Abstract
Over 10 million doses of COVID‐19 vaccines based on RNA technology, viral vectors, recombinant protein, and inactivated virus have been administered worldwide. Although generally very safe, post‐vaccine myocarditis can result from adaptive humoral and cellular, cardiac‐specific inflammation within days and weeks of vaccination. Rates of vaccine‐associated myocarditis vary by age and sex with the highest rates in males between 12 and 39 years. The clinical course is generally mild with rare cases of left ventricular dysfunction, heart failure and arrhythmias. Mild cases are likely underdiagnosed as cardiac magnetic resonance imaging (CMR) is not commonly performed even in suspected cases and not at all in asymptomatic and mildly symptomatic patients. Hospitalization of symptomatic patients with electrocardiographic changes and increased plasma troponin levels is considered necessary in the acute phase to monitor for arrhythmias and potential decline in left ventricular function. In addition to evaluation for symptoms, electrocardiographic changes and elevated troponin levels, CMR is the best non‐invasive diagnostic tool with endomyocardial biopsy being restricted to severe cases with heart failure and/or arrhythmias. The management beyond
guideline‐directed treatment of heart failure and arrhythmias includes non‐specific measures to control pain. Anti‐inflammatory drugs such as non‐steroidal anti‐inflammatory drugs, and corticosteroids have been used in more severe cases, with only anecdotal evidence for their effectiveness. In all age groups studied, the overall risks of SARS‐CoV‐2 infection‐related hospitalization and death are hugely greater than the risks from post‐vaccine myocarditis. This consensus statement serves as a practical resource for physicians in their clinical practice, to understand, diagnose, and manage affected patients. Furthermore, it is intended to stimulate research in this area.
Keywords: COVID‐19, Inflammation, Myocarditis, Outcomes, Pathology, Vaccination
Overview on incidence, diagnosis, and therapy in vaccine related myocarditis.
Introduction
The rapid spread of coronavirus 2019 (COVID‐19), which overwhelmed healthcare systems around the world within weeks, required the rapid development and introduction of novel methods of disease prevention and treatment. Vaccines using a broad range of different technologies have been developed with an unprecedented speed, were tested in large randomized clinical trials and utilized broadly once Emergency Use Authorization had been granted to reduce the spread of the infection with the goal of achieving immunity across the entire population. Indeed, as of this day, more than 10 billion doses have been administered worldwide. 1
While many praised the efficiency with which these novel vaccines have been developed, a considerable percentage of the population remained skeptical, as the concept and mechanisms of some of the most commonly used COVID‐19 vaccines, in particular those using RNA technology, are rather distinct from classic vaccines using better known platforms. These concerns have been further amplified by a surge of reports of vaccine‐related complications after the most commonly used vaccine types against severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) such as adenovirus‐based and mRNA‐based vaccines. 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12
One of the most discussed complications affecting the heart is myocarditis and/or pericarditis. The goal of the current review article is to provide clinicians and scientists with an objective and comprehensive overview of the data regarding the clinical presentation, diagnosis, pathophysiology and management of myocarditis following the most widely utilized COVID‐19 vaccines (Graphical Abstract). Furthermore, the incidence of COVID‐19 vaccine‐related myocarditis is compared with most recent data of COVID‐19 infection‐induced myocarditis. Further, this is brought into perspective with the expected background incidence of the condition collected during years prior to the pandemic.
One of the main goals of this article is to objectively report potential adverse outcomes after vaccination as this is crucial to raise awareness amongst physicians and scientists by providing robust data on the prevalence, incidence and clinical presentation of patients with this potential complication. Phenotypic characterization of the clinical presentation of vaccine‐related myocarditis is also important to identify affected patients early and provide timely therapy where required. This is relevant, as myocarditis can lead to severe arrhythmias and is one of the most common causes of sudden cardiac death in young adults based on autopsy studies. 13 Furthermore, insight into vaccine‐associated myocarditis may help to potentially identify markers for those who will develop this adverse event and to improve the development the next generation of vaccines.
Epidemiology of vaccine‐related myocarditis
Until recently, vaccine‐associated myocarditis has been reported in the literature as a very rare adverse event predominantly in the context of live attenuated smallpox vaccine and has only been described in case reports for other vaccines. 14 The implementation of the COVID‐19 vaccine using novel medical technology (i.e. RNA‐based vaccines) into phase 3 trials and their clinical implementation was followed by population‐based tracking of outcomes and complication rates. For example, the United States deployed the passive reporting Vaccine Adverse Event Reporting System (VAERS) that tracked outcomes in about 200 million individuals, and similar systems are in place in numerous other countries, in particular the United Kingdom and Israel. Using information from this large database, it was quickly recognized that there was a low, but consistent rate of patients presenting with post‐vaccination myocarditis and/or pericarditis. For example, data obtained from VAERS have shown that the incidence peaks in young males of 15–17 years with 105.9 cases per million doses administered and identified the second dose as the highest risk compared to the first dose.
Reporting bias
Several issues must be considered when evaluating the risk of vaccine‐induced myocarditis. First, there is a background risk of myocarditis in the general population as well. It is well known that this risk varies with time and geographic location, reflecting the important, but not exclusive role played by the virus pandemic. Second, the risk of the SARS‐CoV‐2 virus itself has to be considered. 15 It was shown by the US Center for Disease Control and Prevention (CDC) that infection with SARS‐CoV‐2 increases the risk of myocarditis by 16‐fold from 9 cases per 100 000 to 150 cases per 100 000. 16 Third, another crucial issue when considering the prevalence and incidence of vaccine‐associated myocarditis is the fact that the disease presents across a spectrum of severity and symptomatology. Thus, the available data primarily reflect detection of myocarditis in those with more than minimally symptomatic disease and as such the true extent of myocarditis related to mRNA vaccination against SARS‐CoV‐2 is unknown as it would require systematic evaluation by electrocardiogram (ECG), troponin and cardiac magnetic resonance imaging (CMR) and/or endomyocardial biopsy (EMB) data in a much larger population of individuals receiving the vaccine. While CMR is a gold‐standard method to detect myocarditis non‐invasively, it does not provide a definitive diagnosis of myocarditis. Given that there were no control populations, the potential consequences of the vaccines based on CMR may have been potentially overestimated. Having a control population would be valuable in a study that investigates the effect of vaccines by CMR. Rather, the available data primarily reflect the experience of populations eligible to receive vaccine, who have access to care and diagnosis, and who have experienced symptoms within days of receiving a vaccine that led to a physician visit and a diagnostic work‐up. In addition, there is a reporting bias across time as popular media transmitted the concern regarding myocarditis to the general public, increasing the probability that patients with symptoms would seek care. As a result, the US studies tend to primarily reflect passively reported cases gathered through the VAERS, and other such mechanisms. 17
Finally, not all health care, with a few exceptions (see below) were well prepared for close monitoring of post‐vaccination symptoms and complications.
Incidence estimates worldwide
Table 1 summarizes data on COVID‐19 vaccine‐related myocarditis derived from population studies and clinical trials with a focus on mRNA vaccines. 7 , 8 , 9 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 It is worth mentioning that the age and sex distribution of the cohorts as well as differences in diagnosing and defining myocarditis and appropriate reporting do limit the comparability and data interpretation of such studies to an important degree. Indeed, different criteria for the diagnosis of myocarditis had been used, when those registries on vaccination‐related myocarditis were established in various parts of the world. Currently, efforts are underway to develop more uniform criteria for the diagnosis of myocarditis to facilitate comparability and merging of international data in a healthcare crisis such as the COVID‐19 pandemic and to collaborate in research projects at a larger scale.
Table 1.
Summary of studies related to COVID‐19 post‐vaccination myocarditis
| Type of study | Data source | Type of vaccine | Total study population (n) | Total cases of myocarditis (n) | Fulminant myocarditis (n) | Cumulative incidence | Highest incidence | Characteristics of group with highest incidence | Male (%) | Method of diagnosis | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Population study | Danish nationwide registry |
BNT162b2 mRNA vaccine (Pfizer‐BioNTech) mRNA‐1273 vaccine (Moderna) Ad26.COV2.S vaccine (adenovirus, Janssen/Johnson&Johnson) |
4 931 775 3 981 109 (received) BNT162b2 mRNA and mRNA‐1273 vaccine |
69 (among BNT162b2 mRNA and mRNA‐1273 vaccine) | NA | 1.7 per 100 000 individuals after mRNA vaccine | 5.7 per 100 000 individuals after mRNA vaccine |
mRNA‐1273 vaccine in 12–39 years BNT162b2 mRNA vaccine in women 12–39 years |
73 | ICD‐10 codes for myocarditis I40.0, I40.1, I40.9, I41.1, I41.8, I51.4 | Husby et al.19 |
| Population study | KPSC System |
50%: BNT162b2 mRNA vaccine (Pfizer‐BioNTech) |
1 dose: 2 392 924 | 2 | 0 | 0.08 (0.02–0.33) per 100 000 individuals | 0.58 per per 100 000 individuals | 2 doses | 100 | Reports from clinicians to KPSC | Simone et al.20 |
|
50%: mRNA‐1273 vaccine (Moderna) |
2 doses: 2 236 851 | 13 | 0.58 (0.34–1) per 100 000 individuals | ||||||||
| Population study | Beth Israel Deaconess Medical Center; Massachusetts Information System |
BNT162b2 mRNA vaccine (Pfizer‐BioNTech) mRNA‐1273 vaccine (Moderna) Ad26.COV2.S vaccine (adenovirus, Janssen/Johnson&Johnson) ChAdOx1 vaccine (adenovirus, AstraZeneca) |
268 320 | 10 | NA | 3.72 per 100 000 individuals | NA | 2 doses of RNA vaccine | 50 | ESC diagnostic criteria | Farahmand et al.7 |
| Pharmacovigilance study | Moderna global safety database | mRNA‐1273 vaccine (Moderna) | Approx. 151 100 000 | 1439 | 21 | 0.95 per 100 000 individuals | 7.40 per 100 000 individuals |
Male 18–24 years 2 doses |
78 | Collection: mostly based on adverse event reports submitted voluntarily. Verification: definition of the Brighton Collaboration and CDC | Straus et al.18 |
| Population study | Clalit Health Services (largest national healthcare organization) | BNT162b2 mRNA vaccine (Pfizer‐BioNTech) | 2 558 421 | 54 | 1 | 2.13 (1.56–2.70) per 100 000 individuals | 10.69 (6.93–14.46) per 100 000 individuals |
Male 16–29 years |
94 | Screening: ICD‐9 (codes 422, 429.0, 398.0 and 391.2). Verification: definition of the CDC 1 | Witberg et al.8 |
| Population study | Ministry of Health | BNT162b2 mRNA vaccine (Pfizer‐BioNTech) | 1 dose: 5 442 696 | 19 | 1 | 0.34 per 100 000 individuals | 1.91 per 100 000 individuals |
Male 20–24 years |
89 | Screening: ICD‐9 (codes 422.0‐9x and 429.0x). Verification: definition of the Brighton Collaboration | Mevorach et al.9 |
| 2 doses: 5 125 635 | 117 | 2,28 per 100 000 individuals | 15.07 per 100 000 individuals |
Male 16–19 years |
86 | ||||||
| Safety and efficacy study | Multinational, phase 3, placebo‐controlled, observer‐blinded trial, in 16‐year and older individuals | BNT162b2 mRNA vaccine (Pfizer‐BioNTech) | 21 720 | 0 | 0 | 0 | 0 | NA | NA | NA | Polack et al.22 |
| Safety and efficacy study | Ongoing phase 2–3, placebo‐controlled trial, in 12–17‐year‐old adolescents | mRNA‐1273 vaccine (Moderna) | 2489 | 0 | 0 | 0 | 0 | NA | NA | NA | Ali et al.23 |
| Population study | US Military Health System |
BNT162b2 mRNA vaccine (Pfizer‐BioNTech) mRNA‐1273 vaccine (Moderna) |
2 810 000 | 23 | NA | 0.82 per 100 000 doses | 1.9 per 100 000 doses | 2 doses | 100 | Vaccine Adverse Events Reporting System | Montgomery et al.21 |
| Safety and efficacy study | Multinational, placebo‐controlled, observer‐blinded trial, in 12–15‐year‐old adolescents | BNT162b2 mRNA vaccine (Pfizer‐BioNTech) | 2260 | 0 | 0 | 0 | 0 | NA | NA | NA | Frenck et al.24 |
| Safety and efficacy study | Multicentre (US), phase 3, placebo‐controlled, observer‐blinded trial, persons at high risk for SARS‐CoV‐2 infection or its complications | mRNA‐1273 vaccine (Moderna) | 15 210 | 0 | 0 | 0 | 0 | NA | NA | NA | Baden et al.25 |
CDC, Centers for Disease Control and Prevention; ESC, European Society of Cardiology; ICD, International Classification of Diseases; KPSC, Kaiser Permanente Southern California; NA, not available; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2.
Furthermore, proving causality is a particular challenge. A best effort was undertaken to choose studies that are comparable. Population studies included data of several million individuals. A pharmacovigilance study by the company Moderna® that is currently in the preprint stage contains data on more than 151 million individuals. In this study, the incidence of vaccine‐related myocarditis was 0.95 per 100 000 individuals and was highest in men aged 18–24 years, who had received two doses of the vaccine. 18 Similar to that study, young men have also been reported in other population studies as a group at particular high risk, especially after the second dose of an mRNA vaccine. 7 , 8 , 9 , 19 , 20 , 21 A recent head‐to‐head comparison of BNT162b2 and mRNA‐1273 by the Vaccine Safety Datalink (part of a collaboration between the CDC and integrated healthcare organizations) revealed modestly higher risk for myocarditis and pericarditis after mRNA‐1273 than after BNT162b2. 26 Similar findings were observed in a cohort study of claims databases in the United States. 27
A recent population study investigated the risk for myocarditis in 12–15‐year‐old adolescents after receiving BNT162b2. Data for the incidence of hospitalization for myocarditis between June and October 2021 were collected by the Israeli Ministry of Health (IMoH). The risk estimates of myocarditis after the second dose among male recipients were 8.09 cases/100 000 and among female recipients 0.69 cases/100 000. The risk of myocarditis after receipt of the second vaccine among 12–15‐year‐old male adolescents was estimated to be 1/12 361, but 1/144 439 among female adolescents. 28 When interpreting these data, it should be considered that myocarditis was not confirmed by EMB and that only hospitalization records were acquired.
Incidence estimates in the United Kingdom
Patone and colleagues 29 investigated in the English National Immunisation (NIMS) Database of COVID‐19 the development of myocarditis, pericarditis, or arrhythmias post‐vaccination with BNT162b2 mRNA (Pfizer‐BioNTech®), ChAdOx1 adenovirus‐based vaccine (AstraZeneca®), or mRNA‐1273 vaccine (Moderna®). Since some patients in this study tested positive for SARS‐CoV‐2 virus before or after the vaccine, causality is difficult to exclusively attribute to the vaccine, particularly because of the incubation time of the infection of around 5 days and the fact that the immune response to the vaccine also requires a few days. Indeed, some patients may have developed myocarditis due to SARS‐CoV‐2 infection as they had received simultaneously the vaccine. 3 Overall, the authors concluded that there was a small increase in risk of myocarditis within a week of receiving the first dose of both adenovirus and mRNA vaccines and after the second dose of mRNA vaccines, whilst the increase was much greater after SARS‐CoV‐2 infection. Initial safety and efficacy studies including more than 20000 participants did not detect any cases of myocarditis post vaccine, likely due to the smaller sample size and the rare occurrence of this adverse event.
Vaccine rollout and complications in Israel
While clinical trials are the gold standard to estimate both vaccine efficacy and potential side effects of new medical technologies including vaccines, due to their size, they are inherently limited in their ability to detect rare side effects. Thus, continuous post‐marketing surveillance based on observational data is warranted. Such surveillance enabled the IMoH to be the first to report a possible link between myocarditis and mRNA SARS‐CoV‐2 vaccination in June 2021. 30
Israel has been a global source of timely real‐world evidence on vaccine safety and effectiveness 11 , 31 , 32 as its relatively centralized public healthcare system, with four integrated healthcare payer‐provider organizations, allowed very efficient vaccination campaigns. For example, in less than 10 weeks, over half of the Israeli population received the first vaccine dose. 33 Israel was also among the first countries in the world to mass‐vaccinate its population with third and fourth doses.
Importantly, the Israeli healthcare organizations maintain extensive healthcare data repositories within electronic health records (EHR) systems that have been implemented nationally over the last two decades. As the Israeli healthcare organizations serve both as insurers and health providers, their data repositories include both claims data and EHR‐based data of aspects of care. 34 These data repositories were augmented with centralized COVID‐19 data reporting systems that were implemented early in the pandemic by the IMoH, including data on polymerase chain reaction (PCR) tests, COVID‐19 hospitalization and mortality, and later COVID‐19 vaccination.
This unique setting of efficient vaccination campaigns and integrated data repositories allowed identification and characterization of myocarditis side effects following the BNT162b2 mRNA (Pfizer‐BioNTech®) COVID‐19 vaccine primarily used in Israel. Vaccine safety monitoring has been coordinated by a national task force, led by the IMoH. Individual reports from physicians and individual patients on suspected adverse events were reported to the IMoH. On 2 June 2021, the IMoH reported that through this surveillance, 148 events of myocarditis temporally associated with vaccination had been recorded and suggested a possible link between the second vaccine dose and myocarditis, most notably among males aged 16–30 years.
This finding was further verified in a large retrospective vaccine‐safety assessment, using a target trial approach to emulate a clinical trial with retrospective EHR data from Clalit ‐ Israel's largest of four state‐mandated health organizations. 11 Over 800 000 vaccinated individuals aged 16 years and over were matched with unvaccinated individuals based on an extensive set of sociodemographic, geographic and health‐related attributes. The most potentially serious adverse event identified was myocarditis, with 2.7 (95% confidence interval 1.0–4.6) excess cases per 100 000 vaccinated individuals aged 16 and over and occurring more frequently among young males (Figure 1 ). This study also provided context for interpreting these vaccine safety findings, by comparing over 170 000 SARS‐CoV‐2‐infected (and unvaccinated) individuals with uninfected matched controls. The infection was associated with 11.0 (5.6–15.8) excess cases of myocarditis per 100 000 infected individuals.
Figure 1.
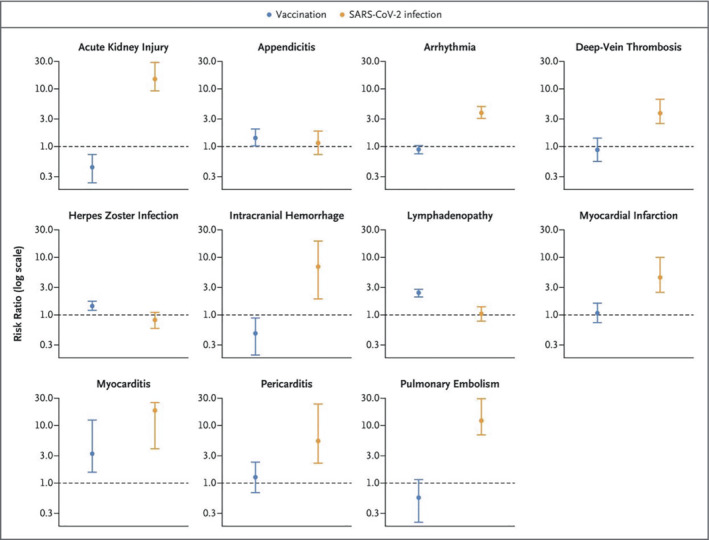
Risk of complications after COVID‐19 vaccine versus with COVID‐19 infection: data were obtained from a national study in Israel. Each cohort consisted of more than 800 000 individuals. Relative risk for developing myocarditis after vaccine was 3.2, while it is 18.3 after getting COVID‐19. From Barda et al. 11
Two assessments based on individual chart‐review case adjudication shed further light on post‐vaccination myocarditis incidence and severity 8 , 9 and confirmed myocarditis cases in excess mainly among young males. The incidence per 100 000 vaccinated males aged 16–29 years was estimated to be 10.7 (6.9–14.5) (Figure 1 ). 8 Both studies found the vast majority of cases to be clinically mild.
Recent data released by the IMoH suggest that the rates of myocarditis following the third dose of the BNT162b2 vaccine were considerably lower than after the second dose in both sexes and different age groups. 35 Data on rates of myocarditis following the fourth vaccine dose are still being collected. Of note, early data further point to the possible benefit of increasing the interval between the first and second mRNA vaccine doses in decreasing the risk of myocarditis. 36
Differences between vaccines
While both adenovirus‐vectored and mRNA vaccines have been associated with myocarditis and pericarditis, mRNA vaccines had the highest incidence. 19 Notably, in large population studies a highly discrepant male: female ratio with involvement of younger individuals, and the higher incidence of myocarditis after the second dose in comparison to the first or third doses was observed worldwide. In addition, possibly due to the higher dose involved, the Moderna vaccine has a higher incidence of post‐vaccine myocarditis compared to the Pfizer vaccine.
Overall, among 18‐ to 29‐year olds in the US, an estimated 22.4 excess cases per million after the second dose of Pfizer‐BioNTech® and 31.2 cases per million second doses after Moderna® vaccine developed myocarditis. In Canada, using passive surveillance reporting systems (CARFISS), among 18‐ to 29‐year olds the myocarditis and/or pericarditis reporting rates after a second dose of Moderna® (140/million doses) was five times higher than Pfizer (25/million doses). 37 In a second Canadian study using enhanced passive surveillance, among 18‐ to 29‐year olds the myocarditis and/or pericarditis reporting rate after a second dose of Moderna® (300/million doses) was five times higher than Pfizer (59/million doses). 38 In the UK, using a passive reporting system, among persons 18–29 years of age, the myocarditis and/or pericarditis reporting rate after a second dose of Moderna® (71/million doses) was 2.5 times higher than Pfizer (24/million doses). In a self‐controlled case series of myocarditis hospitalizations among males below 40 years old, events after a second dose of Moderna® (101/million doses) were over eight times higher than with Pfizer‐BioNTech® (12/million doses). Similarly, rates in Germany were >2 times higher after a second dose of Moderna® (117 per million doses) compared to Pfizer (47/million doses). In France among males 18–24 years of age, that same rate was higher after Moderna® (139/million doses) compared to Pfizer‐BioNTech (43/million doses).
Table 2 illustrates cases of myocarditis for every million second doses of mRNA vaccine administered among persons aged 5 years and older. These are all cases reported in the VAERS and represent cases verified to meet the case definition by provider interview or medical record review. Male adolescents (age 16–17 years) had the highest incidence of myocarditis (75.9 per million doses administered) after receiving the second dose of an mRNA vaccine.
Table 2.
VAERS Reporting Rates of Myocarditis (per million doses administered) after mRNA vaccine, days 0–7
| Vaccine | Age (years) | Males | Females | ||||
|---|---|---|---|---|---|---|---|
| Dose 1 | Dose 2 | Booster | Dose 1 | Dose 2 | Booster | ||
| Pfizer | 5–11 | 0.2 | 2.6 | 0 | 0.2 | 0.7 | 0 |
| Pfizer | 12–15 | 5.3 | 46.4 | 15.3 | 0.7 | 4.1 | 0 |
| Pfizer | 16–17 | 7.2 | 75.9 | 24.1 | 0 | 7.5 | 0 |
| Either | 18–24 | 4.2 | 38.9 | 9.9 | 0.6 | 4.0 | 0.6 |
| Either | 25–29 | 1.8 | 15.2 | 4.8 | 0.4 | 3.5 | 2.0 |
| Either | 30–39 | 1.9 | 7.5 | 1.8 | 0.6 | 0.9 | 0.6 |
| Either | 40–49 | 0.5 | 3.3 | 0.4 | 0.4 | 1.6 | 0.6 |
| Either | 50–64 | 0.5 | 0.7 | 0.4 | 0.6 | 0.5 | 0.1 |
| Either | ≥65 | 0.5 | 0.3 | 0.6 | 0.1 | 0.5 | 0.1 |
Either means either Pfizer or Moderna mRNA vaccine administered. Data as of 26 May 2022.
Bold numbers indicate rates that exceed calculated baseline rate in the population of 0.2–2.2 per million population.
Data presented at the Advisory Committee of the Immunization Practices Committee, June 2022.
In the UK, as of 8 December 2021, there were 507 reports of myocarditis and 365 reports of pericarditis following use of the RNA‐based Pfizer/BioNTech vaccine, 171 reports of myocarditis and 198 reports of pericarditis following use of the adeno‐virus‐based AstraZeneca vaccine, and 111 reports of myocarditis and 63 reports of pericarditis following use of the Moderna vaccine to the Medicines and Healthcare products Regulatory Agency (MHRA). However, many of these are self‐reports, and have not been confirmed by medical adjudication. 39
Overall, we may conclude that the risk of myocarditis is significantly higher after the second dose of an mRNA vaccine, higher in males, and higher after COVID‐19 infection compared to COVID mRNA immunization. A possible explanation of the higher prevalence of such complication in young males may well be related to their higher levels of androgens predisposing to a higher inflammatory and immune response, as also reported in animal models of myocarditis. 40 What remains unclear is whether the prognosis, complications, or severity differ by aetiology.
COVID‐19 myocarditis versus post‐vaccination myocarditis
The prevalence of mRNA vaccine‐associated myocarditis must also be related to the risk of myocarditis after COVID‐19 infection. Recent data presented at the Advisory Committee on Immunization Practices on 4 February 2022, derived from the VAERS database, revealed 359 cases meeting a case definition for myocarditis 0–7 days after vaccination through 13 January 2022, after 164 million administered doses of an mRNA vaccine (Table 3 ). 35
Table 3.
Prevented hospitalizations and excess vaccination‐related myocarditis cases with different COVID‐19 vaccines 35
| Age and sex groups | Hospitalizations prevented | Excess vaccine‐related myocarditis cases |
|---|---|---|
| All 18–39 years old | ||
| mRNA‐1273 vaccine (Moderna®) | 2982 | 33 |
| BNT162b2 mRNA (Pfizer‐BioNTech®) | 2820 | 24 |
| Males 18–39 years old | ||
| mRNA‐1273 vaccine (Moderna®) | 1903 | 68 |
| BNT162b2 mRNA (Pfizer‐BioNTech®) | 1799 | 47 |
The CDC stated that the risk of myocarditis after infection with COVID‐19 was 146 cases per 100 000. The risk was noted to be much higher for males, older adults (age >50 years) and children under 16 years of age. 16 In one study involving healthcare organizations that cover a fifth of the US population, males aged 12–17 years developed myocarditis after COVID‐19 at a rate of about 450 cases per million infections. 16 In males of the same age, after the second dose of mRNA vaccine, 67 cases of myocarditis per million were detected. If myocarditis cases after the first and second dose were added together, 77 cases per million resulted; a rate almost six times lower than after COVID‐19 infection. 16 Another perspective point is a recent study that reported the general effectiveness of the BNT162b2 vaccine in preventing hospitalization for severe COVID‐19 by comparing a vaccinated cohort versus unvaccinated. 41 The effectiveness was reported to be 98% against intensive care unit admission and 98% against COVID‐19 requiring life support. 41
Diagnosis
Clinical presentation of vaccine‐related myocarditis
Early detection of vaccine‐related myocarditis is crucial to prevent potential progression into a more severe clinical course, at a stage when the disease process may still be reversible with appropriate measures. 42
Symptoms typically start within the first few days after administration of the second dose of a vaccine against SARS‐CoV‐2 43 (Table 4 ). Young males are at highest risk for developing myocarditis after COVID‐19 vaccination. 44 Symptoms are generally mild and non‐specific and include chest pain or pressure which may be respiratory‐dependent, shortness of breath, palpitations, malaise, general weakness and fatigue, as well as subfebrile to febrile temperatures. 45 On the other hand, some patients may be asymptomatic, 46 , 47 possibly those with limited pericardial involvement.
Table 4.
Clinical characteristics of vaccination‐related myocarditis
| Symptoms | Signs |
|---|---|
| Chest pain or pressure, may be respiratory‐dependent | Elevated troponins (peak between 48–72 h after symptom onset) |
| Shortness of breath | C‐reactive protein elevation |
| Palpitations | Minor pericardial effusion on transthoracic echocardiography |
| Malaise | Cardiac inflammation on cardiac magnetic resonance imaging |
| General weakness and fatigue |
Electrocardiographic changes (most commonly subtle and non‐specific): Mild diffuse ST‐segment changes PQ segment depressions Non‐specific ST‐segment changes Sinus tachycardia Supraventricular or ventricular arrhythmias (very rare) |
| Subfebrile or febrile temperatures | Clinical signs of heart failure and severe arrhythmias are very rare |
In contrast to virus‐triggered or autoimmune myocarditis, 48 almost all patients who come to medical attention with vaccine‐related myocarditis present with chest pain (95–100%); however, this may reflect a selection bias, reflecting the identification of only symptomatic cases. 47 Troponin levels are elevated in most patients and peak between 48 and 72 h after symptom onset. 21 Inflammatory markers, e.g. C‐reactive protein, may be elevated, especially with concomitant pericarditis. 46 , 47 , 49 , 50 Cardiac inflammation, however, is evident on CMR (see below) in most patients. 4 , 50 , 51 Minor pericardial effusion may be observed, but large effusions are rare. ECG changes are most commonly non‐specific and subtle, such as mild diffuse ST‐segment changes, PQ segment depressions or non‐specific ST‐segment changes, similar to classic myocarditis or myopericarditis (Figure 2 ). Commonly, patients present with sinus tachycardia, while relevant supraventricular or ventricular arrhythmias are very rare. 52 , 53 Most patients with vaccine‐related myocarditis and pericarditis develop mild disease without symptoms of heart failure or fatal arrhythmias. 8 , 29 , 46
Figure 2.
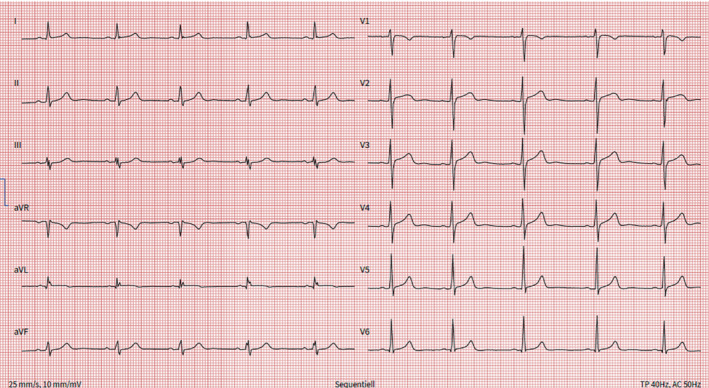
Twelve‐lead electrocardiogram of a 24‐year‐old man who developed severe, stabbing chest pain radiating to both shoulders and markedly elevated high‐sensitivity troponin T levels (peak 635 ng/L) after receiving the BNT162b2 mRNA vaccine as a booster. There are conduction abnormalities (aVL, III, aVF) and non‐specific ST‐segment changes in the precordial leads.
Outcomes are usually favourable. 44 Nevertheless, there have been only few reports in the literature of patients developing severe chest pain, signs and symptoms of heart failure, and haemodynamic instability. 46 , 47 As with background myocarditis, cardiologists must be highly vigilant for severe cases, as fulminant myocarditis associated with lymphocytic, giant cell, and eosinophilic myocarditis has now been reported at the case‐report level, but in a very limited number of cases. 54 , 55 Such patients need early detection and aggressive intervention (see ‘Management of vaccine‐related myocarditis’ section). Indeed, fulminant forms with severe deterioration of contractile function, atrioventricular block, ventricular tachyarrhythmias and syncope, albeit rare, do occur. All these instances may benefit from a morphologic characterization using EMB (see ‘Indications for EMB’ section) as well as molecular studies (PCR for the most common cardiotropic viruses; see ‘Pathology of myocarditis following COVID‐19 vs. COVID‐19 vaccine’ section) to define whether other infectious agents or mechanisms inducing cardiac damage are operating. Indications for treatment with antiviral agents, steroids and/or immunosuppressive therapy may be appropriate in some cases.
A final and crucial consideration is whether myocarditis due to vaccination or by the virus itself increases the long‐term risk of cardiac complications, notably the development of dilated cardiomyopathy or heart failure with preserved ejection fraction. At present no data support such evolution, but it therefore will be crucial to conduct long‐term follow‐up studies of cohorts of individuals who have had virus or vaccine‐induced myocarditis.
Currently, there are no established risk factors to predict outcomes. As in classic myocarditis, patients should be admitted and monitored several days for acute arrhythmias. If patients are clinically improving without evidence for deterioration of cardiac function or major arrhythmias, and troponin levels are decreasing, they may be discharged. Importantly, according to current knowledge, an unfavourable outcome appears very unusual in myocarditis following COVID‐19 vaccination.
Cardiac magnetic resonance imaging
The diagnosis of myocarditis can be challenging, and a definitive diagnosis requires histological confirmation via EMB 56 (see below). CMR offers the non‐invasive assessment of cardiac pathologies including myocarditis, 45 , 57 pericarditis and embolic complications. A position statement by the European Society of Cardiology suggests a diagnosis of clinically suspected myocarditis in the presence of clinical symptoms and at least one of four clinical criteria, one of which is evidence of late gadolinium enhancement (LGE) and/or oedema on CMR. 58 In asymptomatic cases, at least two clinical criteria are required. While CMR has good diagnostic accuracy in ‘infarct‐like’ myocarditis, presenting with chest pain, fever, elevated cardiac enzymes, and ST‐segment elevation on ECG, its diagnostic accuracy appears to be limited in primarily arrhythmic presentations. 59 The International Consensus Group on CMR Diagnosis of Myocarditis published an update of the ‘Lake Louise Criteria’ in 2018, which included oedema, LGE, T1‐, extracellular volume and T2‐mapping techniques as alternative parameters of fibrosis and oedema, with one fibrosis and one oedema criterion required for a CMR diagnosis of myocarditis. 60
Cardiac magnetic resonance studies in patients who have recovered from the acute phase of COVID‐19 have reported widely differing percentages of myocarditis after COVID‐19 ranging from 0% to 60% of patients owing mostly to different diagnostic criteria and patient selection. 61 Indeed, a direct comparison of EMB and CMR in patients post‐COVID‐19 revealed that only a minority of patients fulfilled CMR criteria (updated Lake Louise Criteria) and pathological findings on EMB. 62
Reports of myocarditis after COVID‐19 vaccination 3 , 21 , 63 , 64 are limited by different referral patterns and few clinical findings, and often lacking EMB or CMR confirmation. Indeed, for myocarditis or pericarditis post‐COVID‐19 vaccination, the recent published large observational studies did not systematically incorporate CMR. 5 , 21 Therefore, the true number of asymptomatic myocarditis and/or pericarditis cases after COVID‐19 vaccination based on CMR criteria is currently unknown. According to expert opinions and single centre reports, very often mild abnormalities on CMR are found in symptomatic vaccinated patients (Figures 3 and 4 ). 65 In contrast to COVID‐19 vaccination, myocarditis after vaccination against influenza appears exceedingly rare. 66 If myocarditis is suspected based on cardiac symptoms after vaccination with elevated laboratory values including troponin and N‐terminal pro‐B‐type natriuretic peptide or wall motion abnormalities on echocardiography, the patient should undergo CMR and if indicated EMB (Figure 5 and Table 5 ). 60 , 61 , 62 , 67 , 68
Figure 3.
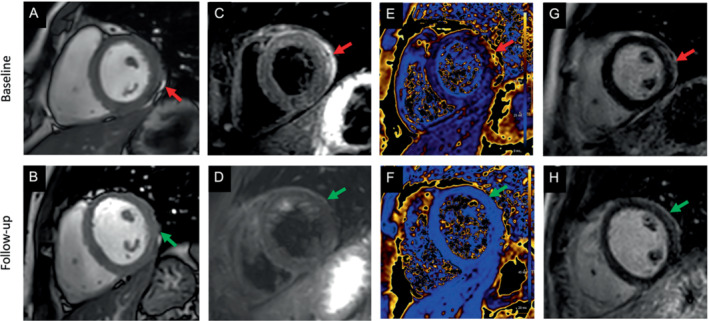
Cardiac magnetic resonance (CMR) images of a patient with signs of a myopericarditis after mRNA SARS‐CoV‐2 vaccination with Spikevax (Moderna). Full description of this case can be found in Jahnke et al. 65 One day after vaccination the patient complained about chest pain and discomfort, shortness of breath, limited physical capacity and malaise. High‐sensitivity troponin T was elevated up to 526 ng/L (normal <14 ng/L). C‐reactive protein, N‐terminal pro‐B‐type natriuretic peptide, electrocardiogram, echocardiography, coronary angiogram and computed tomography pulmonary angiography were normal. CMR was normal for function (including strain) (A), but demonstrated slight pericardial effusion (red arrow in A). T2 weighted images indicated a regional oedema anterolateral/inferolateral (basal) with corresponding elevated quantitative myocardial T2‐mapping parameters up to 70 ms (normal up to 51 ms at 3 Tesla) (C, E – red arrows). Patchy subepicardial late gadolinium enhancement (LGE) indicating inflammatory myocardial necrosis (G). Pericardial enhancement in the T2‐weighted and LGE images in corresponding locations indicated a pericardial involvement as well (C, E). The findings resolved at 4‐month CMR follow‐up (green arrow in B, D, F, H).
Figure 4.
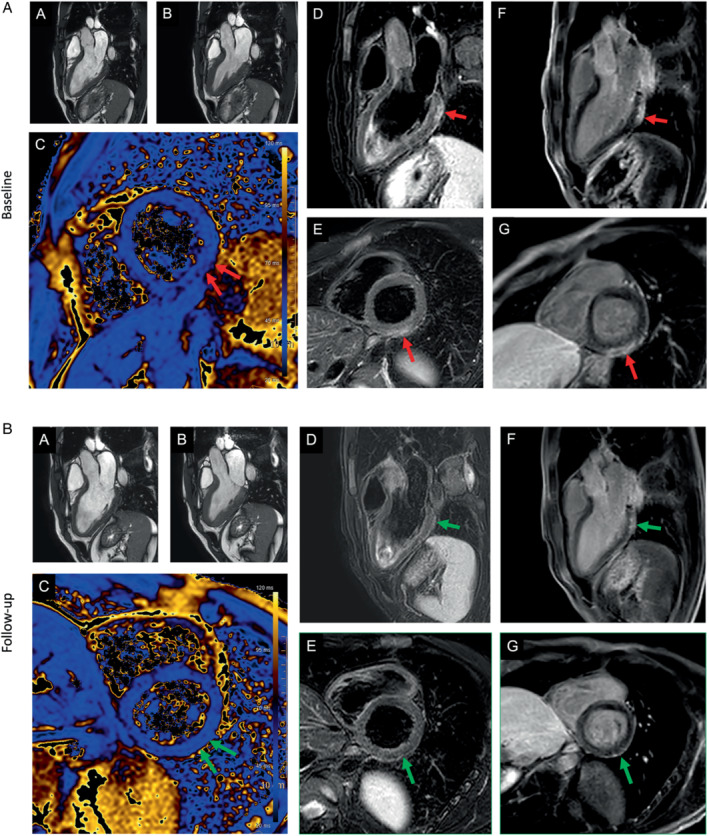
(A) Patient with signs of a myocarditis after mRNA SARS‐CoV‐2 vaccination with Comirnaty (Pfizer‐BioNTech). Three days after the second dose of the vaccine, the patient presented to the emergency room of a referring hospital with chest pain and discomfort, shortness of breath, and decreased exercise capacity. High‐sensitivity troponin T level was elevated at 380 ng/L; N‐terminal pro‐B‐type natriuretic peptide 250 ng/L (<88). Cardiac magnetic resonance (CMR) demonstrated normal left and right ventricular ejection fraction (A, B), with reduced global longitudinal strain. T2‐weighted images indicated a regional oedema inferior/inferolateral (basal) (in D, E) with corresponding elevated quantitative myocardial T2‐mapping parameters (C) and corresponding subepicardial focal late gadolinium enhancement (F, G). (B) The findings at baseline (indicated by red arrow) resolved almost completely (small epicardial fibrosis inferolateral basal) at 4‐month CMR follow‐up (second figure – green arrow). Full description of this case can be found in Jahnke et al. 65
Figure 5.
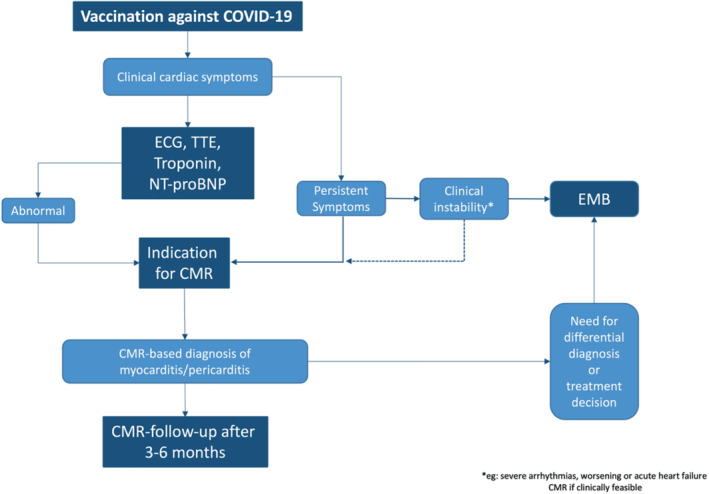
Potential workflow for the use of advanced cardiac magnetic resonance (CMR) in patients post‐vaccination and suspected myo‐/pericarditis. ECG, electrocardiogram; EMB, endomyocardial biopsy; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; TTE, transthoracic echocardiography. Modified from Doeblin and Kelle61
Table 5.
Recommendation for advanced diagnostic workup: endomyocardial biopsy and cardiac magnetic resonance imaging
| Endomyocardial biopsy | Cardiac magnetic resonance imaging (CMR) |
|---|---|
| Acute myocarditis with acute heart failure or cardiogenic shock | CMR scans should be performed for clinical indications according to recent publications on the condition 60 , 61 , 67 |
| Acute myocarditis with ventricular arrhythmias or high‐degree atrioventricular block | Protocols should be adjusted to the clinical scenario, but generally should include standard CINE imaging, T2 (e.g. STIR) oedema imaging, T2 mapping (e.g. T2‐GraSE), pre‐ and post‐contrast T1 mapping (e.g. MOLLI), and late enhancement imaging (e.g. mDIXON). 62 If possible, strain analysis should be performed (based on feature tracking or fSENC/ DENSE) |
| Acute myocarditis or chronic inflammation in the context of peripheral eosinophilia | Vasodilator stress CMR with adenosine or regadenoson may be performed in patients with suspected myocardial ischaemia (e.g. microvascular disease), but should be avoided in the acute stage, particularly in more severe forms |
| Acute myocarditis or dilated cardiomyopathy suspected as chronic inflammatory cardiomyopathy with continuous/recurrent release of inflammatory and cardiac markers | CMR image analysis and measurements should be performed using dedicated CMR post‐processing software 68 |
| When diagnosis has an impact on further therapy | The definite CMR diagnosis of acute myocarditis should be based on the updated ‘Lake Louise Criteria’ requiring findings of myocardial damage (non‐ischaemic late gadolinium enhancement) and oedema with a non‐ischaemic pattern 60 |
Indications for endomyocardial biopsy
Although EMB is rarely indicated and performed in post‐vaccine myocarditis as its course is usually mild, more severe presentations may require EMB. It appears advisable to follow the position statement of the European Society of Cardiology and the scientific statement of the American Heart Association, which were developed for general myocarditis. 58 , 69 Stating that a case‐to‐case evaluation is important, the above mentioned societies consider EMB indicated in clinical scenarios as outlined in Table 5 . 42
Standard EMB 70 and particularly the left ventricular (LV) approach 71 whenever the right ventricle is structurally and functionally normal, are critical for definitive diagnosis of myocarditis and to provide information about its pathophysiology.
Specifically, the morpho‐molecular characteristics of myocardial inflammatory lesions may suggest the mechanism involved. In that regard, the presence of eosinophils has been reported among inflammatory infiltrates of the myocardium suggesting the possibility of a hypersensitivity reaction similar to what has been described for other drugs including the smallpox vaccine. 14 , 72 , 73 , 74 Moreover, analysis of T‐ and B‐cell subsets as well as the presence of giant cells may all be helpful in analysing pathophysiology and clinical course. 75
Pathology of myocarditis following COVID‐19 versus COVID‐19 vaccine
Cardiotropic viruses, such as coxsackieviruses (Figure 6A ), parvovirus B19 and some herpes viruses including human herpesvirus 6 and Epstein–Barr virus, have long been known to induce myocarditis. 58 , 76 Only recently, it has become apparent that infections with coronaviruses including both SARS‐CoV and SARS‐CoV‐2 (Figure 6B,C ) may induce myocarditis. 77
Figure 6.
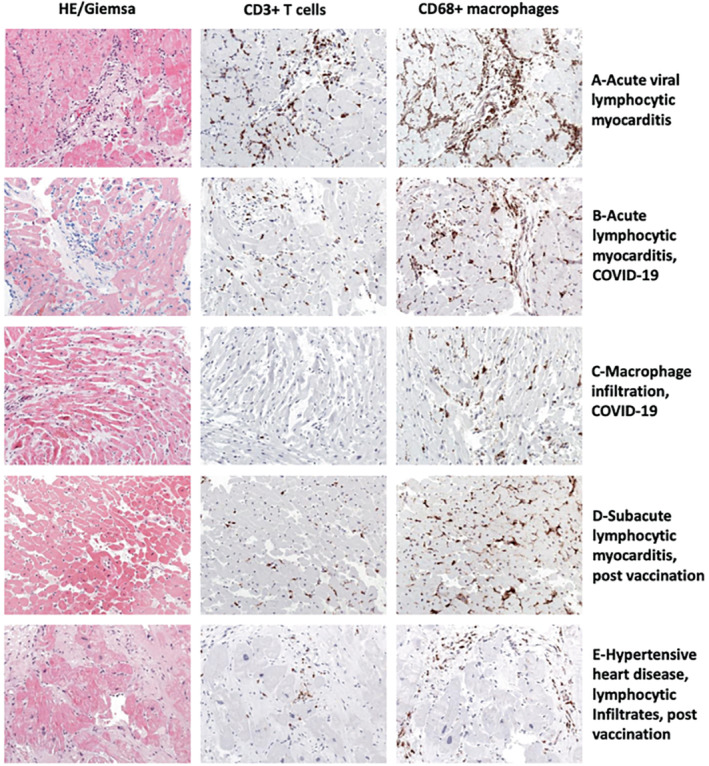
(A) In acute enteroviral myocarditis, myocyte necrosis in the presence of numerous CD3+ T cells and CD68+ macrophages (32‐year‐old male). (B) Some patients with COVID‐19 develop acute/subacute myocarditis (17‐year‐old female). (C) The majority of patients develop low levels of T‐cell infiltrates, but numerous macrophages (38‐year‐old male). Similar findings are observed in mRNA vaccinated patients. Rare cases present with acute/subacute myocarditis (D, 37‐year‐old male) in endomyocardial biopsy, but most patients reveal mild inflammation and suffer from pre‐existing diseases such as hypertensive heart disease (E, 56‐year‐old male). All images magnification ×200.
A variety of pathological mechanisms have been implicated in the induction of myocarditis in the course of SARS virus infections (see also ‘Pathophysiology of vaccine‐related myocarditis’ section). 77 , 78 , 79 , 80 Our current understanding is mainly based on findings on EMB and heart tissue samples obtained at autopsy. Immunohistochemical staining of myocardial tissue of patients who died of SARS‐CoV infection revealed a significant amount of CD68+ macrophages, whereas infiltration by CD3+ T cells was minimal. 81 Similar observations were reported among patients with SARS‐CoV‐2 infection. 77 , 82 , 83 Whereas lymphocytic myocarditis was present in only 14% (n = 3) of patients who died with COVID‐19, interstitial macrophage infiltration was noted in 86% (n = 18) of cases. 84
Despite the fact that in electron microscopy studies SARS‐CoV‐2 viruses were visualized in endothelial cells, 77 cardiomyocytes 15 and macrophages 85 mostly in the early viremic phase of the infection, it is not clear whether SARS‐CoV‐2 can directly induce myocardial injury and, subsequently, inflammation. A direct virus‐mediated injury of any type was not substantiated in 40 hearts of patients who died of COVID‐19. Indeed, all hearts exhibited evidence of both, pre‐existing chronic and acute damage, but only one heart showed signs of myocarditis. 86 The causes of myocardial damage in the context of SARS‐CoV‐2 infection may be more likely multifactorial including systemic inflammation, microembolization and hypoxaemia (for further details see online supplementary Appendix S1 paragraph 1).
In 97 male patients with clinically suspected myocarditis following mRNA vaccination, 7 cases with acute lymphocytic myocarditis (mean age: 34.1 years), one with acute eosinophilic myocarditis (28 years of age) and 25 with a mild healing or chronic lymphocytic myocarditis (mean age: 33.1 years), were diagnosed based on EMB (Klingel K., unpublished data). In patients with myocarditis following vaccination consistently more macrophages than T cells were observed, similar to findings in patients with COVID‐19 (Figure 6D,E ).
Pathophysiology of vaccine‐related myocarditis
It is important to note that the four most widely used vaccines in the West licensed by the regulatory authorities, i.e. BioNTech/Pfizer® (BTN162b2/Comirnaty; EMA: 21.12Ambas.20), Moderna® (mRNA‐1273/ Spikevax; EMA: 6.1.21), as well as the adenoviral vector‐based vaccines of AstraZeneca® (AZD1222/ChAdOx1‐S/Vaxzevria; EMA: 29. 1.21) and Janssen® (Ad26.COV2.S; EMA: 11.3.21), encode very similar forms of the SARS‐CoV‐2 Spike glycoprotein. 87 The proposed mechanisms by which SARS‐CoV‐2 vaccines could induce myocarditis involve activation of both innate and adaptive immune responses against the SARS‐CoV‐2 Spike glycoprotein, but also the recognition of the mRNA itself as an antigen by the immune system. 88 As, similar to viral myocarditis, SARS‐CoV‐2 post‐vaccine myocarditis occurs predominantly in young men, sex hormones may play a role in genetically susceptible individuals (Figure 7 ). 89 , 90 Indeed, viral myocarditis has been associated with genetic variants in genes encoding for different HLA factors, but also for structural proteins of the heart. 48
Figure 7.
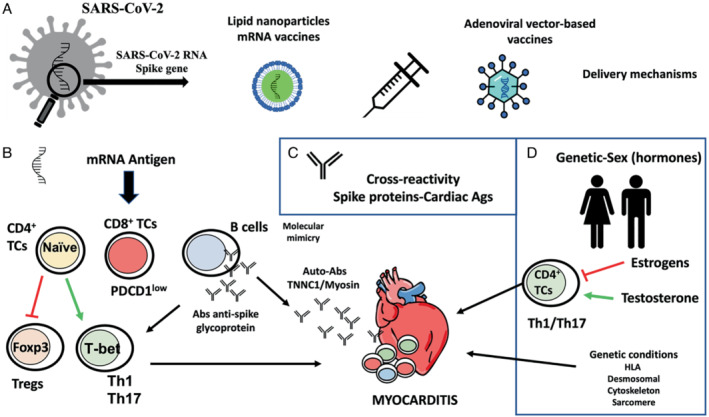
Potential molecular mechanisms for the development of myocarditis following vaccines against COVID‐19. (A) COVID‐19 vaccines are developed from the modified SARS‐CoV‐2 Spike gene. The mRNA vaccines are introduced via lipid nanoparticles, while the adenoviral vector‐based vaccines deliver the Spike sequence as a codon‐optimized DNA. (B) The mRNA can act as an antigen, so it can be recognized by the immune system and activate specific responses of the adaptive immune system. Some of these responses are capable of activating cardiotropic clones of T and B cells triggering cardiac inflammation. (C) Molecular mimicry between Spike glycoprotein and myosin heavy chain or troponin C1, may trigger cross‐reactivity between IgM antibodies against SARS‐CoV‐2 Spike glycoprotein and cardiac autoantigens and potentially induce myocardial inflammation. (D) Development of SARS‐CoV‐2 vaccine myocarditis is associated with young men, suggesting a role for sex hormones. Testosterone activates specific T helper cell responses, whereas oestrogen inhibits pro‐inflammatory T‐cell responses. In addition, viral myocarditis is associated with genetic variants of genes encoding for different HLA factors and structural proteins of the heart.
In the presence of already existing heart‐specific autoimmunity, vaccination can boost self‐reactive T‐cell responses and aggravate preexisting autoimmune heart disease. Such patients are at risk for the development of fulminant and potentially fatal myocarditis. Accordingly, several case reports describe patients with fulminant myocarditis, or distinct myocarditis phenotypes, such as giant cell myocarditis. 55 , 91 , 92
If this hypothesis would be confirmed, the formation of new antigens from the haptenic activity of some components of BNT162b2 mRNA (Pfizer‐BioNTech®) toward cardiomyocyte macromolecules would be indicated and a likely positive response to steroids be suggested (for further details see online supplementary Appendix S1 paragraph 2).
Management of vaccine‐related myocarditis
Heart failure and arrhythmias associated with a reaction to COVID‐19 vaccination should be treated with guideline‐directed therapies, in the first instance with heart failure drugs, i.e. angiotensin‐converting enzyme inhibitors or angiotensin receptor–neprilysin inhibitors, beta‐blockers, mineralocorticoid receptor antagonists, and sodium–glucose cotransporter 2 inhibitors 93 (Table 6 ). Because most cases present with chest pain, acute coronary syndromes should be excluded clinically and in uncertain cases angiographically. 74 Indeed, the majority of mRNA‐related myocarditis cases have normal or near normal LV ejection fraction and symptoms resolve quickly. 8 In the rare cases of fulminant myocarditis, the American Heart Association has given a class of recommendation 1 indication for EMB 69 and for pacemakers in case of higher‐degree atrioventricular block. Mechanical circulatory support and/or extracorporeal membrane oxygenation should be considered (class IIA) with LV dysfunction to unload the left ventricle and provide support as a bridge to recovery. 94
Table 6.
Management of vaccination‐related myocarditis
| Clinical presentation | Treatment |
|---|---|
| Chest pain |
|
| Heart failure with reduced ejection fraction |
|
| Arrhythmias |
|
| Fulminant cases/cardiogenic shock (very rare) |
|
| Higher‐degree atrioventricular block (very rare) |
|
Because the immunological mechanisms of cardiac injury after COVID‐19 vaccine are not well established, the relative risks and benefits of adding anti‐inflammatory treatments in this setting are currently uncertain, although case reports suggest activation of cellular immunity (see ‘Pathology of myocarditis following COVID‐19 vs. COVID‐19 vaccine’ section). 46 , 95 However, the majority of reports do not include data if and what sort of immunosuppressive therapies had been used. One approach that balances the risks and possible benefits of immunosuppression in post‐vaccine‐related myocarditis is a selective use of corticosteroids for a short duration in patients with severely impaired LV function. Hajjo et al. 96 proposed glucocorticoids as preferred treatment based on system biology evidence from the VAERS dataset. These would include the cases that present with cardiogenic shock. 97 , 98 Shared decision making with the patient should be employed when discussing a possible second vaccination or booster dose. Furthermore, monitoring for long‐term sequalae is needed for those who develop post‐acute sequelae of SARS‐CoV‐2 (PASC) or ‘long COVID’ syndrome. 99 , 100 As of today, the epidemiology of PASC remains controversial in the literature 101 and data on its association with vaccination are even more scarce.
In addition to that, an increased risk for post‐COVID‐19 symptoms and conditions has been reported recently for children and adolescents aged 0–17 years, including pulmonary embolism (adjusted hazard ratio 2.01), myocarditis and cardiomyopathy (adjusted hazard ratio 1.99). 102
With regard to when to return to sports activity, it is recommended to follow the general recommendations as for myocarditis, which have been updated in detail recently for COVID‐19 myocarditis. 103 , 104
Finally, with the advent of the Omicron variant associated with a much milder clinical course, the risks and benefits of further vaccination should be discussed particularly with young male patients who had a post‐vaccination myocarditis after the first or second dose, and/or a vaccine with a different platform (such as recombinant S protein) may be an option. In that regard, NVX‐CoV2373 (Novavax) COVID‐19 vaccine is a recombinant Spike (rS) protein nanoparticle vaccine for which an Emergency Use Authorization has been issued by the Food and Drug Administration for primary COVID‐19 immunization of unvaccinated adults aged ≥18 years in July 2022. 105
Required further research
Despite the great strides made, there is still much to understand and that should form the basis for future work.
Mechanistic insights
There is a need to better understand the mechanisms of cardiac injury. Why and how precisely do SARS‐CoV‐2 vaccines cause myocarditis? How does vaccine‐related myo/pericarditis differ from COVID‐19 infection‐related myocarditis? Are there parallels to elevated cytokine levels harming various organs as it has been observed in COVID‐19 or septic patients? Who is at high risk and why? Possibly, some patients may benefit from a lower dosage of the vaccine or concomitant anti‐inflammatory therapy as is routine with certain RNA therapeutics. 106 Further research is urgently needed to identify patients at risk for developing vaccine complications 107 and to determine potential effective (pre‐)medications that would allow for a sufficient immune response to achieve immunity, while preventing excessive immune reactions that may be involved in vaccination‐associated myocarditis.
Future work should also address, if there is a genetic susceptibility, and why it is that mainly younger men are affected? Appropriately, there has been hesitation in undertaking an EMB in all patients affected. Better models are therefore required to establish the causal link as several different pathways are likely to have been affected particularly in the absence of typical ‘control’ cohorts.
Risk profiling
As yet, we do not have a clear sense of the long‐term implications of post‐vaccine myocarditis. Is there an increased arrhythmic risk in particular? The experience from SARS‐CoV‐1 indicates the need for concerted population‐based, systematic national and international level large long‐term registries to determine the scale of the problem and to determine what risk factors best predict long‐term adverse outcomes, so that there is appropriate targeting of those at greatest risk. Ideally, these should include high‐level imaging such as CMR. Much of our current data is from selected countries and we need to better understand the impact across a broader demographic of race, age and sex. The need for this missing data becomes even more pertinent as the push for booster doses accelerates.
Management
There is as yet no consensus on how to manage patients presenting acutely. Whilst guideline‐driven therapy is the overriding mandate, what form should this take and for how long? Secondly, many patients have persistent symptoms yet do not exhibit significant LV dysfunction, troponin rise or arrhythmia nor overt abnormalities on CMR – thus, for those in primary care, this is a real challenge. In those that have developed myocarditis after the first dose of the vaccine, there needs to be more evidence on when they should receive a second dose and importantly, with which agent?
Lessons for future pandemics
Once this pandemic has eased, it will be important to consider how to improve vaccine development for future pandemics – what mitigation steps are required and rather than a uniform approach to all patients would a more nuanced approach have utility based on risk profile, what type of vaccine and what dose to each patient?
Conclusions
COVID‐19 vaccines are overall very safe. There is a low but consistent, tangible rate of post‐vaccination myocarditis and/or pericarditis identified in several national and international level studies. Whilst there can be no room for complacency, fortunately, the majority are mild and not associated with hospitalization or severe complications. This risk has to be balanced against the much greater risk of death, pulmonary, vascular, and cardiac complications by the SARS‐CoV‐2 virus itself. Overall, the risk/benefit ratio hugely remains in favour of vaccination for most classes of ages, especially in older adults. This message is important for both the public and policy makers. For the medical community, a key red flag is in identifying affected individuals (i.e. particularly young men) presenting with chest pain, palpitations, or shortness of breath within 7 days of the second dose of an mRNA vaccine. Management is largely supportive for the majority of affected individuals, unless there is evidence of heart failure or major arrhythmic episodes. Besides pain management, guideline‐driven therapy, potentially complemented by a short course of corticosteroids may need to be considered. Future work should focus on the mechanistic basis for myocarditis and in identifying those at increased risk of adverse outcomes in need of closer long‐term monitoring.
Supporting information
Appendix S1. Supporting Information.
Acknowledgements
The authors met via teleconference on several occasions and the main authors once in person. Each author performed an in‐depth review of the literature on their topic. Finally, each expert presented their interpretation of the literature on their topic to the expert panel and a consensus was made together regarding recommendations. Open Access funding enabled and organized by Projekt DEAL.
Conflict of interest: none declared.
References
- 1. Johns Hopkins Corona Virus Resource Center . COVID‐19 Dashboard. https://coronavirus.jhu.edu/map.html (last accessed 8 September 2022).
- 2. Shay DK, Shimabukuro TT, DeStefano F. Myocarditis occurring after immunization with mRNA‐based COVID‐19 vaccines. JAMA Cardiol. 6:1115–7. [DOI] [PubMed] [Google Scholar]
- 3. Kim HW, Jenista ER, Wendell DC, Azevedo CF, Campbell MJ, Darty SN, et al. Patients with acute myocarditis following mRNA COVID‐19 vaccination. JAMA Cardiol. 2021;6:1196–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shiyovich A, Witberg G, Aviv Y, Eisen A, Orvin K, Wiessman M, et al. Myocarditis following COVID‐19 vaccination: magnetic resonance imaging study. Eur Heart J Cardiovasc Imaging. 2021;23:1075–82. [DOI] [PubMed] [Google Scholar]
- 5. Gargano JW, Wallace M, Hadler SC, Langley G, Su JR, Oster ME, et al. Use of mRNA COVID‐19 vaccine after reports of myocarditis among vaccine recipients: update from the Advisory Committee on Immunization Practices ‐ United States, June 2021. MMWR Morb Mortal Wkly Rep. 2021;70:977–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Perez Y, Levy ER, Joshi AY, Virk A, Rodriguez‐Porcel M, Johnson M, et al. Myocarditis following COVID‐19 mRNA vaccine: a case series and incidence rate determination. Clin Infect Dis. 2021;75:e749–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Farahmand R, Trottier CA, Kannam JP, Ho KKL. Incidence of myopericarditis and myocardial injury in coronavirus disease 2019 vaccinated subjects. Am J Cardiol. 2022;164:123–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Witberg G, Barda N, Hoss S, Richter I, Wiessman M, Aviv Y, et al. Myocarditis after Covid‐19 vaccination in a large health care organization. N Engl J Med. 2021;385:2132–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mevorach D, Anis E, Cedar N, Bromberg M, Haas EJ, Nadir E, et al. Myocarditis after BNT162b2 mRNA vaccine against Covid‐19 in Israel. N Engl J Med. 2021;385:2140–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Viskin D, Topilsky Y, Aviram G, Mann T, Sadon S, Hadad Y, et al. Myocarditis associated with COVID‐19 vaccination: echocardiography, cardiac tomography, and magnetic resonance imaging findings. Circ Cardiovasc Imaging. 2021;14:e013236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Barda N, Dagan N, Ben‐Shlomo Y, Kepten E, Waxman J, Ohana R, et al. Safety of the BNT162b2 mRNA Covid‐19 vaccine in a nationwide setting. N Engl J Med. 2021;385:1078–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gov.UK . Coronavirus vaccine – summary of Tellow Card reporting. https://www.gov.uk/government/publications/coronavirus‐covid‐19‐vaccine‐adverse‐reactions/coronavirus‐vaccine‐summary‐of‐yellow‐card‐reporting (last accessed 8 September 2022).
- 13. Eckart RE, Scoville SL, Campbell CL, Shry EA, Stajduhar KC, Potter RN, et al. Sudden death in young adults: a 25‐year review of autopsies in military recruits. Ann Intern Med. 2004;141:829–34. [DOI] [PubMed] [Google Scholar]
- 14. Eckart RE, Love SS, Atwood JE, Arness MK, Cassimatis DC, Campbell CL, et al. Incidence and follow‐up of inflammatory cardiac complications after smallpox vaccination. J Am Coll Cardiol. 2004;44:201–5. [DOI] [PubMed] [Google Scholar]
- 15. Bojkova D, Wagner JUG, Shumliakivska M, Aslan GS, Saleem U, Hansen A, et al. SARS‐CoV‐2 infects and induces cytotoxic effects in human cardiomyocytes. Cardiovasc Res. 2020;116:2207–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Boehmer TK, Kompaniyets L, Lavery AM, Hsu J, Ko JY, Yusuf H, et al. Association between COVID‐19 and myocarditis using hospital‐based administrative data – United States, March 2020‐January 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1228–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hause AM, Baggs J, Marquez P, Myers TR, Su JR, Blanc PG, et al. Safety monitoring of COVID‐19 vaccine booster doses among adults – United States, September 22, 2021‐February 6, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:249–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Straus W, Urdaneta V, Esposito DB, Mansi JA, Rodriguez CS, Burton P, et al. Myocarditis after mRNA‐1273 vaccination: a population‐based analysis of 151 million vaccine recipients worldwide. medRxiv November 12, 2021. doi. 10.1101/2021.11.11.21265536 [DOI] [Google Scholar]
- 19. Husby A, Hansen JV, Fosbøl E, Thiesson EM, Madsen M, Thomsen RW, et al. SARS‐CoV‐2 vaccination and myocarditis or myopericarditis: population based cohort study. BMJ. 2021;375:e068665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Simone A, Herald J, Chen A, Gulati N, Shen AYJ, Lewin B, et al. Acute myocarditis following COVID‐19 mRNA vaccination in adults aged 18 years or older. JAMA Intern Med. 2021;181:1668–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Montgomery J, Ryan M, Engler R, Hoffman D, McClenathan B, Collins L, et al. Myocarditis following immunization with mRNA COVID‐19 vaccines in members of the US military. JAMA Cardiol. 2021;6:1202–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. C4591001 Clinical Trial Group. Safety and efficacy of the BNT162b2 mRNA Covid‐19 vaccine. N Engl J Med. 2020;383:2603–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ali K, Berman G, Zhou H, Deng W, Faughnan V, Coronado‐Voges M, et al. Evaluation of mRNA‐1273 SARS‐CoV‐2 vaccine in adolescents. N Engl J Med. 2021;385:2241–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Frenck RW Jr, Klein NP, Kitchin N, Gurtman A, Absalon J, Lockhart S, et al. C4591001 Clinical Trial Group. Safety, immunogenicity, and efficacy of the BNT162b2 Covid‐19 vaccine in adolescents. N Engl J Med. 2021;385:239–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, et al. COVE Study Group. Efficacy and safety of the mRNA‐1273 SARS‐CoV‐2 vaccine. N Engl J Med. 2021;384:403–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Goddard K, Lewis N, Fireman B, Weintraub E, Shimabukuro T, Zerbo O, et al. Risk of myocarditis and pericarditis following BNT162b2 and mRNA‐1273 COVID‐19 vaccination. Vaccine. 2022;40:5153–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wong HL, Hu M, Zhou CK, Lloyd PC, Amend KL, Beachler DC, et al. Risk of myocarditis and pericarditis after the COVID‐19 mRNA vaccination in the USA: a cohort study in claims databases. Lancet. 2022;399:2191–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mevorach D, Anis E, Cedar N, Hasin T, Bromberg M, Goldberg L, et al. Myocarditis after BNT162b2 vaccination in Israeli adolescents. N Engl J Med. 2022;386:998–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Patone M, Mei XW, Handunnetthi L, Dixon S, Zaccardi F, Shankar‐Hari M, et al. Risks of myocarditis, pericarditis, and cardiac arrhythmias associated with COVID‐19 vaccination or SARS‐CoV‐2 infection. Nat Med. 2021;28:410–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Vogel G, Couzin‐Frankel J. Israel reports link between rare cases of heart inflammation and COVID‐19 vaccination in young men. Science. 2021. 10.1126/science.abj7796 [DOI] [Google Scholar]
- 31. Dagan N, Barda N, Kepten E, Miron O, Perchik S, Katz MA, et al. BNT162b2 mRNA Covid‐19 vaccine in a Nationwide mass vaccination setting. N Engl J Med. 2021;384:1412–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Barda N, Dagan N, Cohen C, Hernán MA, Lipsitch M, Kohane IS, et al. Effectiveness of a third dose of the BNT162b2 mRNA COVID‐19 vaccine for preventing severe outcomes in Israel: an observational study. Lancet. 2021;398:2093–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Our World in Data . https://ourworldindata.org/vaccination‐israel‐impact (last accessed 8 September 2022).
- 34. Barda N, Dagan N, Balicer RD. BNT162b2 mRNA Covid‐19 vaccine in a nationwide mass vaccination setting. Reply. N Engl J Med. 2021;384:1970. [DOI] [PubMed] [Google Scholar]
- 35. Centers for Disease Control and Prevention . ACIP Presentation Slides: February 4, 2022 Meeting. https://www.cdc.gov/vaccines/acip/meetings/slides‐2022‐02‐04.html (last accessed 8 September 2022).
- 36. Klein N; Kaiser Permanente Vaccine Study Center; Kaiser Permanente Northern California. Myocarditis Analyses in the Vaccine Safety Datalink: Rapid Cycle Analyses and “Head‐to‐Head” Product Comparison. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides‐2022‐02‐04/10‐COVID‐Klein‐508.pdf (last accessed 8 September 2022).
- 37. Abraham N, Spruin S, Rossi T, Fireman B, Zafack J, Blaser C, et al. Myocarditis and/or pericarditis risk after mRNA COVID‐19 vaccination: a Canadian head to head comparison of BNT162b2 and mRNA‐1273 vaccines. Vaccine. 2022;40:4663–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Buchan SA, Seo CY, Johnson C, Alley S, Kwong JC, Nasreen S, et al. Epidemiology of myocarditis and pericarditis following mRNA vaccines in Ontario, Canada: by vaccine product, schedule and interval among adolescents and adults in Ontario, Canada. JAMA Netw Open. 2022;5:e2218505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gov.UK . Information for healthcare professionals on myocarditis and pericarditis following COVID‐19 vaccination. https://www.gov.uk/government/publications/covid‐19‐vaccination‐myocarditis‐and‐pericarditis‐information‐for‐healthcare‐professionals/information‐for‐healthcare‐professionals‐on‐myocarditis‐and‐pericarditis‐following‐covid‐19‐vaccination (last accessed 8 September 2022).
- 40. Huber SA. Increased susceptibility of male BALB/c mice to coxsackievirus B3‐induced myocarditis: role for CD1d. Med Microbiol Immunol. 2005;194:121–7. [DOI] [PubMed] [Google Scholar]
- 41. Olson SM, Newhams MM, Halasa NB, Price AM, Boom JA, Sahni LC, et al.; Overcoming Covid‐19 Investigators . Effectiveness of BNT162b2 vaccine against critical Covid‐19 in adolescents. N Engl J Med. 2022;386:713–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Muller M, Cooper LT, Heidecker B. Diagnosis, risk stratification and management of myocarditis. Heart. 2021;108:1486–97. [DOI] [PubMed] [Google Scholar]
- 43. Woo W, Kim AY, Yon DK, Lee SW, Hwang J, Jacob L, et al. Clinical characteristics and prognostic factors of myocarditis associated with the mRNA COVID‐19 vaccine. J Med Virol. 2022;94:1566–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Oster ME, Shay DK, Su JR, Gee J, Creech CB, Broder KR, et al. Myocarditis cases reported after mRNA‐based COVID‐19 vaccination in the US from December 2020 to August 2021. JAMA. 2022;327:331–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gnecchi M, Moretti F, Bassi EM, Leonardi S, Totaro R, Perotti L, et al. Myocarditis in a 16‐year‐old boy positive for SARS‐CoV‐2. Lancet. 2020;395:e116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Verma AK, Lavine KJ, Lin CY. Myocarditis after Covid‐19 mRNA vaccination. N Engl J Med. 2021;385:1332–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bozkurt B, Kamat I, Hotez PJ. Myocarditis with COVID‐19 mRNA vaccines. Circulation. 2021;144:471–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Heymans S, Eriksson U, Lehtonen J, Cooper LT Jr. The quest for new approaches in myocarditis and inflammatory cardiomyopathy. J Am Coll Cardiol. 2016;68:2348–64. [DOI] [PubMed] [Google Scholar]
- 49. Haaf P, Kuster GM, Mueller C, Berger CT, Monney P, Burger P, et al. The very low risk of myocarditis and pericarditis after mRNA COVID‐19 vaccination should not discourage vaccination. Swiss Med Wkly. 2021;151:w30087. [DOI] [PubMed] [Google Scholar]
- 50. Matta A, Kunadharaju R, Osman M, Jesme C, McMiller Z, Johnson EM, et al. Clinical presentation and outcomes of myocarditis post mRNA vaccination: a meta‐analysis and systematic review. Cureus. 2021;13:e19240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Fronza M, Thavendiranathan P, Chan V, Karur GR, Udell JA, Wald RM, et al. Myocardial injury pattern at MRI in COVID‐19 vaccine‐associated myocarditis. Radiology. 2022;304:553–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Haussner W, DeRosa AP, Haussner D, Tran J, Torres‐Lavoro J, Kamler J, et al. COVID‐19 associated myocarditis: a systematic review. Am J Emerg Med. 2022;51:150–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Murakami Y, Shinohara M, Oka Y, Wada R, Noike R, Ohara H, et al. Myocarditis following a COVID‐19 messenger RNA vaccination: a Japanese case series. Intern Med. 2021;61:501–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Uesako H, Fujikawa H, Hashimoto S, Wakabayashi T. Prominent J waves and ventricular fibrillation due to myocarditis and pericarditis after BNT162b2 mRNA COVID‐19 vaccination. Can J Cardiol. 2022;38:844–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ameratunga R, Woon ST, Sheppard MN, Garland J, Ondruschka B, Wong CX, et al. First identified case of fatal fulminant necrotizing eosinophilic myocarditis following the initial dose of the Pfizer‐BioNTech mRNA COVID‐19 vaccine (BNT162b2, Comirnaty): an extremely rare idiosyncratic hypersensitivity reaction. J Clin Immunol. 2022;42:441–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Tschope C, Ammirati E, Bozkurt B, ALP C, Cooper LT, Felix SB, et al. Myocarditis and inflammatory cardiomyopathy: current evidence and future directions. Nat Rev Cardiol. 2021;18:169–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Luetkens JA, Isaak A, Zimmer S, Nattermann J, Sprinkart AM, Boesecke C, et al. Diffuse myocardial inflammation in COVID‐19 associated myocarditis detected by multiparametric cardiac magnetic resonance imaging. Circ Cardiovasc Imaging. 2020;13:e010897. [DOI] [PubMed] [Google Scholar]
- 58. Caforio AL, Pankuweit S, Arbustini E, Basso C, Gimeno‐Blanes J, Felix SB, et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2013;34:2648a–d. [DOI] [PubMed] [Google Scholar]
- 59. Francone M, Chimenti C, Galea N, Scopelliti F, Verardo R, Galea R, et al. CMR sensitivity varies with clinical presentation and extent of cell necrosis in biopsy‐proven acute myocarditis. JACC Cardiovasc Imaging. 2014;7:254–63. [DOI] [PubMed] [Google Scholar]
- 60. Ferreira VM, Schulz‐Menger J, Holmvang G, Kramer CM, Carbone I, Sechtem U, et al. Cardiovascular magnetic resonance in nonischemic myocardial inflammation: expert recommendations. J Am Coll Cardiol. 2018;72:3158–76. [DOI] [PubMed] [Google Scholar]
- 61. Doeblin P, Kelle S. Going after COVID‐19 myocarditis. Eur Heart J Cardiovasc Imaging. 2021;22:852–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Tanacli R, Doeblin P, Götze C, Zieschang V, Faragli A, Stehning C, et al. COVID‐19 vs. classical myocarditis associated myocardial injury evaluated by cardiac magnetic resonance and endomyocardial biopsy. Front Cardiovasc Med. 2021;8:737257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Marshall M, Ferguson ID, Lewis P, Jaggi P, Gagliardo C, Collins JS, et al. Symptomatic acute myocarditis in 7 adolescents after Pfizer‐BioNTech COVID‐19 vaccination. Pediatrics. 2021;148:e2021052478. [DOI] [PubMed] [Google Scholar]
- 64. Hause AM, Gee J, Baggs J, Abara WE, Marquez P, Thompson D, et al. COVID‐19 vaccine safety in adolescents aged 12‐17 years – United States, December 14, 2020‐July 16, 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1053–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Jahnke C, Doeblin P, Tanacli R, Witt U, Schneider M, Stehning C, et al. Case series of potential cardiac inflammation associated with various SARS‐CoV‐2 vaccinations assessed by cardiac MRI. Front Cardiovasc Med. 2022;9:829392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Nagano N, Yano T, Fujita Y, Koyama M, Hasegawa R, Nakata J, et al. Hemodynamic collapse after influenza vaccination: a vaccine‐induced fulminant myocarditis? Can J Cardiol. 2020;36:1554.e5–7. [DOI] [PubMed] [Google Scholar]
- 67. Kelle S, Bucciarelli‐Ducci C, Judd RM, Kwong RY, Simonetti O, Plein S, et al. Society for Cardiovascular Magnetic Resonance (SCMR) recommended CMR protocols for scanning patients with active or convalescent phase COVID‐19 infection. J Cardiovasc Magn Reson. 2020;22:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Schulz‐Menger J, Bluemke DA, Bremerich J, Flamm SD, Fogel MA, Friedrich MG, et al. Standardized image interpretation and post‐processing in cardiovascular magnetic resonance – 2020 update: Society for Cardiovascular Magnetic Resonance (SCMR): Board of Trustees Task Force on Standardized Post‐Processing. J Cardiovasc Magn Reson. 2020;22:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Bozkurt B, Colvin M, Cook J, Cooper LT, Deswal A, Fonarow GC, et al. Current diagnostic and treatment strategies for specific dilated cardiomyopathies: a scientific statement from the American Heart Association. Circulation. 2016;134:e579–646. [DOI] [PubMed] [Google Scholar]
- 70. Cooper LT, Baughman KL, Feldman AM, Frustaci A, Jessup M, Kuhl U, et al.; American Heart Association, American College of Cardiology, European Society of Cardiology, Heart Failure Society of America, Heart Failure Association of the European Society of Cardiology . The role of endomyocardial biopsy in the management of cardiovascular disease: a scientific statement from the American Heart Association, the American College of Cardiology, and the European Society of Cardiology. Endorsed by the Heart Failure Society of America and the Heart Failure Association of the European Society of Cardiology. J Am Coll Cardiol. 2007;50:1914–31. [DOI] [PubMed] [Google Scholar]
- 71. Chimenti C, Frustaci A. Contribution and risks of left ventricular endomyocardial biopsy in patients with cardiomyopathies: a retrospective study over a 28‐year period. Circulation. 2013;128:1531–41. [DOI] [PubMed] [Google Scholar]
- 72. Casey CG, Iskander JK, Roper MH, Mast EE, Wen XJ, Török TJ, et al. Adverse events associated with smallpox vaccination in the United States, January‐October 2003. JAMA. 2005;294:2734–43. [DOI] [PubMed] [Google Scholar]
- 73. Engler RJ, Nelson MR, Collins LC Jr, Spooner C, Hemann BA, Gibbs BT, et al. A prospective study of the incidence of myocarditis/pericarditis and new onset cardiac symptoms following smallpox and influenza vaccination. PLoS One. 2015;10:e0118283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Ammirati E, Cavalotti C, Milazzo A, Pedrotti P, Soriano F, Schroeder JW, et al. Temporal relation between second dose BNT162b2 mRNA Covid‐19 vaccine and cardiac involvement in a patient with previous SARS‐COV‐2 infection. Int J Cardiol Heart Vasc. 2021;34:100774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Dannebaum R, Suwalski P, Asgharian H, du Zhipei G, Lin H, Weiner J, et al.; Pa‐COVID Study Group . Highly multiplexed immune repertoire sequencing links multiple lymphocyte classes with severity of response to COVID‐19. EClinicalMedicine. 2022;48:101438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Pankuweit S, Klingel K. Viral myocarditis: from experimental models to molecular diagnosis in patients. Heart Fail Rev. 2013;18:683–702. [DOI] [PubMed] [Google Scholar]
- 77. Ho HT, Peischard S, Strutz‐Seebohm N, Klingel K, Seebohm G. Myocardial damage by SARS‐CoV‐2: emerging mechanisms and therapies. Viruses. 2021;13:1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Imazio M, Klingel K, Kindermann I, Brucato A, de Rosa FG, Adler Y, et al. COVID‐19 pandemic and troponin: indirect myocardial injury, myocardial inflammation or myocarditis? Heart. 2020;106:1127–31. [DOI] [PubMed] [Google Scholar]
- 79. Dolhnikoff M, Ferreira Ferranti J, de Almeida Monteiro RA, Duarte‐Neto AN, Soares Gomes‐Gouvêa M, Viu Degaspare N, et al. SARS‐CoV‐2 in cardiac tissue of a child with COVID‐19‐related multisystem inflammatory syndrome. Lancet Child Adolesc Health. 2020;4:790–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Yang L, Han Y, Nilsson‐Payant BE, Gupta V, Wang P, Duan X, et al. A human pluripotent stem cell‐based platform to study SARS‐CoV‐2 tropism and model virus infection in human cells and organoids. Cell Stem Cell. 2020;27:125–36.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Oudit GY, Kassiri Z, Jiang C, Liu PP, Poutanen SM, Penninger JM, et al. SARS‐coronavirus modulation of myocardial ACE2 expression and inflammation in patients with SARS. Eur J Clin Invest. 2009;39:618–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Greulich S, Klingel K. COVID‐19 and myocarditis: findings from cardiac magnetic resonance imaging and endomyocardial biopsies. Hamostaseologie. 2021;41:366–70. [DOI] [PubMed] [Google Scholar]
- 83. Weckbach LT, Schweizer L, Kraechan A, Bieber S, Ishikawa‐Ankerhold H, Hausleiter J, et al.; EMB Study Group . Association of complement and MAPK activation with SARS‐CoV‐2‐associated myocardial inflammation. JAMA Cardiol. 2021;7:286–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Basso C, Leone O, Rizzo S, de Gaspari M, van der Wal AC, Aubry MC, et al. Pathological features of COVID‐19‐associated myocardial injury: a multicentre cardiovascular pathology study. Eur Heart J. 2020;41:3827–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Tavazzi G, Pellegrini C, Maurelli M, Belliato M, Sciutti F, Bottazzi A, et al. Myocardial localization of coronavirus in COVID‐19 cardiogenic shock. Eur J Heart Fail. 2020;22:911–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Dal Ferro M, Bussani R, Paldino A, Nuzzi V, Collesi C, Zentilin L, et al. SARS‐CoV‐2, myocardial injury and inflammation: insights from a large clinical and autopsy study. Clin Res Cardiol. 2021;110:1822–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Hoffmann M, Kleine‐Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, et al. SARS‐CoV‐2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280 e278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Heymans S, Cooper LT. Myocarditis after COVID‐19 mRNA vaccination: clinical observations and potential mechanisms. Nat Rev Cardiol. 2022;19:75–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Caso F, Costa L, Ruscitti P, Navarini L, del Puente A, Giacomelli R, et al. Could SARS‐coronavirus‐2 trigger autoimmune and/or autoinflammatory mechanisms in genetically predisposed subjects? Autoimmun Rev. 2020;19:102524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Weiner J, Suwalski P, Holtgrewe M, Rakitko A, Thibeault C, Müller M, et al. Increased risk of severe clinical course of COVID‐19 in carriers of HLA‐C*04:01. EClinicalMedicine. 2021;40:101099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Abbate A, Gavin J, Madanchi N, Kim C, Shah PR, Klein K, et al. Fulminant myocarditis and systemic hyperinflammation temporally associated with BNT162b2 mRNA COVID‐19 vaccination in two patients. Int J Cardiol. 2021;340:119–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Cui G, Li R, Zhao C, Wang DW. Case report: COVID‐19 vaccination associated fulminant myocarditis. Front Cardiovasc Med. 2021;8:769616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, et al.; ESC Scientific Document Group . 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2022;24:4–131. [DOI] [PubMed] [Google Scholar]
- 94. Kociol RD, Cooper LT, Fang JC, Moslehi JJ, Pang PS, Sabe MA, et al.; American Heart Association Heart Failure and Transplantation Committee of the Council on Clinical Cardiology . Recognition and initial management of fulminant myocarditis: a scientific statement from the American Heart Association. Circulation. 2020;141:e69–92. [DOI] [PubMed] [Google Scholar]
- 95. Murphy JG, Wright RS, Bruce GK, Baddour LM, Farrell MA, Edwards WD, et al. Eosinophilic‐lymphocytic myocarditis after smallpox vaccination. Lancet. 2003;362:1378–80. [DOI] [PubMed] [Google Scholar]
- 96. Hajjo R, Sabbah DA, Bardaweel SK, Tropsha A. Shedding the light on post‐vaccine myocarditis and pericarditis in COVID‐19 and non‐COVID‐19 vaccine recipients. Vaccines. 2021;9:1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Lim Y, Kim MC, Kim KH, Jeong IS, Cho YS, Choi YD, et al. Case report: acute fulminant myocarditis and cardiogenic shock after messenger RNA coronavirus disease 2019 vaccination requiring extracorporeal cardiopulmonary resuscitation. Front Cardiovasc Med. 2021;8:758996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Maki H, Aikawa T, Ibe T, Oyama‐Manabe N, Fujita H. Biventricular systolic dysfunction in acute myocarditis after SARS‐CoV‐2 mRNA‐1273 vaccination. Eur Heart J Cardiovasc Imaging. 2021;23:e87. [DOI] [PubMed] [Google Scholar]
- 99. Ayoubkhani D, Bermingham C, Pouwels KB, Glickman M, Nafilyan V, Zaccardi F, et al. Trajectory of long covid symptoms after covid‐19 vaccination: community based cohort study. BMJ. 2022;377:e069676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Office for National Statistics . Self‐reported long COVID after two doses of a coronavirus (COVID‐19) vaccine in the UK: 26 January 2022. https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/bulletins/selfreportedlongcovidaftertwodosesofacoronaviruscovid19vaccineintheuk/26january2022 (last accessed 8 September 2022).
- 101. Raman B, Bluemke DA, Luscher TF, Neubauer S. Long COVID: post‐acute sequelae of COVID‐19 with a cardiovascular focus. Eur Heart J. 2022;43:1157–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Kompaniyets L, Bull‐Otterson L, Boehmer TK, Baca S, Alvarez P, Hong K, et al. Post‐COVID‐19 symptoms and conditions among children and adolescents – United States, March 1, 2020‐January 31, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:993–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Gluckman TJ, Bhave NM, Allen LA, Chung EH, Spatz ES, Ammirati E, et al. 2022 ACC expert consensus decision pathway on cardiovascular sequelae of COVID‐19 in adults: myocarditis and other myocardial involvement, post‐acute sequelae of SARS‐CoV‐2 infection, and return to play: a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2022;79:1717–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Patriki D, Baltensperger N, Berg J, Cooper LT, Kissel CK, Kottwitz J, et al. A prospective pilot study to identify a myocarditis cohort who may safely resume sports activities 3 months after diagnosis. J Cardiovasc Transl Res. 2021;14:670–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Twentyman E, Wallace M, Roper LE, Anderson TC, Rubis AB, Fleming‐Dutra KE, et al. Interim recommendation of the Advisory Committee on Immunization Practices for use of the Novavax COVID‐19 vaccine in persons aged ≥18 years – United States, July 2022. MMWR Morb Mortal Wkly Rep. 2022;71:988–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Canadian Agency for Drugs and Tehnologies in Health . Pharmacoeconomic Review Report: Patisiran (Onpattro): (Alnylam Netherlands B.V.): Indication: Treatment of polyneuropathy in adult patients with hereditary transthyretin‐mediated amyloidosis CADTH Common Drug Review. August 2019. https://www.ncbi.nlm.nih.gov/books/NBK549694 (last accessed 8 September 2022). [PubMed]
- 107. Poland GA, Kennedy RB. Vaccine safety in an era of novel vaccines: a proposed research agenda. Nat Rev Immunol. 2022;22:203–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supporting Information.


