Abstract
Host genetic factors may be correlated with the severity of coronavirus disease 2019 (COVID‐19). Angiotensin‐converting enzyme 2 (ACE2) plays a vital role in viral cell entrance. The current study aimed to evaluate the association of ACE2 rs2285666 polymorphism and clinical parameters with COVID‐19 mortality. The ACE2 rs2285666 polymorphism was genotyped using the polymerase chain reaction‐restriction fragment length polymorphism in 556 recovered and 522 dead patients. In this study, the frequency of ACE2 rs2285666 CC was significantly higher than TT genotype in dead patients. The multivariate logistic regression analysis results showed that the higher levels of alanine aminotransferase, alkaline phosphatase, creatinine, erythrocyte sedimentation rate, and C‐reactive protein and the low levels of uric acid, cholesterol, low density lipoprotein, 25‐hydroxyvitamin D, real‐time PCR Ct values, and ACE2 rs2285666 CC genotype were associated with increased mortality rates after COVID‐19. In conclusion, our findings demonstrated a possible link between COVID‐19 mortality, clinical parameters, and ACE2 rs2285666 CC. Further research is required to confirm these results.
Keywords: angiotensin‐converting enzyme 2, coronavirus disease 2019, severe acute respiratory syndrome coronavirus 2
1. INTRODUCTION
The current coronavirus disease 2019 (COVID‐19) pandemic has triggered a worldwide crisis that is devastating health organizations (Abdelsattar et al., 2022). The morbidity and mortality of COVID‐19 significantly increase when the disease is combined with pre‐existing risk factors, such as age and underlying diseases. While most infected people recover, even young and unpredictably healthy people can succumb to the sickness (Dong et al., 2020). These findings have prompted how genetic susceptibility could account for differences in COVID‐19 severity. Genetic factors might be associated with high levels of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) transmissions and disease progression in the infected population. However, these determinants remain mostly unknown at this time (Ellinghaus et al., 2020).
Recent studies on the complications and death rates of COVID‐19 show that it is more common in people with cardiovascular diseases (CVDs). Researchers have found similar results in the past when they looked at the links between CVDs and susceptibility to coronavirus infections, such as SARS‐CoV and the Middle East respiratory syndrome coronavirus (MERS‐CoV) (Augustine et al., 2022). Given the high angiotensin‐converting enzyme 2 (ACE2) expression in the heart and the SARS‐CoV‐2 transfection mechanism, it is speculated that the poor prognosis of COVID‐19 patients might be associated with heart damage. Currently, there are two main theories about how the heart might be damaged by SARS‐CoV‐2. First, SARS‐CoV‐2 directly damages the heart in persons without any CVDs; and second, SARS‐CoV‐2 can also contribute to the poor prognoses in persons who already have CVDs (Guo et al., 2020; Inciardi et al., 2020).
SARS‐CoV‐2 infection is primarily dependent on the cellular components of infected hosts. ACE2, located on the heart, respiratory tract, kidney, and oral mucosa, has an essential role in virus entry into host cells (Hoffmann et al., 2020). A splice region variant named rs2285666 (G > A, Intron 3/4) is the best described single nucleotide polymorphism (SNP) in ACE2. Several reports have revealed correlations with diabetes, cerebral stroke, coronary heart disease, and hypertension (Möhlendick et al., 2021). The A‐allele is linked to higher ACE2 levels in the serum of healthy people, subjects with diabetes, and patients with diabetes and cerebral stroke (Wu et al., 2017). It is unclear whether the elevated blood ACE2 levels are linked with A‐allele represents greater membrane‐bound ACE2 in tissues, such as lungs. However, this polymorphism explains the link between COVID‐19 susceptibility and disease progression because the gene ACE2 acts as a cellular receptor for virus entry (Möhlendick et al., 2021).
This study aimed to evaluate if the severity of COVID‐19 could be predicted by genotyping the ACE2 rs2285666 polymorphisms.
2. MATERIALS AND METHODS
2.1. Study population
This study was carried out on 1078 COVID‐19 patients at Pasteur Institute of Iran (PII) during June 2021 to February 2022. Following approval by the PII Ethical Committee (IR PII REC 1400.042), the study was conducted according to the 1975 Declaration of Helsinki and any applicable local regulations. Furthermore, written informed consent was directly obtained from all participants.
In our study, SARS‐CoV‐2 (all samples were alpha variant) was evaluated using reverse transcriptase real‐time polymerase chain reaction (rtReal Time‐PCR) for nasopharyngeal or oropharyngeal swab samples collected from patients. During the rtReal Time‐PCR, the cycle threshold (Ct) determines the detectable level of the viral load in the swap samples.
The exclusion criteria were the patients who had heart disease, chronic kidney disease, liver disease, asthma, chronic obstructive pulmonary disease, cystic fibrosis, cancer, diabetes, human immunodeficiency virus, obesity, and pregnancy.
Among COVID‐19 infected patients, 556 patients (recovered patients) were managed in an outpatient setting, whereas 522 patients (dead patients) needed to be admitted to the hospital.
Recovered patients presented with mild clinical manifestations such as fever and respiratory symptoms, and an X‐ray or computed tomography (CT) revealed no evidence of pneumonia. The dead patients had the following conditions: breathlessness with a respiratory rate below 30, less than 92% oxygen saturation levels at rest in a single finger of one arm, rapid progression of lesions exceeding 50% within 24–48 h, shock, acute respiratory failure requiring assistance from mechanical ventilation, involvement of other organs, and the need for intensive care.
All clinical parameters were taken when the patients visited the hospital before hospitalization. The laboratory test results, including total high density lipoprotein (HDL), low‐density lipoprotein (LDL), triglyceride (TG), cholesterol, fasting blood glucose (FBS), 25‐hydroxyvitamin D, C‐reactive protein (CRP), erythrocyte sedimentation rate (ESR), triiodothyronine (T3), thyroxine (T4), thyroid‐stimulating hormone (TSH), aspartate aminotransferase (AST), alkaline phosphatase (ALP), alanine aminotransferase (ALT), real‐time PCR Ct values, uric acid, serum creatinine, platelets, haemoglobin, and white blood cells (WBC), were obtained from the medical records.
2.2. DNA extraction and ACE2 rs2285666 genotyping
The peripheral blood mononuclear cells (PBMCs) were isolated from the blood samples of patients by Ficoll (Ficoll‐Paque PLUS, GE Healthcare, USA) density gradient centrifugation and were kept at −70°C.
The High Pure PCR Template Preparation Kit (Roche Diagnostics Deutschland GmbH, Mannheim, Germany) was used to separate genomic DNA from the PBMCs of COVID‐19 patients following the manufacturer's instructions. The ACE2 rs2285666 was genotyped by using polymerase chain reaction restriction fragment length polymorphism (PCR‐RFLP) method.
The forward and reverse primers were 5′‐AAACCACTGAAATGACTTACTTACTG‐3′ and 5′‐GCCTCACTGTCCTATGACTTTAT‐3′, respectively. The PCR conditions for the analysis of ACE2 rs2285666 were initial denaturation at 95°C for 5 min, followed by 35 cycles of denaturing at 95°C for 35 s, annealing at 57°C for 35 s, extension at 72°C for 45 s, and final extension at 72°C for 10 min. The PCR products (673 bp) were visualized by electrophoresis on 1.5% agarose gel and were digested with 1 U of AluI (Fermentas, Vilnius, Lithuania) at 37°C for 16 h. The RFLP products were visualized by electrophoresis on 2% agarose gel. The product size was 177 and 496 bp for the TT genotype and 673 bp for the CC genotype. At least 10% of samples were randomly genotyped with the Sanger technique on an ABI 3500 DX Genetic Analyzer (ABI, Thermo Fisher Scientific, Waltham, MA, USA) to confirm the PCR‐RFLP results and were then evaluated using MEGA version 11.0 (https://www.megasoftware.net/).
2.3. Statistical analysis
All the data were examined using the statistical software IBM SPSS for Windows version 22.0 (SPSS. Inc, Chicago, IL, USA). The Shapiro–Wilk test was utilized to evaluate whether continuous variables had a normal distribution. In addition, the Pearson's Chi‐square and Mann–Whitney U tests were used to analyse the data for quantitative and continuous variables. A multivariate logistic regression analysis employing the Hosmer–Lemeshow test was used to evaluate the connection between COVID‐19 resistance and several susceptibility risk factors. A two‐tailed p‐value < .05 was considered statistically significant. The odds ratio (OR) and corresponding confidence interval (CI) of 95% were determined. A two‐tailed p‐value < .05 was considered statistically significant and calculated by comparison among dead and recovered patients. The effect of ACE2 rs2285666 on COVID‐19 mortality was assessed using the area under the curve‐receiver operating characteristic (AUC‐ROC). The inheritance mode analysis in SNPStats software was used to evaluate the relationship between COVID‐19 mortality and ACE2 rs2285666. The Akaike information criterion (AIC) and the Bayesian information criterion (BIC) were used to identify the best fitted model for each SNP (Solé et al., 2006).
3. RESULTS
3.1. Demographic and clinical parameters of COVID‐19 patients
Table 1 shows the clinical and demographic characteristics of the participants. This study was performed on 1078 COVID‐19 participants, who were divided into two groups of recovered (n = 556) and dead (n = 522) patients.
TABLE 1.
Comparison of laboratory parameters between dead and recovered patients infected with coronavirus disease 2019 (COVID‐19)
| Variables | Dead patients (n = 522) | Recovered patients (n = 556) | p‐Value |
|---|---|---|---|
| Mean age ± SD | 57.8 ± 11.3 | 51.5 ± 12.7 | <.001* |
| Gender (male/female) | 280/242 (53.6/46.4%) | 289/267 (52.0/48.0%) | 0.585 |
| ALT, IU/L (mean ± SD) (reference range: 5–40) | 44.3 ± 23.6 | 33.0 ± 24.5 | <.001* |
| AST, IU/L (mean ± SD) (reference range: 5–40) | 37.0 ± 12.9 | 30.7 ± 15.3 | <.001* |
| ALP, IU/L (mean ± SD) (reference range: up to 306) | 201.1 ± 66.0 | 169.2 ± 89.6 | <.001* |
| Cholesterol, mg/dl (mean ± SD) (reference range: 50–200) | 116.5 ± 38.4 | 123.2 ± 36.9 | <.001* |
| TG, mg/dl (mean ± SD) (Reference range: 60–165) | 117.6 ± 42.2 | 132.2 ± 36.9 | .009* |
| LDL, mg/dl (mean ± SD) (Reference range: up to 150) | 71.4 ± 36.7 | 111.4 ± 48.7 | <.001* |
| HDL, mg/dl (mean ± SD) (Reference range: >40) | 30.5 ± 11.0 | 34.5 ± 11.8 | <.001* |
| WBC, 109/L (mean ± SD) (Reference range: 4000–10,000) | 7600.4 ± 2712.8 | 7738.8 ± 2901.1 | .589 |
| CRP, mg/L (mean ± SD) (reference range: < 10 mg/L negative) | 66.6 ± 21.5 | 56.4 ± 20.8 | <.001* |
| ESR, mm/1st h (mean ± SD) (reference range: 0–15) | 55.1 ± 15.8 | 46.4 ± 15.5 | <.001* |
| FBS, mg/dl (mean ± SD) (reference range: 70–100) | 110.2 ± 42.6 | 106.1 ± 41.5 | .002* |
| Platelets × 1000/cumm (mean ± SD) (reference range: 140,000–400,000) | 185 ± 77 | 184 ± 67 | .317 |
| Haemoglobin, g/dl (mean ± SD) (reference range: 12–18) | 14.4 ± 2.1 | 12.4 ± 1.8 | .363 |
| Uric acid, mg/dl (mean ± SD) (reference range: 3.6–6.8) | 3.4 ± 1.2 | 5.8 ± 1.6 | <.001* |
| Creatinine, mg/dl (mean ± SD) (reference range: 0.6–1.4) | 1.1 ± 0.3 | 0.8 ± 0.3 | <.001* |
| T3, ng/dl (mean ± SD) (reference range: 2.3–4.2) | 3.0 ± 1.1 | 2.6 ± 0.9 | .051 |
| T4, μg/dl (mean ± SD) (reference range: 5.6–13.7) | 9.0 ± 4.7 | 8.4 ± 5.8 | .352 |
| TSH, mu/L (mean ± SD) (reference range: 0.4–4.5) | 3.5 ± 1.8 | 3.3 ± 1.6 | .626 |
| 25‐Hydroxy vitamin D, ng/mL (mean ± SD) (Sufficiency: 21–150) | 27.0 ± 10.2 | 36.2 ± 13.5 | <.001* |
| Real‐time PCR Ct values | 12.5 ± 6.9 | 26.1 ± 8.1 | .021* |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; ALP, alkaline phosphatase; TG, triglyceride; LDL, low density lipoprotein; HDL, high density lipoprotein; WBC, white blood cells; CRP, C‐reactive protein; ESR, erythrocyte sedimentation rate; FBS, fasting blood glucose; T3, triiodothyronine; T4, thyroxine; TSH, thyroid‐stimulating hormone; Ct, cycle threshold; SD, standard deviation.
*Statistically significant (< 0.05).
The mean ages of recovered and dead patients were 51.5 ± 12.7 and 57.8 ± 11.3 years, respectively. High levels of ALT (p <.001), AST (p <.001), ALP (p <.001), FBS (p =.002), Cr (p <.001), CRP (p <.001), and ESR (p <.001) and uric acid (p <.001), 25‐hydroxyvitamin D (p <.001), real‐time PCR Ct value (p =.021), TG (p =.025), cholesterol (p =.001), HDL (p <.001), and LDL (p <.001) were associated with increased mortality rate after COVID‐19.
3.2. Correlation of ACE2 rs2285666 and COVID‐19 mortality
The effect of ACE2 rs2285666 on COVID‐19 mortality is depicted in Figure 1. COVID‐19 mortality was significantly higher in patients with ACE2 rs2285666 CC genotypes compared to other genotypes, while recovered patients had TT genotypes (Figure 1a).
FIGURE 1.
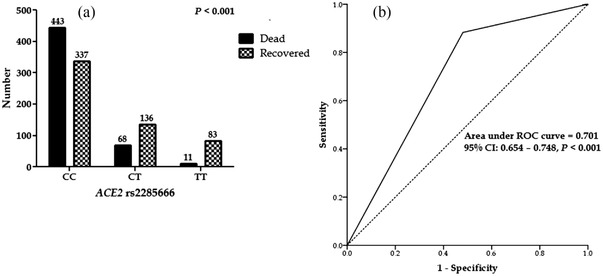
(a) Frequency of ACE2 rs2285666 in coronavirus disease 2019 (COVID‐19) patients. (b) ROC curve with the ACE2 rs2285666 for prediction the mortality rate in COVID‐19 patients
There are four inheritance models including codominant, dominant, recessive, and overdominant in Table 2 that were constructed using the SNPStats software. The model with the lowest AIC and BIC values was considered the best‐fitting inheritance for the relationship between ACE2 rs2285666 and COVID‐19 mortality.
TABLE 2.
ACE2 rs2285666 association with COVID‐19 mortality
| Groups | |||||||
|---|---|---|---|---|---|---|---|
| Model | Genotype | Recovered patients | Dead patients | OR (95% CI) | p‐Value | AIC | BIC |
| Allele | C | 810 (73.0%) | 954 (91.0%) | – | – | – | – |
| T | 302 (18.0%) | 90 (0.9%) | – | – | – | – | |
| Codominant | CC | 337 (60.6%) | 443 (84.9%) | 1.00 |
<.0001 |
1402.3 |
1422.3 |
| CT | 136 (24.5%) | 68 (13.0%) | 0.38 (0.28–0.53) | ||||
| TT | 83 (14.9%) | 11 (2.1%) | 0.10 (0.05–0.19) | ||||
| Dominant | CC | 337 (60.6%) | 443 (84.9%) | 1.00 |
<.0001 |
1417.5 |
1432.4 |
| CT‐TT | 219 (39.4%) | 79 (15.5%) | 0.27 (0.20–0.37) | ||||
| Recessive | CC‐CT | 420 (75.5%) | 511 (97.9%) | 1.00 |
<.0001 |
1442.5 |
1452.4 |
| TT | 83 (14.9%) | 11 (2.1%) | 0.12 (0.06–0.23) | ||||
| Overdominant | CC‐TT | 420 (75.5%) | 454 (87.0%) | 1.00 |
<.0001 |
1475.9 |
1490.8 |
| CT | 136 (24.5%) | 68 (13.0%) | 0.46 (0.34–0.64) | ||||
| Minor allele frequency (T) | 0.18 | 0.09 | – | – | – | – | |
Abbreviations: AIC, Akaike information criterion; BIC, Bayesian information criterion; CI, confidence intervals; OR, odds ratios.
The best‐fitting inheritance model for ACE2 rs2285666 was codominant that had the lowest AIC and BIC values. The CC genotype was related to an increased risk of death. Minor allele frequency (T) in recovered, dead, and all patients was 0.27, 0.09, and 0.18, respectively (Table 2).
Moreover, the AUC‐ROC value for ACE2 rs2285666 was 0.701, indicating that host genetic factors frequently affect viral infection mortality (Figure 1b).
3.3. Factors correlated with COVID‐19 mortality
We used multivariate logistic regression analysis to examine some of the factors associated with COVID‐19 mortality. The mortality rate of COVID‐19 infection was associated to mean age (OR 0.959, 95% CI 0.942–0.977, p <.001), cholesterol (OR 1.039, 95% CI 1.020–1.059, p <.001), LDL (OR 1.016, 95% CI 1.011–1.021, p <.001), uric acid (OR 2.169, 95% CI 1.882–2.498, p <.001), creatinine (OR 0.049, 95% CI 0.025–0.097, p <.001), ESR (OR 0.971, 95% CI 0.957–0.984, p <.001), CRP (OR 0.985, 95% CI 0.976–0.995, p =.004), 25‐hydroxyvitamin D (OR 1.062, 95% CI 1.042–1.082, p <.001), ALT (OR 0.983, 95% CI 0.974–0.993, p =.001), ALP (OR 0.997, 95% CI 0.995–0.100, p <.001), real‐time PCR Ct values (OR 0.912, 95% CI 0.901–0.965, p =.003), and ACE2 rs2285666 CC (OR 7.452, 95% CI 4.867–11.410, p <.001) (Table 3).
TABLE 3.
Factors associated with dead patients infected with coronavirus disease 2019 (COVID‐19)
| Factors | ||
|---|---|---|
| Baseline predictors | OR (95 % CI) | p‐Value |
| Mean age ± SD | 0.959 (0.942−0.977) | <.001* |
| Cholesterol, mg/dl | 1.039 (1.020−1.059) | <.001* |
| LDL, mg/dl | 1.016 (1.011−1.021) | <.001* |
| Uric acid, mg/dl | 2.169 (1.882−2.498) | <.001* |
| Creatinine, mg/dl | 0.049 (0.025−0.097) | <.001* |
| ESR, (mm/1st h) | 0.971 (0.957−0.984) | <.001* |
| CRP, mg/L | 0.985 (0.976−0.995) | .004* |
| 25‐Hydroxyvitamin D, (ng/ml) | 1.062 (1.042−1.082) | <.001* |
| ALT, IU/L | 0.983 (0.974−0.993) | .001* |
| ALP, IU/L | 0.997 (0.995−1.000) | .029* |
| Real‐time PCR Ct values | 0.912 (0.901–0.965) | .003* |
| ACE2 rs2285666 (TT) | 7.452 (4.867–11.410) | <.001* |
Abbreviations: ALT, alanine aminotransferase; ALP, alkaline phosphatase; CI, confidence intervals; LDL, low density lipoprotein; HDL, high density lipoprotein; ESR, erythrocyte sedimentation rate; CRP, C‐reactive protein; Ct, cycle threshold; ACE2, angiotensin‐converting enzyme 2; OR, odds ratios; SD, standard deviation. *Statistically significant (< 0.05).
4. DISCUSSION
This comprehensive investigation aimed to determine if ACE2 rs2285666 polymorphism in COVID‐19 patients is connected with the severity and mortality of SARS‐CoV‐2 infection in Iranian patients. According to our findings, ACE2 rs2285666 polymorphism is associated with COVID‐19 mortality.
Several studies are currently being conducted to investigate the effect of genetic host variables on the risk of SARS‐CoV‐2 infection or the severity of COVID‐19. Scientists have hypothesized that polymorphisms in genes encoding critical components of the renin‐angiotensin system, including ACE or ACE2, may contribute to infection risk (Möhlendick et al., 2021).
The comparison between the two groups of patients revealed a higher mortality rate in the ACE2 rs2285666 CC genotype than the TT genotype, which was in agreement with other studies (Alimoradi et al., 2022; Möhlendick et al., 2021; Srivastava et al., 2020). Several investigations have indicated the relationship between ACE2 rs2285666 CC genotype and the prevalence and risk of SARS‐CoV‐2 infection. However, the effect of this SNP on disease severity or the mortality rate of COVID‐19 was not found (Gómez et al., 2020; Karakaş Çelik et al., 2021). This discrepancy may be related to the sample size, which was larger in our study than in the previous research. Furthermore, the correlation of ACE2 rs2285666 polymorphism with COVID‐19 susceptibility is inconsistent in studies worldwide, which may be due to ethnic variances between groups, as these variants show some population‐based differences (Sarangarajan et al., 2021; Srivastava et al., 2020). Although the gene expression of ACE2 receptor may influence COVID‐19 susceptibility, there is no evidence that ACE2 gene polymorphisms are directly related to COVID‐19 severity (Çelik et al., 2021).
The mechanism behind this discovery is still unknown, and it may even be counterintuitive. Suppose the theory that A‐allele is linked to higher ACE2 serum levels or perhaps cellular ACE2 expression is valid. In that case, A‐allele carriers should be at higher risk if ACE2 is the “receptor” for SARS‐CoV‐2 entrance (Wu et al., 2017). We have no explanation for our findings at this time. However, because our cohort consisted of a highly selected cohort of both dead and recovered patients, it is important to emphasize that it is not representative of the general population.
ACE2 rs2285666 has already been identified as a possible risk factor for hypertension, type 2 diabetes, and coronary artery disease (Asselta et al., 2020; Chaoxin et al., 2013). It has also been observed that patients with higher ACE1 activity (a protein that is similar to ACE2) in conjunction with lower ACE2 activity (i.e., hemizygous C/G‐males or CC/GG females for ACE2 rs2285666) are predisposed to hypertension, mainly in association with cardiovascular risk factors such as age, dyslipidaemia, and diabetes. As a result, it is clear that a low ACE2 level contributes to the severe consequences of SARS‐CoV‐2 infection. As a result, it could also be a risk factor for the comorbidities found in COVID‐19 individuals (Srivastava et al., 2020). The ACE2 controls systemic vascular resistance, blood volume, and therefore cardiovascular homeostasis. ACE2 SNPs such as rs2285666 have previously been indicated to cardiovascular disorders, dyslipidaemia, hypertension, and stroke. People who take ACE inhibitors and ARBs (angiotensin II type I receptor blockers) produce more receptors. Since the SARS‐CoV‐2 attaches to ACE2 receptors in the heart and lungs, this raises the question of whether these people are more likely to get sick. However, the confirmed previous findings suggest that underlying cardiovascular illness is correlated with an increased risk of death among COVID‐19‐infected individuals (Mehra et al., 2020).
The variant ACE2 rs2285666 is positioned at the beginning of intron 3 and can potentially impact gene expression through alternative splicing mechanisms (Li, 2012; Yang et al., 2015). In addition, a connection has been demonstrated between three ACE2 rs2285666 genotypes and the ACE2 protein level evaluated in serum through enzyme‐linked immunoassay, with the A/A genotype having about 50% higher expression than the G/G genotype (Li, 2012). Recently, it was demonstrated that replacing G with A augments the strength of the splicing site by almost 9.2%, resulting in increased protein expression of ACE2 (Asselta et al., 2020). It has also been observed that patients with higher ACE1 activity, as a protein similar to ACE2, in combination with lower ACE2 activity (i.e., CC/GG females or hemizygous C/G‐males for ACE2 rs2285666) are more susceptible to hypertension, especially in association with classical cardiovascular risk factors, including dyslipidaemia, diabetes, and age (Ghafouri‐Fard et al., 2020; Pinheiro et al., 2019). Therefore, it is obvious that a low ACE2 level contributes to the severe repercussions of SARS‐CoV‐2 infection (Samavati & Uhal, 2020; Verdecchia et al., 2020).
It may lead to conflicting findings; however, this should not dismiss the role of ACE2 gene polymorphisms in susceptibility to COVID‐19 or illness severity. The ACE2 enzyme has been linked to significant lung injury and organ regression in people with COVID‐19. Alongside, their receptors are important for SARS‐CoV‐2 entrance into cells, salt and water retention in hypertension, and the stimulation of fibrotic and inflammatory conditions that contribute to cytokine storm (Cafiero et al., 2021). As described for SARS and MERS‐CoV, severe COVID‐19 patients had an uncontrolled systemic inflammatory response known as cytokine release syndrome (CRS). It is caused by the release of large amounts of pro‐inflammatory cytokines such as interleukin‐6 (1L‐6), IL‐1, IL‐17, and tumour necrosis factor‐alpha (TNF‐α) by immune and nonimmune effector cells and appears to contribute to SARS‐CoV‐2 pulmonary inflammation and significant lung damage. Furthermore, hypercoagulation and thrombosis caused by the significant production of pro‐inflammatory cytokines contribute to the mortality of SARS‐CoV‐2‐infected patients (Darif et al., 2021; Montazersaheb et al., 2022). Therefore, a substantial investigation is required to determine the effect of ACE2 gene expression and polymorphism on susceptibility to SARS‐CoV‐2 infection and clinical outcomes in COVID‐19 patients (Mahmood et al., 2022).
The study findings revealed that age, liver enzymes, lipid profile, uric acid, and 25‐hydroxyvitamin D could be considered important risk factors for severe disease in COVID‐19 patients, which may help to determine the risk for severe infection.
The post‐mortem investigation of the liver tissues of patients with SARS showed fatty degeneration and central lobular necrosis, proving that SARS‐CoV‐2 can also target the human liver (Fan et al., 2020). Given the presence of ACE2 receptors in hepatocytes, a direct viral cytotoxic effect cannot be ruled out. A powerful inflammatory response is distinguished by extremely elevated levels of interleukin‐6, a protein implicated in both the inflammatory and repair responses in liver disease (Schaefer et al., 2020).
It has been suggested that cholesterol‐rich microdomains serve as a platform for the efficient interaction of the S protein with the cellular receptor ACE2. Moreover, an increase in the cholesterol content of cell membranes due to serum cholesterol could raise the invasion of pseudo‐SARS‐CoV‐2 and the virus's ability to infect (Glende et al., 2008; Wang et al., 2020). It has been indicated that cholesterol can simultaneously transport ACE2 to an endocytic entry point where viruses may dock, allowing the virus to enter cells more effectively (Wang et al., 2020). As a result, higher levels of lipids could exacerbate the severity of COVID‐19.
There is now sufficient evidence to suggest that 25‐hydroxyvitamin D deficiency impairs respiratory and immune function and increases COVID‐19 severity and death. 25‐Hydroxyvitamin D protection against COVID‐19 is thought to be mediated by interactions with ACE2, reduction of cytokine responses, maintenance of cell junctions, and enhancement of cellular immunity (Grant et al., 2020). ACE2 is the host cell receptor for SARS‐CoV‐2, modulating the ACE2/renin‐angiotensin system. Consequently, it may reduce acute respiratory distress syndrome (ARDS) and acute lung injury produced by SARS‐CoV‐2. 25‐Hydroxyvitamin D has been shown to modulate ACE2, which is a target of SARS‐CoV‐2. It has been hypothesized that 25‐hydroxyvitamin D may help diminish ARDS and acute lung injury caused by SARS‐CoV‐2 by modulating ACE2 (Getachew & Tizabi, 2021).
A high serum uric acid level may cause damage in various body tissues, resulting in elevated ACE2. A high serum uric acid level, caused primarily by decreased urate excretion in urine, may impair ACE2 synthesis in the kidneys due to low urinary urate. Notably, it was recently revealed that uric acid level was a significant predictor of COVID‐19 worsening. A possible interaction between uric acid and ACE2 could be linked to COVID‐19 getting worse. Additional research on the relationship between ACE2 and uric acid is required in the future (Furuhashi et al., 2021; Ishii et al., 2020).
In conclusion, the findings of this study revealed a strong association between the clinical parameters related to the ACE2 gene and the COVID‐19 mortality rate. Furthermore, we demonstrated that the patients with the ACE2 rs2285666 CC genotype were at a higher risk of dying from COVID‐19 than the TT genotype.
AUTHOR CONTRIBUTIONS
Fereshteh Khalilzadeh and Fatemeh Sakhaee performed the experiments and prepared the manuscript. Iraj Ahmadi and Enayat Anvari were involved in clinical sample and data acquisition. Mohammad Saber Zamani and Fatemeh Sakhaee analysed data and interpreted data. Abolfazl Fateh designed and supervised clinical study, interpreted data, read, and approved manuscript. All authors reviewed the manuscript.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
DATA AVAI LABILITY STATEMENT
Data available on request from the authors.
Khalilzadeh, F. , Sakhaee, F. , Sotoodehnejadnematalahi, F. , Zamani, M. S. , Ahmadi, I. , Anvari, E. , & Fateh, A. (2022). Angiotensin‐converting enzyme 2 rs2285666 polymorphism and clinical parameters as the determinants of COVID‐19 severity in Iranian population. International Journal of Immunogenetics, 49, 325–332. 10.1111/iji.12598
Fereshteh Khalilzadeh and Fatemeh Sakhaee contributed equally to this study
REFERENCES
- Abdelsattar, S. , Kasemy, Z. , Ewida, S. , Abo‐Elsoud, R. , Zytoon, A. , Abdelaal, G. , Abdelgawad, A. , Khalil, F. , & Kamel, H. (2022). ACE2 and TMPRSS2 SNPs as determinants of susceptibility to, and severity of, a COVID‐19 infection. British Journal of Biomedical Science, 79, 102383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alimoradi, N. , Sharqi, M. , Firouzabadi, D. , Sadeghi, M. M. , Moezzi, M. I. , & Firouzabadi, N. (2022). SNPs of ACE1 (rs4343) and ACE2 (rs2285666) genes are linked to SARS‐CoV‐2 infection but not with the severity of disease. Virology Journal, 19(1), 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asselta, R. , Paraboschi, E. M. , Mantovani, A. , & Duga, S. (2020). ACE2 and TMPRSS2 variants and expression as candidates to sex and country differences in COVID‐19 severity in Italy. Aging, 12(11), 10087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustine, R. , Abhilash, S. , Nayeem, A. , Salam, S. A. , Augustine, P. , Dan, P. , Maureira, P. , Mraiche, F. , Gentile, C. , & Hansbro, P. M. (2022). Increased complications of COVID‐19 in people with cardiovascular disease: Role of the renin–angiotensin‐aldosterone system (RAAS) dysregulation. Chemico‐Biological Interactions, 351, 109738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cafiero, C. , Rosapepe, F. , Palmirotta, R. , Re, A. , Ottaiano, M. P. , Benincasa, G. , Perone, R. , Varriale, E. , D'Amato, G. , & Cacciamani, A. (2021). Angiotensin system polymorphisms’ in sars‐cov‐2 positive patients: Assessment between symptomatic and asymptomatic patients: A pilot study. Pharmacogenomics and Personalized Medicine, 14, 621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaoxin, J. , Daili, S. , Yanxin, H. , Ruwei, G. , Chenlong, W. , & Yaobin, T. (2013). The influence of angiotensin‐converting enzyme 2 gene polymorphisms on type 2 diabetes mellitus and coronary heart disease. European Review for Medical and Pharmacological Sciences, 17(19), 2654–2659. [PubMed] [Google Scholar]
- Darif, D. , Hammi, I. , Kihel, A. , Saik, I. E. I. , Guessous, F. , & Akarid, K. (2021). The pro‐inflammatory cytokines in COVID‐19 pathogenesis: What goes wrong? Microbial Pathogenesis, 153, 104799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong, Y. , Mo, X. , Hu, Y. , Qi, X. , Jiang, F. , Jiang, Z. , & Tong, S. (2020). Epidemiology of COVID‐19 among children in China. Pediatrics, 145(6), e20200702. [DOI] [PubMed] [Google Scholar]
- Ellinghaus, D. , Degenhardt, F. , Bujanda, L. , & Buti, M. (2020). Genomewide association study of severe Covid‐19 with respiratory failure. NEJM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan, Z. , Chen, L. , Li, J. , Cheng, X. , Yang, J. , Tian, C. , Zhang, Y. , Huang, S. , Liu, Z. , & Cheng, J. (2020). Clinical features of COVID‐19‐related liver functional abnormality. Clinical Gastroenterology and Hepatology, 18(7), 1561–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuhashi, M. , Sakai, A. , Tanaka, M. , Higashiura, Y. , Mori, K. , Koyama, M. , Ohnishi, H. , Saitoh, S. , & Shimamoto, K. (2021). Distinct regulation of U‐ACE2 and P‐ACE2 (urinary and plasma angiotensin‐converting enzyme 2) in a Japanese general population. Hypertension, 78(4), 1138–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getachew, B. , & Tizabi, Y. (2021). Vitamin D and COVID‐19: Role of ACE2, age, gender, and ethnicity. Journal of Medical Virology, 93(9), 5285–5294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghafouri‐Fard, S. , Noroozi, R. , Omrani, M. D. , Branicki, W. , Pośpiech, E. , Sayad, A. , Pyrc, K. , Łabaj, P. P. , Vafaee, R. , & Taheri, M. (2020). Angiotensin converting enzyme: A review on expression profile and its association with human disorders with special focus on SARS‐CoV‐2 infection. Vascular Pharmacology, 130, 106680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glende, J. , Schwegmann‐Wessels, C. , Al‐Falah, M. , Pfefferle, S. , Qu, X. , Deng, H. , Drosten, C. , Naim, H. Y. , & Herrler, G. (2008). Importance of cholesterol‐rich membrane microdomains in the interaction of the S protein of SARS‐coronavirus with the cellular receptor angiotensin‐converting enzyme 2. Virology, 381(2), 215–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez, J. , Albaiceta, G. M. , García‐Clemente, M. , López‐Larrea, C. , Amado‐Rodríguez, L. , Lopez‐Alonso, I. , Hermida, T. , Enriquez, A. I. , Herrero, P. , & Melón, S. (2020). Angiotensin‐converting enzymes (ACE, ACE2) gene variants and COVID‐19 outcome. Gene, 762, 145102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant, W. B. , Lahore, H. , McDonnell, S. L. , Baggerly, C. A. , French, C. B. , Aliano, J. L. , & Bhattoa, H. P. (2020). Evidence that vitamin D supplementation could reduce risk of influenza and COVID‐19 infections and deaths. Nutrients, 12(4), 988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo, T. , Fan, Y. , Chen, M. , Wu, X. , Zhang, L. , He, T. , Wang, H. , Wan, J. , Wang, X. , & Lu, Z. (2020). Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID‐19). JAMA Cardiology, 5(7), 811–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann, M. , Kleine‐Weber, H. , Schroeder, S. , Krüger, N. , Herrler, T. , Erichsen, S. , Schiergens, T. S. , Herrler, G. , Wu, N.‐H. , & Nitsche, A. (2020). SARS‐CoV‐2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell, 181(2), 271–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inciardi, R. M. , Adamo, M. , Lupi, L. , Cani, D. S. , Di Pasquale, M. , Tomasoni, D. , Italia, L. , Zaccone, G. , Tedino, C. , & Fabbricatore, D. (2020). Characteristics and outcomes of patients hospitalized for COVID‐19 and cardiac disease in Northern Italy. European Heart Journal, 41(19), 1821–1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii, M. , Terai, H. , Kabata, H. , Masaki, K. , Chubachi, S. , Tateno, H. , Nakamura, M. , Nishio, K. , Koh, H. , & Watanabe, R. (2020). Clinical characteristics of 345 patients with coronavirus disease 2019 in Japan: A multicenter retrospective study. Journal of Infection, 81(5), e3–e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karakaş Çelik, S. , Çakmak Genç, G. , Pişkin, N. , Açikgöz, B. , Altinsoy, B. , Kurucu İşsiz, B. , & Dursun, A. (2021). Polymorphisms of ACE (I/D) and ACE2 receptor gene (Rs2106809, Rs2285666) are not related to the clinical course of COVID‐19: A case study. Journal of Medical Virology, 93(10), 5947–5952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Y.‐y. (2012). Lack of association of ACE2 G8790A gene mutation with essential hypertension in the Chinese population: A meta‐analysis involving 5260 subjects. Frontiers in Physiology, 3, 364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmood, Z. S. , Fadhil, H. Y. , Hussein, T. A. A. , & Ad'hiah, A. H. (2022). Severity of coronavirus disease 19: Profile of inflammatory markers and ACE (rs4646994) and ACE2 (rs2285666) gene polymorphisms in Iraqi patients. Meta Gene, 30, 101014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehra, M. R. , Desai, S. S. , Kuy, S. , Henry, T. D. , & Patel, A. N. (2020). Cardiovascular disease, drug therapy, and mortality in Covid‐19. The New England Journal of Medicine, 382(25), e102. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Möhlendick, B. , Schönfelder, K. , Breuckmann, K. , Elsner, C. , Babel, N. , Balfanz, P. , Dahl, E. , Dreher, M. , Fistera, D. , & Herbstreit, F. (2021). ACE2 polymorphism and susceptibility for SARS‐CoV‐2 infection and severity of COVID‐19. Pharmacogenetics and Genomics, 31, 165–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montazersaheb, S. , Hosseiniyan Khatibi, S. M. , Hejazi, M. S. , Tarhriz, V. , Farjami, A. , Ghasemian Sorbeni, F. , Farahzadi, R. , & Ghasemnejad, T. (2022). COVID‐19 infection: An overview on cytokine storm and related interventions. Virology Journal, 19(1), 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinheiro, D. S. , Santos, R. S. , Jardim, P. C. V. , Silva, E. G. , Reis, A. A. , Pedrino, G. R. , & Ulhoa, C. J. (2019). The combination of ACE I/D and ACE2 G8790A polymorphisms revels susceptibility to hypertension: A genetic association study in Brazilian patients. PLoS One, 14(8), e0221248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samavati, L. , & Uhal, B. D. (2020). ACE2, much more than just a receptor for SARS‐COV‐2. Frontiers in Cellular and Infection Microbiology, 317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarangarajan, R. , Winn, R. , Kiebish, M. A. , Bountra, C. , Granger, E. , & Narain, N. R. (2021). Ethnic prevalence of angiotensin‐converting enzyme deletion (D) polymorphism and COVID‐19 risk: Rationale for use of angiotensin‐converting enzyme inhibitors/angiotensin receptor blockers. Journal of Racial and Ethnic Health Disparities, 8(4), 973–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer, E. A. , Arvind, A. , Bloom, P. P. , & Chung, R. T. (2020). Interrelationship between coronavirus infection and liver disease. Clinical Liver Disease, 15(5), 175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solé, X. , Guinó, E. , Valls, J. , Iniesta, R. , & Moreno, V. (2006). SNPStats: A web tool for the analysis of association studies. Bioinformatics, 22(15), 1928–1929. [DOI] [PubMed] [Google Scholar]
- Srivastava, A. , Bandopadhyay, A. , Das, D. , Pandey, R. K. , Singh, V. , Khanam, N. , Srivastava, N. , Singh, P. P. , Dubey, P. K. , & Pathak, A. (2020). Genetic association of ACE2 rs2285666 polymorphism with COVID‐19 spatial distribution in India. Frontiers in Genetics, 10, 1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdecchia, P. , Cavallini, C. , Spanevello, A. , & Angeli, F. (2020). The pivotal link between ACE2 deficiency and SARS‐CoV‐2 infection. European Journal of Internal Medicine, 76, 14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, H. , Yuan, Z. , Pavel, M. A. , Hobson, R. , & Hansen, S. B. (2020). The role of high cholesterol in age‐related COVID19 lethality. 10.1101/2020.05.09.086249 [DOI]
- Wu, Y. H. , Li, J. Y. , Wang, C. , Zhang, L. M. , & Qiao, H. (2017). The ACE 2 G8790A polymorphism: Involvement in type 2 diabetes mellitus combined with cerebral stroke. Journal of Clinical Laboratory Analysis, 31(2), e22033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, M. , Zhao, J. , Xing, L. , & Shi, L. (2015). The association between angiotensin‐converting enzyme 2 polymorphisms and essential hypertension risk: A meta‐analysis involving 14,122 patients. Journal of the Renin‐Angiotensin‐Aldosterone System, 16(4), 1240–1244. [DOI] [PubMed] [Google Scholar]


