Abstract
Background
COVID‐19 patients were often transferred to other intensive care units (ICUs) to prevent that ICUs would reach their maximum capacity. However, transferring ICU patients is not free of risk. We aim to compare the characteristics and outcomes of transferred versus non‐transferred COVID‐19 ICU patients in the Netherlands.
Methods
We included adult COVID‐19 patients admitted to Dutch ICUs between March 1, 2020 and July 1, 2021. We compared the patient characteristics and outcomes of non‐transferred and transferred patients and used a Directed Acyclic Graph to identify potential confounders in the relationship between transfer and mortality. We used these confounders in a Cox regression model with left truncation at the day of transfer to analyze the effect of transfers on mortality during the 180 days after ICU admission.
Results
We included 10,209 patients: 7395 non‐transferred and 2814 (27.6%) transferred patients. In both groups, the median age was 64 years. Transferred patients were mostly ventilated at ICU admission (83.7% vs. 56.2%) and included a larger proportion of low‐risk patients (70.3% vs. 66.5% with mortality risk <30%). After adjusting for age, APACHE IV mortality probability, BMI, mechanical ventilation, and vasoactive medication use, the hazard of mortality during the first 180 days was similar for transferred patients compared to non‐transferred patients (HR [95% CI] = 0.99 [0.91–1.08]).
Conclusions
Transferred COVID‐19 patients are more often mechanically ventilated and are less severely ill compared to non‐transferred patients. Furthermore, transferring critically ill COVID‐19 patients in the Netherlands is not associated with mortality during the first 180 days after ICU admission.
Keywords: COVID‐19, intensive care unit, intrahospital transfer, mortality, severity of illness
Editorial Comment.
This nationwide Dutch cohort study provides a description of transferred versus non‐transferred COVID‐19 ICU patients, and found that transfer was not associated with additional risk for mortality. Whether it is indeed safe to transfer ICU patients with COVID‐19 between hospitals or whether it represents confounding by indication is unknown.
1. INTRODUCTION
During the coronavirus disease 2019 (COVID‐19) pandemic, there was a strong focus on accommodating the growing number of COVID‐19 patients on the limited number of available intensive care unit (ICU) beds. Therefore, ICUs expanded their regular number of beds to accommodate all patients. Moreover, to prevent that ICUs would reach their maximum capacity and thereby compromise the quality of provided care, patients were also transferred to other ICUs. 1 , 2 , 3 , 4 , 5
In general, transferring ICU patients is not free of risk. 6 , 7 , 8 Well‐prepared relocations with specialist transportation teams and in accordance with guidelines may reduce the risks associated with patient transfers. 8 , 9 , 10 , 11 , 12 Although individual patients who were transferred in the COVID‐19 period were in general stable enough for the transfer, they were still in need of critical care. Especially mechanically ventilated patients have an increased risk of complications. 7 , 10 , 13 Transferring COVID‐19 patients in particular has an additional challenge of preventing further spread of the coronavirus.
In the Netherlands, ICU‐patient transfers usually take place within the regional acute care networks, but during the pandemic, a national taskforce was specifically appointed to facilitate interregional transfers of COVID‐19 patients. 14 Mobile ICUs in conjunction with specialist transportation teams were used to transport (COVID‐19) patients between hospitals. 5 , 8 The Netherlands is a small country and transport is mainly over road but over larger distances (>100 km), patients were sometimes transferred using helicopters. 15 , 16 , 17
The emphasis of these transfers lied on ensuring equal distribution of resources between hospitals. However, it is unclear which COVID‐19 patients were transferred and whether the transfer of these patients was associated with higher or lower mortality at the hospital and in the months after discharge. In this study, we aimed to compare the characteristics and outcomes of transferred and non‐transferred COVID‐19 ICU patients during the COVID‐19 epidemic in the Netherlands.
2. MATERIALS AND METHODS
2.1. Data source
Data were obtained from the Dutch National Intensive Care Evaluation (NICE) registry, a quality registry covering all Dutch ICUs. 18 , 19 All ICUs collect at least the minimal dataset (MDS) for each admitted patient. The MDS describes the severity of illness based on, among others, demographics, comorbidities, the Acute Physiology and Chronic Health Evaluation (APACHE) IV model 20 as well as outcome measures such as ICU and in‐hospital mortality, length of stay, and ICU readmission.
On behalf of the Dutch government, the NICE registry additionally collected information on the COVID‐19 patients admitted to the ICU or general ward in a separate registration module. This made it possible to track the patients transferred between the hospitals and calculate the total length of stay over multiple admission periods and overall mortality. The study dataset was anonymized and consisted of, among others, the COVID‐19 status of the patient (i.e., “laboratory confirmed,” “CT‐scan confirmed,” “suspected,” or “negative”), all consecutive ICU admission and discharge dates, APACHE IV severity of illness score, also known as the APACHE III severity of illness score, APACHE IV mortality probability based on the first ICU admission and the final hospital mortality status of the last hospital in case of transfer. In addition, death events after hospital discharge were established using the Vektis claims database. Health insurance is mandatory for all Dutch inhabitants and almost all (97%) of the Dutch inhabitants have private healthcare insurance. 21 The Vektis databases contain reimbursement data on all medical treatments paid for by Dutch insurance companies, as well as demographic information, such as gender, date of birth, and date of death, for all registered residents of the Netherlands. 22 For all deceased Dutch patients in the study period, we received the date of death from Vektis and this made it possible to derive the mortality during the first 180 days following ICU admission.
2.2. Inclusion and exclusion criteria
We included COVID‐19 patients admitted to Dutch ICUs between March 1, 2020, and July 1, 2021. Patients with a positive SARS‐CoV‐2 RT‐PCR test (i.e., reverse transcription‐polymerase chain reaction [RT‐PCR] on a nasopharyngeal swab) or a CT‐scan consistent with COVID‐19 (i.e., a CO‐RADS score of ≥4) in combination with the clinical diagnosis of viral pneumonia as described in Prokop et al. 23 during their (first) ICU admission were included. Patients with an ICU length of stay shorter than 4 h, missing core clinical MDS data, and patients with admission type elective or emergency surgery were excluded. Patients with a medical admission type and a non‐COVID‐19 related APACHE IV diagnosis were also excluded (Table S1). Last, patients for whom the APACHE IV exclusion criteria were applicable, were excluded from all analyses which include the APACHE IV mortality probability. These criteria included: patients with an ICU length‐of‐stay less than 4 h or longer than 1 year; readmissions; patients with missing admission diagnosis or admission type; patients with burns; transplant patients or Coronary Care Unit or recovery patients. 20
For each patient, we labeled whether they were transferred between ICUs or not. We considered a transfer as a continuation of ICU treatment in an ICU of a different hospital. Of note: Patients who were transferred from a general ward of a hospital to an ICU of another hospital, or vice versa, were not considered as (ICU) transfers. Patients for whom the difference between the discharged date in the first ICU and admission date in the second ICU was less than 3 days, presumably due to administrative errors, were considered as transfers. Patients readmitted to an ICU after three or more days were considered new admissions.
2.3. Ethics approval
The medical ethics review committee of the Amsterdam University Medical Centers reviewed the proposal for this study and waived the need for informed consent (reference number W21_371 # 21.412).
2.4. Statistical analyses
Categorical variables are presented using absolute and relative frequencies while continuous variables are presented using mean and standard deviation or median and interquartile range (IQR) depending on their distribution. Baseline patient characteristics and outcomes were compared between the two patient groups (non‐transferred and transferred patients). Furthermore, we used chord diagrams to display the influx and efflux of patients to and from each type of hospital (i.e., teaching academic hospital, a hospital directly affiliated to a university; teaching non‐academic hospital, a non‐university hospital that is linked with a medical school, where medical students and newly qualified doctors receive practical training; and general peripheral hospitals which are all non‐teaching hospitals). Thereby, we stratified the transferred patients in age groups (<60 and ≥60 years) and severity of illness groups (<30% vs. ≥30%–70% vs. ≥70% APACHE IV risk of mortality) to illustrate potential different transfer patterns for young versus older patients and less and more severely ill patients.
We assessed crude cumulative mortality risks by Kaplan–Meier survival estimates for the transferred and non‐transferred patients. We used left‐truncation by excluding the follow‐up time between ICU admission and moment of transfer for the transferred patients. This was done to tackle the problem of “immortal time bias,” that is, those who were transferred were not observable at risk of death during the time interval between admission and transfer, and this may involve an artificial survival time advantage for those who were transferred. A multivariable Cox regression model was used to analyze the risk of transfer on mortality during the first 180 days after admission. The adjusted hazard ratio with 95% confidence interval was calculated. To determine the patient characteristics to adjust for, we drew a Directed Acyclic Graph (DAG) to make a representation of the direct causal effects of one patient characteristic on another. 24 The patient characteristics we considered were based on literature and availability in our dataset: age, APACHE IV mortality probability, BMI, diabetes, gender, mechanical ventilation in the first 24 h of ICU admission, and use of vasoactive medication. The identified factors that may confound the association between transfer and mortality were added to the Cox regression model as covariates. The continuous covariates were included in the model as splines.
All statistical analyses were performed using R version 3.6.0. The DAG was drawn and analyzed in DAGitty version 3.0. 25
3. RESULTS
Between March 1, 2020, and July 1, 2021, 12,670 patients with a positive COVID‐19 status were admitted to one of the 73 Dutch ICUs in 68 hospitals that treated COVID‐19 ICU patients in the Netherlands. Of these, 1943 patients had an ICU length of stay shorter than 4 h or had no clinical data available in the NICE database and were therefore excluded. Furthermore, 165 surgery admissions, 349 patients with a non‐COVID‐related APACHE IV reason for admission diagnoses, and four patients without a hospital mortality status were excluded. Of the remaining 10,038 patients, 7244 (72.2%) were classified as non‐transferred patients while 2794 (27.8%) were classified as transferred (Figure 1). An additional 171 patients met at least one of the APACHE IV exclusion criteria and were excluded from all analyses concerning the APACHE IV mortality probability.
FIGURE 1.
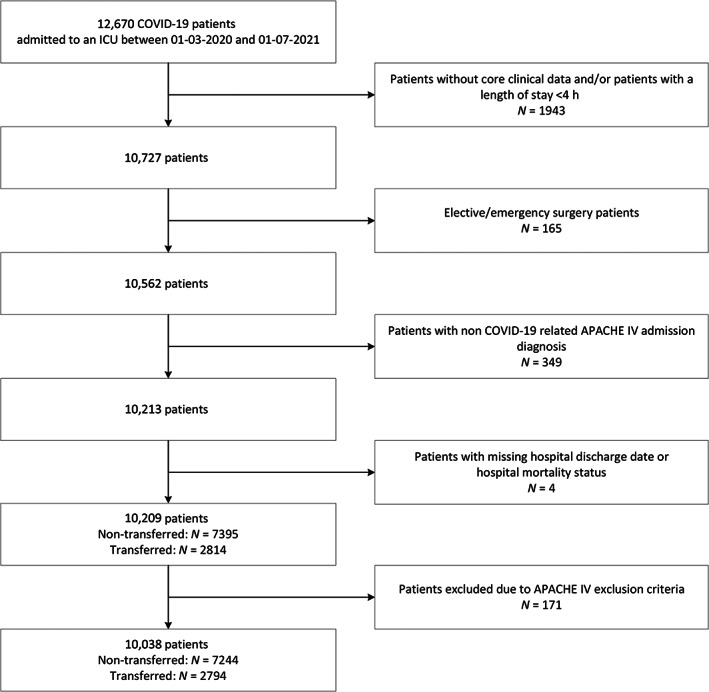
Patient selection. Flowchart illustrating the patient inclusion and exclusion criteria for this study. APACHE, Acute Physiology and Chronic Health Evaluation; COVID‐19, coronavirus disease 2019; ICU, intensive care unit
3.1. Patient characteristics
The characteristics and outcomes of the included patients are shown in Table 1. Most patients were male (69.2% and 72.2% in the non‐transferred and transferred group, respectively). The median age in both groups was 64 years. The proportion of patients who received mechanical ventilation in the first 24 h of ICU admission was higher in the transferred group than in the non‐transferred group (83.7% vs. 56.2%). The non‐transferred group had a higher proportion of high‐risk patients (i.e., APACHE IV mortality probability >70%). Most transferred patients had no comorbidity (63.3% vs. 57.4%), but both patient groups had the same median APACHE IV mortality probability (20%). In both groups, the proportion of hospital mortality was comparable (27.6% of non‐transferred patients and 26.8% of transferred patients), while the transferred patients had a longer median ICU length of stay compared to the non‐transferred patients (18 vs. 10 days). The percentages for the 180‐day mortality of the non‐transferred and the transferred group were also comparable (23.6% vs. 23%).
TABLE 1.
Characteristics and outcomes of non‐transferred and transferred COVID‐19 patients
| Non‐transferred | Transferred | |
|---|---|---|
| Number of patients, N (%) | 7395 (72.4) | 2814 (27.6) |
| Sex = Male, N (%) | 5118 (69.2) | 2033 (72.2) |
| Age, median (IQR) | 64.0 (56.0–72.0) | 64.0 (57.0–71.0) |
| Age group | ||
| <40 | 318 (4.3) | 87 (3.1) |
| 40–45 | 206 (2.8) | 60 (2.1) |
| 45–50 | 381 (5.2) | 148 (5.3) |
| 50–55 | 701 (9.5) | 278 (9.9) |
| 55–60 | 998 (13.5) | 367 (13) |
| 60–65 | 1119 (15.1) | 486 (17.3) |
| 65–70 | 1285 (17.4) | 514 (18.3) |
| 70–75 | 1356 (18.3) | 542 (19.3) |
| 75–80 | 806 (10.9) | 280 (10) |
| 80–85 | 204 (2.8) | 50 (1.8) |
| ≥85 | 21 (0.3) | 2 (0.1) |
| Mechanical ventilation in 1st 24 h of ICU admission, N (%) | 4159 (56.2) | 2354 (83.7) |
| APACHE III‐score, median (IQR) | 60.0 (49.0–72.0) | 58.0 (47.0–69.0) |
| APACHE IV mortality probability, median (IQR) | 0.2 (0.1–0.4) | 0.2 (0.1–0.3) |
| APACHE IV mortality probability | ||
| <0.3 | 4814 (66.5) | 1964 (70.3) |
| 0.3–0.7 | 2175 (30) | 779 (27.9) |
| ≥0.7 | 255 (3.5) | 51 (1.8) |
| BMI, median (IQR) | 28.6 (25.7–32.4) | 28.7 (26.0–32.4) |
| BMI group | ||
| <18.5 | 33 (0.4) | 4 (0.1) |
| 18.5–25 | 1413 (19.1) | 511 (18.2) |
| 25–30 | 3000 (40.6) | 1169 (41.5) |
| 30–35 | 1833 (24.8) | 727 (25.8) |
| 35–40 | 744 (10.1) | 285 (10.1) |
| >40 | 372 (5) | 118 (4.2) |
| Vasoactive medication = yes, N (%) | 3351 (45.3) | 1878 (66.7) |
| Acute renal failure = yes, N (%) | 545 (7.4) | 130 (4.6) |
| Comorbidities | ||
| Diabetes = yes, N (%) | 1740 (23.5) | 589 (20.9) |
| Immunological insufficiency = yes, N (%) | 753 (10.2) | 185 (6.6) |
| Renal failure = yes, N (%) | 337 (4.6) | 73 (2.6) |
| COPD/Respiratory insufficiency = yes, N (%) | 968 (13.1) | 346 (12.3) |
| Malignancy/hematological malignancy = yes, N (%) | 204 (2.8) | 33 (1.2) |
| Comorbidities | ||
| None | 4243 (57.4) | 1782 (63.3) |
| 1 | 2314 (31.3) | 848 (30.1) |
| >1 | 838 (11.3) | 184 (6.5) |
| Died in hospital, N (%) | 2040 (27.6) | 754 (26.8) |
| Died 180 days after ICU admission, N (%) | 2142 (29.0) | 787 (28.0) |
| ICU length of stay in 1st ICU before transfer, median (IQR) | ‐ | 3.0 (2.0–5.0) |
| Total ICU length of stay, median (IQR) | 10.0 (5.0–19.0) | 18.0 (11.0–32.0) |
| Hospital length of stay in days, median (IQR) | 18.0 (11.0–31.0) | 26.0 (17.0–42.0) |
Abbreviations: APACHE, Acute Physiology and Chronic Health Evaluation; BMI, body mass index; COPD, chronic obstructive pulmonary disease; ICU, intensive care unit; IQR, interquartile range.
3.2. Transfer patterns
Figure 2 displays the number of transfers between hospital types. Most transfers of COVID‐19 patients took place from non‐academic teaching hospitals to academic hospitals (20.4%, N = 573), from general peripheral hospitals to academic hospitals (19.8%, N = 557), and between non‐academic teaching hospitals (16.4%, N = 470). There was also a small number (N = 46) of COVID‐19 ICU patients transferred from Dutch ICUs to German ICUs, indicated as “other” in Figure 2 (see also Table S2).
FIGURE 2.
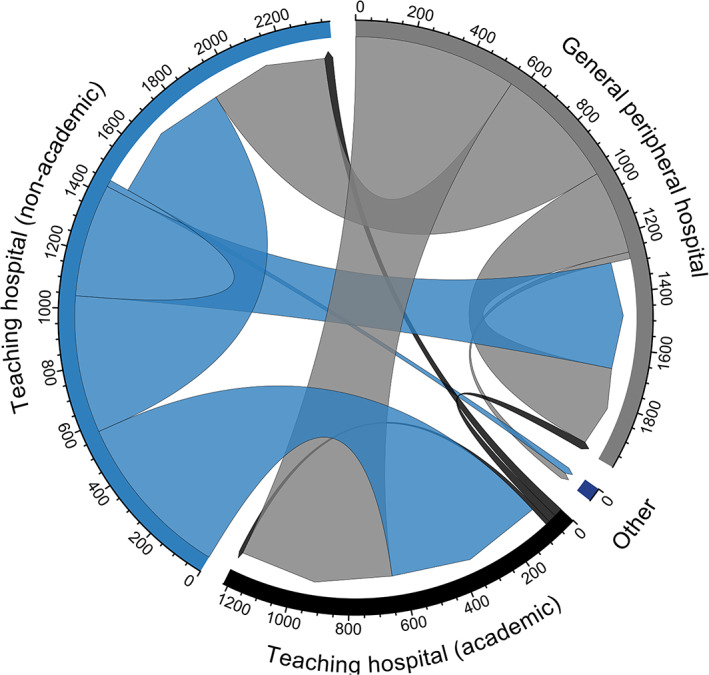
Number of COVID‐19 transfers between hospital types. (Gray) Number of transfers from general peripheral hospitals to other hospital types. For example, at the top right >550 patients were transferred from general peripheral hospitals to academic teaching hospitals and >250 patients were transferred within general peripheral hospitals. (Black) Number of transfers from teaching hospitals (academic) to other hospital types. For example, <100 patients were transferred from academic teaching hospitals to general peripheral hospitals, non‐academic teaching hospitals, and other academic hospitals. (Blue) Number of transfers from teaching hospitals (non‐academic) to other hospital types. For example, from the bottom left >550 patients are transferred from non‐academic teaching hospitals to academic hospitals
Considering the patients' age, we see that the largest proportions of transfers in the patient group younger than 60 took place from general peripheral hospitals to academic hospitals and from non‐academic teaching hospitals to academic hospitals (21.9% and 21.7%, Figure S1). In the group 60 or older, we see a similar pattern (Figure S1 and Table S3).
Figure S2 shows the transfer patterns between different hospital types stratified by APACHE IV mortality probability. In the high‐risk group, the number of transferred patients (N = 51) was much lower than in the middle (N = 779) and low‐risk (N = 1964) groups. A total of 20.9% of the low‐risk patients and 19.4% of the middle‐risk patients were transferred from non‐academic teaching hospitals to academic hospitals while 27.5% of high‐risk patients were transferred between non‐academic teaching hospitals (Table S4).
3.3. 180‐day mortality
The crude probabilities of survival of transferred patients and non‐transferred patients at 180 days after ICU admission were comparable (Figure 3).
FIGURE 3.
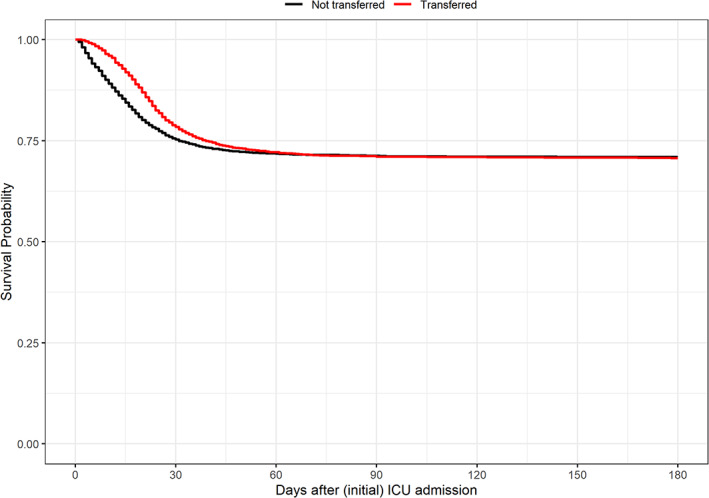
180‐day survival of transferred and non‐transferred COVID‐19 patients. Kaplan–Meier survival curves of transferred (red) and non‐transferred (black) COVID‐19 patients. Left truncation was applied, so the survival curve of the non‐transferred patients starts at the first day of intensive care unit (ICU) admission while the survival curve of the transferred patients starts at the day of the first ICU transfer
According to the unadjusted left‐truncated model, the hazards of mortality in the transferred group were comparable to those in the non‐transferred group (HR [95% CI] = 1.02 [0.94–1.11]). Based on the resulting DAG (Figure S3), the following variables were identified as potential confounders: age, BMI, mechanical ventilation in 1st 24 h of ICU admission, and vasoactive medication. After correcting for these factors, the hazard ratio of mortality for transferred COVID‐19 patients compared to non‐transferred COVID‐19 patients remained similar (HRadj [95% CI] = 0.99 [0.91–1.08]) (Table 2).
TABLE 2.
Results of Cox regression models on the association of transfer of COVID‐19 patients on 180‐day mortality
| Cox model | Covariate | HR (95% CI) | HRadj a (95% CI) |
|---|---|---|---|
| Overall | Transferred | 1.02 (0.94–1.11) | 0.99 (0.91–1.08) |
| <30 days | Transferred | ‐ | 0.86 (0.78–0.95) |
| ≥30 days | Transferred | ‐ | 1.67 (1.40–1.99) |
Abbreviations: CI, confidence interval; HR, hazard ratio; HRadj, adjusted hazard ratio.
Model adjusted for age, APACHE IV mortality probability, BMI, mechanical ventilation in 1st 24 h of ICU admission and vasoactive medication.
Figure 3 also shows a difference between both survival curves that declines during the follow‐up, which makes the assumption of proportional hazards questionable. This prompted us to do a post hoc analysis by calculating the adjusted hazard ratio's for different periods separately. An interaction term of {time × transfer} was added to the left truncated adjusted Cox model. This interaction term consisted of “transfer” (yes/no) with a dichotomous period variable indicating the period before 30 days or after 30 days. The HRadj before the 30‐day period was 0.86 (0.78–0.95) and the HRadj after 30 days was 1.67 (1.40–1.99).
4. DISCUSSION
In this study, we compared the characteristics and outcomes of transferred and non‐transferred COVID‐19 patients in the Netherlands. We found that transferred COVID‐19 patients were mostly ventilated in the first 24 h of ICU admission, had a larger proportion of low‐risk patients, and had a longer ICU length of stay compared to non‐transferred patients. Most of the patients in the transferred group had no comorbidities and this group had the same median APACHE IV mortality probability as non‐transferred patients. Most transfers of COVID‐19 patients took place from non‐academic teaching hospitals or peripheral hospitals to academic hospitals. We also found that transferring critically ill COVID‐19 patients is not associated with increased 180‐day mortality.
The adjusted hazard ratio before 30 days (0.86 [0.78–0.95]) indicates a more favorable outcome for the transferred patients compared to the non‐transferred patients. The hazard ratio after 30 days (1.67 [1.40–1.99]) shows the opposite. We have made the distinction between <30 days and ≥30 days because we see that the Kaplan–Meier curves of the transferred and non‐transferred group converge and at roughly the 30‐day point the steepness (which indicates the hazard) of the curves change. This means that the HR is not constant during the follow‐up, and this violation of the proportional hazards assumption was taken into account by inclusion of a term for interaction of {time × transfer status}. From the hazard ratio's <30 days and ≥30 days we see that the hazard of death during the first 30 days is more favorable in the transferred group compared to the non‐transferred group, and thereafter this is reversed. So, there is a survival benefit during the first 30 days among those with early transfer compared to non‐transferred controls. At later stages, with increasing numbers of those with later transfer, there is a survival disadvantage compared to non‐transferred controls. However, the difference between early and late stages is subtle. The Kaplan–Meier curves fully converge around Day 60, and, thus, there is hardly any difference in eventual survival prospect between transferred and non‐transferred patients.
Our finding that transferring critically ill COVID‐19 patients is not associated with (180‐day) mortality was similar to previous studies, which focused on critically ill patients in general. 10 , 13 , 26 Although they do not report on mortality after discharge, they found neither an association between intrahospital transfer and hospital mortality nor found a statistically significant difference in hospital mortality between transferred and non‐transferred patients. A study, Painvin et al., which does focus on COVID‐19 patients specifically, also found that transferring COVID‐19 patients did not lead to higher mortality. 4 They corrected for the occurrence of acute kidney injury, number of proning sessions and duration of mechanical ventilation. We only have information on whether the patient was ventilated (at admission/in the first 24 h of admission), so we could not fully repeat their analyses. Yet, we used clinical input and DAG analyses to identify and adjust for important confounders available in our dataset.
A strength of our study is that we could obtain a large and nationwide sample of COVID‐19 patients in the Netherlands and track in which hospitals each patient was (consecutively) admitted. Additionally, we repeated our analyses without the ICU patients that were discharged from the ICU within the first day or the first 3 days of ICU admission; herewith, excluding the patients who otherwise had no chance of being transferred to another ICU. The results from these additional confirmed our initial results.
This study also has several limitations. First, no distinction could be made between transfers due to limited ICU capacity or transfers due to the complexity of the patient's disease and the continuation of care in a more specialized ICU. However, due to the national policy to transfer to ensure equal distribution of resources between hospitals, we assume that most of the transfers during the COVID‐19 surges were triggered by the limited ICU capacity. Second, patients were classified into two groups “transferred” and “non‐transferred” and in the transferred group, no further distinction was made between how often a patient was transferred, as the proportion of patients transferred more than once was low (3.8%, Figure S4). A transfer to and back from an ICU in another hospital could indicate that the initial transferring ICU temporarily had limited capacity or that a patient temporarily went to another hospital for (specialized) treatment, however, not all transferred patients receiving (more specialized) treatment in another hospital were transferred back to the initial ICU. Third, we did not take the distance between hospitals into account in our analyses. In general, the larger the distance between hospitals, the more time is needed to transfer the patient but this also depends on transportation mode. We were unable to include this information in our models because information on different modes of transportation (by road or by air) was not available. Fourth, it is reasonable to assume that intensivists decide which patient can be or need to be transferred to another ICU based on information that is not available in our dataset. Although we robustly identified potential confounders by modeling confounders by a DAG, there is a risk of residual confounding as our dataset only included a limited number of data items. Last, we had no information on whether incidents or deaths occurred during the patient's transfer. In this study, we focused on 180‐day mortality as the outcome and found no association between transfer and mortality. However, some studies like Schwebel et al. found that although there were no associations between transferring critically ill patients on mortality, there were occurrences of complications such as pneumothorax, ventilator‐associated pneumonia, hypoglycemia and hyperglycemia. 13 This could mean that we should also consider that transferring patients also has potential risk of complications.
Although our results suggest that in a surge of COVID‐19 cases or maybe even other infectious outbreaks in the future and when resources are limited, patients can be transferred to other hospitals, we have also seen that the transferred patient group have a longer median length of stay and thus use more resources. Further research into possible subgroup effects could be useful to determine the effects of specific COVID‐19 patient groups on hospital‐ or long‐term mortality and resource use.
5. CONCLUSIONS
Transferred COVID‐19 patients are more often mechanically ventilated at admission, are less severely ill but have a longer ICU stay compared to non‐transferred patients. Furthermore, the transfer of critically ill COVID‐19 patients in the Netherlands is not associated with 180‐day mortality.
AUTHOR CONTRIBUTION
Safira A. Wortel: Conceptualization, Data curation, Formal analysis, Methodology, Writing ‐ original draft. Ferishta Bakhshi‐Raiez: Conceptualization, Data curation, Methodology, Writing ‐ review & editing. Fabian Termorshuizen: Conceptualization, Methodology, Writing ‐ review & editing. Dylan W. deLange: Conceptualization, Writing ‐ review & editing. Dave A. Dongelmans: Conceptualization, Writing ‐ review & editing. Nicolette F. de Keizer: Conceptualization, Methodology, Resources, Supervision, Writing ‐ review & editing.
FUNDING INFORMATION
This research was funded by the Netherlands Organization for Health Research and Development (ZonMw) COVID‐19 Program in the bottom‐up focus area 1 “Predictive diagnostics and treatment” for theme 3 “Risk analysis and prognostics” (project number 10430 01 201 0011: IRIS). The funder had no role in the design of the study or in the writing of the manuscript.
CONFLICT OF INTEREST
Nicolette F. de Keizer, Dylan W. de Lange, and Dave A. Dongelmans are members of the board of the Dutch National Intensive Care Evaluation (NICE) foundation. The NICE foundation pays the Department of Medical Informatics, Amsterdam UMC, for processing data of all Dutch ICUs into audit and feedback information. Safira A. Wortel, Nicolette F. de Keizer, Fabian Termorshuizen, and Ferishta Bakhshi‐Raiez are employees of the Department of Medical Informatics and work on the NICE project.
Supporting information
Table S1 Included APACHE IV admission diagnoses
Table S2 Transfers between hospital types
Figure S1: Proportions of COVID‐19 transfers between hospital types stratified by age groups <60 and ≥60 years
Table S3 Transfer between hospital Types stratified by age groups
Figure S2 Proportions of intra‐hospital transfers of COVID‐19 patients between hospital types stratified by APACHE IV mortality probability groups <0.3, between 0.3 and 0.7 and ≥0.7
Table S4 Transfer between hospital Types stratified by APACHE IV mortality probability
Figure S3: Directed Acyclic Graph—(A) Unadjusted and (B) adjusted for age, APACHE IV mortality probability, BMI, Mechanical ventilation in first 24 h of ICU admission and vasoactive medication
Figure S4 Transfer patterns and their frequency. A, B, C, and D represent ICUs in a distinct hospital. Of the 10,209 COVID‐19 patients, 7395 were not transferred (72.4%), 2424 were transferred once (23.7%) while 390 patients were transferred more than once (3.8%).
ACKNOWLEDGMENTS
The authors would like to thank all the Dutch hospitals for their enormous efforts to collect data during the COVID‐19 pandemic. The authors would also like to thank Eric van der Zwan for his assistance in the record linkage for this study.
Wortel SA, Bakhshi‐Raiez F, Termorshuizen F, et al. Comparison of patient characteristics and long‐term mortality between transferred and non‐transferred COVID‐19 patients in Dutch intensive care units: A national cohort study. Acta Anaesthesiol Scand. 2022;66(9):1107‐1115. doi: 10.1111/aas.14129
Funding information ZonMw, Grant/Award Number: 10430 01 201 0011
Contributor Information
Safira A. Wortel, Email: s.a.wortel@amsterdamumc.nl.
the Dutch COVID‐19 Research Consortium:
M. S. Arbous, M. G. W. Barnas, A. J. G. H. Bindels, D. P. Boer, R. J. Bosman, G. B. Brunnekreef, M. Th. de Bruin, M. de Graaff, R. M. de Jong, A. R. de Meijer, W. de Ruijter, R. de Waal, A. Dijkhuizen, T. P. J. Dormans, A. Draisma, I. Drogt, B. J. W. Eikemans, P. W. G. Elbers, J. L. Epker, M. L. Erkamp, B. Festen‐Spanjer, T. Frenzel, D. Gommers, N. C. Gritters, I. Z. Hené, M. Hoeksema, J. W. M. Holtkamp, M. E. Hoogendoorn, A. P. I. Houwink, C. J. M. G. Jacobs, I. T. A. Janssen, H. Kieft, M. P. Koetsier, T. J. J. Koning, N. Kusadasi, J. A. Lens, J. G. Lutisan, D. J. Mehagnoul‐Schipper, D. Moolenaar, F. Nooteboom, R. V. Pruijsten, D. Ramnarain, A. C. Reidinga, E. Rengers, A. A. Rijkeboer, F. W. Rozendaal, R. M. Schnabel, V. M. Silderhuis, J. J. Spijkstra, P. Spronk, L. F. te Velde, L. C. Urlings‐Strop, B. C. T. van Bussel, A. E. van den Berg, R. van den Berg, P. H. J. van der Voort, E. M. van Driel, L. van Gulik, F. M. van Iersel, M. van Lieshout, E. R. van Slobbe‐Bijlsma, M. van Tellingen, J. Vandeputte, D. P. Verbiest, D. J. Versluis, E. Verweij, M. Vrolijk‐de Mos, and R. M. J. Wesselink
REFERENCES
- 1. The Dutch COVID‐19 Research Consortium . One year of COVID‐19 in The Netherlands ‐ a Dutch narrative. Neth J Crit Care. 2021;29:78‐84. [Google Scholar]
- 2. Mazzoli CA, Gamberini L, Lupi C, et al. Interhospital transfer of critically ill COVID‐19 patients: preliminary considerations from the Emilia‐Romagna experience. Air Med J. 2020;39(5):423‐426. [Google Scholar]
- 3. Pett E, Leung HL, Taylor E, et al. Critical care transfers and COVID‐19: managing capacity challenges through critical care networks. J Intensive Care Soc. 2022;23:203‐209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Painvin B, Messet H, Rodriguez M, et al. Inter‐hospital transport of critically ill patients to manage the intensive care unit surge during the COVID‐19 pandemic in France. Ann Intensive Care. 2021;11(1):1‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Berkeveld E, Mikdad S, Zandbergen HR, et al. Experience of the coronavirus disease (COVID‐19) patient care in the Amsterdam region: optimization of acute care organization. Disaster Med Public Health Prep. 2020;1–5:1194‐1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Beckmann U, Gillies DM, Berenholtz SM, Wu AW, Pronovost P. Incidents relating to the intra‐hospital transfer of critically ill patients. Intensive Care Med. 2004;30(8):1579‐1585. [DOI] [PubMed] [Google Scholar]
- 7. Parmentier‐Decrucq E, Poissy J, Favory R, et al. Adverse events during intrahospital transport of critically ill patients: incidence and risk factors. Ann Intensive Care. 2013;3(1):1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. van Lieshout EJ, Binnekade J, Reussien E, et al. Nurses versus physician‐led interhospital critical care transport: a randomized non‐inferiority trial. Intensive Care Med. 2016;42(7):1146‐1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bellingan G, Olivier T, Batson S, Webb A. Comparison of a specialist retrieval team with current United Kingdom practice for the transport of critically ill patients. Intensive Care Med. 2000;26(6):740‐744. [DOI] [PubMed] [Google Scholar]
- 10. Janz DR, Khan YA, Mooney JL, et al. Effect of interhospital ICU relocation on patient physiology and clinical outcomes. J Intensive Care Med. 2019;34(11–12):1010‐1016. [DOI] [PubMed] [Google Scholar]
- 11. Warren J, Fromm RE Jr, Orr RA, Rotello LC, Horst HM, Medicine AC of CC . Guidelines for the inter‐and intrahospital transport of critically ill patients. Crit Care Med. 2004;32(1):256‐262. [DOI] [PubMed] [Google Scholar]
- 12. Bergman L, Pettersson M, Chaboyer W, Carlström E, Ringdal M. Improving quality and safety during intrahospital transport of critically ill patients: a critical incident study. Aust Crit Care. 2020;33(1):12‐19. [DOI] [PubMed] [Google Scholar]
- 13. Schwebel C, Clec'h C, Magne S, et al. Safety of intrahospital transport in ventilated critically ill patients: a multicenter cohort study. Crit Care Med. 2013;41(8):1919‐1928. [DOI] [PubMed] [Google Scholar]
- 14. LCPS . Landelijk Coördinatiecentrum Patiënten Spreiding [Internet]. LCPS; 2022. Accessed April 10, 2022. https://lcps.nu/ [Google Scholar]
- 15. van Geffen G‐J, Spoelder E, Tacken M, Slagt C. Snel en efficiënt transport van coronapatiënten per helikopter; Helikopter Mobiel Medisch Team ondersteunt MICU‐transporten per helikopter [Internet]. Medisch Contact; 2021. Accessed April 10, 2022. https://www.medischcontact.nl/nieuws/laatste-nieuws/artikel/snel-en-efficient-transport-van-coronapatienten-per-helikopter-.htm [Google Scholar]
- 16. Spoelder EJ, Tacken MC, van Geffen G‐J, Slagt C. Helicopter transport of critical care COVID‐19 patients in The Netherlands: protection against COVID‐19 exposure‐a challenge to critical care retrieval personnel in a novel operation. Scand J Trauma Resusc Emerg Med. 2021;29(1):1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rikken QG, Mikdad S, Mota MTC, et al. Operational experience of the Dutch helicopter emergency medical services (HEMS) during the initial phase of the COVID‐19 pandemic: jeopardy on the prehospital care system? Eur J Trauma Emerg Surg. 2021;47(3):703‐711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. van de Klundert N, Holman R, Dongelmans DA, de Keizer NF. Data resource profile: the Dutch National Intensive Care Evaluation (NICE) registry of admissions to adult intensive care units. Int J Epidemiol. 2015;44(6):1850‐1850h. [DOI] [PubMed] [Google Scholar]
- 19. Dutch National Intensive Care Evaluation [Internet]. Stichting NICE; 2022. Accessed April 10, 2022. https://stichting-nice.nl/ [Google Scholar]
- 20. Zimmerman JE, Kramer AA, McNair DS, Malila FM. Acute Physiology and Chronic Health Evaluation (APACHE) IV: hospital mortality assessment for today's critically ill patients. Crit Care Med. 2006;34(5):1297‐1310. [DOI] [PubMed] [Google Scholar]
- 21. Centraal Bureau voor de Statistiek (CBS) StatLine . Basisverzekering (Zvw); kosten per persoon, inkomen [Internet]. Accessed April 10, 2022. https://opendata.cbs.nl/statline/#/CBS/nl/dataset/81827NED/table?ts=1649399662543
- 22. Vektis . Business intelligence centrum voor de zorg | Vektis.nl [Internet]. Vektis; 2022. Accessed April 10, 2022. https://www.vektis.nl [Google Scholar]
- 23. Prokop M, Van Everdingen W, van Rees Vellinga T, et al. CO‐RADS: a categorical CT assessment scheme for patients suspected of having COVID‐19—definition and evaluation. Radiology. 2020;296(2):E97‐E104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Greenland S, Pearl J, Robins JM. Causal diagrams for epidemiologic research. Epidemiology. 1999;10:37‐48. [PubMed] [Google Scholar]
- 25. Textor J, van der Zander B, Gilthorpe MS, Liśkiewicz M, Ellison GT. Robust causal inference using Directed Acyclic Graphs: the R package ‘dagitty’. Int J Epidemiol. 2016;45(6):1887‐1894. [DOI] [PubMed] [Google Scholar]
- 26. Barratt H, Harrison DA, Rowan KM, Raine R. Effect of non‐clinical inter‐hospital critical care unit to unit transfer of critically ill patients: a propensity‐matched cohort analysis. Crit Care. 2012;16(5):1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Included APACHE IV admission diagnoses
Table S2 Transfers between hospital types
Figure S1: Proportions of COVID‐19 transfers between hospital types stratified by age groups <60 and ≥60 years
Table S3 Transfer between hospital Types stratified by age groups
Figure S2 Proportions of intra‐hospital transfers of COVID‐19 patients between hospital types stratified by APACHE IV mortality probability groups <0.3, between 0.3 and 0.7 and ≥0.7
Table S4 Transfer between hospital Types stratified by APACHE IV mortality probability
Figure S3: Directed Acyclic Graph—(A) Unadjusted and (B) adjusted for age, APACHE IV mortality probability, BMI, Mechanical ventilation in first 24 h of ICU admission and vasoactive medication
Figure S4 Transfer patterns and their frequency. A, B, C, and D represent ICUs in a distinct hospital. Of the 10,209 COVID‐19 patients, 7395 were not transferred (72.4%), 2424 were transferred once (23.7%) while 390 patients were transferred more than once (3.8%).


