Abbreviations
- CBC
Complete blood counts
- COVID‐19
Coronavirus Disease 2019
- EOS
Eosinophils
- HB
Hemoglobin
- IgG
Immunoglobulin‐G
- LYM
Lymphocyte
- MONO
Monocytes
- NEU
Neutrophil
- PLT
Platelet
- SARS‐CoV‐2
Severe Acute Respiratory Syndrome Coronavirus‐2
- THC
Tetrahydrocannabinol
- WBC
White blood cells
Dear Editor
On July 29, 2021, the Israeli Ministry of Health approved the third anti‐Coronavirus disease 2019(COVID‐19) vaccination (3rd‐BNT162b2‐booster‐dose), leading to a sharp daily drop in diagnosed positive COVID‐19 cases and mortality rates in Israel [1]. The booster dose increased severe acute respiratory syndrome coronavirus‐2(SARS‐CoV‐2) neutralization efficiency approximately a hundred‐fold compared to individuals receiving only the second dose, providing significant protection against infection [2].
Due to their immunocompromised condition, cancer patients may be more susceptible and generally more vulnerable to infections [3]. Indeed, due to the chronic weakening of their immune system, cancer patients are at higher risk of developing severe clinical outcomes from SARS‐CoV‐2 infection and are associated with an increased risk of morbidity and mortality [3]. Cancer patients treated with anticancer drugs or undergoing major surgery have double the risk of developing a severe illness, hospitalization, and death due to COVID‐19 [3, 4]. Early studies on cancer patients in Israel who received the second BNT162b‐booster‐dose indicated a noticeable lag in antibody production compared to controls, despite the comparable seroconversion rate tested four weeks after administration [5]. No adverse effects or interaction between immunoglobulin G (IgG) levels and active anticancer therapies, such as chemotherapy or radiation, were reported [4]. Recently, a study evaluating immune response 4‐weeks after administration by cancer patients receiving the 3rd‐BNT162b2‐booster‐dose indicated an efficient anti‐COVID‐19 immunity when neither gender nor chemotherapy status was associated with higher antibody levels [6, 7]. Although supporting the 3rd‐BNT162b2‐booster‐dose for actively treated cancer patients, significantly lower anti‐COVID‐19 IgG levels were noted in cancer patients compared to the control group.
Cannabis may potently suppress humoral immunity, and antigen‐specific antibody production since natural or synthetic tetrahydrocannabinol (THC) derivatives can hinder humoral and cell‐mediated immunity [8]. The American Society of Clinical Oncology (ASCO) estimated that by 2021, 20% to 40% of cancer patients were consuming some form of cannabis either during or after treatment. In response to the lack of prior reports on anti‐COVID‐19 antibody production among cannabis users in combination with cannabis’ potent immunosuppressive properties and its consumption by cancer patients, we see an urgent and immediate need to test the effect of cannabis consumption on anti‐COVID‐19 immunity in cancer patients following the 3rd‐BNT162b2‐booster‐dose. The study methods are provided in the Supplementary Material.
We monitored humoral immunity after the 3rd‐BNT162b2‐booster‐dose to assess cancer patients’ vaccine‐derived antibody production compared to non‐cancer (cancer‐free) donors. Unlike the recent 3rd‐BNT162b2‐booster‐dose reports measuring anti‐COVID‐19 IgG immediately after administration (3 to 4 weeks) and before the total increase in antibodies levels, affinity, and avidity (from 30 to 120 days), our measurements were taken between 31 to 120 days after the 3rd‐BNT162b2‐booster‐dose, after a full IgG avidity maturation (high‐affinity IgG occurrence) humoral response. To enable an appropriate uniformed and matched comparison representation between different groups (i.e., cancer vs. non‐cancer donors and cannabis users vs. non‐users), we assessed the anti‐COVID‐19 antibody titer of all samples using a unified, standardized and authorized immunoassay.
We assessed IgG titers of cancer vs. cancer‐free non‐cancer (Supplementary Material) donors and cannabis users vs. non‐users (Supplementary Table S1). A total of 154 participants were grouped into the following groups: cancer (n = 62); cancer + cannabis users (n = 25); non‐cancer (n = 46); non‐cancer + cannabis users (n = 21). The median age was 62.0 years ([interquartile range (IQR).25 = 53, IQR.75 = 71]), and the study contained 85(55%) females. Within the cancer groups, 60 patients had metastatic disease. The most frequent treatment was chemotherapy (n = 47), followed by chemotherapy and biological treatment (n = 11). Co‐morbidities among cancer patients were equally distributed between cannabis users and non‐users. Among cannabis users, the mean (standard deviation) monthly dosage of non‐cancer + cannabis use was 32.38 g/month (± 14.80) and 28.40 g/month (± 18.64) for cancer + cannabis use (P = 0.433) (Supplementary Table S1). All participants were tested for IgG levels at a single time point (between day 31 and day 122 after the 3rd‐BNT162b2‐booster‐dose (mean 73 days [IQR25 = 63, IQR75 = 73]).
Our results showed that mean IgG titers were equivalent over all the groups considered in this study (Supplementary Table S1 and Figure 1A). Overall, donors’ health conditions (i.e., cancer vs. non‐cancer) and cannabis use (i.e., users vs. non‐users) were not significant factors of variance for IgG titers (two‐way ANOVA for cannabis use P = 0.643, health conditions P = 0.681, interaction between cannabis use and health conditions P = 0.090). We also tested for other possible sources of IgG variance, specifically treatments, tumor extent, and type of cancer. We found no significant association or correlation between these variables or the time of blood sampling to IgG titers (Figure 1B).
FIGURE 1.
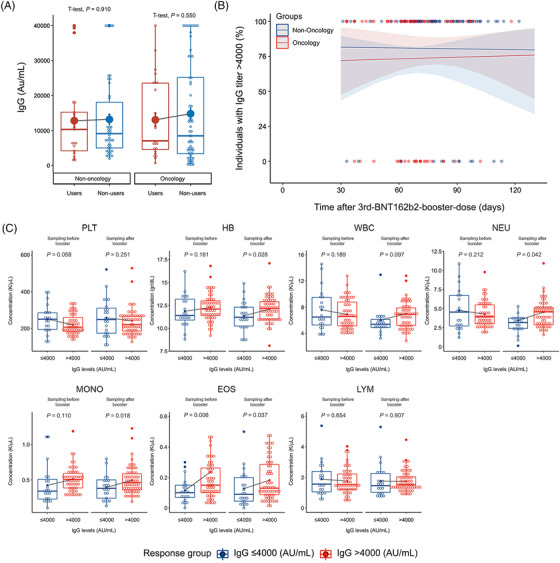
IgG titer levels and CBC parameters over various groups. (A) IgG titers (Au/mL) in relation to two factors of variance, cannabis use (user, non‐users) and cancer status (non‐cancer, cancer). On each box plot, the central mark indicates the median, and the bottom and top edges of the box indicate the 25th and 75th percentiles, respectively. Means are plotted individually using the ‘full dots’ marker symbol. (B) Incidence (%) of high responders (Ig > 4000) over time (days from vaccination to the measurement of anti‐COVID‐19 immunoglobulin G [IgG]). (C) Correlation of CBC levels and cell counts, in relation to two factors of variance, IgG response (high responders > 4000 AU/mL vs. low responders ≤4000 AU/mL) and time of measurement (Sampling before booster BNT162b2 booster dose vs. Sampling after booster after BNT162b2 booster dose). Each box represents a different tested blood component. The left side of each box shows measurements before the BNT162b2 booster dose (Sampling before booster), and the right side shows measurements after the BNT162b2 booster dose (Sampling after booster). Platelet (PLT), Hemoglobin (HB), White blood cells (WBC), Neutrophil (NEU), Monocytes (MONO), Eosinophils (EOS), Lymphocyte (LYM)
Since there were no significant differences in IgG levels between initial study donor grouping (as in Figure 1), we next dichotomized all donors according to IgG titer levels (without any previous correlation to initial grouping). We defined IgG = 4000 AU/mL as a threshold of 10% of the IgG maximum titer value. Thus, the groups were: 3rd‐BNT162b2‐booster‐dose low responders (IgG ≤4000 AU/mL) and high responders (IgG > 4000 AU/mL) (Supplementary Table S2). Since low responders may be at higher risk of COVID‐19 infection due to the low anti‐COVID‐19 IgG titers, we tested whether this group was associated with any distinct complete blood count (CBC) features (Supplementary Table S3). Blood samples were taken before the 3rd‐BNT162b2‐booster‐dose (designated as Sampling before booster) and again after 3rd‐BNT162b2‐booster‐dose administration (designated as Sampling after booster). Notably, levels of eosinophils (EOS) were the only CBC variable showing a significant difference between low and high responders both before the 3rd‐BNT162b2‐booster‐dose (P = 0.006) and after the 3rd‐BNT162b2‐booster‐dose (P = 0.037) (Supplementary Table S3 and Figure 1C). Levels of hemoglobin (HB, P = 0.028), neutrophils (Neu, P < 0.042), and monocytes (Mono, P = 0.018) also showed a significant variation but only after the 3rd‐BNT162b2‐booster‐dose (Figure 1C). Thus, circulating EOS levels may serve as a clinical biomarker predicting IgG response (pre‐and post‐booster dose), while the other laboratory parameter prediction efficiency may be less clinically relevant when trying to identify individuals at higher risk of severe COVID‐19 disease in a pre‐vaccinated population. Analyzing the correlation between CBC variables and mainly circulating levels of peripheral eosinophils and their relationship with COVID‐19 protective immunity and IgG production is now under large‐scale research population survey in our lab. Even though data are currently minimal and largely unexplained, low circulating eosinophil count or eosinopenia in COVID‐19 patients correlates with critical disease progression and, most importantly, higher COVID‐19 mortality rates [9]. Likewise, eosinophil count was recently suggested as a predictor of COVID‐19 re‐infection and may also assist in predicting elderly COVID‐19 patients’ transfer to intensive care units [10].
In contrast to earlier studies reporting antibody production at early time points, our results indicate that vaccine‐induced IgG production was equally effective in cancer patients and control groups. We also assessed the possible interaction between cannabis consumption and anti‐COVID‐19 vaccination and demonstrated no significant impact on anti‐COVID‐19 IgG production by cannabis consumption. Thus, our findings imply that patients can keep integrating cannabis during anticancer therapy without any major concern of compromising anti‐COVID‐19 protective immunity. Our report also sheds light on the unexplained correlation between peripheral blood eosinophil and improved COVID‐19 outcome by linking it to anti‐COVID‐19 IgG levels and possibly humoral immune response; without any association to cannabis use or cancer treatment; which deserves further attention and additional studies.
AUTHOR CONTRIBUTIONS
RO, AA, KM, AD, EH, and CR performed CBC blood processing analysis and documentation. KM, SH, SY, MC, AAM, and BSG were responsible for donor recruitment, monitoring patients, and collecting and assembling clinical data and blood samples. CPS, CI, and BSG performed statistics and data analysis, generated the figures, and wrote the manuscript. CI and BSG conceived, designed, supervised, and sponsored the study.
COMPETING INTERESTS
The authors declare that they have no competing interests.
FUNDING INFORMATION
This study is funded by the Ministry of Health (Jerusalem, Israel) grant number: 3000015198 and the Israel Cancer Association grant number 2020002.
AVAILABILITY OF DATA AND MATERIALS
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
All study donors signed an informed consent form included in the study protocol, which had been authorized and approved by the Institutional Ethics Committee (no. 0133‐21‐EMC).
CONSENT FOR PUBLICATION
All study donors signed an informed consent form included in the study protocol, which had been authorized and approved by the Institutional Ethics Committee (0133‐21‐EMC).
Supporting information
Supporting Information
ACKNOWLEDGEMENTS
We would also like to thank Ms. Tami Appelbaum for her major help with linguistic and manuscript editing.
Contributor Information
Cohen Idan, Email: idan5161@gmail.com.
Bar‐Sela Gil, Email: gil_ba@clalit.org.il.
REFERENCES
- 1. Barda N, Dagan N, Cohen C, Hernan MA, Lipsitch M, Kohane IS, et al. Effectiveness of a third dose of the BNT162b2 mRNA COVID‐19 vaccine for preventing severe outcomes in Israel: An observational study. Lancet. 2021;398(10316):2093–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nemet I, Kliker L, Lustig Y, Zuckerman N, Erster O, Cohen C, et al. Third BNT162b2 vaccination neutralization of SARS‐CoV‐2 omicron infection. N Engl J Med. 2022;386(5):492–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dai M, Liu D, Liu M, Zhou F, Li G, Chen Z, et al. Patients with cancer appear more vulnerable to SARS‐CoV‐2: A multicenter study during the COVID‐19 outbreak. Cancer Discov. 2020;10(6):783–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fendler A, de Vries EGE, GeurtsvanKessel CH, Haanen JB, Wormann B, Turajlic S, et al. COVID‐19 vaccines in patients with cancer: Immunogenicity, efficacy and safety. Nat Rev Clin Oncol. 2022;19(6):385–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Goshen‐Lago T, Waldhorn I, Holland R, Szwarcwort‐Cohen M, Reiner‐Benaim A, Shachor‐Meyouhas Y, et al. Serologic Status and Toxic Effects of the SARS‐CoV‐2 BNT162b2 Vaccine in Patients Undergoing Treatment for Cancer. JAMA Oncol. 2021;7(10):1507–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ligumsky H, Dor H, Etan T, Golomb I, Nikolaevski‐Berlin A, Greenberg I, et al. Immunogenicity and safety of BNT162b2 mRNA vaccine booster in actively treated patients with cancer. Lancet Oncol. 2022;23(2):193–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shapiro LC, Thakkar A, Campbell ST, Forest SK, Pradhan K, Gonzalez‐Lugo JD, et al. Efficacy of booster doses in augmenting waning immune responses to COVID‐19 vaccine in patients with cancer. Cancer Cell. 2022;40(1):3–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Smith SH, Harris LS, Uwaydah IM, Munson AE. Structure‐activity relationships of natural and synthetic cannabinoids in suppression of humoral and cell‐mediated immunity. J Pharmacol Exp Ther. 1978;207(1):165–70. [PubMed] [Google Scholar]
- 9. Yan B, Yang J, Xie Y, & Tang X. Relationship between blood eosinophil levels and COVID‐19 mortality. World Allergy Organ J. 2021;14(3):100521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Li X, Yin D, Yang Y, Bi C, Wang Z, Ma G, et al. Eosinophil: A nonnegligible predictor in COVID‐19 Re‐positive patients. Front Immunol. 2021;12:690653. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.


