Abstract
Objectives
Recent studies suggested that the expansion of long‐standing multiple sclerosis (MS) lesions and an enlargement of choroid plexus may be linked to chronic inflammation and microglial activation. We investigated the potential association between plexus volume and subsequent lesion expansion in patients with relapsing‐remitting MS.
Methods
Pre‐ and post‐gadolinium 3D‐T1, 3D FLAIR and diffusion tensor images were acquired from 49 patients. Choroid plexus (CP) volume (normalised by Total Intracranial Volume, TIV) and lesion activity were analysed between baseline and 48 months. In addition, plexus volume was measured in 40 healthy controls of similar age and gender.
Results
Baseline CP/TIV ratio was significantly larger in RRMS patients compared to normal controls (p < 0.001). CP/TIV ratio remained stable in RRMS patients during follow‐up period. There was a strong correlation between baseline CP/TIV ratio and subsequent rate of chronic lesion expansion (p < 0.001), which was stronger in close proximity to CSF. A cut‐off of 98 × 10−5 CP/TIV ratio predicted future lesion expansion with a sensitivity of 85% and specificity of 76%. CP/TIV ratio larger than a cut‐off was associated with >8‐fold increased risk of chronic lesion expansion. Baseline CP/TIV ratio was also associated with change in Mean Diffusivity (MD) inside of chronic lesions. Furthermore, baseline CP/TIV ratio significantly correlated with central brain atrophy. There was, however, no correlation between CP/TIV ratio and volume of new lesions.
Interpretation
Our data demonstrate that baseline CP/TIV ratio predicts subsequent expansion of chronic periventricular MS lesions and associated tissue damage within and outside of chronic lesions.
Introduction
Expansion of chronic MS lesions, caused by slow‐burning inflammation at the lesion rim, has been implicated as one of the main forces driving MS progression. 1 , 2 , 3 , 4 We recently demonstrated that lesion expansion is most noticeable in close proximity to ventricles, suggesting a link between slow burning inflammation and cerebrospinal fluid (CSF). 5
It is well established that the choroid plexus, located within the ventricular system of the central nervous system (CNS), plays an important role in the implementation of the immune response within the CNS. 6 The plexus is essential for the production of CSF, 7 but also responsible for regulating entry of immune cells and specific molecules to the brain parenchyma, providing a gateway for antigen trafficking between the CSF and the vascular circulation. 8 , 9
Several recent publications have demonstrated an association between plexus volume and the degree of inflammation in brain tissue, suggesting that plexus volume may serve as a surrogate marker for MS disease activity. 10 , 11 , 12
Therefore, the aim of the current study is to investigate the potential association between plexus volume and subsequent lesion expansion in patients with relapsing‐remitting MS (RRMS).
Methods
The study was approved by the University of Sydney and Macquarie University Human Research Ethics Committees and followed the tenets of the Declaration of Helsinki. Written informed consent was obtained from all participants.
Subjects
Forty‐nine consecutive patients with established RRMS from MADMS study (National MS Society grant, G‐1508‐05946), who reached 5 years of follow‐up were enrolled in this retrospective longitudinal analysis. MS was diagnosed according to the revised McDonald 2010 criteria. 13 Patients underwent MRI scans and clinical assessment at 0, 12 and 60 months. Patients were relapse‐free and their therapy did not change for at least 6 months prior to enrolment. In addition, no steroid treatment was administered for at least 6 months prior to enrolment. The main analysis was performed between 12 months (termed ‘baseline’) and 60 months (termed ‘follow‐up’), while scans performed at 0 months (termed ‘pre‐study scans’) were used to identify (and exclude) newly developed lesions at the start of the study (i.e. between 0 and 12 months). 3
At the start of the study, 25 patients were on low‐efficacy treatment (injectables, such as interferon and glatiramer acetate, teriflunomide and dimethyl‐fumarate), 14 21 patients were receiving high‐efficacy drugs (fingolimod, natalizumab and alemtuzumab) 14 and three patients were treatment‐free. During the study period (i.e. between baseline and follow‐up visits), 16 patients changed the treatment category. By the end of the study period, 14 patients remained on low‐efficacy treatment and 32 patients were on high‐efficacy drugs. The decision to modify the therapy was taken by the treating physician based on combination of clinical and radiological activity. Overall, 16 patients did not change treatment during the follow‐up. In addition, plexus volume from 40 healthy controls of similar age and gender composition was also analysed.
MRI protocol and analysis
MRI was performed using a 3T GE Discovery MR750 scanner (GE Medical Systems, Milwaukee, WI). The following MRI sequences were acquired: Pre‐ and post‐contrast (gadolinium) Sagittal 3D T1, FLAIR CUBE, diffusion‐weighted MRI. JIM 7 software (Xinapse Systems, Essex, UK) was used to segment individual lesions (using co‐registered T2 FLAIR images). Expansion of chronic lesions was estimated using custom‐build software, as previously described. 15 Specific acquisition parameters and MRI image processing are presented in Supplementary material.
Choroid plexus within lateral ventricles was semi‐automatically segmented on co‐registered T1 images (Fig. 1) using JIM 9 software (Xinapse Systems, Essex, UK) by a trained analyst (AK) at baseline and follow‐up. The analyst, who was blinded to the clinical and lesional data ensured the correct contour was selected by the software and performed manual adjustments where needed. To estimate intra‐rater variability segmentation was performed twice with an interval of 2 weeks and intraclass correlation coefficient (ICC) was calculated.
Figure 1.
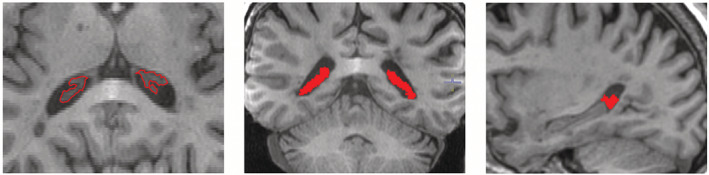
Example of plexus delineation using T1 image. JIM9‐assisted contouring was performed on axial image and verified using coronal and sagittal views.
To reduce inter‐subject variability CP volume was normalised by Total Intracranial Volume (TIV) using FreeSurfer software (http://surfer.nmr.mgh.harvard.edu/), as previously suggested 12 Therefore, CP/TIV ratio (×1000) will be referred in this paper as CP volume.
To examine the effect of distance from CSF on the relationship between plexus volume and lesion expansion, periventricular brain tissue was separated into two 5‐mm‐wide bands, one closer to CSF (1–5 mm from ventricle) and one more remote (6–10 mm) using the DiL option in FSL and the rate of lesion expansion and its relationship with plexus volume for each band measured independently. For band‐based analysis, the number of voxels at baseline and follow‐up within a single band was estimated for each patient in 3D space. The rate of expansion (percentage‐wise) within the band was then calculated as a relative change in number of lesional voxels attributed to lesion expansion occurring during the follow‐up. 3
We have previously ascribed the association between expansion of chronic lesions and lesional or brain tissue loss to axonal and neuronal death following transection of axons at the expanding rim caused by slow‐burning inflammation. 3 , 16 Therefore, to investigate whether individual differences in CP/TIV ratio translate to neuro‐axonal loss, we examined the potential association of plexus volume with markers of lesional tissue damage and central brain atrophy. Volumetric change in ventricles was used as a measure of central brain atrophy (CBA). 17 , 18 It was calculated by multiplying baseline ventricle volume (as segmented by SIENAX) by the percentage ventricular volume change (as calculated by VIENA, a tool part of FSL). 19 This measure of CBA is believed to be more sensitive to periventricular white matter loss associated with MS lesions and less affected by grey matter changes. 17 , 18 The degree of tissue damage in chronic lesions was assessed by mean diffusivity (MD), as established before. 20 , 21 Progressive tissue destruction was measured as an increase in MD between baseline and follow‐up timepoints 16 , 21 using the baseline lesion mask, which was adjusted to correct for brain atrophy‐related displacement of lesions at follow‐up, as described previously. 3
Statistics
Statistical analysis was performed using SPSS 22.0 (SPSS, Chicago, IL, USA). Pearson correlation coefficient was used to measure statistical dependence between two numerical variables, while Spearman correlation was used for ordinal variables. For partial correlation, data were adjusted for age, gender, disease duration, baseline lesion volume and ventricular size. p < 0.05 was considered statistically significant. Shapiro–Wilk test was used to test for normal distribution. Comparisons between groups were made using Student's t‐test. Longitudinal changes were assessed using a paired two‐sample t‐test. Fisher's exact test was used for categorical data. To investigate how well plexus volume predicts tissue loss inside chronic lesions and brain atrophy after 4 years of follow‐up, we performed a linear regression analysis that included age, gender, disease duration, baseline ventricular volume, baseline lesion volume and volume of new (confluent and free standing) lesions. Bonferroni adjustment was used to correct p‐value for multiple variables.
Based on degree of chronic lesion volume increase, patients were separated into two groups: patients who demonstrated significant (i.e. >10%) lesion expansion and patients with stable chronic lesion volume (lesion expansion <10%). 15 Receiver operating characteristic (ROC) curve analysis was performed to determine the sensitivity and specificity of baseline plexus volume in predicting future lesion expansion, while the hazard ratio was calculated to estimate the relative risk of chronic lesion expansion in patients who demonstrated plexus enlargement.
In addition, to estimate an effect of choroid plexus on central brain atrophy and consider age‐related progression of ventricular expansion in healthy subjects of 3.5% per year, 22 , 23 patients were separated into two groups: patients with ventricular expansion similar to that of healthy controls (i.e. expansion <14% in 4 years) and patients with ventricular expansion exceeding healthy controls (i.e. expansion >14% in 4 years).
Results
Forty‐nine consecutive MS patients enrolled in longitudinal study of axonal loss in MS who reached 5 years of follow‐up were included in the study. Demographic and lesion data in presented in Table 1.
Table 1.
Demographic, clinical and imaging data.
| RRMS patients | Normal controls | |
|---|---|---|
| Total number of subjects | 49 | 40 |
| Age at enrolment, years, (mean ± SD) | 41.4 ± 9.1 | 37.4 ± 7.7 |
| Gender | 20 M/29F | 16 M/24F |
| Disease duration at enrolment, years (mean ± SD) | 5.6 ± 4.5 | n/a |
| EDSS at baseline, median (range) | 1.0 (0–5) | n/a |
| EDSS at follow‐up, median (range) | 1.0 (0–6) | n/a |
| No. of patients on stable treatment | 31 | n/a |
| Lesion volume (mm3): | ||
| Baseline | 5738 ± −5180 | n/a |
| Follow‐up | 6520 ± 5681 | n/a |
| New lesions volume, (mm3) | 231 ± 234 | n/a |
| Expanding lesion volume, (mm3) | 555 ± 775 | n/a |
Example of plexus delineation is shown in Figure 1. There was high correspondence between two rounds of plexus masking (intra‐rater ICC = 0.96, 95% Confidence interval: 0.77–0.98).
Baseline CP/TIV ratio was significantly larger in RRMS patients compared to normal controls (95.8 ± 32.1 vs. 75.2 ± 12.1, p < 0.001).
No correlation was found between baseline CP/TIV ratio and baseline lesion volume (r = 0.25, p = 0.08), ventricle volume (r = 0.24, p = 0.1), gender (r = 0.16, p = 0.27 Spearman) or disease duration (r = 0.08, p = 0.6). There was also no significant difference in baseline CP/TIV ratio between the two treatment groups (87.4 and 101 for low‐efficacy and high‐efficacy treatment groups, respectively, p = 0.3). CP/TIV ratio, however, was significantly associated with baseline EDSS (r = 0.44, p = 0.01) and patient age (r = 0.32, p = 0.02).
There was no significant change in CP/TIV ratio during follow‐up (p = 0.45, paired t‐test).
Baseline CP/TIV ratio associated with subsequent expansion of chronic lesions
We observed a strong association between baseline CP/TIV ratio and subsequent rate of lesion expansion (r = 0.77, p < 0.001). This correlation remained unchanged after adjusting for age, gender, disease duration, ventricular volume and baseline lesion volume (partial correlation r = 0.74, p < 0.001) (Fig. 2). Sub‐analysis of patients who remained on stable treatment during the entire study period (n = 33) also did not materially change the relationship between baseline CP/TIV ratio and subsequent lesion expansion when adjustment was made for treatment group (partial correlation, r = 0.77, p < 0.001).
Figure 2.
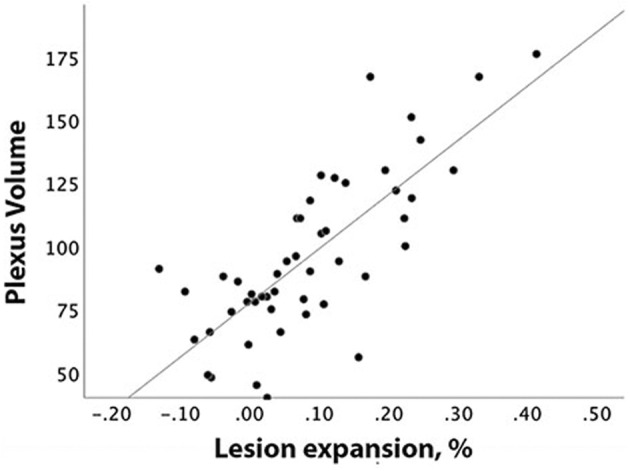
Correlation between baseline plexus volume and patient‐based percentage of chronic lesion expansion.
In addition, baseline CP/TIV ratio correlated with the change in MD inside expanding area of chronic lesions (r = 0.55, p < 0.001).
There was, however, no significant correlation between baseline CP/TIV ratio and volume of new (combined confluent and free‐standing) lesions (r = 0.08, p = 0.5, partial correlation r = 0.011, p = 0.4). Variation in CP/TIV ratio during follow‐up did not correlate with volume of new lesions or the rate of chronic lesion expansion (r = 0.01, p = 0.9 and r = 0.14, p = 0.4).
There were 20 patients who demonstrated significant (i.e. >10%) lesion expansion and 29 patients with stable chronic lesion volume (lesion expansion <10%). Patient‐based receiver operating characteristic (ROC) curve analysis of baseline CP/TIV ratio (area under the curve = 0.89) yielded a cut‐off of 98 × 10−5 plexus/TIV ratio, which discriminated between patients who have and those who do not have future lesion expansion with a sensitivity of 85% and specificity of 76% (Fig. 3).
Figure 3.
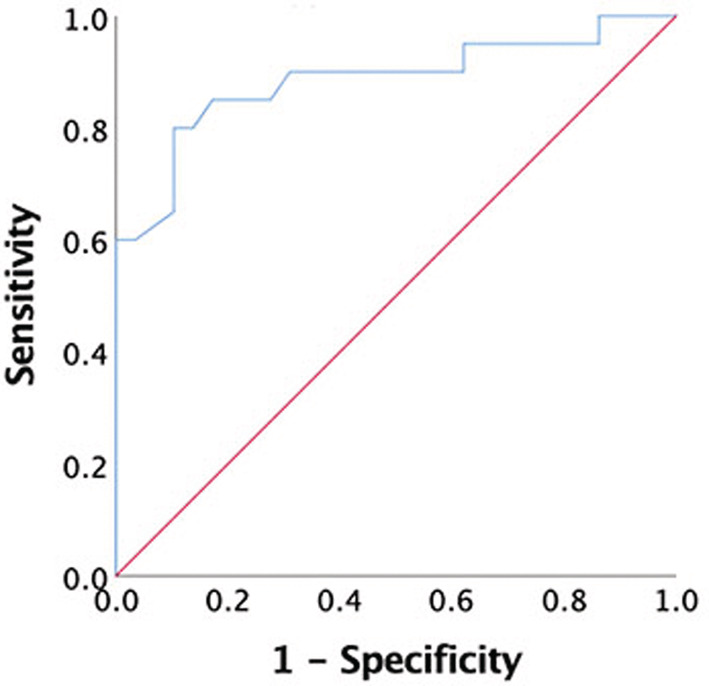
ROC curve showing sensitivity and specificity of baseline plexus volume in detecting expansion of chronic lesions.
CP/TIV ratio larger than a cut‐off calculated using ROC analysis (i.e. 98 × 10−5 plexus/TIV ratio) was associated with >8‐fold increased risk of chronic lesion expansion (hazard ratio for lesion expansion comparing enlarged vs. non‐enlarged plexus = 8.4; 95% confidence interval (CI): 2.8 to 25.0).
CP/TIV ratio correlates with tissue damage inside MS lesions and loss of extra‐lesional brain tissue
CP/TIV ratio at baseline demonstrated a significant correlation with baseline MD in lesional core (r = 0.58, p < 0.001, partial correlation adjusted for age, gender, duration, ventricular volume and baseline lesion volume, r = 0.42, p = 0.004), which increased at follow‐up (r = 0.66, p < 0.001, partial correlation r = 0.60, p < 0.001).
Remarkably, baseline CP/TIV ratio also significantly correlated with change in MD in lesional core during the study period (r = 0.67, p < 0.001, partial correlation, 0.66, p < 0.001 respectively). Furthermore, baseline CP/TIV ratio also showed a similar association with ventricular enlargement: (r = 0.57, p < 0.001, partial correlation: r = 0.51, p < 0.001). There was, however, no association observed between CP/TIV ratio and EDSS change during follow‐up.
Patient‐based analysis of patients grouped according to the rate of central brain atrophy demonstrated significantly larger baseline plexus volume in patients with a higher rate of ventricular expansion (112.4+/−40.3 vs. 85.7+/− 29.7 respectively, p = 0.01) (Fig. 4).
Figure 4.
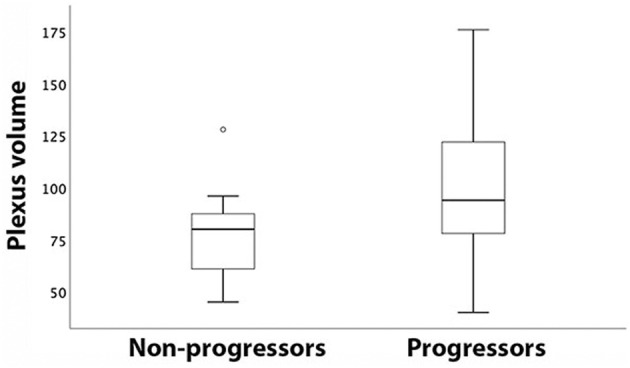
Box‐plot of plexus volume in patients with stable (non‐progressors) and expanding (progressors) ventricles.
In accordance with our previous publications, 15 , 21 we found a significant correlation between volume of lesion expansion and baseline MD in the lesional core (r = 0.47, p < 0.001, partial correlation adjusted for age, gender, duration r = 0.40, p = 0.005), which also increased at follow‐up (r = 0.61, p < 0.001, partial correlation adjusted for age, gender, duration, r = 0.57, p < 0.001). Moreover, expansion of chronic lesions correlated with the change in MD during the study period (r = 0.63, p < 0.001, partial correlation adjusted for age, gender, duration, r = 0.62, p < 0.001 respectively). Similarly strong relationships were observed between lesion expansion and CBA (r = 0.71, p < 0.001, partial: r = 0.67, p < 0.001).
Linear regression model
To investigate further the impact of CP/TIV ratio on predicting future tissue loss inside (MD change) and outside (brain atrophy) of chronic lesions, a linear regression model was performed.
Change in MD inside chronic lesions (model R 2 = 0.51, p < 0.001) was driven almost entirely by baseline CP/TIV ratio (Standardised coefficient Beta: 0.62, p < 0.001 and 0.25, p = 0.02 for CP/TIV ratio and volume of new lesions respectively). Contribution of CP/TIV ratio remained significant after correction for multiple variables was applied (p < 0.01). Other variables did not reach significance (Fig. 5A). Removal of baseline plexus volume had a dramatic effect on the model predictive power (R 2 = 0.13, p = 0.01, F change <0.001).
Figure 5.
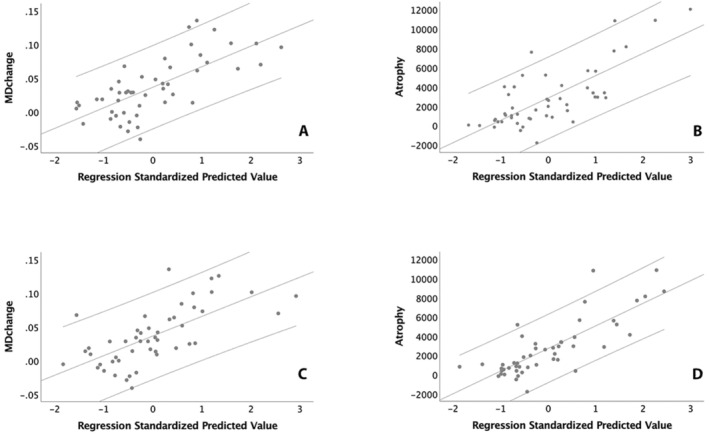
(A, B) Linear regression analysis of baseline plexus volume versus lesional MD (A) and brain atrophy (B). (C, D) Linear regression analysis of expansion of chronic lesions versus lesional MD (C) and brain atrophy (B).
For the central atrophy model (R 2 = 0.56, p < 0.001), baseline CP/TIV ratio was also the main contributor to the tissue loss outside of lesions (Standardised coefficient Beta: 0.44, 0.35 and 0.31 for plexus volume, volume of new lesions and baseline ventricular volume respectively, p < 0.001, 0.001 and 0.003 respectively) (Fig. 5B). All three variables remained significant after applying Bonferroni correction (p < 0.01, p = 0.008 and p = 0.024 respectively). Again, the exclusion of baseline CP/TIV ratio significantly reduced the predictive power of the model (R 2 = 0.37, p < 0.001, F change <0.001).
The addition of lesion expansion as a dependent variable to progressive lesional MD change or brain atrophy models revealed a high degree of collinearity between the lesion expansion rate and baseline CP/TIV ratio (variance proportions 0.98 and 0.56 respectively). As a result, the substitution of CP/TIV ratio with the rate of chronic lesion expansion did not materially change the model's predictive power. Thus, using lesion expansion during the follow‐up period rather than baseline CP/TIV ratio for modelling MD change in the lesional core demonstrated the predictive power of R 2 = 0.46, p < 0.001 and was driven by the volume of lesion expansion and new lesions (Standardised coefficient Beta: 0.58, p < 0.001 and 0.26, p = 0.02 for lesion expansion and new lesions respectively) (Fig. 5C). The contribution of lesion expansion volume remained significant after correction for multiple variables was applied (p < 0.01). Modelling central brain atrophy using a similar substitution revealed R 2 = 0.63 (p < 0.001), with lesion expansion, volume of new lesions and ventricular volume showing significant contribution (Standardised coefficient Beta: 0.67, 0.26 and 0.25, p < 0.001 0.007 and 0.015 respectively) (Fig. 5D). Lesion expansion remained significant after Bonferroni correction (p < 0.01).
Correlation between CP/TIV ratio and lesion expansion is related to the distance from CSF
We measured the rate of lesion expansion and its relationship with CP/TIV ratio in the two bands independently. While CP/TIV ratio explained 50% of the variability in the rate of lesion expansion in the band closer to ventricles, this relationship was significantly weaker (p = 0.01) for the more distal band (r = 0.71, p < 0.001 vs. r = 37, p = 0.01 in close and remote rings respectively) (Fig. 6).
Figure 6.
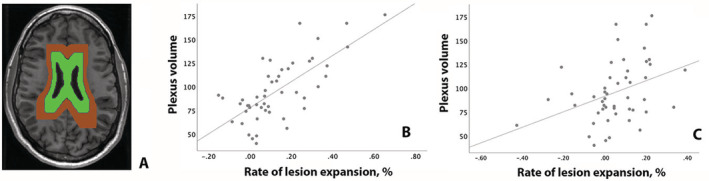
Analysis of association between plexus volume and chronic lesion expansion at a different distance from ventricles. (A) Example of two‐bands mask. (B) Correlation between plexus volume and lesion expansion in the band adjacent to ventricles (green). (C) Correlation between plexus volume and lesion expansion in the more distal band (brown).
Discussion
In accordance with recently published studies, we found a significantly larger volume of choroid plexus (as measured by CP/TIV ratio) in our cohort of RRMS patients compared to normal controls. 24
CP/TIV ratio correlates with subsequent expansion of chronic lesion
Slow‐burning inflammatory demyelination at the edge of chronic MS lesions (and lesion expansion as its imaging equivalent) has been implicated as a major factor contributing to disease evolution, including progressive neurodegeneration, brain atrophy and disability worsening. 1 , 2 , 3 Here we explored the relationship between the rate of expansion in chronic MS lesions and CP/TIV ratio in cohort of RRMS patients. We demonstrated a strong association between the size of the choroid plexus at baseline and subsequent expansion of chronic lesions. This relationship remained unchanged after adjustment was made for age, gender, disease duration, ventricular volume and baseline lesion volume or by analysing patients who remained on stable treatment during the study. A close link between baseline CP/TIV ratio and lesion expansion was also supported by the observation that the association between the two is stronger in close proximity to the ventricles, where the plexus resides. Furthermore, the predictive value of CP/TIV ratio in identifying future lesion expansion was corroborated by the ROC analysis, which revealed that a cut‐off of 98 × 10−5 plexus/TIV ratio predicted which patients would exhibit future lesion expansion with a sensitivity of 85% and specificity of 76%. Patients with baseline CP/TIV ratio above this threshold had an 8‐fold increased risk of chronic lesion expansion. In addition, degree of tissue loss within expanding part of chronic lesions (as measured by an increase in MD) was also positively linked to the size of the plexus at baseline.
Surprisingly, we found that despite strong association between baseline plexus/TIV ratio and rate of chronic lesions enlargement, plexus/TIV ratio itself remains stable during 4 years of follow‐up. This implies that plexus enlargement may occur early in the course of MS, which is also supported by recent study of Ricigliano et al. which demonstrated enlarged choroid plexus even at presymtomatic stage of the disease. 12 , 25
Stability of plexus and its weak association with baseline lesion volume (as was also observed in a recent study by Müller et al. 24 ) and with the volume and severity of new lesions implies that, at least in our cohort, increased plexus size relates to a pathogenesis that is discrete from acute inflammatory demyelination, supporting, therefore, the notion that different mechanisms are likely to be responsible for acute and smouldering inflammation. 1 , 2 , 15 , 26 , 27
The essential role of the choroid plexus in maintaining the blood–CSF barrier and modulation of inflammatory cells trafficking into the central nervous system has recently attracted the attention of MS researchers. 28 , 29 It has become clear that the plexus not only actively participates in acute inflammatory processes including antigen presentation and recruitment of peripheral inflammatory cells, 30 but remains chronically inflamed, even in long‐standing MS. 8
Several recent studies have examined the size of the choroid plexus in MS patients and its potential relationship with disease progression. Ricigliano et al. 12 demonstrated a larger volume, and increased inflammation, of the choroid plexus compared with normal controls, particularly in the relapsing‐remitting stage of the disease. The authors also reported an association of larger plexus size with measures of disease activity, including relapse rate or the number of enhancing lesions and parenchymal neuroinflammation. A close relationship between enlargement of choroid plexus and neuroinflammation in both MS and experimental models of demyelination (cuprizone) and experimental autoimmune encephalomyelitis (EAE) has also been demonstrated in another study 10 in which choroid plexus volume outperformed conventional MRI biomarkers in predicting EDSS progression.
Various hypotheses have been proposed to explain plexus enlargement in MS. Inflammation through activation and infiltration of immune cells, 28 endothelial immune proliferation, increase in permeability of the Blood‐CSF Barrier, 10 CSF hypersecretion, 31 oxidative stress 32 and oedema 33 have all been suggested as potential contributing factors. Furthermore, it was recently demonstrated that plexus volume is related to the increased activity of key innate immune cells in the CNS, namely astrocytes and microglia. 10
Interestingly, microglial activation also plays a central role in sustaining slow‐burning inflammation at the rim of chronic lesions, 34 , 35 , 36 , 37 , 38 which may suggest a shared pathomechanism between plexus inflammation and lesion expansion. Thus, for instance, it is feasible that soluble cytotoxic factors and/or inflammatory mediators produced by long‐standing intrathecal inflammation (including choroid plexus, but also meningeal infiltrates and interstitial fluid 9 ) could diffuse from CSF into the periventricular white matter, forming /sustaining a permanent inflammatory environment in and around the ventricles, thereby driving slow‐burning inflammation at the rim of chronic periventricular lesions. 39 , 40 , 41
This concept is supported by the recently described periventricular gradient of chronic lesion expansion, which demonstrated a decrease in a rate of chronic lesion enlargement as distance from the CSF (and plexus) increases 5 ; and by the observation reported in this study that the association between baseline plexus volume and lesion expansion is stronger in close proximity to the CSF. Notably, the periventricular gradient of lesion expansion is strikingly similar to the pattern of microstructural damage in the white matter described near the ventricles and in the most external cortical layers (i.e. adjacent to CSF‐filled ventricular and subarachnoid spaces), which is believed to be mediated by innate immune cell activation. 40 , 41 , 42 , 43 This common mechanism of inflammation, found in both CSF and meninges, 9 , 44 has recently prompted a reappraisal of the pathogenesis of MS, particularly of its chronic progressive stage. 45
While the possibility that perilesional inflammation causes diffusion of oxidative factors from the brain parenchyma into the CSF compartment, reaching the CP epithelium and leading to CP inflammation and expansion cannot be completely eliminated, 29 our longitudinal data suggest that this ‘reverse causation’ is unlikely.
The association between CP/TIV ratio and brain tissue loss inside lesions and in NAWM is likely to be mediated by rim inflammation
We previously reported a close association of chronic lesion expansion with a progressive increase in MD inside established lesions and with brain atrophy; this finding was replicated in the current study. Progressive tissue damage inside expanding lesions has also been reported by other groups. 2 , 46 The mechanism of MD change inside chronic lesions was attributed to widening of the extracellular space following Wallerian and retrograde degeneration of the intra‐lesional part of axons transected at the lesion rim (as a result of slow‐burning inflammation). 15 , 16 Similarly, degeneration of the extra‐lesional component of the same axons leads to the elimination of their associated axolemmae and myelin sheaths from the white matter, neuronal death and ultimately to brain atrophy.
The current study demonstrated that baseline CP/TIV ratio also significantly correlates with progressive change in isotropic water diffusion (MD) inside chronic lesions and with central brain atrophy (as measured by ventricular enlargement) over 4 years of follow‐up. In addition, group analysis demonstrated a significantly higher CP/TIV ratio in patients with progressive central brain atrophy compared to ‘non‐progressors’.
However, considering the strong correlations between baseline CP/TIV ratio and subsequent chronic lesion expansion on one hand, and between the latter and measures of tissue loss on the other, we hypothesise that the observed association between plexus metrics and progressive change in lesional MD or brain atrophy is likely to be an indirect consequence of the relationship between the plexus and lesion expansion.
Furthermore, the high collinearity and virtually interchangeable character of baseline CP/TIV ratio and subsequent expansion of chronic lesions in models predicting progressive brain damage (as shown by Linear Regression analysis) also lend a strong support to the notion that, while baseline plexus CP/TIV ratio explains about 50% of the variability in tissue damage both within and outside of chronic lesions in following 4 years, the mechanism of this association is likely to be operating via inflammatory activity at the rim of chronic lesions and accompanying lesion expansion.
The limitations of our study are relatively small sample size and heterogeneity of disease‐modifying therapies. We admit that the switch to a potentially more effective DMT during the study may also potentially influence the result. This study (due to its small sample size) was not designed to assess the effect of various DMT on the relationship between plexus and MS lesions. However, while we recognise that treatment may potentially affect the relationship between plexus and lesion expansion, the sub‐analysis of patients who remained on stable treatment adjusted for treatment type was not materially different from the main result (it must also be noticed that majority of patients on stable treatment enrolled in this study were receiving high‐efficacy therapy). In addition, there was also no significant difference in baseline CP/TIV ratio between the two treatment groups, although the sample size for this analysis was very small. Recently described transient fluctuation in ventricular volume, which may potentially affect the accuracy of plexus measurement, was also not taken into account due to long inter‐test intervals. 47
We were not able to demonstrate a correlation between plexus volume and EDSS change. This may reflect a lack of significant progression of EDSS over the course of the study or insensitivity of the EDSS to periventricular lesional damage.
In summary, our study demonstrated strong correlations of normalised size of choroid plexus with (1) subsequent expansion of chronic periventricular MS lesions; (2) associated axonal loss within established lesions and (3) central brain atrophy. While the molecular mechanisms underpinning these relations require further investigation, the longitudinal nature of the study, the strength of the reported associations and the higher strength of correlation between plexus volume and lesion expansion in close proximity to CSF/plexus, together with the observed temporal sequence of events argue in favour of a causal relationship between the volume of choroid plexus and subsequent lesion expansion. 48
Conflict of Interest
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Supporting information
Supplementary S1 Specific MRI parameters and image processing.
Acknowledgements
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Supported by the National Multiple Sclerosis Society (NMSS), Novartis Save Neuron Grant, Sydney Eye Hospital foundation grant and Sydney Medical School.
Funding Statement
This work was funded by National Multiple Sclerosis Society grant RD4716A6/3.
References
- 1. Dal‐Bianco A, Grabner G, Kronnerwetter C, et al. Slow expansion of multiple sclerosis iron rim lesions: pathology and 7 T magnetic resonance imaging. Acta Neuropathol. 2017;133(1):25‐42. doi: 10.1007/s00401-016-1636-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Elliott C, Belachew S, Wolinsky JS, et al. Chronic white matter lesion activity predicts clinical progression in primary progressive multiple sclerosis. Brain. 2019;142(9):2787‐2799. doi: 10.1093/brain/awz212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Klistorner S, Barnett MH, Yiannikas C, et al. Expansion of chronic lesions is linked to disease progression in relapsing‐remitting multiple sclerosis patients. Mult Scler J. 2021;27(10):1533‐1542. doi: 10.1177/1352458520974357 [DOI] [PubMed] [Google Scholar]
- 4. Maggi P, Kuhle J, Schädelin S, et al. Chronic white matter inflammation and serum neurofilament levels in multiple sclerosis. Neurology. 2021;97:e543‐e553. doi: 10.1212/wnl.0000000000012326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Klistorner SA, Barnett MH, Graham SL, Wang C, Klistorner A. The expansion and severity of chronic MS lesions follows a periventricular gradient. MSJ. 2022;28:1504‐1514. [DOI] [PubMed] [Google Scholar]
- 6. Mihaljevic S, Michalicova A, Bhide M, Kovac A. Pathophysiology of the choroid plexus in brain diseases. Gen Physiol Biophys. 2021;40:443‐462. doi: 10.4149/gpb_2021032 [DOI] [PubMed] [Google Scholar]
- 7. Hofman FM, Chen TC. Choroid plexus: structure and function. In: Neman J, Chen TC, eds. The Choroid Plexus and Cerebrospinal Fluid. : Elsevier Inc.; 2016:29–40. doi: 10.1016/B978-0-12-801740-1/00003-2. [DOI] [Google Scholar]
- 8. Vercellino M, Votta B, Condello C, et al. Involvement of the choroid plexus in multiple sclerosis autoimmune inflammation: a neuropathological study. J Neuroimmunol. 2008;199(1–2):133‐141. doi: 10.1016/j.jneuroim.2008.04.035 [DOI] [PubMed] [Google Scholar]
- 9. Monaco S, Nicholas R, Reynolds R, Magliozzi R. Intrathecal inflammation in progressive multiple sclerosis. Int J Mol Sci. 2020;21(21):1‐11. doi: 10.3390/ijms21218217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fleischer V, Gonzalez‐Escamilla G, Ciolac D, et al. Translational value of choroid plexus imaging for tracking neuroinflammation in mice and humans. Proc Natl Acad Sci USA. 2021;118(36):1‐12. doi: 10.1073/pnas.2025000118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Manouchehri N, Stüve O. Choroid plexus volumetrics and brain inflammation in multiple sclerosis. Proc Natl Acad Sci USA. 2021;118(40):10‐12. doi: 10.1073/pnas.2115221118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ricigliano VA, Morena E, Colombi A, et al. Choroid plexus enlargement in inflammatory multiple sclerosis. Radiology. 2021;301:166‐177. [DOI] [PubMed] [Google Scholar]
- 13. Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011;69(2):292‐302. doi: 10.1002/ana.22366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Samjoo IA, Worthington E, Drudge C, et al. Efficacy classification of modern therapies in multiple sclerosis. J Comp Eff Res. 2021;10(6):495‐507. doi: 10.2217/cer-2020-0267 [DOI] [PubMed] [Google Scholar]
- 15. Klistorner SA, Barnett M, Yiannikas C, et al. Expansion of chronic lesions is linked to disease progression in relapsing‐remitting MS patients. Mult Scler. 2021;10:1533‐1542. [DOI] [PubMed] [Google Scholar]
- 16. Wang C, Barnett MH, Yiannikas C, et al. Lesion activity and chronic demyelination are the major determinants of brain atrophy in MS. Neurol Neuroimmunol NeuroInflammation. 2019;6(5):1‐9. doi: 10.1212/NXI.0000000000000593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dwyer M, Silva D, Bergsland N, et al. Neurological software tool for reliable atrophy measurement (NeuroSTREAM) of the lateral ventricles on clinical‐quality T2‐FLAIR MRI scans in multiple sclerosis. Neuroimage Clin. 2017;15:769‐779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kalkers N, Vrenken H, Uitdehaag B, Polman C, Barkhof F. Brain atrophy in multiple sclerosis: impact of lesions and of damage of whole brain tissue. Mult Scler. 2002;8(5):410‐414. doi: 10.1191/1352458502ms833oa [DOI] [PubMed] [Google Scholar]
- 19. Smith SM, Zhang Y, Jenkinson M, et al. Accurate, robust and automated longitudinal and cross‐sectional brain change analysis. Neuroimage. 2002;17:479‐489. [DOI] [PubMed] [Google Scholar]
- 20. Castriota‐scanderbeg A, Fasano F, Hagberg G, Nocentini U, Filippi M, Caltagirone C. Coefficient Dav is more sensitive than fractional anisotropy in monitoring progression of irreversible tissue damage in focal nonactive multiple sclerosis lesions. AJNR Am J Neuroradiol 2003;24:663–670. [PMC free article] [PubMed] [Google Scholar]
- 21. Klistorner A, Wang C, Yiannikas C, et al. Evidence of progressive tissue loss in the core of chronic MS lesions: a longitudinal DTI study. NeuroImage Clin. 2018;17:1028‐1035. doi: 10.1016/j.nicl.2017.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Opfer R, Ostwaldt AC, Sormani MP, et al. Estimates of age‐dependent cutoffs for pathological brain volume loss using SIENA/FSL—a longitudinal brain volumetry study in healthy adults. Neurobiol Aging. 2018;65:1‐6. doi: 10.1016/j.neurobiolaging.2017.12.024 [DOI] [PubMed] [Google Scholar]
- 23. Dwyer MG, Hagemeier J, Bergsland N, et al. Establishing pathological cut‐offs for lateral ventricular volume expansion rates. NeuroImage Clin. 2018;18(February):494‐501. doi: 10.1016/j.nicl.2018.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Muller J, Sinnecker T, Wendebourg M, Schlger M, Yaldizi O. Choroid plexus volume in multiple sclerosis vs neuromyelitis optica spectrum disorder. Neurol Neuroimmunol Neuroinflam. 2022;9:e1147. doi: 10.1212/NXI.0000000000001147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ricigliano V, Louapre C, Poirion É. An imaging signature in choroid plexuses in pre‐symptomatic multiple sclerosis. Present Annu Meet Eur Charcot Found. 2021; Poster‐online. [Google Scholar]
- 26. Geladaris A, Häusler D, Weber MS. Microglia: the missing link to decipher and therapeutically control MS progression? Int J Mol Sci. 2021;22(7):3461. doi: 10.3390/ijms22073461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Absinta M, Sati P, Reich DS. Advanced MRI and staging of multiple sclerosis lesions. Nat Publ gr. 2016;12(6):358‐368. doi: 10.1038/nrneurol.2016.59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rodríguez‐Lorenzo S, Konings J, Van Der Pol S, et al. Inflammation of the choroid plexus in progressive multiple sclerosis: accumulation of granulocytes and T cells. Acta Neuropathol Commun. 2020;8(1):1‐13. doi: 10.1186/s40478-020-00899-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rodríguez‐Lorenzo S, Ferreira Francisco DM, Vos R, et al. Altered secretory and neuroprotective function of the choroid plexus in progressive multiple sclerosis. Acta Neuropathol Commun. 2020;8(1):1‐13. doi: 10.1186/s40478-020-00903-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dixon GA, Pérez CA. Multiple sclerosis and the choroid plexus: emerging concepts of disease immunopathophysiology. Pediatr Neurol. 2020;103:65‐75. doi: 10.1016/j.pediatrneurol.2019.08.007 [DOI] [PubMed] [Google Scholar]
- 31. Barkho BZ, Monuki ES. Proliferation of cultured mouse choroid plexus epithelial cells. PLoS One. 2015;10(3):1‐14. doi: 10.1371/journal.pone.0121738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Campbell G, Kraytsberg E, Krishnan K, et al. Clonal expansion of mitochondrial DNA deletions in multiple sclerosis. Acta Neuropathol. 2012;124(1):209‐220. doi: 10.1007/s00401-012-1001-9.Clonal [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cardia E, Molina D, Abbate F, et al. Morphological modifications of the choroid plexus in a rodent model of acute ventriculitis induced by gram‐negative liquoral sepsis ‐ possible implications in the pathophysiology of hypersecretory hydrocephalus. Childs Nerv Syst. 1995;11(9):511‐516. doi: 10.1007/BF00822840 [DOI] [PubMed] [Google Scholar]
- 34. Prineas JW, Kwon EE, Cho E, et al. Immunopathology of secondary‐progressive multiple sclerosis. Ann Neurol. 2006;50:646‐657. doi: 10.1002/ana.1255 [DOI] [PubMed] [Google Scholar]
- 35. Lassmann H. New concepts on progressive multiple sclerosis. Cur Neurol Neurosci Reports. 2007;7:239‐244. [DOI] [PubMed] [Google Scholar]
- 36. Absinta M, Maric D, Gharagozloo M, et al. A lymphocyte–microglia–astrocyte axis in chronic active multiple sclerosis. Nature. 2021;597:709‐714. doi: 10.1038/s41586-021-03892-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Frischer JM, Weigand SD, Yong G, et al. Clinical and pathological insights into the dynamic nature of the white matter multiple sclerosis plaque. Ann Neurol. 2016;78(5):710‐721. doi: 10.1002/ana.24497.Clinical [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Datta G, Colasanti A, Rabiner EA, et al. Neuroinflammation and its relationship to changes in brain volume and white matter lesions in multiple sclerosis. Brain. 2017;140:2927‐2938. doi: 10.1093/brain/awx228 [DOI] [PubMed] [Google Scholar]
- 39. Magliozzi R, Scalfari A, Pisani AI, et al. The CSF profile linked to cortical damage predicts multiple sclerosis activity. Ann Neurol. 2020;88(3):562‐573. doi: 10.1002/ana.25786 [DOI] [PubMed] [Google Scholar]
- 40. Magliozzi R, Howell OW, Reeves C, et al. A gradient of neuronal loss and meningeal inflammation in multiple sclerosis. Ann Neurol. 2010;68(4):477‐493. doi: 10.1002/ana.22230 [DOI] [PubMed] [Google Scholar]
- 41. Poirion E, Tonietto M, Lejeune FX, et al. Structural and clinical correlates of a periventricular gradient of neuroinflammation in multiple sclerosis. Neurology. 2021;96(14):e1865‐e1875. doi: 10.1212/WNL.0000000000011700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Liu Z, Pardini M, Yaldizli O, et al. Magnetization transfer ratio measures in normal‐appearing white matter show periventricular gradient abnormalities in multiple sclerosis. Brain. 2015;138(5):1239‐1246. doi: 10.1093/brain/awv065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Brown JWL, Pardini M, Brownlee WJ, et al. An abnormal periventricular magnetization transfer ratio gradient occurs early in multiple sclerosis. Brain. 2017;140:387‐398. doi: 10.1093/brain/aww296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Pardini M, Petracca M, Harel A, et al. The relationship between cortical lesions and periventricular NAWM abnormalities suggests a shared mechanism of injury in primary‐progressive MS. NeuroImage Clin. 2017;16:111‐115. doi: 10.1016/j.nicl.2017.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Pardini M, Brown JWL, Magliozzi R, Reynolds R, Chard DT. Surface‐in pathology in multiple sclerosis: a new view on pathogenesis? Brain. 2021;144:1646‐1654. doi: 10.1093/brain/awab025 [DOI] [PubMed] [Google Scholar]
- 46. Elliott C, Arnold DL, Chen H, et al. Patterning chronic active demyelination in slowly expanding/evolving white matter MS lesions. Am J Neuroradiol. 2020;41(9):1‐8. doi: 10.3174/ajnr.A6742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Millward JM, Delgado PR, Smorodchenko A, et al. Transient enlargement of brain ventricles during relapsing‐remitting multiple sclerosis and experimental autoimmune encephalomyelitis. JCI Insight. 2020;5(21):1‐21. doi: 10.1172/jci.insight.140040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hill A. The environment and disease: association or causation? Proc R Soc Med. 1965;58:295‐300. doi: 10.1016/S0079-7421(08)60562-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary S1 Specific MRI parameters and image processing.


