1. INTRODUCTION
Olfactory dysfunction (OD) impacts patients’ lives, and the causes for this are multiple. 1 It results in decreased flavor perception, and the inability to recognize spoiled food, hazardous odors, or volatile chemicals poses a health and safety risk. Compared with other causes of olfactory disorders, postinfectious OD is associated with a higher level of quality of life (QoL) impairment. Pre–coronavirus disease 2019 (COVID‐19) studies confirmed that QoL is reduced in patients with OD and recent studies show the impact of COVID‐19–related OD on patients’ QoL both in the short 2 and medium term. 3 To date, its long‐term consequences on QoL remain partially unexplored. We investigated by means of Sniffin’ Sticks (SS) test and validated QoL questionnaires the effects of persistent COVID‐19–related OD on QoL in patients with a history of OD longer than 1 year.
2. METHODS
We conducted a cross‐sectional study including patients with a history of mild to moderate COVID‐19 referred to our long‐COVID smell clinic between December 2020 and April 2022 for persistent COVID‐19–related OD. Patients with a preexisting history of OD or other pathologies known to affect olfaction were not included. The study was conducted in accordance with the 1996 Declaration of Helsinki and approved by the research ethics committee (reference 14/SC/1180).
Detailed characteristics of the population are reported in Table 1. Olfaction was assessed using the SS extended test, while self‐assessment of smell was performed using a visual analog scale (sVAS). 4 The 36‐Item Short Form Health Survey (SF‐36) was chosen for the measurement of QoL, while the brief version of the Questionnaire of Olfactory Disorders‐Negative Statements (brief QOD‐NS) 5 was used to quantify the smell loss symptoms’ effect on patients’ QoL. Sinonasal symptoms were evaluated using the Sino‐Nasal Outcome Test‐22 (SNOT‐22).
TABLE 1.
Detailed characteristics and differences in olfaction and questionnaires scores for normosmic and dysosmic patients
| Normosmia (n = 14) | Dysosmia (n = 46) | p Value | |
|---|---|---|---|
| General characteristics | |||
| Age (mean ± SD) (years) | 44.4 ± 12.3 | 43.0 ± 13.1 | 0.71 |
| Sex, n (%) | |||
| Men | 7 (50.0) | 13 (28.3) | 0.19 |
| Women | 7 (50.0) | 33 (71.7) | |
| Duration of smell loss (mean ± SD) (days) | 405.4 ± 151.8 | 431.1 ± 198.5 | 0.66 |
| Parosmia, n (%) | |||
| No | 2 (14.3) | 8 (17.4) | >0.99 |
| Yes | 12 (85.7) | 38 (82.6) | |
| Phantosmia, n (%) | |||
| No | 10 (71.4) | 29 (63.0) | 0.75 |
| Yes | 4 (28.6) | 17 (37.0) | |
| Smoking status, n (%) | |||
| Never smoked | 11 (78.6) | 38 (82.6) | |
| Have smoked | 3 (21.4) | 8 (17.4) | 0.71 |
| Current | 1 (7.1) | 4 (8.7) | |
| Quit | 2 (14.3) | 4 (8.7) | |
| Comorbidities, n (%) | |||
| None | 9 (64.3) | 33 (71.7) | 0.74 |
| Yes | 5 (35.7) | 13 (28.3) | |
| Hypothyroidism | 2 | 2 | |
| Hypertension | 1 | 3 | |
| Hyperlipidemia | 1 | 2 | |
| Diabetes | 1 | 2 | |
| Allergic rhinitis | 1 | 1 | |
| Migraine | 2 | 0 | |
| Others | 3 | 15 | |
| Previous nasal operations, n (%) | |||
| No | 14 (100.0) | 39 (84.8) | 0.18 |
| Yes | 0 (0) | 7 (15.2) | |
| Rhinitis, n (%) | |||
| No | 8 (57.1) | 35 (76.1) | 0.19 |
| Yes | 6 (42.9) | 11 (23.9) | |
| Medication use, n (%) | |||
| None | 13 (92.9) | 37 (80.4) | 0.43 |
| Yes | 1 (7.1) | 9 (19.6) | |
| α‐Blockers | 0 | 0 | |
| Sartans | 0 | 0 | |
| Dicumarolics | 0 | 0 | |
| Antiplatelet drugs | 0 | 2 | |
| Biguanides | 0 | 0 | |
| Antidepressants | 0 | 2 | |
| Others | 1 | 8 | |
| Olfactory test and PROMs | |||
| Sniffin’ Sticks score (median [IQR]) | |||
| TDI | 32.0 [31.4 – 33.6] | 23.3 [20.4 – 28.3] | <0.0001**** |
| Threshold | 6.8 [5.8 – 7.6] | 4.6 [1.4 – 5.5] | <0.0001**** |
| Discrimination | 14.0 [13.0 – 15.0] | 10.0 [8.0 – 12.0] | <0.0001**** |
| Identification | 12.0 [11.0 – 13.0] | 10.0 [7.8 – 11.0] | 0.0002*** |
| SNOT‐22 (median [IQR]) | |||
| Total SNOT‐22 score | 16.5 [10.0 – 30.5] | 23.0 [12.5 – 46.0] | 0.23 |
| Rhinologic symptoms a | 4.5 [1.0 – 6.0] | 2.0 [0 – 5.0] | 0.11 |
| Extranasal rhinologic symptoms | 0 [0 – 3.0] | 0 [0 – 1.0] | 0.45 |
| Ear/facial symptoms | 0 [0 – 4.3] | 1.0 [0 – 4.0] | 0.49 |
| Psychological dysfunction | 5.0 [1.8 – 10.0] | 10.5 [5.0 – 21.3] | 0.03* |
| Sleep dysfunction | 4.0 [2.3 – 10.3] | 9.5 [1.3 – 16.0] | 0.24 |
| Loss of smell or taste | 3.0 [2.8 – 4.0] | 4.0 [3.0 – 5.0] | 0.01** |
| SF‐36 (mean ± SD or median [IQR]) b , (%) | |||
| Physical functioning | 95.0 [85.0 – 100] | 95.0 [80.0 – 100] | 0.82 |
| Role limitations because of physical health | 100 [100 – 100] | 100 [43.8 – 100] | 0.11 |
| Role limitations because of emotional problems | 100 [66.7 – 100] | 66.7 [25.0 – 100] | 0.07 |
| Energy/fatigue | 67.9 ± 12.7 | 46.5 ± 18.1 | 0.0001**** |
| Emotional well‐being | 77.4 ± 16.4 | 63.2 ± 18.2 | 0.01** |
| Social functioning | 84.8 ± 20.3 | 68.8 ± 28.6 | 0.05* |
| Pain | 90.0 [67.5 – 92.5] | 85.0 [67.5 – 100] | 0.80 |
| General health | 74.6 ± 12.9 | 65.2 ± 21.8 | 0.05* |
| QOD‐NS (mean ± SD) | 15.1 ± 4.8 | 8.5 ± 5.5 | 0.0001**** |
| sVAS (mean ± SD) | 7.5 ± 0.5 | 3.3 ± 2.2 | <0.0001**** |
Significant p values in bold. Levels of significance *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001, **** p ≤ 0.0001.
The item “loss of smell or taste” was excluded from the rhinologic symptoms domain and presented separately.
Abbreviations: PROMs, patient‐reported outcome measures; QOD‐NS, brief version of the Questionnaire of Olfactory Disorders‐Negative Statements; SF‐36, 36‐Item Short Form Health Survey; SNOT‐22, Sino‐Nasal Outcome Test‐22; sVAS, visual analog scale for sense of smell (0 represents “sense of smell absent” and 10 “sense of smell not affected”).
Results of mean ± standard deviation (SD) or median [interquartile range (IQR)] have been reported according to data distribution (normal vs non‐normal distribution).
Variables were compared using unpaired t test and Mann‐Whitney test. Fisher exact test was used to investigate associations between variables in normosmic and dysosmic patients. Linear regression analysis was used to explore correlations between odor threshold (T), odor discrimination (D), and odor identification (I) (TDI) scores and questionnaire outcomes.
3. RESULTS
Sixty patients completed the assessment and were included in the study (Table1). None of the patients had other long‐haul COVID‐19 symptoms apart from OD. Patients were categorized into three groups based on their TDI score: 14 normosmics (patients who reported OD but showed normal SS scores [TDI ≥30.75]), 39 hyposmics (16 < TDI < 30.75), and seven anosmics (TDI ≤16). For comparative analysis, patients with hyposmia and anosmia were combined and classified as “dysosmics” (n = 46).
No differences in the general characteristics and qualitative OD (parosmia/phantosmia) were observed between normosmics and dysmosmics. Dysosmics showed significantly lower scores (worse outcomes) in the SF‐36 domains “energy/fatigue,” “emotional well‐being,” “social functioning,” and “general health,” and brief QOD‐NS, sVAS, and SS scores, with threshold being the most affected SS subtest. Similarly, dysosmics had higher scores (worse outcomes) in SNOT‐22 subdomains “psychological dysfunction” and “loss of smell or taste” (Table 1, Figure 1). A significant linear correlation was found between the SS and SF‐36 domain “energy/fatigue” (p < 0.01 for all but identification, which was not significant) and sVAS (p = 0.03 for identification and p < 0.01 for the remaining SS scores).
FIGURE 1.
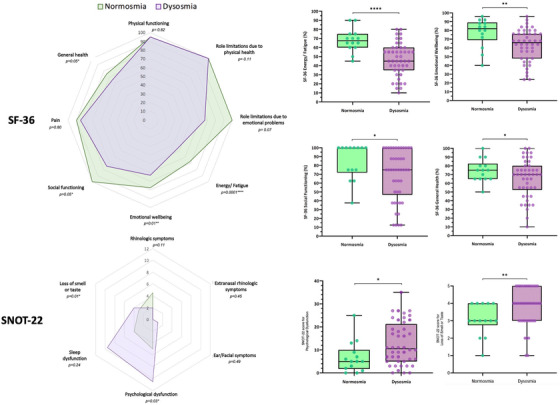
Results from 36‐Item Short Form Health Survey (SF‐36) and Sino‐Nasal Outcome Test‐22 (SNOT‐22) radar charts (left) showing median scores for normosmic and dysosmic patients and their p values. Box plots (right) demonstrate SF‐36 and SNOT‐22 domains with significant differences between the two groups.
4. DISCUSSION
Our study offers the longest follow‐up (14 months) at which effects of COVID‐19–related OD on QoL have been measured. Dysosmic patients demonstrated worse QoL scores when compared with normosmics, especially in the SF‐36 domains “energy/fatigue,” “emotional well‐being,” and “social functioning,” which confirms the difficulties these patients report in their everyday activities. 6 However, when we looked at the SF‐36 scores of those who recovered their sense of smell (normosmics), these were found to be within the normative values for the UK population, 7 suggesting how olfactory recovery could contribute to improving QoL.
Our data highlight a significantly higher psychological dysfunction in dysosmic patients according to SNOT‐22 when compared with normosmics, corroborating previous findings demonstrated both in the medium 8 and long term. 9 At 14 months from OD onset, dysosmic patients continued to have lower SS scores with a linear correlation demonstrated with the SF‐36 domain “energy/fatigue,” which could reflect the general level of debility commonly reported by dysosmic patients. However, both the identification and, more significantly, the threshold scores were found to be outside SS normative values also in the normosmic group. This confirms how threshold remains the most affected task in COVID‐19–related OD both in the medium 4 and long term. 6
Qualitative disorders (parosmia/phantosmia) have been reported to be more associated with severe reduction in QoL than purely quantitative disorders, and the fact that QoL in our normosmic group was within the normal range despite a similar rate of parosmia/phantosmia in the two groups may be caused by either a less severe qualitative OD in patients who recovered their sense of smell or the adoption of more efficient compensatory strategies to cope with parosmia.
Dysosmic patients demonstrated lower scores at the brief QOD‐NS, confirming its usefulness in discriminating patients at a higher risk of QoL detriments related to OD. 10 A significant linear correlation between sVAS and SS (both TDI and SS subtests scores) was also observed in our analysis, replicating previous results. 4
A limit of the study is the lack of a control group of patients with no history of OD with or without previous COVID‐19. However, we could speculate that our normosmic group offers similar characteristics, whereas QoL scores were found to be within the normal range. Because of the cross‐sectional nature of the study and the fact that it included only patients with reported OD, results need to be verified in wider populations.
In conclusion, patients with persistent OD show worse QoL scores than those who have recovered sense of smell, suggesting how olfactory recovery could contribute to QoL improvement. Threshold remains the most affected task in the long term, which strengthens the hypothesis that SARS‐CoV‐2 targets the olfactory epithelium.
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest.
Pendolino AL, Tan HQM, Choi D, Ottaviano G, Andrews PJ. Long‐term quality‐of‐life impairment in patients with more than 1‐year COVID‐19–related olfactory dysfunction. Int Forum Allergy Rhinol. 2022;1‐5. 10.1002/alr.23071
REFERENCES
- 1. Patel ZM, Holbrook EH, Turner JH, et al. International consensus statement on allergy and rhinology: olfaction. Int Forum Allergy Rhinol. 2022;12(4):327‐680. 10.1002/alr.22929 [DOI] [PubMed] [Google Scholar]
- 2. AlShakhs A, Almomen A, AlYaeesh I, et al. The association of smell and taste dysfunction with COVID19, and their functional impacts. Indian J Otolaryngol Head Neck Surg. 2021:1‐6. doi:10.1007/s12070‐020‐02330‐w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Otte MS, Haehner A, Bork ML, Klussmann JP, Luers JC, Hummel T. Impact of COVID‐19‐mediated olfactory loss on quality of life. ORL J Otorhinolaryngol Relat Spec. 2022:1‐6. doi:10.1159/000523893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bordin A, Mucignat‐Caretta C, Gaudioso P, et al. Comparison of self‐reported symptoms and psychophysical tests in coronavirus disease 2019 (COVID‐19) subjects experiencing long‐term olfactory dysfunction: a 6‐month follow‐up study. Int Forum Allergy Rhinol. 2021;11(11):1592‐1595. doi:10.1002/alr.22828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mattos JL, Edwards C, Schlosser RJ, et al. A brief version of the questionnaire of olfactory disorders in patients with chronic rhinosinusitis. Int Forum Allergy Rhinol. 2019;9(10):1144‐1150. doi:10.1002/alr.22392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Boscolo‐Rizzo P, Hummel T, Hopkins C, et al. High prevalence of long‐term olfactory, gustatory, and chemesthesis dysfunction in post‐COVID‐19 patients: a matched case‐control study with one‐year follow‐up using a comprehensive psychophysical evaluation. Rhinology. 2021;59(6):517‐527. doi:10.4193/Rhin21.249 [DOI] [PubMed] [Google Scholar]
- 7. Lloyd A. Assessment of the SF‐36 version 2 in the United Kingdom. J Epidemiol Community Health. 1999;53(10):651‐652. doi:10.1136/jech.53.10.651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vaira LA, Gessa C, Deiana G, et al. The effects of persistent olfactory and gustatory dysfunctions on quality of life in long‐COVID‐19 patients. Life (Basel). 2022;12(2):141. doi:10.3390/life12020141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tan HQM, Pendolino AL, Andrews PJ, Choi D. Prevalence of olfactory dysfunction and quality of life in hospitalised patients 1 year after SARS‐CoV‐2 infection: a cohort study. BMJ Open. 2022;12(1):e054598. doi:10.1136/bmjopen‐2021‐054598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liu DT, Prem B, Sharma G, Kaiser J, Besser G, Mueller CA. Depression symptoms and olfactory‐related quality of life. Laryngoscope. 2022. doi:10.1002/lary.30122 [DOI] [PMC free article] [PubMed] [Google Scholar]


