Abstract
Tocilizumab is an interleukin (IL)‐6 receptor inhibitor that has been proposed as a therapeutic agent for treating coronavirus disease 2019 (COVID‐19). The aim of this umbrella review was to determine the efficacy of tocilizumab in treating COVID‐19, and to provide an overview of all systematic reviews on this topic. We systematically searched PubMed, Scopus, the Web of Science collection, the Cochrane library, Epistemonikos, and Google Scholar, as well as the medRxiv preprint server. These databases were searched up to 30 September 2021, using the following keywords: ‘SARS‐CoV‐2’, ‘COVID‐19’, ‘tocilizumab’, ‘RHPM‐1’, ‘systematic review’, and ‘meta‐analysis’. Studies were included if they were systematic reviews (with or without meta‐analysis) investigating the efficacy or safety of tocilizumab in confirmed COVID‐19 patients. The AMSTAR 2 checklist was used to assess quality of the included articles, while publication bias was examined using Egger's test. A total of 50 eligible systematic reviews were included. The pooled estimates showed significant reductions in clinical failure (risk ratio (RR) 0.75; 95% confidence interval (CI), 0.61–0.93), deaths (RR 0.78; 95%CI, 0.71–0.85) and the need for mechanical ventilation (RR 0.77; 95%CI, 0.64–0.92) for those receiving tocilizumab compared with the control group. Also, an emerging survival benefit was demonstrated for those who received tocilizumab, over those in the control group (adjusted hazard ratio (aHR) 0.52; 95%CI, 0.43–0.63). In addition, tocilizumab substantially increased the number of ventilator‐free days, compared with the control treatments (weighted mean difference (WMD) 3.38; 95%CI, 0.51–6.25). Furthermore, lymphocyte count (WMD 0.26 × 109/L; 95%CI, 0.14–0.37), IL‐6 (WMD 176.99 pg/mL; 95%CI, 76.34–277.64) and D‐dimer (WMD 741.08 ng/mL; 95%CI, 109.42–1372.75) were all significantly elevated in those receiving tocilizumab. However, the level of lactate dehydrogenase (LDH) (WMD −30.88 U/L; 95%CI, −51.52, −10.24) and C‐reactive protein (CRP) (WMD ‐104.83 mg/L; 95%CI, −133.21, −76.46) were both significantly lower after treatment with tocilizumab. Tocilizumab treatment reduced the risk of intubation, mortality and the length of hospital stay, without increasing the risk of superimposed infections in COVID‐19 patients. Therefore, tocilizumab can be considered an effective therapeutic agent for treating patients with COVID‐19.
Keywords: COVID‐19, efficacy, interleukin 6, SARS‐CoV‐2, tocilizumab, umbrella review
Abbreviations
- aHR
adjusted hazard ratio
- AMSTAR 2
A Measurement Tool to Assess Systematic Reviews 2
- CAR
chimaeric antigen receptor
- CI
confidence interval
- COVID‐19
coronavirus disease 2019
- CRP
C‐reactive protein
- CRS
cytokine release syndrome
- ICU
intensive care unit
- IL
interleukin
- IQR
interquartile range
- LDH
lactate dehydrogenase
- MD
mean difference
- MRA
myeloma receptor antibody
- OR
odds ratio
- RCT
randomized controlled trial
- RR
risk ratio
- SARS‐CoV‐2
severe acute respiratory syndrome coronavirus 2
- SD
standard deviation
- WBC
white blood cell
- WMD
weighted mean difference
1. INTRODUCTION
In late 2019, a pandemic of novel viral pneumonia occurred in China, which was later named coronavirus disease 2019 (COVID‐19). 1 The alarming progression of the disease, and the severity of its clinical manifestations, motivated many researchers to try to develop vaccines and therapeutic approaches for controlling or treating this disease. 2 Several serology analyses have shown that patients with severe COVID‐19 manifest higher serum Interleukin (IL)‐6 levels, in comparison with those with milder forms of the disease, suggesting that elevated levels of IL‐6 might be associated with greater disease severity and worse outcomes. 3 , 4 , 5 This hypothesis raised hopes that IL‐6 receptor inhibitors would be effective in treating COVID‐19. 6
Tocilizumab, also known as myeloma receptor antibody (MRA), is a recombinant humanised antibody of the IgG1 subclass that acts as an IL‐6 receptor inhibitor. 7 Its main use is for the treatment of autoimmune disorders, such as rheumatoid arthritis and systemic juvenile idiopathic arthritis. 8 , 9 Tocilizumab has also been found to be effective in treating cytokine release syndrome (CRS), which is associated with some types of immunotherapy, such as chimaeric antigen receptor (CAR)‐T cell therapy. 10 These observations form the basis for considering tocilizumab as a therapeutic agent for COVID‐19. 11
There have been several systematic reviews and meta‐analyses which have investigated the efficacy of tocilizumab for treating COVID‐19, but they have reached different conclusions. For example, a living systematic review and meta‐analysis showed that tocilizumab had no effect on the risk of short‐term mortality. 12 In contrast, another meta‐analysis revealed that all‐cause mortality was significantly lower in those receiving tocilizumab. 13 Therefore, we conducted the present umbrella review in order to comprehensively evaluate all available evidence regarding the efficacy of tocilizumab in the treatment of COVID‐19.
2. METHOD
This umbrella review aimed to provide a comprehensive overview and to critically appraise the existing systematic reviews, which evaluated the efficacy and safety of tocilizumab treatment in patients with COVID‐19. Our primary outcome was to evaluate the occurrence of “clinical failure”, which was defined as requiring intubation, admission to an intensive care unit (ICU), or death. Also, we evaluated the overall death rate. The secondary outcomes included the need for mechanical ventilation, risk of ICU admission, hospital discharge rate, presence of a super‐infection, length of the hospital stay, length of the ICU stay, number of ventilator‐free days, and changes in laboratory parameters.
2.1. Systematic search
The following databases were searched up to 30 September 2021: PubMed, Scopus, Web of Science collection, Cochrane library, Epistemonikos, and medRxiv. In addition, the first 100 pages of the Google Scholar search engine were manually searched to identify additional eligible studies. There were no limitations or restrictions used in any of the search fields, such as language, date and study type. Furthermore, backward and forward citation searching of all included studies were performed to discover whether there were any additional relevant articles. The search strategy comprised a combination of the following keywords (SARS‐CoV‐2 OR COVID‐19) AND (tocilizumab OR RHPM‐1) AND (systematic review OR meta‐analysis). A detailed description of the search strategy used in each database is presented in Table S1.
2.2. Selection of meta‐analyses
All of the articles identified through the electronic and manual searches were exported to EndNote 20. After removing duplicates, two groups of authors independently screened the title and abstracts of the articles and excluded those that were irrelevant. In the next step, the same groups reviewed the full‐texts of the remaining papers, in accordance with the eligibility criteria. Any discrepancies between the two groups were resolved by consulting other authors. Studies were included if they were: (1) conducted on patients with confirmed COVID‐19, based on serological, molecular, or computed tomography (CT)‐scan techniques; (2) used tocilizumab as the intervention; (3) used a standard of care treatment or placebo for the control group; (4) reported at least one of the outcomes of interest (i.e. clinical failure, overall death, need for mechanical ventilation, risk of ICU admission, hospital discharge rate, super‐infection, length of the hospital stay, ICU stay, ventilator‐free days, and changes in laboratory parameters); and (5) conducted a systematic review, with or without a meta‐analysis. Studies were excluded if they were: (1) cross‐sectional, case‐control, cohort or clinical trials; (2) living systematic reviews and review articles that did not used a systematic approach (e.g. rapid or scoping reviews); (3) systematic reviews on preclinical or animal studies; and (4) investigated the effectiveness of tocilizumab combined with other IL‐6 inhibitor therapies.
All eligible meta‐analyses were reviewed and the primary studies were identified. Individual primary studies were selected for the recalculation of the summary effect, based on the following criteria: (1) retrospective and prospective observational studies with a matched control group, in terms of disease severity (i.e. similar proportions of patients receiving respiratory support in both the experimental and control groups), (2) randomized controlled trials, or (3) single‐group studies which assessed the pattern of changes in laboratory measures before and after tocilizumab therapy.
2.3. Data extraction
Data extraction was conducted using previously designed Microsoft Office Excel forms. Two researchers independently obtained the following information from each included study: (1) basic information about the study, including the first author's name, year of publication and the journal; (2) search date and names of the databases searched, number of included studies, total number of participants, study designs of the included studies, tools used for assessing the risk of bias, age and sex of the included participants, general summary, and summary effect size (95% confidence interval (CI)) for each outcome. Disagreements were resolved by discussing or consulting a third author and all of the extracted data were double‐checked by other reviewers.
2.4. Methodological quality
Two authors independently assessed the risk of bias and the quality of the included articles using the “A Measurement Tool to Assess Systematic Reviews 2 (AMSTAR 2)” checklist. 14 This checklist consists of 16 items, of which seven are considered critical domains: protocol registration, adequacy of the literature search, justification for excluding individual studies, risk of bias from the individual studies being included, appropriateness of the meta‐analytical methods, consideration of the risk of bias when interpreting the results of the review, and assessment of the presence and likely impact of publication bias. The checklist does not create an overall score, but provides a total rating based on weaknesses detected in the critical domains. The overall confidence in the results of the review can be qualitatively rated as either “high” (no or one non‐critical weakness), “moderate” (more than one non‐critical weakness), “low” (one critical flaw, with or without non‐critical weaknesses), and “critically low” (more than one critical flaw, with or without non‐critical weaknesses). A third reviewer was consulted to resolve any discrepancies between the two authors.
2.5. Statistical analysis
The crude data, multivariable adjusted hazard ratios (aHRs) that controlled for any confounders, and their 95% CIs were extracted from all primary studies included in the selected meta‐analyses. Following this, we performed our own meta‐analysis using the DerSimonian and Laird random‐effects method. The binary outcomes were examined using summary risk ratios (RRs) and aHRs, while for continuous outcomes weighted mean differences (WMDs) and their corresponding 95% CIs were recalculated. Furthermore, whenever the continuous variables were reported as a median with a range or interquartile range (IQR), we converted them to a mean and standard deviation (SD) using the method proposed by Lue et al. and Wan et al. 15 , 16
When recalculating the summary effect sizes, primary studies were excluded if: (1) the retrospective and prospective observational studies had unmatched control groups, (2) they were not conducted in the general population, such as studies that recruited COVID‐19 patients with specific underlying disorders, or (3) they only reported unadjusted HRs. We excluded the aforementioned primary studies from the meta‐analyses and then reanalysed the effect sizes using the random‐effects model. This approach helped to ensure that the general population was targeted and that the risk of selection bias was minimised among the primary studies included. This is because tocilizumab was more likely to be given to those who presented with more severe forms of the disease, which may lead to a higher proportion of negative outcomes in the intervention group, relative to the controls.
The between study heterogeneity was assessed for each meta‐analysis by estimating I 2 statistics and their 95% CIs, 17 while publication bias was examined using Egger's test. 18 All analyses were performed in STATA Statistical Software, version 17 (Stata Corporation, College Station, TX, USA). Statistical significance was defined as p‐value <0.05.
3. RESULTS
3.1. Literature search
The systematic search identified a total of 709 records, which came from PubMed (n = 94), Scopus (n = 279), the Web of Science collection (n = 80), the Cochrane library (n = 1), Epistemonikos (n = 139), and medRxiv (n = 116). Following the removal of 197 duplicate records, the remaining 512 studies were screened and 93 publications were selected for full text review. One article was not accessible and thus it was excluded. 19 After evaluating the other 92 articles for eligibility, 42 were excluded for the following reasons: one study investigated tocilizumab combined with another IL‐6 inhibitor, 20 two discussed therapies other than tocilizumab, 21 , 22 37 were not systematic reviews, 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 and two were systematic reviews of preclinical or animal studies. 60 , 61 Finally, 50 articles met the eligibility criteria and were included in the present umbrella review 6 , 28 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83 , 84 , 85 , 86 , 87 , 88 , 89 , 90 , 91 , 92 , 93 , 94 , 95 , 96 , 97 , 98 , 99 , 100 , 101 , 102 , 103 , 104 , 105 , 106 , 107 , 108 , 109 (Figure 1).
FIGURE 1.
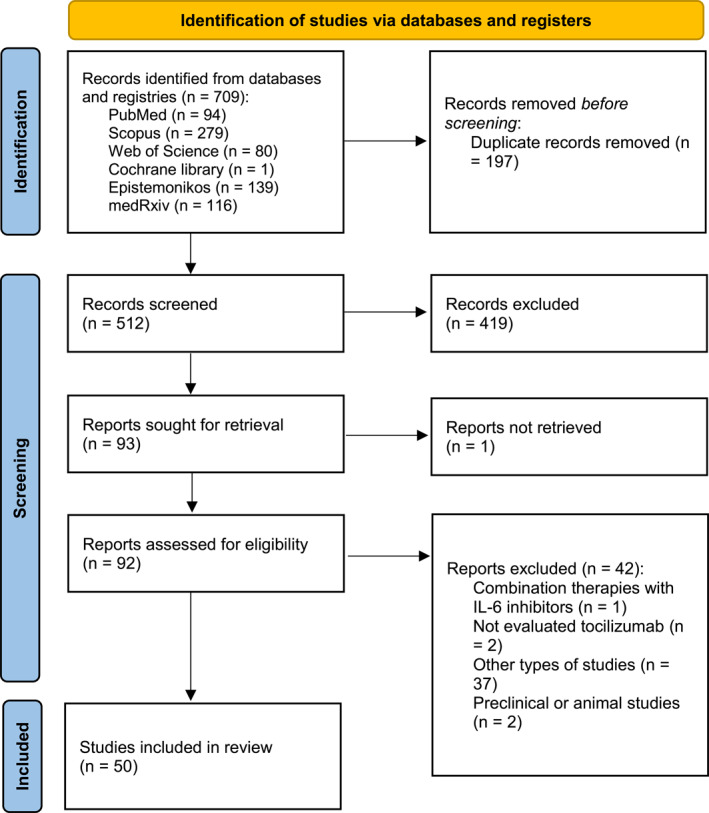
Study selection process
3.2. Characteristics of the studies included in the meta‐analysis
The 50 included studies were comprised of nine preprints and 41 published articles, which appeared in 37 different journals. They were all published in English and published in 2020 and 2021. Two studies did not report the search date, while in the remaining 48 comprehensive searches were performed from 1 December 2019 up to May 2021. Table 1 summarises the characteristics of the included studies.
TABLE 1.
Characteristics of the systematic reviews included in the present umbrella review
| Study identification | Journal | Search date | Searched databases | Number of included studies/total number of participants | Study design of included studies | Tools for assessment of risk of bias | Age of included participants | Sex of included participants (the proportion of females) |
|---|---|---|---|---|---|---|---|---|
| Nugroho et al. 2021 74 | F1000Research | 2 November 2020 | PubMed, Embase, Medline, and Cochrane | 26 studies/2112 in intervention group and 6160 in control group | Cohort, case‐control, and RCT | NOS | A mean/median age from 29 to 77 in intervention group and from 21 to 73.5 in controls | Not reported |
| Aziz et al. 2020 62 | Journal of Medical Virology | 1 January 2020 to 23 July 2020 | PubMed/MEDLINE, Embase, Lit‐COVID, WHO COVID, Cochrane, and Web of Science | 23 studies/6279 patients (1897 in tocilizumab + standard therapy group and 4382 in standard therapy alone) | RCT and cohort studies | NOS, NCOS and ROBINS‐I | 62.2 ± 4.7 years in tocilizumab + standard therapy group and 64.8 ± 4.1 years in standard therapy group | 39.4% in tocilizumab + standard therapy group and 28.0% in standard therapy group |
| Conti et al. 2021 110 | Journal of Personalised Medicine | May 2021 | PubMed, Scopus, Embase, Cochrane, WILEY, and ClinicalTrials.gov | 47 studies/9640 patients (3085 in tocilizumab + standard therapy and 6355 patients in standard therapy alone) | Observational studies and RCT | NOS | The mean age of all participants was 64.44 ± 13.89 years (range 53–78 years) | Not reported |
| Elangovan et al. 2020 66 | Preprint | 1 August 2020 | PubMed, medRxiv and Scopus | 29 studies/684 patients | RCT, nRCT, and observational studies (cohort study and case series) | RoB 2.0 and ROBINS‐I tools, NOS | Not reported | Not reported |
| Gupta et al. 2021 111 | Journal of Investigative Medicine | 19 April 2021 | Medline (PubMed), Embase, Google Scholar, and Cochrane | 6 studies/3013 patients (1651 in tocilizumab + standard therapy and 1362 patients in standard therapy alone) | RCT | RoB 2 | Not reported | Not reported |
| Hariyanto et al. 2020 112 | Drug Research | 1 November 2020 | PubMed and Europe PMC | 38 studies/13,412 patients (4090 in tocilizumab group and 9322 in non‐tocilizumab group) | RCT, nRCT, and observational studies (cohort study and case control) | NOS | Adult patients (at least 18 years old) | Not reported |
| Klopfenstein et al. 2021 70 | Infectious Diseases and Therapy | 27 April 2021 | Medline, the Cochrane Library, and Embase | 9 studies/6482 patients (3357 in tocilizumab group and 3125 in placebo group) | RCT | RoB 2.0 | Not reported | Not reported |
| Kyriakopoulos et al. 2021 113 | Preprint | 31 March 2021 | MEDLINE, CENTRAL and medRxiv | 52 studies/27,004 patients (8048 in tocilizumab group and 18,956 in non‐tocilizumab group) | RCT and observational studies | RoB 2.0 and ROBINS‐I tools | Not reported | Not reported |
| Podmore et al. 2021 63 | Preprint | July 2020 to 1 March 2021 | Embase and PubMed | 41 studies/Not reported | RCT and observational studies | ROBINS‐I tool | Not reported | Not reported |
| Wafa et al. 2021 114 | Preprint | January 25 to 5 February 2021 | PubMed (MEDLINE), Science Direct, Cochrane Library, ProQuest and Springer | 10 studies/Not reported | RCT | RoB 2.0 | Not reported | Not reported |
| Kotak et al. 2020 115 | Cureus | 29 June 2020 | PubMed and Cochrane | 7 studies/766 patients (351 in the tocilizumab group and 414 in the control group) | Observational studies | NOS | Not reported | Not reported |
| Abdulrahman et al. 2021 116 | Preprint | 3 January 2021 | PUBMED, EMBASE, and medRxiv | 9 studies/6326 patients (3272 in the tocilizumab group and 3054 in the control group) | RCT | RoB 2.0 | Adult patients (at least 18 years old) | Not reported |
| Lan et al. 2020 72 | International Journal of Antimicrobial Agents | 24 May 2020 | PubMed, Cochrane Library, Embase, medRxiv and bioRxiv. | 7 studies/592 patients (240 in the tocilizumab group and 352 in the control group) | Observational studies | NOS | Adult patients (at least 18 years old) | Not reported |
| Rezaei et al. 2021 75 | Expert Review of Clinical Immunology | 26 December 2020 | PubMed, Embase, CENTRAL, ClinicalTrials.gov, Scopus, and preprints | 73 studies (45 comparative studies and 28 single‐arm studies)/Not reported | Comparative studies (RCT, case– control studies), single‐arm observational studies | RoB 2.0 and NOS | 63.14 ± 5.2 years | 36% |
| Chandrasekar et al. 2020 117 | Journal of Medical Virology | 1 December 2019 to 11 May 2020. | PubMed/MEDLINE, Embase, Cochrane Central, Google Scholar, MedRxiv | 29 studies/5207 patients 3624 patients in the intervention arm (mean age: 55.9 ± 8.4 years, 62% males) and 1583 patients (mean age: 52.5 ± 8.5 years, 60.7% males) in the control arm | RCT, prospective cohorts, retrospective cohorts, and case series | RoB 2.0 | 55.9 ± 8.4 in intervention arm and 52.5 ± 8.5 in the control arm | 62% in intervention arm and 60.7% in the control arm |
| Rubio‐Rivas et al. 2021 118 | The Journal of Human Pharmacology and Drug Therapy | 1 January 2020 to 13 April 2021 | PubMed/MEDLINE, and Scopus | 64 studies/20,616 patients (7668 in tocilizumab + standard therapy and 12,948 in standard therapy alone) | RCT and observational studies | NOS and RoB 2.0 | 62.4 ±15.1 in tocilizumab group and not reported in control group | 31.2% in tocilizumab group and not reported in control group |
| Tleyjeh et al. 2021 12 | Clinical Microbiology and Infection | 8 October 2020 | Ovid MEDLINE(R) and Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations and Daily, Ovid Embase, Ovid Cochrane Central Register of Controlled Trials, Ovid Cochrane Database of Systematic Reviews, Web of Science, Scopus up, preprint servers and Google | 24 studies (five RCTs and 19 cohorts)/1325 patients in RCTs and 9850 in cohorts (detailed number of patients in intervention and control group was not reported) | RCT and cohort | RoB 2.0 and ROBINS‐I tools | Not reported | Not reported |
| Viswanatha et al. 2021 119 | Clinical and Experimental Rheumatology | 1 January 2020 to 30 September 2020 | PubMed, Scopus, CENTRAL, and Google scholar | 24 studies/5686 patients (1841in tocilizumab + standard therapy and 3454 in standard therapy alone) | Comparative studies (RCT, case– control studies) | NOS, RoB 2.0 | Not reported | Not reported |
| Wei et al. 2021 79 | Infectious Disease of Poverty | 20 March 2020 | PubMed, PMC, Scopus, Google Scholar, and Web of Science | 25 studies/10,201 patients (3135 in tocilizumab + standard therapy and 7066 in standard therapy alone) | RCT and observational studies | RoB 2.0 | Not reported | Not reported |
| Zhao, J. et al. 2020 120 | Critical Care | 25 July 2020 | PubMed, Embase, Medline, Cochrane, and CNKI | 10 studies/1675 patients (675 patients in tocilizumab + standard therapy, while 1000 in standard therapy alone) | RCT and cohort | Not reported | older/elderly (mean/median age ≥52 years) | Not reported |
| Berardicurti et al. 2020 121 | Clinical and Experimental Rheumatology | 1 January 2020 to 21 July 2020 | MEDLINE, Cochrane Library, SCOPUS, and Web of Science | 22 studies/1520 tocilizumab‐treated patients | RCT and observational studies | NOS | 61 years (95% CI: 59–64) | 29% |
| Boregowda et al. 2020 122 | Frontiers in Medicine | December 2019 to 14 June 2020. | PubMed, Embase, Cochrane library, Web of Science, and MedRxiv | 16 studies/3641 (1153 in tocilizumab + standard therapy and 2488 in standard therapy alone) | RCT and observational studies | ROBINS‐I | Not reported | 36% (38.3% in standard therapy group and 31.3% in tocilizumab group) |
| Campbell et al. 2021 123 | Frontiers in Medicine | 1 January 2020 to 25 February 2021 | The global WHO database of Individual Case Safety Reports (ICSRs)/adverse drug reactions (ADRs) (“VigiBase”), searching Medline, Embase, and Web of Science | 72 studies/Not reported | Cohort | RoB 2.0 | Not reported | Not reported |
| Chen et al. 2020 124 | Leukemia | 1 January 2020 to 27 October 2020 | PubMed, Web of Science and Medline | 32 studies/11,487 patients (detailed number of patients in intervention and control group was not reported) | RCT and observational studies | NOS, Jadad scale | Not reported | Not reported |
| Han et al. 2021 125 | Frontiers in Pharmacology | 10 August 2020 | PubMed, EMBASE, ISI Web of Science, Cochrane library, ongoing clinical trial registries (clinicaltrials.gov), and preprint servers (medRxiv, ChinaXiv) | 33 studies/5630 patients (2132 in anti‐IL‐6 signalling (anti‐IL6/IL‐6 R/JAK) agents + standard therapy and 3498 in standard therapy alone) | RCT, and observational studies (cohort study and case control) | The MINORS index, NOS, RoB 2.0 | Not reported | Not reported |
| Kaye et al. 2021 94 | PeerJ | 4 August 2020 | PubMed and SearchWorks | 34 studies (16 case‐control studies and 18 uncontrolled trials)/1008 in tocilizumab + standard therapy and 1537 in standard therapy alone) | Case‐control studies and uncontrolled studies | Not reported | Not reported | |
| Khan et al. 2021 126 | Respiratory Infection | 7 January 2021 | MEDLINE, and EMBASE and ongoing clinical trial registries (clinicaltrials.gov and EU Clinical Trials Register) | 70 studies/20,972 patients (6563 in tocilizumab + standard therapy and 14,409 in standard therapy alone) | RCT and observational studies | RoB 2.0 | Not reported | Not reported |
| Peng et al. 2021 100 | Reviews in Medical Virology | 1 January 2020 to 20 December 2020 | PubMed, Embase Medline, Web of Science and MedRxiv | 29 studies/Sample size not reported | Observational studies | Not conducted | Not reported | Not reported |
| Petrelli et al. 2021 127 | World Journal of Methodology | 9 June 2020 | PubMed, EMBASE, SCOPUS, Web of Science, MedRxiv, Science Direct, and the Cochrane Library | 33 studies/13,476 patients (3264 in tocilizumab + standard therapy and 10,212 in standard therapy alone) | RCT and observational studies | ROBIN‐I, NOS | Median: 62 | Not reported |
| Pinzon et al. 2021 128 | Journal of Infection and Public Health | November 2020 | PubMed and medRxiv | 16 studies/Not reported | RCT and observational studies | OCEBM | Not reported | Not reported |
| Singh et al. 2020 129 | Preprint | 4 June 2020 | PubMed, The Cochrane Central Register of Controlled Trials, preprint server (medRxiv) and international clinical trial register (clinicaltrials.gov) | 13 studies/2750 patients (819 in tocilizumab + standard therapy and 1931 in standard therapy alone) | RCT, nRCT, and observational studies | ROBIN‐I, RoB 2.0 | Not reported | Not reported |
| Zhao et al. 2021 130 | European Journal of Clinical Pharmacology | 27 September 2020 | PubMed, Embase, Medline, and Cochrane | 19 studies/2493 patients (detailed number of patients in intervention and control group was not reported) | Observational studies | Not conducted | Not reported | Not reported |
| Alunno et al. 2021 82 | Clinical Science | 11 December 2020 | MEDLINE, Embase, The Cochrane Database of Systematic Reviews, CENTRAL and CINAHL | 4 studies/Not reported | RCT | RoB 2.0. | Not reported | Not reported |
| Elsokary et al. 2020 131 | Preprint | 26 August 2020 | ClinicalTrial.gov, ProQuest, PubMed, Embase, Cochrane, Google Scholar, Science direct, Chinese Clinical Trial Registry (ChiCTR), and medRxiv | 6 studies/1473 patients (472 in tocilizumab + standard therapy and 1001 in standard therapy alone) | Cohort studies | ROBIN‐I | Not reported | Not reported |
| Lin et al. 2021 85 | International Immunopharmacology | 20 February 2021 | PubMed, Embase, Cochrane Library, Clinicaltrials.gov, WHO International Clinical Trials Registry Platform and the preprint server of medRxiv | 8 studies/6314 patients (3267 in tocilizumab + standard therapy and 3047 in placebo or standard therapy alone) | RCT | RoB 2.0. | The mean or medium age ranged from 56 to 64 years | 40% |
| Mathew et al. 2020 132 | Open access journal of biomedical science | May 25 to 16 June 2020 | PubMed and Google Scholar | 14 studies/Not reported | RCT, cohorts, case reports and case series. | Not conducted | Not reported | Not reported |
| Misra et al. 2020 86 | European Journal of Clinical Investigation | 29 June 2020 | PubMed, EMBASE, Medline, Google Scholar, Cochrane library and clinicaltrials.gov | 4 studies/806 patients (294 in tocilizumab + standard therapy group and 512 in standard therapy alone) | RCT, nRCT, cohort and case‐control studies | RoB 2.0., NOS | Not reported | Not reported |
| Ogiji et al. 2020 133 | EAS Journal of Pharmacy and Pharmacology | 25 May 2020 | PubMed, Google Scholar, Scopus | 18 studies/Not reported | Cohort and case‐control studies, case reports and case series | Joanna Briggs Institute's critical appraisal checklist | Not reported | Not reported |
| Putman et al. 2021 134 | Arthritis & Rheumatology | 19 March 2020 and 7 May 2020 | Ovid Medline and E‐pub Ahead of Print, In‐Process & Other Non‐Indexed Citations, and Daily, Ovid Embase, Ovid Cochrane Central Register of Controlled Trials, Ovid Cochrane Database of Systematic Reviews, Scopus, Web of Science, and ClinicalTrials.Gov | 7 studies/339 patients (detailed number of patients in intervention and control group was not reported) | Cohort and case series | NOS | Not reported | Not reported |
| Russell et al. 2020 89 | Ecancermedicalscience | Not reported | Ovid MEDLINE | Not reported | Not reported | Not conducted | Not reported | Not reported |
| Talaie et al. 2020 135 | DARU Journal of Pharmaceutical Sciences | 1 July 2020 | PubMed, Embase, Scopus, Cochrane, and Scholar | 11 studies/Not reported | All types of studies | RoB 2.0.,NOS, NIH Quality Assessment Tool | Not reported | Not reported |
| Zhang et al. 2020 136 | Frontiers in Public Health | 19 December 2020 | WHO COVID‐19 Global Research Database, PubMed, PubMed Central, LitCovid, Proquest Central and Ovid | Not reported | RCT | RoB 2.0. | Not reported | Not reported |
| Antwi‐Amoabeng et al. 2020 137 | Journal of Medical Virology | 27 April 2020 | PubMed, Embase, and Medline | 11 studies/29 patients | Case reports and case series | ROBIN‐I | 63 ±12 | 17.2% |
| Chibber et al. 2020 138 | European Journal of Pharmacology | Not reported | Google Scholar, Embase, and PubMed | Not reported | Not reported | Not conducted | Not reported | Not reported |
| Kim et al. 2020 33 | PLOS MEDICINE | the beginning of 2020 to 24 August 2020 | PubMed, Google Scholar, MEDLINE, the Cochrane Library, medRxiv, SSRN, WHO International Clinical Trials Registry Platform, and ClinicalTrials.gov | 9 studies/Not reported | RCT and observational studies | RoB 2.0. | Not reported | Not reported |
| Mahroum et al. 2021 107 | International Journal of Environmental Research and Public Health | 20 July 2020 | PubMed, MEDLINE, Scopus, medRxiv and SSRN | 39 studies/15,531 patients (3657 in tocilizumab + standard therapy group and 11,874 in standard therapy alone) | Case control, cohort | NOS | Ranged from 55 to 76.8 | Not reported |
| Martinez‐Vizcaino et al. 2020 108 | Preprint | 22 April 2020 | International Clinical Trials Registry Platform (WHO‐ICTRP) | 10 studies/2175 patients | RCT | Not conducted | Not reported | Not reported |
| POZO et al. 2020 139 | European Review for Medical and Pharmacological Sciences | 18 April 2020 | PubMed, Web of Science, Scopus, and clinicaltrials.gov | 13 studies/Not reported | RCT and observational studies | Not conducted | Not reported | Not reported |
| Qayyumi et al. 2020 140 | Cancer Research, Statistics, and Treatment | 23 May 2020 | PubMed, Embase, and Google Scholar | One study/21 patients | Case series | Not conducted | Not reported | Not reported |
| Zeraatkar et al. 2021 141 | Preprint | 9 October 2020 | Medline Ovid, PubMed, PubMed Central, Embase, CAB Abstracts, Global Health, PsycInfo, Cochrane 138 Library, Scopus, Academic Search Complete, Africa Wide Information, CINAHL, ProQuest Central, SciFinder, the Virtual Health Library, LitCovid, WHO and CDC Covid‐19 website, Eurosurveillance, China CDC Weekly, Homeland Security Digital Library, ClinicalTrials.gov, bioRxiv, medRxiv, chemRxiv, and SSRN | 20 trials/7608 patients | RCT | RoB 2.0. | Ranged from 42.1 to 69.8 | Not reported |
Abbreviations: CDC, Centres for Disease Control and Prevention; NOS, The Newcastle‐Ottawa scale; nRCT, non‐randomized controlled trials; RCT, Randomized controlled trials; RoB 2.0, Cochrane risk of bias tool for RCTs; ROBINS‐I, Risk of bias in nonrandomised studies of Interventions; WHO, World Health Organization.
A total of 70 primary studies were included in the published meta‐analyses, including 55 retrospective cohorts, 11 randomized control trials (RCTs), and four prospective cohorts. Thirty‐eight primary studies provided a matched control group and 37 studies reported the adjusted multivariate effect sizes. There were 24 studies conducted in the USA, 12 in Italy, 10 in Spain, and 24 in other countries.
3.3. Primary outcomes
3.3.1. Tocilizumab administration and risk of intubation, admission to ICU, or death
The first outcome was the combined outcome of either intubation, admission to ICU, or death, which was collectively called clinical failure. Ten publications, which were comprised of six retrospective studies, one prospective cohort study, and three clinical trials (n = 3318), were used for recalculating the summary aHR. The results of the pooled estimate showed that there was a significant 58% reduction in this composite outcome in the group receiving tocilizumab, relative to the control group (aHR 0.42; 95%CI, 0.30–0.59, I 2 = 61.0%). In a subgroup analysis, by study type, the risk of clinical failure was greatly reduced for the treatment group in retrospective cohort studies (aHR 0.31; 95%CI, 0.19–0.51, I 2 = 68.7%) and RCTs (aHR 0.62; 95%CI, 0.43–0.89, I 2 = 0.0%), but not for prospective cohorts (aHR 0.65; 95%CI, 0.23–1.83, I 2=NA) (Figure 2).
FIGURE 2.
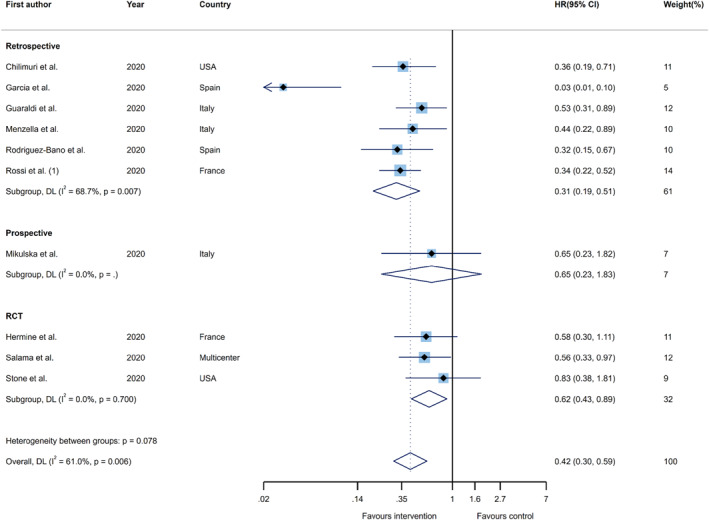
Forest plots of the pooled estimates for hazard ratios on the association between tocilizumab administration and risk of clinical failure (i.e. intubation, admission to ICU, or death) by study type. hazard ratio (HR); confidence interval (CI); DerSimonian and Laird (DL); randomized controlled trial (RCT)
In order to recalculate the summary effect for clinical failure, in terms of RR, 5140 patients from ten studies (two retrospective cohorts and eight RCTs) were enroled. Participants who received tocilizumab had an overall significant 25% lower risk of clinical failure, as compared to their counterparts in the control group (RR 0.75; 95%CI, 0.61–0.93, I 2 = 44.1%). Furthermore, the advantage of tocilizumab administration in reducing the risk of clinical failure ranged from a 19% reduction in clinical trials (RR 0.81; 95%CI, 0.69–0.95, I 2 = 21.2%) to a 65% reduction in retrospective studies (RR 0.35; 95%CI, 0.13–0.99, I 2 = 49.6%) (Figure 3).
FIGURE 3.
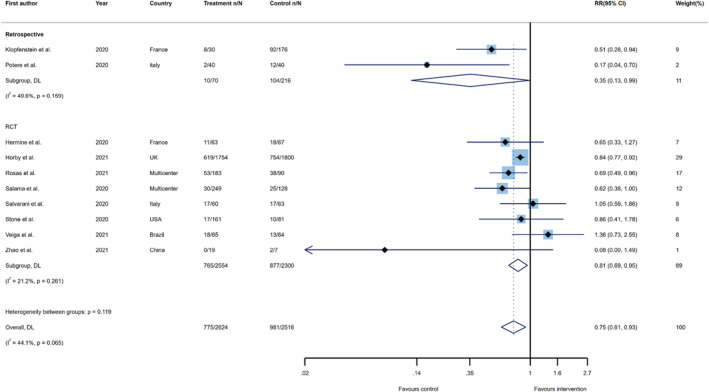
Forest plots of the summary effects for risk ratios on the association between tocilizumab administration and risk of clinical failure (i.e. intubation, admission to ICU, or death) by study type. risk ratio (RR); confidence interval (CI); DerSimonian and Laird (DL); randomized controlled trial (RCT)
3.3.2. Tocilizumab administration and the overall risk of mortality
A total of 33 primary studies, consisting of 18,538 participants, reported aHRs for mortality. These studies were comprised of 30 retrospective studies and three RCTs. After recalculating the summary effect, an emerging survival benefit was demonstrated for those receiving tocilizumab over the control group (aHR 0.52; 95%CI, 0.43–0.63, I 2 = 74.0%). When the pooled estimates were stratified, based on the study design, the summary effects remained statistically significant, with a larger benefit being found in retrospective studies (aHR 0.50; 95%CI, 0.41–0.61, I 2 = 75.9%), relative to clinical trials (aHR 0.67; 95%CI, 0.53–0.86, I 2 = 0.3%) (Figure 4).
FIGURE 4.
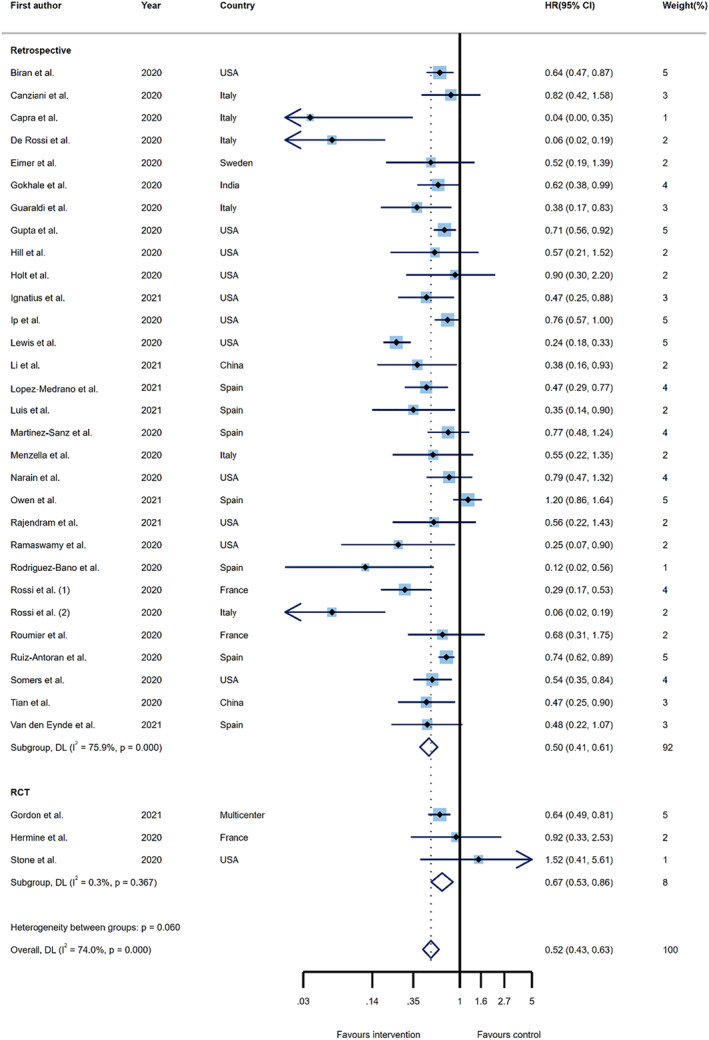
Forest plots of the pooled estimates for hazard ratios on the association between tocilizumab administration and risk of overall mortality by study type. hazard ratio (HR); confidence interval (CI); DerSimonian and Laird (DL); randomized controlled trial (RCT)
In the next step, the mortality RRs were reanalysed using 38 primary studies, which included data for 16,072 COVID‐19 patients. Twenty‐eight publications were retrospective observational studies, one was a prospective cohort, and nine were RCT. Tocilizumab administration resulted in substantially lower odds of death, when compared to the control group (RR 0.78; 95%CI, 0.71–0.85, I 2 = 40.8%). Analysing the results by study design, tocilizumab therapy was associated with a lower risk of mortality, compared to the control groups, in retrospective studies (27%), prospective studies (80%), and RCTs (11%). The differences were statistically significant for all types of study designs (RR 0.73; 95%CI, 0.66–0.81, I 2 = 29.9%; RR 0.20; 95%CI, 0.04–0.93, I 2=NA; and RR 0.89; 95%CI, 0.80–0.98, I 2 = 5.9%, respectively) (Figure 5).
FIGURE 5.
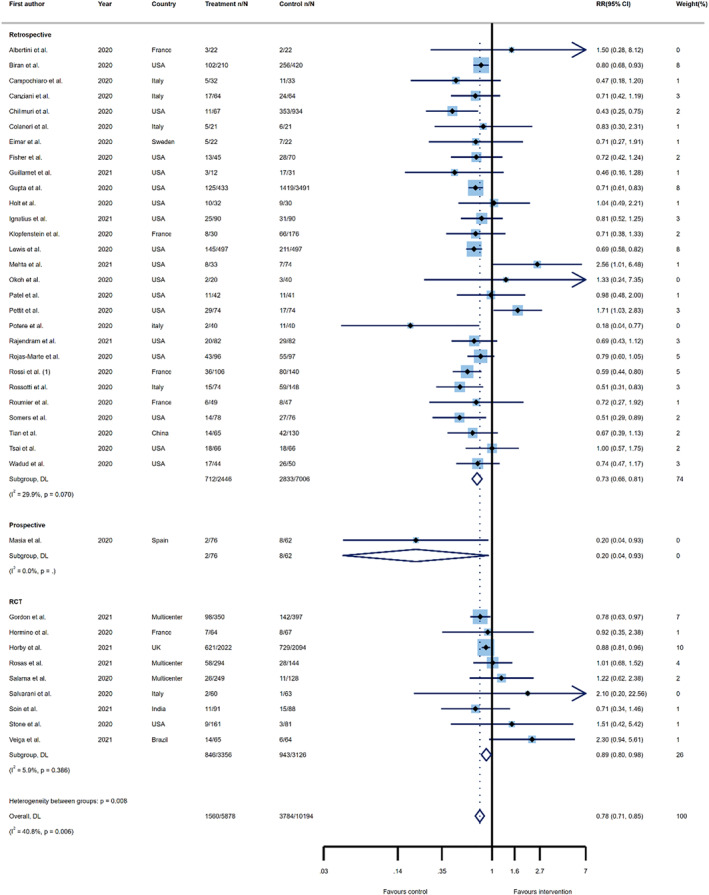
Forest plots of the summary effects for risk ratios on the association between tocilizumab administration and risk of overall mortality by study type. risk ratio (RR); confidence interval (CI); DerSimonian and Laird (DL); randomized controlled trial (RCT)
3.4. Secondary outcomes
3.4.1. Tocilizumab administration and the need for mechanical ventilation
Eight primary retrospective studies and seven RCTs, with a total population of 5792 COVID‐19 patients, were used to recalculate the RR for requiring mechanical ventilation. Patients who were given tocilizumab had a significantly lower risk of requiring mechanical ventilation, than those who were treated with the control group medications (RR 0.77; 95%CI, 0.64–0.92, I 2 = 44.9%). However, although the beneficial impact of tocilizumab was found in clinical trials (RR 0.79; 95%CI, 0.71–0.89, I 2 = 0.0%), this was not the case in retrospective studies (RR 0.72; 95%CI, 0.43–1.21, I 2 = 68.5%) (Figure S1).
3.4.2. Tocilizumab administration and the risk of ICU admission
The effect of tocilizumab on the probability of being admitted to ICU was examined in eight publications, which were comprised of three retrospective studies, one prospective cohort, and four RCTs (a total of 1052 COVID‐19 patients). Reanalysis of the summary effect revealed that tocilizumab did not reduce the overall risk of ICU admission (RR 0.85; 95%CI, 0.65–1.11, I 2 = 57.7%). Furthermore, the sub‐group analysis showed no reduced risk for retrospective cohorts, prospective cohorts or clinical trials (RR 0.77; 95%CI, 0.36–1.63, I 2 = 70.8%; RR 0.92; 95%CI 0.38–2.24, I 2=NA; and RR 0.79; 95%CI, 0.51–1.23, I 2 = 67.8%, respectively), in comparison to the control groups (Figure S2).
3.4.3. Tocilizumab administration and hospital discharge
The outcome of being discharged from hospital after receiving tocilizumab, in comparison with the control treatments, was assessed in 15 primary investigations (11 retrospective cohorts and four RCTs) that recruited a total of 7159 COVID‐19 patients. In general, administration of tocilizumab resulted in a significant higher rate of hospital discharge, relative to the control group (RR 1.12; 95%CI, 1.03–1.22, I 2 = 64.1%). Moreover, the subgroup analysis showed that although tocilizumab improved the chances of hospital discharge in patients enrolled in retrospective cohort studies (RR 1.23; 95%CI, 1.04–1.45, I 2 = 66.3%), no significant differences were found in RCTs (RR 1.07; 95%CI, 0.98–1.16, I 2 = 61.9%) (Figure S3).
3.4.4. Tocilizumab administration and the risk of superadded infection
The summary effect was recalculated for 23 studies (8684 patients), in order to estimate the impact of tocilizumab therapy on the risk of superadded infections. No significant association was found between the administration of tocilizumab and an elevated risk of secondary infection (RR 1.00; 95%CI, 0.80–1.26, I 2 = 77.1%). In both subgroups, which consisted of 16 retrospective cohorts and seven RCTs, there was no evidence that tocilizumab was related to a higher rate of co‐infections (RR 1.13; 95%CI, 0.86–1.48, I 2 = 80.4% and RR 0.75; 95%CI, 0.54–1.04, I 2 = 31.3%, respectively) (Figure S4).
3.4.5. Tocilizumab administration and the length of the hospital stay, ICU stay, and ventilator‐free days
The summary effects of the continuous outcomes were recalculated, in terms of the impact of tocilizumab therapy on the: length of hospital stay (10 studies), length of the ICU stay (five studies), and number of ventilator‐free days (six studies). The pooled estimation revealed that receiving tocilizumab increased the number of ventilator free days, compared to the control treatments (WMD 3.38; 95%CI, 0.51–6.25, I 2 = 75.8%). In contrast, no significant relationship was found between tocilizumab treatment and the length of the hospital or ICU stays (WMD −0.19; 95%CI, −3.34, 2.95; I 2 = 97.3% and WMD −0.49; 95%CI, −7.88, 6.91, I 2 = 97.6%, respectively) (Figure S5–S7).
3.4.6. Tocilizumab administration and the laboratory parameters
Data on the laboratory measures before and after tocilizumab therapy were available for the levels of: white blood cells (WBC), neutrophils, lymphocytes, IL‐6, lactate dehydrogenase (LDH), C‐reactive protein (CRP), D‐dimer, and ferritin. The time intervals between the baseline measurements and those after tocilizumab administration ranged from five to 14 days. Following the reanalysis of the summary effects, the level of lymphocytes (WMD 0.26 × 109/L; 95%CI, 0.14–0.37, I 2 = 45.1%), IL‐6 (WMD 176.99 pg/mL; 95%CI, 76.34–277.64, I 2 = 94.3%), and D‐dimer (WMD 741.08 ng/mL; 95%CI, 109.42–1372.75, I 2 = 75.8%) were significantly higher after administration of tocilizumab. In contrast, the levels of LDH (WMD −30.88 U/L; 95%CI, −51.52, −10.24, I 2 = 0.0%) and CRP (WMD −104.83 mg/L; 95%CI, −133.21, −76.46, I 2 = 91.3%) were significantly lower after tocilizumab administration (Figure S8–S15).
3.5. Publication bias
There was evidence of publication bias (Egger's p‐value <0.05) for the outcomes of mortality (p = 0.017), level of IL‐6 (p = 0.012), and level of CRP (p = 0.003). In contrast, publication bias was not found for: clinical failure (effect size of aHR, p = 0.368 and RR p = 0.129), mortality (effect size of RR p = 0.719), the need for mechanical ventilation (p = 0.439), ICU admission (p = 0.106), hospital discharge rate (p = 0.269), superadded infection (p = 0.192), length of hospital stay (p = 0.417), length of ICU stay (p = 0.128), length of ventilator‐free days (p = 0.758), WBC count (p = 0.461), neutrophil count (p = 0.648), lymphocyte count (p = 0.295), level of LDH (p = 0.950), level of ferritin (p = 0.481), and level of D‐dimer (p = 0.423).
3.6. Quality assessment
The results of the quality assessment showed that 29 (58%) were critically low, 12 (24%) were low, eight (1 were moderate, and 1 (2%) study was high quality. Among the critical domains, the most common problem was not taking into account the risk of bias when interpreting the results. Among the non‐critical domains, most studies did not report the source(s) of funding for their study (Table S2).
4. DISCUSSION
The present umbrella review found that tocilizumab administration significantly reduced the risk of requiring mechanical ventilation and dying in COVID‐19 patients. Moreover, tocilizumab significantly increased the likelihood of hospital discharge and a higher number of ventilator‐free days, without increasing the risk of super‐imposed infections. In terms of the effects of tocilizumab treatment on laboratory measures, it significantly increased lymphocytes, IL‐6 and D‐dimer, and decreased LDH and CRP levels.
We found that tocilizumab treatment significantly decreased the risk of mortality by 48%. In addition, the risk of clinical failure, which was defined as a combination of intubation, ICU admission, or death, was 0.42 times lower in the tocilizumab group than among the controls. A systematic review of hospitalised COVID‐19 patients showed that remdesivir decreased the 14‐day mortality rate of COVID‐19 patients by 36%, but not the 28‐day mortality rate (RR = 1.14, 95%CI: 1.06, 1.22). 142 Furthermore, treatment with favipiravir showed no significant difference from the control group, in terms of COVID‐19 mortality (RR 1.19; 95%CI, 0.85–1.66). 143 Moreover, an umbrella review revealed that treating COVID‐19 patients with convalescent plasma significantly reduced the mortality rate, compared with the controls. 144 Another umbrella review, on the efficacy of hydroxychloroquine or chloroquine in patients with COVID‐19, showed there was a lack of consistency in the clinical efficacy reported by the included articles. 145 A network meta‐analysis on the efficacy of anti‐severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) antibodies for treating COVID‐19 revealed that bamlanivimab + etesevimab decreased mortality by 87% (95%CI, 0.02–0.77). 146 The differences in the mortality outcomes between the numerous strategies for treating COVID‐19 could be due to differences in the inclusion criteria between the studies and features of the eligible populations, such as the prevalence of various underlying diseases and previous infection with SARS‐CoV‐2. However, comparing our results with the previously published studies shows that tocilizumab appears to be one of the most effective therapies for reducing COVID‐19 mortality.
Tocilizumab reduced the need for mechanical ventilation by 23%, although it was not significantly associated with a reduced risk of ICU admission. In comparison, Deng et al. found no significant differences in the incidence of mechanical ventilation, ICU admission, and duration of ICU hospitalisation in COVID‐19 patients treated with anti‐SARS‐CoV‐2 antibodies (e.g., monoclonal antibodies and intravenous immunoglobulins) and those in the control group. 146 Furthermore, research by Yu et al. found that sarilumab was not significantly associated with a reduced risk of invasive mechanical ventilation (RR 1.15; 95%CI, 0.38–3.51), whereas tocilizumab reduced the risk by 21% (RR 0.79; 95%CI, 0.71–0.88). 147 The differences between the two may be due to the limited number of studies which have evaluated the effects of sarilumab, compared with tocilizumab, or the different mechanisms of action. Sarilumab blocks IL‐6 and IL‐6 receptors, but tocilizumab is only an IL‐6 receptor antagonist. 148
A systematic review evaluating the effects of five pharmacologic interventions (i.e., anti‐inflammatory, antiviral, antiparasitic, antibody, and antibiotics) on the length of hospital stay showed that anti‐inflammatory (mean difference (MD) −1.41; 95%CI, −1.75, −1.07) and antiparasitic drugs (MD −0.65; 95%CI, −1.26, −0.03) significantly reduced the length of hospital stay. 149 However, in the present study we did not find any significant differences between the tocilizumab and control groups, in terms of the length of hospital stay or ICU stay (p > 0.05). This discrepancy could be as a result of the different aims, methodologies, and therapies included in these two studies. Similar to our findings, in a review of 13 articles and 4770 patients, Ebrahimi Chaharom and colleagues showed that corticosteroids did not significantly reduce the duration of hospital stay (odds ratio (OR) 1.56; 95%CI, −0.29, 3.41). 150 A network meta‐analysis on 196 trials, which included 76,767 participants and compared the efficacy of different COVID‐19 treatments, revealed that IL‐6 inhibitors significantly reduced the risk of mechanical ventilation (OR 0.72; 95%CI, 0.57–0.90) and also the length of hospital stay (MD −4.5; 95%CI, −6.7, −2.3), while no significant differences were found in the time to symptom resolution (MD −0.7; 95%CI, −2.7, 1.7) or the number of ventilator‐free days (MD 1.6; 95%CI, −0.2, 3.3). 151 Similarly, we found a reduced risk of mechanical ventilation for those receiving tocilizumab (RR 0.77; 95%CI, 0.64–0.92), but in contrast we found a significant increase in the number of ventilator‐free days (WMD 3.38; 95%CI, 0.51–6.25). These inconsistencies could be explained by differences in the number of included studies and due to the inclusion of all types of IL‐6 inhibitors, compared with our study which only included tocilizumab. Moreover, the above‐mentioned study showed no significant difference in the occurrence of adverse events between IL‐6 inhibitors and the control group (MD −4.0; 95%CI, −9.0, 67.0), 151 while our study also found no increased risk for superadded infection in those treated with tocilizumab (RR 1.00; 95%CI, 0.80–1.26).
COVID‐19 has been associated with increased platelet levels and CRP, as well as decreased lymphocytes. 152 Tocilizumab, which is also used to treat rheumatologic diseases like rheumatoid arthritis, has been found to reduce CRP and erythrocyte sedimentation levels. 153 The results of a systematic review of 11 studies, including 29 patients, showed that IL‐6 and CRP levels were significantly higher and lower, respectively, after tocilizumab treatment (p = 0.002 for IL‐6 and p < 0.0001 for CRP). 103 In addition, the results of another meta‐analysis showed that tocilizumab was associated with significant reductions in CRP (MD −106.69 mg/L; 95%CI, −146.90, −66.49), D‐dimer (MD −3.06 mg/L; 95%CI, −5.81, −0.31), ferritin (MD 532.80 ng/ml; 95%CI, −810.93, −254.67), and procalcitonin (MD −0.67 ng/ml; 95%CI, −1.13, −0.22), while significantly increasing lymphocyte counts (MD 360/μl; 95%CI, 0.18, 0.54). 61 In accordance with previous findings, we also found a substantial increase in lymphocyte count, IL‐6 and D‐dimer level, as well as a decrease in CRP.
The quality assessment of the studies included in our research, using AMSTAR 2, showed that most of the included studies had low and critically low quality. Similarly, an umbrella review which summarised the systematic reviews on the clinical presentations of COVID‐19, diagnostic tools, therapeutic modalities and laboratory and radiologic findings, reported that all of the articles included had critically low ratings, based on AMSTAR 2. 154 Moreover, concordant findings were also made by studies reviewing the effectiveness of chloroquine, hydroxychloroquine and convalescent plasma for treating COVID‐19. 144 , 155 Perhaps one explanation of these somewhat surprizing findings is that early in the COVID‐19 pandemic, study quality was not adequately assessed during the peer‐review process. 156
To best of our knowledge, this is the first umbrella review on the efficacy of tocilizumab for treating COVID‐19. This article consolidates the knowledge by providing a comprehensive summary of the most up‐to‐date evidence for one of the most promising options for treating COVID‐19. Nevertheless, this study has several limitations which should be considered when interpreting the results and/or using this information in clinical practice. Firstly, we used AMSTAR 2 to assess the quality of the included articles, but this approach has some limitations. For instance, due to the pressing need for scientific papers during the COVID‐19 crisis, several studies might not have reported some methodological details that are important for quality assessment. Secondly, several primary studies where included in more than one systematic review. We included all of these in our study, but the overlapping data were not included when calculating the pooled effect sizes. Thirdly, although we systematically searched the above‐mentioned databases and conducted an extensive search for grey literature, there is still a chance that some articles were missed. Fourthly, we conducted subgroup analysis only by study design. Past medical history, geographical region or disease severity, which are important prognostic factors for COVID‐19, were not included in the analysis. 157 Fifthly, most of the studies did not report the number of participants by sex and age group, so we were not able to perform subgroup on the effects of tocilizumab administration by age and sex. Sixthly, we included preprints in the study. Since preprints have not yet been peer‐reviewed, this might lead to bias in the findings. Seventhly, some of the laboratory parameters, like creatinine kinase which can be used as a prognostic factor, were not included in the present study. 158 Eighthly, the protocol of the study was not registered in PROSPERO, although it was submitted to the relevant university committee.
5. CONCLUSIONS
This umbrella review found that tocilizumab reduced the risk of intubation and mortality, lead to an earlier discharge from hospital and did not increase the risk of a super‐imposed infection. Therefore, tocilizumab can be considered a successful treatment strategy and should be included in guidelines for treating COVID‐19 patients. Nevertheless, the quality of the included articles was generally low and further high quality primary studies, in particular RCTs, are needed. Furthermore, a future umbrella review is needed to examine the safety of tocilizumab for treating COVID‐19 patients in more detail.
CONFLICT OF INTEREST
No conflict of interest declared.
ETHICS STATEMENT
The present study was approved by the ethics committee of the Shahid Beheshti University of Medical Sciences (IR.SBMU.RETECH.REC.1401.316).
AUTHOR CONTRIBUTION
Maryam Noori, Seyed Aria Nejadghaderi, Saeid Safiri, Shahnam Arshi and Ali‐Asghar Kolahi conceptualised the topic; Maryam Noori searched the databases; Mohammad Mahdi Rezaei Tolzali, Pourya Shokri and Shayan Rahmani performed screening and full‐text review; Mohammad Mahdi Rezaei Tolzali, Pourya Shokri, Shayan Rahmani, Shokoufeh Khanzadeh, and Seyed Aria Nejadghaderi performed data extraction and quality assessment; Maryam Noori performed statistical analysis; Maryam Noori, Asra Fazlollahi, Seyed Aria Nejadghaderi, Kuljit Singh and Mark J. M. Sullman prepared the first draft of the manuscript; Saeid Safiri, Shahnam Arshi and Ali‐Asghar Kolahi supervised this project. All authors reviewed and approved the final version of the manuscript.
Supporting information
Supplementary Material
ACKNOWLEDGEMENTS
We would like to acknowledge the support of the Shahid Beheshti University of Medical Sciences, Tehran, Iran. We also thank Seyed Ehsan Mousavi and Hanieh Marandi for their valuable efforts in the screening of the studies. The present study was supported by Shahid Beheshti University of Medical Sciences, Tehran, Iran (Grant No 43002212).
Rezaei Tolzali MM, Noori M, Shokri P, et al. Efficacy of tocilizumab in the treatment of COVID‐19: an umbrella review. Rev Med Virol. 2022;e2388. 10.1002/rmv.2388
Mohammad Mahdi Rezaei Tolzali, Maryam Noori and Pourya Shokri contributed equally to this work.
None of the authors listed on the manuscript are employed by a government agency that has a primary function other than research and/or education. Also, none of the authors are submitting this manuscript as an official representative or on behalf of the government.
Contributor Information
Ali‐Asghar Kolahi, Email: a.kolahi@sbmu.ac.ir.
Shahnam Arshi, Email: s.arshi@sbmu.ac.ir.
Saeid Safiri, Email: safiris@tbzmed.ac.ir.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
REFERENCES
- 1. Hamam H. COVID‐19 Surprised Us and Empowered Technology to Be its Own Master. Vol 3. Latin American Science, Technology and Society; 2020:272‐281. [Google Scholar]
- 2. Stasi C, Fallani S, Voller F, Silvestri C. Treatment for COVID‐19: an overview. Eur J Pharmacol. 2020;889:173644. 10.1016/j.ejphar.2020.173644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cortegiani A, Ippolito M, Greco M, et al. Rationale and evidence on the use of tocilizumab in COVID‐19: a systematic review. Pulmonology. 2021;27(1):52‐66. 10.1016/j.pulmoe.2020.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054‐1062. 10.1016/s0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID‐19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46(5):846‐848. 10.1007/s00134-020-05991-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Luo P, Liu Y, Qiu L, Liu X, Liu D, Li J. Tocilizumab treatment in COVID‐19: a single center experience. J Med Virol. 2020;92(7):814‐818. 10.1002/jmv.25801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Venkiteshwaran A. Tocilizumab. MABs. 2009;1(5):432‐438. 10.4161/mabs.1.5.9497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. De Benedetti F, Brunner HI, Ruperto N, et al. Randomized trial of tocilizumab in systemic juvenile idiopathic arthritis. N Engl J Med. 2012;367(25):2385‐2395. 10.1056/nejmoa1112802 [DOI] [PubMed] [Google Scholar]
- 9. Singh JA, Beg S, Lopez‐Olivo MA. Tocilizumab for rheumatoid arthritis. Cochrane Database Syst Rev. 2010;7:Cd008331. [DOI] [PubMed] [Google Scholar]
- 10. Maude SL, Frey N, Shaw PA, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371(16):1507‐1517. 10.1056/nejmoa1407222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Xu X, Han M, Li T, et al. Effective treatment of severe COVID‐19 patients with tocilizumab. Proc Natl Acad Sci U. S. A. 2020;117(20):10970‐10975. 10.1073/pnas.2005615117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tleyjeh IM, Kashour Z, Damlaj M, et al. Efficacy and safety of tocilizumab in COVID‐19 patients: a living systematic review and meta‐analysis. Clin Microbiol Infect. 2021;27(2):215‐227. 10.1016/j.cmi.2020.10.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vela D, Vela‐Gaxha Z, Rexhepi M, Olloni R, Hyseni V, Nallbani R. Efficacy and safety of tocilizumab versus standard care/placebo in patients with COVID‐19; a systematic review and meta‐analysis of randomized clinical trials. Br J Clin Pharmacol. 2022;88(5):1955‐1963. 10.1111/bcp.15124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shea BJ, Reeves BC, Wells G, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non‐randomised studies of healthcare interventions, or both. BMJ. 2017;358:j4008. 10.1136/bmj.j4008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Luo D, Wan X, Liu J, Tong T. Optimally estimating the sample mean from the sample size, median, mid‐range, and/or mid‐quartile range. Stat Methods Med Res. 2018;27(6):1785‐1805. 10.1177/0962280216669183 [DOI] [PubMed] [Google Scholar]
- 16. Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14(1):1‐13. 10.1186/1471-2288-14-135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ. 2003;327(7414):557‐560. 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Egger M, Smith GD, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629‐634. 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Banerjee S, Mahapatra AK. Systematic Review on Treatment Trials of Tocilizumab‐ A Repurposing Drug against COVID‐19. Reviews on Recent Clinical Trials; 2021. [DOI] [PubMed]
- 20. Milagre ST, Pereira AA, de Oliveira Andrade A, et al. Effectiveness and Quality Analysis of Methods in Studies for the Treatment of COVID‐19. Research on Biomedical Engineering; 2021. [Google Scholar]
- 21. Amir S, Seyedeh FM, Mehran NB, Hossein PE. The effect of proposed drugs in the treatment of patients with COVID‐ 19: a systematic review. Yafteh. 2020;22(3). [Google Scholar]
- 22. Raja MA, Mendoza MA, Villavicencio A, et al. COVID‐19 in solid organ transplant recipients: a systematic review and meta‐analysis of current literature. Transplant Rev. 2021;35(1):100588. 10.1016/j.trre.2020.100588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Khiali S, Khani E, Entezari‐Maleki T. A comprehensive review of tocilizumab in COVID‐19 acute respiratory distress syndrome. J Clin Pharmacol. 2020;60(9):1131‐1146. 10.1002/jcph.1693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Abubakar AR, Sani IH, Godman B, et al. Systematic review on the therapeutic options for COVID‐19: clinical evidence of drug efficacy and implications. Infect Drug Resist. 2020;13:4673‐4695. 10.2147/idr.s289037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Alzghari SK, Acuña VS. Supportive treatment with tocilizumab for COVID‐19: a systematic review. J Clin Virol. 2020;127:104380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bokharee N, Khan YH, Khokhar A, Mallhi TH, Alotaibi NH, Rasheed M. Pharmacological interventions for COVID‐19: a systematic review of observational studies and clinical trials. Expert Rev Anti‐Infect Ther. [DOI] [PubMed] [Google Scholar]
- 27. Cantini F, Goletti D, Petrone L, Fard SN, Niccoli L, Foti R. Immune therapy, or antiviral therapy, or both for COVID‐19: a systematic review. Drugs. 2020;80(18):1929‐1946. 10.1007/s40265-020-01421-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Del Pozo JSG, Galindo MF, Nava E, Jordan J. A systematic review on the efficacy and safety of IL‐6 modulatory drugs in the treatment of COVID‐19 patients. Eur Rev Med Pharmacol Sci. 2020;24(13):7475‐7484. [DOI] [PubMed] [Google Scholar]
- 29. Elavarasi A, Sahoo RK, Seth T, et al. Anti‐interleukin‐6 therapies for Covid‐19: a systematic review, critical appraisal and meta‐analysis. NMJI (Natl Med J India). 2020;33(3):152‐157. 10.4103/0970-258x.288119 [DOI] [PubMed] [Google Scholar]
- 30. Coomes EA, Hourmazd H. Interleukin‐6 in COVID‐19: A Systematic Review and Meta‐Analysis. medRxiv; 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fernando SM, Rochwerg B. COVID‐19, tocilizumab reduces all‐cause mortality at 28 d. Ann Intern Med. 2021;174(6):Jc63. 10.7326/acpj202106150-063 [DOI] [PubMed] [Google Scholar]
- 32. Ghazvini K, Karbalaei M, Keikha M. What are the clinical benefits of tocilizumab for COVID‐19 patients? Evidence from available case‐control studies. Pharmacien Hospitalier et Clinicien. 2021;56(2):217‐221. 10.1016/j.phclin.2020.11.003 [DOI] [Google Scholar]
- 33. Kim MS, An MH, Kim WJ, Hwang TH. Comparative efficacy and safety of pharmacological interventions for the treatment of COVID‐19: a systematic review and network meta‐analysis. PLoS Med. 2020;17(12):e1003501. 10.1371/journal.pmed.1003501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kim MS, An MH, Kim WJ, Hwang TH. Comparative Efficacy and Safety of Pharmacological Interventions for the Treatment of COVID‐19: A Systematic Review and Network Meta‐Analysis of Confounder‐Adjusted 20212 Hospitalized Patients. medRxiv; 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mahmud S, Nagraj S, Karia R, et al. 140: efficacy and safety of tocilizumab in hospitalized COVID‐19 patients: a systematic review. Crit Care Med. 2021;49(1):55‐None. 10.1097/01.ccm.0000726448.40803.c6 [DOI] [Google Scholar]
- 36. Mansourabadi AH, Sadeghalvad M, Mohammadi‐Motlagh HR, Rezaei N. The Immune System as a Target for Therapy of SARS‐CoV‐2: A Systematic Review of the Current Immunotherapies for COVID‐19. Life Sciences; 2020: 258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Meyerowitz EA, Sen P, Schoenfeld SR, et al. Immunomodulation as treatment for severe coronavirus disease 2019: a systematic review of current modalities and future directions. Clin Infect Dis. 2021;72(12):E1130‐E1143. 10.1093/cid/ciaa1759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Reza S, Sara P, Roya S, Ali S. Anti‐inflammatory, Immunomodulatory Agents as Potential Strategies against COVID‐19: A Systematic Review; 2020.
- 39. Russell B, Moss C, George G, et al. Associations between immune‐suppressive and stimulating drugs and novel COVID‐19—a systematic review of current evidence. ecancer. 2020;4(14):022. 10.3332/ecancer.2020.1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Siordia JA, Bernaba M, Yoshino K, et al. Systematic and statistical review of coronavirus disease 19 treatment trials. SN Comprehensive Clinical Medicine. 2020;2(8):1‐12. 10.1007/s42399-020-00399-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ahmad A, Rehman MU, Alkharfy KM. An alternative approach to minimize the risk of coronavirus (Covid‐19) and similar infections. Eur Rev Med Pharmacol Sci. 2020;24(7):4030‐4034. [DOI] [PubMed] [Google Scholar]
- 42. Awasthi S, Wagner T, Venkatakrishnan A, et al. Plasma IL‐6 levels following corticosteroid therapy as an indicator of ICU length of stay in critically ill COVID‐19 patients. medRxiv. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Dixit SB, Zirpe KG, Kulkarni AP, et al. Current approaches to Covid‐19: therapy and prevention. Indian J Crit Care Med. 2020;24(9):838‐846. 10.5005/jp-journals-10071-23470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gudadappanavar AM, Benni J. An evidence‐based systematic review on emerging therapeutic and preventive strategies to treat novel coronavirus (SARS‐CoV‐2) during an outbreak scenario. J Basic Clin Physiol Pharmacol. 2020;31(6). 10.1515/jbcpp-2020-0113 [DOI] [PubMed] [Google Scholar]
- 45. Karateev DE, Luchikhina EL. Иммуномодулирующая медикаментозная терапия при заболевании, вызванном инфекцией SARS‐CoV‐2 (COVID‐19). Almanac Clin Med. 2020;48:51‐67. 10.18786/2072-0505-2020-48-036 [DOI] [Google Scholar]
- 46. Kim MS, Kim WJ, An MH. Comparative efficacy and safety of pharmacological managements for hospitalized COVID‐19 patients: protocol for systematic review and trade‐off network meta‐analysis. medRxiv. 2020. [Google Scholar]
- 47. Yang C, Liu E, Liu M. Safety concerns regarding concomitant use of tocilizumab and glucocorticoids in COVID‐19 patients. In: Proceedings of the National Academy of Sciences of the United States of America. Vol 117; 2020:30025‐30026. 10.1073/pnas.2009253117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zhang C, Jin H, Wen Y, Yin G. A systematic review and network meta‐analysis for COVID‐19 treatments. medRxiv. 2020. [Google Scholar]
- 49. Zhou Z, Price CC. Overview on the use of IL‐6 agents in the treatment of patients with cytokine release syndrome (CRS) and pneumonitis related to COVID‐19 disease. Expet Opin Invest Drugs. 2020;29(12):1407‐1412. 10.1080/13543784.2020.1840549 [DOI] [PubMed] [Google Scholar]
- 50. Albuquerque AM, Tramujas L, Sewanan LR, Brophy JM. Tocilizumab in COVID‐19: a Bayesian reanalysis of recovery. medRxiv. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Almarzooq AA. Interleukin‐6 receptor genetic variation and tocilizumab treatment response to COVID‐19. medRxiv. 2021. [Google Scholar]
- 52. Alvarez‐Mon M, Asunsolo A, Sanz J, et al. Tocilizumab efficacy in COVID‐19 patients is associated with respiratory severity‐based stages. medRxiv. 2021. [Google Scholar]
- 53. Atzeni F, Masala IF, Rodríguez‐Carrio J, et al. The rheumatology drugs for COVID‐19 management: which and when? J Clin Med. 2021;10(4):1‐21. 10.3390/jcm10040783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Godolphin PJ, Fisher DJ, Berry LR, et al. Association between tocilizumab, sarilumab and all‐cause mortality at 28 days in hospitalized patients with COVID‐19: a network meta‐analysis. medRxiv. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Group RC, Horby PW, Pessoa‐Amorim G, et al. Tocilizumab in patients admitted to hospital with COVID‐19 (RECOVERY): preliminary results of a randomised, controlled, open‐label, platform trial. medRxiv. 2021. [Google Scholar]
- 56. Kyriazopoulou E, Huet T, Cavalli G, et al. Effect of anakinra on mortality in COVID‐19: a patient level meta‐analysis. medRxiv. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Leung E, Jorgensen SCJ, Crass RL, et al. Pharmacokinetic/pharmacodynamic considerations of alternate dosing strategies of tocilizumab in COVID‐19. medRxiv. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Marra F, Smolders EJ, El‐Sherif O, et al. Recommendations for dosing of repurposed COVID‐19 medications in patients with renal and Hepatic impairment. Drugs RD. 2021;21(1):9‐27. 10.1007/s40268-020-00333-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Yakar Hİ, Pazarli AC, İnönü Köseoğlu H, Kanbay A. The effect of tocilizumab on severe Covid‐19 infection: review of current evidence. Tuberk ve Toraks. 2021;69(1):74‐83. 10.5578/tt.20219909 [DOI] [PubMed] [Google Scholar]
- 60. Hariyanto TI, Kurniawan A. Tocilizumab administration is associated with reduction in biomarkers of coronavirus disease 2019 (COVID‐19) infection. J Med Virol. 2020;93(3):1832‐1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ivan Hariyanto T, Kurniawan A. Tocilizumab administration is associated with the reduction in biomarkers of coronavirus disease 2019 infection. J Med Virol. 2021;93(3):1832‐1836. 10.1002/jmv.26698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Aziz M, Haghbin H, Abu Sitta E, et al. Efficacy of tocilizumab in COVID‐19: a systematic review and meta‐analysis. J Med Virol. 2021;93(3):1620‐1630. 10.1002/jmv.26509 [DOI] [PubMed] [Google Scholar]
- 63. Bélène P, Nawab Q, Alessandra L, et al. Tocilizumab and Mortality in Hospitalised Patients with Covid‐19. A Systematic Review Comparing Randomised Trials with Observational Studies. medRxiv; 2021. [Google Scholar]
- 64. Christos K, Georgios N, Athena G, Haralampos M, Evangelos E, Konstantinos K. Systematic Review and Meta‐Analysis of Tocilizumab Administration for the Treatment of Hospitalised Patients with COVID‐19. SSRN; 2021. [Google Scholar]
- 65. Conti V, Corbi G, Sellitto C, et al. Effect of tocilizumab in reducing the mortality rate in COVID‐19 patients: a systematic review with meta‐analysis. J Personalized Med. 2021;11(7):628. 10.3390/jpm11070628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Elangovan EJ, Kumar VS, Kathiravan A, Mallampalli R, Thomas T, Subramaniyam G. Rationale and Prognosis of Repurposed Drugs with Risk Stratification of COVID‐19 Patients Requiring Oxygen Supplementation: A Systematic Review and Meta‐Analysis. medRxiv; 2020. [Google Scholar]
- 67. Gupta S, Padappayil RP, Bansal A, Daouk S, Brown B. Tocilizumab in patients hospitalized with COVID‐19 pneumonia: systematic review and meta‐analysis of randomized controlled trials. J Invest Med. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Hariyanto TI, Hardyson W, Kurniawan A. Efficacy and Safety of Tocilizumab for Coronavirus Disease 2019 (Covid‐19) Patients: A Systematic Review and Meta‐Analysis. Drug research; 2021. [DOI] [PubMed] [Google Scholar]
- 69. Ifan Ali W, Nando Reza P, David Setyo B, Henry S, Alfian Nur R, Citrawati Dyah Kencono W. The Efficacy and Safety of Monoclonal Antibody Treatments against COVID‐19: A Systematic Review and Meta‐Analysis of Randomized Clinical Trials. medRxiv; 2021. [Google Scholar]
- 70. Klopfenstein T, Gendrin V, Gerazime A, et al. Systematic review and subgroup meta‐analysis of randomized trials to determine tocilizumab's place in COVID‐19 pneumonia. Infect Dis Ther. 2021;10(3):1195‐1213. 10.1007/s40121-021-00488-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Kotak S, Khatri M, Malik M, et al. Use of tocilizumab in COVID‐19: a systematic review and meta‐analysis of current evidence. Cureus. 2020;12(10):e10869. 10.7759/cureus.10869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Lan SH, Lai CC, Huang HT, Chang SP, Lu LC, Hsueh PR. Tocilizumab for severe COVID‐19: a systematic review and meta‐analysis. Int J Antimicrob Agents. 2020;56(3):106103. 10.1016/j.ijantimicag.2020.106103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Li Z, Che Q, Li M, et al. Efficacy and Safety of Tocilizumab in Patients with COVID‐19: A Systematic Review and Meta‐Analysis. ResearchSquare; 2020. [Google Scholar]
- 74. Nugroho CW, Suryantoro SD, Yuliasih Y, et al. Optimal use of tocilizumab for severe and critical COVID‐19: a systematic review and meta‐analysis. F1000Research. 2021;10:73. 10.12688/f1000research.45046.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Rezaei S, Fatemi B, Karimi Majd Z, et al. Efficacy and safety of tocilizumab in severe and critical COVID‐19: a systematic review and meta‐analysis. Expet Rev Clin Immunol. 2021;17(5):1‐13. 10.1080/1744666x.2021.1908128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Rubio‐Rivas M, Forero CG, Mora‐Luján JM, et al. Beneficial and harmful outcomes of tocilizumab in severe COVID‐19: a systematic review and meta‐analysis. Pharmacotherapy. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Thoguluva Chandrasekar V, Venkatesalu B, Patel HK, Spadaccini M, Manteuffel J, Ramesh M. Systematic review and meta‐analysis of effectiveness of treatment options against SARS‐CoV‐2 infection. J Med Virol. 2021;93(2):775‐785. 10.1002/jmv.26302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Viswanatha GL, Anjana Male C, Shylaja H. Efficacy and safety of tocilizumab in the management of COVID‐19: a systematic review and meta‐analysis of observational studies. Clin Exp Rheumatol. 2021. [DOI] [PubMed] [Google Scholar]
- 79. Wei Q, Lin H, Wei RG, et al. Tocilizumab treatment for COVID‐19 patients: a systematic review and meta‐analysis. Infect Dis Poverty. 2021;10(1):71. 10.1186/s40249-021-00857-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Zhao J, Cui W, Tian BP. Efficacy of tocilizumab treatment in severely ill COVID‐19 patients. Crit Care. 2020;24(1):524. 10.1186/s13054-020-03224-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Zhao M, Lu J, Tang Y, Dai Y, Zhou J, Wu Y. Tocilizumab for treating COVID‐19: a systemic review and meta‐analysis of retrospective studies. Eur J Clin Pharmacol. 2020;77(3):311‐319. 10.1007/s00228-020-03017-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Alunno A, Najm A, Mariette X, et al. Immunomodulatory therapies for SARS‐CoV‐2 infection: a systematic literature review to inform EULAR points to consider. Ann Rheum Dis. 2021;80(6):803‐815. 10.1136/annrheumdis-2020-219725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Chenyang Z, Huaqing J, Yifeng W, Guosheng Y. A Systematic Review and Network Meta‐Analysis for COVID‐19 Treatments. medRxiv; 2020. [Google Scholar]
- 84. Emeka O, Casimir O, Uchenna E, et al. Prospects of monoclonal antibodies in COVID‐19 treatment: a systematic review. Author. 2020. [Google Scholar]
- 85. Lin WT, Hung SH, Lai CC, Wang CY, Chen CH. The effect of tocilizumab on COVID‐19 patient mortality: a systematic review and meta‐analysis of randomized controlled trials. Int Immunopharm. 2021;96:107602. 10.1016/j.intimp.2021.107602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Misra S, Nath M, Hadda V, Vibha D. Efficacy of various treatment modalities for nCOV‐2019: a systematic review and meta‐analysis. Eur J Clin Invest. 2020;50(11):e13383. 10.1111/eci.13383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Mohamed E, Hozaifa E, Ahmed E, Mahmoud A. Tocilizumab in Treatment of Severe COVID‐19 Patients: A Systematic Review and Meta‐Analysis of Cohort Studies. Authorea; 2020. [Google Scholar]
- 88. Putman M, Chock YPE, Tam H, et al. Antirheumatic disease therapies for the treatment of COVID‐19: a systematic review and meta‐analysis. Arthritis Rheumatol. 2021;73(1):36‐47. 10.1002/art.41469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Russell B, Moss C, George G, et al. Associations between immune‐suppressive and stimulating drugs and novel COVID‐19‐a systematic review of current evidence. Ecancermedicalscience. 2020;14:1‐43. 10.3332/ecancer.2020.1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Talaie H, Hosseini SM, Nazari M, et al. Is there any potential management against COVID‐19? a systematic review and meta‐analysis. Daru J Fac Pharm. 2020;28(2):1‐13. Tehran University of Medical Sciences. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Berardicurti O, Ruscitti P, Ursini F, et al. Mortality in tocilizumab‐treated patients with COVID‐19: a systematic review and meta‐analysis. Clin Exp Rheumatol. 2020;38(6):1247‐1254. [PubMed] [Google Scholar]
- 92. Boregowda U, Nanjappa A, Perisetti A, Gajendran M, Kutti Sridharan G, Goyal H. Addition of tocilizumab to the standard of care reduces mortality in severe COVID‐19: a systematic review and meta‐analysis. Front Med. 2020;7:586221. 10.3389/fmed.2020.586221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Han Q, Guo M, Zheng Y, et al. Current evidence of interleukin‐6 signaling inhibitors in patients with COVID‐19: a systematic review and meta‐analysis. Front Pharmacol. 2020;11:615972. 10.3389/fphar.2020.615972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Kaye AG, Siegel R. The efficacy of IL‐6 inhibitor Tocilizumab in reducing severe COVID‐19 mortality: a systematic review. Peer J. 2020;8:e10322. 10.7717/peerj.10322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Singh S, Khera D, Chugh A, Mittal N, Mittal R, Chugh V. Efficacy and Safety of Tocilizumab in the Treatment of SARS‐CoV‐2: A Systematic Review and Meta‐Analysis. ResearchSquare; 2020. [Google Scholar]
- 96. Abdulrahman B, Aletreby W, Mady A, et al. Tocilizumab effect in COVID‐19 hospitalized patients: a systematic review and meta‐analysis of randomized control trials. medRxiv. 2021. [Google Scholar]
- 97. Campbell C, Andersson M, Ansari MA, et al. Risk of reactivation of hepatitis B virus (HBV) and tuberculosis (TB) and complications of hepatitis C virus (HCV) following Tocilizumab therapy: a systematic review to inform risk assessment in the COVID era. medRxiv. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Chen CX, Hu F, Wei J, et al. Systematic Review and Meta‐Analysis of Tocilizumab in Persons with Coronavirus Disease‐2019 (COVID‐19); 2021. [DOI] [PMC free article] [PubMed]
- 99. Khan FA, Stewart I, Fabbri L, et al. Systematic Review and Meta‐Analysis of Anakinra, Sarilumab, Siltuximab and Tocilizumab for COVID‐19. Thorax; 2021. [DOI] [PubMed] [Google Scholar]
- 100. Peng J, Fu M, Mei H, et al. Efficacy and secondary infection risk of tocilizumab, sarilumab and anakinra in COVID‐19 patients: a systematic review and meta‐analysis. Rev Med Virol. 2021;32(3):e2295. 10.1002/rmv.2295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Petrelli F, Cherri S, Ghidini M, Perego G, Ghidini A, Zaniboni A. Tocilizumab as treatment for COVID‐19: a systematic review and meta‐analysis. World J Methodol. 2021;11(3):95‐109. 10.5662/wjm.v11.i3.95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Pinzon RT, Wijaya VO, Buana RB. Interleukin‐6 (IL‐6) inhibitors as therapeutic agents for coronavirus disease 2019 (COVID‐19): a systematic review and meta‐analysis. Journal Infect Public Health. 2021;14(8):1001‐1009. 10.1016/j.jiph.2021.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Antwi‐Amoabeng D, Kanji Z, Ford B, Beutler BD, Riddle MS, Siddiqui F. Clinical outcomes in COVID‐19 patients treated with tocilizumab: an individual patient data systematic review. J Med Virol. 2020;92(11):2516‐2522. 10.1002/jmv.26038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Burhanuddin Q, Florida S, Arjun S, Vidisha T, Pankaj C. Management of COVID‐19: a brief overview of the various treatment strategies. Cancer, Research, Statistics and Treatment. 2020;3(2):233. 10.4103/crst.crst_187_20 [DOI] [Google Scholar]
- 105. Chibber P, Haq SA, Ahmed I, Andrabi NI, Singh G. Advances in the possible treatment of COVID‐19: a review. Eur J Pharmacol. 2020:883. 10.1016/j.ejphar.2020.173372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Kim MS, An MH, Kim WJ, Hwang T‐H. Comparative efficacy and safety of pharmacological interventions for the treatment of COVID‐19: a systematic review and network meta‐analysis of confounder‐adjusted 20212 hospitalized patients. medRxiv. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Mahroum N, Watad A, Bridgewood C, et al. Systematic review and meta‐analysis of tocilizumab therapy versus standard of care in over 15,000 COVID‐19 pneumonia patients during the first eight months of the pandemic. Int J Environ Res Publ Health. 2021;18(17):9149. 10.3390/ijerph18179149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Martinez‐Vizcaino V, Mesas AE, Cavero‐Redondo I, et al. The race to find a sars‐cov‐2 drug can only be won by a few chosen drugs: a systematic review of registers of clinical trials of drugs aimed at preventing or treating Covid‐19. medRxiv. 2020. [Google Scholar]
- 109. Zeraatkar D, Cusano E, Martinez JPD, et al. Tocilizumab and sarilumab alone or in combination with corticosteroids for COVID‐19: a systematic review and network meta‐analysis. medRxiv. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Conti V, Corbi G, Sellitto C, et al. Effect of tocilizumab in reducing the mortality rate in Covid‐19 patients: a systematic review with meta‐analysis. J Personalized Med. 2021;11(7):628. 10.3390/jpm11070628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Gupta S, Padappayil RP, Bansal A, Daouk S, Brown B. Tocilizumab in patients hospitalized with COVID‐19 pneumonia: systematic review and meta‐analysis of randomized controlled trials. J Invest Med. 2022;70(1):55‐60. 10.1136/jim-2021-002001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Hariyanto TI, Hardyson W, Kurniawan A. Efficacy and safety of tocilizumab for coronavirus disease 2019 (Covid‐19) patients: a systematic review and meta‐analysis. Drug Research. 2021;71(05):265‐274. 10.1055/a-1336-2371 [DOI] [PubMed] [Google Scholar]
- 113. Kyriakopoulos C, Ntritsos G, Gogali A, Milionis H, Evangelou E, Kostikas K. Systematic review and meta‐analysis of tocilizumab administration for the treatment of Hospitalised patients with COVID‐19. SSRN; 2021. [DOI] [PMC free article] [PubMed]
- 114. Wafa IA, Pratama NR, Budi DS, Sutanto H, Rosyid AN, Wungu CDK. The Efficacy and Safety of Monoclonal Antibody Treatments against COVID‐19: A Systematic Review and Meta‐Analysis of Randomized Clinical Trials. medRxiv; 2021. [PubMed] [Google Scholar]
- 115. Kotak S, Khatri M, Malik M, et al. Use of tocilizumab in COVID‐19: a systematic review and meta‐analysis of current evidence. Cureus. 2020;12(10):e10869. 10.7759/cureus.10869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Abdulrahman B, Aletreby W, Mady A, et al. Tocilizumab Effect in COVID‐19 Hospitalized Patients: A Systematic Review and Meta‐Analysis of Randomized Control Trials. medRxiv; 2021. [Google Scholar]
- 117. Thoguluva Chandrasekar V, Venkatesalu B, Patel HK, Spadaccini M, Manteuffel J, Ramesh M. Systematic review and meta‐analysis of effectiveness of treatment options against SARS‐CoV‐2 infection. J Med Virol. 2021;93(2):775‐785. 10.1002/jmv.26302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Rubio‐Rivas M, Forero CG, Mora‐Luján JM, et al. Beneficial and harmful outcomes of tocilizumab in severe COVID‐19: a systematic review and meta‐analysis. Pharmacotherapy. 2021;41(11):884‐906. 10.1002/phar.2627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Viswanatha GL, Anjana Male C, Shylaja H. Efficacy and safety of tocilizumab in the management of COVID‐19: a systematic review and meta‐analysis of observational studies. Clin Exp Rheumatol. 2021. [DOI] [PubMed]
- 120. Zhao J, Cui W, Tian BP. Efficacy of tocilizumab treatment in severely ill COVID‐19 patients. Crit Care. 2020;24(1):1‐4. 10.1186/s13054-020-03224-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Berardicurti O, Ruscitti P, Ursini F, et al. Mortality in tocilizumab‐treated patients with COVID‐19: a systematic review and meta‐analysis. Clin Exp Rheumatol. 2020;38(6):1247‐1254. [PubMed] [Google Scholar]
- 122. Boregowda U, Perisetti A, Nanjappa A, Gajendran M, Kutti Sridharan G, Goyal H. Addition of tocilizumab to the standard of care reduces mortality in severe COVID‐19: a systematic review and meta‐analysis. Front Med. 2020;7:602. 10.3389/fmed.2020.586221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Campbell C, Andersson MI, Ansari MA, et al. Risk of reactivation of hepatitis B virus (HBV) and tuberculosis (TB) and complications of hepatitis C virus (HCV) following tocilizumab therapy: a systematic review to inform risk assessment in the COVID‐19 era. Front Med. 2021;8:1346. 10.3389/fmed.2021.706482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Chen CX, Hu F, Wei J, et al. Systematic review and meta‐analysis of tocilizumab in persons with coronavirus disease‐2019 (COVID‐19). Leukemia. 2021;35(6):1661‐1670. 10.1038/s41375-021-01264-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Han Q, Guo M, Zheng Y, et al. Current evidence of interleukin‐6 signaling inhibitors in patients with COVID‐19: a systematic review and meta‐analysis. Front Pharmacol. 2020;11:615972. 10.3389/fphar.2020.615972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Khan FA, Stewart I, Fabbri L, et al. Systematic review and meta‐analysis of anakinra, sarilumab, siltuximab and tocilizumab for COVID‐19. Thorax. 2021;76(9):907‐919. 10.1136/thoraxjnl-2020-215266 [DOI] [PubMed] [Google Scholar]
- 127. Petrelli F, Cherri S, Ghidini M, Perego G, Ghidini A, Zaniboni A. Tocilizumab as treatment for COVID‐19: a systematic review and meta‐analysis. World J Methodol. 2021;11(3):95‐109. 10.5662/wjm.v11.i3.95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Pinzon RT, Wijaya VO, Buana RB. Interleukin‐6 (IL‐6) inhibitors as therapeutic agents for coronavirus disease 2019 (COVID‐19): a systematic review and meta‐analysis. Journal Infect Public Health. 2021;14(8):1001‐1009. 10.1016/j.jiph.2021.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Singh S, Khera D, Chugh A, Mittal N, Mittal R, Chugh VK. Efficacy and Safety of Tocilizumab in the Treatment of SARS‐CoV‐2: A Systematic Review and Meta‐Analysis. ResearchSquare. 2020.
- 130. Zhao M, Lu J, Tang Y, Dai Y, Zhou J, Wu Y. Tocilizumab for treating COVID‐19: a systemic review and meta‐analysis of retrospective studies. Eur J Clin Pharmacol. 2021;77(3):311‐319. 10.1007/s00228-020-03017-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Elsokary M, Elsawah H, Elshafie A, Abdallah M. Tocilizumab in treatment of severe COVID‐19 patients: a systematic review and meta‐analysis of cohort studies. Authorea Prepr. 2020. [Google Scholar]
- 132. Mathew SR. Comparison of tocilizumab and convalescent Plasma Therapy for COVID‐19: A Systematic Review; 2020.
- 133. Ogiji E, Ofor C, Ezenkwa U, et al. Prospects of monoclonal antibodies in COVID‐19 treatment: a systematic review. Authorea Prepr. 2020. [Google Scholar]
- 134. Putman M, Chock YPE, Tam H, et al. Antirheumatic disease therapies for the treatment of COVID‐19: a systematic review and meta‐analysis. Arthritis Rheumatol. 2021;73(1):36‐47. 10.1002/art.41469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Talaie H, Hosseini SM, Nazari M, et al. Is there any potential management against COVID‐19? a systematic review and meta‐analysis. DARU J Pharm Sci. 2020;28(2):765‐777. 10.1007/s40199-020-00367-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Zhang C, Jin H, Wen YF, Yin G. Efficacy of COVID‐19 treatments: a Bayesian network meta‐analysis of randomized controlled trials. Front Public Health. 2021;9. 10.3389/fpubh.2021.729559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Antwi‐Amoabeng D, Kanji Z, Ford B, Beutler BD, Riddle MS, Siddiqui F. Clinical outcomes in COVID‐19 patients treated with tocilizumab: an individual patient data systematic review. J Med Virol. 2020;92(11):2516‐2522. 10.1002/jmv.26038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Chibber P, Haq SA, Ahmed I, Andrabi NI, Singh G. Advances in the possible treatment of COVID‐19: a review. Eur J Pharmacol. 2020;883:173372. 10.1016/j.ejphar.2020.173372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Del Pozo JS‐G, Galindo MF, Nava E, Jordán J. A systematic review on the efficacy and safety of IL‐6 modulatory drugs in the treatment of COVID‐19 patients. Eur Rev Med Pharmacol Sci. 2020;24(13):7475‐7484. [DOI] [PubMed] [Google Scholar]
- 140. Qayyumi B, Sharin Singh A, Tuljapurkar V, Chaturvedi P. Management of COVID‐19: a brief overview of the various treatment strategies. Cancer Research, Statistics, and Treatment. 2020;3(2):233. 10.4103/crst.crst_187_20 [DOI] [Google Scholar]
- 141. Zeraatkar D, Cusano E, Martinez JPD, et al. Tocilizumab and Sarilumab Alone or in Combination with Corticosteroids for COVID‐19: A Systematic Review and Network Meta‐Analysis. medRxiv; 2021. [DOI] [PMC free article] [PubMed]
- 142. Elsawah HK, Elsokary MA, Abdallah MS, ElShafie AH. Efficacy and safety of remdesivir in hospitalized Covid‐19 patients: systematic review and meta‐analysis including network meta‐analysis. Rev Med Virol. 2021;31(4):e2187. 10.1002/rmv.2187 [DOI] [PubMed] [Google Scholar]
- 143. Hung DT, Ghula S, Aziz JMA, et al. The efficacy and adverse effects of favipiravir on patients with COVID‐19: a systematic review and meta‐analysis of published clinical trials and observational studies. Int J Infect Dis. 2022;120:217‐227. 10.1016/j.ijid.2022.04.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Franchini M, Corsini F, Focosi D, Cruciani M. Safety and efficacy of convalescent plasma in COVID‐19: an overview of systematic reviews. Diagnostics. 2021;11(9):1663. 10.3390/diagnostics11091663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Shahsavarinia K, Ghojazadeh M, Sanaie S, et al. Clinical efficacy of hydroxychloroquine or chloroquine in patients with COVID‐19: an umbrella review. Pharmaceut Sci. 2021;27(4):481‐488. 10.34172/ps.2021.66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Deng J, Heybati K, Ramaraju HB, Zhou F, Rayner D, Heybati S. Differential efficacy and safety of anti‐SARS‐CoV‐2 antibody therapies for the management of COVID‐19: a systematic review and network meta‐analysis. Infection. 2022. 10.1007/s15010-022-01825-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Yu S‐Y, Koh D‐H, Choi M, et al. Clinical efficacy and safety of interleukin‐6 receptor antagonists (tocilizumab and sarilumab) in patients with COVID‐19: a systematic review and meta‐analysis. Emerg Microb Infect. 2022;11(1):1154‐1165. 10.1080/22221751.2022.2059405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Montazersaheb S, Hosseiniyan Khatibi SM, Hejazi MS, et al. COVID‐19 infection: an overview on cytokine storm and related interventions. Virology J. 2022;19(1):92. 10.1186/s12985-022-01814-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Akbari A, Razmi M, Sedaghat A, et al. Comparative effectiveness of pharmacological interventions on mortality and the average length of hospital stay of patients with COVID‐19: a systematic review and meta‐analysis of randomized controlled trials. Expert Rev Anti‐infect Ther. 2022;20(4):585‐609. 10.1080/14787210.2022.1997587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Ebrahimi Chaharom F, Pourafkari L, Ebrahimi Chaharom AA, Nader ND. Effects of corticosteroids on Covid‐19 patients: a systematic review and meta‐analysis on clinical outcomes. Pulm Pharmacol Therapeut. 2022;72:102107. 10.1016/j.pupt.2021.102107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Siemieniuk RAC, Bartoszko JJ, Ge L, et al. Drug treatments for Covid‐19: living systematic review and network meta‐analysis. BMJ. 2020;370:m2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Pormohammad A, Ghorbani S, Baradaran B, et al. Clinical characteristics, laboratory findings, radiographic signs and outcomes of 61,742 patients with confirmed COVID‐19 infection: a systematic review and meta‐analysis. Microb Pathog. 2020;147:104390. 10.1016/j.micpath.2020.104390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Navarro‐Millán I, Singh JA, Curtis JR. Systematic review of tocilizumab for rheumatoid arthritis: a new biologic agent targeting the interleukin‐6 receptor. Clin Therapeut. 2012;34(4):788‐802.e3. 10.1016/j.clinthera.2012.02.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Borges do Nascimento IJ, O’Mathúna DP, von Groote TC, et al. Coronavirus disease (COVID‐19) pandemic: an overview of systematic reviews. BMC Infect Dis. 2021;21(1):525. 10.1186/s12879-021-06214-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Chivese T, Musa OAH, Hindy G, et al. Efficacy of chloroquine and hydroxychloroquine in treating COVID‐19 infection: a meta‐review of systematic reviews and an updated meta‐analysis. Trav Med Infect Dis. 2021;43:102135. 10.1016/j.tmaid.2021.102135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Jung RG, Di Santo P, Clifford C, et al. Methodological quality of COVID‐19 clinical research. Nat Commun. 2021;12(1):943. 10.1038/s41467-021-21220-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Treskova‐Schwarzbach M, Haas L, Reda S, et al. Pre‐existing health conditions and severe COVID‐19 outcomes: an umbrella review approach and meta‐analysis of global evidence. BMC Med. 2021;19(1):212. 10.1186/s12916-021-02058-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Kiss S, Gede N, Hegyi P, et al. Early changes in laboratory parameters are predictors of mortality and ICU admission in patients with COVID‐19: a systematic review and meta‐analysis. Med Microbiol Immunol. 2021;210(1):33‐47. 10.1007/s00430-020-00696-w [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, upon reasonable request.


