Abstract
Patients infected with SARS‐CoV‐2 have varying manifestations of cardiac involvement. We report four patients presenting with symptomatic cardiac sarcoidosis (CS) or giant cell myocarditis (GCM) 1–8 months after mild COVID‐19. All patients received immunosuppressive therapy and improved gradually within the following months. The possible temporal association between the CS/GCM and COVID‐19 infection might suggest that COVID‐19 could be a trigger for granulomatous myocarditis.
Keywords: Cardiac sarcoidosis, Giant cell myocarditis, COVID‐19, Heart failure
Introduction
Patients infected with SARS‐CoV‐2 have varying manifestations of cardiac involvement. Here, we describe the clinical presentation and management of four cases with granulomatous myocarditis [sarcoidosis or giant cell myocarditis (GCM)], 1–8 months after mild COVID‐19 infection (Supporting Information, Table S1 ).
Case report
Patient 1
A 35‐year‐old previously healthy male suffered from COVID‐19 infection in December 2020. Initial symptoms were fever and myalgia, but later, he developed worsening dyspnoea. He was admitted in January 2021 and diagnosed with severe heart failure and non‐sustained ventricular tachycardia (NSVT). Troponin I (TnI) and N‐terminal pro‐BNP (NT‐proBNP) were significantly elevated, and COVID‐19 PCR test at this point was negative. Cardiac magnetic resonance imaging (cMRI) showed severe biventricular dysfunction and acute myocardial inflammation (Figure 1 ). 18F‐Fluorodeoxyglucose positron emission tomography/computed tomography (18F‐FDG PET/CT) confirmed the presence of widespread myocardial inflammation as well as uptake in the mediastinal and abdominal lymph nodes, suggesting systemic sarcoidosis with cardiac involvement.
Figure 1.
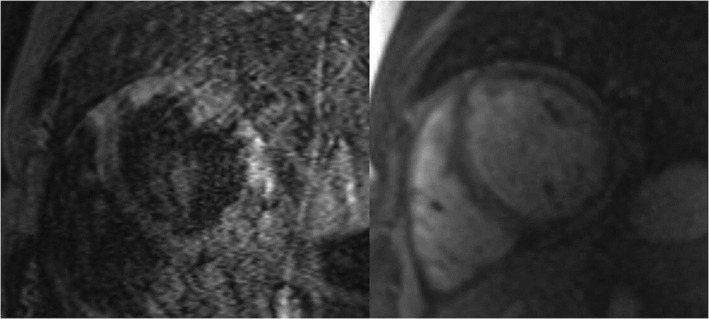
Cardiac magnetic resonance imaging images. T2 short tau inversion recovery (STIR) image showing signs of myocardial oedema (left) and late gadolinium enhancement (LGE) image showing widespread, midwall, non‐ischaemic‐type LGE on both ventricles (right).
The final diagnosis of cardiac sarcoidosis (CS) was based on endomyocardial biopsy (EMB), which showed well‐formed non‐necrotizing granulomas and solitary giant cells (Figure 2 ). Pulse‐dose steroids were administered for 3 days followed by oral prednisolone tapered down slowly over a year, and a cardiac resynchronization therapy defibrillator (CRT‐D) was implanted.
Figure 2.
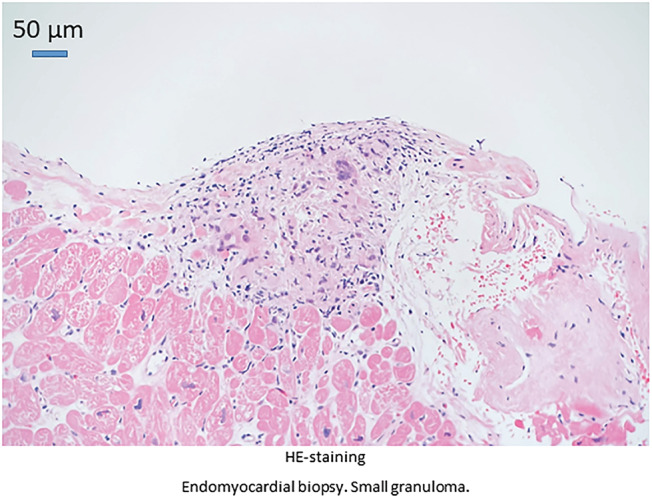
Endomyocardial biopsy demonstrating a small granuloma. Haematoxylin and eosin (H&E) staining.
A follow‐up 18F‐FDG PET in April 2021 showed decreased extent and intensity of fluorodeoxyglucose (FDG) uptake consistent with attenuated inflammatory activity (Figure 3 ). On the latest follow‐up in August 2021, left ventricular ejection fraction (LVEF) had stabilized at 35%, symptoms had improved to New York Heart Association (NYHA) II, and TnI was effectively decreased. According to our protocol, the patient was continued on 10 mg oral prednisolone daily.
Figure 3.
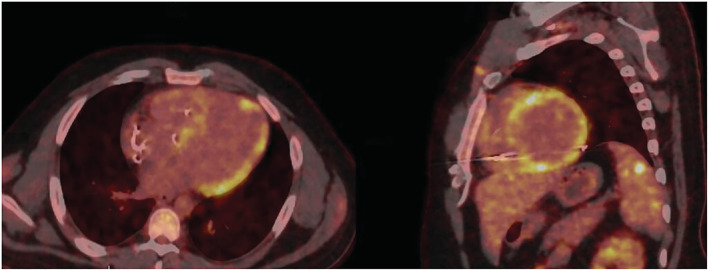
Cardiac 18F‐fluorodeoxyglucose positron emission tomography/computed tomography (18F‐FDG PET/CT) images showing patchy fluorodeoxyglucose (FDG) uptake in the left ventricle.
Patient 2
A 53‐year‐old, previously healthy female had COVID‐19 infection in April 2020 with mild symptoms. However, she developed progressive dyspnoea on exertion in the following months. In December 2020, she was urgently admitted to the hospital due to new‐onset chest pain. Interestingly, electrocardiogram (ECG) showed arrhythmogenic right ventricular dysplasia (ARVD)‐type ECG features (Figure 4 ). Coronary angiography showed atheromatous coronaries but no stenoses. cMRI showed mild left ventricular (LV) dysfunction, marked right ventricular (RV) dysfunction, and RV dilation with extensive late gadolinium enhancement (LGE) of the left ventricle (Figure 5 ). The finding did not fulfil minor nor major criteria for ARVD.
Figure 4.
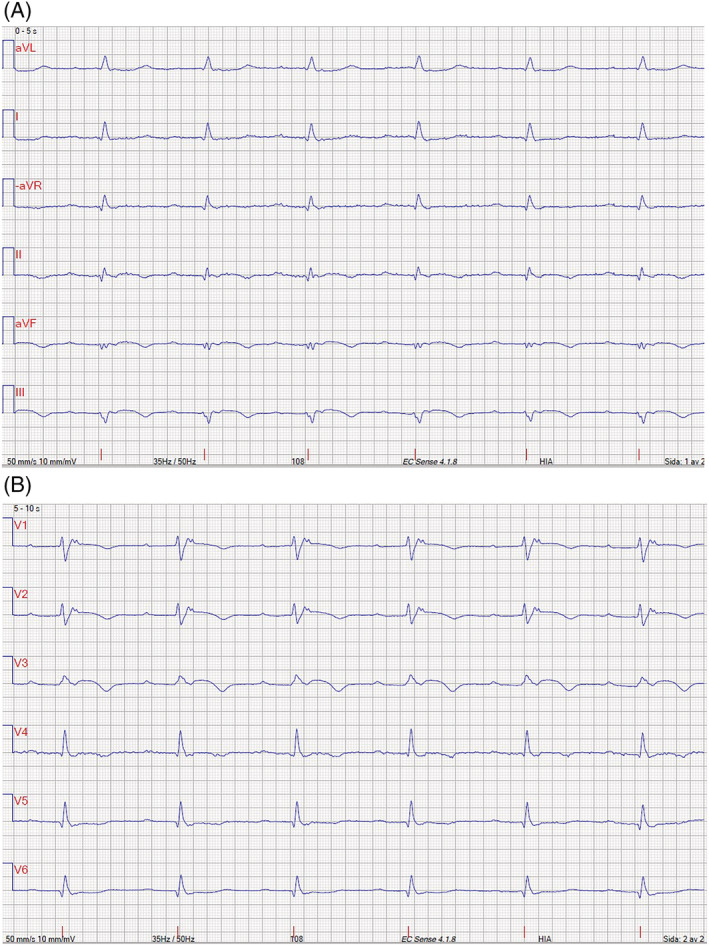
(A) Electrocardiogram (ECG) limb leads showing first‐degree atrioventricular (AV) block, low QRS amplitude, and T‐wave inversions in inferior leads. (B) ECG precordial leads showing first‐degree AV block, T‐wave inversions in right precordial leads V1–3, and epsilon wave. Recording speed was 50 mm/s.
Figure 5.
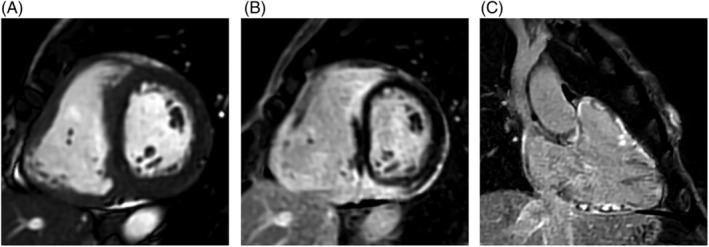
Cardiac magnetic resonance imaging (MRI) images. (A) Steady‐state free precession (SSFP) sagittal functional image showing a thickened right ventricular myocardium, (B) 2D phase‐sensitive inversion recovery (PSIR) sagittal image showing extensive biventricular delayed enhancement involving mainly the septum and adjacent right ventricle (RV), and (C) 2D PSIR projection of the inflow and outflow tract of the RV showing extensive delayed enhancement involving the RV.
The diagnosis of GCM was based on EMB results, showing signs of extensive myocyte destruction, necrosis, abundant giant cells, and eosinophils (Figure 6 ). In one of the biopsies, there was a loose granuloma formation. Given the abysmal prognosis of untreated GCM, treatment included steroids, tacrolimus, and mycophenolate mofetil, and an implantable cardioverter defibrillator (ICD) was implanted. A control EMB after 1 month showed fibrosis without inflammation. Follow‐ups revealed robust treatment response with no ventricular arrhythmias.
Figure 6.
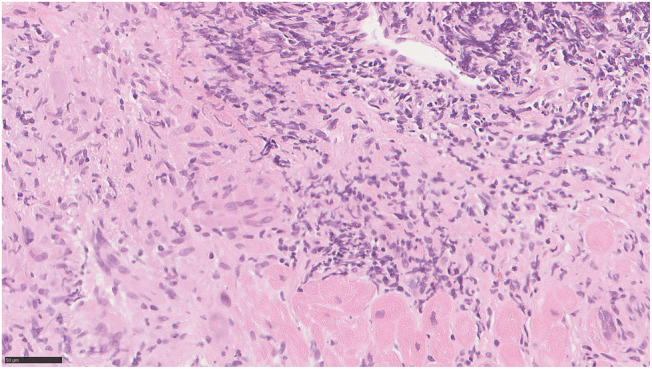
Endomyocardial biopsy demonstrating loss of myocytes, diffuse infiltration of macrophages, lymphocytes, a few eosinophilic leucocytes, and some giant cells. Haematoxylin and eosin staining; bar corresponds to 50 μm.
Patient 3
A 47‐year‐old female with hypothyroidism had COVID‐19 in December 2020. Symptoms were mild with fever and palpitations. After 3 months, the patient sought help at her general practitioners due to worsening palpitations and progressive dyspnoea. The initial ECG showed NSVT, which resulted in an immediate hospitalization. Coronary CT angiogram was normal. cMRI revealed signs of extensive myocardial inflammation.
The first EMB revealed no signs of inflammation. However, a second magnetic resonance imaging (MRI)‐guided EMB of the inferior part of the septum showed signs consistent with GCM (Figure 7 ). She was treated with a similar regimen as Patient 2 including an ICD. During the first month, the ICD detected frequent NSVTs, and amiodarone was added to the drug regimen. Patient is doing well, with only minor symptoms with NYHA I–II with no arrythmias.
Figure 7.
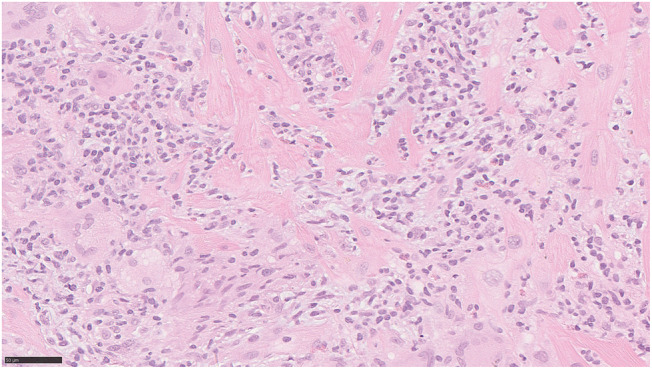
Endomyocardial biopsy demonstrating myocyte destruction and diffuse infiltration of macrophages, lymphocytes, a few eosinophilic leucocytes, and some giant cells. Haematoxylin and eosin staining; bar corresponds to 50 μm.
Patient 4
A 40‐year‐old, previously healthy male had COVID‐19 in early March 2022 with mild symptoms. He initially recovered but was admitted in mid‐April for sustained ventricular tachycardias (VTs). cMRI demonstrated severe LV dysfunction and myocardial oedema. EMB confirmed the diagnosis of GCM. He quickly developed cardiogenic shock and was placed on venoarterial extracorporeal membrane oxygenation (VA‐ECMO). He was started on triple immunosuppressive therapy and was weaned of extracorporeal membrane oxygenation (ECMO) after 8 days. ICD was implanted for secondary prevention. The tendency to VTs was decreased by the use of amiodarone–mexiletine–bisoprolol combination, and he was discharged in stable condition in late May 2022.
Discussion
We describe four patients with granulomatous myocarditis as a sequela of a mild COVID‐19 infection. Cardiac manifestations of heart failure and/or ventricular tachycardia occurred 1, 3, and 8 months after COVID‐19 infection. All patients had a characteristic late gadolinium enhancement pattern on cMRI, as well as consistent 18F‐FDG PET/CT findings in the case of CS. EMB of the RV septum showed well‐formed granulomas in one patient and giant cells and eosinophils in three other patients. Tests for SARS‐CoV‐2 in the myocardium were negative. After the biopsy results, immunosuppressive therapy was started with a favourable response. As granulomatous myocarditis is an exceedingly rare disease, we speculate that rather than being just a random event, CS/GCM developed due to previous SARS‐CoV‐2 infection in these four cases.
In Patient 1, CS might have been smouldering even before the COVID‐19 onset.
Patients infected with SARS‐CoV‐2 have varying manifestations of cardiac involvement. Myocardial injury is very common as measured by elevated troponin levels but does not distinguish among the causes of injury such as myocarditis, microvascular dysfunction, small vessel cardiac vasculitis, and right heart strain due to pulmonary embolism. Myocarditis is often suspected but rarely if ever histologically confirmed even in autopsy studies. 1 In clinical and autopsy series, only a few cases found histological evidence for lymphocytic infiltrates or SARS‐CoV‐2 genome in the heart, 2 which is in line with our own unpublished results. Based on the current available evidence, acute myocarditis is rare in patients with COVID‐19 infection, on the order of ~1–2%. 3
Little is known regarding the long‐term implications of COVID‐19 on the heart. 4 Pericarditis has been seen late after an acute infection, 5 whereas the findings of late gadolinium enhancement of the myocardium on cMRI have unknown long‐term consequences. Taken together, a variety of laboratory and imaging abnormalities are seen in post‐acute COVID‐19, although many of the cMRI findings are not detected at a significantly higher rate than among healthy controls in some studies. 6
Granulomatous inflammation has been described after a SARS‐CoV infection in the heart, 7 skin, 8 , 9 lymph nodes, 10 and Löfgren's syndrome, which is an acute form of sarcoidosis. 11 A delayed immunological response triggered by viral infection might give rise to granulomatous lesions. Other data suggest that together with innate immune response factors, deficient clearance of bacterial and/or viral and/or inorganic particles might be associated with granulomatous myocarditis. 12 The presence of viruses in the myocardium can potentially trigger a hypersensitivity‐type reaction, which can initiate CS and GCM. Based on possible temporal relationship between COVID‐19 and the development of symptomatic CS/GCM in our four cases, we speculate that the viral infection acted only as initiating factor. For Patient 2, we do believe that longer time between the COVID‐19 infection and diagnosis of GCM is due to diagnostic delay. The mechanism of granulomatous inflammation in these cases is unclear, but inflammation and a dysregulated immune response following SARS‐CoV‐2 infection might prompt other environmental triggers to cause the observed pathologies in predisposed individuals. Future studies will be needed to determine how common granulomatous inflammation in the heart is following a COVID‐19 infection.
Conflict of interest
None declared.
Supporting information
Table S1. Overview of disease manifestation and clinical findings for patient 1–4.
Acknowledgements
We express our gratitude to the patients for participating in this study. We thank Kristiina Nokelainen for skilled technical assistance with immunostainings.
Bollano, E. , Polte, C. L. , Mäyränpää, M. I. , Oldfors, A. , Bergh, N. , Lehtonen, J. , and Kandolin, R. (2022) Cardiac sarcoidosis and giant cell myocarditis after COVID‐19 infection. ESC Heart Failure, 9: 4298–4303. 10.1002/ehf2.14088.
References
- 1. Escher F, Pietsch H, Aleshcheva G, Bock T, Baumeier C, Elsaesser A, Wenzel P, Hamm C, Westenfeld R, Schultheiss M, Gross U, Morawietz L, Schultheiss HP. Detection of viral SARS‐CoV‐2 genomes and histopathological changes in endomyocardial biopsies. ESC Heart Fail. 2020; 7: 2440–2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Basso C, Leone O, Rizzo S, de Gaspari M, van der Wal AC, Aubry MC, Bois MC, Lin PT, Maleszewski JJ, Stone JR. Pathological features of COVID‐19‐associated myocardial injury: a multicentre cardiovascular pathology study. Eur Heart J. 2020; 41: 3827–3835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kawakami R, Sakamoto A, Kawai K, Gianatti A, Pellegrini D, Nasr A, Kutys B, Guo L, Cornelissen A, Mori M, Sato Y, Pescetelli I, Brivio M, Romero M, Guagliumi G, Virmani R, Finn AV. Pathological evidence for SARS‐CoV‐2 as a cause of myocarditis: JACC review topic of the week. J Am Coll Cardiol. 2021; 77: 314–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Satterfield BA, Bhatt DL, Gersh BJ. Cardiac involvement in the long‐term implications of COVID‐19. Nat Rev Cardiol. 2021; 19: 332–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Carubbi F, Alunno A, Leone S, di Gregorio N, Mancini B, Viscido A, del Pinto R, Cicogna S, Grassi D, Ferri C. Pericarditis after SARS‐CoV‐2 infection: another pebble in the mosaic of long COVID? Viruses. 2021; 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Joy G, Artico J, Kurdi H, Seraphim A, Lau C, Thornton GD, Oliveira MF, Adam RD, Aziminia N, Menacho K, Chacko L, Brown JT, Patel RK, Shiwani H, Bhuva A, Augusto JB, Andiapen M, McKnight A, Noursadeghi M, Pierce I, Evain T, Captur G, Davies RH, Greenwood JP, Fontana M, Kellman P, Schelbert EB, Treibel TA, Manisty C, Moon JC, COVIDsortium Investigators . Prospective case‐control study of cardiovascular abnormalities 6 months following mild COVID‐19 in healthcare workers. J Am Coll Cardiol Img. 2021; 14: 2155–2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bearse M, Hung YP, Krauson AJ, Bonanno L, Boyraz B, Harris CK, Helland TL, Hilburn CF, Hutchison B, Jobbagy S, Marshall MS, Shepherd DJ, Villalba JA, Delfino I, Mendez‐Pena J, Chebib I, Newton‐Cheh C, Stone JR. Factors associated with myocardial SARS‐CoV‐2 infection, myocarditis, and cardiac inflammation in patients with COVID‐19. Mod Pathol. 2021; 34: 1345–1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Polat Ekinci A, Buyukbabani N, Mese S, Pehlivan G, Okumuş NG, Ağaçfidan A, Özkaya E. COVID‐19‐triggered sarcoidal granulomas mimicking scar sarcoidosis. J Eur Acad Dermatol Venereol. 2021; 35: e477–e480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Behbahani S, Baltz JO, Droms R, Deng AC, Amano SU, Levin NA, O'Brien MC, Wiss K. Sarcoid‐like reaction in a patient recovering from coronavirus disease 19 pneumonia. JAAD Case Rep. 2020; 6: 915–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sekulic M, Harper H, Nezami BG, Shen DL, Sekulic SP, Koeth AT, Harding CV, Gilmore H, Sadri N. Molecular detection of SARS‐CoV‐2 infection in FFPE samples and histopathologic findings in fatal SARS‐CoV‐2 cases. Am J Clin Pathol. 2020; 154: 190–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mihalov P, Krajcovicova E, Kacerova H, Sabaka P. Lofgren syndrome in close temporal association with mild COVID‐19—case report. IDCases. 2021; 26: e01291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Calender A, Israel‐Biet D, Valeyre D, Pacheco Y. Modeling potential autophagy pathways in COVID‐19 and sarcoidosis. Trends Immunol. 2020; 41: 856–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Overview of disease manifestation and clinical findings for patient 1–4.


