Summary
Obstructive sleep apnea (OSA) is characterised by recurring episodes of upper airway obstruction during sleep and the fundamental abnormality reflects the inability of the upper airway dilating muscles to withstand the negative forces generated within the upper airway during inspiration. Factors that result in narrowing of the oropharynx such as abnormal craniofacial anatomy, soft tissue accumulation in the neck, and rostral fluid shift in the recumbent position increase the collapsing forces within the airway. The counteracting forces of upper airway dilating muscles, especially the genioglossus, are negatively influenced by sleep onset, inadequacy of the genioglossus responsiveness, ventilatory instability, especially post arousal, and loop gain. OSA is frequently associated with comorbidities that include metabolic, cardiovascular, renal, pulmonary, and neuropsychiatric, and there is growing evidence of bidirectional relationships between OSA and comorbidity, especially for heart failure, metabolic syndrome, and stroke. A detailed understanding of the complex pathophysiology of OSA encourages the development of therapies targeted at pathophysiological endotypes and facilitates a move towards precision medicine as a potential alternative to continuous positive airway pressure therapy in selected patients.
Keywords: comorbidity, diagnosis, epidemiology, obstructive sleep apnea, pathophysiology, treatment
1. INTRODUCTION
Obstructive sleep apnea (OSA) is characterised by recurring episodes of upper airway obstruction during sleep, leading to markedly reduced (hypopnea) or absent (apnea) airflow at the nose and mouth. The condition is usually associated with loud snoring and intermittent hypoxaemia, and apneas are typically terminated by brief micro‐arousals, which result in sleep fragmentation and diminished amounts of slow‐wave sleep (SWS) and rapid‐eye‐movement (REM) sleep (Deegan & McNicholas, 1995). Patients with OSA are usually unaware of this sleep disturbance but the changes in sleep architecture contribute significantly to the prominent symptoms of unrefreshing sleep and excessive daytime sleepiness (EDS) typically reported by many of these patients (Levy et al., 2015). Furthermore, the intermittent hypoxaemia and sleep fragmentation associated with OSA generate cell and molecular responses that generate systemic inflammation, sympathetic excitation, and other responses that predispose to comorbidities, especially cardiometabolic and neuropsychiatric (McNicholas, 2019).
While anecdotal reports on breathing stoppages during sleep were already published long ago, a full account of the OSA syndrome was given for the first time by Guilleminault et al. in the 1970s. In several seminal papers, clinical manifestations and typical findings on polysomnography (PSG) were described (Guilleminault et al., 1976). These authors and other independent research teams confirmed that previously unexplained medical conditions originate in respiratory problems occurring during nocturnal sleep. Current evidence indicates that OSA is common in the community and has a considerable impact on public health. Strong associations have been shown between OSA and drowsiness‐related motor vehicle accidents (Bonsignore et al., 2019). There is accumulating evidence that untreated OSA is associated with hypertension and constitutes a risk for cardiovascular disease (McNicholas et al.,2007). For these reasons, adequate management of OSA proves a major concern for public health authorities.
2. PATHOPHYSIOLOGY
While the detailed pathophysiology of OSA is complex, the fundamental abnormality reflects the inability of the upper airway dilating muscles to withstand the negative force generated within the upper airway during inspiration (Figure 1). In the normal setting, upper airway dilating muscles contract in a co‐ordinated manner that is timed with each inspiration, thus counteracting the negative pressure that is generated within the upper airway during inspiration. Factors that increase this negative pressure or diminish the efficacy of dilating muscle contraction upset this balance and predispose to upper airway obstruction (Deegan & McNicholas, 1995). Narrowing of the upper airway increases upper airway negative pressure during inspiration, thus promoting collapse. Factors contributing to narrowing include craniofacial bony morphology, soft tissue accumulation from obesity or adenotonsillar hypertrophy, and transient factors such as fluid accumulation that gravitates towards the neck in the recumbent position.
FIGURE 1.
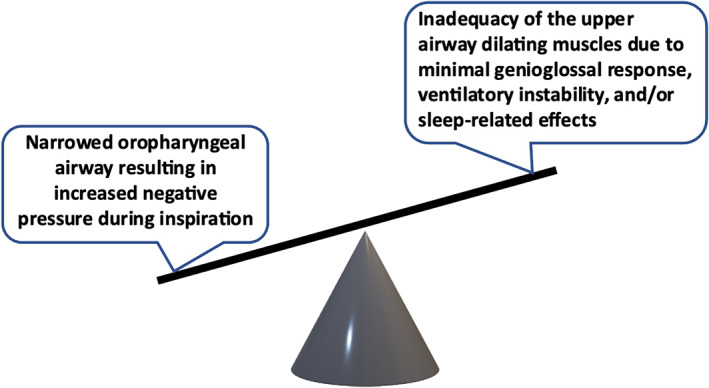
Balance of forces affecting the patency of the upper airway. Factors resulting in increased negative intrapharyngeal pressure and factors that reduce dilating muscle contraction together promote airway collapse
2.1. Upper airway narrowing
Most patients with OSA demonstrate a narrowed oropharyngeal airway that can be clinically assessed by the Mallampati score (Yu & Rosen, 2020), and genetic factors play a major role (Chi et al., 2014). Cephalometric and computed tomography (CT) studies demonstrate bony dimensions in the lower face and neck that result in upper airway narrowing (Neelapu et al., 2017), and clinical assessment demonstrates micro‐ or retrognathia in many of these patients (McNicholas, 2008a). Children with the Robin sequence or Treacher‐Collins syndrome are especially prone to OSA because of bony changes to the lower face and/or mandible that result in structural narrowing of the oropharyngeal airway (Tan, Kheirandish‐Gozal et al., 2016).
Soft tissue accumulation in and around the upper airway, such as with obesity and adenotonsillar hypertrophy, can predispose to OSA by narrowing the oropharyngeal lumen. Fat in the neck results in oropharyngeal narrowing and abdominal obesity reduces traction on the upper airway, which further predisposes to increased collapsibility (Deegan & McNicholas, 1995). Adenotonsillar hypertrophy is an important contributing factor in paediatric OSA, often in association with obesity (Dayyat et al., 2009).
Fluid accumulation in patients with congestive heart failure (CHF) and end‐stage renal failure, predisposes to OSA by nocturnal redistribution of fluid in the recumbent position to the parapharyngeal soft tissues, which increases upper airway resistance and collapsibility (Lyons et al., 2017; White & Bradley, 2013).
Nasal obstruction, especially variable nasal obstruction as in rhinitis, contributes to the pathophysiology of OSA (McNicholas, 2008b; McNicholas et al., 1982) and intranasal corticosteroids have been reported to reduce the apnea–hypopnea index (AHI) in patients with rhinitis and mild to moderate OSA (Kiely et al., 2004). The supine posture also has an adverse effect on upper airway patency (Yildirim et al., 1991), largely due to gravitational forces.
2.2. Upper airway dilator muscle function
Oropharyngeal airway patency is dependent on pharyngeal dilator muscles, especially the genioglossus, which act to stiffen the collapsible segment during inspiration (Deegan & McNicholas, 1995). These muscles contract in a phasic manner that is co‐ordinated with inspiration, which precedes diaphragmatic contraction by milliseconds (Strohl et al., 1980). Activity of these upper airway muscles is modulated by chemical stimuli, vagal input, changes in upper airway pressure, and baroreceptor activity (Brouillette & Thach, 1980).
In OSA, a narrowed upper airway generates greater inspiratory collapsing force, which requires more forceful dilating muscle contraction to maintain airway patency. Dilating muscle activity is higher than normal subjects during wakefulness and diminishes to a greater extent during sleep, thus predisposing to obstruction (Mezzanotte et al., 1996), especially in REM sleep (Carberry et al., 2016). Overall, the deficit in OSA relates to inadequate compensation in the face of increased inspiratory negative pressure rather than a primary deficiency in muscle function, which is compounded by these being skeletal muscles, resulting in a greater decrement during sleep than the diaphragm.
2.3. Respiratory control
The pattern of recurring apnea frequently observed in OSA supports an instability of ventilatory control similar to periodic breathing and upper airway obstruction is most likely when diaphragmatic and genioglossal inspiratory electromyogram (EMG) activity is at the lowest point of the cycle (Deegan & McNicholas, 1995). EMG activity progressively increases through the later stages of apnea and apnea termination is followed by hyperventilation for several breaths, after which both EMGs then decrease in activity, which predisposes to further obstruction (Dempsey et al., 2010).
2.4. Apnea threshold
Normal subjects demonstrate fluctuations in ventilation associated with the transition from wakefulness to non‐REM sleep, which is due to a reduction in the carbon dioxide (CO2) drive to breathe and the exposing of a sensitive apneic threshold that is critically CO2 dependent (Phillipson, 1978). In OSA, this threshold is amplified by post‐apnea hyperventilation, resulting in CO2 reduction, and predisposing to further apnea (Dempsey et al., 2010). Additional factors that may contribute to further apnea post hyperventilation include lung stretch receptor and baroreceptor stimulation (Deegan & McNicholas, 1995).
2.5. Loop gain
The predisposition to apnea associated with recurring cycles of hyper‐ and hypoventilation during sleep varies relating to loop gain, which refers to the gain of the negative feedback loop that regulates ventilation in response to a ventilatory disturbance. A high loop gain occurs where the magnitude of the increase in ventilation following apnea is high, thus increasing ventilatory system instability and increasing the likelihood of subsequent apnea (Dempsey et al., 2010). Two types of respiratory control system gain are evident, namely plant gain, which relates to the background drive to breathe, and controller gain, which relates to chemoreponsiveness (Dempsey et al., 2010). A reduced ventilatory drive and associated hypoventilation increases susceptibility to apnea by requiring only small transient ventilatory overshoots to reach the apneic threshold (high plant gain). Controller gain describes the slope of the ventilatory response to CO2 and an increased slope results in an increased susceptibility to apnea even in the setting of background hyperventilation and low plant gain.
2.6. Arousal
Termination of apnea is usually associated with brain arousal, which may be an important protective mechanism (Eckert & Malhotra, 2008), but may also contribute to the pathophysiology of OSA by predisposing to further upper airway collapse because of increased post‐apneic hyperventilation (Jordan et al., 2007; McNicholas, 1998). The intensity of respiratory cortical arousals appears to be a distinct pathophysiological feature that is associated with disease severity in patients with OSA (Bahr et al., 2021). Increasing ventilatory effort appears to be the most important arousal stimulus (Deegan & McNicholas, 1995).
The arousal response varies in patients with OSA and can be quantified by the arousal threshold, which can be assessed noninvasively by PSG (Sands et al., 2018b). A low arousal threshold is an important potential contributing factor to OSA pathophysiology and may represent a therapeutic target in selected patients (Eckert et al., 2011).
2.7. Integrated pathophysiology and implications for treatment
The relevance of physiological, non‐anatomical factors in the pathophysiology of OSA has generated major interest in recent years (Randerath et al., 2018) and in one study of subjects with and without OSA, similar proportions of subjects, roughly one‐third each, had the endotypic traits of a minimal genioglossus muscle responsiveness during sleep, a low arousal threshold, or a high loop gain, with 28% of subjects having more than one of these traits (Eckert et al., 2013).
While the basic deficit of increased upper airway collapsibility in OSA can be readily reversed by continuous positive airway pressure (CPAP) therapy, a detailed understanding of the pathophysiology opens the potential for other management options (Schutz et al., 2021). Inadequate upper airway dilating muscle compensation may be improved by targeted pharmacotherapy (Taranto‐Montemurro et al., 2019). Sleep‐induced reduction in respiratory motor neurone output can be reversed by electrical stimulation of the hypogossal nerve (Strollo et al., 2014). Acetazolamide may benefit OSA in selected patients with a high loop gain (Edwards et al., 2012) and zolpidem increases sleep efficiency and the respiratory arousal threshold (Messineo et al., 2020).
3. DIAGNOSIS
The high global prevalence of OSA (Benjafield et al., 2019) represents a challenge for diagnosis as the current “gold standard” diagnostic test is PSG in a sleep laboratory, which is labour intensive and expensive (Kapur et al., 2017). Disease severity is currently measured by the AHI as determined from a sleep study. However, there is a poor relationship between daytime symptoms such as sleepiness and the severity of OSA recorded in a sleep study (Deegan & McNicholas, 1996), and there is a growing trend to move away from the AHI as the principle measure of OSA severity towards a more personalised approach to OSA diagnosis and treatment (Pevernagie et al., 2020), by considering individual risk factors, clinical history and comorbid disease in the diagnosis and treatment of OSA (Randerath et al., 2018). Other signals such as heart rate variability, pulse transit time, expanded analysis of the oximetry signal, and the use of biomotion sensors may allow for an improved and phenotypic diagnosis (McNicholas, 2021). Home sleep apnea testing (HSAT) and wearable technologies may help improve access to diagnosis (O'Mahony et al., 2020), and advances in telemedicine help to strengthen inter‐departmental collaboration, thus improving the overall care of patients with OSA (O'Donnell et al., 2020).
Patients with OSA typically present with EDS, frequent awakenings, bed partner reports of frequent choking/gasping during sleep, and loud snoring (Kapur et al., 2017), in addition to morning headache, dry mouth, nonrestorative sleep, and may have hypertension or other comorbidity (McNicholas, 2008a). However, many patients do not report EDS or fatigue with some patients reporting only minimal symptoms (Heinzer et al., 2015), which have important implications for management and disease outcomes (Gagnadoux et al., 2016).
The Epworth Sleepiness Scale (ESS) is the most widely used clinical tool to evaluate subjective sleepiness, but correlates poorly with AHI (Kingshott et al., 1995) and is open to reporting bias. Daytime symptoms in OSA are influenced by age, gender, and the presence of other comorbidities (Levy et al., 2015), particularly depression and insomnia, and other symptoms such as fatigue and tiredness may be equally important in certain groups. Anthropometric and other objective variables such as age, sex, body mass index (BMI), neck circumference and co‐morbidities may be more reliable than subjective variables such as snoring and EDS in predicting OSA (Ustun et al., 2016). The identification of clinically significant OSA may be improved by the inclusion of relevant comorbidities, especially the loss of nocturnal blood pressure (BP) dipping (Crinion et al., 2017).
3.1. Polysomnography
Sleep staging remains fundamentally based on the scoring rules established by Rechtschaffen and Kales in 1968 (Rechtschaffen & Kales, 1968) and the scoring of sleep‐disordered breathing (SDB) events are based on the so‐called Chicago Criteria introduced in 1999, which also proposed a severity grading for OSA based on the AHI (Flemons et al., 1999). However, developments in technology and signal analysis in more recent years expand and enhance the information available from the PSG.
Although the traditional “gold standard” PSG fails to demonstrate an individual's susceptibility for systemic effects of intermittent hypoxaemia or to provide insight into the underlying pathophysiology. The oxygen desaturation index (ODI) may be a stronger and more reliable predictor of adverse cardiovascular outcomes than the AHI (Tkacova et al., 2014) and is easier to measure. Furthermore, the ODI and other variables of oxygen desaturation, such as the cumulative time spent below oxygen saturation levels such as 90% (CT90), minimal oxygen saturation, and mean oxygen desaturation also provide important clinical information to better address disease severity and risk of comorbidity in patients with comparable AHI (Dewan et al., 2015).
3.2. Home sleep apnea testing
Home sleep apnea testing can be performed in the patient's home, is cheaper and more convenient than PSG. HSAT will record between four and seven variables that include respiratory effort, airflow, heart rate or electrocardiograph (ECG), arterial oxygen saturation, snoring, body position and movement. Newer devices have improved diagnostic accuracy and have been validated against PSG (Collop et al., 2011). Also, HSAT can record several nights of sleep, which is advantageous as night‐to‐night variability is a feature of OSA and is most relevant in patients with mild disease (Guerrero et al., 2014). However, HSAT devices underestimate the AHI due to their inability to record total sleep time and detect arousals (Escourrou et al., 2015). Fewer physiological variables are measured which can miss co‐existing sleep disorders such as insomnia, periodic limb movements, and parasomnias.
3.3. Clinical and pathophysiological phenotypes
Clusters of different clinical phenotypes can be identified among the broad population of patients presenting for assessment of OSA (Bailly et al., 2020). Furthermore, certain pathophysiological traits that are very common in OSA such as loss of nocturnal dipping of BP have significant implications for the development of associated comorbidity (Crinion et al., 2017). Thus, whatever sleep study is employed in the assessment of suspected OSA, the findings must be integrated into the overall assessment of the patient as regards clinical significance, and management should be linked to the underlying phenotype where symptom profile and additional factors to the AHI such as acute systemic effects and associated relevant comorbidity are factored into the decision‐making process (Randerath et al., 2018).
3.4. New technologies
New measurements and technologies may better assess the various pathophysiological mechanisms underlying OSA, such as loop gain, arousal threshold, and anatomical factors. Reliable techniques to monitor sleep structure and arousals outside conventional EEG, such as arterial tonometry, require further evaluation and development, in addition to assessment of autonomic state and cardiovascular events associated with OSA events, such as ECG algorithms, heart rate variability, arterial tonometry, and capnography, which are currently omitted from conventional PSG (Khoo & Chalacheva, 2016). Further development of existing signals, such as cordless portable acoustic devices, allow enhanced diagnostic potential (Abbasi, 2017; Alshaer et al., 2016). There is a need to identify and validate physiological signals during wakefulness or sleep that may be useful in predicting cardiovascular risk.
The potential role of new technologies, like smartphone‐based applications (Ko et al., 2015) and consumer‐focused wearable devices (Haghayegh et al., 2019), are not yet sufficiently elaborated, and healthcare systems often do not integrate objective information provided by the patient from self‐made home recordings such as from smartphone applications.
Several key questions can be considered for the optimum diagnosis of a clinically significant OSA:
What are the most appropriate diagnostic criteria to evaluate suspected OSA?
Is the current severity grading of OSA appropriate given the high general population prevalence of elevated AHI?
Is ODI and/or other measures of oxygen desaturation more relevant than the AHI in assessing OSA severity, especially regarding comorbidity risk?
How should variables such as sleepiness and non‐dipping nocturnal BP be integrated into the clinical diagnosis?
4. COMORBIDITIES
Obstructive sleep apnea is independently associated with many comorbidities, including cardiovascular, metabolic, renal, and neuropsychiatric (McNicholas, 2019). However, many studies were cross‐sectional in design, which limits the ability to evaluate causality, and there is growing evidence of a bi‐directional relationship between OSA and comorbidity (Gleeson & McNicholas, 2022). Furthermore, most reports evaluating links between OSA and comorbidity have used the AHI as the principal metric of OSA severity, and there is growing evidence that the relationship of OSA with comorbidity should take into account additional variables such as acute and chronic systemic effects (Randerath et al., 2018). Figure 2 illustrates the theoretical relationship between respiratory events, their systemic effects, and potential end‐organ impact.
FIGURE 2.
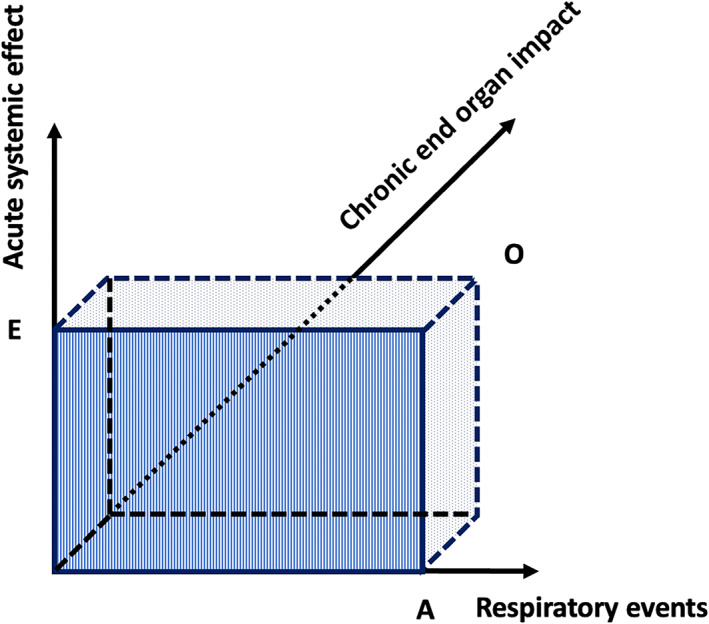
Three‐dimensional model of obstructive sleep apnea (OSA) disease severity. The x‐axis represents the amount of respiratory events (A) in an overnight sleep period. While this number is usually expressed as the apnea–hypopnea index in clinical studies, the absolute count of events is considered in this model. The y‐axis represents an acute systemic effect (E) induced by a respiratory event, e.g. a certain degree of hypoxaemia. The hatched area A*E represents the integration of all events with their associated systemic effects, being the nightly exposure to adverse effects of OSA. The nightly exposure inflicts repetitive strain to the end‐organ systems. The z‐axis represents a chronic end‐organ impact (O) of OSA, e.g. cognitive impairment, arterial hypertension, cardiovascular damage, insulin resistance, etc. Due to interindividual differences in susceptibility, the end‐organ impact may be variable among patients with OSA for similar levels of exposure. The dotted volume A*E*O represents the relation between the three dimensions. Based on postulated differences in susceptibility, the disease spectrum may vary from low exposure/high impact to high exposure/low impact. The dashed aspect of the boundaries indicates that the end‐organ impact of OSA is as yet difficult to assess. This is due to uncertainty regarding susceptibility on the one hand and to possible confounding effects of other disease processes on the other. Figure reproduced from (Randerath et al., 2018) with permission from the publisher
4.1. Cardiovascular disease
Obstructive sleep apnea is proposed as an independent risk factor for cardiovascular disease (McNicholas et al., 2007) and potential mechanisms include intermittent hypoxaemia (Ryan et al., 2005), sleep fragmentation (Carreras et al., 2014), exaggerated intra‐thoracic pressure swings (Bradley et al., 2001), sympathetic excitation (Jullian‐Desayes et al., 2015), inflammation (Ryan et al., 2009), and oxidative stress (Eltzschig & Eckle, 2011). However, these potential mechanistic relationships are complex, and, for example, there is evidence that mild hypoxaemia may be cardioprotective (Almendros et al., 2014). Furthermore, randomised controlled trials (RCTs) of positive pressure therapy of OSA have failed to show convincing evidence of benefit in the secondary prevention of cardiovascular disease (McEvoy et al., 2016; Peker et al., 2016), which challenges the notion that OSA is an independent contributor to cardiovascular morbidity. As further explained in the treatment section below, the relationship of OSA to cardiovascular disease and its outcomes remains uncertain.
4.1.1. Hypertension
Obstructive sleep apnea is a risk factor for systemic hypertension, often with a non‐dipping nocturnal BP profile (Parati et al., 2013), including data from the Sleep Heart Health Study (Nieto et al., 2000) and the Wisconsin Sleep Cohort Study (Peppard et al., 2000). A meta‐analysis of seven prospective studies confirmed an independent relationship with incident hypertension in a dose‐dependent fashion (Xia et al., 2018), which is often associated with resistance to conventional anti‐hypertensive treatment (Hou et al., 2018). Indeed, non‐dipping nocturnal BP is highly predictive of OSA, independent of symptom profile (Crinion et al., 2019), and REM‐associated OSA is independently associated with incident non‐dipping BP (Mokhlesi et al., 2015). Sympathetic excitation appears to be the principal pathogenic mechanism with possible additional involvement of renin–angiotensin–aldosterone system dysfunction (Crinion et al., 2021). Incident hypertension has also been independently associated with mild OSA, especially in patients aged <60 years (Vgontzas et al., 2019).
Continuous PAP therapy reduces BP, especially in younger subjects and those with uncontrolled hypertension or severe oxygen desaturation (Pengo et al., 2020), and in more CPAP‐compliant patients (Levy & McNicholas, 2013). CPAP may also restore the nocturnal dipping pattern in normotensive patients with OSA (Crinion et al., 2021; Sapina‐Beltran et al., 2019).
4.1.2. Congestive heart failure
Congestive heart failure has a bi‐directional relationship with sleep disordered breathing and is associated with both OSA and central sleep apnea (CSA). Typically, CSA in CHF is characterised by a central breathing pause that occurs during the decrescendo portion of the cyclic respiratory pattern (Naughton et al., 1993). OSA is associated with increased risk of incident heart failure (Gottlieb et al., 2010), which is proportional to OSA severity, and may be linked to major swings in intrathoracic pressure resulting in increased cardiac preload and afterload (Nicholl et al., 2014). The severity of intermittent hypoxaemia is a stronger predictor of outcome in patients with CHF than the AHI (Watanabe et al., 2017). CHF predisposes to OSA by nocturnal redistribution of fluid in the recumbent position to the parapharyngeal soft tissue, which increases upper airway resistance and collapsibility (White & Bradley, 2013).
4.1.3. Coronary artery disease
The evidence for OSA as an independent risk factor for coronary artery disease is equivocal and is stronger for males than females (Dong et al., 2013; Gottlieb et al., 2010; Hla et al., 2015). Furthermore, CPAP therapy has not been associated with improved outcomes in patients presenting with acute coronary syndrome (Sanchez‐de‐la‐Torre et al., 2020), except in those presenting with a first episode (Zapater et al., 2020).
4.1.4. Atrial fibrillation (AF)
Obstructive sleep apnea is a significant risk factor for the development and recurrence of AF (Linz et al., 2018) and international guidelines for the management of AF recommend diagnosis and treatment of OSA (Calkins et al., 2018; Kirchhof et al., 2016), as untreated disease has been shown to reduce the efficacy of both pharmacological and catheter‐based anti‐arrhythmic therapy. A meta‐analysis of seven prospective cohort studies found that CPAP therapy was associated with a reduction in AF recurrence, irrespective of whether they underwent pulmonary vein isolation (Shukla et al., 2015).
4.1.5. Stroke
The relationship between OSA and stroke is complex and bi‐directional (Bassetti et al., 2020). After adjusting for potential confounders such as age, sex, BMI, smoking, hypertension and diabetes, untreated OSA conveys a two‐fold increased risk of stroke incidence (Yaggi et al., 2005), and this increased risk is confirmed by large prospective population studies such as the Sleep Heart Health Study (Redline et al., 2010) and the Wisconsin Cohort Study, which also reported that 25% of strokes occurred during the night (Young, 2009). The SAVE trial (ClinicalTrials.gov Identifier: NCT00738179) reported no reduction in stroke rate with CPAP therapy (McEvoy et al., 2016), but a more recent post hoc analysis of this trial identified self‐reported snoring as a risk factor for incident stroke (Li et al., 2020), and CPAP compliance of >4 h may provide benefit (Parra et al., 2015). Randomised trials support an improvement in both short‐ and long‐term functional outcomes with CPAP therapy in patients with OSA after stroke (Brill et al., 2018). Stroke may predispose to OSA by impairment of breathing control mechanisms at a central level and/or adversely affecting upper airway muscle function, and OSA is highly prevalent after stroke (Alexiev et al., 2018).
4.2. Diabetes mellitus
Obstructive sleep apnea is independently associated with diabetes mellitus (Reutrakul & Mokhlesi, 2017). Several cross‐sectional cohort studies, including the European Sleep Apnea Database (ESADA) (Kent et al., 2014a, 2014b), have demonstrated an independent relationship with type 2 diabetes and insulin resistance, which are also supported by long‐term follow‐up studies (Kendzerska et al., 2014; Lindberg et al., 2012). Potential mechanisms of diabetes and insulin resistance include intermittent hypoxaemia and sleep fragmentation leading to sympathetic excitation and inflammation (Reutrakul & Mokhlesi, 2017).
Some consequences of diabetes mellitus could predispose to OSA, including neuropathy affecting the upper airway muscles, and disturbances in ventilatory control. However, the impact of CPAP therapy on glycaemic control is uncertain (Chirinos et al., 2014; Reutrakul & Mokhlesi, 2017).
4.3. Renal dysfunction
Renal disease and OSA have a bi‐directional relationship (Gleeson & McNicholas, 2022). OSA is up to 10‐times more prevalent in patients with chronic kidney disease (CKD) than the general population (Hanly, 2004), and potential contributing factors include increased chemoreflex sensitivity, reduced clearance of uraemic toxins, and hypervolaemia (Abuyassin et al., 2015). Fluid accumulation with associated nocturnal redistribution in the recumbent position, similar to CHF, is a key factor in end‐stage renal disease, as evidenced by the beneficial impact on OSA of fluid removal during dialysis (Lyons et al., 2015).
Obstructive sleep apnea can also contribute to CKD (Ahmed et al., 2011), and possible mechanisms include hypertension and intra‐renal hypoxaemia with glomerular hyperfiltration (Abuyassin et al., 2015). However, retrospective data analysis from the Wisconsin Sleep Cohort reported that OSA did not accelerate kidney function decline over time (Canales et al., 2018) and long‐term follow‐up of CPAP therapy in patients with OSA have shown no sustained benefit to renal function (Loffler et al., 2017; Rimke et al., 2021).
4.4. Depression
Depression and OSA may exhibit similar symptoms, including poor concentration, memory, and fatigue, which complicate their clinical assessment and diagnosis. The prevalence of depression in OSA ranges from 20% to 40% (Bixler et al., 2017) and there appears to be an increased odds ratio of depression with increasing severity of SDB (Peppard et al., 2006). A meta‐analysis of 22 RCTs reported that therapy of OSA resulted in a dose‐dependent improvement in depressive symptomatology using recognised depression scales (Povitz et al., 2014).
5. EPIDEMIOLOGY
The first major epidemiological study on OSA was published in 1993. Using PSG, Young et al. assessed the prevalence of OSA in the Wisconsin Sleep Cohort, a representative sample from the employed population (Young et al., 1993). The estimated prevalence of OSA, defined as an AHI of ≥5 events/h, was found to be 24% in men and 9% in women. Based on the combined presence of EDS and an increased AHI, it was estimated that 2% of women and 4% of men met the minimal diagnostic criteria for OSA disorder. The relevance of having OSA without EDS, which occurred in the majority of subjects, was not further addressed. Also, it was not explored whether OSA and EDS were causally related. To test this relationship, an interventional study would have been required.
Participants from the abovementioned cohort were invited for repeat studies at 4‐year intervals. In a subsequent investigation, the data from the 1988–1994 period were compared with the 2007–2010 period, and the prevalence of OSA was assessed based on an AHI of ≥15 events/h, representing moderate‐to‐severe OSA (Peppard et al., 2013). The prevalence rates of moderate‐to‐severe OSA in the latter period were 10% among men aged 30–49 years; 17% among men aged 50–70 years; 3% among women aged 30–49 years; and 9% among women aged 50–70 years. As in the foregoing study, the prevalence of an increased AHI was higher than the prevalence of EDS. A direct comparison between both studies was not feasible because of differences in assessment of subjective sleepiness. Substantial increases in OSA prevalence were seen over the span of two decades, ranging between 14% and 55% depending on the subgroup. The rise in OSA figures was ascribed to the surge of prevalent obesity.
The HypnoLaus study is a large ongoing survey on the prevalence of OSA in the region of Lausanne, Switzerland (Heinzer et al., 2015). As in the latest Wisconsin study, an AHI of ≥15 events/h is used for identifying OSA. However, the method used for hypopnea scoring is different in the study design of these cohorts, the Wisconsin study applying a 4% desaturation criterion and the HypnoLaus study being compliant with the 2012 American Academy of Sleep Medicine (AASM) scoring criteria (Berry et al., 2012). Heinzer et al. found that the prevalence of OSA in their population‐based sample was 23.4% in women and 49.7% in men. It was suggested that this high rate might be attributable to the increased sensitivity of the applied recording techniques and scoring criteria. Indeed, the 2012 AASM scoring system is much more prone to detecting hypopneas and thus produces higher AHI values. In keeping with the American studies, only a minority of men and women with an AHI of ≥15 events/h had concomitant EDS. In a subsequent analysis, Heinzer et al. applied prevailing AASM criteria of an AHI of ≥5 events/h plus symptoms and/or comorbidities (AASM, 2014; Heinzer et al., 2016) to test the occurrence of OSA in their population (Heinzer, Marti‐Soler, & Haba‐Rubio, 2016). The estimated prevalence was 79.2% in men and 54.3% in women. It was concluded that these overwhelming figures were probably not indicative of an OSA epidemic, but rather pointed to the unrealistic definition of OSA issued by the AASM.
Recently, a literature search was performed to try and estimate the global prevalence of OSA in individuals aged 30–69 years (Benjafield et al., 2019). The AASM 2012 scoring criteria were used as a reference for assessing the AHI, and a conversion algorithm was created for studies that had applied other criteria to allow determination of equivalent AHIs. Reliable prevalence data from 16 countries were extrapolated to other countries. Adjustments were based on population similarities, considering distributions of BMI, race, and geographical proximity. It was estimated that, globally, 936 million men and women of the considered age group have at least mild OSA and 425 million have moderate‐to‐severe OSA. The prevalence was highest in China, followed by the USA, Brazil, and India.
The use of the AHI as a primary predictor for identifying clinically relevant OSA in the general population is questionable. In all demographic studies to date, the prevalence of an increased AHI proves significantly higher than the associated prevalence of self‐reported EDS. Whether people from the community in whom an increased AHI is found are at risk of having or developing OSA‐related medical problems has not been established. If this risk would be substantial, the implementation of OSA screening programmes would be justified. The US Preventive Services Task Force have investigated this issue and have advised against population surveys for OSA in adults who are asymptomatic or who have unrecognised symptoms (Bibbins‐Domingo et al., 2017). The task force was unable to determine the magnitude of the benefits versus harms of screening for OSA. For case finding to be appropriate, the essence of OSA as a clinically relevant disease must be defined beyond the AHI. With the introduction of a valid clinical definition, biases in future epidemiological studies could be avoided (Holley & Phillips, 2018).
6. PHENOTYPES
Endotypes and phenotypes of SDB have been systematically studied in recent years (Edwards, Redline, Sands, & Owens, 2019). An “endotype” represents a particular mechanism that causes a physiological or metabolic disturbance in certain organ systems. As explained in the pathophysiology section above, several endotypes, in the sense of pathophysiological traits, have been distinguished in OSA. Evidence is accumulating that physiologically targeted treatment for OSA may effectively decrease the AHI and thus lower the pathophysiological burden of the disorder (Eckert, 2018).
While the term “phenotype” can have different meanings (and is sometimes used to also denote “endotype”), it usually refers to a combination of disease characteristics, in relation to clinically meaningful attributes (symptoms, treatment response, health outcomes, quality of life) that can be used to distinguish certain categories of patients from others (Zinchuk et al., 2017). Because OSA embodies a complex and heterogeneous disorder, several cluster analyses have been performed to discern clinical subtypes (Zinchuk & Yaggi, 2020). While certain profiles emerge from these studies, the method for subtyping clinical features is as yet not suitable to predict clinical outcomes in individual patients.
Originally, three symptomatic forms of OSA have been observed, namely patients with disturbed sleep, minimal symptoms, and EDS (Ye et al., 2014). These findings have been reproduced and expanded in a subsequent international, multicentric study (Keenan et al., 2018). In addition to the three basic clusters two other categories were recognised, i.e., upper airway symptoms dominant and sleepiness dominant subtypes. Similar average AHI values were found in both study samples and across clusters indicating that clinical phenotypes cannot be differentiated by the AHI. While the AHI fell short in predicting cardiovascular outcomes, a multisite study in a US Veteran cohort demonstrated that certain physiological endotypes, assessed by PSG, captured risk of adverse cardiovascular outcomes (Zinchuk & Yaggi, 2019).
Regarding cardiovascular outcomes, mixed results have been shown. There is evidence that the sleepy OSA phenotype is associated with worse clinical outcome and higher comorbidity (Randerath et al., 2018) and other phenotypes such as non‐dipping nocturnal BP have a high diagnostic prediction and are associated with a higher risk of comorbidity (Crinion et al., 2019). A recent survey from the USA seems to confirm the cardiovascular implications of EDS in OSA. Using data from the Sleep Heart Health Study, the excessively sleepy subtype was found to be most strongly associated with prevalent heart failure and with incident cardiovascular disease (Mazzotti et al., 2019). In contrast, the disturbed sleep or “insomnia” subtype, a predominant cluster in a survey of ESADA, was more frequently linked with cardiovascular comorbidity than the sleepy phenotype, despite lower mean AHI values (Saaresranta et al., 2016).
Of note, OSA is not only a complex and heterogeneous disorder, but may also co‐occur with other highly prevalent sleep disorders (Pepin et al., 2018). Unhealthy lifestyle habits and manifestations of medical and psychiatric comorbidities may further blend in with the palette of symptoms, thereby generating mixed phenotypes. The challenge for future research lies in the identification of unique markers of OSA to estimate its relative contribution in these composite conditions, and to predict response to targeted treatment more reliably (Edwards et al., 2019; Pevernagie, 2021).
7. TREATMENT
The aim of treating SDB is to maintain the patency of the upper airway, to stabilise the breathing pattern and to ensure adequate ventilation of the lungs. Because OSA and CSA frequently co‐occur, and share clinical and pathogenetic features (Dempsey et al., 2014), certain therapeutic strategies are common to both.
Anatomical traits compromising the patency of the upper airway are predominant in OSA. These traits are related to the surrounding musculoskeletal structure, body mass and muscular responsiveness. Non‐anatomical traits including arousability and loop gain may be present in both OSA and CSA. Habits such as alcohol use, sleep medication, lifestyle, body position while sleeping may influence both anatomical and non‐anatomical traits and thus the severity of the breathing disturbance. Part of the treatment plan is to address these variables, by which the AHI can readily be improved (Kuna et al., 2013; Strobel & Rosen, 1996).
Supplemental oxygen has been used for treating respiratory sleep disorders with varying degrees of success. Nocturnal oxygen therapy (NOT) may be beneficial for treating CSA with Cheyne‐Stokes respiration in heart failure (Aurora et al., 2012). Increasing the arterial PO2 is thought to dampen the respiratory drive, thus reducing minute ventilation and increasing CO2 reserves (Bordier et al., 2016). While supplemental oxygen is not recommended as a first‐line treatment for OSA, it may be co‐administered to reduce residual hypoxaemia in patients using CPAP. Promise lies in the identification of NOT‐responsiveness in patients with OSA, based on the assessment of endotypic traits using diagnostic PSG (Sands et al., 2018a). Combination of NOT with non‐CPAP treatment modalities might also improve clinical outcomes (Edwards et al., 2016).
Several pharmacological agents have been tested in different types of SDB. While some drugs may target treatable traits of OSA or CSA, there are as yet no compounds that are licensed in this domain (Gaisl et al., 2019). Acetazolamide, a carbonic anhydrase inhibitor, is known to reduce loop gain of the ventilatory control (Edwards et al., 2012), making it suitable for treating both CSA and OSA. A meta‐analysis of nine studies in patients with heart failure and CSA confirmed a modest, but statistically significant decrease of AHI (Wongboonsin et al., 2019). In OSA, the AHI may also be lowered, but daytime sleepiness and other clinical outcomes have not been shown to improve (Gaisl et al., 2019). Because long‐term studies with acetazolamide are lacking, its role in the extended treatment of respiratory sleep disorders is uncertain.
For a long time, hypnotics were believed to have adverse effects on respiration during sleep. Recent studies shed a different light on this conception. The effects of hypnotics in CSA are not well‐known, although some beneficial effect on the AHI may be anticipated due to inhibition of arousal and increase of CO2 stores. In OSA, interest was revived in the therapeutic potential of drugs that suppress arousability and that might reduce the AHI in patients with OSA with a low arousal threshold (Eckert et al., 2011). Against expectation, zolpidem was not found to increase the AHI, but to promote a positive effect by stimulating genioglossus activity during sleep in patients with OSA (Carberry et al., 2017). Due to a lack of randomised trials, no recommendations can presently be made regarding the selection of patients with OSA who could benefit from treatment with hypnotic drugs (Carberry et al., 2018).
The use of devices that deliver PAP to the airways is the mainstay of treating OSA (Sullivan et al., 1981). Besides mechanically stabilising the upper airway, PAP devices may have other effects on the respiratory system, such as increasing the end‐expiratory lung volume and improving stability of the central respiratory drive. These mechanisms may also be beneficial in heart failure with CSA and Cheyne‐Stokes respiration, as some of these patients are responsive to CPAP treatment (Bradley et al., 2005).
Several RCTs on PAP therapy have demonstrated a significant improvement of EDS. This effect is translated into a reduced risk of motor vehicle accidents, because adequately treated patients with OSA are no longer sleepy at the wheel (Antonopoulos, Sergentanis, Daskalopoulou, & Petridou, 2011). Studies regarding effects of PAP treatment on arterial hypertension have demonstrated modest improvement, as explained above. However, trials testing secondary prevention of cardiovascular disease in patients with OSA by applying PAP therapy have not shown convincing evidence of benefit (Abuzaid et al., 2017).
Problems with acceptance and tolerance may compromise CPAP treatment compliance. Adequate compliance, defined as CPAP use for ≥4 h/night for ≥70% of nights, was reported insufficient in >40% of patients with OSA in the initial treatment phase (Weaver & Grunstein, 2008). Many factors determine compliance with CPAP therapy. OSA disease severity and symptomatic benefit predict better outcomes, whereas adverse physical and psychological effects are linked with unsatisfactory results. Patient education and supportive interventions are paramount to treatment success (Smith et al., 2009). However, absence of any symptomatic improvement despite sufficient compliance should prompt considering other diagnoses and may justify discontinuation of CPAP therapy.
Mandibular repositioning devices (MAD) are increasingly used for treating primary snoring and OSA (Ramar et al., 2015). This therapy has been proven effective in patients with OSA who have a mildly to moderately elevated AHI. Healthy dentition is required, as the appliance must be anchored to the teeth of the upper and lower jaw. MAD therapy generally reduces the AHI and improves EDS. In studies comparing CPAP and MAD therapy, the effects on the AHI and EDS were better in the former group, but seemed similar in patients with milder OSA receiving either CPAP or MAD (Sharples et al., 2016).
Last but not least, surgical procedures for OSA can be applied in carefully selected patients. For a thorough review of these procedures the reader is referred elsewhere (Pevernagie et al., 2021). Below is a concise description of the most common interventions.
Nasal surgery aims at improving the endonasal lumen to facilitate nasal breathing. It usually has only a minor effect on OSA severity, but may improve compliance to PAP therapy in patients with OSA with nasal obstruction (Randerath et al., 2011).
Pharyngeal surgery comprises resection of adenoids and/or tonsils when these structures are enlarged, as well as remodelling of pharyngeal structures in patients with OSA, preferably selected after drug‐induced sedation endoscopy (Rashwan et al., 2018).
Hypoglossal nerve stimulation is a relatively new surgical technique based on electrical stimulation of a branch of the hypoglossal nerve that induces tongue protrusion. A set of components is implanted, including a pulse generator, a sensor‐lead monitoring inspiration and a stimulation electrode leading to the hypoglossal nerve. Candidates for this treatment option are symptomatic patients with OSA who are intolerant to PAP therapy (Strollo et al., 2014).
In patients with cranio‐skeletal deficiencies, maxillomandibular advancement surgery may offer a permanent remedy for OSA. The procedure consists of a bilateral sagittal split osteotomy of the mandibula and a Le Fort I osteotomy of the maxilla. Both structures are advanced by ~10 mm and fixed in this new position. Patients with OSA who have been appropriately selected for this intervention may have a lasting improvement in EDS and AHI, among other variables (Camacho et al., 2019).
Finally, bariatric surgery can be an appropriate remedy for obese patients with OSA in whom dietary regimens have failed. Overall, bariatric surgery is successful in reducing weight and the AHI, but in some patients persistent symptoms of OSA may prompt continuation of specific treatment for OSA (Greenburg et al., 2009).
8. FUTURE CHALLENGES
Despite extensive knowledge on the pathophysiological mechanisms and the adverse systemic effects of SDB, only small and/or nonsignificant results from CPAP therapy have been demonstrated in clinical trials aiming to reduce the cardiovascular burden in patients with OSA. These findings are paradoxical and prompt further consideration. It can no longer be denied that problems with the basic concept of OSA must be addressed as an obligatory step for respiratory sleep medicine to advance. Two aspects should be considered in particular: the causality issue and differences in individual susceptibility of systemic effects of OSA.
It is commonly assumed that the co‐occurrence of symptoms and signs suggestive of OSA with an increased AHI implies a causal relationship. However, many symptoms and signs of OSA are nonspecific (e.g., disturbed sleep, EDS, fatigue, high BP) and may fit other diagnoses as well (e.g., insomnia, narcolepsy, sleep restriction, primary hypertension). Therefore, the association between clinical manifestations and pathophysiological findings in OSA may due to coincidence (Figure 3). In that instance, treatment of OSA will be ineffective. Symptomatic response to therapy is a factor that corroborates causality, but treatment responsiveness is as yet difficult to predict.
FIGURE 3.
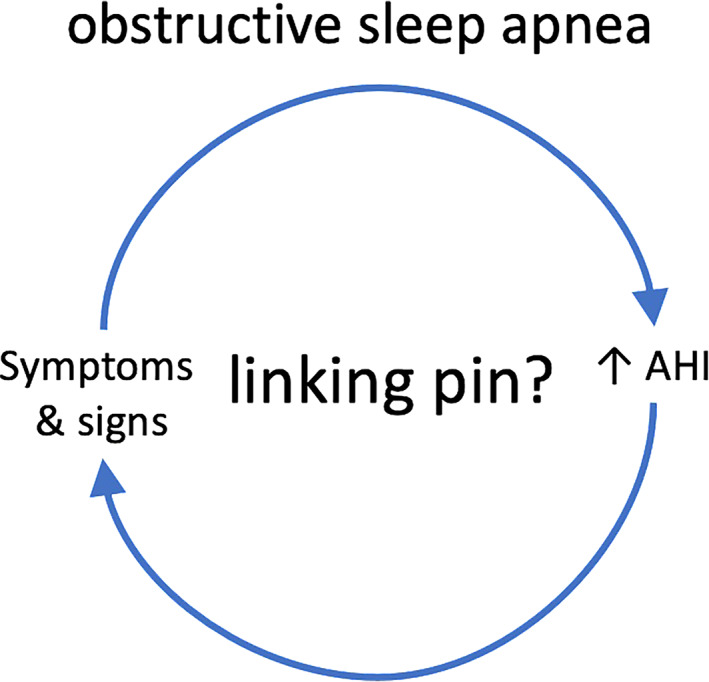
Reconsidering the conventional obstructive sleep apnea model. The assumption that an increased apnea–hypopnea index (AHI) is causally linked to symptoms and signs is an heuristic that has been introduced by the Stanford school in the 1970s. However, because both elements of this relationship are highly prevalent in the general population, the default hypothesis should rather be that the association is based on coincidence. Other factors have to be introduced into this model to enhance specificity, i.e., to increase the degree of certainty regarding causality
Another plausible explanation for equivocal therapeutic results may lie in differences in individual susceptibility to systemic effects of OSA (Pevernagie et al., 2020; Randerath et al., 2018) (Figure 2). Some subjects may be vulnerable, whereas others may be relatively resistant, irrespective of the AHI value or even the degree of hypoxaemia. Thus, disease severity may be disparate among subjects with similar levels of exposure. Consequently, results of treatment may average out to naught as categories with various degrees of disease severity (despite equivalent AHI or ODI values) are being recruited in clinical trials (Pevernagie, 2021).
Future research should be aimed to resolve unanswered questions regarding OSA diagnosis and management (McNicholas et al., 2018) (Figure 4). New approaches to syndrome definition are required that consider different clinical OSA phenotypes. Certain comorbidities strongly associated with OSA could also be included in severity grading, if a causal association can be demonstrated. New diagnostic approaches are required that incorporate novel technologies to provide surrogates for sleep structure, to gauge exposure to systemic effects of OSA, and to identify specific biomarkers for disease classification. Indeed, both causality and susceptibility issues may be settled by finding markers that enhance specificity in the relationship between pathophysiological endotypes and clinical phenotypes. While useful markers can conceivably be derived from PSG (Gauld & Micoulaud‐Franchi, 2021; Lechat et al., 2022; Lim et al., 2020; Pepin et al., 2018), it will be compulsory to adapt the conventional sleep diagnostic approach to techniques for ambulatory and multi‐night assessment.
FIGURE 4.
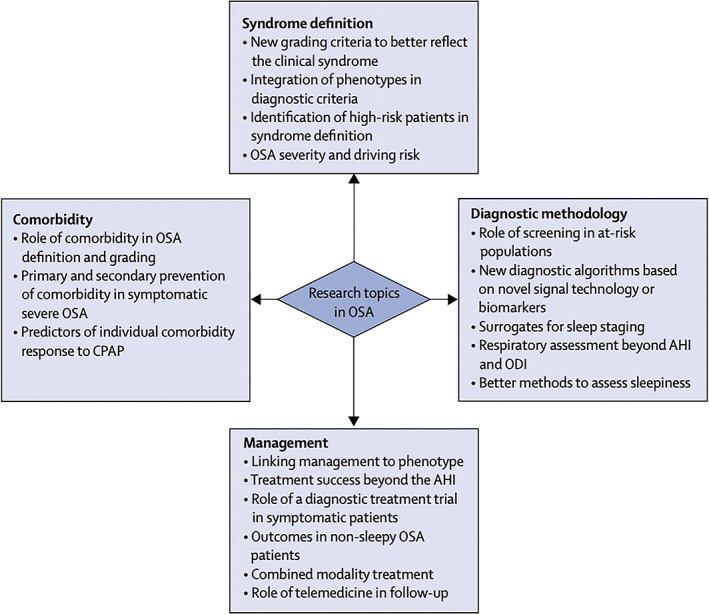
Proposed topics for future research in obstructive sleep apnoea (OSA). Research topics in OSA should be elaborated in four major domains: syndrome definition, diagnostic methodology, management, and clarifying the role of comorbidity. Figure reproduced from (McNicholas et al., 2018) with permission from the publisher. AHI, apnea–hypopnea index. CPAP, continuous positive airway pressure; ODI, oxygen desaturation index
Eventually, these efforts will give way to a new framework for personalised treatment and precision medicine, not only by linking pathophysiology to phenotype, but also by providing markers that identify treatable traits suitable for targeted treatment (Bonsignore et al., 2017; Pevernagie, 2021; Pien et al., 2018). As OSA is a complex and heterogeneous disease that may overlap with other sleep disorders, a differentiated approach is mandatory. This observation underlines the importance of clinical sleep medicine, because of its integrative methodology and added value for managing person‐specific sleep disorders.
AUTHOR CONTRIBUTION
Each author contributed equally to the preparation and writing of this paper.
ACKNOWLEDGEMENTS
Open access funding provided by IReL. [Correction added on 1 June 2022, after first online publication: IReL funding statement has been added.]
McNicholas, W. T. , & Pevernagie, D. (2022). Obstructive sleep apnea: transition from pathophysiology to an integrative disease model. Journal of Sleep Research, 31(4), e13616. 10.1111/jsr.13616
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- AASM (2014). Obstructive sleep apnea, adult. In Sateia M. (Ed.), The international classification of sleep disorders (3rd ed., pp. 53–62). American Academy of Sleep Medicine. [Google Scholar]
- Abbasi, J. (2017). In‐home, over‐the‐counter Sleep apnea sensor on the horizon. Jama‐Journal of the American Medical Association, 317(22), 2271. 10.1001/jama.2017.6928 [DOI] [PubMed] [Google Scholar]
- Abuyassin, B. , Sharma, K. , Ayas, N. T. , & Laher, I. (2015). Obstructive Sleep apnea and kidney disease: A potential bidirectional relationship? Journal of Clinical Sleep Medicine, 11(8), 915–924. 10.5664/jcsm.4946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abuzaid, A. S. , Al Ashry, H. S. , Elbadawi, A. , Ld, H. , Saad, M. , Elgendy, I. Y. , Elgendy, A. , Mahmoud, A. N. , Mentias, A. , Baraka, A. , & Lal, C. (2017). Meta‐analysis of cardiovascular outcomes with continuous positive airway pressure therapy in patients with obstructive Sleep apnea. The American Journal of Cardiology, 120(4), 693–699. 10.1016/j.amjcard.2017.05.042 [DOI] [PubMed] [Google Scholar]
- Ahmed, S. B. , Ronksley, P. E. , Hemmelgarn, B. R. , Tsai, W. H. , Manns, B. J. , Tonelli, M. , Klarenbach, S. W. , Chin, R. , Clement, F. M. , & Hanly, P. J. (2011). Nocturnal hypoxia and loss of kidney function. PLoS One, 6(4), e19029. 10.1371/journal.pone.0019029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexiev, F. , Brill, A. K. , Ott, S. R. , Duss, S. , Schmidt, M. , & Bassetti, C. L. (2018). Sleep‐disordered breathing and stroke: Chicken or egg? Journal of Thoracic Disease, 10(Suppl 34), S4244–S4252. 10.21037/jtd.2018.12.66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almendros, I. , Wang, Y. , & Gozal, D. (2014). The polymorphic and contradictory aspects of intermittent hypoxia. American Journal of Physiology. Lung Cellular and Molecular Physiology, 307(2), L129–L140. 10.1152/ajplung.00089.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alshaer, H. , Fernie, G. R. , Tseng, W. H. , & Bradley, T. D. (2016). Comparison of in‐laboratory and home diagnosis of sleep apnea using a cordless portable acoustic device. Sleep Medicine, 22, 91–96. 10.1016/j.sleep.2015.11.003 [DOI] [PubMed] [Google Scholar]
- Antonopoulos, C. N. , Sergentanis, T. N. , Daskalopoulou, S. S. , & Petridou, E. T. (2011). Nasal continuous positive airway pressure (nCPAP) treatment for obstructive sleep apnea, road traffic accidents and driving simulator performance: A meta‐analysis. Sleep Medicine Reviews, 15(5), 301–310. 10.1016/j.smrv.2010.10.002 [DOI] [PubMed] [Google Scholar]
- Aurora, R. N. , Chowdhuri, S. , Ramar, K. , Bista, S. R. , Casey, K. R. , Lamm, C. I. , Kristo, D. A. , Mallea, J. M. , Rowley, J. A. , Zak, R. S. , & Tracy, S. L. (2012). The treatment of central sleep apnea syndromes in adults: Practice parameters with an evidence‐based literature review and meta‐analyses. Sleep, 35(1), 17–40. 10.5665/sleep.1580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahr, K. , Geisler, V. , Huppertz, T. , Groppa, S. , Matthias, C. , Gouveris, H. , & Muthuraman, M. (2021). Intensity of respiratory cortical arousals is a distinct pathophysiologic feature and is associated with disease severity in obstructive Sleep apnea patients. Brain Sciences, 11(3), 1–17. 10.3390/brainsci11030282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailly, S. , Grote, L. , Hedner, J. , Schiza, S. , McNicholas, W. T. , Basoglu, O. K. , Lombardi, C. , Dogas, Z. , Roisman, G. , Pataka, A. , Bonsignore, M. R. , Pepin, J. L. , & ESADA Study Group . (2020). Clusters of sleep apnoea phenotypes: A large pan‐European study from the European Sleep Apnoea database (ESADA). Respirology, 26, 378–387. 10.1111/resp.13969 [DOI] [PubMed] [Google Scholar]
- Bassetti, C. L. A. , Randerath, W. , Vignatelli, L. , Ferini‐Strambi, L. , Brill, A. K. , Bonsignore, M. R. , Grote, L. , Jennum, P. , Leys, D. , Minnerup, J. , Nobili, L. , Tonia, T. , Morgan, R. , Kerry, J. , Riha, R. , McNicholas, W. T. , & Papavasileiou, V. (2020). EAN/ERS/ESO/ESRS statement on the impact of sleep disorders on risk and outcome of stroke. European Journal of Neurology, 27(7), 1117–1136. 10.1111/ene.14201 [DOI] [PubMed] [Google Scholar]
- Benjafield, A. V. , Ayas, N. T. , Eastwood, P. R. , Heinzer, R. , Ip, M. S. M. , Morrell, M. J. , Nunez, C. M. , Patel, S. R. , Penzel, T. , Pépin, J. L. , Peppard, P. E. , Sinha, S. , Tufik, S. , Valentine, K. , & Malhotra, A. (2019). Estimation of the global prevalence and burden of obstructive sleep apnoea: A literature‐based analysis. The Lancet Respiratory Medicine, 7(8), 687–698. 10.1016/s2213-2600(19)30198-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry, R. B. , Brooks, R. , Gamaldo, C. E. , Harding, S. M. , Marcus, C. L. , & Vaughn, B. V. (2012). The AASM manual for the scoring of sleep and associated events. Rules, terminology and technical specifications. Version 2.0. American Academy of Sleep Medicine. [Google Scholar]
- Bibbins‐Domingo, K. , Grossman, D. C. , Curry, S. J. , Davidson, K. W. , Epling, J. W., Jr. , Garcia, F. A. , Herzstein, J. , Kemper, A. R. , Krist, A. H. , Kurth, A. E. , Landefeld, C. S. , Mangione, C. M. , Phillips, W. R. , Phipps, M. G. , Pignone, M. P. , Silverstein, M. , & Tseng, C. W. (2017). Screening for obstructive Sleep apnea in adults: US preventive services task force recommendation statement. JAMA, 317(4), 407–414. 10.1001/jama.2016.20325 [DOI] [PubMed] [Google Scholar]
- Bixler, E. O. , Gaines, J. , & Vgontzas, A. N. (2017). Obstructive sleep apnoea and depression: Is there an association? The European Respiratory Journal, 49(6), 1700858. 10.1183/13993003.00858-2017 [DOI] [PubMed] [Google Scholar]
- Bonsignore, M. R. , Marrone, O. , & Fanfulla, F. (2019). Sleep apnea, sleepiness, and driving risk. Sleep Medicine Clinics, 14(4), 431–439. 10.1016/j.jsmc.2019.08.001 [DOI] [PubMed] [Google Scholar]
- Bonsignore, M. R. , Suarez Giron, M. C. , Marrone, O. , Castrogiovanni, A. , & Montserrat, J. M. (2017). Personalised medicine in sleep respiratory disorders: Focus on obstructive sleep apnoea diagnosis and treatment. European Respiratory Review, 26(146), 170069. 10.1183/16000617.0069-2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordier, P. , Lataste, A. , Hofmann, P. , Robert, F. , & Bourenane, G. (2016). Nocturnal oxygen therapy in patients with chronic heart failure and sleep apnea: A systematic review. Sleep Medicine, 17, 149–157. 10.1016/j.sleep.2015.10.017 [DOI] [PubMed] [Google Scholar]
- Bradley, T. D. , Hall, M. J. , Ando, S. , & Floras, J. S. (2001). Hemodynamic effects of simulated obstructive apneas in humans with and without heart failure. Chest, 119(6), 1827–1835. 10.1378/chest.119.6.1827 [DOI] [PubMed] [Google Scholar]
- Bradley, T. D., Logan, A. G., Kimoff, R. J., Series, F., Morrison, D., Ferguson, K., Belenkie, I., Pfeifer, M., Fleetham, J., Hanly, P. Smilovitch, M. Tomlinson, G., Floras, J. S., CANPAP Investigators (2005). Continuous positive airway pressure for central sleep apnea and heart failure. The New England Journal of Medicine, 353(19), 2025–2033. doi: 10.1056/NEJMoa051001. [DOI] [PubMed]
- Brill, A. K. , Horvath, T. , Seiler, A. , Camilo, M. , Haynes, A. G. , Ott, S. R. , Egger, M. , & Bassetti, C. L. (2018). CPAP as treatment of sleep apnea after stroke: A meta‐analysis of randomized trials. Neurology, 90(14), e1222–e1230. 10.1212/WNL.0000000000005262 [DOI] [PubMed] [Google Scholar]
- Brouillette, R. T. , & Thach, B. T. (1980). Control of genioglossus muscle inspiratory activity. Journal of Applied Physiology: Respiratory, Environmental and Exercise Physiology, 49(5), 801–808. 10.1152/jappl.1980.49.5.801 [DOI] [PubMed] [Google Scholar]
- Calkins, H. , Hindricks, G. , Cappato, R. , Kim, Y. H. , Saad, E. B. , Aguinaga, L. , Akar, J. G. , Badhwar, V. , Brugada, J. , Camm, J. , Chen, P.‐S. , Chen, S.‐A. , Chung, M. K. , Nielsen, J. C. , Curtis, A. B. , Davies, D. W. , Day, J. D. , d'Avila, A. , de Groot, N. M. S. N. , … Yamane, T. (2018). 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Europace, 20(1), e1–e160. 10.1093/europace/eux274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camacho, M. , Noller, M. W. , Del Do, M. , Wei, J. M. , Gouveia, C. J. , Zaghi, S. , Boyd, S. B. , & Guilleminault, C. (2019). Long‐term results for Maxillomandibular advancement to treat obstructive Sleep apnea: A meta‐analysis. Otolaryngology and Head and Neck Surgery, 160(4), 580–593. 10.1177/0194599818815158 [DOI] [PubMed] [Google Scholar]
- Canales, M. T. , Hagen, E. W. , Barnet, J. H. , Peppard, P. E. , & Derose, S. F. (2018). Sleep apnea and kidney function trajectory: Results from a 20‐year longitudinal study of healthy middle‐aged adults. Sleep, 41(1), 1–8. 10.1093/sleep/zsx181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carberry, J. C. , Amatoury, J. , & Eckert, D. J. (2018). Personalized management approach for OSA. Chest, 153(3), 744–755. 10.1016/j.chest.2017.06.011 [DOI] [PubMed] [Google Scholar]
- Carberry, J. C. , Fisher, L. P. , Grunstein, R. R. , Gandevia, S. C. , McKenzie, D. K. , Butler, J. E. , & Eckert, D. J. (2017). Role of common hypnotics on the phenotypic causes of obstructive sleep apnoea: Paradoxical effects of zolpidem. The European Respiratory Journal, 50(6), 1701344. 10.1183/13993003.01344-2017 [DOI] [PubMed] [Google Scholar]
- Carberry, J. C. , Jordan, A. S. , White, D. P. , Wellman, A. , & Eckert, D. J. (2016). Upper airway collapsibility (Pcrit) and pharyngeal dilator muscle activity are Sleep stage dependent. Sleep, 39(3), 511–521. 10.5665/sleep.5516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carreras, A. , Zhang, S. X. , Peris, E. , Qiao, Z. , Gileles‐Hillel, A. , Li, R. C. , Wang, Y. , & Gozal, D. (2014). Chronic sleep fragmentation induces endothelial dysfunction and structural vascular changes in mice. Sleep, 37(11), 1817–1824. 10.5665/sleep.4178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi, L. , Comyn, F. L. , Keenan, B. T. , Cater, J. , Maislin, G. , Pack, A. I. , & Schwab, R. J. (2014). Heritability of craniofacial structures in normal subjects and patients with sleep apnea. Sleep, 37(10), 1689–1698. 10.5665/sleep.4082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirinos, J. A. , Gurubhagavatula, I. , Teff, K. , Rader, D. J. , Wadden, T. A. , Townsend, R. , Foster, G. D. , Maislin, G. , Saif, H. , Broderick, P. , Chittams, J. , Hanlon, A. L. , & Pack, A. I. (2014). CPAP, weight loss, or both for obstructive sleep apnea. The New England Journal of Medicine, 370(24), 2265–2275. 10.1056/NEJMoa1306187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collop, N. A. , Tracy, S. L. , Kapur, V. , Mehra, R. , Kuhlmann, D. , Fleishman, S. A. , & Ojile, J. M. (2011). Obstructive sleep apnea devices for out‐of‐center (OOC) testing: Technology evaluation. Journal of Clinical Sleep Medicine, 7(5), 531–548. 10.5664/jcsm.1328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crinion, S. J. , Kleinerova, J. , Kent, B. , Nolan, G. , Taylor, C. T. , Ryan, S. , & McNicholas, W. T. (2021). Non‐dipping nocturnal blood pressure correlates with obstructive sleep apnoea severity in normotensive subjects and may reverse with therapy. ERJ Open Research, 7(3), 1–9. 10.1183/23120541.00338-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crinion, S. J. , Ryan, S. , Kleinerova, J. , Kent, B. D. , Gallagher, J. , Ledwidge, M. , McDonald, K. , & McNicholas, W. T. (2019). Nondipping nocturnal blood pressure predicts Sleep apnea in patients with hypertension. Journal of Clinical Sleep Medicine, 15(7), 957–963. 10.5664/jcsm.7870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crinion, S. J. , Ryan, S. , & McNicholas, W. T. (2017). Obstructive sleep apnoea as a cause of nocturnal nondipping blood pressure: Recent evidence regarding clinical importance and underlying mechanisms. The European Respiratory Journal, 49(1), 1601818. 10.1183/13993003.01818-2016 [DOI] [PubMed] [Google Scholar]
- Dayyat, E. , Kheirandish‐Gozal, L. , Sans Capdevila, O. , Maarafeya, M. M. A. , & Gozal, D. (2009). Obstructive sleep apnea in children: Relative contributions of body mass index and adenotonsillar hypertrophy. Chest, 136(1), 137–144. 10.1378/chest.08-2568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deegan, P. C. , & McNicholas, W. T. (1995). Pathophysiology of obstructive sleep apnoea. The European Respiratory Journal, 8(7), 1161–1178. 10.1183/09031936.95.08071161 [DOI] [PubMed] [Google Scholar]
- Deegan, P. C. , & McNicholas, W. T. (1996). Predictive value of clinical features for the obstructive sleep apnoea syndrome. The European Respiratory Journal, 9(1), 117–124 Retrieved from http://erj.ersjournals.com/cgi/content/abstract/9/1/117 [DOI] [PubMed] [Google Scholar]
- Dempsey, J. A. , Veasey, S. C. , Morgan, B. J. , & O'Donnell, C. P. (2010). Pathophysiology of sleep apnea. Physiological Reviews, 90(1), 47–112. 10.1152/physrev.00043.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempsey, J. A. , Xie, A. , Patz, D. S. , & Wang, D. (2014). Physiology in medicine: Obstructive sleep apnea pathogenesis and treatment–considerations beyond airway anatomy. Journal of Applied Physiology (Bethesda, MD: 1985), 116(1), 3–12. 10.1152/japplphysiol.01054.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewan, N. A. , Nieto, F. J. , & Somers, V. K. (2015). Intermittent hypoxemia and OSA: Implications for comorbidities. Chest, 147(1), 266–274. 10.1378/chest.14-0500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong, J. Y. , Zhang, Y. H. , & Qin, L. Q. (2013). Obstructive sleep apnea and cardiovascular risk: Meta‐analysis of prospective cohort studies. Atherosclerosis, 229(2), 489–495. 10.1016/j.atherosclerosis.2013.04.026 [DOI] [PubMed] [Google Scholar]
- Eckert, D. J. (2018). Phenotypic approaches to obstructive sleep apnoea ‐ new pathways for targeted therapy. Sleep Medicine Reviews, 37, 45–59. 10.1016/j.smrv.2016.12.003 [DOI] [PubMed] [Google Scholar]
- Eckert, D. J. , & Malhotra, A. (2008). Pathophysiology of adult obstructive sleep apnea. Proceedings of the American Thoracic Society, 5(2), 144–153. 10.1513/pats.200707-114MG [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert, D. J. , Owens, R. L. , Kehlmann, G. B. , Wellman, A. , Rahangdale, S. , Yim‐Yeh, S. , White, D. P. , & Malhotra, A. (2011). Eszopiclone increases the respiratory arousal threshold and lowers the apnoea/hypopnoea index in obstructive sleep apnoea patients with a low arousal threshold. Clinical Science (London, England), 120(12), 505–514. 10.1042/cs20100588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert, D. J. , White, D. P. , Jordan, A. S. , Malhotra, A. , & Wellman, A. (2013). Defining phenotypic causes of obstructive sleep apnea. Identification of novel therapeutic targets. American Journal of Respiratory and Critical Care Medicine, 188(8), 996–1004. 10.1164/rccm.201303-0448OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards, B. A. , Redline, S. , Sands, S. A. , & Owens, R. L. (2019). More than the sum of the respiratory events: Personalized medicine approaches for obstructive Sleep apnea. American Journal of Respiratory and Critical Care Medicine, 200(6), 691–703. 10.1164/rccm.201901-0014TR [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards, B. A. , Sands, S. A. , Eckert, D. J. , White, D. P. , Butler, J. P. , Owens, R. L. , Malhotra, A. , & Wellman, A. (2012). Acetazolamide improves loop gain but not the other physiological traits causing obstructive sleep apnoea. The Journal of Physiology, 590(5), 1199–1211. 10.1113/jphysiol.2011.223925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards, B. A. , Sands, S. A. , Owens, R. L. , Eckert, D. J. , Landry, S. , White, D. P. , Malhotra, A. , & Wellman, A. (2016). The combination of supplemental oxygen and a hypnotic markedly improves obstructive Sleep apnea in patients with a mild to moderate upper airway collapsibility. Sleep, 39(11), 1973–1983. 10.5665/sleep.6226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eltzschig, H. K. , & Eckle, T. (2011). Ischemia and reperfusion – from mechanism to translation. Nature Medicine, 17(11), 1391–1401. 10.1038/nm.2507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escourrou, P. , Grote, L. , Penzel, T. , McNicholas, W. T. , Verbraecken, J. , Tkacova, R. , Riha, R. L. , Hedner, J. , & Group, E. S . (2015). The diagnostic method has a strong influence on classification of obstructive sleep apnea. Journal of Sleep Research, 24(6), 730–738. 10.1111/jsr.12318 [DOI] [PubMed] [Google Scholar]
- Flemons, W. W. , Buysse, D. , Redline, S. , Pack, A. , Strohl, K. , Wheatley, J. , Young, T. , Douglas, N. , Levy, P. , Mcnicholas, W. , Fleetham, J. , White, D. , Schmidt Nowarra, W. , Carley, D. , Romaniuk, J. , & European Respiratory, S. Australasian Sleep, A. American Thoracic Societ . (1999). Sleep‐related breathing disorders in adults: Recommendations for syndrome definition and measurement techniques in clinical research. Sleep, 22(5), 667–689 Retrieved from <Go to ISI>://000081894700013. [PubMed] [Google Scholar]
- Gagnadoux, F. , Le Vaillant, M. , Paris, A. , Pigeanne, T. , Leclair‐Visonneau, L. , Bizieux‐Thaminy, A. , Alizon, C. , Humeau, M.‐P. , Nguyen, X.‐L. , Rouault, B. , Trzepizur, W. , Meslier, N. , & Institut de Recherche en Santé Respiratoire des Pays de la Loire Sleep Cohort Group . (2016). Relationship between OSA clinical phenotypes and CPAP treatment outcomes. Chest, 149(1), 288–290. 10.1016/j.chest.2015.09.032 [DOI] [PubMed] [Google Scholar]
- Gaisl, T. , Haile, S. R. , Thiel, S. , Osswald, M. , & Kohler, M. (2019). Efficacy of pharmacotherapy for OSA in adults: A systematic review and network meta‐analysis. Sleep Medicine Reviews, 46, 74–86. 10.1016/j.smrv.2019.04.009 [DOI] [PubMed] [Google Scholar]
- Gauld, C. , & Micoulaud‐Franchi, J. A. (2021). Why could sleep medicine never do without polysomnography? Journal of Sleep Research, e13541. 10.1111/jsr.13541 [DOI] [PubMed] [Google Scholar]
- Gleeson, M. , & McNicholas, W. T. (2022). Bidirectional relationships of comorbidity with obstructive sleep apnoea . Eur Respir Rev., 31, 1–15. 10.1183/16000617.0256-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb, D. J. , Yenokyan, G. , Newman, A. B. , O'Connor, G. T. , Punjabi, N. M. , Quan, S. F. , Redline, S. , Resnick, H. E. , Tong, E. K. , Diener‐West, M. , & Shahar, E. (2010). Prospective study of obstructive sleep apnea and incident coronary heart disease and heart failure: The sleep heart health study. Circulation, 122(4), 352–360. 10.1161/CIRCULATIONAHA.109.901801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenburg, D. L. , Lettieri, C. J. , & Eliasson, A. H. (2009). Effects of surgical weight loss on measures of obstructive sleep apnea: A meta‐analysis. The American Journal of Medicine, 122(6), 535–542. 10.1016/j.amjmed.2008.10.037 [DOI] [PubMed] [Google Scholar]
- Guerrero, A. , Embid, C. , Isetta, V. , Farre, R. , Duran‐Cantolla, J. , Parra, O. , Barbé, F. , Montserrat, J. M. , & Masa, J. F. (2014). Management of sleep apnea without high pretest probability or with comorbidities by three nights of portable sleep monitoring. Sleep, 37(8), 1363–1373. 10.5665/sleep.3932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilleminault, C. , Tilkian, A. , & Dement, W. C. (1976). The sleep apnea syndromes. Annual Review of Medicine, 27, 465–484. 10.1146/annurev.me.27.020176.002341 [DOI] [PubMed] [Google Scholar]
- Haghayegh, S. , Khoshnevis, S. , Smolensky, M. H. , Diller, K. R. , & Castriotta, R. J. (2019). Accuracy of wristband Fitbit models in assessing Sleep: Systematic review and meta‐analysis. Journal of Medical Internet Research, 21(11), e16273. 10.2196/16273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanly, P. (2004). Sleep apnea and daytime sleepiness in end‐stage renal disease. Seminars in Dialysis, 17(2), 109–114. 10.1111/j.0894-0959.2004.17206.x [DOI] [PubMed] [Google Scholar]
- Heinzer, R. , Vat, S. , Marques‐Vidal, P. , Marti‐Soler, H. , Andries, D. , Tobback, N. , Mooser, V. , Preisig, M. , Malhotra, A. , Waeber, G. , Vollenweider, P. , Tafti, M. , & Haba‐Rubio, J. (2015). Prevalence of sleep‐disordered breathing in the general population: The HypnoLaus study. The Lancet Respiratory Medicine, 3(4), 310–318. 10.1016/s2213-2600(15)00043-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hla, K. M. , Young, T. , Hagen, E. W. , Stein, J. H. , Finn, L. A. , Nieto, F. J. , & Peppard, P. E. (2015). Coronary heart disease incidence in sleep disordered breathing: The Wisconsin Sleep cohort study. Sleep, 38(5), 677–684. 10.5665/sleep.4654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holley, A. B. , & Phillips, B. (2018). The next 25 years of obstructive Sleep apnea epidemiology‐Don't keep repeating past mistakes. American Journal of Respiratory and Critical Care Medicine, 198(3), 410–411. 10.1164/rccm.201802-0315LE [DOI] [PubMed] [Google Scholar]
- Hou, H. , Zhao, Y. , Yu, W. , Dong, H. , Xue, X. , Ding, J. , Xing, W. , & Wang, W. (2018). Association of obstructive sleep apnea with hypertension: A systematic review and meta‐analysis. Journal of Global Health, 8(1), 010405. 10.7189/jogh.08.010405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan, A. S. , Wellman, A. , Heinzer, R. C. , Lo, Y. L. , Schory, K. , Dover, L. , Gautam, S. , Malhotra, A. , & White, D. P. (2007). Mechanisms used to restore ventilation after partial upper airway collapse during sleep in humans. Thorax, 62(10), 861–867. 10.1136/thx.2006.070300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jullian‐Desayes, I. , Joyeux‐Faure, M. , Tamisier, R. , Launois, S. , Borel, A. L. , Levy, P. , & Pepin, J. L. (2015). Impact of obstructive sleep apnea treatment by continuous positive airway pressure on cardiometabolic biomarkers: A systematic review from sham CPAP randomized controlled trials. Sleep Medicine Reviews, 21, 23–38. 10.1016/j.smrv.2014.07.004 [DOI] [PubMed] [Google Scholar]
- Kapur, V. K. , Auckley, D. H. , Chowdhuri, S. , Kuhlmann, D. C. , Mehra, R. , Ramar, K. , & Harrod, C. G. (2017). Clinical practice guideline for diagnostic testing for adult obstructive Sleep apnea: An American Academy of Sleep medicine clinical practice guideline. Journal of Clinical Sleep Medicine, 13(3), 479–504. 10.5664/jcsm.6506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keenan, B. T. , Kim, J. , Singh, B. , Bittencourt, L. , Chen, N. H. , Cistulli, P. A. , Magalang, U. J. , McArdle, N. , Mindel, J. W. , Benediktsdottir, B. , Arnardottir, E. S. , Prochnow, L. K. , Penzel, T. , Sanner, B. , Schwab, R. J. , Shin, C. , Sutherland, K. , Tufik, S. , Maislin, G. , … Pack, A. I. (2018). Recognizable clinical subtypes of obstructive sleep apnea across international sleep centers: A cluster analysis. Sleep, 41(3), 1–14. 10.1093/sleep/zsx214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendzerska, T. , Gershon, A. S. , Hawker, G. , Tomlinson, G. , & Leung, R. S. (2014). Obstructive sleep apnea and incident diabetes. A historical cohort study. American Journal of Respiratory and Critical Care Medicine, 190(2), 218–225. 10.1164/rccm.201312-2209OC [DOI] [PubMed] [Google Scholar]
- Kent, B. D. , Grote, L. , Bonsignore, M. R. , Saaresranta, T. , Verbraecken, J. , Levy, P. , Sliwinski, P. , Tkacova, R. , Kvamme, J.‐A. , Fietze, I. , Hedner, J. , McNicholas, W. T. , & European Sleep Apnoea Database, c . (2014a). Sleep apnoea severity independently predicts glycaemic health in nondiabetic subjects: The ESADA study. The European Respiratory Journal, 44(1), 130–139. 10.1183/09031936.00162713 [DOI] [PubMed] [Google Scholar]
- Kent, B. D. , Grote, L. , Ryan, S. , Pepin, J. L. , Bonsignore, M. R. , Tkacova, R. , Saaresranta, T. , Verbraecken, J. , Lévy, P. , Hedner, J. , & McNicholas, W. T. (2014b). Diabetes mellitus prevalence and control in sleep‐disordered breathing: The European Sleep apnea cohort (ESADA) study. Chest, 146(4), 982–990. 10.1378/chest.13-2403 [DOI] [PubMed] [Google Scholar]
- Khoo, M. C. K. , & Chalacheva, P. (2016). Model‐derived markers of autonomic cardiovascular dysfunction in Sleep‐disordered breathing. Sleep Medicine Clinics, 11(4), 489–501. 10.1016/j.jsmc.2016.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiely, J. L. , Nolan, P. , & McNicholas, W. T. (2004). Intranasal corticosteroid therapy for obstructive sleep apnoea in patients with co‐existing rhinitis. Thorax, 59(1), 50–55 Retrieved from http://thorax.bmj.com/cgi/content/abstract/59/1/50 [PMC free article] [PubMed] [Google Scholar]
- Kingshott, R. N. , Sime, P. J. , Engleman, H. M. , & Douglas, N. J. (1995). Self assessment of daytime sleepiness: Patient versus partner. Thorax, 50(9), 994–995 Retrieved from http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1021317/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchhof, P. , Benussi, S. , Kotecha, D. , Ahlsson, A. , Atar, D. , Casadei, B. , Castella, M. , Diener, H. C. , Heidbuchel, H. , Hendriks, J. , Hindricks, G. , Manolis, A. S. , Oldgren, J. , Popescu, B. A. , Schotten, U. , Van Putte, B. , Vardas, P. , & Group, E. S. C. S. D . (2016). 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. European Heart Journal, 37(38), 2893–2962. 10.1093/eurheartj/ehw210 [DOI] [PubMed] [Google Scholar]
- Ko, P. R. , Kientz, J. A. , Choe, E. K. , Kay, M. , Landis, C. A. , & Watson, N. F. (2015). Consumer Sleep technologies: A review of the landscape. Journal of Clinical Sleep Medicine, 11(12), 1455–1461. 10.5664/jcsm.5288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuna, S. T. , Reboussin, D. M. , Borradaile, K. E. , Sanders, M. H. , Millman, R. P. , Zammit, G. , Newman, A. B. , Wadden, T. A. , Jakicic, J. M. , Wing, R. R. , Pi‐Sunyer, F. X. , Foster, G. D. , & Sleep, A. R. G. o. t. L. A. R. G. (2013). Long‐term effect of weight loss on obstructive sleep apnea severity in obese patients with type 2 diabetes. Sleep, 36(5), 641–649A. 10.5665/sleep.2618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechat, B. , Hirotsu, C. , Appleton, S. , Younes, M. , Adams, R. J. , Vakulin, A. , Hansen, K. , Zajamsek, B. , Wittert, G. , Catcheside, P. , Heinzer, R. , & Eckert, D. J. (2022). A novel EEG marker predicts perceived SLEEPINESS AND poor sleep quality. Sleep, 1–9. 10.1093/sleep/zsac051 [DOI] [PubMed] [Google Scholar]
- Levy, P. , Kohler, M. , McNicholas, W. T. , Barbe, F. , McEvoy, R. D. , Somers, V. K. , Lavie, L. , & Pepin, J. L. (2015). Obstructive sleep apnoea syndrome. Nature Reviews. Disease Primers, 1, 15015. 10.1038/nrdp.2015.15 [DOI] [PubMed] [Google Scholar]
- Levy, P. , & McNicholas, W. T. (2013). Sleep apnoea and hypertension: Time for recommendations. The European Respiratory Journal, 41(3), 505–506. 10.1183/09031936.00007213 [DOI] [PubMed] [Google Scholar]
- Li, J. , McEvoy, R. D. , Zheng, D. , Loffler, K. A. , Wang, X. , Redline, S. , Woodman, R. J. , & Anderson, C. S. (2020). Self‐reported snoring patterns predict stroke events in high‐risk patients with OSA: Post hoc analyses of the SAVE study. Chest, 158(5), 2146–2154. 10.1016/j.chest.2020.05.615 [DOI] [PubMed] [Google Scholar]
- Lim, D. C. , Mazzotti, D. R. , Sutherland, K. , Mindel, J. W. , Kim, J. , Cistulli, P. A. , Magalang, U. J. , Pack, A. I. , de Chazal, P. , Penzel, T. , & Investigators, S. (2020). Reinventing polysomnography in the age of precision medicine. Sleep Medicine Reviews, 52, 101313. 10.1016/j.smrv.2020.101313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg, E. , Theorell‐Haglow, J. , Svensson, M. , Gislason, T. , Berne, C. , & Janson, C. (2012). Sleep apnea and glucose metabolism: A long‐term follow‐up in a community‐based sample. Chest, 142(4), 935–942. 10.1378/chest.11-1844 [DOI] [PubMed] [Google Scholar]
- Linz, D. , McEvoy, R. D. , Cowie, M. R. , Somers, V. K. , Nattel, S. , Levy, P. , Kalmann, J. M. , & Sanders, P. (2018). Associations of obstructive Sleep apnea with atrial fibrillation and continuous positive airway pressure treatment: A review. JAMA Cardiology, 3(6), 532–540. 10.1001/jamacardio.2018.0095 [DOI] [PubMed] [Google Scholar]
- Loffler, K. A. , Heeley, E. , Freed, R. , Anderson, C. S. , Brockway, B. , Corbett, A. , Chang, C. L. , Douglas, J. A. , Ferrier, K. , Graham, N. , Hamilton, G. S. , Hlavac, M. , McArdle, N. , McLachlan, J. , Mukherjee, S. , Naughton, M. T. , Thien, F. , Young, A. , Grunstein, R. R. , … Investigators, S. (2017). Effect of obstructive Sleep apnea treatment on renal function in patients with cardiovascular disease. American Journal of Respiratory and Critical Care Medicine, 196(11), 1456–1462. 10.1164/rccm.201703-0603OC [DOI] [PubMed] [Google Scholar]
- Lyons, O. D. , Chan, C. T. , Yadollahi, A. , & Bradley, T. D. (2015). Effect of ultrafiltration on sleep apnea and sleep structure in patients with end‐stage renal disease. American Journal of Respiratory and Critical Care Medicine, 191(11), 1287–1294. 10.1164/rccm.201412-2288OC [DOI] [PubMed] [Google Scholar]
- Lyons, O. D. , Inami, T. , Perger, E. , Yadollahi, A. , Chan, C. T. , & Bradley, T. D. (2017). The effect of fluid overload on sleep apnoea severity in haemodialysis patients. The European Respiratory Journal, 49(4), 1601789. 10.1183/13993003.01789-2016 [DOI] [PubMed] [Google Scholar]
- Mazzotti, D. R. , Keenan, B. T. , Lim, D. C. , Gottlieb, D. J. , Kim, J. , & Pack, A. I. (2019). Symptom subtypes of obstructive Sleep apnea predict incidence of cardiovascular outcomes. American Journal of Respiratory and Critical Care Medicine, 200(4), 493–506. 10.1164/rccm.201808-1509OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEvoy, R. D. , Antic, N. A. , Heeley, E. , Luo, Y. , Ou, Q. , Zhang, X. , Mediano, O. , Chen, R. , Drager, L. F. , Liu, Z. , Chen, G. , Du, B. , McArdle, N. , Mukherjee, S. , Tripathi, M. , Billot, L. , Li, Q. , Lorenzi‐Filho, G. , Barbe, F. , … Coordinators . (2016). CPAP for prevention of cardiovascular events in obstructive Sleep apnea. The New England Journal of Medicine, 375(10), 919–931. 10.1056/NEJMoa1606599 [DOI] [PubMed] [Google Scholar]
- McNicholas, W. T. (1998). Arousal in the sleep apnoea syndrome: A mixed blessing? Eur Resp J, 12(6), 1239–1241 Retrieved from http://erj.ersjournals.com [DOI] [PubMed] [Google Scholar]
- McNicholas, W. T. (2008a). Diagnosis of obstructive sleep apnea in adults. Proceedings of the American Thoracic Society, 5(2), 154–160. 10.1513/pats.200708-118MG [DOI] [PubMed] [Google Scholar]
- McNicholas, W. T. (2008b). The nose and OSA: Variable nasal obstruction may be more important in pathophysiology than fixed obstruction. European Respiratory Journal, 32(1), 3–8. 10.1183/09031936.00050208 [DOI] [PubMed] [Google Scholar]
- McNicholas, W. T. (2019). Obstructive sleep apnoea and comorbidity ‐ an overview of the association and impact of continuous positive airway pressure therapy. Expert Review of Respiratory Medicine, 13(3), 251–261. 10.1080/17476348.2019.1575204 [DOI] [PubMed] [Google Scholar]
- McNicholas, W. T. (2021). Getting more from the Sleep recording. Sleep Medicine Clinics, 16(4), 567–574. 10.1016/j.jsmc.2021.08.001 [DOI] [PubMed] [Google Scholar]
- McNicholas, W. T. , Bassetti, C. L. , Ferini‐Strambi, L. , Pepin, J. L. , Pevernagie, D. , Verbraecken, J. , Randerath, W. , & Baveno Working Group, m . (2018). Challenges in obstructive sleep apnoea. The Lancet Respiratory Medicine, 6(3), 170–172. 10.1016/S2213-2600(18)30059-6 [DOI] [PubMed] [Google Scholar]
- McNicholas, W. T. , Bonsigore, M. R. , & Management Committee of E. C. A. B . (2007). Sleep apnoea as an independent risk factor for cardiovascular disease: Current evidence, basic mechanisms and research priorities. The European Respiratory Journal, 29(1), 156–178. 10.1183/09031936.00027406 [DOI] [PubMed] [Google Scholar]
- McNicholas, W. T. , Tarlo, S. , Cole, P. , Zamel, N. , Rutherford, R. , Griffin, D. , & Phillipson, E. A. (1982). Obstructive apneas during Sleep in patients with seasonal allergic rhinitis. American Review of Respiratory Disease, 126(4), 625–628 Retrieved from <Go to ISI>://A1982PK18400007. [DOI] [PubMed] [Google Scholar]
- Messineo, L. , Eckert, D. J. , Lim, R. , Chiang, A. , Azarbarzin, A. , Carter, S. G. , & Carberry, J. C. (2020). Zolpidem increases sleep efficiency and the respiratory arousal threshold without changing sleep apnoea severity and pharyngeal muscle activity. The Journal of Physiology, 598(20), 4681–4692. 10.1113/JP280173 [DOI] [PubMed] [Google Scholar]
- Mezzanotte, W. S. , Tangel, D. J. , & White, D. P. (1996). Influence of sleep onset on upper‐airway muscle activity in apnea patients versus normal controls. American Journal of Respiratory and Critical Care Medicine, 153(6 Pt 1), 1880–1887. 10.1164/ajrccm.153.6.8665050 [DOI] [PubMed] [Google Scholar]
- Mokhlesi, B. , Hagen, E. W. , Finn, L. A. , Hla, K. M. , Carter, J. R. , & Peppard, P. E. (2015). Obstructive sleep apnoea during REM sleep and incident non‐dipping of nocturnal blood pressure: A longitudinal analysis of the Wisconsin Sleep cohort. Thorax, 70(11), 1062–1069. 10.1136/thoraxjnl-2015-207231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naughton, M. , Benard, D. , Tam, A. , Rutherford, R. , & Bradley, T. D. (1993). Role of hyperventilation in the pathogenesis of central sleep apneas in patients with congestive heart failure. The American Review of Respiratory Disease, 148(2), 330–338. 10.1164/ajrccm/148.2.330 [DOI] [PubMed] [Google Scholar]
- Neelapu, B. C. , Kharbanda, O. P. , Sardana, H. K. , Balachandran, R. , Sardana, V. , Kapoor, P. , Gupta, A. , & Vasamsetti, S. (2017). Craniofacial and upper airway morphology in adult obstructive sleep apnea patients: A systematic review and meta‐analysis of cephalometric studies. Sleep Medicine Reviews, 31, 79–90. 10.1016/j.smrv.2016.01.007 [DOI] [PubMed] [Google Scholar]
- Nicholl, D. D. , Hanly, P. J. , Poulin, M. J. , Handley, G. B. , Hemmelgarn, B. R. , Sola, D. Y. , & Ahmed, S. B. (2014). Evaluation of continuous positive airway pressure therapy on renin‐angiotensin system activity in obstructive sleep apnea. American Journal of Respiratory and Critical Care Medicine, 190(5), 572–580. 10.1164/rccm.201403-0526OC [DOI] [PubMed] [Google Scholar]
- Nieto, F. J. , Young, T. B. , Lind, B. K. , Shahar, E. , Samet, J. M. , Redline, S. , D'Agostino, R. B. , Newman, A. B. , Lebowitz, M. D. , & Pickering, T. G. (2000). Association of sleep‐disordered breathing, sleep apnea, and hypertension in a large community‐based study. Sleep Heart Health Study. JAMA, 283(14), 1829–1836. 10.1001/jama.283.14.1829 [DOI] [PubMed] [Google Scholar]
- O'Donnell, C. , Ryan, S. , & McNicholas, W. T. (2020). The impact of telehealth on the Organization of the Health System and Integrated Care. Sleep Medicine Clinics, 15(3), 431–440. 10.1016/j.jsmc.2020.06.003 [DOI] [PubMed] [Google Scholar]
- O'Mahony, A. M. , Garvey, J. F. , & McNicholas, W. T. (2020). Technologic advances in the assessment and management of obstructive sleep apnoea beyond the apnoea‐hypopnoea index: A narrative review. Journal of Thoracic Disease, 12(9), 5020–5038. 10.21037/jtd-sleep-2020-003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parati, G. , Lombardi, C. , Hedner, J. , Bonsignore, M. R. , Grote, L. , Tkacova, R. , Lévy, P. , Riha, R. , Bassetti, C. , Narkiewicz, K. , Mancia, G. , McNicholas, W. T. , & members, E. C. A. B . (2013). Recommendations for the management of patients with obstructive sleep apnoea and hypertension. The European Respiratory Journal, 41(3), 523–538. 10.1183/09031936.00226711 [DOI] [PubMed] [Google Scholar]
- Parra, O. , Sanchez‐Armengol, A. , Capote, F. , Bonnin, M. , Arboix, A. , Campos‐Rodriguez, F. , Pérez‐Ronchel, J. , Durán‐Cantolla, J. , Martínez‐Null, C. , de la Peña, M. , Jiménez, M. C. , Masa, F. , Casadon, I. , Alonso, M. L. , & Macarron, J. L. (2015). Efficacy of continuous positive airway pressure treatment on 5‐year survival in patients with ischaemic stroke and obstructive sleep apnea: A randomized controlled trial. Journal of Sleep Research, 24(1), 47–53. 10.1111/jsr.12181 [DOI] [PubMed] [Google Scholar]
- Peker, Y. , Glantz, H. , Eulenburg, C. , Wegscheider, K. , Herlitz, J. , & Thunstrom, E. (2016). Effect of positive airway pressure on cardiovascular outcomes in coronary artery disease patients with nonsleepy obstructive Sleep apnea. The RICCADSA randomized controlled trial. American Journal of Respiratory and Critical Care Medicine, 194(5), 613–620. 10.1164/rccm.201601-0088OC [DOI] [PubMed] [Google Scholar]
- Pengo, M. F. , Soranna, D. , Giontella, A. , Perger, E. , Mattaliano, P. , Schwarz, E. I. , Lombardi, C. , Bilo, G. , Zambon, A. , Steier, J. , Parati, G. , Minuz, P. , & Fava, C. (2020). Obstructive sleep apnoea treatment and blood pressure: Which phenotypes predict a response? A systematic review and meta‐analysis. The European Respiratory Journal, 55(5), 1901945. 10.1183/13993003.01945-2019 [DOI] [PubMed] [Google Scholar]
- Pepin, J. L. , Bailly, S. , & Tamisier, R. (2018). Incorporating polysomnography into obstructive sleep apnoea phenotyping: Moving towards personalised medicine for OSA. Thorax, 73(5), 409–411. 10.1136/thoraxjnl-2017-210943 [DOI] [PubMed] [Google Scholar]
- Peppard, P. E. , Szklo‐Coxe, M. , Hla, K. M. , & Young, T. (2006). Longitudinal association of sleep‐related breathing disorder and depression. Archives of Internal Medicine, 166(16), 1709–1715. 10.1001/archinte.166.16.1709 [DOI] [PubMed] [Google Scholar]
- Peppard, P. E. , Young, T. , Barnet, J. H. , Palta, M. , Hagen, E. W. , & Hla, K. M. (2013). Increased prevalence of sleep‐disordered breathing in adults. American Journal of Epidemiology, 177(9), 1006–1014. 10.1093/aje/kws342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peppard, P. E. , Young, T. , Palta, M. , & Skatrud, J. (2000). Prospective study of the association between sleep‐disordered breathing and hypertension. The New England Journal of Medicine, 342(19), 1378–1384. 10.1056/NEJM200005113421901 [DOI] [PubMed] [Google Scholar]
- Pevernagie, D. (2021). Future treatment of Sleep disorders: Syndromic approach versus Management of Treatable Traits? Sleep Medicine Clinics, 16(3), 465–473. 10.1016/j.jsmc.2021.05.005 [DOI] [PubMed] [Google Scholar]
- Pevernagie, D. , Gnidovec‐Strazisar, B. , Grote, L. , Heinzer, R. , McNicholas, W. T. , Penzel, T. , Randerath, W. , Schiza, S. , Verbraecken, J. , & Arnardottir, E. S. (2020). On the rise and fall of the apnea‐hypopnea index: A historical review and critical appraisal. Journal of Sleep Research, 29(4), e13066. 10.1111/jsr.13066 [DOI] [PubMed] [Google Scholar]
- Pevernagie, D. , Sastry, M. , & van Maanen, P. (2021). Sleep‐related breathing disorders – treatment of sleep‐disordered breathing. In Bassetti C. L. A., McNicholas W. T., Paunio T., & Peigneux P. (Eds.), Sleep medicine textbook (2nd ed., pp. 357–378). European Sleep Society. [Google Scholar]
- Phillipson, E. A. (1978). Control of breathing during sleep. The American Review of Respiratory Disease, 118(5), 909–939. 10.1164/arrd.1978.118.5.909 [DOI] [PubMed] [Google Scholar]
- Pien, G. W. , Ye, L. , Keenan, B. T. , Maislin, G. , Bjornsdottir, E. , Arnardottir, E. S. , Benediktsdottir, B. , Gislason, T. , & Pack, A. I. (2018). Changing faces of obstructive Sleep apnea: Treatment effects by cluster designation in the Icelandic Sleep apnea cohort. Sleep, 41(3), 1–13. 10.1093/sleep/zsx201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Povitz, M. , Bolo, C. E. , Heitman, S. J. , Tsai, W. H. , Wang, J. , & James, M. T. (2014). Effect of treatment of obstructive sleep apnea on depressive symptoms: Systematic review and meta‐analysis. PLoS Medicine, 11(11), e1001762. 10.1371/journal.pmed.1001762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramar, K. , Dort, L. C. , Katz, S. G. , Lettieri, C. J. , Harrod, C. G. , Thomas, S. M. , & Chervin, R. D. (2015). Clinical practice guideline for the treatment of obstructive Sleep apnea and snoring with Oral appliance therapy: An update for 2015. Journal of Clinical Sleep Medicine, 11(7), 773–827. 10.5664/jcsm.4858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randerath, W. , Bassetti, C. L. , Bonsignore, M. R. , Farre, R. , Ferini‐Strambi, L. , Grote, L. , Hedner, J. , Kohler, M. , Martinez‐Garcia, M. A. , Mihaicuta, S. , Montserrat, J. , Pepin, J. L. , Pevernagie, D. , Pizza, F. , Polo, O. , Riha, R. , Ryan, S. , Verbraecken, J. , & McNicholas, W. T. (2018). Challenges and perspectives in obstructive sleep apnoea: Report by an ad hoc working group of the Sleep disordered breathing Group of the European Respiratory Society and the European Sleep Research Society. The European Respiratory Journal, 52(3), 1702616. 10.1183/13993003.02616-2017 [DOI] [PubMed] [Google Scholar]
- Randerath, W. , Verbraecken, J. , Andreas, S. , Bettega, G. , Boudewyns, A. , Hamans, E. , Jalbert, F. , Paoli, J. R. , Sanner, B. , Smith, I. , Stuck, B. A. , Lacassagne, L. , Marklund, M. , Maurer, J. T. , Pepin, J. L. , Valipour, A. , Verse, T. , Fietze, I. , & European Respiratory Society task force on non, C. t. i. s. a . (2011). Non‐CPAP therapies in obstructive sleep apnoea. The European Respiratory Journal, 37(5), 1000–1028. 10.1183/09031936.00099710 [DOI] [PubMed] [Google Scholar]
- Rashwan, M. S. , Montevecchi, F. , Cammaroto, G. , Badr El Deen, M. , Iskander, N. , El Hennawi, D. , El Tabbakh, M. , Meccariello, G. , Gobbi, R. , Stomeo, F. , & Vicini, C. (2018). Evolution of soft palate surgery techniques for obstructive sleep apnea patients: A comparative study for single‐level palatal surgeries. Clinical Otolaryngology, 43(2), 584–590. 10.1111/coa.13027 [DOI] [PubMed] [Google Scholar]
- Rechtschaffen, A. , & Kales, A. (1968). A manual of standardized terminology. US Government Printing Office, Public Health Service, Washington. [Google Scholar]
- Redline, S. , Yenokyan, G. , Gottlieb, D. J. , Shahar, E. , O'Connor, G. T. , Resnick, H. E. , Diener‐West, M. , Sanders, M. H. , Wolf, P. A. , Geraghty, E. M. , Ali, T. , Lebowitz, M. , & Punjabi, N. M. (2010). Obstructive sleep apnea‐hypopnea and incident stroke: The sleep heart health study. American Journal of Respiratory and Critical Care Medicine, 182(2), 269–277. 10.1164/rccm.200911-1746OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reutrakul, S. , & Mokhlesi, B. (2017). Obstructive Sleep apnea and diabetes: A state of the art review. Chest, 152(5), 1070–1086. 10.1016/j.chest.2017.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimke, A. N. , Ahmed, S. B. , Turin, T. C. , Pendharkar, S. R. , Raneri, J. K. , Lynch, E. J. , & Hanly, P. J. (2021). Effect of CPAP therapy on kidney function in patients with chronic kidney disease: A pilot randomized controlled trial. Chest, 159(5), 2008–2019. 10.1016/j.chest.2020.11.052 [DOI] [PubMed] [Google Scholar]
- Ryan, S. , Taylor, C. T. , & McNicholas, W. T. (2005). Selective activation of inflammatory pathways by intermittent hypoxia in obstructive sleep apnea syndrome. Circulation, 112(17), 2660–2667. 10.1161/CIRCULATIONAHA.105.556746 [DOI] [PubMed] [Google Scholar]
- Ryan, S. , Taylor, C. T. , & McNicholas, W. T. (2009). Systemic inflammation: A key factor in the pathogenesis of cardiovascular complications in obstructive sleep apnoea syndrome? Thorax, 64(7), 631–636. 10.1136/thx.2008.105577 [DOI] [PubMed] [Google Scholar]
- Saaresranta, T. , Hedner, J. , Bonsignore, M. R. , Riha, R. L. , McNicholas, W. T. , Penzel, T. , Anttalainen, U. , Kvamme, J. A. , Pretl, M. , Sliwinski, P. , Verbraecken, J. , Grote, L. , & Group, E. S . (2016). Clinical phenotypes and comorbidity in European Sleep Apnoea patients. PLoS One, 11(10), e0163439. 10.1371/journal.pone.0163439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez‐de‐la‐Torre, M. , Sanchez‐de‐la‐Torre, A. , Bertran, S. , Abad, J. , Duran‐Cantolla, J. , Cabriada, V. , Mediano, O. , Masdeu, M. J. , Alonso, M. L. , Masa, J. F. , Barceló, A. , de la Peña, M. , Mayos, M. , Coloma, R. , Montserrat, J. M. , Chiner, E. , Perelló, S. , Rubinós, G. , Mínguez, O. , … Spanish Sleep, N. (2020). Effect of obstructive sleep apnoea and its treatment with continuous positive airway pressure on the prevalence of cardiovascular events in patients with acute coronary syndrome (ISAACC study): A randomised controlled trial. The Lancet Respiratory Medicine, 8(4), 359–367. 10.1016/S2213-2600(19)30271-1 [DOI] [PubMed] [Google Scholar]
- Sands, S. A. , Edwards, B. A. , Terrill, P. I. , Butler, J. P. , Owens, R. L. , Taranto‐Montemurro, L. , Azarbarzin, A. , Marques, M. , Hess, L. B. , Smales, E. T. , de Melo, C. M. , White, D. P. , Malhotra, A. , & Wellman, A. (2018a). Identifying obstructive sleep apnoea patients responsive to supplemental oxygen therapy. The European Respiratory Journal, 52(3), 1800674. 10.1183/13993003.00674-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sands, S. A. , Terrill, P. I. , Edwards, B. A. , Taranto Montemurro, L. , Azarbarzin, A. , Marques, M. , de Melo, C. M. , Loring, S. H. , Butler, J. P. , White, D. P. , & Wellman, A. (2018b). Quantifying the arousal threshold using polysomnography in obstructive Sleep apnea. Sleep, 41(1), 1–9. 10.1093/sleep/zsx183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapina‐Beltran, E. , Santamaria‐Martos, F. , Benitez, I. , Torres, G. , Masa, J. F. , Sanchez‐de‐la‐Torre, M. , Barbé, F. , & Dalmases, M. (2019). Normotensive patients with obstructive sleep apnoea: Changes in 24‐h ambulatory blood pressure monitoring with continuous positive airway pressure treatment. Journal of Hypertension, 37(4), 720–727. 10.1097/HJH.0000000000001934 [DOI] [PubMed] [Google Scholar]
- Schutz, S. G. , Dunn, A. , Braley, T. J. , Pitt, B. , & Shelgikar, A. V. (2021). New frontiers in pharmacologic obstructive sleep apnea treatment: A narrative review. Sleep Medicine Reviews, 57, 101473. 10.1016/j.smrv.2021.101473 [DOI] [PubMed] [Google Scholar]
- Sharples, L. D. , Clutterbuck‐James, A. L. , Glover, M. J. , Bennett, M. S. , Chadwick, R. , Pittman, M. A. , & Quinnell, T. G. (2016). Meta‐analysis of randomised controlled trials of oral mandibular advancement devices and continuous positive airway pressure for obstructive sleep apnoea‐hypopnoea. Sleep Medicine Reviews, 27, 108–124. 10.1016/j.smrv.2015.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla, A. , Aizer, A. , Holmes, D. , Fowler, S. , Park, D. S. , Bernstein, S. , Bernstein, N. , & Chinitz, L. (2015). Effect of obstructive Sleep apnea treatment on atrial fibrillation recurrence: A meta‐analysis. JACC: Clinical Electrophysiology, 1, 41–51. 10.1016/j.jacep.2015.02.014 [DOI] [PubMed] [Google Scholar]
- Smith, I. , Nadig, V. , & Lasserson, T. J. (2009). Educational, supportive and behavioural interventions to improve usage of continuous positive airway pressure machines for adults with obstructive sleep apnoea. The Cochrane Database of Systematic Review, Rev(2), CD007736. 10.1002/14651858.CD007736 [DOI] [PubMed] [Google Scholar]
- Strobel, R. J. , & Rosen, R. C. (1996). Obesity and weight loss in obstructive sleep apnea: A critical review. Sleep, 19(2), 104–115. 10.1093/sleep/19.2.104 [DOI] [PubMed] [Google Scholar]
- Strohl, K. P. , Hensley, M. J. , Hallett, M. , Saunders, N. A. , & Ingram, R. H., Jr. (1980). Activation of upper airway muscles before onset of inspiration in normal humans. Journal of Applied Physiology: Respiratory, Environmental and Exercise Physiology, 49(4), 638–642. 10.1152/jappl.1980.49.4.638 [DOI] [PubMed] [Google Scholar]
- Strollo, P. J., Jr. , Soose, R. J. , Maurer, J. T. , de Vries, N. , Cornelius, J. , Froymovich, O. , Hanson, R. D. , Padhya, T. A. , Steward, D. L. , Gillespie, M. B. , Woodson, B. T. , Van de Heyning, P. H. , Goetting, M. G. , Vanderveken, O. M. , Feldman, N. , Knaack, L. , Strohl, K. P. , & Group, S. T . (2014). Upper‐airway stimulation for obstructive sleep apnea. The New England Journal of Medicine, 370(2), 139–149. 10.1056/NEJMoa1308659 [DOI] [PubMed] [Google Scholar]
- Sullivan, C. E. , Issa, F. G. , Berthon‐Jones, M. , & Eves, L. (1981). Reversal of obstructive sleep apnoea by continuous positive airway pressure applied through the nares. Lancet, 1(8225), 862–865. 10.1016/s0140-6736(81)92140-1 [DOI] [PubMed] [Google Scholar]
- Tan, H. L. , Kheirandish‐Gozal, L. , Abel, F. , & Gozal, D. (2016). Craniofacial syndromes and sleep‐related breathing disorders. Sleep Medicine Reviews, 27, 74–88. 10.1016/j.smrv.2015.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taranto‐Montemurro, L. , Messineo, L. , Sands, S. A. , Azarbarzin, A. , Marques, M. , Edwards, B. A. , Eckert, D. J. , White, D. P. , & Wellman, A. (2019). The combination of Atomoxetine and oxybutynin greatly reduces obstructive Sleep apnea severity. A randomized, placebo‐controlled, double‐blind crossover trial. American Journal of Respiratory and Critical Care Medicine, 199(10), 1267–1276. 10.1164/rccm.201808-1493OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tkacova, R. , McNicholas, W. T. , Javorsky, M. , Fietze, I. , Sliwinski, P. , Parati, G. , Grote, L. , Hedner, J. , & European Sleep Apnoea Database study, c . (2014). Nocturnal intermittent hypoxia predicts prevalent hypertension in the European Sleep Apnoea database cohort study. The European Respiratory Journal, 44(4), 931–941. 10.1183/09031936.00225113 [DOI] [PubMed] [Google Scholar]
- Ustun, B. , Westover, M. B. , Rudin, C. , & Bianchi, M. T. (2016). Clinical prediction models for sleep apnea: The importance of medical history over symptoms. Journal of Clinical Sleep Medicine, 12(2), 161–168. 10.5664/jcsm.5476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vgontzas, A. N. , Li, Y. , He, F. , Fernandez‐Mendoza, J. , Gaines, J. , Liao, D. , Basta, M. , & Bixler, E. O. (2019). Mild‐to‐moderate sleep apnea is associated with incident hypertension: Age effect. Sleep, 42(4), 1–7. 10.1093/sleep/zsy265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe, E. , Kiyono, K. , Matsui, S. , Somers, V. K. , Sano, K. , Hayano, J. , Ichikawa, T. , Kawai, M. , Harada, M. , & Ozaki, Y. (2017). Prognostic importance of novel oxygen desaturation metrics in patients with heart failure and central Sleep apnea. Journal of Cardiac Failure, 23(2), 131–137. 10.1016/j.cardfail.2016.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver, T. E. , & Grunstein, R. R. (2008). Adherence to continuous positive airway pressure therapy: The challenge to effective treatment. Proceedings of the American Thoracic Society, 5(2), 173–178. 10.1513/pats.200708-119MG [DOI] [PMC free article] [PubMed] [Google Scholar]
- White, L. H. , & Bradley, T. D. (2013). Role of nocturnal rostral fluid shift in the pathogenesis of obstructive and central sleep apnoea. The Journal of Physiology, 591(5), 1179–1193. 10.1113/jphysiol.2012.245159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wongboonsin, J. , Thongprayoon, C. , Bathini, T. , Ungprasert, P. , Aeddula, N. R. , Mao, M. A. , & Cheungpasitporn, W. (2019). Acetazolamide therapy in patients with heart failure: A meta‐analysis. Journal of Clinical Medicine, 8(3), 1–12. 10.3390/jcm8030349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia, W. , Huang, Y. , Peng, B. , Zhang, X. , Wu, Q. , Sang, Y. , Luo, Y. , Liu, X. , Chen, Q. , & Tian, K. (2018). Relationship between obstructive sleep apnoea syndrome and essential hypertension: A dose‐response meta‐analysis. Sleep Medicine, 47, 11–18. 10.1016/j.sleep.2018.03.016 [DOI] [PubMed] [Google Scholar]
- Yaggi, H. K. , Concato, J. , Kernan, W. N. , Lichtman, J. H. , Brass, L. M. , & Mohsenin, V. (2005). Obstructive sleep apnea as a risk factor for stroke and death. The New England Journal of Medicine, 353(19), 2034–2041. 10.1056/NEJMoa043104 [DOI] [PubMed] [Google Scholar]
- Ye, L. , Pien, G. W. , Ratcliffe, S. J. , Bjornsdottir, E. , Arnardottir, E. S. , Pack, A. I. , Benediktsdottir, B. , & Gislason, T. (2014). The different clinical faces of obstructive sleep apnoea: A cluster analysis. The European Respiratory Journal, 44(6), 1600–1607. 10.1183/09031936.00032314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yildirim, N. , Fitzpatrick, M. F. , Whyte, K. F. , Jalleh, R. , Wightman, A. J. , & Douglas, N. J. (1991). The effect of posture on upper airway dimensions in normal subjects and in patients with the sleep apnea/hypopnea syndrome. The American Review of Respiratory Disease, 144(4), 845–847. 10.1164/ajrccm/144.4.845 [DOI] [PubMed] [Google Scholar]
- Young, T. (2009). Rationale, design and findings from the Wisconsin Sleep cohort study: Toward understanding the total societal burden of sleep disordered breathing. Sleep Medicine Clinics, 4(1), 37–46. 10.1016/j.jsmc.2008.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young, T. , Palta, M. , Dempsey, J. , Skatrud, J. , Weber, S. , & Badr, S. (1993). The occurrence of sleep‐disordered breathing among middle‐aged adults. The New England Journal of Medicine, 328(17), 1230–1235. 10.1056/NEJM199304293281704 [DOI] [PubMed] [Google Scholar]
- Yu, J. L. , & Rosen, I. (2020). Utility of the modified Mallampati grade and Friedman tongue position in the assessment of obstructive sleep apnea. Journal of Clinical Sleep Medicine, 16(2), 303–308. 10.5664/jcsm.8188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zapater, A. , Sanchez‐de‐la‐Torre, M. , Benitez, I. D. , Targa, A. , Bertran, S. , Torres, G. , Aldomà, A. , De Batlle, J. , Abad, J. , Duran‐Cantolla, J. , Cabriada‐Nuño, V. , Mediano, O. , Masdeu, M. J. , Muñoz, C. , Masa, J. F. , De la Peña, M. , Mayos, M. , Coloma, R. , Montserrat, J. M. , … Spanish Sleep, N. (2020). The effect of Sleep apnea on cardiovascular events in different acute coronary syndrome phenotypes. American Journal of Respiratory and Critical Care Medicine, 202(12), 1698–1706. 10.1164/rccm.202004-1127OC [DOI] [PubMed] [Google Scholar]
- Zinchuk, A. V. , Gentry, M. J. , Concato, J. , & Yaggi, H. K. (2017). Phenotypes in obstructive sleep apnea: A definition, examples and evolution of approaches. Sleep Medicine Reviews, 35, 113–123. 10.1016/j.smrv.2016.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinchuk, A. V. , & Yaggi, H. K. (2019). Sleep apnea heterogeneity, phenotypes, and cardiovascular risk. Implications for trial design and precision Sleep medicine. American Journal of Respiratory and Critical Care Medicine, 200(4), 412–413. 10.1164/rccm.201903-0545ED [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinchuk, A. V. , & Yaggi, H. K. (2020). Phenotypic subtypes of OSA: A challenge and opportunity for precision medicine. Chest, 157(2), 403–420. 10.1016/j.chest.2019.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


