Abstract
Despite extensive research on cancer care during the COVID‐19 pandemic, evidence on the impact on prediagnostic time intervals is lacking. To better understand how COVID‐19 changed the pathway to diagnosis of cancer, we examined the length of intervals from symptom onset to diagnosis for 13 common cancer types with known clinical stage over 1‐year nonpandemic period (March 2019 to March 2020; N = 844) and three biannual COVID periods (March 2020 to September 2021; N = 1172). We analyzed the patient interval (from first symptoms to presentation to a physician), the primary care/emergency department interval (from presentation with relevant symptoms to a primary care or emergency department physician to referral to a hospital‐based diagnosis center) and the hospital interval (from referral to diagnosis). Compared to nonpandemic data, there were significant changes across COVID periods. The pandemic mostly impacted patient intervals for cancers diagnosed over the first 6 months after onset in March 2020. Overall median patient intervals were longest in the early COVID period (39 [IQR 22‐64] days) and shortest in the nonpandemic period (20 [IQR 13‐30] days; Kruskal‐Wallis test [χ 2], P < .0001). Differences in clinical stage between periods were relevant, with cancers from the mid‐period (September 2020 to March 2021) showing the most advanced stage. A shift to later stage was plausibly a result of delayed intervals in the early COVID period. Since intervals are eventually relevant to prognosis, our results provide a baseline against which the impact of improvement strategies to minimize the negative outcomes of COVID‐19‐associated cancer delays can be assessed and implemented.
Keywords: COVID‐19, diagnostic interval, patient interval, primary care interval, stage
What's new?
The time interval from symptom onset to cancer diagnosis is shaped by multiple factors, including access to healthcare. During the ongoing COVID‐19 pandemic, healthcare access has at times been severely restricted. How this has affected time to cancer diagnosis remains unknown. Here, time intervals from symptom onset to diagnosis were analyzed for 13 cancer types from March 2019 through September 2021. Median intervals increased markedly over the first six months of the pandemic. Cancers from the mid‐period, September 2020 to March 2021, were diagnosed at more advanced stages, a shift likely related to delayed diagnosis in the early pandemic period.
Abbreviations
- COVID‐19
coronavirus disease 2019
- ED
emergency department
- IQR
interquartile range
- PC
primary care
1. INTRODUCTION
In contrast to asymptomatic detection via screening interventions or detection as an incidental finding during an unrelated procedure, patients are most commonly diagnosed with cancer after presenting with suspected symptoms to physicians at primary care (PC). 1 , 2 Research on suspected cancers has expanded since 1999/2000 when, in the United Kingdom, concerns about delays in diagnosis prompted the implementation of urgent referral pathways. 3 , 4 Studies have shown that increased use of urgent referrals from PC is associated with a reduction in late‐stage disease and mortality for most cancer types. 5 , 6 , 7 Aware of the prognostic relevance of earlier‐stage diagnosis, the international research community created standards that could be examined and compared between healthcare systems. The result was the publication in 2012 of the Aarhus statement, which provides definitions for time points and intervals in the route to diagnosis of cancer. 8 With the date of first symptom as the first individual time point in the pathway and the date of diagnosis as the last, three main intervals were defined: the patient interval, from first symptom to first presentation to a physician, the PC interval, from first presentation to a PC physician to first referral to another physician (typically, at secondary care) for further diagnostic activity and management and the secondary care interval, from first referral to treatment start. Additionally, the time elapsed between first presentation and date of diagnosis makes the commonly used diagnostic interval. 8
The intervals in the patient journey from symptom onset to diagnosis can be shaped by patient‐, physician‐ and healthcare system‐related factors, 9 with implications for clinical outcomes. 10 For example, longer intervals to care‐seeking for cancer symptoms are associated with advanced stage at diagnosis, particularly in low‐resource communities with psychological, social and cultural barriers for contacting healthcare providers. 11 , 12 To date, however, high‐quality evidence on the association between intervals and outcomes is limited. A challenge in such evaluation is the waiting‐time paradox, by which shorter diagnostic intervals in more unwell patients with aggressive disease are associated with poorer outcomes compared to those experiencing longer intervals. 2 , 10 This phenomenon was considered in a systematic review in 2015 that examined the association between time to diagnosis or treatment and outcomes across a wide range of cancer types. 13 A total of 209 studies were analyzed including those accounting for the waiting‐time paradox, and results were arranged according to the type of association, namely, positive (evidence of shorter/longer intervals being associated with more/less favorable outcomes), negative or no association. Despite data heterogeneity, there were more reports of a positive association between shorter times to diagnosis and more favorable outcomes (improved earlier‐stage diagnosis, survival and quality of life) for colorectal, breast, head and neck, melanoma and testicular cancers. Whereas a more limited number of studies showed a positive association for prostate, pancreatic and bladder cancers, there was no association or it was negative for others, including lung cancer. 13 More recently, a systematic review evaluating the secondary care interval for non‐small cell lung cancer (from referral to first treatment receipt) suggested an association between shorter times to treatment and improved outcomes in early‐stage disease, mostly in patients having surgery. 14
Against this background, it must be stressed that diagnosing cancer at a treatable stage will be feasible as long as a number of investigative tests are available to clinicians evaluating suspected patients. 15 However, owing to well‐known overshadowing, lack of access to healthcare facilities during the ongoing coronavirus disease 2019 (COVID‐19) pandemic meant that key investigations were delayed or canceled even for patients with cancer symptoms who were subsequently diagnosed with cancer. 16 By monitoring the weekly diagnostic activity of a quick diagnosis center at a tertiary hospital during the pandemic and comparing it with nonpandemic data, we reported that, as volumes declined, waiting times to endoscopic, imaging and biopsy/cytology procedures increased significantly. As a result, both for cancer and noncancer patients, diagnosis was markedly delayed. 17 Although changes in referral patterns and cancer detection rates following the pandemic declaration are well documented, evidence on its impact on Aarhus statement‐defined time intervals from symptom onset to diagnosis is lacking. We therefore set out to examine differences in the length of intervals in the diagnostic pathway for a range of common cancers in patients presenting with suspected symptoms before and during the course of the pandemic.
2. METHODS
2.1. Design and setting
For this retrospective study, we included patients with a new diagnosis of cancer who had been consecutively referred to a quick diagnosis center at an academic hospital in Barcelona between 1 March 2019 and 1 September 2021. This tertiary public hospital provides complex healthcare services for patients attended at PC centers, the emergency department (ED) and others referred from secondary‐level hospitals. Based on a daycare facility, the diagnosis center evaluates patients with symptoms potentially indicative of cancer. Primary care constitutes the referral source for 40% to 45% of patients and 20% to 25% of them are diagnosed with cancer. 17 Further, up to 30% of patients referred from the ED, the main source for referral, have a diagnosis of cancer. 18 The operating procedures, staffing and referral criteria have been described elsewhere. 19
2.2. Patient population
The study population involved individuals aged 18 years and older from the catchment area of the hospital (“Example” borough; population 540 000) who were diagnosed with one of the following solid tumor sites: pancreas, colon, rectum, lung, esophagus, stomach, breast, head and neck, kidney, urinary tract and bladder, ovary, endometrium and liver. Kidney, urinary tract and bladder cancers were considered jointly as were colorectal cancers. These cancers were selected as they are the most common nonhematologic cancers with symptomatic presentation in Spain, representing 65.8% of all incident cancers globally, 20 they span a broad range of clinical aggressiveness, and they are managed and treated in most qualified institutions and departments.
2.3. Study periods
The first COVID‐19 case in Spain was detected on 31 January 2020 (25 February 2020 in Catalonia). By the spring of 2020, the country was one of the most severely affected in Europe. 21 The pandemic periods of the study were established considering the timeline of the COVID‐19 pandemic in Barcelona and Spain and the ongoing situation at the hospital. 22 The early period (March to September 2020) paralleled the first pandemic wave in Catalonia, characterized by the extreme pressure on the healthcare system and the first lockdown on 14 March 2020. 23 The mid‐period (September 2020 to March 2021) concurred with the second (October to December 2020) and third (January to March 2021) pandemic waves, with different levels of restrictions and delivery, as of 27 December 2020, of the first COVID‐19 vaccines for specific population groups. The late period (March to September 2021) correlated with the fourth (March to July 2021) and fifth (July to October 2021) pandemic waves, characterized by an overall decline in the number of new cases but emergence, as of early July 2021, of the COVID‐19 delta variant, which mostly affected youngsters and young people with a low vaccination coverage. 22 , 24 We also defined a nonpandemic or pre‐COVID period, running from March 2019 to March 2020.
2.4. Time intervals
The primary outcome was the length of time spent within time points recognized as significant markers on the prediagnostic pathway of cancer from first symptoms to diagnosis. 8 The study intervals were defined considering the waiting‐time targets set in the Strategy on Cancer of the National Health System. 25 These targets are based on information collected by the own public body through nationwide audits and available evidence. Three intervals were analyzed: the patient interval, from first recorded symptom (see below) to presentation to a healthcare provider; the PC/ED interval, from presentation to PC or ED to referral to the diagnosis center and the hospital interval, from referral to date of diagnosis (first confirmatory histology report).
2.5. Database
Information on the variables of interest was extracted from the e‐health records of the hospital and PC centers and a deidentified database was created. Data quality was assessed through external audits, which validated the variables in the e‐health record system. In addition to the patient, PC/ED and hospital interval, audited variables included tumor‐specific, demographic and clinical variables. Stage at diagnosis was categorized according to the Union for International Cancer Control TNM classification (I, II, III and stage IV or metastatic). 26 Demographic variables were abstracted and included age at diagnosis (continuous variable with age groups: 18‐49 years, 50‐59 years, 60‐69 years, 70‐79 years, 80‐89, ≥90 years); sex (male, female); ethnicity (White, non‐White); household income (median income in patient's zip code: highest, intermediate‐high, intermediate‐low, lowest) and median education level (highest, intermediate‐high, intermediate‐low, lowest). Clinical variables were abstracted and included previous history of cancer and any evidence of relapse at follow‐up; Charlson's comorbidity score (0, 1, 2, ≥3) 27 ; alcohol consumption (none, excessive, normal limits); smoking status (none, current smoker, former smoker) and presenting symptoms. Information on new‐onset symptoms was captured from e‐health records and referral letters sent by PC and ED physicians, and were elicited prospectively (before diagnosis) during healthcare encounters using a list of 17 symptoms and signs (or abnormal findings), namely, dysphagia, rectal bleeding, palpable abdominal mass, abdominal pain, hepatomegaly/liver mass, change in bowel habit, exudative ascites, hemoptysis, dyspnea, cough, lump/lymphadenopathy, breast lump/mass, hematuria, metrorrhagia, weight loss, iron‐deficiency anemia and suspected cancer in imaging investigation (related to presenting symptoms or as an incidental finding).
2.6. Inclusion and exclusion criteria
Only patients with a histologically confirmed diagnosis of cancer were eligible. Screen‐detected cases, patients not fulfilling the original referral criteria for evaluation and those with an overall performance status precluding proper management at an ambulatory diagnosis center were excluded. 28 Exclusion criteria also applied to cancers with missing or unknown intervals, histology and staging in e‐health records, stage 0 cancers, patients with severe acute respiratory syndrome coronavirus 2 infection and those who died or were lost to follow‐up before diagnosis.
2.7. Statistical analyses
For all cancer types, time intervals were computed from March 2019 to March 2020 and for each COVID period from March 2020 to September 2021. Since the graphical analysis of intervals revealed skewed distributions (confirmed with the Kolmogorov‐Smirnov test), medians with interquartile ranges (IQR 25‐75) are reported. Overall and for each cancer site, differences in intervals between periods were determined with the Kruskal‐Wallis nonparametric analysis of variance test and, when such test revealed significant differences at the 0.05 level, both the Dunn's test and the Wilcoxon rank‐sum test were run to determine exactly which groups were different. Differences in demographics, clinical variables and stage at diagnosis were assessed with t‐tests or the Mann‐Whitney U test for continuous data and the Fisher's exact test or the χ 2 test for categorical data and frequencies. A two‐sided P‐value of .05 or less was characterized as statistically significant. Statistical analyses were done using OriginPro 2022 (OriginLab, Northampton, Massachusetts) and GraphPad Prism v9.3.0 (GraphPad Software, San Diego, California).
3. RESULTS
The flow of patient selection by study period is outlined in Figure 1. There were 909 patients who were initially eligible in the nonpandemic period, 332 in the early period, 387 in the mid‐period and 562 in the late period. After applying the exclusion criteria, a total of 844, 306, 351 and 515 patients with solid tumors, respectively, were selected for definitive analysis.
FIGURE 1.
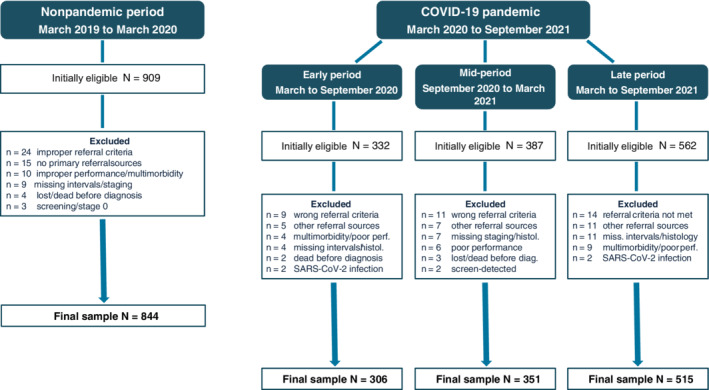
Flowchart of patient selection by study period. SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2
3.1. Cancer sites
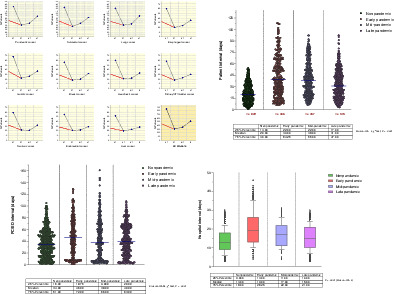
The number of cancers diagnosed in each period can be seen in Figure 2 and Table S1. Although for each tumor site, numbers decreased appreciably in the early and mid‐period and rebounded in the late period, percentages showed little variation throughout the duration of the study. Most common cancers across periods were pancreatic, colorectal and lung cancer. Of 844 pre‐COVID patients, 130 (15.4%) were diagnosed with pancreatic cancer, 125 (14.8%) with colorectal cancer and 121 (14.3%) with lung cancer. Across COVID periods, the percentage of pancreatic cancers ranged from 16.3% (50/306) in the early to 17.1% (88/515) in the late period. For colorectal cancer, it ranged from 14.4% in the early to 15.9% in the late period and, for lung cancer, from 16.3% to 17.3%, respectively. The number and percentages of less common cancers is shown in Figure 2 and Table S1. Gastric and esophageal cancer were relatively common, being diagnosed in 8.1% and 7.1% of nonpandemic patients, respectively. Across COVID periods, 7.2% to 7.4% of patients were diagnosed with gastric cancer and 6.5% to 6.8% with esophageal cancer. Liver cancers were the least common of the study and showed similar percentages across periods.
FIGURE 2.
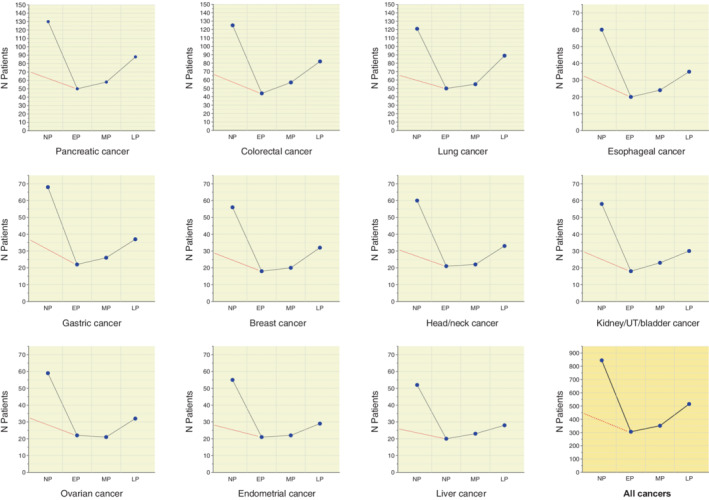
Layout of multiple plots representing the 11 cancers types of the study and their number (blue symbols) over 1‐year nonpandemic and three COVID periods. The red dotted lines in each plot connect the number of cases in the 6‐month nonpandemic period (March to September 2019) to the number in the early pandemic period. EP, early pandemic period; LP, late pandemic period; MP, mid‐pandemic period; NP, nonpandemic period; UT, urinary tract
3.2. Patient characteristics
The general characteristics of patient cohorts are shown in Table 1. The ED was the main referral source over the four periods, most notably in the mid‐period (58.1% of referrals). Mean age ranged from 66.8 (SD 12.7) years in the nonpandemic to 69.1 (14) years in the late period. Nonpandemic patients had significantly lower income and education levels and less comorbidities than late period patients. There were fewer smokers among nonpandemic and mid‐period patients than among those from the late period. Weight loss was the main presenting symptom in each period, ranging from 33.6% in the nonpandemic to 37.9% in the mid‐period. Common clinical manifestations across periods also included abdominal pain, suspected cancer in imaging investigation and change in bowel habit. Exudative ascites and respiratory symptoms including hemoptysis, dyspnea and cough were more common among late period patients (Table 1).
TABLE 1.
Patient characteristics by study period
| Pre‐COVID period, N = 844 | Early period, N = 306 | Mid‐period, N = 351 | Late period, N = 515 | |
|---|---|---|---|---|
| Sex, n (%) a | 674 | 245 | 288 | 422 |
| Females | 328 (48.7) | 116 (47.4) | 138 (47.9) | 198 (46.9) |
| Males | 346 (51.3) | 129 (52.7) | 150 (52.1) | 224 (53.1) |
| Age at diagnosis, years [mean (SD)] | 66.8 (12.7) | 68.4 (11.2) | 67.7 (13.3) | 69.1 (14) |
| 40‐49 | 10.1 | 2 | 10 | 9.7 |
| 50‐59 | 24.1 | 24.8 | 21.9 | 20.4 |
| 60‐69 | 21.6 | 26.1 | 20.8 | 22.7 |
| 70‐79 | 22.9 | 25.2 | 24.8 | 18.5 |
| 80‐89 | 21.4 | 21.9 | 18.5 | 20.6 |
| ≥90 | 0 | 0 | 4 | 8.2 |
| Median age, years (IQR) | 67 (55‐78) | 69 (59‐78) | 68 (55‐78) | 68 (57‐81) |
| Referral pathway, n (%) | ||||
| Emergency department | 466 (55.2) | 176 (57.5) | 204 (58.1) | 290 (56.3) |
| Primary care | 378 (44.8) | 130 (42.5) | 147 (41.9) | 225 (43.7) |
| Household income, n (%) | ||||
| Highest | 150 (17.8) | 62 (20.3) | 67 (19.1) | 109 (21.2) |
| Intermediate‐high | 245 (29) | 98 (32) | 109 (31.1) | 175 (34) |
| Intermediate‐low | 279 (33.1) | 90 (29.4) | 108 (30.8) | 143 (27.8) |
| Lowest | 170 (20.1) | 56 (18.3) | 67 (19.1) | 88 (17.1) |
| Education level, n (%) | ||||
| Highest | 80 (9.5) | 34 (11.1) | 36 (10.3) | 62 (12) |
| Intermediate‐high | 195 (23.1) | 77 (25.2) | 85 (24.2) | 140 (27.2) |
| Intermediate‐low | 357 (42.3) | 127 (41.5) | 147 (41.9) | 204 (39.6) |
| Lowest | 212 (25.1) | 68 (22.2) | 83 (23.7) | 109 (21.2) |
| Ethnic origin, n (%) | ||||
| White | 789 (93.5) | 290 (94.8) | 330 (94) | 490 (95.2) |
| Non‐White | 55 (6.5) | 16 (5.2) | 21 (6) | 25 (4.9) |
| Previous history of cancer, n (%) | ||||
| Yes | 89 (10.5) | 41 (13.4) | 39 (11.1) | 73 (14.2) |
| Relapse | 6 (6.7) | 3 (4.9) | 0 (0) | 4 (5.5) |
| Median follow‐up, months (IQR) | 44 (36‐53) | 39 (33‐46) | 46 (41‐51) | 43 (35‐51) |
| Charlson's index, n (%) | ||||
| 0‐1 | 624 (73.9) | 215 (70.3) | 255 (72.7) | 356 (69.1) |
| 2 | 183 (21.7) | 70 (22.9) | 78 (22.2) | 119 (23.1) |
| ≥3 | 37 (4.4) | 21 (6.9) | 18 (5.1) | 40 (7.8) |
| Alcohol intake, n (%) | ||||
| Normal limits | 222 (26.3) | 88 (28.8) | 93 (26.5) | 147 (28.5) |
| Excessive | 64 (7.6) | 28 (9.2) | 30 (8.6) | 49 (9.5) |
| None | 558 (66.1) | 190 (62.1) | 228 (65) | 319 (61.2) |
| Smoking, n (%) | ||||
| None | 399 (47.3) | 128 (41.8) | 158 (45) | 198 (38.5) |
| Current smoker | 240 (28.4) | 95 (31.1) | 103 (29.3) | 170 (33) |
| Former smoker | 205 (24.3) | 83 (27.1) | 90 (25.6) | 147 (28.5) |
| Presenting symptoms/signs, n (%) | ||||
| Dysphagia | 56 (6.6) | 18 (5.9) | 25 (7.1) | 33 (6.4) |
| Rectal bleeding | 43 (5.1) | 12 (3.9) | 26 (7.4) | 28 (5.4) |
| Palpable abdominal mass | 30 (3.6) | 9 (2.9) | 9 (2.6) | 20 (3.9) |
| Abdominal pain | 193 (22.9) | 79 (25.8) | 96 (27.4) | 145 (28.2) |
| Hepatomegaly/liver mass | 57 (6.8) | 24 (7.8) | 28 (8) | 34 (6.6) |
| Change in bowel habit | 86 (10.2) | 26 (8.5) | 39 (11.1) | 55 (10.7) |
| Exudative ascites | 41 (4.9) | 11 (3.6) | 14 (4) | 34 (6.6) |
| Hemoptysis | 45 (5.3) | 20 (6.5) | 17 (4.8) | 37 (7.2) |
| Dyspnea | 43 (5.1) | 19 (6.2) | 23 (6.6) | 39 (7.6) |
| Cough | 74 (8.8) | 36 (11.8) | 34 (9.7) | 67 (13) |
| Lump/lymphadenopathy | 68 (8.1) | 24 (7.8) | 24 (6.8) | 45 (8.7) |
| Breast lump/mass | 46 (5.5) | 15 (4.9) | 14 (4) | 26 (5.1) |
| Hematuria | 27 (3.2) | 9 (2.9) | 15 (4.3) | 17 (3.3) |
| Metrorrhagia | 52 (6.2) | 22 (7.2) | 20 (5.7) | 25 (4.9) |
| Weight loss | 284 (33.6) | 114 (37.3) | 133 (37.9) | 183 (35.5) |
| Iron‐deficiency anemia | 43 (5.1) | 14 (4.6) | 22 (6.3) | 30 (5.8) |
| Suspected cancer in imaging investigation | 78 (9.2) | 31 (10.1) | 30 (8.6) | 57 (11.1) |
In italics, number of females and males in each period after excluding breast, ovarian and endometrial cancers.
3.3. Time intervals
Median intervals differed by cancer type and by study period. Across periods, gastric, lung and pancreatic cancers had the longest intervals, whereas kidney/urinary tract/bladder, endometrial, head/neck and esophageal cancers had the shortest. While median intervals for nonpandemic cancers were shorter than in any other COVID period, early period cancers had the longest (Tables 2, S2 and S3).
TABLE 2.
Distribution of median patient intervals for each cancer type over 1‐year nonpandemic and three biannual COVID periods
| Cancer site | Patient interval (days) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nonpandemic period | Early period | Mid‐period | Late period | |||||||||
| N | Median | IQR | N | Median | IQR | N | Median | IQR | N | Median | IQR | |
| Pancreatic | 130 | 18 | 11‐27 | 50 | 52 | 26‐72 | 58 | 45 | 29‐63 | 88 | 38 | 17‐50.5 |
| Colorectal | 125 | 25 | 16‐36 | 44 | 48 | 21.5‐67.5 | 57 | 42 | 24‐65 | 82 | 37 | 24‐46 |
| Lung | 121 | 23 | 18‐38 | 50 | 60 | 31‐84 | 55 | 52 | 31‐69 | 89 | 46 | 25‐59 |
| Esophageal | 60 | 20 | 8.5‐23 | 20 | 28 | 17.5‐44 | 24 | 25 | 15‐38 | 35 | 22 | 9‐31 |
| Gastric | 68 | 24 | 15‐36.5 | 22 | 66 | 28‐100 | 26 | 57 | 29‐81 | 37 | 53 | 36‐74 |
| Breast | 56 | 22 | 16.5‐32 | 18 | 31 | 26‐39 | 20 | 27 | 15‐39.5 | 32 | 25 | 16.5‐36 |
| Head/neck | 60 | 21.5 | 10‐30.5 | 21 | 28 | 18‐38 | 22 | 24 | 17‐40 | 33 | 22 | 13‐26 |
| KUB | 58 | 16 | 11‐24 | 18 | 25 | 11‐46 | 23 | 22 | 18‐43 | 30 | 17 | 14‐28 |
| Ovarian | 59 | 21 | 16‐29 | 22 | 50 | 26‐54 | 21 | 43 | 35‐77 | 32 | 37 | 23.5‐47.5 |
| Endometrial | 55 | 15 | 9‐20 | 21 | 22 | 17‐30 | 22 | 18 | 14‐30 | 29 | 17 | 12‐25 |
| Liver | 52 | 19 | 8.5‐23.5 | 20 | 43 | 28.5‐54 | 23 | 39 | 29‐48 | 28 | 34 | 22‐48.5 |
Note: Just to highlight numbers of cancers in each period
Abbreviations: IQR, interquartile range; KUB, kidney, urinary tract and bladder.
At the 0.05 level, overall median patient intervals were longest in the early (39 [IQR 22‐64] days) and shortest in the nonpandemic period (20 [IQR 13‐30] days; Kruskal‐Wallis, χ 2 test, P < .0001; Figure 3). For each cancer type, median patient intervals in the early period were significantly longer than in the nonpandemic period, most notably for gastric (66 [IQR 28‐100] vs 24 [IQR 15‐36.5] days, respectively; P = .0002), lung (60 [IQR 31‐84] vs 23 [IQR 18‐38] days, respectively; P < .0001) and pancreatic cancer (52 [IQR 26‐72] vs 18 [IQR 11‐27] days, respectively; P < .0001; Table 2).
FIGURE 3.
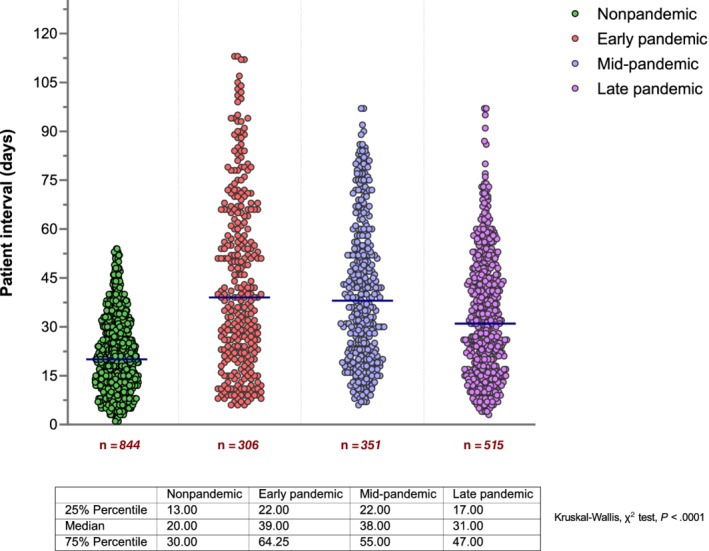
Scatter dot plots showing overall patient intervals in the pre‐COVID, early, mid and late pandemic periods. Horizontal lines denote the median of each group. Each dot (green, red, violet and purple) represents the value of a single patient. Red numbers below the x axis are the number of cancers in each period
Statistical differences were also observed in overall median PC/ED intervals, which were longer in early and late periods (46 [IQR 19‐72] and 40 [IQR 20‐63] days, respectively) and shortest in the nonpandemic period (34 [IQR 16‐51] days; Kruskal‐Wallis, χ 2 test, P < .0001; Figure 4). For five cancer types (pancreatic, colorectal, gastric, ovarian and liver), median PC/ED intervals in the early period were significantly longer than in the nonpandemic period. Unlike patient intervals, however, differences for other cancers were modest (Table S2, Figure S1).
FIGURE 4.
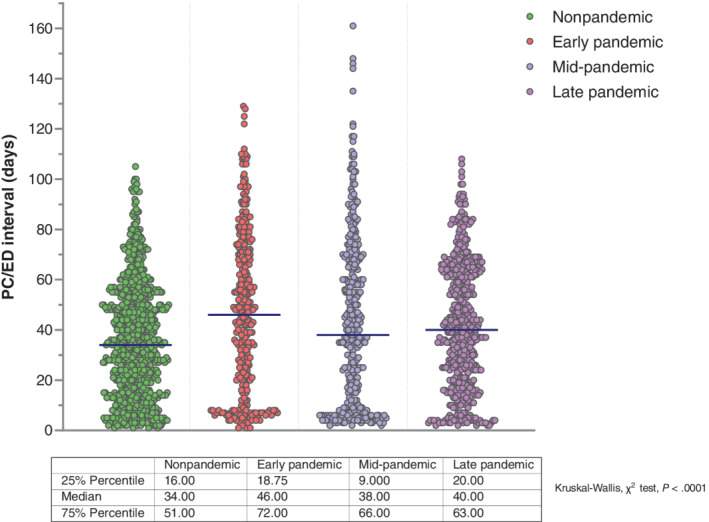
Overall median primary care/emergency department intervals across study periods. The dots in scatter plots represent the values of individual patients and illustrate data patterns when taken as a whole. Median values in each plot are represented by dark blue horizontal lines
Analysis of hospital intervals revealed significant differences between periods, both overall (P < .0001 on Kruskal‐Wallis test) and by cancer type, with longest median intervals for early period cancers and shortest for nonpandemic cancers (Figure S2). Early period cancers with longest hospital intervals were gastric (25 [IQR 15‐38] vs 15 [IQR 9‐20] days in nonpandemic; P = .0002), pancreatic (24 [IQR 12‐32] vs 16 [IQR 10‐22] days in nonpandemic; P = .001) and lung cancer (23 [IQR 14‐26] vs 15 [IQR 10‐20] days, respectively; P = .003; Table S3). Hospital intervals for ovarian and liver cancers were also significantly longer in the early compared to the nonpandemic period.
3.4. Presenting symptoms and intervals
The nature and relative frequency of presenting symptoms of each cancer type in each period are displayed as two‐dimensional heat maps (Figure S3). For some cancers, most patients presented with one alarm symptom, without relevant differences between periods. Alarm symptoms across periods included metrorrhagia (85%‐100% of endometrial cancer patients), hematuria (85%‐100% of kidney/urinary tract/bladder cancer patients), dysphagia (83%‐94% of esophageal cancer patients) and breast lump/mass and neck lump/lymph node for breast and head/neck cancer patients, respectively. In contrast, cancers with a broader range of presenting symptoms, mostly nonalarm symptoms, across periods included pancreatic cancer [abdominal pain (range 48%‐58%); weight loss (30%‐34%); change in bowel habit (12%‐20%)], gastric cancer [iron‐deficiency anemia (17%‐21%); weight loss (15%‐18%); exudative ascites (22% of mid‐period cases)] and ovarian cancer. Other cancers presented with a combination of nonspecific and one or two alarm symptoms including colorectal and lung cancer (Figure S3). As shown in Tables 2, S2 and S3, median patient, PC/ED and hospital intervals for cancers with broader symptom signatures were in general longer, especially in the early COVID period, than those with narrow signatures.
3.5. Stage at diagnosis
Investigation of clinical stage uncovered significant differences between periods. Figure S4 is a visual representation of differences in stage between periods for each cancer type taking as baseline the 6‐month nonpandemic period March to September 2019 (ie, away from pandemic onset; N = 449). Table S4 shows the number and proportion of tumors by stage at diagnosis over the four study periods (March 2019 to September 2021). Compared to nonpandemic, mid‐period cancers had the most advanced stage. In particular, 84.5% of pancreatic, 70.9% of lung and 69.2% of gastric cancers were diagnosed at stage IV in the mid‐period, from 68.5%, 51.2% and 50%, respectively, in the nonpandemic period. Overall, 51.3% of mid‐period cancers were diagnosed at stage IV, from 38.4% and 41.8% in the nonpandemic and early COVID periods, respectively. Although late period cancers were less advanced than those from the mid‐period, 47% were still diagnosed with stage IV disease. For some cancers, differences between periods were notable for both stage III and IV. Specifically, 36.8% and 38.6% of colorectal cancers from the mid‐period were diagnosed at stage III and IV, respectively, compared to 27.2% and 24.8% from the nonpandemic period. Similar differences were observed for ovarian and liver cancers but were less pronounced for other cancers (Table S4, Figure S4).
Clinical stage was examined according to referral pathways (ie, mode of presentation). Table S5 shows the distribution of cancers from each period broken down by referral pathway and stage at diagnosis. Throughout March 2019 to September 2021, patients presenting as emergencies had more advanced stages than those referred from PC, with significant differences between them. Considering only stage III to IV disease, ovarian, colorectal, gastric, lung and pancreatic cancers had the highest rate of emergency diagnosis. Notably, there were no differences in referral pathways between periods. For stage III to IV disease, ED referrals ranged from 62.6% in the nonpandemic to 65.2% in the mid‐period, and PC referrals ranged from 34.8% in the mid‐period to 37.4% in the nonpandemic period (Table S5).
4. DISCUSSION
4.1. Principal findings
This investigation provides insights into how COVID‐19 changed time intervals in the pathway from symptom onset to diagnosis across 13 major cancer types over three biannual COVID periods extending from March 2020 up until September 2021. Despite the study population was relatively heterogeneous, our data indicate that prediagnostic intervals across pandemic periods were longer than over 1‐year nonpandemic period. Although significant changes occurred through all COVID periods, the impact was more marked for patient intervals for cancers diagnosed over the first 6 months of the pandemic. For all cancers combined, median patient intervals in the early pandemic period were 95% longer than in the nonpandemic period. Though PC/ED and hospital intervals were also significantly longer in the early compared to the nonpandemic period, relative percent changes were smaller. Combined with the fact that mid‐period cancers had the most advanced stage at diagnosis, these results suggest a shift to later‐stage disease from delayed intervals in the earlier period.
4.2. Findings in context
The present findings add to prior evidence about prediagnostic cancer care during the pandemic by describing and comparing the length of time intervals across a range of common cancers. This fills an important gap in the literature, which has primarily focused on trends in new cancer diagnosis. As reported by authors from different countries and by our group, declining trends have been attributed to a combination of patient and health system factors, most notably a decrease in the number of patients presenting in PC, a decrease in the number of early referrals from primary to secondary care, and major setbacks at secondary care with hospitals reassigning healthcare resources to pandemic preparedness. 17 , 18 , 29 , 30 , 31 , 32 , 33 , 34 Consistent with reported findings, the number of cancers in our study decreased from an average of 70 per month in the pre‐COVID period to 51 per month in the early period, or a relative reduction of 27.1%. An upward trend was observed afterwards into the mid‐period, in which the monthly average was 59, representing a 15.7% reduction compared to the nonpandemic average.
Our results suggest an impact of COVID‐19 on stage at diagnosis, with significant differences between periods. Some evidence exists on stage‐shift to later‐stage disease due to the pandemic. By investigating the stage distribution of lung cancer in 554 patients between 1 July 2019 and 31 March 2021, a retrospective study at a large hospital in New York found that, in comparison with the distribution in nonpandemic months and the second quarter of 2020, which included the lockdown period, the proportion of stage IV cancers diagnosed during the quarters succeeding gradual resumption (October 2020 to March 2021) increased significantly. 35 Arguably, an apparent stage‐shift was a consequence of lockdown‐related delays and the reported exponential nature of lung cancer growth and time to progression. Interestingly, a recent study analyzing circulating concentrations of tumor DNA in newly diagnosed metastatic colorectal cancer before vs after lockdown in France found that patients diagnosed after it had a greater tumor burden (defined as statistically higher concentrations of tumor DNA) than those diagnosed before, and that the median survival of patients with high tumor DNA concentrations was lower than of those with lower concentrations. 36 Throughout the duration of our study, cancer patients were more likely to be diagnosed at stage III to IV, which is consistent with previous publications of the diagnosis center of our institution. Of note, cancers from the mid‐period were more likely to be diagnosed at stage III to IV than nonpandemic and early period cancers (80% vs 61% and 67%, respectively). Since the overall median length of patient, PC/ED and hospital intervals was longest in the early pandemic period, such delays could be a relevant factor in why stage at diagnosis differed between periods.
While diagnostic delays in cancer have come sharply into focus during the pandemic, this is, as far as we know, the first report to assess the impact on time points and intervals in the pathway to diagnosis. Building on previous research about the relative contribution of the patient and PC interval in the overall length of the pre‐referral interval (from symptom onset to referral), 37 we found that, though differing by cancer type, overall median PC/ED intervals in the nonpandemic period were 70% longer than median patient intervals. Specifically, the PC/ED interval was nearly 3‐fold greater than the patient interval for pancreatic cancer and 2‐fold greater for gastric, lung, kidney/urinary tract/bladder and endometrial cancers. However, owing to the length of patient intervals in the early COVID period, the relative increase of PC/ED intervals during this period was only 18%. On closer examination of nonpandemic data, it became apparent that median PC/ED intervals contrasted sharply with median PC intervals reported in other world regions. 37 , 38 , 39 By analyzing nearly 11 000 patients aged at least 15 years who presented with symptoms to PC in England and were subsequently diagnosed with one of 28 common and less common cancers, data from a national audit showed that, while varying by cancer type, median patient intervals were significantly longer than PC intervals for 18 of 28 cancer types, which included those analyzed here. 37 More recent evidence on lung cancer patients from 10 states in Australia, Canada and Europe showed median patient intervals which, in four states (Wales, Scotland, Sweden and Canada), were similar to the median patient interval of the pre‐COVID period of our study. However, the median PC/ED interval for our lung cancer patients (46 days) compares unfavorably with PC intervals reported elsewhere, with Canadian cancer patients having the longest (29 and 30 days in two jurisdictions). 38 A similar pattern was reported for esophageal and gastric cancers in a retrospective cohort study from the Netherlands. Whereas patient and secondary care intervals were similar or longer than patient and hospital intervals for our nonpandemic esophageal and gastric cancers, median PC intervals of the Dutch study were substantially shorter than PC/ED intervals reported here. 39
Consistent with the Spanish Strategy on Cancer, most patients from the nonpandemic period met the target for the maximum time between first appointment at secondary care and a histological diagnosis (≤15 days). However, observed PC/ED intervals are not nearly the target time between PC suspicion and first hospital visit (≤7 days). 25 Possible explanations for the excessive length of PC/ED intervals across periods and their substantial dilation during the early pandemic period should be sought at the own particularities of the Spanish health system and its debilitated response to cope with COVID‐19 when the burden was among the greatest worldwide. 40 Notwithstanding a relatively high level of efficiency, with health indicators better than predicted according to the country's socio‐demographic index, the healthcare system's workforce and capacity have been reduced as a result of a decade of underinvestment that followed the 2008 financial crisis. 21 An overly hospital‐centered healthcare model and a weak coordination between primary and specialized care are additional shortcomings that have negatively impacted the continuity of care. 41
In our study, intervals for cancers presenting with alarm symptoms were substantially shorter, most notably in the early pandemic period, that those presenting with a broader range of symptoms, either nonspecific or with a few alarm symptoms. Nicholson et al recently investigated whether the decline in urgent referrals for suspected cancer during the first pandemic wave in England was a result of fewer patients consulting with PC vs fewer PC referrals. 34 Twenty‐eight clinical features of cancer were matched with eight cancer‐specific urgent referral pathways. While overall consultations decreased by 24.2%, urgent referrals for the selected clinical features decreased only by 10.5%. Interestingly, consultations rates for alarm symptoms such as hematuria, dysphagia or breast lumps were the first to return to expected rates in the period following lockdown. In contrast, with some exception, consultations for nonspecific clinical features such as abdominal pain or change in bowel habit recovered much slower. 34
A salient finding was the stage distribution of cancers by mode of presentation. While such distribution across periods was similar, emergency presenters were significantly more likely than those referred from PC to be diagnosed at a later stage, mainly stage IV. This finding is consistent with published data showing that diagnosis of cancer as an emergency is associated with poorer outcomes including advanced‐stage disease. 42 In an analysis of routes to diagnosis by stage for 10 cancer sites in England, the proportion of stage IV cancers among patients with an emergency diagnosis was 30%, whereas it was 17% among those diagnosed via fast‐track referrals, and 14% for nonurgent referrals. 43
4.3. Strengths and limitations
In the interest of evaluating the impact of COVID‐19 on time intervals from symptom onset to diagnosis, our study used real‐time evidence mapped according to a published framework and compared four cohorts of cancer patients over 12 months before and 18 months after onset of pandemic. As such, this is the main strength of the study. Aspects unique to the analysis are the eligibility criteria, with a study population restricted to adults with 13 types of common cancers, and a sufficiently detailed dataset to enable intervals to be analyzed as a continuous variable in compliance with recommendations in the Aarhus statement. 8 Whereas studies on cancer staging and intervals abound in missing information that may confound and bias the results, only patients with a full degree of stage completeness were included, which is an additional strength.
Several limitations must be acknowledged, however. First, we examined data at one tertiary academic institution in an area of Northeastern Spain which was heavily impacted by COVID‐19. To learn whether the results are generalizable, the investigation should be replicated in multiple centers, both nationally in Spain and internationally. Such replication might address the impact of local factors and strengthen the quality of data. Second, patient changes in health‐seeking behavior during the pandemic were not examined. While we have previously reported about how the number of patients seeking healthcare for suspected symptoms declined massively during Spanish lockdown, 17 , 18 a population‐based survey of the Spanish population shed light on anticipated help‐seeking attitudes for cancer symptoms before and after the pandemic. 44 Third, although investigating possible changes on treatment due to the pandemic and survival rates would have enriched the study, its focus was on how COVID‐19 affected prediagnostic pathways and intervals. Similarly, it was not designed to explain the relationship between symptom appearance and outcomes.
4.4. Implications
For patients presenting with symptoms, timeliness of cancer diagnosis is determined by the length of the patient and PC interval. 9 Although in Spain evidence about such intervals across the range of cancers reported is lacking, clarifying how they were adversely shaped by the pandemic can inform priorities for future research and policy strategies to enable timely access to diagnosis as healthcare services are further protected from COVID‐19. Beyond national boundaries, our results are timely in light of ongoing pandemic and may act as a proof of concept for the association between time intervals and stage at diagnosis, relatively unexplored so far. Considering that intervals are eventually relevant to prognosis, 45 the study provides a baseline against which the impact of subsequent initiatives to counteract the adverse outcomes of pandemic‐associated cancer delays can be evaluated and implemented.
5. CONCLUSION
The COVID‐19 pandemic provided an unusual opportunity to examine the impact on key time components of the patient route from first symptoms to diagnosis of cancer. This research showed that the length of patient, PC/ED and hospital intervals across 13 cancer sites increased over a period of 18 months following the start of pandemic in March 2020. COVID‐19 mostly impacted patient intervals, both overall and by cancer type, over the first 6 months. When comparing stage at diagnosis between periods, we found that mid‐period cancers were most advanced. Plausibly, a shift from earlier to later stage was a result of delayed intervals in the early pandemic period on top of a weakened response of the system.
Added to the body of research showing that reducing intervals improves outcomes including survival and clinical stage, these results justify improvement efforts in the early diagnosis of symptom‐detected cancers and provide pointers to where strategies for implementation of change might be best targeted as the pandemic goes on.
AUTHOR CONTRIBUTIONS
Xavier Bosch: Designed the work, acquired data and wrote the article. Elisabet Montori‐Palacin: Acquired data and played an important role in interpreting the results. Rosa Martínez‐Ferrer: Acquired data and interpreted the results. Anna Aldea: Acquired data and interpreted the results. Pedro Moreno: Contributed to the design of the study and interpreted the results. Alfonso López‐Soto: Played an important role in the design of the work and in drafting the article. All authors critically revised the article, approved the final version and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The work reported in the article has been performed by the authors, unless clearly specified in the text.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
ETHICS STATEMENT
The study was approved by the ethical review committee at the Hospital Clínic of Barcelona. Formal consent was not required due to the retrospective design and de‐identification of study subjects. The research reported here adhered to the precepts of the Declaration of Helsinki.
Supporting information
Appendix S1 Supporting Information.
Bosch X, Montori‐Palacin E, Martínez‐Ferrer R, Aldea A, Moreno P, López‐Soto A. Time intervals in the care pathway to cancer diagnosis during the COVID‐19 pandemic: A large retrospective study from a high‐volume center. Int J Cancer. 2022;1‐12. doi: 10.1002/ijc.34260
DATA AVAILABILITY STATEMENT
The data that support the findings of our study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Koo MM, Hamilton W, Walter FM, Rubin GP, Lyratzopoulos G. Symptom signatures and diagnostic timeliness in cancer patients: a review of current evidence. Neoplasia. 2018;20(2):165‐174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Koo MM, Unger‐Saldaña K, Mwaka AD, et al. Conceptual framework to guide early diagnosis programs for symptomatic cancer as part of global cancer control. JCO Glob Oncol. 2021;7:35‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Emery JD, Shaw K, Williams B, et al. The role of primary care in early detection and follow‐up of cancer. Nat Rev Clin Oncol. 2014;11(1):38‐48. [DOI] [PubMed] [Google Scholar]
- 4. Richards M, Thorlby R, Fisher R, Torton C. Unfinished business: an assessment of the national approach to improving cancer services in England 1995‐2015. Health Foundation; 2018. https://www.healthorguk/publications/unfinished-business. Accessed March 17, 2022.
- 5. Meechan D, Gildea C, Hollingworth L, Richards MA, Riley D, Rubin G. Variation in use of the 2‐week referral pathway for suspected cancer: a cross‐sectional analysis. Br J Gen Pract. 2012;62(602):e590‐e597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Møller H, Gildea C, Meechan D, Rubin G, Round T, Vedsted P. Use of the English urgent referral pathway for suspected cancer and mortality in patients with cancer: cohort study. BMJ. 2015;351:h5102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Round T, Gildea C, Ashworth M, Møller H. Association between use of urgent suspected cancer referral and mortality and stage at diagnosis: a 5‐year national cohort study. Br J Gen Pract. 2020;70(695):e389‐e398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Weller D, Vedsted P, Rubin G, et al. The Aarhus statement: improving design and reporting of studies on early cancer diagnosis. Br J Cancer. 2012;106(7):1262‐1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Swann R, McPhail S, Witt J, et al. Diagnosing cancer in primary care: results from the National Cancer Diagnosis Audit. Br J Gen Pract. 2018;68(666):e63‐e72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tørring ML, Falborg AZ, Jensen H, et al. Advanced‐stage cancer and time to diagnosis: an international cancer benchmarking partnership (ICBP) cross‐sectional study. Eur J Cancer Care (Engl). 2019;28(5):e13100. [DOI] [PubMed] [Google Scholar]
- 11. Lyratzopoulos G, Liu MP, Abel GA, Wardle J, Keating NL. The association between fatalistic beliefs and late stage at diagnosis of lung and colorectal cancer. Cancer Epidemiol Biomarkers Prev. 2015;24(4):720‐726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Anakwenze C, Bhatia R, Rate W, et al. Factors related to advanced stage of cancer presentation in Botswana. J Glob Oncol. 2018;4:1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Neal RD, Tharmanathan P, France B, et al. Is increased time to diagnosis and treatment in symptomatic cancer associated with poorer outcomes? Systematic review. Br J Cancer. 2015;112:S92‐S107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hall H, Tocock A, Burdett S, et al. Association between time‐to‐treatment and outcomes in non‐small cell lung cancer: a systematic review. Thorax. 2021;77:762‐768. doi: 10.1136/thoraxjnl-2021-216865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ott JJ, Ullrich A, Miller AB. The importance of early symptom recognition in the context of early detection and cancer survival. Eur J Cancer. 2009;45(16):2743‐2748. [DOI] [PubMed] [Google Scholar]
- 16. Fleming KA, Horton S, Wilson ML, et al. The Lancet Commission on diagnostics: transforming access to diagnostics. Lancet. 2021;398(10315):1997‐2050. [Erratum in: Lancet. 2021;398(10315):1964]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bosch X, Torres M, Moreno P, López‐Soto A. Delays in cancer diagnostic testing at a quick referral unit in Spain during COVID‐19. Diagnostics (Basel). 2021;11(11):2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bosch X, Capdevila A, Grafia I, Ladino A, Moreno PJ, López‐Soto A. The impact of Covid‐19 on patients with suspected cancer: an analysis of ED presentation and referrals to a quick diagnosis unit. Am J Emerg Med. 2021;48:1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bosch X, Jordán A, Coca A, López‐Soto A. Quick diagnosis units versus hospitalization for the diagnosis of potentially severe diseases in Spain. J Hosp Med. 2012;7(1):41‐47. [DOI] [PubMed] [Google Scholar]
- 20. Ferlay J, Ervik M, Lam F, Colombet M, Mery L, Piñeros M, Znaor A, Soerjomataram I, Bray F. Global cancer observatory: cancer today. International Agency for Research on Cancer; 2020. https://gco.iarc.fr/today. Accessed March 17, 2022.
- 21. The Lancet Public Health . COVID‐19 in Spain: a predictable storm? Lancet Public Health. 2020;5(11):e568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. COVID Data (2020‐2022). Epidemiological and healthcare situation in Catalonia. Agency of Health Quality and Assessment of Catalonia (AQuAS) Generalitat de Catalunya. https://dadescovidcat/critics?lang=eng. Accessed June 19, 2022.
- 23. Decree of the Presidency of March 14, 2020. Real Decreto 463/2020, de 14 de marzo, por el que se declara el estado de alarma para la gestión de la situación de crisis sanitaria ocasionada por el COVID‐19, BOE‐A‐2020‐3692 2020. https://www.boees/eli/es/rd/2020/03/14/463. Accessed March 17, 2022.
- 24. Singanayagam A, Hakki S, Dunning J, et al. Community transmission and viral load kinetics of the SARS‐CoV‐2 delta (B.1.617.2) variant in vaccinated and unvaccinated individuals in the UK: a prospective, longitudinal, cohort study. Lancet Infect Dis. 2022;22(2):183‐195. [Erratum in: Lancet Infect Dis. 2021;21(12):e363]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Strategy on Cancer of the National Health System (2021 Update). Spanish Health Ministry; 2021. https://wwwsanidadgobes/organizacion/sns/planCalidadSNS/pdf/Estrategia_en_cancer_del_Sistema_Nacional_de_Salud_Actualizacion_2021pdf. Accessed March 17, 2022.
- 26. Brierley JD, Gospodarowicz MK, Wittekind C, eds. TNM Classification of Malignant Tumours. 8th ed. Chichester: Wiley‐Blackwell; 2017:272. [Google Scholar]
- 27. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373‐383. [DOI] [PubMed] [Google Scholar]
- 28. Bosch X, Aibar J, Capell S, Coca A, López‐Soto A. Quick diagnosis units: a potentially useful alternative to conventional hospitalisation. Med J Aust. 2009;191(9):496‐498. [DOI] [PubMed] [Google Scholar]
- 29. Richards M, Anderson M, Carter P, Ebert BL, Mossialos E. The impact of the COVID‐19 pandemic on cancer care. Nat Cancer. 2020;1:1‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Morris EJA, Goldacre R, Spata E, et al. Impact of the COVID‐19 pandemic on the detection and management of colorectal cancer in England: a population‐based study. Lancet Gastroenterol Hepatol. 2021;6(3):199‐208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rutter MD, Brookes M, Lee TJ, Rogers P, Sharp L. Impact of the COVID‐19 pandemic on UK endoscopic activity and cancer detection: a national endoscopy database analysis. Gut. 2021;70(3):537‐543. [DOI] [PubMed] [Google Scholar]
- 32. Ranganathan P, Sengar M, Chinnaswamy G, et al. National cancer grid of India. Impact of COVID‐19 on cancer care in India: a cohort study. Lancet Oncol. 2021;22(7):970‐976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kaufman HW, Chen Z, Niles J, Fesko Y. Changes in the number of US patients with newly identified cancer before and during the coronavirus disease 2019 (COVID‐19) pandemic. JAMA Netw Open. 2020;3(8):e2017267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nicholson BD, Ordóñez‐Mena JM, Lay‐Flurrie S, et al. Consultations for clinical features of possible cancer and associated urgent referrals before and during the COVID‐19 pandemic: an observational cohort study from English primary care. Br J Cancer. 2022;126(6):948‐956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mynard N, Saxena A, Mavracick A, et al. Lung cancer stage shift as a result of COVID‐19 lockdowns in New York City, a brief report. Clin Lung Cancer. 2021;23:e238‐e242. doi: 10.1016/j.cllc.2021.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Thierry AR, Pastor B, Pisareva E, et al. Association of COVID‐19 lockdown with the tumor burden in patients with newly diagnosed metastatic colorectal cancer. JAMA Netw Open. 2021;4(9):e2124483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lyratzopoulos G, Saunders CL, Abel GA, et al. The relative length of the patient and the primary care interval in patients with 28 common and rarer cancers. Br J Cancer. 2015;112:S35‐S40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Menon U, Vedsted P, Zalounina Falborg A, et al. Time intervals and routes to diagnosis for lung cancer in 10 jurisdictions: cross‐sectional study findings from the international cancer benchmarking partnership (ICBP). BMJ Open. 2019;9(11):e025895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. van Erp NF, Helsper CW, Slottje P, et al. Time to diagnosis of symptomatic gastric and oesophageal cancer in The Netherlands: where is the room for improvement? United Eur Gastroenterol J. 2020;8(5):607‐620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Legido‐Quigley H, Mateos‐García JT, Campos VR, Gea‐Sánchez M, Muntaner C, McKee M. The resilience of the Spanish health system against the COVID‐19 pandemic. Lancet Public Health. 2020;5(5):e251‐e252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. López‐Casasnovas G, Francesc‐López Seguí F, Arasanz‐Goset A. Sustainability and resilience in the Spanish health system. The Partnership for Health System Sustainability and Resilience (PHSSR). London School of Economics and Political Science; 2021. https://www.3.weforum.org/docs/WEF_PHSSR_Spain_Report.pdf. Accessed March 17, 2022.
- 42. Zhou Y, Abel GA, Hamilton W, et al. Diagnosis of cancer as an emergency: a critical review of current evidence. Nat Rev Clin Oncol. 2017;14(1):45‐56. [DOI] [PubMed] [Google Scholar]
- 43. National Cancer Intelligence Network . Routes to diagnosis of cancer by stage 2012‐2013; 2016. http://www.ncin.org.uk/view?rid=3071. Accessed March 17, 2022.
- 44. Petrova D, Pollán M, Rodriguez‐Barranco M, Garrido D, Borrás JM, Sánchez MJ. Anticipated help‐seeking for cancer symptoms before and after the coronavirus pandemic: results from the Onco‐barometer population survey in Spain. Br J Cancer. 2021;124(12):2017‐2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hanna TP, King WD, Thibodeau S, et al. Mortality due to cancer treatment delay: systematic review and meta‐analysis. BMJ. 2020;371:m4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Supporting Information.
Data Availability Statement
The data that support the findings of our study are available from the corresponding author upon reasonable request.


