Abstract
There is a global concern about the safety of COVID‐19 vaccines associated with platelet function. However, their long‐term effects on overall platelet activity remain poorly understood. Here we address this problem by image‐based single‐cell profiling and temporal monitoring of circulating platelet aggregates in the blood of healthy human subjects, before and after they received multiple Pfizer‐BioNTech (BNT162b2) vaccine doses over a time span of nearly 1 year. Results show no significant or persisting platelet aggregation trends following the vaccine doses, indicating that any effects of vaccinations on platelet turnover, platelet activation, platelet aggregation, and platelet‐leukocyte interaction was insignificant.
Keywords: COVID‐19, mRNA vaccine, platelet aggregation, thrombosis
1. INTRODUCTION
mRNA COVID‐19 vaccines, namely the Pfizer‐BioNTech (BNT162b2) and Moderna (mRNA‐1273) vaccines, have been highly effective worldwide in preventing infection, serious illness, and death [1, 2]. On the other hand, there is a global concern about their safety, in response to multiple reports of rare but serious adverse effects after administration of the vaccines. These effects include myocarditis, lymphadenopathy, thrombocytopenia, pulmonary embolism, and myocardial infarction [3]. To date, the effects of mRNA vaccinations on the immune response, in particular the neutralizing antibody response of lymphocytes and monocytes to SARS‐CoV‐2, have been well studied [1, 2, 4, 5]. However, the effects of mRNA vaccinations on platelet function are somewhat overlooked, despite evidence that platelets play an important role in the immune response by releasing several mediators or expressing adhesion and immune receptors on the platelet surface [6]. While the rapid platelet‐immune crosstalk after mRNA vaccinations has been studied [7], the long‐term effects of mRNA vaccinations on overall platelet activity (i.e., platelet activation, platelet aggregation, and platelet‐leukocyte interaction) remain poorly understood, due to the lack of tools available to study platelet activity with high resolution and statistical accuracy [8, 9].
In this Brief Report, we investigate the long‐term effects of Pfizer‐BioNTech (BNT162b2) mRNA vaccinations on platelet activity by image‐based single‐cell profiling and temporal monitoring of circulating platelet aggregates in the blood of healthy human subjects, before and after they received multiple Pfizer‐BioNTech (BNT162b2) vaccine doses over a time span of nearly 1 year. To achieve this, we used an optical frequency‐division‐multiplexed (FDM) microscope on a hydrodynamic‐focusing microfluidic chip, which enabled high‐throughput, blur‐free, bright‐field image acquisition at single‐cell resolution [8, 9, 10]. As shown in Figure 1A, our experimental procedure consisted of: (i) periodic sampling of blood (1 ml) from healthy subjects; (ii) sample processing and image data acquisition (25,000 images per blood sample); and (iii) digital image processing and statistical analysis. As shown in Figure 1B, blood sampling was performed at a frequency of several times per month per subject, in order to observe temporal changes in the concentration and size distribution of single platelets and platelet aggregates. Negative control image data were obtained from the same subjects before their vaccine doses, under the same sample preparation and image acquisition conditions. The objective area in Figure 1B is defined by the area of a detected event (e.g., a single platelet, a platelet aggregate, a residual blood component, or cell debris) within each image. Transient fluctuations in the concentration of platelet aggregates were caused by factors such as daily variations in sample processing or the physiological conditions of the subjects.
FIGURE 1.
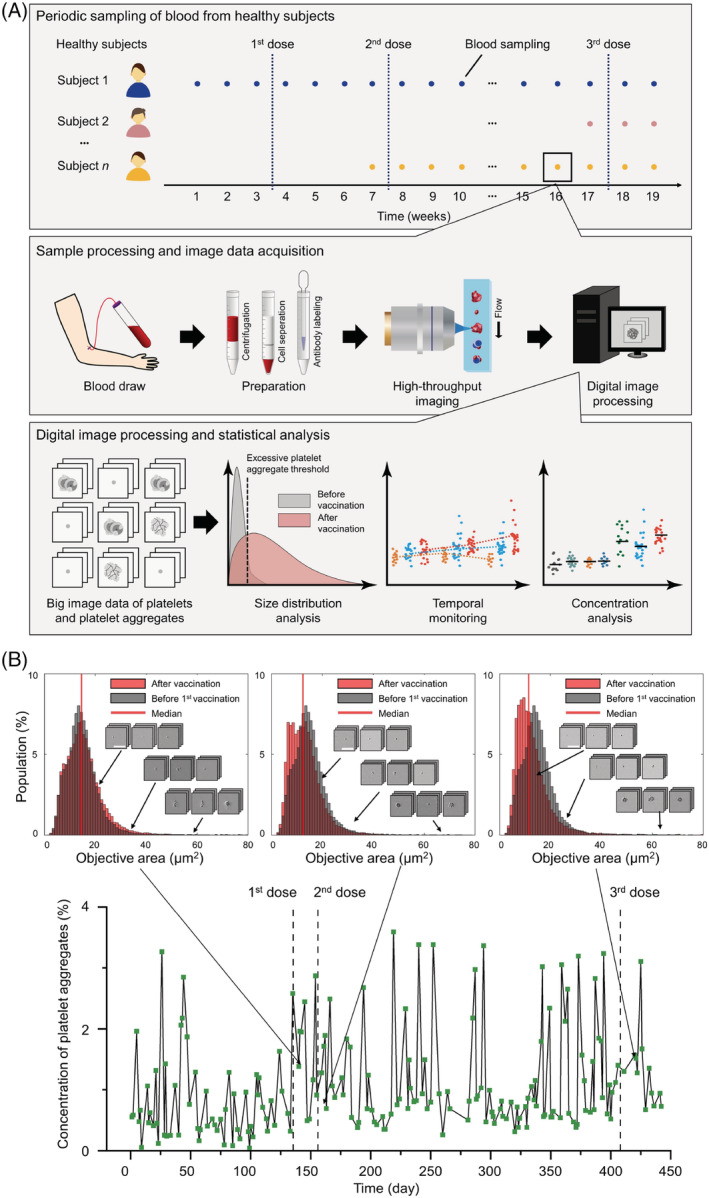
Monitoring of platelet activity in the blood of healthy subjects before and after Pfizer‐BioNTech vaccine doses. (A) Experimental procedure. (B) Concentration of circulating platelet aggregates in a female subject. Insets show the size distribution and typical images of single platelets and platelet aggregates. Scale bars, 30 μm [Color figure can be viewed at wileyonlinelibrary.com]
2. RESULTS
Figure 2A shows the concentrations of platelet aggregates in the blood samples of healthy subjects (n = 11) monitored continuously over 11 months, during which they received vaccine doses. The concentration of platelet aggregates is defined as the ratio of the number of acquired images containing platelet aggregates to the total number of acquired images for each blood sample and serves as a direct indicator of platelet activity. Data point colors identify subjects. The concentration of platelet aggregates in all subjects remained low (less than 4%), with modest transient variations within each subject. To investigate the potential vaccine‐induced activation of leukocytes (e.g., neutrophils), we classified the platelet aggregates in Figure 2A into platelet–platelet and platelet‐leukocyte aggregates. Figure 2B shows that the concentration of platelet‐leukocyte aggregates was also low for all subjects (less than 3%). Importantly, despite the subjects' immune response to the vaccination indicated by expected changes in the lymphocyte and monocyte counts (Figure 2C) [1, 2, 4, 5], these platelet aggregate data (Figure 2A,B), combined with small variations in the platelet count, mean platelet volume (MPV), platelet distribution width (PDW), and immature platelet fraction (IPF) (Figure 2C), indicate no significant or persisting trends following vaccine administration. This suggests that any effect of vaccinations on platelet turnover, activation, or aggregation was insignificant.
FIGURE 2.
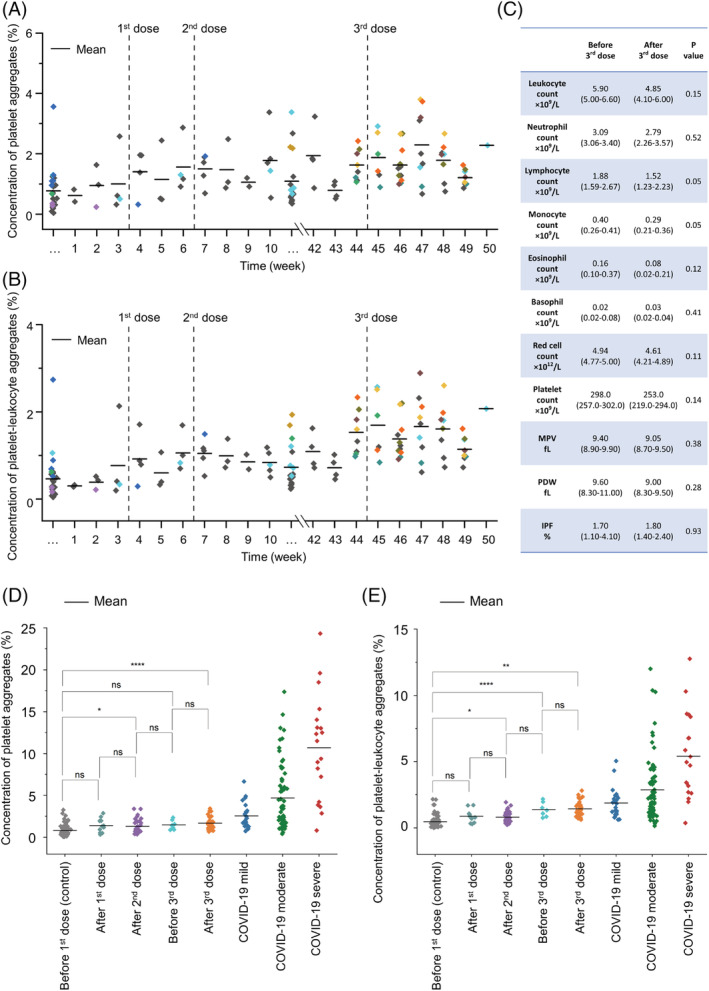
Statistical results. (A) Monitored concentrations of circulating platelet aggregates in healthy subjects. (B) Monitored concentrations of circulating platelet‐leukocyte aggregates in healthy subjects. (C) Laboratory test results. (D) Concentrations of circulating platelet aggregates in healthy subjects (for five vaccination periods), compared with those in COVID‐19 patients. (E) Concentrations of circulating platelet‐leukocyte aggregates in healthy subjects in comparison with those in COVID‐19 patients. Ns: P > 0.05; *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001; ****P ≤ 0.0001 [Color figure can be viewed at wileyonlinelibrary.com]
To better understand the temporal data in Figure 2A,B, we segmented them into five periods: (i) before the first dose (about 3 months); (ii) after the first dose (about 1 month); (iii) after the second dose (about 2 months); (iv) before the third dose (about 1 month); and (v) after the third dose (about 1 month). The data in the first period serve as a negative control. Figure 2D,E show the concentration in each period of platelet and platelet‐leukocyte aggregates, respectively. Notably, there are no statistically‐significant differences in the concentrations of platelet or platelet‐leukocyte aggregates between consecutive periods. While there was a statistically‐significant difference in the concentrations of platelet aggregates between the negative control period (i) and periods (iii) and (v), there was no such difference between period (i) and periods (ii) and (iv). These results indicate that the vaccinations did not have long‐term effects on platelet activity. Short‐term transient effects occurring 1–2 months after the vaccination were presumably due to the vaccination‐induced elevations of soluble platelet activation markers such as soluble P‐selectin [11, 12], which may lead to the increase in concentrations of platelet aggregates. The fluctuations in the concentrations of platelet‐leukocyte aggregates in Figure 2E can be attributed to the immediate immune response after the vaccination, the variation of the physical conditions of each subject, and the subject‐to‐subject differences. Although the underlying mechanisms of vaccine‐induced platelet activity and COVID‐19‐associated thromboinflammation are different, the figure also shows for comparison the average peak concentrations of platelet aggregates in mild (n = 23), moderate (n = 68), and severe (n = 19) hospitalized COVID‐19 patients. These data were obtained using the same imaging platform [8]. The mild, moderate, and severe patient groups correspond to those requiring no oxygen therapy, those requiring oxygen therapy, and those requiring mechanical ventilation or extracorporeal membrane oxygenation for respiratory support, respectively. As shown in Figure 2D,E, the average concentrations of aggregates in the healthy subjects (across all five periods) were found to be below those of the COVID‐19 patient groups. Moreover, Figure 2E shows that the interaction between platelets and leukocytes was not significantly activated by the vaccinations.
3. DISCUSSION
To the best of our knowledge, this is the first longitudinal examination of platelet activity in healthy subjects before and after multiple mRNA COVID‐19 vaccinations. Our method of image‐based profiling, temporal monitoring, and big data analysis has provided previously inaccessible insight into platelet activity related to immune response. Our work suggests that measuring the concentrations of platelet aggregates and platelet‐leukocyte aggregates is a potentially effective approach to evaluate platelet activity after vaccination. This method is a promising tool for evaluating long‐term response to mRNA vaccinations, thus facilitating new vaccine testing and more. Yet, there are a few caveats to mention. First, we focused on the Pfizer‐BioNTech (BNT162b2) vaccine in this study because it is the dominant COVID‐19 vaccine in Japan, and the Moderna vaccine was not available at the University of Tokyo Hospital. Second, since the blood samples were taken from each healthy subject at the timing when they were able to cooperate on this study, the blood sampling period and frequency were not consistent. Third, our cross‐study comparisons between healthy subjects and COVID‐19 patients may be confounded as measurement conditions between the two studies were not strictly controlled.
4. MATERIALS AND METHODS
4.1. Experiment overview
This study was conducted with the approval of the Institutional Ethics Committee in the School of Medicine at the University of Tokyo (no. 11049, no. 11344) in compliance with the relevant guidelines and regulations. Informed consent for participation in the study was obtained from the patients using an opt‐out process on the webpage of the University of Tokyo Hospital. Patients who refused participation in our study were excluded. Written informed consent was also obtained from the healthy subjects. The demographics of the healthy subjects are as follows: age 25–63, n = 4 (male), n = 7 (female). Blood samples (with 3.2% citrate) from the healthy subjects were drawn multiple times on different dates, according to the availability of healthy subjects.
4.2. Sample preparation
Single platelets and platelet aggregates were enriched from whole blood by density‐gradient centrifugation to maximize the efficiency of detecting platelets and platelet aggregates, as described in our previous report [8]. For analyzing the concentration of platelet aggregates, 500 μl of blood was diluted with 5 ml of saline. Platelets were isolated by using Lymphoprep (STEMCELLS, ST07851), a density‐gradient medium, based on the protocol provided by the vendor. Specifically, the diluted blood was layered on the top of Lymphoprep medium and centrifuged at 800 g for 20 min. After the centrifugation, 500 μl of the sample was taken from the mononuclear layer. Platelets were immunofluorescently labeled by adding 10 μl of anti‐CD61‐PE (Beckman Coulter, IM3605) and 5 μl of anti‐CD45‐PC7 (Beckman Coulter, IM3548) to the blood sample to ensure the detection of all platelets or platelet aggregates in the sample. Then, 500 μl of 2% paraformaldehyde (Wako, 163–20,145) was added for fixation. Without the fixation, platelet aggregates in the sample would be dismantled. Therefore, the fixation process was performed at least within 4 h after the blood draw. The entire sample preparation was performed at room temperature.
4.3. FDM microscopy
The FDM microscope is a high‐speed, blur‐free, bright‐field imaging system based on a spatially distributed optical frequency comb as the optical source and a single‐pixel photodetector as the image sensor. Since the optical frequency comb is composed of multiple beams which are spatially distributed, it is capable of simultaneously interrogating the one‐dimensional spatial profile of a target object (e.g., a platelet, a platelet aggregate). In addition, since each discrete beam of the optical frequency comb is tagged by a different modulation frequency, a spatial‐profile‐encoded image can be retrieved by performing Fourier transformation on the time‐domain waveform detected by the single‐pixel detector. We used a continuous‐wave laser (Cobolt Calypso, 491 nm, 100 mW) as the laser source. Emitted light from the laser was split by a beam splitter, deflected and frequency‐shifted by acousto‐optic deflectors (Brimrose TED‐150‐100‐488, 100‐MHz bandwidth), and recombined by another beam splitter. The resultant optical frequency comb was focused by an objective lens (Olympus UPLSAPO20X, NA:0.75) onto objects (e.g., single platelets, platelet aggregates) flowing at 1 m/s in a customized hydrodynamic‐focusing microfluidic channel (Hamamatsu Photonics). Light transmitted through the flowing objects was collected by an avalanche photodiode (Thorlabs APD430A/M) and processed by a home‐made LabVIEW program (LabVIEW 2016) to reconstruct the bright‐field images. The line scan rate, spatial resolution, field of view, and number of pixels after making pixel aspect ratio corrections were 3 MHz, 0.8 μm, 53.6 μm × 53.6 μm, and 67 × 67 pixels, respectively. Fluorescence emitted from platelets labeled by anti‐CD61‐PE was also collected and used for triggering image acquisitions. Fluorescence emitted from leukocytes labeled by anti‐CD45‐PC7 was collected to verify the image‐based identification of platelet‐leukocyte aggregates. Image acquisition was performed at a high throughput of 100–300 events per sec (eps), where an event is defined as a single platelet or a platelet aggregate since red blood cells, leukocytes, and cell debris were not detected as events. The throughput was chosen to avoid clogging the microchannel, although the theoretical throughput of the machine was >10,000 eps.
4.4. COVID‐19 patients
The COVID‐19 patients used in this study were clinically diagnosed with COVID‐19 based on their reverse transcription polymerase chain reaction (RT‐PCR) test results. Blood samples were collected as residual coagulation test samples (with 3.2% citrate) after the completion of requested clinical laboratory tests at the University of Tokyo Hospital. Blood cells in the samples were stored at room temperature.
4.5. Clinical laboratory tests
Hematological tests (leukocyte count, neutrophil count, lymphocyte count, eosinophil count, basophil count, red cell count, platelet count, MPV, PDW, and IPF) were performed using an XN hematology analyzer (Sysmex, Japan) on the blood samples from the healthy subjects before and after the third vaccine dose.
4.6. Statistical analysis
The concentrations of platelet aggregates were determined by calculating the ratio of the number of platelet aggregate images to the total number of acquired images. Outliers, which were defined as values that lied outside of the specified range (mean ± 3 SDs), were excluded from the data analysis. The composition of platelet aggregates was identified by analyzing the morphology of platelet aggregates in their images using custom codes in MATLAB and Python 3. Specifically, the objective area was segmented using edge detection algorithms. Then, the number and area of the detected objects in the segmentation region were calculated. The classification algorithm was developed based on the observation of cell images and the signal from anti‐CD45‐PC7. If the size of the whole segmented area was greater than 48 μm2, the image was defined as an image of a platelet aggregate. If the number of objects was greater than 2 and there was a single object with an area greater than 32 μm2, the image was defined as an image of a platelet‐leukocyte aggregate. Statistical analysis was performed using the Kruskal‐Wallis ANOVA test followed by the Bonferroni test with significance levels at 0.05.
4.7. Comparison between healthy subjects and COVID‐19 patients
To compare the concentrations of platelet aggregates in the blood samples of the healthy subjects and the hospitalized COVID‐19 patients, we used the highest concentrations of platelet aggregates during the hospital stay of the patients, for the following reasons. First, according to our earlier report [8], their platelet aggregate concentrations typically reach the highest levels ~1 week after their initial symptoms appeared. The prognosis of the patients is somewhat predictable, depending on the concentration of platelet aggregates, and can hence be used as a good indicator of the condition of the patients. Second, in the report [8], we also showed strong correlations between the concentration of platelet aggregates and the severity, mortality, respiratory condition, and vascular endothelial dysfunction level of the patients.
AUTHOR CONTRIBUTIONS
Keisuke Goda conceived the concept of the work. Yuqi Zhou, Masako Nishikawa, Hiroshi Kanno, Ruoxi Yang, Yuma Ibayashi, and Walker Peterson conducted the experiments and data analysis. Yuqi Zhou, Masako Nishikawa, Hiroshi Kanno, Ruoxi Yang, Yuma Ibayashi, Ting‐Hui Xiao, Nao Nitta, Shigeki Miyata, Yogen Kanthi, Gustavo K. Rohde, Yutaka Yatomi, Kyoji Moriya, Maik Herbig, and Keisuke Goda interpreted the results. Ting‐Hui Xiao, Yutaka Yatomi, and Keisuke Goda supervised the work.
CONFLICT OF INTEREST
Nao Nitta and Keisuke Goda are shareholders of CYBO.
5.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1002/cyto.a.24677.
Supporting information
MIFlowCyt: MIFlowCyt Item Checklist.
Appendix S1 Supplementary Information.
ACKNOWLEDGMENTS
This work was supported by AMED JP20wm0325021, JSPS Core‐to‐Core Program, JSPS‐NUS/NTU Bilateral Joint Research Program 2021, JSPS KAKENHI grant numbers 19H05633, 20H00317, and 21K15640, ImPACT Program, White Rock Foundation, Ogasawara Foundation, Nakatani Foundation, Konica Minolta Foundation, Charitable Trust Laboratory Medicine Research Foundation of Japan, and Chemo‐Sero‐Therapeutic Research Institute. We thank Prof. Masayuki Miyasaka at Osaka University for discussion.
Zhou Y, Nishikawa M, Kanno H, Yang R, Ibayashi Y, Xiao T‐H, et al. Long‐term effects of Pfizer‐BioNTech COVID‐19 vaccinations on platelets. Cytometry. 2022. 10.1002/cyto.a.24677
Funding information Charitable Trust Laboratory Medicine Research Foundation of Japan; Chemo‐Sero‐Therapeutic Research Institute; ImPACT Program; Japan Agency for Medical Research and Development, Grant/Award Number: JP20wm0325021; JSPS KAKENHI, Grant/Award Numbers: 19H05633, 20H00317, 21K15640; JSPS‐NUS/NTU Bilateral Joint Research Program 2021; Konica Minolta Foundation; Nakatani Foundation for Advancement of Measuring Technologies in Biomedical Engineering; Ogasawara Foundation for the Promotion of Science and Engineering; White Rock Foundation; National Institutes of Health, USA, Grant/Award Number: GM130825
Contributor Information
Ting‐Hui Xiao, Email: xiaoth@chem.s.u-tokyo.ac.jp.
Keisuke Goda, Email: goda@chem.s.u-tokyo.ac.jp, Email: goda@chem.s.u-tokyo.ac.jp.
REFERENCES
- 1. Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the BNT162b2 mRNA Covid‐19 vaccine. N Engl J Med. 2020;383:2603–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pardi N, Hogan MJ, Porter FW, Weissman D. mRNA vaccines – a new era in vaccinology. Nat Rev Drug Discov. 2018;17:261–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Klein NP, Lewis N, Goddard K, Fireman B, Zerbo O, Hanson KE, et al. Suveillance for adverse events after COVID‐19 mRNA vaccination. JAMA. 2021;326:1390–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jeyanathan M, Afkhami S, Smaill F, Miller MS, Lichty BD, Xing Z. Immunological considerations for COVID‐19 vaccine strategies. Nat Rev Immunol. 2020;20:615–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wang Y, Zhang Z, Luo J, Han X, Wei Y, Wei X. mRNA vaccine: a potential therapeutic strategy. Mol Cancer. 2021;20:33. 10.1186/s12943-021-01311-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gaertner F, Massberg S. Patrolling the vascular borders: platelets in immunity to infection and cancer. Nat Rev Immunol. 2019;19:747–60. [DOI] [PubMed] [Google Scholar]
- 7. Flego D, Cesaroni S, Romiti GF, Corica B, Marrapodi R, Scafa N, et al. Platelet and immune signature associated with a rapid response to the BNT162b2 mRNA COVID‐19 vaccine. J Thromb Haemost. 2022;20:961–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nishikawa M, Kanno H, Zhou Y, Xiao T, Suzuki T, Ibayashi Y, et al. Massive image‐based single‐cell profiling reveals high levels of circulating platelet aggregates in patients with COVID‐19. Nat Commun. 2021;12:7135. 10.1038/s41467-021-27378-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nitta N, Sugimura T, Isozaki A, Mikami H, Hirak K, Sakuma S, et al. Intelligent image‐activated cell sorting. Cell. 2018;175:266–76. [DOI] [PubMed] [Google Scholar]
- 10. Zhou Y, Yasumoto A, Lei C, Huang C‐J, Kobayashi H, Wu Y, et al. Intelligent classification of platelet aggregates by agonist type. Elife. 2020;9:e52938. 10.7554/eLife.52938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Petito E, Colonna E, Falcinelli E, Mezzasoma AM, Cesari E, Giglio E, et al. Anti‐severe acute respiratory syndrome coronavirus‐2 adenoviral‐vector vaccines trigger subclinical antiplatelet autoimmunity and increase of soluble platelet activation markers. Br J Haematol. 2022;198:257–66. 10.1111/bjh.18245 [DOI] [PubMed] [Google Scholar]
- 12. Campello E, Bulato C, Simion C, Spiezia L, Radu CM, Gavasso S, et al. Assessing clinically meaningful hypercoagulability after COVID‐19 vaccination: a longitudinal study. Thromb Haemost. 2022. 10.1055/a-1788-5206 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
MIFlowCyt: MIFlowCyt Item Checklist.
Appendix S1 Supplementary Information.


