S2. There were five epi waves during the observation period, 18 and the use of remdesivir increased in the later waves.
Table 1.
Characteristics of COVID‐19 patients who were treated in the intensive care unit (N = 168)
| Total N = 168 | Control (N = 35) | Remdesivir use, interval ≤9 days (N = 96) | Remdesivir use, interval 10+ days (N = 37) | p Value | |
|---|---|---|---|---|---|
| Sex, n (%) | |||||
| Female | 32 (19.0) | 5 (14.3) | 18 (18.8) | 9 (24.3) | 0.55 |
| Male | 136 (81.0) | 30 (85.7) | 78 (81.2) | 28 (75.7) | |
| Mean (SD), age (years) | 60.9 (13.5) | 63.9 (14.6) | 59.3 (13.4) | 62.4 (12.5) | 0.17 |
| Age (years), n (%) | |||||
| −39 | 9 (5.4) | 2 (5.7) | 6 (6.2) | 1 (2.7) | 0.34 |
| 40–64 | 87 (51.8) | 14 (40.0) | 55 (57.3) | 18 (48.6) | |
| 65+ | 72 (42.9) | 19 (54.3) | 35 (36.5) | 18 (48.6) | |
| Transferred from other hospitals, n (%) | 102 (60.7) | 22 (62.9) | 55 (57.3) | 25 (67.6) | 0.53 |
| Median (IQR) length of stay in the previous hospital (days) | 4 (2–7) | 4.5 (3–6) | 4 (2–6) | 5 (3–8) | 0.21 |
| BMI, n (%) | |||||
| Obese 30+ (kg/m2) | 29 (17.3) | 3 (8.6) | 20 (20.8) | 6 (16.2) | 0.25 |
| Comorbidities, n (%) | |||||
| Type 2 diabetes | 50 (29.8) | 19 (54.3) | 23 (24.0) | 8 (21.6) | 0.002* |
| Cardiovascular disease | 16 (9.5) | 10 (28.6) | 6 (6.2) | 0 (0.0) | <0.001* |
| Cerebrovascular disease | 12 (7.1) | 2 (5.7) | 8 (8.3) | 2 (5.4) | 0.79 |
| Chronic respiratory disease | 11 (6.5) | 5 (14.3) | 5 (5.2) | 1 (2.7) | 0.10 |
| Cancer | 11 (6.5) | 2 (5.7) | 7 (7.3) | 2 (5.4) | 0.90 |
| Median (IQR) number of comorbidities | 0 (0–1) | 1 (1–2) | 0* (0–1) | 0* (0–1) | <0.001* |
| Smoking status, n (%) | |||||
| Never | 59 (35.1) | 10 (28.6) | 36 (37.5) | 13 (35.1) | 0.53 |
| Current or past | 85 (50.6) | 18 (51.4) | 50 (52.1) | 17 (45.9) | |
| Missing | 24 (14.3) | 7 (20.0) | 10 (10.4) | 7 (18.9) | |
| Mean (SD) interval of onset to admission (days) | 8.3 (4.2) | 7.3 (5.2) | 7.2 (3.6) | 11.9* (2.2) | <0.001* |
| Date of admission, n (%) | |||||
| 1st–2nd wave (April– October/2020) | 48 (28.6) | 16 (45.7) | 17 (17.7) | 15 (40.5) | <0.001* |
| 3rd wave (November/2020–February/2021) | 37 (22.0) | 11 (31.4) | 20 (20.8) | 6 (16.2) | |
| 4th wave (March‐Jun/2021) | 36 (21.4) | 6 (17.1) | 26 (27.1) | 4 (10.8) | |
| 5th wave (July–November/2021) | 47 (28.0) | 2 (5.7) | 33 (34.4) | 12 (32.4) |
Note: Pearson's χ 2 test for categorical variables. ANOVA followed by Dunnett's test or Kruskal–Wallis test followed by Dunn's test (with the control group as the reference, respectively) for continuous variables.
Abbreviations: ANOVA, analysis of variance; BMI, body mass index; COVID‐19, coronavirus disease 2019; SD, standard deviation.
p < 0.05.
There was no significant difference in the lung total capacity volume among the groups (Table 2). The control group showed low levels of hemoglobin, albumin, and estimated glomerular filtration rate (eGFR), and high levels of C‐reactive protein and d‐dimer compared to the group of remdesivir use (interval ≤9 days).
Table 2.
Radiological and laboratory findings of COVID‐19 patients who were treated in the intensive care unit (N = 168)
| Total (N = 168) | Control (N = 35) | Remdesivir use, interval ≤9 days (N = 96) | Remdesivir use, interval 10+ days (N = 37) | p Value | ||
|---|---|---|---|---|---|---|
| Total opacity volume (%) tertiles, n (%) [Q1–3, each median (range)] | ||||||
| Q1: 19.6 (0.18–30.8) | 56 (33.3) | 15 (42.9) | 31 (32.3) | 10 (27.0) | 0.28 | |
| Q2: 43.6 (31.2–56.4) | 56 (33.3) | 8 (22.9) | 37 (38.5) | 11 (29.7) | ||
| Q3: 65.5 (56.5–94.4) | 56 (33.3) | 12 (34.3) | 28 (29.2) | 16 (43.2) | ||
| Laboratory data | ||||||
| WBC (×1000/μl), mean (SD) | 8.85 (4.69) | 9.92 (5.64) | 8.37 (4.60) | 9.10 (3.79) | 0.23 | |
| NLR, mean (SD) | 14.4 (15.2) | 17.0 (17.2) | 13.9 (15.3) | 13.4 (12.8) | 0.53 | |
| Hb (g/dl), mean (SD) | 13.7 (2.2) | 12.5 (2.4) | 14.2* (1.9) | 13.6* (2.0) | <0.001* | |
| <10 (g/dl), n (%) | 8 (4.8) | 5 (14.3) | 2 (2.1) | 1 (2.7) | 0.012* | |
| Plt (×10 000/μl), mean (SD) | 21.6 (9.7) | 19.8 (9.8) | 21.1 (9.7) | 24.8 (9.1) | 0.061 | |
| CRP (mg/L), mean (SD) | 11.00 (7.85) | 14.18 (9.03) | 9.68* (6.99) | 11.36 (8.07) | 0.013* | |
| 10+ (mg/L), n (%) | 79 (47.0) | 21 (60.0) | 39 (40.6) | 19 (51.4) | 0.12 | |
| Alb (g/dl), mean (SD) | 2.9 (0.5) | 2.6 (0.8) | 3.0* (0.4) | 2.8 (0.4) | 0.003* | |
| <3 (g/dl), n (%) | 99 (58.9) | 23 (65.7) | 52 (54.2) | 24 (64.9) | 0.35 | |
| LDH (U/L), median (IQR) | 442.5 (375.5–599) | 402 (308–482) | 479 (375.5–634.5) | 441 (387–578) | 0.076 | |
| AST (U/L), median (IQR) | 46 (32–63.5) | 36 (24–52) | 48* (38‐64) | 46 (35–60) | 0.047* | |
| ALT (U/L), median (IQR) | 37 (22–56.5) | 23 (14–49) | 38* (26.5–57.5) | 41* (26–57) | 0.023* | |
| 115+ (IU/L), n (%) | 11 (6.5) | 3 (8.6) | 5 (5.2) | 3 (8.1) | 0.72 | |
| eGFR (ml/min/1.73 m2), mean (SD) | 68.7 (32.4) | 39.6 (38.6) | 74.6* (24.0) | 80.7* (29.4) | <0.001* | |
| <30 (ml/min/1.73 m2), n (%) | 21 (12.5) | 18 (51.4) | 2 (2.1) | 1 (2.7) | <0.001* | |
| d‐dimer (μg/ml), median (IQR) | 1.62 (0.72–4.36) | 3.17 (1.3–8.1) | 1.15* (0.54–2.2) | 2.13 (0.93–6.01) | <0.001* | |
| 0.5 (μg/ml), n (%) | 141 (83.9) | 34 (97.1) | 74 (77.1) | 33 (89.2) | 0.013* | |
Note: Pearson's χ 2 test for categorical variables. ANOVA followed by Dunnett's test or Kruskal–Wallis test followed by Dunn's test (with the control group as the reference, respectively) for continuous variables.
Abbreviations: ANOVA, analysis of variance; Alb, albumin; ALT, alanine aminotransferase; AST, aspartate aminotransferase; COVID‐19, coronavirus disease 2019; Cre, creatinine; CRP, C‐reactive protein; eGFR, estimated glomerular filtration rate; Hb, hemoglobin; LDH, lactate dehydrogenase; NLR, neutrophil–lymphocyte ratio; Plt, platelets; SD, standard deviation; WBC, white blood cell.
p < 0.05.
At baseline day, 131 (78.0%) patients required high flow oxygen (HFO) or IMV support (Table 3). The control group had a high prevalence of continuous renal replacement therapy (54.3%). The mean (SD) intervals from onset to intubation and ECMO implementation were 8.9 (±4.0) and 15.8 (±8.3) days, respectively. The severity assessment scores were assessed on initial ICU admission and were highest in the control group. Dexamethasone was used concomitantly in more than 90% of the groups treated with remdesivir, while the control group used experimental therapies other than remdesivir, such as favipiravir. The in‐hospital mortality rate for the entire period was 32/168 (19.0%), which did not significantly vary by wave (p = 0.534). The mortality rate in the control group was 45.7% (16/35) compared to 10.4% (10/96) and 16.2% (6/37) in the group of remdesivir use within 9 days or remdesivir use with an interval of 10+ days, respectively. Median (IQR) time to discharge, back transfer, and death were 20 (15–25.5) days, 12.5 (6–26) days, and 26.5 (19–41) days, respectively. At the time of back transfer of the 96 patients, 86 (89.6%) were no oxygen or low‐flow oxygen demand, 2 (2.1%) were high‐flow oxygen demand, and 8 (8.3%) required tracheostomy with positive pressure ventilation. Of 143 intubated patients, 104 (72.2%) patients were weaned from IMV after a median (IQR) of 7 (4.5–12) days from intubation, 6 of whom were back transferred before tracheostomy closure.
Table 3.
Clinical disease grades and treatment of COVID‐19 patients who were treated in the intensive care unit (N = 168)
| Total (N = 168) | Control (N = 35) | Remdesivir use, interval ≤9 days (N = 96) | Remdesivir use, interval 10+ days (N = 37) | p Value | |
|---|---|---|---|---|---|
| Oxygen demand at baseline, n (%) | |||||
| Low flow oxygen or oxygen free | 37 (22.0) | 9 (25.7) | 21 (21.9) | 7 (18.9) | 0.78 |
| High flow oxygen or mechanical ventilator | 131 (78.0) | 26 (74.3) | 75 (78.1) | 30 (81.1) | |
| Severity assessment at first ICU stay | |||||
| SOFA score, median (IQR) | 5 (3–7) | 7 (5–10) | 4* (3–6) | 5* (3–6) | <0.001* |
| APACHE II score, mean (SD) | 14.1 (7.0) | 19.3 (7.7) | 12.3* (6.3) | 14.0* (5.7) | <0.001* |
| Treatment period (days), median (IQR) | |||||
| ICU stay | 10 (6–20.5) | 13 (8–30) | 9 (5.5–18) | 10 (5–18) | 0.11 |
| Intensive care, n (%) | |||||
| Mechanical ventilator | 143 (85.1) | 32 (91.4) | 81 (84.4) | 30 (81.1) | 0.45 |
| ECMO | 23 (13.7) | 3 (8.6) | 16 (16.7) | 4 (10.8) | 0.42 |
| CRRT | 36 (21.4) | 19 (54.3) | 13 (13.5) | 4 (10.8) | <0.001* |
| Medication, n (%) | |||||
| Dexamethasone | 149 (88.7) | 22 (62.9) | 91 (94.8) | 36 (97.3) | <0.001* |
| mPSL pulse | 51 (30.4) | 16 (45.7) | 23 (24.0) | 12 (32.4) | 0.054 |
| Favipiravir | 43 (25.6) | 17 (48.6) | 17 (17.7) | 9 (24.3) | 0.002* |
| Tocilizumab | 25 (14.9) | 9 (25.7) | 11 (11.5) | 5 (13.5) | 0.12 |
| Baricitinib | 38 (22.6) | 0 (0.0) | 30 (31.2) | 8 (21.6) | <0.001* |
| Complications, n (%) | |||||
| Arrhythmia | 34 (20.2) | 11 (31.4) | 18 (18.8) | 5 (13.5) | 0.14 |
| DVT or PE | 10 (6.0) | 5 (14.3) | 4 (4.2) | 1 (2.7) | 0.061 |
| Mediastinal emphysema | 7 (4.2) | 1 (2.9) | 4 (4.2) | 2 (5.4) | 0.86 |
| Pneumothorax | 8 (4.8) | 1 (2.9) | 6 (6.2) | 1 (2.7) | 0.58 |
| VAP | 63 (37.5) | 11 (31.4) | 35 (36.5) | 17 (45.9) | 0.42 |
| BSI | 47 (28.0) | 13 (37.1) | 22 (22.9) | 12 (32.4) | 0.22 |
| Median (IQR) observational period (days) | 17.5 (9–27.5) | 22 (13–31) | 18 (9–29.5) | 14 (7–22) | 0.066 |
| Prognosis, n (%) | |||||
| Discharge home | 40 (23.8) | 9 (25.7) | 25 (26.0) | 6 (16.2) | <0.001* |
| Back transfer | 96 (57.1) | 10 (28.6) | 61 (63.5) | 25 (67.6) | |
| Death | 32 (19.0) | 16 (45.7) | 10 (10.4) | 6 (16.2) | |
Note: Pearson's χ 2 test for categorical variables. ANOVA followed by Dunnett's test or Kruskal–Wallis test followed by Dunn's test (with the control group as the reference, respectively) for continuous variables.
Abbreviations: ANOVA, analysis of variance; APACHE, acute physiology and chronic health evaluation; BSI, bloodstream infections; COVID‐19, coronavirus disease 2019; CRRT, continuous renal replacement therapy; ECMO, extracorporeal membrane oxygenation; DVT, deep venous thrombosis; mPSL, methylprednisolone; PE, pulmonary embolism; SD, standard deviation; SOFA, sequential organ failure assessment; VAP, ventilator‐associated pneumonia.
p < 0.05.
3.2. Survival analysis
Kaplan–Meier curves (Figure 1) revealed a significantly higher survival rate for the group of remdesivir use for an interval of ≤9 days (log‐rank p < 0.001), while the survival curve of remdesivir use for an interval 10+ days group crossed that of the control group (log‐rank p = 0.415). Adjusting for age and sex by Cox proportional hazard regression (Table 4), there was a significant reduction in in‐hospital mortality among remdesivir‐treated patients (interval ≤9 days) compared with the control group (hazard ratio [HR]: 0.25; 95% confidence interval [CI]: 0.111–0.564). After further adjustment for the number of comorbidities, the date of admission, renal dysfunction, liver dysfunction, baseline oxygen demands, and chest CT opacity volume, remdesivir use (interval ≤9 days) was associated with reduced risk for in‐hospital mortality more so than the control group (HR: 0.10; 95% CI: 0.025–0.428). Conversely, the association of remdesivir use (interval 10+ days) with in‐hospital mortality was not significant (HR: 0.42; 95% CI: 0.117–1.524).
Figure 1.
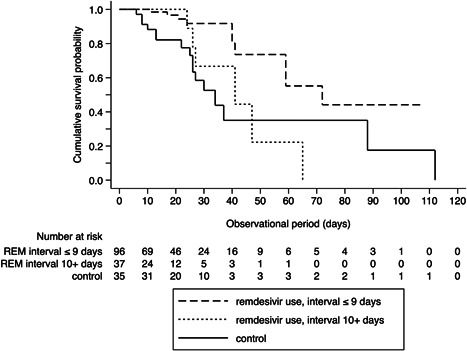
Kaplan–Meier survival curves of patients comparing between patients treated within 9 days from symptom onset, after 10 days from symptom onset and control. *Statistically significant using Bonferroni‐adjusted α critical value = 0.025. Control versus remdesivir (REM) use (interval ≤9 days): log rank p < 0.001*; control versus remdesivir use (interval 10+ days): log rank p = 0.415.
Table 4.
Association of remdesivir and mortality of COVID‐19 patients who were treated in the intensive care unit in Cox hazard models (N = 168)
| Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|
| Variables | HR | 95% CI | HR | 95% CI | HR | 95% CI |
| Control | Ref. | Ref. | Ref. | |||
| Remdesivir, interval ≤9 days | 0.25* | 0.111–0.564 | 0.16* | 0.052–0.478 | 0.10* | 0.025–0.428 |
| Remdesivir, interval 10+ days | 0.73 | 0.279–1.911 | 0.57 | 0.194–1.671 | 0.42 | 0.117–1.524 |
| Age (years) | ||||||
| −39 | Ref. | Ref. | Ref. | |||
| 40–64 | 0.22 | 0.024–2.082 | 0.21 | 0.019–2.391 | 0.18 | 0.013–2.367 |
| 65+ | 0.65 | 0.076–5.551 | 1.04 | 0.097–11.131 | 0.78 | 0.059–10.366 |
| Sex | ||||||
| Female | Ref. | Ref. | Ref. | |||
| Male | 0.83 | 0.302–2.304 | 1.29 | 0.387–4.300 | 1.18 | 0.340–4.106 |
| Number of comorbidities | ||||||
| 0 | Ref. | Ref. | ||||
| 1 | 3.02* | 1.123–8.111 | 3.62* | 1.244–10.562 | ||
| 2+ | 3.13* | 1.088–8.986 | 5.53* | 1.747–17.526 | ||
| Date of admission | ||||||
| 1st–2nd wave | Ref. | Ref. | ||||
| 3rd wave | 1.85 | 0.656–5.218 | 2.40 | 0.736–7.802 | ||
| 4th wave | 1.34 | 0.371–4.868 | 1.97 | 0.439–8.842 | ||
| 5th wave | 5.74* | 1.423–23.146 | 7.04* | 1.311–37.821 | ||
| eGFR (ml/min/1.73 m2) | ||||||
| 30+ | Ref. | |||||
| <30 | 0.82 | 0.276–2.415 | ||||
| ALT (IU/L) | ||||||
| <115 | Ref. | |||||
| 115+ | 3.27 | 0.497–21.557 | ||||
| Baseline oxygen demands | ||||||
| Low flow oxygen or oxygen free | Ref. | |||||
| High flow oxygen or Ventilator | 2.11 | 0.559–7.951 | ||||
| Lung opacity volume (%) tertiles | ||||||
| Q1: median 19.6 (0.18–30.8) | Ref. | |||||
| Q2: median 43.6 (31.2–56.4) | 3.10* | 1.011–9.479 | ||||
| Q3: median 65.5 (56.5–94.4) | 1.14 | 0.357–3.645 | ||||
Note: First–second wave (April–October/2020), second wave (November/2020–February/2021), fourth wave (March–June/2021), fifth wave (July–November/2021). Model 1: Adjusted for age and sex. Model 2: Additionally adjusted for the number of risk comorbidities and the date of admission. Model 3: Additionally adjusted for renal dysfunction, liver dysfunction, baseline oxygen demand, and lung opacity volume.
Abbreviations: ALT, alanine aminotransferase; CI, confidence interval; COVID‐19, coronavirus disease 2019; eGFR, Estimation of glomerular filtration rate; HR, hazard ratio.
p < 0.05.
3.3. Adverse events of remdesivir
Eight patients who did not complete remdesivir for less than 5 days, due to adverse events including allergic‐type reaction (rash) (n = 1), decrease in eGFR <30 ml/min/1.73 m2 (n = 2), and due to recovered respiratory status (n = 5). During treatment with remdesivir, the incidence of AKI was 15.0% (20/133) and liver injury was 20.3% (27/133).
3.4. Sensitivity analysis
Excluding the 41 patients (n = 127), Kaplan–Meier analysis revealed a significantly lower in‐hospital mortality rate for patients who used remdesivir within 9 days from symptom onset compared to the control group (log‐rank test: p < 0.001; Figure 2). Multivariate Cox proportional hazard regression of the full model showed that remdesivir use (interval ≤9 days) showed a 90% reduction of mortality risk (HR: 0.11; 95% CI: 0.022–0.572) compared to the control group. Conversely, the association of remdesivir use (interval 10+ days) with in‐hospital mortality was not significant (HR: 0.86; 95% CI: 0.236–3.107).
Figure 2.
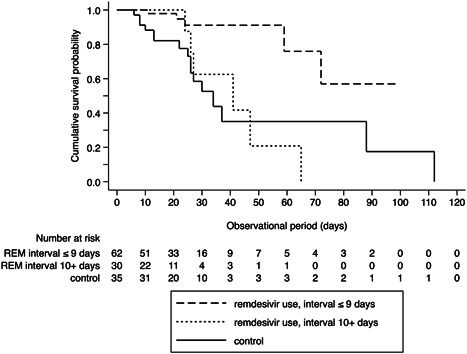
Sensitivity analysis: Kaplan–Meier survival curves excluding 39 patients who had started remdesivir (REM) before admission to Tokyo Medical and Dental University Hospital and 2 patients whose computed tomography scan for pneumonia evaluation was taken after the start of remdesivir. *Statistically significant using Bonferroni‐adjusted α critical value = 0.025. Control versus remdesivir use (interval ≤9 days): Log rank p < 0.001*; control versus remdesivir use (interval 10+ days): log rank p = 0.548.
4. DISCUSSION
Among COVID‐19 patients who received corticosteroids, remdesivir use within 9 days from symptom onset was effective to reduce in‐hospital mortality in an actual ICU setting. Conversely, the association of remdesivir use with 10 days after symptom onset and in‐hospital mortality was not effective in reducing mortality. Current findings were inconsistent with those of previous RCTs, such as RCTs 1 , 2 , 4 , 19 and the primary outcome of a large WHO‐sponsored RCT, 3 which showed that remdesivir did not statistically improve mortality rates compared to placebo or standard care only. However, Adaptive COVID‐19 Treatment Trial (ACTT‐1), 1 international Phase III study, and propensity score matching (PSM) study in the United States 20 showed a nonsignificant trend toward lower risk of mortality in remdesivir use. Additionally, in a large PSM study from the Premier Healthcare Database in the United States (28 855 pairs), remdesivir was reported to be associated with a reduction in mortality at 14 and 28 days (HR: 0.89; 95% CI: 0.82–0.96). Another PSM that included COVID‐19 patients with pneumonia in several countries 22 also showed that Day 28 all‐cause mortality rate was significantly lower in the remdesivir cohort than in the nonremdesivir cohort (odds ratio: 0.67; 95% CI: 0.47–0.95). Thus, while remdesivir may not have sufficient efficacy, as shown in previous RCTs, it is effective, as shown in previous observational studies (which is consistent with the current study).
The effect size shown in the present study was larger than the previous studies, which may be attributed to racial differences. In ACTT‐1, 1 more than 50% of the patients were Caucasian and only 12.7% were Asian. In one PSM study, 20 80% of patients were non‐Caucasian but Black or Latinx. The PSM studies that reported the mortality benefit of remdesivir 21 , 22 had a matched population of almost 70% Caucasian. There was an RCT of remdesivir targeting Asians (China) in the early stages of the SARS‐CoV‐2 epidemic 4 ; however, only moderate cases were enrolled, which were different from the target cases of this study. The present study included Japanese and 4.8% of other Asians who had severe to critical COVID‐19, indicating that remdesivir is associated with decreased mortality in these populations.
Another possible explanation for the larger effect size could be the severity of cases. Previous studies 20 , 21 , 22 included only 24.5%–30.5% of participants who required HFO or IMV support at baseline. Even in two RCTs that enrolled patients with pneumonia and SpO2 94% or lower, 1 , 19 the number of patients requiring HFO or IMV at enrollment was limited (40‐45%). In contrast, 78.0% of our samples were patients with HFO or IMV support at baseline. A PSM study with subgroup analysis for HFO and IMV/ECMO at baseline, 22 with a mean duration of symptoms of remdesivir‐treated patients before 9.1 days, found no mortality benefit. However, another large PSM study, 21 which excluded patients transferred from other hospitals and with remdesivir likely to be administered relatively early, showed an association between remdesivir and a reduction in mortality. In addition, the RCT from China 4 reported a trend toward mortality benefit in a subgroup of patients treated with remdesivir within 10 days. Meanwhile, the ACTT‐1 1 showed a statistically significant association with remdesivir and rate ratio for recovery only in the subgroup who underwent randomization during the first 10 days after the onset in a post hoc analysis. These data indicate that remdesivir is more effective when administered early from the onset. Although the ACTT‐1 1 did not show a recovery effect of remdesivir in HFO and IMV/ECMO cases, it is hypothesized that the effectiveness of remdesivir could have been indicated in the present study by considering the timing of treatment.
In addition, the use of corticosteroids may change the effect size of remdesivir. That is, the ACTT‐1 1 and the DisCoVeRy trial 19 also included corticosteroid‐free cases, but we targeted only patients who received corticosteroids. In this study, which included a large number of patients with severe pneumonia, there may have been a synergistic effect of the corticosteroid combination. 23
The reason why remdesivir was effective for the patients <9 days from onset, but not for 10+ days, may be due to the viral load of SARS‐CoV‐2. In a report of necropsy of SARS‐CoV‐2‐infected rhesus macaques 7 days after remdesivir administration, 11 remdesivir metabolites were found in the lower respiratory tract and had lower lung viral loads and reduced lung damage, which indicates that it is desirable to administer remdesivir before or immediately after lung damage. A peak viral load in the respiratory tract was reported to be occurring around the 10th day of illness in the SARS‐CoV 24 and around 5–6th day in the SARS‐CoV‐2. 25 One RCT 26 found that remdesivir treatment for COVID‐19 outpatients at risk of severe disease within 7 days of onset significantly reduced COVID‐19‐related hospitalization. Furthermore, as the observational study from India reported that remdesivir treatment interval ≤9 days from symptom onset had a significantly lower all‐cause mortality than those with interval 10+ days, 5 it is estimated that remdesivir should be administered within a few days of the peak SARS‐CoV‐2 viral load in the respiratory tract. On IMV requiring patients, observational study from Italy 27 reported an interaction between the time elapsed from intubation to remdesivir use and discharge. In the present study, the median (IQR) time from onset to intubation for all intubated patients was 8.9 (±4.0) days, and the proportion of patients who received remdesivir before intubation was significantly higher in the ≤9 days group, 91.4%, compared with 63.3% in the 10+ days group (p < 0.001). Similar to the previous observational study, early remdesivir treatment was also associated with improved survival in this population. This trend remained consistent in the sensitivity analysis.
In the clinical practice, we controlled the effect of degree of pneumonia, one of the criteria for remdesivir administration, although previous studies did not account for it. In the present study, CT opacity volume was significantly wider when baseline oxygen demand was HFO or IMV (median 51.8% vs. 21.5%, p < 0.01). However, in Cox's full model, there was no increase in the effect size of HR with increasing opacity volume quantile. Previous studies have reported a correlation between the timing of CT examination and the opacity volume, 28 and lung damage may differs between ground‐glass opacity and consolidation. Hence, it is necessary to consider not only the volume of opacity but also the concentration and timing of CT examination to estimate the severity of pneumonia from CT images of the lungs.
Those who had ALT or aspartate transaminase >5 times the upper normal limit or creatinine clearance <50 ml/min using the Cockcroft–Gault equation was excluded from ACTT‐1 1 due to the hepatic and renal dysfunction criteria for the administration of remdesivir. Although the present study included a patient population that was excluded from the ACTT‐1, 1 during treatment with remdesivir, the incidence of AKI and liver injury was 15%–20% and only 3 (1.8%) patients discontinued treatment within 5 days due to side effects. As the liver and renal dysfunction is a limiting factor for remdesivir use, it is likely that some populations could have benefited from remdesivir with detailed monitoring by blood sampling.
There are several limitations in the present study. First, the sample size was relatively small and was limited to a single center. However, continuous observation at the same facility allowed us to assess the outcome under a consistent treatment central member. Second, we have censored transfers and discharges in the time‐to‐event analysis. In fact, the risk of death after discharge or transfer is lower for patients who continue to be treated in the ICU. Although the risk was not completely eliminated, 90% of patients had recovered to low‐flow oxygen at the time of transfer, with the mortality rate after ICU discharge in COVID‐19 to be only 1% within the first year. 29 Therefore, it is not considered a major bias to treat discharged or back‐transferred patients as censored. Third, SARS‐CoV‐2 variant and vaccine information could not be adjusted due to limited data. Therefore, indirect adjustment by epi waves and vaccination coverage proportion was adopted. Despite these limitations, the present study is the first to show the effectiveness of remdesivir on mortality in routine clinical practice in the Japanese ICU. It provides strong evidence of the importance of considering the interval since symptom onset of illness when using remdesivir for critically ill patients.
5. CONCLUSIONS
Previous RCTs did not show a survival benefit of remdesivir, but if early administration was possible, real clinical practice has shown that survival benefits even in critically ill patients.
AUTHOR CONTRIBUTIONS
Mariko Hanafusa: Conceptualization; methodology; formal analysis; writing–original draft. Nobutoshi Nawa: Formal analysis; writing–review and editing. Yuki Goto: Investigation; resources. Tomoki Kawahara: Resources; formal analysis. Shigeru Miyamae: Investigation; resources; writing–review and editing. Yutaka Ueki: Investigation; resources; writing–review and editing. Nobuyuki Nosaka: Writing–review and editing. Kenji Wakabayashi: Writing–review and editing. Shuji Tohda: Investigation; resources; writing–review and editing. Ukihide Tateishi: Investigation; resources; writing–review and editing. Takeo Fujiwara: Conceptualization; writing–review and editing, supervision. All authors have read and agreed to the published version of the manuscript.
ETHICS STATEMENT
Considering the retrospective study design and complete anonymization, the patients included in the study were not required to give their consent to participate in the study. We applied opt‐out method to obtain consent on this study by posting the research content on the website of the TMDU Bioethics Research Center. Ethical approval was obtained from Ethics Committee at TMDU (M2021‐167).
Supporting information
Supplementary information.
ACKNOWLEDGMENTS
The authors are grateful to the members of the Department of Diagnostic Radiology, Tokyo Medical and Dental University, Koichiro Kimura, Takuya Adachi, Kanae Takahashi, Megumi Uemichi, Hiroto Hada, Ryosuke Kaho, Kohei Maehara, Takuya Watanabe, Takumi Hiraishi, and Kosuke Inoue for providing the quantitative volume data of lung in this paper.
Hanafusa M, Nawa N, Goto Y, et al. Effectiveness of remdesivir with corticosteroids for COVID‐19 patients in intensive care unit: a hospital‐based observational study. J Med Virol. 2022;1‐10. 10.1002/jmv.28168
DATA AVAILABILITY STATEMENT
The data are available on reasonable request from the corresponding author. For privacy restrictions, the data are not publicly available.
REFERENCES
- 1. Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of Covid‐19—final report. N Engl J Med. 2020;383(19):1813‐1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Spinner CD, Gottlieb RL, Criner GJ, et al. Effect of remdesivir vs standard care on clinical status at 11 days in patients with moderate COVID‐19: a randomized clinical trial. JAMA. 2020;324(11):1048‐1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Consortium WHOST, Pan H, Peto R, et al. Repurposed antiviral drugs for Covid‐19—interim WHO solidarity trial results. N Engl J Med. 2021;384(6):497‐511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wang Y, Zhang D, Du G, et al. Remdesivir in adults with severe COVID‐19: a randomised, double‐blind, placebo‐controlled, multicentre trial. Lancet. 2020;395(10236):1569‐1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mehta RM, Bansal S, Bysani S, Kalpakam H. A shorter symptom onset to remdesivir treatment (SORT) interval is associated with a lower mortality in moderate‐to‐severe COVID‐19: a real‐world analysis. Int J Infect Dis. 2021;106:71‐77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ogura T, Ohshimo S, Liu K, Iwashita Y, Hashimoto S, Takeda S. Establishment of a disaster management‐like system for COVID‐19 patients requiring veno‐venous extracorporeal membrane oxygenation in Japan. Membranes. 2021;11(8):625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. ECMOnet J. COVID‐19 critical care status from CRoss Icu Searchable Information System (CRISIS). 2022. [Google Scholar]
- 8. Armstrong RA, Kane AD, Cook TM. Outcomes from intensive care in patients with COVID‐19: a systematic review and meta‐analysis of observational studies. Anaesthesia. 2020;75(10):1340‐1349. [DOI] [PubMed] [Google Scholar]
- 9. Pasquini Z, Montalti R, Temperoni C, et al. Effectiveness of remdesivir in patients with COVID‐19 under mechanical ventilation in an Italian ICU. J Antimicrob Chemother. 2020;75(11):3359‐3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lim ZJ, Subramaniam A, Ponnapa Reddy M, et al. Case fatality rates for patients with COVID‐19 requiring invasive mechanical ventilation. A meta‐analysis. Am J Respir Crit Care Med. 2021;203(1):54‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Williamson BN, Feldmann F, Schwarz B, et al. Clinical benefit of remdesivir in rhesus macaques infected with SARS‐CoV‐2. Nature. 2020;585(7824):273‐276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Takahashi M, Fujioka T, Horii T, et al. Can deep learning‐based volumetric analysis predict oxygen demand increase in patients with COVID‐19 pneumonia? Medicina. 2021;57(11):1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Arru C, Ebrahimian S, Falaschi Z, et al. Comparison of deep learning, radiomics and subjective assessment of chest CT findings in SARS‐CoV‐2 pneumonia. Clin Imaging. 2021;80:58‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. World Health Organization. Corticosteroids for COVID‐19. 2020. [Google Scholar]
- 15. Doi K, Nishida O, Shigematsu T, et al. The Japanese Clinical Practice Guideline for acute kidney injury 2016. J Intensive Care. 2018;6:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ishii E, Nawa N, Matsui H, Otomo Y, Fujiwara T. Comparison of disease patterns and outcomes between non‐Japanese and Japanese patients at a single tertiary emergency care center in Japan. J Epidemiol. 2022;32(2):80‐88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Terada M, Ohtsu H, Saito S, et al. Risk factors for severity on admission and the disease progression during hospitalisation in a large cohort of patients with COVID‐19 in Japan. BMJ Open. 2021;11(6):e047007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. The Ministry of Health LaW, Japan. Visualizing the Data: Information on COVID‐19 Infections. 2022. [Google Scholar]
- 19. Ader F, Bouscambert‐Duchamp M, Hites M, et al. Remdesivir plus standard of care versus standard of care alone for the treatment of patients admitted to hospital with COVID‐19 (DisCoVeRy): a phase 3, randomised, controlled, open‐label trial. Lancet Infect Dis. 2022;22(2):209‐221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Garibaldi BT, Wang K, Robinson ML, et al. Comparison of time to clinical improvement with vs without remdesivir treatment in hospitalized patients with COVID‐19. JAMA Netw Open. 2021;4(3):e213071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mozaffari E, Chandak A, Zhang Z, et al. Remdesivir treatment in hospitalized patients with COVID‐19: a comparative analysis of in‐hospital all‐cause mortality in a large multi‐center observational cohort. Clin Infect Dis. 2022;75(1):e450‐e458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Olender SA, Walunas TL, Martinez E, et al. Remdesivir versus standard‐of‐care for severe coronavirus disease 2019 infection: an analysis of 28‐day mortality. Open Forum Infect Dis. 2021;8(7):ofab278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Group RC, Horby P, Lim WS, et al. Dexamethasone in hospitalized patients with Covid‐19. N Engl J Med. 2021;384(8):693‐704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Peiris JS, Chu CM, Cheng VC, et al. Clinical progression and viral load in a community outbreak of coronavirus‐associated SARS pneumonia: a prospective study. Lancet. 2003;361(9371):1767‐1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pan Y, Zhang D, Yang P, Poon LLM, Wang Q. Viral load of SARS‐CoV‐2 in clinical samples. Lancet Infect Dis. 2020;20(4):411‐412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gottlieb RL, Vaca CE, Paredes R, et al. Early remdesivir to prevent progression to severe Covid‐19 in outpatients. N Engl J Med. 2022;386(4):305‐315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lapadula G, Bernasconi DP, Bellani G, et al. Remdesivir use in patients requiring mechanical ventilation due to COVID‐19. Open Forum Infect Dis. 2020;7(11):ofaa481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pan F, Ye T, Sun P, et al. Time course of lung changes at chest CT during recovery from coronavirus disease 2019 (COVID‐19). Radiology. 2020;295(3):715‐721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ceccato A, Perez‐Arnal R, Motos A, Barbe F, Torres A, Ciberes UC. One‐year mortality after ICU admission due to COVID‐19 infection. Intensive Care Med. 2022;48:366‐368. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information.
Data Availability Statement
The data are available on reasonable request from the corresponding author. For privacy restrictions, the data are not publicly available.


