Key messages.
Chronic spontaneous urticaria cases are emerging after repeated doses of SARS‐CoV‐2 mRNA vaccines.
Sensitization to SARS‐CoV‐2 mRNA vaccines and polysorbate‐80 has been found in our population.
Further studies are needed to confirm these findings.
To the editor,
The Swiss regulatory agency approved BNT 162b2 from Pfizer‐BioNTech and mRNA‐1273 from Moderna in December 2020 and January 2021, respectively. A third vaccine dose (booster) was recommended by the end of October 2021. Although the vast majority of persons immunized had a good tolerance to the vaccine, a few experienced unexpected adverse reactions, which in rare cases were severe, including hypersensitivity reactions. Skin reactions are the most frequently observed unexpected adverse events after SARS‐CoV‐2 mRNA vaccines, mostly large local reactions, delayed morbilliform rashes and delayed urticaria. We were surprised by the emergence of an unprecedented number of cases of chronic urticaria and angioedema in the second year of the vaccine campaign for SARS‐CoV‐2. All cases that occurred during the first quarter of 2022 were in patients who had received repeated vaccine doses in the previous days to weeks.
All patients had been referred between January and April 2022 to clinical practices in Southern Switzerland for an allergy work‐up after having developed chronic urticaria. They fulfilled the European Academy of Allergy and Clinical Immunology diagnostic criteria for chronic spontaneous urticaria (CSU), defined by the presence of recurrent or persistent urticarial lesions with or without angioedema for more than >6 weeks. We were also able to perform basophil activation tests (BAT) to previously administered mRNA vaccines and main excipients as well as a CU‐BAT in selected patients (ADR‐AC Laboratories).
Regional ethics committee reviewed and approved the manuscript (Cantonal Ethics Committee Req‐2022‐00359).
We report a series of 32 patients having developed chronic urticaria within days after repeated immunization with SARS‐CoV‐2 mRNA vaccines. The patient's characteristics are shown in Table 1. Mean age was 44.4 ± 13.1 years and 61% were females. Eight (25%) patients were atopic and four (13%) had a history of dermatologic conditions (pityriasis; chronic itching without urticaria; palmoplantar dyshidrosis; history of chronic spontaneous urticaria that had subsided several years before presentation). No patient had a history of drug allergy. Patients did not report of chronic recurrent NSAIDs intake. Symptoms started after a third dose of SARS‐CoV‐2 mRNA vaccine in 30 (94%) patients, which proved to be an mRNA‐1273 booster in 29 (91%). After a symptom‐free interval of at least 48 h, urticaria developed at a median of 10 (IQR 4) days after immunization. None of our patients had presented urticarial lesions or other types of skin lesion after previous vaccine doses. Some had experienced mild adverse reactions, such as those typically reported (injection‐site pain, headache, chills, fever and nausea). 1 While urticaria was the leading symptom in all, four (13%) patients additionally presented angioedema. None presented vasculitic features. Eighteen (56%) patients had no obvious triggers for urticaria. Those with triggers had dermographism (34%), cholinergic (6%) or pressure (3%) urticaria. Four (12%) patients had a concomitant, symptomatic but uncomplicated SARS‐CoV‐2 infection confirmed by a positive PCR test temporarily correlated with symptoms onset.
TABLE 1.
Characteristics of 32 patients with chronic urticaria after repeated immunization with mRNA vaccines for SARS‐CoV‐2
| Pat. | Sex | Age (years) | History of atopy | Vaccine | dose | Days to urticaria onset after immunization | Sympto‐matic SARS‐CoV‐2 infection | BAT to mRNA vaccine | BAT on donor basophils |
|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 42 | No | BNT 162b2 | 3rd | 30 | Yes | N/D | N/D |
| 2 | M | 16 | No | BNT 162b2 | 2nd | 30 | No | N/D | N/D |
| 3 | M | 34 | No | mRNA‐1273 | 3rd | 14 | No | Positive | Negative |
| 5 | F | 36 | No | mRNA‐1273 | 3rd | 10 | No | Positive | Negative |
| 6 | M | 42 | No | mRNA‐1273 | 3rd | 6 | No | N/D | N/D |
| 7 | F | 21 | Yes | mRNA‐1273 | 3rd | 10 | No | N/D | N/D |
| 8 | F | 50 | No | mRNA‐1273 | 3rd | 9 | No | N/D | N/D |
| 9 | F | 32 | Yes | mRNA‐1273 | 3rd | 11 | No | N/D | N/D |
| 10 | M | 46 | Yes | mRNA‐1273 | 3rd | 10 | Yes | N/D | N/D |
| 11 | F | 27 | Yes | mRNA‐1273 | 3rd | 14 | No | N/D | N/D |
| 12 | 63 | Yes | mRNA‐1273 | 3rd | 11 | No | N/D | N/D | |
| 13 | F | 56 | No | mRNA‐1273 | 3rd | 15 | No | N/D | N/D |
| 14 | M | 34 | No | mRNA‐1273 | 3rd | 3 | No | Positive | Negative |
| 15 | F | 46 | No | mRNA‐1273 | 3rd | 34 | No | Positive | N/D |
| 16 | M | 44 | No | mRNA‐1273 | 3rd | 11 | No | N/D | N/D |
| 17 | F | 54 | Yes | mRNA‐1273 | 3rd | 7 | No | N/D | N/D |
| 18 | F | 40 | No | mRNA‐1273 | 3rd | 10 | Yes | N/D | N/D |
| 19 | F | 45 | No | mRNA‐1273 | 3rd | 10 | No | N/D | N/D |
| 20 | F | 47 | No | mRNA‐1273 | 3rd | 10 | Yes | Positive | N/D |
| 21 | M | 38 | No | mRNA‐1273 | 3rd | 11 | No | N/D | N/D |
| 22 | F | 48 | Yes | mRNA‐1273 | 3rd | 10 | No | Positive | Negative |
| 23 | F | 34 | No | mRNA‐1273 | 3rd | 10 | No | N/D | N/D |
| 24 | F | 41 | No | mRNA‐1273 | 3rd | 10 | No | Positive | Negative |
| 25 | F | 38 | No | mRNA‐1273 | 3rd | 9 | No | N/D | N/D |
| 26 | F | 63 | No | mRNA‐1273 | 3rd | 14 | No | N/D | N/D |
| 27 | M | 38 | No | mRNA‐1273 | 3rd | 10 | No | N/D | N/D |
| 28 | M | 61 | No | mRNA‐1273 | 3rd | 4 | No | N/D | N/D |
| 29 | M | 37 | No | mRNA‐1273 | 3rd | 14 | No | N/D | N/D |
| 30 | F | 66 | No | mRNA‐1273 | 2nd | 32 | No | N/D | N/D |
| 31 | M | 44 | No | mRNA‐1273 | 3rd | 21 | No | N/D | N/D |
| 32 | M | 64 | No | mRNA‐1273 | 3rd | 10 | No | N/D | N/D |
Abbreviations: BAT, basophil activation test; F, female; M, male; N/D, not done.
Of the patients in which data on the initial urticaria management were available, 25 (78%) responded to a single daily dose regime of antihistamines (anti‐H1). Five (16%) patients needed anti‐H1 up dosing and six (19%) additional systemic glucocorticosteroids. None of our patients did receive treatment with omalizumab or other monoclonal antibodies given the good response and symptom control with first‐line treatment.
To date, after an observation period of 3 months, only two (6%) patients are in complete remission, after urticaria persisting for 8 and 12 weeks, respectively. One patient with initial remission presents recurrent urticarial upon sunlight exposure. To date, the two patients with onset of symptoms after the second dose did not receive a third dose of vaccine.
We performed BAT testing in seven patients (ADR‐AC laboratory, Bern, Switzerland) as shown and explained in Figure 1. Basophils activation monitored with CD63 (±IL‐3) and CD203c up‐regulation to the mRNA vaccine was found in all seven patients, and four reacted as well to polysorbate‐80 (contained in Janssen‐Cilag AG vaccine), but none reacted to linear polyethylene glycol‐2000 (PEG‐2000; contained in mRNA‐1273 and BNT 162b2) and trometamol (contained in mRNA‐1273). Besides the seven patients tested in BAT, sera of five patients were analysed for stimulating heterologous basophils. Here, the ability of patient's sera to induce CD63 in basophils from a donor was measured by flow cytometry. 2 This test allows to identify endogenous factors like anti‐FCεRI antibodies, which can constantly activate mastcells in CSU. Nevertheless, none of them showed convincing stimulatory properties (data not shown), suggesting that an autoimmune reaction is unlikely in these patients. CSU related to vaccination was only 1% and the mechanism was hypothesized by the authors as triggering autoimmunity.
FIGURE 1.
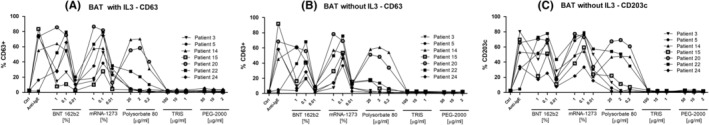
Result of basophil activation test to BNT 162b2, mRNA‐1273, Polysorbate‐80, TRIS, PEG‐2000 in 6 patients (patients 3, 5, 14, 15, 20, 22, 24). Hundred microliters of peripheral blood per test was incubated with or without IL‐3 (1 ng/ml) with different concentration of vaccines (1%, 0.1%, 0.01%) or Polysorbate‐80 (20, 10 and, 2 μg/ml), or TRIS (100, 10 or 1 μg/ml) or PEG‐2000 (50, 10, 2) for 30 min at 37°C, along with anti‐CCR3 PE and anti‐CD63 FITC, anti‐CD203 APC antibodies (Biolegend). Control conditions included a medium‐only negative control, a positive control, involving the crosslinking of the high‐affinity Fc epsilon receptor (anti‐FcERIIgE, Beckmann‐Coulter). Flow cytometry data were collected on NovoCyte flow cytometer (Agilent). After stimulation and staining, basophiles (gated by CCR3+ expression) were analysed for their degranulation (CD63) in the presence of IL‐3 (A), and for their degranulation (CD63, B) and activation (CD203c, C) in the absence of IL‐3. The percentage of event positive for the corresponding marker in CCR3+ gated events is presented for each tested patient. CCR3, C‐C chemokine receptor type 3; CD, cluster of differentiate; IL, interleukin; PEG, polyethylene glycol; TRIS, tromethamine.
In our series of 32 patients with chronic urticaria after mRNA SARS‐CoV‐2 vaccination, most developed symptoms after a third dose (booster), within a median time of 10 days. CSU is a relatively common condition with an estimated prevalence of 0.7%. 3 We were, however, intrigued by a sharp increase in cases that coincided with the booster vaccination campaign. Vaccines have been described as potential precipitants of CSU. In 2018, Magen et al. have reported that 14 cases of CSU temporarily correlated with HBV, HPV, influenza vaccine and DPT occurring a mean of 8 days from immunization. 4 The potential risk that vaccination with SARS‐CoV‐2 vaccine might precipitate onset of CSU in predisposed but still asymptomatic patients was invoked by experts. Several cases of CSU potentially triggered by immunization to SARS‐CoV‐2 are reported: A young man presented chronic urticaria 8 weeks after the second dose of BNT 162b2. 5 An otherwise healthy woman presented with urticaria for 4 weeks with persistent dermographism 12 days after the third dose (booster) with mRNA‐1273. 6 In a recent retrospective study, 32 patients with new‐onset persistent urticaria and CSU in remission that relapsed within 3 months from BNT 162b2 mRNA vaccination were analysed. 7 A positive autologous serum skin test and low basophil count in the peripheral blood were positively associated with the likelihood of CSU recurrence after vaccination with BNT162b2 mRNA.
The fact that our patients had tolerated well previous vaccine doses argues against simple triggering of underlying CSU. Indeed, only one patient had a history of CSU that did not flare after previous immunizations. Furthermore, no basophil activation by patient's serum factors could be identified. This may suggest that patients become sensitized to vaccine compounds after repeated immunization or that the antigens and antibodies triggered by the vaccine cause urticarial lesions in the context of immune complexes due to hyperimmunization. Urticaria and angioedema, however, were the only manifestations in our patients, without other signs in favour of a serum sickness‐like reaction. Some patients had uncomplicated COVID‐19 preceding the onset of urticaria, raising the question of a para‐infectious urticaria. SARS‐CoV‐infection, however, was documented in only four patients, and while we cannot rule out asymptomatic infection in the remaining 28 cases, it seems an unlikely explanation. While allergy skin tests could not be performed in our patients due to ongoing urticaria and anti‐H1 treatment, BAT assessed in seven patients were positive to both tested mRNA vaccines. Interestingly, more than half of patients showed a marked basophil activation with polysorbate‐80, a pegylated derivative of sorbitol commonly used as solubilizer or emulsifier. As all four hydroxyl groups of sorbitol are pegylated, these results might indicate sensitization against the pegylated structures present in both mRNA vaccines. While there are concerns about the specificity of BAT in assessing hypersensitivity, all 14 controls performed in the same lab including 4 previously vaccinated patients had negative BAT to the vaccines, showing good specificity. 8 Further studies are needed to assess the potential of sensitization to polysorbate‐80 and/or PEG after repeated immunization with these excipients. Indeed, once sensitized, such patients could present a type 1 hypersensitivity manifesting as chronic urticaria, possibly sustained by the same allergenic compounds present in a large number of processed foods, cosmetics, and drugs. A type 3 hypersensivity has also been evoked in these patients as repeated doses of SARS‐CoV2 mRNA vaccine could lead to an immune complex‐linked complement activation with a continuous C3a‐ and C5a‐driven basophil activation. 9
In conclusion, our observation of persistent urticaria fulfilling criteria for CSU in close temporal relation with repeated vaccination against SARS‐CoV2 suggests a link between the two, which could be of allergic nature. The potential sensitization to vaccine excipients highlighted by the CD63 activation on basophils in some patients not only to the whole vaccine, but frequently also to polysorbate‐80, urges the need for more studies investigating the possibility of sensitization to vaccine excipients by repeated immunization in predisposed individuals.
AUTHOR CONTRIBUTION
All authors have made significant contribution in conception of the present work and critically reviewed the manuscript.
CONFLICTS OF INTEREST
All the authors have no conflicts of interest to declare. All co‐authors have seen and agreed with the contents of the manuscript. There is no financial interest to report.
ACKNOWLEDGEMENT
Open access funding provided by Universita della Svizzera italiana.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1. Reports of suspected adverse reactions to COVID‐19 vaccines in Switzerland – update. Accessed November 26, 2021. https://www.swissmedic.ch/swissmedic/en/home/news/coronavirus‐covid‐19/covid‐19‐vaccines‐safety‐update‐9.html
- 2. Gentinetta T, Pecaric‐Petkovic T, Wan D, et al. Individual IL‐3 priming is crucial for consistent in vitro activation of donor basophils in patients with chronic urticaria. J Allergy Clin Immunol. 2011;128(6):1227‐1234.e5. [DOI] [PubMed] [Google Scholar]
- 3. Fricke J, Ávila G, Keller T, et al. Prevalence of chronic urticaria in children and adults across the globe: systematic review with meta‐analysis. Allergy. 2020;75(2):423‐432. [DOI] [PubMed] [Google Scholar]
- 4. Magen E, Shalom G, Waitman DA, Kahan NR. Chronic spontaneous urticaria following vaccination. Int J Adv Res. 2018;6(2):1434‐1439. [Google Scholar]
- 5. Thomas J, Thomas G, Chatim A, Shukla P, Mardiney M. Chronic spontaneous urticaria after COVID‐19 vaccine. Cureus. 2021;13(9):e18102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wolfson AR, Freeman EE, Blumenthal KG. Urticaria 12 days after COVID‐19 mRNA booster vaccination. JAMA. 2022;327(17):1702‐1703. [DOI] [PubMed] [Google Scholar]
- 7. Magen E, Yakov A, Green I, Israel A, Vinker S, Merzon E. Chronic spontaneous urticaria after BNT162b2 mRNA (Pfizer‐BioNTech) vaccination against SARS‐CoV‐2. Allergy Asthma Proc. 2022;43(1):30‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Stehlin F, Mahdi‐Aljedani R, Canton L, et al. Intradermal testing with COVID‐19 mRNA vaccines predicts tolerance. Front Allergy. 2022;3:818049. doi: 10.3389/falgy.2022.818049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bonam SR, Chauvin C, Levillayer L, Mathew MJ, Sakuntabhai A, Bayry J. SARS‐CoV‐2 induces cytokine responses in human basophils. Front Immunol. 2022;13:838448. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


