Abstract
Background
Plateletpheresis involves platelet separation and collection from whole blood while other blood cells are returned to the donor. Because platelets are replaced faster than red blood cells, as many as 24 donations can be done annually. However, some frequent apheresis platelet donors (>20 donations annually) display severe plateletpheresis‐associated lymphopenia; in particular, CD4+T but not B cell numbers are decreased. COVID‐19 vaccination thereby provides a model to assess whether lymphopenic platelet donors present compromised humoral immune responses.
Study Design and Methods
We assessed vaccine responses following 2 doses of COVID‐19 vaccination in a cohort of 43 plateletpheresis donors with a range of pre‐vaccination CD4+T cell counts (76–1537 cells/μl). In addition to baseline T cell measurements, antibody binding assays to full‐length Spike and the Receptor Binding Domain (RBD) were performed pre‐ and post‐vaccination. Furthermore, pseudo‐particle neutralization and antibody‐dependent cellular cytotoxicity assays were conducted to measure antibody functionality.
Results
Participants were stratified into two groups: <400 CD4/μl (n = 27) and ≥ 400 CD4/μl (n = 16). Following the first dose, 79% seroconverted within the <400 CD4/μl group compared to 87% in the ≥400 CD4/μl group; all donors were seropositive post‐second dose with significant increases in antibody levels. Importantly differences in CD4+ T cell levels minimally impacted neutralization, Spike recognition, and IgG Fc‐mediated effector functions.
Discussion
Overall, our results indicate that lymphopenic plateletpheresis donors do not exhibit significant immune dysfunction; they have retained the T and B cell functionality necessary for potent antibody responses after vaccination.
Keywords: COVID‐19, donor health, humoral responses, lymphopenia, plateletpheresis
Abbreviations
- COVID‐19
Coronavirus disease 2019
- SARS‐CoV‐2
Severe acute respiratory syndrom coronavirus 2
- VOC
Variants of Concern
- Ig(A, G, M)
Immunoglobulin A, G or M
- ELISA
Enzyme Linked Immunosorbent Assay
- ADCC
Antibody Dependent Cellular Cytotoxicity
- RBD
Receptor Binding Domain
1. INTRODUCTION
Platelets are used for the treatment of thrombocytopenia and other related blood and clotting abnormalities. While platelets can be recovered from whole blood donations, the preferred method of collection is apheresis which allows donors to donate as many as 24 times annually. 1 The process of plateletpheresis involves platelet extraction from whole blood by an apheresis machine and the subsequent return of other blood components to the donor.
Recent literature highlighted a striking correlation between the frequency of platelet donations by apheresis and lymphopenia. 2 , 3 In particular, several apheresis donors with lifetime donations of more than fifty exhibited severe T cell lymphopenia. 2 , 3 One observational study reported that the decline in CD4+T cells and/or CD8+T cells had a modest contribution to immunosuppression‐related or opportunistic infections. 4 In contrast, while Gansner et al 3 showed that lymphopenia was associated with a reduction in the CD4+T helper 17 subsets (Th17), key mediators of mucosal immunity against pulmonary pathogens, 5 their results and evidence from analysis of samples from individuals a year after ceasing platelet donations, suggest functional immune responses are maintained in these individuals. 2 , 3
Exposure to SARS coronavirus‐2 (SARS‐CoV‐2), the aetiologic agent responsible for the COVID‐19 pandemic, 6 can result in individuals suffering anything from asymptomatic infection to severe respiratory failure such as acute respiratory distress syndrome (ARDS) leading to death. 7 SARS‐CoV‐2 infections continue to be a major health burden globally. However, modern medicine facilitated the rapid development of promising treatments and importantly, COVID‐19 vaccines. 8 , 9 , 10 To date there are ten COVID‐19 vaccines that have been granted Emergency Use Authorization (EUA) by the World Health Organization. 9 These include protein subunit vaccines, the widely administered mRNA vaccines, recombinant adenoviral vector vaccines as well as inactivated whole virus vaccines. 10 Others are currently in various phases of development or authorization.
Evidence in the literature supports the existence of strong T cell immunity in convalescent individuals, 11 and the retention of broad cross‐reactive responses to multiple variants one year post‐initial exposure has also been reported. 12 Furthermore SARS‐CoV‐2 memory T cell responses have been speculated to be important in alleviating disease severity. 13 COVID‐19 vaccination is shown to provide adequate boosting of the humoral response nearing that of natural infection in healthy individuals. 14 , 15 , 16 To our knowledge, none have thus far reported on immune responses of lymphopenic plateletpheresis donors following vaccination. Considering the critical role of CD4+T cells in the adaptive humoral response, 17 we sought to determine the ability of these donors to mount an immune response to COVID‐19 vaccination.
2. STUDY DESIGN AND METHODS
2.1. Recruitment, sample collection, and ethics statement
Apheresis platelet donors who donate more than 5 times annually on the Trima Accel automated blood collection system (Terumo BCT, Lakewood, CO) were recruited for the study after informed consent. Recruitment commenced just before the vaccination campaign in May 2021 in the Province of Québec, Canada. A total of 48 donors were contacted and all agreed to participate; however, 4 were excluded from the study because of prior COVID‐19 infection and one was excluded because of an inadequate pre‐vaccination sample leading, which prevented the initial CD4 count from being established. Initial blood samples were obtained from the remaining 43 donors at recruitment (baseline‐V0), before platelet donation. Individuals were administered 2 doses of mRNA vaccines (Pfizer or Moderna), AstraZeneca, or a combination of both (Table 1) with an approximate 11‐week interval between doses 1 and 2. Follow‐up samples were obtained at subsequent visits for platelet donation, at least 3 weeks after receiving a vaccine dose: approximately 5‐weeks post‐dose 1 (V1) and 6‐weeks post‐dose 2 (V2). The blood samples were taken before each platelet donation for platelet enumeration (mandatory analysis at each donation) and used for T cell analysis and plasma recovery. Recovered plasma was stored frozen until required and subsequently heat‐inactivated for 1 h at 56°C before use. All work was conducted in accordance with the Declaration of Helsinki in terms of informed consent and study approval by the Héma‐Québec Ethics Committee (CER 2021‐002).
TABLE 1.
Cohort characteristics
| <400 CD4+ cells/μl | ≥400 CD4+ cells/μl | p value | |
|---|---|---|---|
| (n = 27) | (n = 16) | ||
| Age, year | |||
| Median, (IQR) | 68 (64.5–72) | 62 (55.5–68.5) | 0.0073 |
| Sex | |||
| Female | 2 (7.4) | 0 (0%) | 0.5216 |
| Male | 25 (92.6) | 16 (100%) | |
| Lifetime donations | |||
| Median, (IQR) | 166 (106–251.5) | 24 (9.75–44) | <0.0001 |
| Vaccine administered a | |||
| Pfizer | 16 (64.0) | 6 (46.2) | 0.5678 |
| Moderna | 3 (12.0) | 2 (15.4) | |
| AstraZeneca | 3 (12.0) | 1 (7.6) | |
| Mixed | 3 (12.0) | 4 (30.8) | |
| Interval between doses | |||
| Median (Days), (IQR) | 77 (68–82) | 71 (62–79) | 0.139 |
Note: Data represented by n (percentage) or median (first quartile and third quartile). Mann Whitney was used to compare age, interval between doses, and lifetime donations. Exact Fischer tests to compare sex and vaccines administered.
Vaccination information was missing for 2 and 3 donors in the <400 CD4+ cells/μl and the ≥400 CD4/μl group, respectively.
2.2. Blood counts
Complete blood counts and differentials were established using a Coulter Ac·T 5diff AL hematology analyzer (Beckman‐Coulter Life Sciences, Mississauga, ON, Canada). CD4+ and CD8+T cell counts were determined by flow cytometry on a BD Accuri C6 using the CD4 FITC/CD8 PE/CD3 PerCP BD Tritest (BD Biosciences, Mississauga, ON, Canada) according to the manufacturer's instructions.
2.3. Plasmids and monoclonal antibodies
The plasmids encoding the SARS‐CoV‐2 Spike variants D614G, B.1.617.2 (Delta) B.1.1.529 (Omicron) and the green fluorescence protein expressor (pIRES2‐eGFP; Clontech, Mountain View, CA) were previously described. 18 , 19 , 20 The lentiviral vector pNL4.3 R‐E Luc used to generate pseudoviruses was obtained from NIH AIDS Reagent Program. The CR3022 antibody which recognizes both the SARS‐CoV and the SARS‐CoV‐2 Spike proteins 21 and the conformationally‐independent anti‐S2 antibody CV3‐25 18 , 19 , 22 , 23 , 24 were used for normalization in various assays where indicated.
2.4. Cell lines
293 T human embryonic kidney cells (obtained from ATCC, Manassas, VA) were maintained in Dulbecco's modified Eagle's medium (DMEM) (Wisent, Montreal, QC, Canada) containing 5% fetal bovine serum (VWR) and 100 mg/mL of penicillin–streptomycin (Wisent). The 293 T‐ACE2 cell line, CEM.NKr CCR5+ cells, and CEM.NKr cells stably expressing a GFP‐tagged full‐length SARS‐CoV‐2 Spike (CEM.NKr.SARS‐CoV‐2.Spike cells) have been previously reported. 25 , 26 , 27 All cells were maintained at 37°C under 5% CO2.
2.5. Enzyme‐linked immunosorbent assays (ELISA)
The in‐house ELISA targeting the receptor‐binding domain (RBD) of the SARS‐CoV‐2 spike protein used for the determination of total RBD‐specific immunoglobulins (Ig) was described before. 28 The optical density (OD) threshold for seropositivity was set at 0.230 to obtain a sensitivity of 98.9% and a specificity of 98.5%. 29 The assay was also adapted to analyze the three main classes of Ig (IgA, IgG, and IgM). Plasma samples were diluted at 1:100 for total Ig determination, 1:400 for IgA and IgM, and 1:800 for IgG. Anti‐human polyvalent IgA + IgG + IgM (H + L), IgA alpha chain specific, IgG Fcγ specific, or IgM Fc5μ specific conjugated to HRP (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA) were used as secondary antibodies in the corresponding ELISA. To calculate the RBD‐avidity index, a stringent ELISA incorporating the chaotropic agent urea at 8 M compared to 0 M, was performed as previously described 15 and the avidity index was calculated as reported. 15 , 30
2.6. Pseudovirus neutralization assay
Pseudovirus neutralization assays were performed as previously. 15 , 18 , 31 Briefly pseudoviruses expressing indicated Spike glycoprotein (D614G, Delta or Omicron) 20 , 25 were incubated with plasma (1:50; 1:250; 1:1250; 1:6250; 1:31250) for 1 h at 37°C, then added to 293 T‐ACE2 target cells. 25 Cells were cultured for 48 h at 37°C and then lysed with 30 μl of passive lysis buffer (Promega, Madison, WI). Following one freeze–thaw cycle, luciferase activity was measured on an LB942 TriStar luminometer (Berthold Technologies, Bad Wildbad, Germany) after the addition of 100 μl of luciferin buffer (15 mM MgSO4, 15 mM KH2PO4 [pH 7.8], 1 mM ATP, and 1 mM dithiothreitol) and 50 μl of 1 mM d‐luciferin potassium salt (Prolume, Pinetop, AZ). The neutralization half‐maximal inhibitory dilution (ID50) represents the plasma dilution required to inhibit 50% of the infection of 293 T‐ACE2 cells.
2.7. Cell surface spike recognition and flow cytometry analysis
293 T were transfected with full‐length SARS‐CoV‐2 Spikes and GFP expressor using the calcium‐phosphate method as previously described. 25 48 h post‐transfection, Spike‐expressing 293 T cells were stained with the control CV3‐25 (5 mg/mL) or plasma from vaccinated individuals (1:250 dilution) for 45 min at 37°C. AlexaFluor‐647‐conjugated goat anti‐human IgG (1:1000 dilution) (Thermo Fisher, Mississauga, ON, Canada) were used as secondary antibodies. The percentage of GFP + Spike‐expressing cells was determined by gating the living cell population based on viability dye staining (Aqua Vivid, Invitrogen, Walthem, MA). Samples were acquired on an LSR II cytometer (BD Biosciences), and data analysis was performed using FlowJo v10.7.1 (Tree Star, Woodburn, OR). Median fluorescence intensities (MFI) obtained with plasma were normalized to the MFI obtained with CV3‐25 and presented as a percentage of CV3‐25 binding, as reported. 19 , 22 , 23 , 24
2.8. Antibody‐dependent cellular cytotoxicity (ADCC) assay
Anti‐SARS‐CoV‐2 ADCC assays were performed as reported. 26 , 27 Briefly, plasma from donors (1:500) was incubated at 37°C for 5 h with target cells (comprising parental CEM.NKr CCR5+ cells mixed at a 1:1 with GFP‐tagged CEM.NKr.SARS‐CoV‐2.Spike cells) and effector cells [overnight rested peripheral blood mononuclear cells] for 5 hr, before being fixed in a 2% PBS‐formaldehyde solution. All samples were acquired on an LSRII cytometer (BD Biosciences) and data analysis was performed using FlowJo v10.7.1 (Tree Star). ADCC activity was calculated as previously reported. 26 The specificity threshold was established using the following formula: mean of pre‐pandemic SARS‐CoV‐2 negative plasma +3 standard deviation of the mean of pre‐pandemic SARS‐CoV‐2 negative plasma.
2.9. Statistical analysis
Two‐tailed non‐parametric tests were used when appropriate with a significance threshold established at α = 0.05. Mann Whitney test was used to compare the two groups. Kruskal‐Wallis test followed by Dunn's post hoc test was used for independent samples to compare groups under different conditions. Friedman test for dependent samples was used to compare participants at different time points. All analyses were performed using GraphPad Prism software version 9.3.0 (San Diego, CA). Finally, data visualization/network correlation analysis for the joint representation of the study variables was made using circular edge bundling graphs generated in an undirected mode in program R v.4.1.2 and RStudio v. 2021.09.0 using ggraph, igraph, tidyverse, and RColorBrewer packages. 32 Edges are only shown if p < 0.05, and nodes are sized according to the sum of the connecting edges' absolute r values. Nodes are color‐coded according to the groups of variables.
3. RESULTS
3.1. Frequent plateletpheresis donors exhibit reduction in CD4 + and CD8 + T cell counts
A cohort of 43 plateletpheresis donors was recruited for the study and subsequently classified into two groups; the CD4‐low group consisted of individuals with laboratory ranges of less than 400 CD4+T cells/μl of blood whereas subjects with levels ranging equal to or above 400 were considered to be in the normal range (CD4‐high group) (Table 1; Figure 1). Only infection‐naïve participants were included in the study. Participants were asked to confirm their vaccination date by email; none of them reported SARS‐CoV‐2 infection during the study period. All participants provided at least 2 samples; 35 provided 3 samples (pre‐vaccination, post‐1st and post‐2nd dose) while the post‐1st dose sample was missing for 6 participants (3 from the CD4‐low and 3 from the CD4‐high group) and the post‐2nd dose sample was missing for two participants (both from the CD4‐high group). More than 50% of study participants had been administered the mRNA vaccine, Pfizer, whereas only a small proportion received Moderna (~11%), AstraZeneca (~10%), or a mixed dose (~16%) which comprised of either one of the mRNA vaccines with AstraZeneca.
FIGURE 1.
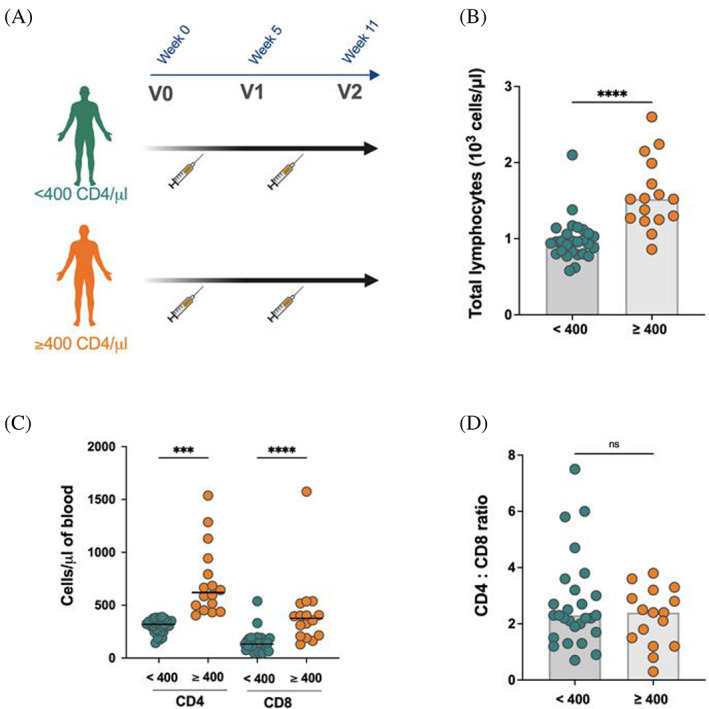
Lymphocyte counts in plateletpheresis donors. (A) Apheresis donor cohort design delineating the stratification into CD4‐low (<400 CD4+T cells/μl of blood) (n = 27) and CD4‐high (>400 CD4+Tcells/μl of blood) (n = 16) groups and highlighting the time of sample collection relative to COVID‐19 vaccination; (B) Total lymphocyte counts in CD4‐low and CD4‐high groups; statistical significance was tested using two‐tailed Mann Whitney test (C) levels of CD4+T cells and CD8+T cells in CD4‐low and CD4‐high groups; statistical significance was tested using two‐tailed Kruskal‐Wallis with post‐test Dunn's correction (D) comparison of the CD4:CD8 ratio between CD4‐low and CD4‐high groups; each symbol represents one donor. Bars (B, D) and lines (C) represent the median. Statistical significance was tested using the two‐tailed Mann–Whitney test (***p < 0.001; ****p < 0.0001; ns, non‐significant). [Color figure can be viewed at wileyonlinelibrary.com]
Overall, total lymphocyte counts in the CD4‐low group were significantly lower compared to the CD4‐high group (p < 0.0001) (Figure 1B). This translated to significantly lower CD4+T cells (p < 0.001) and CD8+T cells (p < 0.0001) (Figure 1C), consistent with previous reports. 2 , 3 Strikingly, individuals within the CD4‐low group had a seven‐fold higher frequency of donations (p < 0.0001) (Table 1), supporting current literature that frequent plateletpheresis donations are indeed associated with declining T cell numbers. 2 , 3 , 4 , 33 Of note, the CD4:CD8 ratio, which is often utilized in the context of HIV‐related immunosuppression as a surrogate to assess the overall strength of immune function, 34 did not significantly differ between the two groups (Figure 1D).
3.2. Lymphopenic platelet donors develop normal levels of anti‐RBD antibodies following SARS‐CoV‐2 vaccination
We first measured the presence of antibodies (total Ig, IgM, IgG, and IgA) recognizing the receptor binding domain (RBD) of SARS‐CoV‐2 Spike at V0, V1, and V2 (Figure 2) using an in‐house RBD‐ELISA. 28 As expected, anti‐RBD levels were low for total immunoglobulins at V0 (Figure 2A). The levels of IgM, IgG, and IgA (Figure 2B–D) were also low at V0 and did not significantly differ between both groups. Following dose 1, there was a statistically significant increase in total immunoglobulins that recognized the RBD for both groups (Figure 2A), which was further increased following the second dose. We observed a slightly higher response in the CD4‐high group, but this difference was not statistically significant. In agreement with a robust isotype switch, the second dose boosted levels of anti‐RBD IgG approximately ten‐fold for the CD4‐low group and five‐fold for the CD4‐high group (Figure 2C), with only 1.5‐fold induction of anti‐RBD‐IgA (Figure 2D) and almost negligible induction of IgM (Figure 2B). These results are consistent with antibody maturation as also shown by the significant increase in anti‐RBD avidity that was similar in both groups (Figure 2E). In agreement with this interpretation, recent work showed that anti‐RBD avidity correlated with B cell class switch 30 indicative of maturation within B cell germinal centers. 35 , 36 Altogether, these results suggest that antibody maturation is minimally impacted in the CD4‐low group.
FIGURE 2.
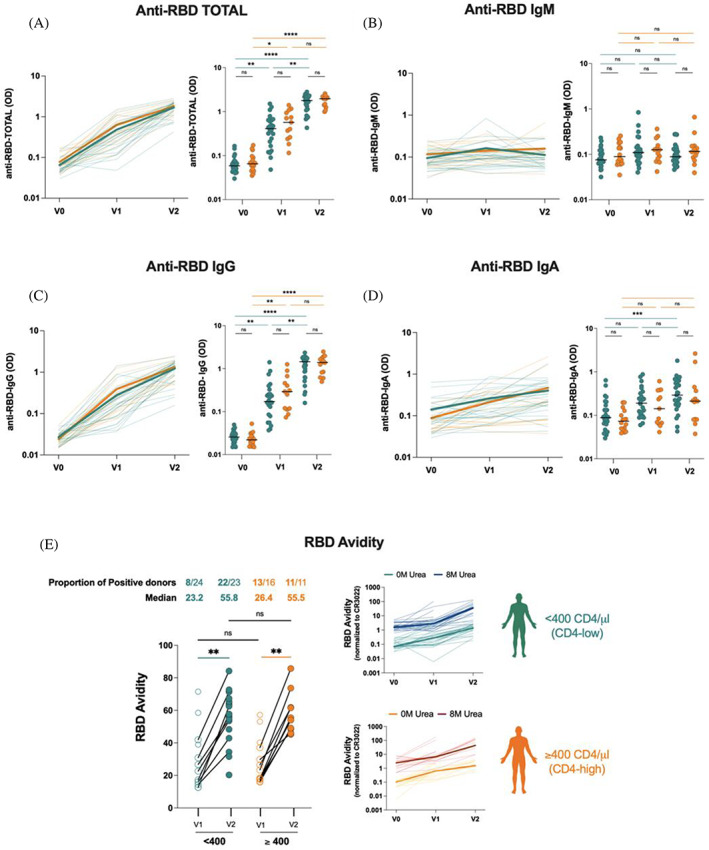
Elicitation of anti‐RBD antibodies in plateletpheresis donors. Indirect ELISAs were performed to determine the presence of (a) Total, (B) IgM, (C) IgG, and (D) IgA RBD‐specific antibodies in plasma before (V0) and after each dose of COVID‐19 vaccine (V1, V2). Curves in the left panels show the ODs for each donor at each time point. Bold lines represent the median OD values for both groups. (E) a modified ELISA comprising 8 M urea was performed to assess the strength of binding of antibodies within the plasma of platelet donors. (left panel) avidity index measured post‐dose 1 (V1) and post‐dose 2 (V2) are represented by the before‐after symbols. Each symbol represents one donor. (right panel) line graphs showing binding with (8 M) or without (0 M) urea for both groups. Curves represent each donor at each timepoint with bold lines representing the median. Statistical significance was tested using two‐tailed Kruskal‐Wallis tests with Dunn's correction (*p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001; ns, non‐significant). [Color figure can be viewed at wileyonlinelibrary.com]
3.3. Lymphopenic plateletpheresis donors exhibit comparable functional responses against D614G, omicron, and Delta
The emergence of variants of concern globally and in particular the persistence and improved transmissibility of the B.1.1.529 variant (Omicron) 37 , 38 continues to be of major public health concern. Using our previously described flow cytometry‐based assay for cell surface recognition of Spike, 14 , 19 , 24 we assessed recognition of D614G, Delta, and Omicron. Analysis showed that while there were low baseline levels of D614G Spike recognition before vaccination, there was a log increase in levels of antibodies at V1, which was further elevated tenfold in both groups at V2 (Figure 3A). Again, there were no significant differences in anti‐Spike recognition between both groups. Similar patterns were observed for Delta (Figure 3B) and Omicron (Figure 3C) Spikes, although the absolute levels of anti‐Spike were lower at V2 for both groups (especially for Omicron) compared to D614G (Figure S1), consistent with previous reports. 14 In agreement with recent observations, 14 , 39 , 40 , 41 , 42 , 43 Spike‐specific antibodies elicited shortly after vaccination fail to present robust neutralization (Figure 3D–F). However, as shown in Figure 3D, the second vaccine dose was associated with a marked increase in D614G neutralization. Neutralization increased approximately two‐fold in the CD4‐low group and five‐fold in the CD4‐high group (the between‐group difference was not significant). Similar neutralization trends were observed for pseudoviral particles bearing the Delta (Figure 3E) and Omicron Spikes (Figure 3F). Consistent with Delta and Omicron resistance to vaccine‐elicited antibodies, these Variants of concern (VOC) were less susceptible to neutralization even at V2 (Figure 3E,F).
FIGURE 3.
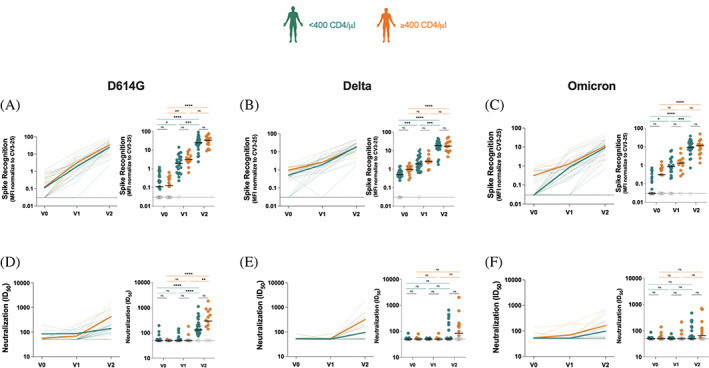
Evolution of spike recognition and neutralization activity of antibodies in plateletpheresis donors. (A)–(C) 293 T cells were transfected with a full‐length spike from (A) D614G, (B) Delta, and (C) omicron variants and stained with the CV3‐25 ab or with plasma from platelet donors. Values represent MFI normalized to CV3‐25 binding. Curves in the left‐panels show the MFI for each donor at each time point. Bold lines represent the median MFI for both groups. Threshold levels for seropositivity (0.03) are represented by dotted lines. (D)–(F) neutralization activity was determined by incubating pseudoviruses bearing (D) D614G, (E) Delta, or (F) omicron spikes with serial dilutions of plasma at 37°C for 1 h prior to infecting 293 T‐ACE2 cells. The neutralization half‐maximal inhibitory dilution (ID50) was determined using non‐linear regression in GraphPad Prism software. Curves in the left‐panels show the ID50 for each donor at each time point. Bold lines represent the median ID50 for both groups. Threshold levels for positive detection are represented by the dotted line. Statistical significance was tested using two‐tailed Kruskal‐Wallis tests with Dunn's correction (*p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001; ns, non‐significant). [Color figure can be viewed at wileyonlinelibrary.com]
3.4. Fc‐effector activities of antibodies are not compromised in lymphopenic plateletpheresis donors
Weak and/or declining neutralizing activity of vaccine‐elicited antibodies 14 , 39 , 40 , 43 , 44 despite effective and prolonged Spike recognition 14 has been previously reported. The role of Fc‐mediated effector functions in defense against SARS‐CoV‐2 has recently been highlighted with Spike recognition correlating positively with Fc‐mediator functionality. 14 , 27 Given that plasma from both groups recognized the membrane‐bound full‐length Spike, we assessed their potential to mediate ADCC, as recently reported for healthy vaccinated and/or previously infected individuals. 14 , 23 , 26 , 27 As expected, there was minimal ADCC activity at V0, which increased for only a few individuals in both groups at V1 (Figure 4 ). However, the second dose boosted ADCC activity more profoundly, with median ADCC values of ~42% (range 3.82%–50.62%) and ~45% (range 29.07%–54.67%) for CD4‐low and CD4‐high groups respectively. We observed no statistically significant differences between ADCC activity of both groups, thus indicating that the lymphopenia observed in some frequent platelet donors does not negatively impact the development of functional vaccine‐elicited antibodies.
FIGURE 4.
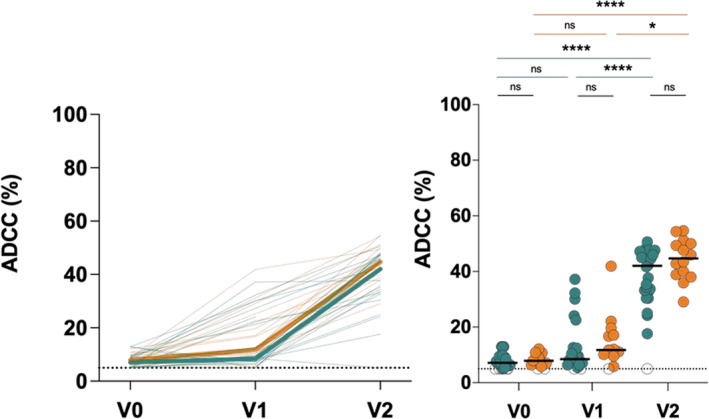
Fc‐effector function of antibodies in plateletpheresis donors. A flow cytometry‐based ADCC assay was performed using target cells comprising CEM‐NKr parental cells and CEM.NKr‐S cells were mixed in a 1:1 ratio and effector cells from uninfected donors were incubated with plasma from platelet donors. Curves in the left‐panels and dots in the right‐panels show the percent (%) ADCC for each donor at each time point. Bold lines represent the median percent ADCC for both groups. Threshold levels for positive ADCC are represented by the dotted line. Statistical significance was tested using two‐tailed Kruskal‐Wallis tests with Dunn's correction (*p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001; ns, non‐significant). [Color figure can be viewed at wileyonlinelibrary.com]
3.5. Integrated analysis of vaccine‐induced responses
To investigate the relationships between the study variables and immune responses, we performed network correlation analyses, which were separately done for both groups (Figure 5). Each edge‐bundling plot depicts the strength of connectedness between different study variables (age, sex, lymphocyte counts, etc) and measured responses (avidity, neutralization, etc) allowing visual representation of relationships between parameters and the Spearman's rank correlation coefficient, r (shown as blue or red for negative or positive pairwise correlations, respectively). In the CD4‐low data set (Figure 5A), the correlation network is more balanced where neutralization responses, particularly at V2, are more densely connected to the broad variety of binding responses compared to the CD4‐high data. In the CD4‐high data set (Figure 5B), there is a strong, focused network involving several binding responses (ie Spike and anti‐RBD recognition). This pattern is highlighted upon segregation based on different timepoints (Figure S2). Overall, though levels of CD4+T cells appear to influence neutralization levels/potency in particular (Figure 3), this does not critically alter the associations between vaccine‐induced responses.
FIGURE 5.
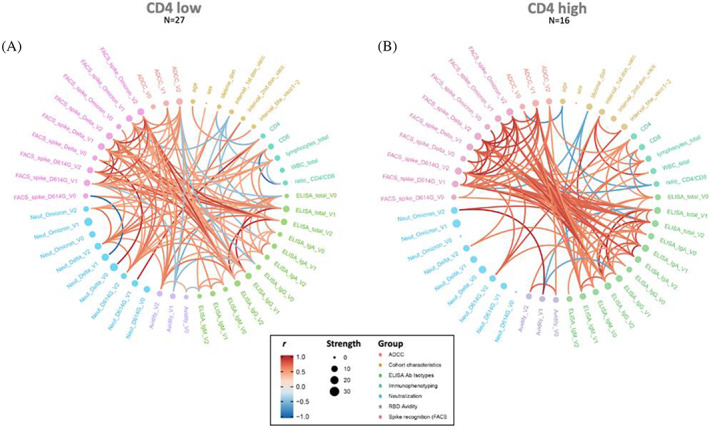
Network model of correlations between humoral responses in CD4‐low and CD4‐high plateletpheresis donors. Circular edge bundling plots where red and blue edges represent positive and negative pairwise correlations between connected parameters, respectively. Only significant correlations (p < 0.05, Spearman rank test) are displayed. Nodes are color‐coded based on the grouping of variables according to the legend (where r is the Spearman correlation coefficient; group categorizes the different variables). Node size corresponds to the degree of relatedness of correlations. Edge bundling plots are shown for correlation analyses using both CD4‐low (a) and CD4‐high (B) data sets. [Color figure can be viewed at wileyonlinelibrary.com]
4. DISCUSSION
Platelet transfusion has been an integral component of effective therapy for patients suffering from various hematologic malignancies and thrombocytopenias. 45 , 46 Platelet collection is a major activity of blood organizations; an adequate supply of platelet concentrates to fulfill the needs of thrombocytopenic patients requires continuous efforts in donor recruitment and retention. Recent literature on lymphopenia associated with frequent platelet donations 2 , 3 , 4 , 33 raised important questions related to donor health and more specifically their ability to mount adequate immune responses. The COVID‐19 vaccination campaign provided a convenient proxy for assessing immune function in our cohort of frequent plateletpheresis donors.
Our cohort of apheresis platelet donors was stratified into two groups: the CD4‐low group comprised of individuals with less than 400 CD4+T cells/μl of blood whereas those with levels equal to or above 400 CD4+T cells/μl were designated as being in the CD4‐high group. Individuals were vaccinated with one of the three main vaccines rolled out early in the pandemic: Pfizer, Moderna, and AstraZeneca, with only a small proportion from both groups receiving a mixed dose. The majority of donors received the mRNA vaccines with a comparable dosage of vaccine types for the two groups.
Consistent with the study from Kaufman's group, 3 we observed that the frequency of donations was associated with the reduction in CD4+ and CD8+T cell counts in peripheral blood; however, this depletion minimally impacted the humoral immune response. In agreement with earlier work on the response to COVID‐19 vaccination, 15 , 31 , 47 , 48 , 49 there were heightened IgG responses in both groups following vaccination, concomitant with an effective maturation of the immune response as revealed by an increase in the avidity index. Emergence of the highly transmissible Omicron also allowed us to expand on these findings, as recent work 50 demonstrated that ancestral SARS‐CoV‐2‐specific T cells cross‐recognized Omicron. We observed that vaccination elicited SARS‐CoV‐2‐specific antibodies recognized Spike from Omicron as well as D614G and Delta but also exhibited neutralization and Fc‐effector functions comparable to those elicited in individuals without lymphopenia. Importantly, as depicted in Figures 2A,C all donors, including those with the lowest CD4 counts, mounted anti‐RBD IgG responses comparable to the CD4‐high group at V2. Additionally, integrated analyses of all study variables revealed concerted responses for the CD4‐low group intriguingly associated with neutralization. However, tighter networks of linear correlations existed for both groups with respect to Spike recognition, anti‐RBD binding, and ADCC supporting the effective establishment of the immune response even in plateletpheresis lymphopenic individuals.
The most likely explanation for plateletpheresis‐associated lymphopenia is the retention of a significant number of blood cells in the leukoreduction system chamber of the Trima Accel collection system at the end of the procedure; the majority of sequestered cells being lymphocytes. 51 The human body contains approximately 460 × 109 lymphocytes 52 and only 2%–3% of total lymphocytes have been estimated to reside in peripheral blood, the remainder being located in lymphoid tissues and organs such as lymph nodes, spleen, and others. 53 Earlier studies on blood lymphocyte composition in pathological or non‐pathological conditions suggested that blood represents a distinct compartment in terms of lymphocyte composition. 54 Of note, initiation of T cell‐dependent immune responses occurs in lymphoid organs, following the presentation of pathogen‐derived peptides by antigen‐presenting cells. Activated follicular CD4+T cells provide critical signals for B cell activation, proliferation, and differentiation leading to the formation of germinal centers in secondary lymphoid organs where maturation of the adaptive immune response occurs. 55 Therefore, the reduction in peripheral blood CD4+T cells is most likely not predictive of immune dysfunction in affected individuals as supported by the data presented herein as well as previous reports. 2 , 3 , 33
Nevertheless, individuals with plateletpheresis‐associated lymphopenia who may be unaware of their status might still be referred for hematology consultations and sometimes invasive examinations after routine blood tests due to their low lymphocyte counts, when their condition can be explained by the frequency of voluntary platelet donations. Importantly, lymphocyte analysis of 15 former apheresis donors showed that a small proportion was lymphopenic at least a year after ceasing platelet donation. 2 Whether lymphopenia persists in our cohort after ceasing plateletpheresis remains to be determined.
Overall, our work supports that lymphopenic platelet donors do not present significant immune dysfunction as, despite low CD4+T cell counts, COVID‐19 vaccines elicited moderate to high levels of antibodies which also translated to efficient neutralization and Fc‐mediator functions, indicating that these individuals retain CD4+T and B cell interactions required for potent antibody responses.
FUNDING INFORMATION
Funding received from Ministère de l'Économie et de l'Innovation du Québec, by the Fondation du CHUM, by a CIHR foundation grant no.352417, by a CIHR operating grant no.177958, and by an Exceptional Fund COVID‐19 from the Canada Foundation for Innovation (CFI) no.41027 to Andrés Finzi Work on variants was supported by the Sentinelle COVID Quebec network (LSPQ and FRQS) to Andrés Finzi. Andrés Finzi is the recipient of Canada Research Chair on Retroviral Entry no. RCHS0235 950‐232424. Annemarie Eare Laumaea was supported by a MITACS Accélération postdoctoral fellowship.
CONFLICT OF INTEREST
The authors have disclosed no conflicts of interest.
Supporting information
Figure S1. Spike recognition and neutralization activity of antibodies within the plasma of apheresis platelet donors at each time point
Figure S2. Network model of correlations between humoral responses in CD4‐ low and CD4‐high separated based on timepoints
ACKNOWLEDGMENTS
The authors are grateful to the Héma‐Québec plateletpheresis donors who participated in this study. The authors thank the CRCHUM BSL3 and Flow Cytometry Platforms for technical assistance. We thank Dr. M. Gordon Joyce (U.S. MHRP) for the monoclonal antibody CR3022.
Laumaea AE, Lewin A, Chatterjee D, Marchitto L, Ding S, Gendron‐Lepage G, et al. COVID‐19 vaccine humoral response in frequent platelet donors with plateletpheresis‐associated lymphopenia. Transfusion. 2022;62(9):1779–1790. 10.1111/trf.17037
Funding information Canada Foundation for Innovation, Grant/Award Number: 41027; Canadian Institutes of Health Research, Grant/Award Numbers: 177958, 352417; Fondation du CHUM; Ministere de l'economie et de l'Innovation du Quebec
Contributor Information
Andrés Finzi, Email: andres.finzi@umontreal.ca.
Renée Bazin, Email: renee.bazin@hema-quebec.qc.ca.
REFERENCES
- 1. Hema Québec. Platelet Donation. 2022. [cited 2022 7th April ]. Available from: https://www.hema-quebec.qc.ca/sang/donneur-sang/types-de-don/don-de-plaquettes/index.en.html.
- 2. Rahmani M, Fortin BM, Berliner N, Issa N, Neuberg D, Kaufman RM, et al. CD4+ T‐cell lymphopenia in frequent platelet donors who have ceased platelet donation for at least 1 year. Transfusion. 2019;59(5):1644–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gansner JM, Rahmani M, Jonsson AH, Fortin BM, Brimah I, Ellis M, et al. Plateletpheresis‐associated lymphopenia in frequent platelet donors. Blood. 2019;133(6):605–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhao J, Gabriel E, Norda R, Höglund P, Baden L, Diedrich BA, et al. Frequent platelet donation is associated with lymphopenia and risk of infections: a nationwide cohort study. Transfusion. 2021;61(2):464–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dubin PJ, Kolls JK. Th17 cytokines and mucosal immunity. Immunol Rev. 2008;226:160–71. [DOI] [PubMed] [Google Scholar]
- 6. Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhang X, Tan Y, Ling Y, Lu G, Liu F, Yi Z, et al. Viral and host factors related to the clinical outcome of COVID‐19. Nature. 2020;583(7816):437–40. [DOI] [PubMed] [Google Scholar]
- 8. WHO. Therapeutics and COVID‐19: Living guideline 2022. [cited 2022 7th April ]. Available from: https://www.who.int/publications/i/item/WHO-2019-nCoV-therapeutics-2022.2. [PubMed]
- 9. WHO . Coronavirus disease (COVID‐19): Vaccines. 2022. [cited 2022 7th April ]. Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/question-and-answers-hub/q-a-detail/coronavirus-disease-(covid-19)-vaccines?adgroupsurvey=%7badgroupsurvey%7d&gclid=CjwKCAiAo4OQBhBBEiwA5KWu_7jdY4HvxtIgd0W569nAq4gBjZIMPYv5EzwANN13Eyw-nQRxl4TbnBoCF8wQAvD_BwE.
- 10. WHO . Status_COVID_VAX: WHO; 2022. [cited 2022 7th April ]. Available from: https://extranet.who.int/pqweb/sites/default/files/documents/Status_COVID_VAX_16Feb2021.pdf.
- 11. Sekine T, Perez‐Potti A, Rivera‐Ballesteros O, Strålin K, Gorin JB, Olsson A, et al. Robust T cell immunity in convalescent individuals with asymptomatic or mild COVID‐19. Cell. 2020;183(1):158–68.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Guo L, Wang G, Wang Y, et al. SARS‐CoV‐2‐specific antibody and T‐cell responses 1 year after infection in people recovered from COVID‐19: a longitudinal cohort study. Lancet Microbe. 2022; E348–E356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Laurén I, Havervall S, Ng H, Lord M, Pettke A, Greilert‐Norin N, et al. Long‐term SARS‐CoV‐2‐specific and cross‐reactive cellular immune responses correlate with humoral responses, disease severity, and symptomatology. Immun Inflamm Dis. 2022;10(4):e595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tauzin A, Nayrac M, Benlarbi M, Gong SY, Gasser R, Beaudoin‐Bussières G, et al. A single dose of the SARS‐CoV‐2 vaccine BNT162b2 elicits fc‐mediated antibody effector functions and T cell responses. Cell Host Microbe. 2021;29(7):1137–50 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tauzin A, Gong SY, Beaudoin‐Bussieres G, et al. Strong humoral immune responses against SARS‐CoV‐2 spike after BNT162b2 mRNA vaccination with a 16‐week interval between doses. Cell Host Microbe. 2022;30(1):97–109 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Krammer F, Srivastava K, Alshammary H, Amoako AA, Awawda MH, Beach KF, et al. Antibody responses in seropositive persons after a single dose of SARS‐CoV‐2 mRNA vaccine. N Engl J Med. 2021;384(14):1372–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Swain SL, McKinstry KK, Strutt TM. Expanding roles for CD4+ T cells in immunity to viruses. Nat Rev Immunol. 2012;12(2):136–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chatterjee D, Tauzin A, Marchitto L, Gong SY, Boutin M, Bourassa C, et al. SARS‐CoV‐2 omicron spike recognition by plasma from individuals receiving BNT162b2 mRNA vaccination with a 16‐week interval between doses. Cell Rep. 2022;38(9):110429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gong SY, Chatterjee D, Richard J, Prévost J, Tauzin A, Gasser R, et al. Contribution of single mutations to selected SARS‐CoV‐2 emerging variants spike antigenicity. Virology. 2021;563:134–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Beaudoin‐Bussieres G, Laumaea A, Anand SP, et al. Decline of humoral responses against SARS‐CoV‐2 spike in convalescent individuals. MBio. 2020;11(5):e02590–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tian X, Li C, Huang A, Xia S, Lu S, Shi Z, et al. Potent binding of 2019 novel coronavirus spike protein by a SARS coronavirus‐specific human monoclonal antibody. Emerging Microbes Infections. 2020;9(1):382–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Li W, Chen Y, Prevost J, et al. Structural basis and mode of action for two broadly neutralizing antibodies against SARS‐CoV‐2 emerging variants of concern. Cell Rep. 2022;38(2):110210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ullah I, Prevost J, Ladinsky MS, et al. Live imaging of SARS‐CoV‐2 infection in mice reveals that neutralizing antibodies require Fc function for optimal efficacy. Immunity. 2021;54(9):2143–58 e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Prevost J, Richard J, Gasser R, et al. Impact of temperature on the affinity of SARS‐CoV‐2 spike glycoprotein for host ACE2. J Biol Chem. 2021;297(4):101151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Prévost J, Gasser R, Beaudoin‐Bussières G, Richard J, Duerr R, Laumaea A, et al. Cross‐sectional evaluation of humoral responses against SARS‐CoV‐2 spike. Cell Rep Med. 2020;1(7):100126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Beaudoin‐Bussieres G, Richard J, Prevost J, Goyette G, Finzi A. A new flow cytometry assay to measure antibody‐dependent cellular cytotoxicity against SARS‐CoV‐2 spike‐expressing cells. STAR Protoc. 2021;2(4):100851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Anand SP, Prevost J, Nayrac M, et al. Longitudinal analysis of humoral immunity against SARS‐CoV‐2 spike in convalescent individuals up to 8 months post‐symptom onset. Cell Rep Med. 2021;2(6):100290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Perreault J, Tremblay T, Fournier MJ, Drouin M, Beaudoin‐Bussières G, Prévost J, et al. Waning of SARS‐CoV‐2 RBD antibodies in longitudinal convalescent plasma samples within 4 months after symptom onset. Blood. 2020;136(22):2588–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lewin A, Therrien R, De Serres G, et al. SARS‐CoV‐2 seroprevalence among blood donors in Québec, and analysis of symptoms associated with seropositivity: a nested case‐control study. Can J Public Health. 2021;112(4):576–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tauzin A, Gendron‐Lepage G, Nayrac M, Anand SP, Bourassa C, Medjahed H, et al. Evolution of anti‐RBD IgG avidity following SARS‐CoV‐2 infection. Viruses. 2022;14(3):532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gasser R, Cloutier M, Prevost J, et al. Major role of IgM in the neutralizing activity of convalescent plasma against SARS‐CoV‐2. Cell Rep. 2021;34(9):108790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. R Core Team . R: a language and environment for statistical computing: R Foundation for statistical computing; 2017. [cited 2022 2nd May]. Available from: https://www.R-project.org/.
- 33. Thuer L, Brosig A, Hutchinson JA, Hähnel V, Offner R, Burkhardt R, et al. Total platelet donation count and donation frequency are determinants of plateletpheresis‐associated lymphopenia. Transfusion. 2021;61(11):3161–73. [DOI] [PubMed] [Google Scholar]
- 34. McBride JA, Striker R. Imbalance in the game of T cells: what can the CD4/CD8 T‐cell ratio tell us about HIV and health? PLoS Pathog. 2017;13(11):e1006624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mesin L, Ersching J, Victora GD. Germinal center B cell dynamics. Immunity. 2016;45(3):471–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hartley GE, Edwards ESJ, Aui PM, Varese N, Stojanovic S, McMahon J, et al. Rapid generation of durable B cell memory to SARS‐CoV‐2 spike and nucleocapsid proteins in COVID‐19 and convalescence. Sci Immunol. 2020;5(54):eabf8891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Torjesen I. Covid‐19: omicron may be more transmissible than other variants and partly resistant to existing vaccines, scientists fear. BMJ. 2021;375:n2943. [DOI] [PubMed] [Google Scholar]
- 38. Araf Y, Akter F, Tang YD, Fatemi R, Parvez MSA, Zheng C, et al. Omicron variant of SARS‐CoV‐2: genomics, transmissibility, and responses to current COVID‐19 vaccines. J Med Virol. 2022;94:1825–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Planas D, Veyer D, Baidaliuk A, Staropoli I, Guivel‐Benhassine F, Rajah MM, et al. Reduced sensitivity of SARS‐CoV‐2 variant Delta to antibody neutralization. Nature. 2021;596(7871):276–80. [DOI] [PubMed] [Google Scholar]
- 40. Collier DA, De Marco A, Ferreira I, et al. Sensitivity of SARS‐CoV‐2 B.1.1.7 to mRNA vaccine‐elicited antibodies. Nature. 2021;593(7857):136–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ellebedy A, Turner J, O'Halloran J, et al. SARS‐CoV‐2 mRNA vaccines induce a robust germinal Centre reaction in humans. 2021.
- 42. Goel RR, Apostolidis SA, Painter MM, Mathew D, Pattekar A, Kuthuru O, et al. Distinct antibody and memory B cell responses in SARS‐CoV‐2 naïve and recovered individuals after mRNA vaccination. Science Immunol. 2021;6(58):eabi6950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sahin U, Muik A, Derhovanessian E, Vogler I, Kranz LM, Vormehr M, et al. COVID‐19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses. Nature. 2020;586(7830):594–9. [DOI] [PubMed] [Google Scholar]
- 44. Wheatley AK, Juno JA, Wang JJ, Selva KJ, Reynaldi A, Tan HX, et al. Evolution of immune responses to SARS‐CoV‐2 in mild‐moderate COVID‐19. Nat Commun. 2021;12(1):1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Webert KE, Alam AQ, Charge SB, Sheffield WP. Platelet utilization: a Canadian Blood Services research and development symposium. Transfus Med Rev. 2014;28(2):84–97. [DOI] [PubMed] [Google Scholar]
- 46. Vinholt PJ. The role of platelets in bleeding in patients with thrombocytopenia and hematological disease. Clin Chem Lab Med. 2019;57(12):1808–17. [DOI] [PubMed] [Google Scholar]
- 47. Levin EG, Lustig Y, Cohen C, Fluss R, Indenbaum V, Amit S, et al. Waning immune humoral response to BNT162b2 Covid‐19 vaccine over 6 months. N Engl J Med. 2021;385(24):e84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ward H, Whitaker M, Flower B, Tang SN, Atchison C, Darzi A, et al. Population antibody responses following COVID‐19 vaccination in 212,102 individuals. Nat Commun. 2022;13(1):907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wei J, Pouwels KB, Stoesser N, Matthews PC, Diamond I, Studley R, et al. Antibody responses and correlates of protection in the general population after two doses of the ChAdOx1 or BNT162b2 vaccines. Nat Med. 2022;28:1072–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Gao Y, Cai C, Grifoni A, Müller TR, Niessl J, Olofsson A, et al. Ancestral SARS‐CoV‐2‐specific T cells cross‐recognize the omicron variant. Nat Med. 2022;28(3):472–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Néron S, Thibault L, Dussault N, Côté G, Ducas É, Pineault N, et al. Characterization of mononuclear cells remaining in the leukoreduction system chambers of apheresis instruments after routine platelet collection: a new source of viable human blood cells. Transfusion. 2007;47(6):1042–9. [DOI] [PubMed] [Google Scholar]
- 52. Trepel F. Number and distribution of lymphocytes in man. Crit Anal Klin Wochenschr. 1974;52(11):511–5. [DOI] [PubMed] [Google Scholar]
- 53. Ganusov VV, De Boer RJ. Do most lymphocytes in humans really reside in the gut? Trends Immunol. 2007;28(12):514–8. [DOI] [PubMed] [Google Scholar]
- 54. Westermann J, Pabst R. Lymphocyte subsets in the blood: a diagnostic window on the lymphoid system? Immunol Today. 1990;11(11):406–10. [DOI] [PubMed] [Google Scholar]
- 55. MacLennan ICM. Germinal centers. Annu Rev Immunol. 1994;12(1):117–39. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Spike recognition and neutralization activity of antibodies within the plasma of apheresis platelet donors at each time point
Figure S2. Network model of correlations between humoral responses in CD4‐ low and CD4‐high separated based on timepoints


