Abstract
Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), the causative agent of the currently ongoing coronavirus disease 2019 (COVID‐19) pandemic, has posed a serious threat to global public health. Recently, several SARS‐CoV‐2 variants of concern (VOCs) have emerged and caused numerous cases of reinfection in convalescent COVID‐19 patients, as well as breakthrough infections in vaccinated individuals. This calls for the development of broad‐spectrum antiviral drugs to combat SARS‐CoV‐2 and its VOCs. Pan‐coronavirus fusion inhibitors, targeting the conserved heptad repeat 1 (HR1) in spike protein S2 subunit, can broadly and potently inhibit infection of SARS‐CoV‐2 and its variants, as well as other human coronaviruses. In this review, we summarized the most recent development of pan‐coronavirus fusion inhibitors, such as EK1, EK1C4, and EKL1C, and highlighted their potential application in combating current COVID‐19 infection and reinfection, as well as future emerging coronavirus infectious diseases.
Keywords: coronavirus, COVID‐19, EK1, fusion inhibitor, heptad repeat 1, lipopeptide
1. INTRODUCTION
The seven coronaviruses (CoVs) infecting human beings are named human CoVs (HCoVs). 1 Among them, severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), Middle East respiratory syndrome coronavirus (MERS‐CoV), and SARS coronavirus (SARS‐CoV) belong to the group of highly pathogenic CoVs, which can cause serious disease and death. 1 In contrast, HCoV‐229E, HCoV‐OC43, HCoV‐NL63, and HCoV‐HKU1 belong to the group of low‐pathogenicity HCoVs since they usually cause mild disease in humans. 2
Coronavirus disease 2019 (COVID‐19), caused by SARS‐CoV‐2 infection, 3 has resulted in about 585 million confirmed cases and about 6.4 million deaths worldwide as of August 13, 2022 (https://covid19.who.int). A series of COVID‐19 vaccines and antiviral drugs have been developed to combat the COVID‐19 pandemic. 2 , 4 , 5 However, some emerging SARS‐CoV‐2 variants of concern (VOCs), especially Omicron and its sublineages, have breached the protective efficacy provided by the current COVID‐19 vaccines and therapeutic antibodies. 6 , 7 , 8 , 9 , 10 In addition, MERS‐CoV is still circulating in the Middle East region and has the potential to coinfect people, together with SARS‐CoV‐2 VOCs. 11 Other animal‐derived CoVs may also cross species and cause disease in humans. 12
These data call for the development of pan‐coronavirus (pan‐CoV) vaccines and therapeutics to protect people from infection of SARS‐CoV‐2 and its variants, as well as other emerging and reemerging HCoVs. 13 , 14 , 15 The heptad repeat 1 (HR1) domain in the S2 subunit of spike (S) protein of HCoVs is a vulnerable and conserved target for the development of pan‐CoV fusion inhibitors (Figure 1A). 15 , 16 , 17 A series of potent pan‐CoV fusion inhibitors targeting HR1 have been developed. In this review, we discussed their progress and assessed their potential application in the treatment and/or prevention of COVID‐19 in the current pandemic and other coronavirus infectious diseases that may emerge in the future.
Figure 1.
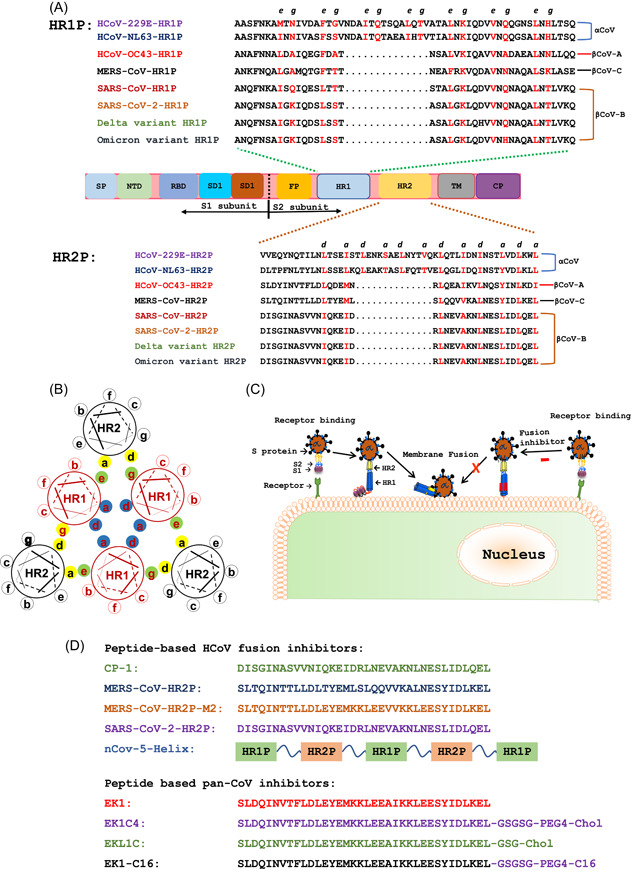
Sequences of peptides derived from the HR1 and HR2 domains and pan‐CoV fusion inhibitors and their mechanisms of action. (A) Functional domains of human CoV S protein and peptides derived from the HR1 and HR2 domains. Spike protein consists of the S1 and S2 subunits. Signal peptide (SP), N‐terminal domain (NTD), receptor‐binding domain (RBD), and subdomains 1 and 2 (SD1 and SD2) are located in the S1 subunit. Fusion peptide (FP), heptad repeat 1 (HR1), heptad repeat 2 (HR2), transmembrane region (TM), and cytoplasmic domain (CP) are located in the S2 subunit. Key amino acid residues at the e and g sites in the HR1 and a and d positions in the HR2‐helical wheels responsible for HR1−HR2 interaction are shown in red. (B) Key amino acid residues involved in the interaction between HR1 and HR2 helices to form 6‐HB. Residues located at the a and d positions in one HR1 helical wheel (shown as blue shadow) interact with those at the d and a positions in another HR1 helical wheel, respectively, to form an internal trimer, while the residues at e and g positions (green shadow) interact with those at a and d positions (yellow shadow) in the HR2 helices to form 6‐HB. (C) Viral fusion and entry mediated by S protein and the mechanism of action of pan‐CoV fusion inhibitors. The viral entry of HCoVs is initiated by binding of RBD in the S1 subunit to the receptor on the host cell, followed by 6‐HB formation via HR1−HR2 interaction of HR1 and HR2 domains in the S2 subunit. Pan‐CoV fusion inhibitors can inhibit viral fusion with and entry into the host cell. (D) Sequences of peptides and lipopeptides with pan‐CoV fusion inhibitory activity. HCoVs, human coronaviruses; pan‐CoV, pan‐coronavirus; 6‐HB, six‐helix bundle.
2. HUMAN COV S PROTEIN IS A KEY TARGET PROTEIN FOR THE DEVELOPMENT OF ANTIVIRALS
HCoV S protein, which is responsible for viral entry, contains several important targets for the development of antivirals or vaccines. 18 , 19 The S protein consists of S1 and S2 subunits. S1 subunit contains a signal peptide (SP), N‐terminal domain (NTD), receptor‐binding domain (RBD), and subdomains 1 and 2 (SD1 and SD2), while the S2 subunit is composed of fusion peptide (FP), HR1, heptad repeat 2 (HR2), transmembrane region (TM), and cytoplasmic domain (CP) (Figure 1A).
The RBD in S1 subunit is responsible for viral receptor engagement through its recognition of and interaction with host cell receptor(s). Therefore, RBD is a classically vulnerable target for the development of viral attachment inhibitors, including neutralizing antibodies (nAbs). 20 Most SARS‐CoV‐2 nAbs block viral entry into host cells by targeting RBD and NTD in the S1 subunit. 20 However, because RBD and NTD have higher mutational frequency than other S protein components, the inhibitory efficacy of these nAbs against different SARS‐CoV‐2 variants (especially, VOCs) is variable. For example, most FDA‐approved nAbs for SARS‐CoV‐2 (WT), such as bamlanivimab, imdevimab, and casirivimab, have shown little efficacy against the Omicron variant and its sublineages. 21 Since RBD sequences are highly variable among different HCoVs, it is difficult to generate a broadly nAb against divergent HCoVs. Thus, it is essential to identify a still more conserved target in S protein for the development of more potent pan‐CoV entry inhibitors or nAbs.
HR1 and HR2 regions in the S2 subunit of HCoVs play a crucial role in mediating viral fusion with and entry into host cells. Formation of the six‐helix bundle (6‐HB) by the interaction between HR1 and HR2 domains can bring viral and cellular membranes together for fusion, resulting in viral entry into host cells for replication. Both HR1 and HR2 regions are relatively conserved in the S protein of HCoVs. 22 , 23 Particularly, amino acid residues at key sites, that is, e and g positions in HR1 helical wheels and a and d positions in HR2 helical wheels, which are directly involved in HR1−HR2 interaction, are the most conserved (Figure 1A,B). Therefore, HR1 and HR2 domains have served as vital targets for the development of broad‐spectrum HCoV fusion inhibitors. 16 , 17 , 22 Synthetic peptides with their sequence derived from the HR2 region can competitively block the interaction of viral HR2 with HR1 domain to form viral 6‐HB and inhibit viral fusion and infection (Figure 1C).
3. DEVELOPMENT OF PEPTIDE‐BASED SARS‐COV, MERS‐COV, AND SARS‐COV‐2 FUSION INHIBITORS
During the outbreak of SARS in 2003, our team developed and reported the first peptide‐based SARS‐CoV fusion inhibitor, CP‐1, 24 which is derived from the HR2 domain in the S2 subunit of SARS‐CoV S protein. CP‐1 can interact with the viral HR1 domain to form heterologous 6‐HB, thus blocking the formation of viral homologous 6‐HB, and inhibiting SARS‐CoV fusion with and entry into host cells expressing human angiotensin‐converting enzyme 2 (hACE2), the receptor of SARS‐CoV. 24 Using a similar approach, we designed and identified a peptide derived from the HR2 domain of MERS‐CoV S protein, designated MERS‐HR2P, which exhibited potent inhibitory activity against MERS‐CoV S protein‐mediated membrane fusion and infection. 25 Using a double E‐K mutation strategy, we developed an optimized peptide, HR2P‐M2, with improved stability, solubility, and antiviral activity. 25 In the human dipeptidyl peptidase 4 (DPP4)‐transduced mouse model, 26 intranasal administration of HR2P‐M2 could significantly reduce viral titer in lung tissue. The combination of HR2P‐M2 with m336 mAb, targeting MERS‐CoV S protein RBD, or with interferon β, exhibited potent synergism in inhibiting MERS‐CoV infection. 26 , 27
At the beginning of the SARS‐CoV‐2 outbreak, we promptly designed and developed a peptide derived from the SARS‐CoV‐2 S protein HR2 domain, 2019‐nCoV‐HR2P, which could interact with the peptide derived from the HR1 domain, 2019‐nCoV‐HR1P, to form 6‐HB. 28 We found that 2019‐nCoV‐HR2P possessed potent inhibitory activity on SARS‐CoV‐2 pseudovirus infection and S protein‐mediated cell−cell fusion. These findings suggested that 2019‐nCoV‐HR2P could be further developed as nasal spray and inhalation formulations to respectively prevent and treat infection by SARS‐CoV‐2 and its variants. 28 In addition, Porotto et al. 29 , 30 have reported that a SARS‐CoV‐2 HR2‐derived lipopeptide, [SARSHRC‐PEG4]2‐chol is able to broadly and effectively inhibit infection by SARS‐CoV‐2 and its variants, including Omicron variant, with IC50 values at the low nanomolar level.
In addition to fusion inhibitors targeting the HR1 domain, those targeting the HR2 domain can also disrupt viral 6‐HB formation and inhibit viral fusion and entry. Root et al. 31 designed a small recombinant protein, denoted 5‐Helix, in which 3 NHR (HR1) and 2 CHR (HR2) segments of HIV‐1 gp41 were alternately linked (N‐C‐N‐C‐N) using short peptide sequences. 5‐Helix binds viral CHR (HR2) to form heterologous 6‐HB, thus inhibiting gp41‐mediated virus‐cell fusion and HIV‐1 infection. Using a similar approach, we recently designed and constructed a recombinant protein‐based SARS‐CoV‐2 fusion inhibitor, nCoV‐5‐Helix, consisting of 3 HR1 and 2 HR2 fragments. Similarly, nCoV‐5‐Helix could interact with the HR2 domain of the viral S2 subunit to block viral homologous 6‐HB formation and inhibit viral S‐mediated cell−cell fusion. The nCoV‐5‐Helix is highly effective in inhibiting infection by pseudotyped and authentic SARS‐CoV‐2 wild‐type strain and its Delta variant, suggesting potential for further development to prevent and treat infection by SARS‐CoV‐2 and its variants. 32
4. DEVELOPMENT OF PAN‐COV FUSION INHIBITORS
Since 2015 when MERS‐CoV spread from the Middle East to Asia, particularly South Korea and China, our team has been devoted to the research and development of pan‐CoV fusion inhibitors based on our previous experience and platforms in developing HIV, SARS‐CoV, and MERS‐CoV fusion inhibitors, as summarized above, to combat any potential outbreak of emerging and reemerging highly pathogenic HCoVs.
4.1. Peptide‐based pan‐CoV fusion inhibitors
Our team previously screened 5 HR1 peptides and 5 HR2 peptides derived from the HR1 and HR2 domains in S proteins of 5 HCoVs (MERS‐CoV, SARS‐CoV, HCoV‐229E, HCoV‐NL63, and HCoV‐OC43) (Figure 1A), respectively, against cell−cell fusion mediated by S protein of each HCoV. In 2019, we reported that only OC43‐HR2P could inhibit cell−cell fusion mediated by the S proteins of all 5 HCoVs, suggesting that OC43‐HR2P had achieved broad‐spectrum anti‐HCoV activity. 17 We then modified the sequence of OC43‐HR2P through E‐K mutation to generate an optimized peptide, termed as EK1, with increased solubility and stability and improved fusion inhibitory activity (Figure 1D). 17 Indeed, EK1 was found to be more stable, soluble and effective than OC43‐HR2P against infection of multiple HCoVs, including MERS‐CoV, SARS‐CoV, HCoV‐OC43, HCoV‐229E, and HCoV‐NL‐63, as well as some SARS‐related CoVs (SARSr‐CoVs) from bats.
In early 2020, we reported that EK1 was also effective against SARS‐CoV‐2 S‐mediated cell−cell fusion and infection of both pseudotyped and authentic SARS‐CoV‐2. Intranasal administration of EK1 to hACE2‐transgenic mice effectively protected them against SARS‐CoV‐2 infection. 33 , 34 Importantly, EK1 could also broadly inhibit infection by divergent SARS‐CoV‐2 VOCs, particularly the Omicron variant which is resistant to most SARS‐CoV‐2 nAbs. 33 , 34 Currently, EK1 in an inhalation formulation is under phase I clinical trials in China.
4.2. Lipopeptide‐based pan‐CoV fusion inhibitors
Lipid modification of antiviral peptides is an important strategy to enhance the antiviral activity of these antiviral peptides. 35 , 36 Unlike regular HCoV fusion inhibitory peptides which mainly inhibit cytoplasmic membrane fusion, lipid‐conjugated HCoV fusion inhibitory peptides can inhibit both cytoplasmic and endosomal membrane fusion. 36 Using a similar approach, we designed a series of PEGylated, cholesterol‐modified EK1 peptides and assessed their antiviral activity. We found that one of these, EK1C4, exhibited the most potent HCoV inhibitory activity with IC50 values at a low nanomolar level, more than 200‐fold more potent than that of EK1 peptide (Table 1). 35 EK1C4 could also effectively protect hACE2‐transgenic mice from SARS‐CoV‐2 infection and protect newborn mice from HCoV‐OC43 infection. 34 , 35 Interestingly, EK1C4 could effectively inhibit antibody‐mediated enhancement of SARS‐CoV‐2 infection. 37
Table 1.
Inhibitory activity of EK1 peptide and EK1‐based lipopeptides against SARS‐CoV‐2 and its variants
| EK1 (IC50: nM) | EK1C4 (IC50: nM) | EKL1C (IC50: nM) | EK1‐C16 (IC50: nM) | |
|---|---|---|---|---|
| SARS‐CoV‐2 (PsV/Caco2) | 1270 | 5.8 | 49.0 | 480 |
| Alpha variant (PsV/Caco2) | 1240 | 5.5 | 12.0 | 190 |
| Gamma variant (PsV/Caco2) | 1250 | 6.6 | 46.0 | 260 |
| Delta variant (PsV/Caco2) | 430 | 9.8 | 32.0 | 110 |
| Omicron variant (live virus/Vero‐TMPRSS‐2) | 1138 | 85.0 | 182.2 | 750 |
| Omicron variant (PsV/Caco2) | 309 | 8.6 | 26.1 | 230 |
Note: Data are from our previously published papers.
Abbreviation: IC50, half maximal inhibitory concentration; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2.
It was reported that PEG linkers in peptide drugs could reduce stability or induce anti‐PEG antibodies in vivo, resulting in adverse effects. 38 To avoid these problems, we designed and synthesized a series of dePEGylated, cholesterol‐modified EK1 peptides and found that EKL1C exhibited the most potent inhibitory activity against infection of SARS‐CoV‐2 and its mutants, as well as other HCoVs tested. Importantly, this dePEGylated lipopeptide possessed remarkably stronger resistance to proteolytic enzymes and higher thermostability than the PEGylated, cholesterol‐conjugated peptide EK1C4. 39 However, while no cholesterol‐conjugated peptide or protein drugs have been approved for clinical use so far, a palmitic acid‐conjugated peptide, as an HIV vaccine, has been reported in phase II clinical trials, suggesting that the palmitic acid‐conjugated peptide is safe for use in humans. 23 , 40 Therefore, we have also designed and synthesized a palmitic acid (C16)‐conjugated EK1 peptide, EK1‐C16, and demonstrated that this lipopeptide is also very effective against infection of SARS‐CoV‐2 and its VOCs, including Omicron, as well as other HCoVs. 23 Overall, these EK1‐based lipopeptides are outstanding candidates for clinical development.
4.3. Long‐acting pan‐CoV fusion inhibitors
To increase the half‐life of EK1 peptide in vivo, we used a fibronectin type III domain (FN3) linked with EK1 peptide to construct a recombinant long‐acting protein, termed FL‐EK1. 41 FL‐EK1 retains potent inhibitory activity and can inhibit infection from SARS‐CoV‐2 and other HCoVs. More importantly, FL‐EK1 possesses good pharmacokinetic profiles, and its half‐life in mice has a 15‐fold increase compared to the original EK1 peptide. FL‐EK1 has proven potential to be developed as a long‐acting pan‐CoV fusion inhibitor.
Other strategies to increase the half‐life of EK1 peptide are also worthy of further studies. For example, IgG Fc‐binding peptide conjugation has been used to increase the half‐life of HIV fusion inhibitors. 42 Therefore, this strategy might also increase the half‐life of pan‐CoV fusion inhibitors.
4.4. Cyclic peptidomimetic‐based fusion inhibitors with potential oral availability
Most recently, we have identified a cyclic peptidomimetic, S‐20‐1, through screening of a one‐bead‐two‐compound (OBTC) cyclic γ‐AApeptide library and modification of the compound. 43 We found that S‐20‐1 effectively inhibited infection of SARS‐CoV‐2 and its variants, as well as SARS‐CoV, MERS‐CoV, HCoV‐OC43, HCoV‐NL63, and HCoV‐229E, by targeting the HR1 domain in S2 subunit and RBD in S1 subunit of HCoV S protein. Intranasal administration of S‐20‐1 protected mice from infection by the challenged HCoV‐OC43 or SARS‐CoV‐2. S‐20‐1 is resistant to proteolytic degradation, has a long half‐life, and possesses potential oral bioavailability. Therefore, S‐20‐1 has potential for further development as an orally applied therapeutic and prophylactic against SARS‐CoV‐2 and other emerging and reemerging HCoVs. 43
4.5. Small‐molecule compound‐based fusion inhibitors
Several small‐molecule compounds, including some repurposed drugs, were reported to block 6‐HB formation and inhibit viral fusion and entry. For example, Itraconazole (ITZ) and Estradiol benzoate (EB), the clinically approved drugs for the treatment of patients with fungal infections and prostate cancer, respectively, can interact with the HR1 domain in S proteins of SARS‐CoV, MERS‐CoV, and SARS‐CoV‐2, thus inhibiting the entry and infection of these three HCoVs. 44 Posaconazole, an FDA‐approved antifungal drug, was reported to broadly inhibit fusion, entry, and infection of SARS‐CoV‐2 and its variants by binding the conserved E‐L‐L motif in the HR2 domain of SARS‐CoV‐2 responsible for stabilizing post‐fusion 6‐HB structure. 45 Some naturally occurring biflavone‐based anti‐HIV agents, for example, hinokiflavone and robustaflavone, could interact strongly with the residues in HR1 and HR2 regions of SARS‐CoV‐2 S protein and block the 6‐HB formation, thus inhibiting SARS‐CoV‐2‐target cell membrane fusion. 46 Virtual screening studies have demonstrated that some phytochemicals, such as Isopomiferin and lycopene, can interact with the HR1 domain with high binding affinity, thus having the potential to inhibit fusion and entry of SARS‐CoV‐2. 47
We have recently reported that analogs of furanyl methylidene rhodanine, such as FD012, possess broad‐spectrum inhibitory and inactivating activities against class I, II, and III enveloped viruses. 48 Like the pan‐CoV fusion inhibitor EK1, FD012 can also inhibit the fusion and entry of all HCoVs tested, including SARS‐CoV‐2 and its variants. Interestingly, FD012 inhibits viral entry and infection by competitively blocking viral 6‐HB formation and inactivates virions by targeting viral membranes, 48 suggesting further development of one of these compounds as an antiviral drug against SARS‐CoV‐2 and other enveloped viruses.
5. PROSPECTS AND CONCLUSIONS
Unlike the exposed RBD in the native state of HCoV S protein, both HR1 and HR2 domains are located in the stem region in S2 subunit of S protein, which harbors significant steric hindrance to access by nAbs and possesses low immunogenicity to elicit nAbs. Therefore, very few nAbs targeting the S2 subunit have been reported to date, such as 76E1, S2P6, and 28D9. 49 , 50 , 51 , 52 Some S2‐targeted nAbs showed potent inhibitory activity against divergent HCoVs, 50 but some of them could only neutralize infection by CoVs in the same genera. 51 , 52
In contrast, peptide‐based fusion inhibitors can easily gain access to and interact with the HR1 or HR2 domain at the fusion‐intermediate stage to block 6‐HB formation and thus inhibit viral fusion with and entry into host cells. More importantly, both HR1 and HR2 domains are more conserved, compared with NTD and RBD, thus serving as important targets for the development of broad‐spectrum anti‐HCoV agents. 2 , 15 , 16 , 22
As therapeutics and prophylactics against SARS‐CoV‐2 infection, peptide‐based fusion inhibitors have several advantages over nAbs (Table 2). First, peptide‐based pan‐CoV fusion inhibitors have a broader antiviral activity than nAbs because pan‐CoV fusion inhibitors target the more conserved HR1 or HR2 domain, compared to NTD or RBD, while nAbs mainly target the less conserved NTD or RBD. Second, the production cost of synthetic peptides is much lower than that of nAbs. 53 Third, both peptides and lipopeptides can be stored and transported at regular temperature, while nAbs must be stored and transported at low temperature. 39 Fourth, peptides can be used in inhalation formation for treatment of COVID‐19, 29 , 34 while it is difficult to prepare antibody inhalation formation because of the large molecular size of IgG (>150 Kd). Although the half‐life of peptides is generally much shorter than that of nAbs, this may not be a significant problem in the short‐term 1‐ or 2‐week use of peptide drugs for urgent treatment of COVID‐19 at the early stage of infection.
Table 2.
Comparison of pan‐CoV fusion inhibitors and SARS‐CoV‐2 nAbs
| Pan‐CoV fusion inhibitors | SARS‐CoV‐2 nAbs | |
|---|---|---|
| Target | HR1/HR2 in S2 subunit | RBD/NTD in S1 subunit |
| Conservation of the target | High | Low |
| Spectrum | Broad | Narrow |
| Production cost | Low | High |
| Storage/shipping at | Room temperature | Low temperature |
| Use in inhalation formulation | Feasible | Difficult |
| Effectiveness | High | High |
| Safety | High | High |
| Half‐life | Short | Long |
Abbreviations: HR1, heptad repeat 1; nAbs, neutralizing antibodies; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2.
Like nAbs drugs, peptide‐based pan‐CoV fusion inhibitors can also be used in combination with drugs having other mechanisms of action, such as RdRp or main protease inhibitors, to achieve more efficient antiviral activity. 2 , 26 , 27 It is anticipated that more potent pan‐CoV fusion inhibitors targeting the HR1 and/or HR2 domain can be developed in the near future for the prevention and treatment of infection by SARS‐CoV‐2 and its variants, as well as emerging and reemerging highly pathogenic HCoVs.
AUTHOR CONTRIBUTIONS
Qiaoshuai Lan wrote the draft manuscript. Lijue Wang and Fanke Jiao drew figures. Shuai Xia, Lu Lu, and Shibo Jiang reviewed and edited the manuscript and figures. All authors reviewed and approved the manuscript.
CONFLICTS OF INTEREST
S. J., L. L., and S. X. are inventors of some patents related to the SARS‐CoV‐2 fusion inhibitors described in this review. The remaining authors declare no conflict of interest.
ACKNOWLEDGMENT
This study was supported by the National Natural Science Foundation of China (92169112 and 82161138002 to S. J.), National Key Research and Development Program of China (2021YFC2300703 to L. L.), and the Shanghai Municipal Science and Technology Major Project (ZD2021CY001 to S. J. and L. L.).
Lan Q, Wang L, Jiao F, Lu L, Xia S, Jiang S. Pan‐coronavirus fusion inhibitors to combat COVID‐19 and other emerging coronavirus infectious diseases. J Med Virol. 2022;1‐8. 10.1002/jmv.28143
Contributor Information
Shuai Xia, Email: sxia15@fudan.edu.cn.
Shibo Jiang, Email: shibojiang@fudan.edu.cn.
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no data sets were generated or analyzed during the current study.
REFERENCES
- 1. Hu B, Guo H, Zhou P, Shi ZL. Characteristics of SARS‐CoV‐2 and COVID‐19. Nat Rev Microbiol. 2021;19(3):141‐154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lan Q, Xia S, Lu L. Coronavirus entry inhibitors. Adv Exp Med Biol. 2022;1366:101‐121. [DOI] [PubMed] [Google Scholar]
- 3. Zhou P, Yang XL, Wang XG, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270‐273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Xiang R, Yu Z, Wang Y, et al. Recent advances in developing small‐molecule inhibitors against SARS‐CoV‐2. Acta Pharm Sin B. 2021;12(4):1591‐1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhou H, Møhlenberg M, Thakor JC, et al. Sensitivity to vaccines, therapeutic antibodies, and viral entry inhibitors and advances to counter the SARS‐CoV‐2 omicron variant. Clin Microbiol Rev. 2022. 10.1128/cmr.00014-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Harvey WT, Carabelli AM, Jackson B, et al. SARS‐CoV‐2 variants, spike mutations and immune escape. Nat Rev Microbiol. 2021;19(7):409‐424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Iketani S, Liu L, Guo Y, et al. Antibody evasion properties of SARS‐CoV‐2 Omicron sublineages. Nature. 2022;604(7906):553‐556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Planas D, Veyer D, Baidaliuk A, et al. Reduced sensitivity of SARS‐CoV‐2 variant Delta to antibody neutralization. Nature. 2021;596(7871):276‐280. [DOI] [PubMed] [Google Scholar]
- 9. Wang X, Zhao X, Cui Y, et al. Neutralization of distinct Omicron sublineages by longitudinal vaccination sera. J Med Virol. 2022;94:5090‐5092. [DOI] [PubMed] [Google Scholar]
- 10. Xia S, Wang L, Zhu Y, Lu L, Jiang S. Origin, virological features, immune evasion and intervention of SARS‐CoV‐2 Omicron sublineages. Signal Transduct Target Ther. 2022;7(1):241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Khan A, Nafisah SB, Mzahim B, et al. MERS‐CoV in the COVID‐19 era: update from Saudi Arabia, 2019−2020. East Mediterr Health J. 2021;27(11):1109‐1113. [DOI] [PubMed] [Google Scholar]
- 12. Xiao K, Zhai J, Feng Y, et al. Isolation of SARS‐CoV‐2‐related coronavirus from Malayan pangolins. Nature. 2020;583(7815):286‐289. [DOI] [PubMed] [Google Scholar]
- 13. Su S, Li W, Jiang S. Developing pan‐β‐coronavirus vaccines against emerging SARS‐CoV‐2 variants of concern. Trends Immunol. 2022;43(3):170‐172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang X, Xia S, Zhu Y, Lu L, Jiang S. Pan‐coronavirus fusion inhibitors as the hope for today and tomorrow. Protein Cell. 2021;12(2):84‐88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cao M, Su X, Jiang S. Broad‐spectrum anti‐coronavirus vaccines and therapeutics to combat the current COVID‐19 pandemic and future coronavirus disease outbreaks. Stem Cell Rep. 2021;16(3):398‐411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lu L, Su S, Yang H, Jiang S. Antivirals with common targets against highly pathogenic viruses. Cell. 2021;184(6):1604‐1620. [DOI] [PubMed] [Google Scholar]
- 17. Xia S, Yan L, Xu W, et al. A pan‐coronavirus fusion inhibitor targeting the HR1 domain of human coronavirus spike. Sci Adv. 2019;5(4):eaav4580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Du L, Yang Y, Zhou Y, Lu L, Li F, Jiang S. MERS‐CoV spike protein: a key target for antivirals. Expert Opin Ther Targets. 2017;21(2):131‐143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhu G, Zhu C, Zhu Y, Sun F. Minireview of progress in the structural study of SARS‐CoV‐2 proteins. Curr Res Microb Sci. 2020;1:53‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yu F, Xiang R, Deng X, et al. Receptor‐binding domain‐specific human neutralizing monoclonal antibodies against SARS‐CoV and SARS‐CoV‐2. Signal Transduct Target Ther. 2020;5(1):212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Planas D, Saunders N, Maes P, et al. Considerable escape of SARS‐CoV‐2 Omicron to antibody neutralization. Nature. 2021;602(7898):671‐675. [DOI] [PubMed] [Google Scholar]
- 22. Yang K, Wang C, White KI, Pfuetzner RA, Esquivies L, Brunger AT. Structural conservation among variants of the SARS‐CoV‐2 spike postfusion bundle. Proc Natl Acad Sci USA. 2022;119(16):e2119467119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lan Q, Chan JF‐W, Xu W, et al. A palmitic acid‐conjugated, peptide‐based pan‐CoV fusion inhibitor potently inhibits infection of SARS‐CoV‐2 omicron and other variants of concern. Viruses. 2022;14(3):549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Liu S, Xiao G, Chen Y, et al. Interaction between heptad repeat 1 and 2 regions in spike protein of SARS‐associated coronavirus: implications for virus fusogenic mechanism and identification of fusion inhibitors. Lancet. 2004;363(9413):938‐947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lu L, Liu Q, Zhu Y, et al. Structure‐based discovery of middle east respiratory syndrome coronavirus fusion inhibitor. Nat Commun. 2014;5(1):3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Channappanavar R, Lu L, Xia S, et al. Protective effect of intranasal regimens containing peptidic middle east respiratory syndrome coronavirus fusion inhibitor against MERS‐CoV infection. J Infect Dis. 2015;212(12):1894‐1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wang C, Hua C, Xia S, Li W, Lu L, Jiang S. Combining a fusion inhibitory peptide targeting the MERS‐CoV S2 protein HR1 domain and a neutralizing antibody specific for the S1 protein receptor‐binding domain (RBD) showed potent synergism against pseudotyped MERS‐CoV with or without mutations in RBD. Viruses. 2019;11(1):30672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Xia S, Zhu Y, Liu M, et al. Fusion mechanism of 2019‐nCoV and fusion inhibitors targeting HR1 domain in spike protein. Cell Mol Immunol. 2020;17(7):765‐767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. de Vries RD, Schmitz KS, Bovier FT, et al. Intranasal fusion inhibitory lipopeptide prevents direct‐contact SARS‐CoV‐2 transmission in ferrets. Science. 2021;371(6536):1379‐1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Schmitz KS, Geers D, de Vries RD, et al. Potency of fusion‐inhibitory lipopeptides against SARS‐CoV‐2 variants of concern. mBio. 2022;13(3):e0124922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Root MJ, Kay MS, Kim PS. Protein design of an HIV‐1 entry inhibitor. Science. 2001;291(5505):884‐888. [DOI] [PubMed] [Google Scholar]
- 32. Xing L, Xu X, Xu W, et al. A five‐helix‐based SARS‐CoV‐2 fusion inhibitor targeting heptad repeat 2 domain against SARS‐CoV‐2 and its variants of concern. Viruses. 2022;14(3):597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Xia S, Chan JF, Wang L, et al. Peptide‐based pan‐CoV fusion inhibitors maintain high potency against SARS‐CoV‐2 Omicron variant. Cell Res. 2022;32(4):1‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Xia S, Lan Q, Zhu Y, et al. Structural and functional basis for pan‐CoV fusion inhibitors against SARS‐CoV‐2 and its variants with preclinical evaluation. Signal Transduct Target Ther. 2021;6(1):288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Xia S, Liu M, Wang C, et al. Inhibition of SARS‐CoV‐2 (previously 2019‐nCoV) infection by a highly potent pan‐coronavirus fusion inhibitor targeting its spike protein that harbors a high capacity to mediate membrane fusion. Cell Res. 2020;30(4):343‐355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Park JE, Gallagher T. Lipidation increases antiviral activities of coronavirus fusion‐inhibiting peptides. Virology. 2017;511:9‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wang Z, Deng T, Zhang Y, et al. ACE2 can act as the secondary receptor in the FcγR‐dependent ADE of SARS‐CoV‐2 infection. iScience. 2022;25(1):103720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Garay RP, El‐Gewely R, Armstrong JK, Garratty G, Richette P. Antibodies against polyethylene glycol in healthy subjects and in patients treated with PEG‐conjugated agents. Expert Opin Drug Deliv. 2012;9(11):1319‐1323. [DOI] [PubMed] [Google Scholar]
- 39. Zhou J, Xu W, Liu Z, et al. A highly potent and stable pan‐coronavirus fusion inhibitor as a candidate prophylactic and therapeutic for COVID‐19 and other coronavirus diseases. Acta Pharm Sin B. 2021;12(4):1652‐1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Salmon‐Céron D, Durier C, Desaint C, et al. Immunogenicity and safety of an HIV‐1 lipopeptide vaccine in healthy adults: a phase 2 placebo‐controlled ANRS trial. AIDS. 2010;24(14):2211‐2223. [DOI] [PubMed] [Google Scholar]
- 41. Duan Q, Xia S, Jiao F, et al. A modified fibronectin type III domain‐conjugated, long‐acting pan‐coronavirus fusion inhibitor with extended half‐life. Viruses. 2022;14(4):655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bi W, Xu W, Cheng L, et al. IgG Fc‐binding motif‐conjugated HIV‐1 fusion inhibitor exhibits improved potency and in vivo half‐life: potential application in combination with broad neutralizing antibodies. PLoS Pathog. 2019;15(12):e1008082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Xue S, Wang X, Wang L, et al. A novel cyclic γ‐AApeptide‐based long‐acting pan‐coronavirus fusion inhibitor with potential oral bioavailability by targeting two sites in spike protein. Cell Discov. 2022;8:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yang C, Pan X, Huang Y, et al. Drug repurposing of itraconazole and estradiol benzoate against COVID‐19 by blocking SARS‐CoV‐2 spike protein‐mediated membrane fusion. Adv Ther. 2021;4(5):2000224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jana ID, Bhttacharya P, Mayilsamy K, et al. Targeting an evolutionarily conserved “E‐L‐L” motif in the spike protein to develop a small molecule fusion inhibitor against SARS‐CoV‐2. bioRxiv. 2022. 10.1101/2022.03.16.484554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mondal S, Karmakar A, Mallick T, Begum NA. Exploring the efficacy of naturally occurring biflavone based antioxidants towards the inhibition of the SARS‐CoV‐2 spike glycoprotein mediated membrane fusion. Virology. 2021;556:133‐139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Majeed A, Hussain W, Yasmin F, Akhtar A, Rasool N. Virtual screening of phytochemicals by targeting HR1 domain of SARS‐CoV‐2 S protein: molecular docking, molecular dynamics simulations, and DFT studies. BioMed Res Int. 2021;2021:6661191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Pu J, He X, Xu W, et al. The analogs of furanyl methylidene rhodanine exhibit broad‐spectrum inhibitory and inactivating activities against enveloped viruses, including SARS‐CoV‐2 and its variants. Viruses. 2022;14(3):489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hurlburt NK, Homad LJ, Sinha I, et al. Structural definition of a pan‐sarbecovirus neutralizing epitope on the spike S2 subunit. Commun Biol. 2022;5(1):342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sun X, Yi C, Zhu Y, et al. Neutralization mechanism of a human antibody with pan‐coronavirus reactivity including SARS‐CoV‐2. Nat Microbiol. 2022;7(7):1063‐1074. [DOI] [PubMed] [Google Scholar]
- 51. Pinto D, Sauer MM, Czudnochowski N, et al. Broad betacoronavirus neutralization by a stem helix‐specific human antibody. Science. 2021;373(6559):1109‐1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wang C, van Haperen R, Gutiérrez‐Álvarez J, et al. A conserved immunogenic and vulnerable site on the coronavirus spike protein delineated by cross‐reactive monoclonal antibodies. Nat Commun. 2021;12(1):1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Murugan NA, Raja KMP, Saraswathi NT. Peptide‐based antiviral drugs. Adv Exp Med Biol. 2021;1322:261‐284. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no data sets were generated or analyzed during the current study.


