Abstract
Aim
Cardiogenic shock (CS) is a hemodynamically complex multisystem syndrome associated with persistently high morbidity and mortality. As CS is characterized by progressive failure to provide adequate systemic perfusion, supporting end‐organ perfusion using mechanical circulatory support (MCS) seems intriguing. Since most patients with CS present in the catheterization laboratory, percutaneously implantable systems have the widest adoption in the field. We evaluated feasibility, outcomes, and complications after the introduction of a full‐percutaneous program for both the Impella CP device and venoarterial extracorporeal membrane oxygenator (VA‐ECMO).
Methods
PREPARE CardShock (PRospective REgistry of PAtients in REfractory cardiogenic shock) is a prospective single‐center registry, including 248 consecutive patients between May 2019 and April 2021, who underwent cardiac catheterization and displayed advanced cardiogenic shock. The median age was 70 (58–77) years and 28% were female. Sixty‐five percent of the cases had cardiac arrest, of which 66% were out‐of‐hospital cardiac arrest. A local standard operating procedure (SOP) indicating indications as well as relative and absolute contraindications for different means of MCS (Impella CP or VA‐ECMO) was used to guide MCS use. The primary endpoint was in‐hospital death and secondary endpoints were spontaneous myocardial infarction and major bleedings during the hospital stay.
Results
Overall mortality was 50.4% with a median survival of 2 (0–6) days. Significant independent predictors of mortality were cardiac arrest during the index event (odds ratio [OR] with 95% confidence interval [CI]: 2.53 [1.43–4.51]; p = 0.001), age > 65 years (OR: 2.05 [1.03–4.09]; p = 0.036]), pH < 7.30 (OR: 2.69 [1.56–4.66]; p < 0.001), and lactate levels > 2 mmol/L (OR: 4.51 [2.37–8.65]; p < 0.001).
Conclusions
Conclusive SOPs assist target‐orientated MCS use in CS. This study provides guidance on the implementation, validation, and modification of newly established MCS programs to aid centers that are establishing such programs.
Keywords: cardiogenic shock, ECMO, impella, mechanical support, mortality, vasopressor
1. INTRODUCTION
Cardiogenic shock (CS) results in progressively diminished cardiac output, end‐organ hypoperfusion, and hypoxia, requiring pharmacological and/or mechanical support for circulation. 1 , 2 Acute myocardial infarction (MI) is the primary cause 3 in the vast majority of cases; however, various other causes, such as arrhythmias, valve disease, myocarditis, decompensating chronic heart failure, or pulmonary embolism can lead to a sudden decline of cardiac function, too. Even though immediate revascularization strategies have led to a marked improvement in the prognosis of MI‐associated CS, its intrahospital mortality is still high at around 50%. 4
As CS is defined as a vicious cycle with progressive failure to provide adequate systemic perfusion, its breakthrough by supporting end‐organ perfusion using mechanical circulatory support (MCS) seems intriguing. Since most patients with MI‐associated CS are presented in the catheterization laboratory, percutaneously implantable systems have the widest adoption in the field.
Historically, the intra‐aortic balloon pump (IABP) was one of the most used devices over the last decades because it was easy to implant through a relatively small access of 7.5 Fr. However, its support was limited with not more than 0.5 L/min absolute increase, and it predominantly played a role in decreasing afterload. Obviously, this was insufficient to provide any survival benefit in true CS, as demonstrated by multicentric randomized trials and meta‐analyses. 5 , 6 Therefore, the technology became abandoned for this indication.
More powerful support is offered by the Impella microaxial pump, reaching up to ~3.5 L/min with the percutaneously implantable device. 7 This is a marked difference as compared to IABP, offering potentially almost complete “replacement” of the circulation for certain patients, as well as efficient unloading of the left ventricle. However, data are still limited to demonstrate clinical benefit.
Venoarterial extracorporeal membrane oxygenator (VA‐ECMO) has, as compared to the systems above, the advantage of providing a complete replacement of the circulation with around 5 L/min and replacement of the oxygenation and decarboxylation allowing to bridge patients with refractory CS to recovery, to transplantation, to long‐term MCS, or to withdrawal in case of futility. 8 Although outcome data in patients treated with VA‐ECMO are still scarce and prospective randomized trials have to be performed. 9 , 10 VA‐ECMO is broadly established due to its enormous support potential, notwithstanding invasiveness, costs, and potential complications that necessitate a rigorous patients selection, and is often implanted by surgical approach. 11 As increasing afterload of the left ventricle due to the retrograde aortic flow sometimes requires additional unloading of the left ventricle as with an additional Impella pump, this further increases invasiveness and costs.
In the present PREPARE CardShock (PRospective REgistry of PAtients in REfractory cardiogenic shock) single‐center registry, we evaluated the first 2 years’ feasibility, outcomes, and complications after the introduction of a full‐percutaneous program for both the Impella device and VA‐ECMO, and present an adaptation of a CS treatment algorithm. Thus, our manuscript provides guidance on the implementation, validation, and modification of newly established MCS programs to aid centers that are establishing such programs.
2. METHODS
PREPARE CardShock is a prospective single‐center registry, including all consecutive patients between May 2019 and April 2021, who underwent cardiac catheterization at the University Heart Center Graz, Austria. At our center, we treat ~1.200 acute coronary syndrome/year. Patients included in the registry required any vasopressor support during the catheterization due to hemodynamic deterioration, matching Society for Cardiovascular Angiography and Interventions (SCAI) classification C to E. 12 The majority of patients were in stage E, indicating a severely ill cohort (see Supporting Information: Table 1).
During the recruiting period, a standard operating procedure (SOP) was in use at the study center, defining relative and absolute contraindications for the use of MCS. These contraindications included patient characteristics, such as age, and comorbidities; markers of circulatory deficiency, such as serum lactate and pH; and procedural aspects, such as no‐flow time and duration of cardiopulmonary resuscitation (Table 1).
Table 1.
Relative and absolute contraindications for MCS use (local SOP during the conduct of the registry)
| CPR | No CPR | |
|---|---|---|
| Relative contraindication | ||
| Biological age (years) | >60 | >70 |
| Absolute contraindications | ||
| Initial pH value | <6.8 | <7.1 |
| Initial lactate (mmol/L) | >20 | >15 |
| Limiting comorbidities |
|
|
| Furthermore |
|
|
|
‐ | |
Abbreviations: COPD, chronic obstructive pulmonary disease; CPR, cardiopulmonary resuscitation; MCS, mechanical circulatory support; ROSC, return of spontaneous circulation; SOP, standard operating procedure.
MCS devices used in this study were the Impella CP® (Abiomed) pump and the Xenios ECMO platform (Xenios). All devices were implanted by the interventional cardiologist via femoral access. In VA‐ECMO cases, the arterial cannula was connected to an antegrade arterial sheath (6F) to ensure adequate perfusion of the leg. Choice of MCS was based on the assessment of residual circulation, as well as the ventilation capacity. Patients with residual, moderate‐to‐severe circulatory reserve qualified primarily for Impella, while patients with severe to no circulatory reserve qualified for VA‐ECMO. Patients, with marked oxygenation insufficiency, expressed by the Horowitz index, qualified for VA‐ECMO.
2.1. Follow‐up
Periprocedural and in‐hospital data were collected prospectively based on a case report form. Missing data were completed based on an electronic patient management system (openMEDOCS, KAGes), which is used by all state hospitals throughout the state of Styria, representing ~85% of all acute medicine beds.
The primary endpoint was in‐hospital death and secondary endpoints were spontaneous angiographically validated MI or major, disabling, or life‐threatening bleeding according to Bleeding Academic Research Consortium (BARC) criteria during the hospital stay. 13 Additionally, length of mechanical ventilation, stay at the intensive care unit, and total duration of hospitalization were assessed.
2.2. Statistics
Data are presented in mean ± standard deviation or median (interquartile range [IQR]), as appropriate. Normal distribution was tested using Pearson omnibus normality test. Continuous variables were compared by unpaired Student's t test or Mann–Whitney U test as appropriate. Categorical variables were compared with Fisher's exact or χ 2 test, as appropriate, and results are expressed in odds ratio (OR) with a 95% confidence interval (CI). A probability value of p < 0.05 was considered significant.
The research ethics board of the Medical University of Graz provided ethics approval for the study (31‐323 ex 18/19) and the study was conducted in accordance with the Declaration of Helsinki.
3. RESULTS
3.1. Overall population
Overall, 248 consecutive patients with cardiogenic shock and vasopressor use in the cath lab were included in this prospective registry. The median age was 70 (58–77) years, ranging from 37 to 94 years and 28% were female. Sixty‐five percent of the cases had cardiac arrest, of which 66% were out‐of‐hospital cardiac arrest. Patients with cardiac arrest were younger than patients without cardiac arrest (median age: 67 [57–77] vs. 72 [65–78] years, respectively; p = 0.003) and exhibited higher body mass indexes (27.7 [24.8–30.9] vs. 25.3 [23.4–29.3]; p = 0.002). The percentage of male patients tended to be higher in the cardiac arrest group (75.8% vs. 64.4%; p = 0.076). Patient characteristics are shown in Table 2 and their correlation to specific culprit vessels is shown in Table 2 of the Supporting Information. Comparing single‐vessel to multivessel diseased patients, classical cardiovascular risk factors were more frequent in multivessel patients, whereas single‐vessel disease patients suffered from resuscitation and intubation more often (Supporting Information: Table 3).
Table 2.
Patients characteristics
| Characteristics of included patients | All patients (n = 248) | CPR patients (n = 161) | no CPR patients (n = 87) | CPR versus no CPR (OR [95% CI]) |
|---|---|---|---|---|
| Age (years), median (IQR) | 70 (58–77) | 67 (57–77) | 72 (65–78) | p = 0.003 |
| Age > 60 years, n (%) | 179 (72.2) | 106 (65.8) | 73 (83.9) | OR: 0.37 [0.18–0.75]; p = 0.003 |
| Age > 70 years, n (%) | 123 (49.6) | 69 (42.9) | 54 (62.1) | OR: 0.46 [0.26–0.81]; p = 0.005 |
| BMI, median (IQR) | 26.8 (24.5–30.2) | 27.7 (24.8–30.9) | 25.3 (23.4–29.3) | p = 0.002 |
| Female gender, n (%) | 70 (28.2) | 39 (24.2) | 31 (35.6) | OR: 0.58 [0.31–1.06]; p = 0.076 |
| Known diabetes, n (%) | 56 (22.6) | 33 (20.5) | 23 (26.4) | OR: 0.72 [0.37–1.38]; p = 0.340 |
| Known hypertension, n (%) | 133 (53.6) | 78 (48.4) | 55 (63.2) | OR: 0.55 [0.31–0.96]; p = 0.033 |
| Known dyslipidemia, n (%) | 81 (32.7) | 48 (29.8) | 33 (37.9) | OR: 0.70 [0.39–1.25]; p = 0.204 |
| Known smoker/ex smoker, n (%) | 60 (24.2) | 35 (21.7) | 25 (28.7) | OR: 0.69 [0.36–1.31]; p = 0.277 |
| Known prior MI, n (%) | 45 (18.1) | 27 (16.8) | 18 (20.7) | OR: 0.77 [0.38–1.58]; p = 0.491 |
| Known prior PCI, n (%) | 51 (20.6) | 33 (20.5) | 18 (20.7) | OR: 0.99 [0.50–1.98]; p = 1 |
| Etiology of CS, n (%) | ||||
| Acute MI | 175 (70.6) | 109 (67.7) | 66 (75.9) | OR: 0.67 [0.35–1.25]; p = 0.192 |
| Others | 73 (29.4) | 52 (32.3) | 21 (24.1) | |
| Initial lactate (mmol/L), median (IQR) | 3.4 (1.7–6.2) | 4.5 (2.2–7.9) | 2.0 (1.2–4.4) | p = 0.004 |
| Initial pH value, median (IQR) | 7.28 (7.16–7.39) | 7.24 (7.13–7.34) | 7.37 (7.27–7.42) | p < 0.001 |
| Initial RRsyst. (mmHg), median (IQR) | 99 (80–115) | 99 (80–116) | 97 (82–112) | p = 0.660 |
| Intubated at arrival, I (%) | 159 (64.1) | 141 (87.6) | 18 (20.7) | OR: 27.03 [12.75–58.18]; p < 0.001 |
| Decision to implant MCS device, I (%) | 48 (19.4) | 24 (14.9) | 24 (27.6) | |
| Successful implantation, I (%) | 47 (97.9) | 24 (100) | 23 (95.8) | OR: 0.46 [0.23–0.91]; p = 0.019 |
| SOP for MCS use fulfilled, I (%) | 42 (87.5) | 20 (83.3) | 22 (91.7) | |
| Length of CCU stay (days), median (IQR) | 4 (2–9) | 5 (1–12) | 4 (2–8) | p = 0.244 |
| Length of CCU stay after surviving Day 1 (days), median (IQR) | 5 (3–12) | 7 (3–16) | 4 (3–8) | p = 0.013 |
| Length of hospital stay (days), median (IQR) | 9 (2–19) | 7 (1–19) | 10 (6–16) | p = 0.372 |
| Respirator duration (days), median (IQR) | 2 (0–7) | 3 (0–7) | 0 (0–3) | p = 0.010 |
| Bleeding event, n (%) | 24 (9.7) | 17 (11.8) | 9 (10.3) | OR: 1.17 [0.45–3.10]; p = 0.827 |
| Intrahospital mortality, n (%) | 125 (50.4) | 94 (58.4) | 31 (35.6) | OR: 2.53 [1.43–4.51]; p = 0.001 |
Abbreviations: CCU, cardiac care unit; CI, confidence interval; CPR, cardiopulmonary resuscitation; CS, cardiogenic shock; IQR, interquartile range; MCS, mechanical circulatory support; MI, myocardial infarction; OR, odds ratio; PCI, percutaneous coronary intervention.
3.2. Outcome in CS patients
Overall mortality was 50.4% with a median survival of 2 (0–6) days. As some of the patients died within the first few hours, mortality was also separately analyzed for those patients surviving the day of admission. In these patients, the median survival was 4 (2–12) days. Significant independent predictors of mortality were cardiac arrest during the index event (OR with 95% CI: 2.53 [1.43–4.51]; p = 0.001), age > 65 years (OR: 2.05 [1.03–4.09]; p = 0.036]) or age > 75 years (OR: 2.62 [1.45–4.74]; p = 0.001), pH < 7.30 (OR: 2.69 [1.56–4.66]; p < 0.001), pH < 7.2 (OR: 2.84 [1.57–5.16]; p < 0.001; pH < 7.0 (OR: 24.63 [3.43–505.93]; p < 0.001), respectively. Lactate levels > 2 mmol/L resulted in an OR of 4.51 (2.37–8.65); p < 0.001, which increases to 4.68 (2.64–8.35); p > 0.001 in patients with lactate > 4.0 mmol/L upon arrival. Supporting Information: Table 4 indicates that intrahospital mortality in our cohort was 100% in patients with an initial lactate > 15mmol/L and there was only one patient surviving with initial pH < 7.0. This patient suffered from pulmonary embolism and presented with severe hypercapnia shifting pH toward low values. Importantly, there was no significant age‐related mortality considering patients with CS but no previous cardiac arrest, although this might be caused by the limited number of patients in this registry.
Length of intensive care unit (ICU) stay only became significantly longer in cardiopulmonary resuscitation (CPR) patients compared to non‐CPR patients if patients who died until the first morning after the index event were excluded accounting for the even higher morbidity of the CPR patients. Not surprisingly, ICU stay was also longer in SCAI E patients compared to SCAI C patients (see Supporting Information: Table 1). This is also reflected by longer median times on respirators in the post‐CPR group 3 (0–7) versus = (0–3) days in the non‐CPR group, respectively (p = 0.01). Median total hospital stay was 9 (IQR: 2–19) days and major bleeding complications were detected in 9.7% of patients without significant differences between CPR and non‐CPR patients.
3.3. Use of MCS in CS patients
Forty‐eight of the 248 patients were treated with an MCS system. Among patients with MCS, 20 received VA‐ECMO and 24 patients received Impella. Four patients were primarily treated with Impella, but then upgraded to ECMELLA, the combination of the two systems, due to persistent shock and/or additionally developing respiratory failure. MCS was implanted successfully in all but one patient (with severe peripheral artery disease) accounting for a 98% success rate in all cases. Bleedings BARC ≥ 2 were more frequent in the MCS group (15/48) compared to the non‐MCS group (11/200) (OR: 7.81 [3.06–20.18]; p < 0.001).
With respect to the use of MCS, the vast majority of 88% of the cases fulfilled the predefined SOP indications. Among the six patients being supplied with an MCS despite violating any predefined criterion for MCS use, three patients had pH below the threshold, one patient had lactate levels above the threshold, one patient had prolonged CPR duration, and one patient did not receive bystander CPR. The age of these patients tended to be lower than the overall average (62 ± 7 vs. 68 ± 12 years, respectively; p = 0.108). Still, five of these six patients died in the hospital, while the only surviving patient suffered a noncoronary index event, namely acute pulmonary embolism.
Patients' characteristics differed considerably between the different devices used as outlined in Table 3, with the most severely ill patients in the VA‐ECMO group as expressed by the highest lactate levels, lowest pH values, and the highest rate of CPR before MCS implantation. These differences likely impacted the outcome. The survival rate was 4/20 (20%) in VA‐ECMO patients, 13/24 (54%) in Impella patients, and 3/4 (75%) in Ecmella patients (Impella was always the first device in these four patients). Thus, overall MCS survival was 20/48 (42%).
Table 3.
Characteristics of MCS patients
| VA‐ECMO (n = 20) | Impella (n = 24) | ECMELLA (n = 4) | VA‐ECMO versus Impella and ECMELLA (OR [95% CI]) | |
|---|---|---|---|---|
| Age (years), median (IQR) | 65 (57–71) | 69 (61–74) | 55 (50–65) | p = 0.575 |
| BMI, median (IQR) | 28.1 (24.7–29.8) | 27.4 (24.5–30.6) | 24.5 (23.4–25.9) | p = 0.592 |
| Gender, n (%) | ||||
| Male | 16 (80) | 16 (66.7) | 3 (75) | OR: 1.89 [0.42–9.12]; p = 0.512 |
| Female | 4 (20) | 8 (33.3) | 1 (25) | |
| Etiology of CS, n (%) | ||||
| Acute MI | 16 (80) | 16 (66.6) | 3 (75) | OR: 1.89 [0.42–9.12]; p = 0.512 |
| Others | 4 (20) | 8 (33.3) | 1 (25) | |
| CPR, n (%) | ||||
| Yes | 15 (75) | 8 (33.3) | 1 (25) | OR: 6.33 [1.50–28.64]; p = 0.008 |
| No | 5 (25) | 16 (66.6) | 3 (75) | |
| Lactate (mmol/L), median (IQR) | ||||
| Initial | 6.3 (3.2–11.1) | 2.7 (1.4–6.1) | 2.3 (1.7–7.2) | p = 0.082 |
| After 4 h | 5.6 (2.8–10.9) | 2.0 (1.1–4.2) | 4.2 (1.5–10.7) | p = 0.128 |
| pH value, median (IQR) | ||||
| Initial | 7.24 (7.07–7.30) | 7.35 (7.19–7.41) | 7.31 (7.27–7.37) | p = 0.038 |
| After 4 h | 7.32 (7.23–7.40) | 7.39 (7.33–7.46) | 7.32 (7.27–7.40) | p = 0.064 |
| RRsyst. (mmHg), median (IQR) | ||||
| Initial | 89 (83–100) | 100 (83–120) | 84 (76–93) | p = 0.191 |
| After 4 h | 98 (90–107) | 111 (101–120) | 96 (82–112) | p = 0.198 |
| Intubated at arrival, n (%) | 15 (75) | 11 (45.8) | 3 (75) | OR: 3.00 [0.73–12.82]; p = 0.134 |
Abbreviations: BMI, body mass index; CI, confidence interval; CPR, cardiopulmonary resuscitation; CS, cardiogenic shock; IQR, interquartile range; MCS, mechanical circulatory support; MI, myocardial infarction; OR, odds ratio; VA‐ECMO, venoarterial extracorporeal membrane oxygenator.
3.4. Patients' characteristics and use of MCS
Age, gender distribution, and BMI as well as the rate of MI and its subgroups of ST‐elevation MI and non‐ST‐elevation MI were not significantly different between patients treated with MCS or not. Also, the rate of the previous resuscitation only tended to be higher in the group not treated with MCS, which was likely triggered by the lower cut‐off for age in patients with prior CPR. The choice of MCS system was also not significantly affected by these baseline parameters. The results validated the accuracy of the SOP as a guide for MCS use as only one patient received a VA‐ECMO and survived despite violating its predefined thresholds. As pulmonary embolism with consecutive hypercapnia and low initial pH was the underlying cause, this implantation can be considered a reasonable exception made by the treating physician.
4. DISCUSSION
The present study prospectively analyzed all patients referred for cardiac catheterization, who suffered from CS prior to or during the procedure. Despite the best medical care and access to various MCS systems, the mortality of cardiogenic shock remained high. Mortality rates were markedly dependent on various clinical and procedural parameters, which are in line with previous studies. 14 , 15 , 16 , 17 , 18 , 19 , 20 While overall mortality was 50%, elevated lactate > 4 mmol/L, low pH value < 7.30, and previous CPR were associated with higher mortality rates; lower age appeared as a favorable predictor.
The rate of MCS use in our cohort was 20% of all cardiogenic shock patients, which is comparable with contemporary landmark trials like CULPRIT‐shock (Culprit Lesion Only PCI Versus Multivessel PCI in Cardiogenic Shock) cohort. 21 With respect to other high‐volume centers (i.e., more than 100 CS patients yearly 20 ), the rate of MCS use in our cohort has slightly exceeded the commonly reported proportion of 10%–20%. Within a nationwide registry in Denmark, numbers are also slightly lower, 22 although these results may be biased by the inclusion of centers without access to MCS. Still, our data demonstrate that the implemented SOP was well adopted, considering the low number of outliners. In addition, data suggest that SOP was also adequately defined, since the mortality rate in the few outliers was as high as 90%, while among those treated according to the SOP with or without MCS, the mortality was markedly lower with 47.4%. On the other hand, only a total of 10/248 patients died and did not receive MCS therapy despite no predefined contraindications but due to the treating physician's decision based on comorbidities and frailty of the patients.
We found that the strongest predictors of in‐hospital mortality were reduced pH, high lactate levels, and older age.
Within the group of MCS patients, the outcome was considerably worse in the VA‐ECMO group compared to the Impella group. But it is important to point out the differences in indication (i.e., complete cardiopulmonary replacement vs. circulatory support). Patients in the VA‐ECMO group revealed markedly worse baseline status, including higher baseline lactate and lower initial pH, which are per se predictors of higher in‐hospital mortality rates. As numbers in these two device groups (20 vs. 24 patients) were rather low, propensity score‐matched analysis was not feasible. With respect to previous studies, both systems can mediate beneficial effects. For instance, a recent analysis for Impella use in MI‐induced refractory cardiogenic shock displayed profoundly improved 30‐day survival in a propensity score‐matched comparison potentially triggered by more complete revascularisation in the Impella arm with significantly more treated vessels and cumulative longer stent length. This might be due to more stable conditions of the patients on Impella support during the percutaneous coronary intervention (PCI). Nevertheless, the authors also report higher complication rates in the Impella group, namely, higher rates of hemolysis and bleedings. 23 Any major bleedings are considered the most relevant complication in MCS use with respect to prognosis almost doubling mortality rates in a recent retrospective analysis. 24
In addition, an important aspect to be considered is that VA‐ECMO might further impair left ventricular function due to increased afterload. Therefore, in several centers upgrading to ECMELLA with implantation of an additional Impella system is a kind of routine, from which a certain combination of “organ perfusion and cardiac protection” effect can be expected. Data from a recent international multicentre cohort study supports this view describing significantly improved 30‐day survival. 25 This effect seems to be even more pronounced in more severe shock indicated by lactate levels > 5 mmol/L as well as in patients after cardiac arrest and resuscitation. These improvements in prognosis were present despite a higher rate of severe bleedings and access site‐related ischemia. Since our cohort includes only four patients with ECMELLA, assessment of this aspect remains beyond the scope of the present analysis. Right heart catheterization with a detailed assessment of hemodynamic parameters, such as actual cardiac output, pulmonary pressure, and resistance could allow a better understanding of the circulatory status and a more sophisticated decision between different means of MCS. However, in clinical practice, this might be feasible rather in a subacutely progressive circulatory insufficiency than in an emergent cardiogenic shock.
Timing of MCS in CS remains an open question; however, accumulating evidence supports initiation even before PCI in MI 26 , 27 for the Impella pump and a recent study using VA‐ECMO in refractory MI‐induced cardiogenic shock also found beneficial effects for the insertion before PCI. 28
Although some studies report only a minor impact of age with respect to prognosis in cardiogenic shock, 29 , 30 this might be due to selection bias as older patients in these studies displayed less comorbidities, indicating a highly selected group of older patients. More likely, increasing age is associated with higher mortality in CS, regardless of shock severity as shown in a recent analysis of a large cohort of the Cardiogenic Shock Work Group registry, 31 a cohort of shock patients out of the Utah Cardiac Recovery shock database, 32 broad analysis of risk scores based on the CULPRIT‐shock cohort 33 and most other trials and registries. 26 , 34 , 35 These findings are supported by our observation, too, as age was found to be a strong predictor of mortality, especially in patients with cardiac arrest. Accordingly, considering the poor outcomes in the older population, indications for MCS use should be critically assessed and should be restricted to selected patients who may benefit from more aggressive treatment strategies despite advanced age.
Our program was set up as an entirely percutaneous approach, and implantation, as well as treatment in the ICU, were performed by cardiologists only. Even though this approach was proven to be feasible, decision‐making and treatment in cardiogenic shock have been shown to further improve within shock teams. 36 We therefore adapted implantation procedures following this 2‐year observation period and perfusionists are on site during implantation and priming of the system and regular joint rounds are done while the MCS systems are running on the cardiac care unit.
The results of this cohort support the notion that defined cut‐offs for at least lactate, pH, and age are useful in guiding the use of MCS in cardiac arrest (CA) patients. However, individual patient selection is still crucial as several underlying diseases might affect single cut‐off values (hypercapnia with low pH values in chronic obstructive pulmonary disease patients or in pulmonary embolism or high lactate levels in sports‐related cardiac arrest) or have a much better prognosis with MCS as shown in case reports and registries for myocarditis. 37 , 38 Few available reports also suppose better prognosis in patients with cardiac arrest during exercise despite high lactate levels, which likely derive in parts from the previous exercise instead of CA‐induced organ damage. 39 , 40
In addition, it is important to emphasize that the clinical setting, namely, acute coronary syndrome versus noncoronary event versus procedural complications should be considered as different entities with potentially different treatment cut‐offs. Based on the data presented and the available literature, we propose an algorithm to replace the pure contraindications of the formerly used SOP as shown in Table 1 and outlined in Figure 1. Its purpose is to guide decisions on MCS use in cardiogenic shock with a more liberal MCS access in high‐risk PCI and peri‐interventional complications but even more restrictive use in patients with low pH, high lactate, and older resuscitated patients.
Figure 1.
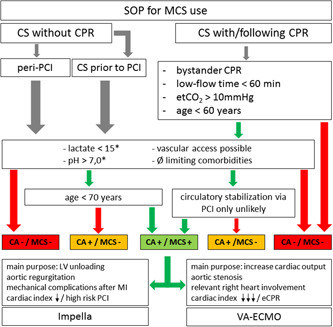
Use of mechanical assist systems in cardiogenic shock. CA, cardiac arrest; (e)CPR, (extracorporeal) cardiopulmonary resuscitation; CS, cardiogenic shock; LV, left ventricle; MCS, mechanical circulatory support; MI, myocardial infarction; PCI, percutaneous coronary intervention; RH, right heart. Green arrow: applicable; red arrow: not applicable. *At admission in cath lab/hospital, relevant hypercapnia to be excluded when pH < 7.0; prior lysis is a contraindication for VA ECMO; pulmonary embolism and cardiac arrest during sports to be considered as more liberal indications for MCS implantation; Impella only is not appropriate in CS without spontaneous circulation. [Color figure can be viewed at wileyonlinelibrary.com]
4.1. Limitations
The presented data refers to a single large center experience. Despite the fact that outcome data is comparable to most other large trials and registries, local particularities must always be considered. The SOP used to guide MCS implantation was broadly communicated within the entire team, but there was no systematic use upon the arrival of individual patients. While the usage of noradrenaline was an inclusion criterion and therefore per se true for all patients, neither the exact dosage nor the administration of other usages of catecholamines was recorded.
Transthoracic echocardiography is an important tool in the diagnostic armamentarium for the cardiogenic shock to have a general understanding of overall systolic function, valvular diseases, or any mechanical complications or reasons for hemodynamic deterioration. This has been performed in all cases; however, no quantitative measures were recorded that could be sufficient for a more detailed scientific analysis.
5. CONCLUSION
Mortality in cardiogenic shock is high and depends strongly on circumstances, such as required CPR or the place of onset, patients' perfusion as indicated by pH and serum lactate, as well as patients' age and comorbidities. Taking these factors into consideration within an SOP supports quick decision‐making within a shock team to supply the most adequate therapy including MCS. The PREPARE Card Shock registry provides guidance on the implementation, validation, and modification of newly established MCS programs to aid centers that are establishing such programs.
CONFLICT OF INTEREST
D. v. L., G. G. T., and S. P. received speaker fees from Abiomed. The remaining authors declare no conflict of interest.
Supporting information
Supporting information.
Supporting information.
Supporting information.
Supporting information.
ACKNOWLEDGMENTS
We would like to thank Klemens Ablasser, Egbert Bisping, Eva Buschmann, Robert Gasser, Stefan Harb, Susanne Knopper, Wolfgang Muckenauer, Roman Weixler, and Robert Zweiker for their tremendous support toward the registry clinically as well as scientifically.
von Lewinski D, Herold L, Stoffel C, et al. PRospective REgistry of PAtients in REfractory cardiogenic shock—The PREPARE CardShock registry. Catheter Cardiovasc Interv. 2022;100:319‐327. 10.1002/ccd.30327
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1. van Diepen S, Katz JN, Albert NM, et al. Contemporary management of cardiogenic shock: a scientific statement from the American Heart Association. Circulation. 2017;136:232‐268. [DOI] [PubMed] [Google Scholar]
- 2. Long A, Baran DA. Lingua franca of cardiogenic shock: speaking the same language. Front Cardiovasc Med. 2021;8:691232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Harjola VP, Lassus J, Sionis A, et al. Clinical picture and risk prediction of short‐term mortality in cardiogenic shock. Eur J Heart Fail. 2015;17(5):501‐509. [DOI] [PubMed] [Google Scholar]
- 4. Hochman JS, Sleeper LA, Webb JG, et al. Early revascularization in acute myocardial infarction complicated by cardiogenic shock. SHOCK Investigators. Should We Emergently Revascularize Occluded Coronaries for Cardiogenic Shock. N Engl J Med. 1999;341(9):625‐634. [DOI] [PubMed] [Google Scholar]
- 5. Thiele H, Zeymer U, Neumann F‐J, et al. Intraaortic balloon support for myocardial infarction with cardiogenic shock. N Engl J Med. 2012;367(14):1287‐1296. [DOI] [PubMed] [Google Scholar]
- 6. Shi Y, Wang Y, Sun X, et al. Effects of mechanical circulatory support devices in patients with acute myocardial infarction undergoing stent implantation: a systematic review and meta‐analysis of randomised controlled trials. BMJ Open. 2021;11(6):044072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Burzotta F, Russo G, Previ L, Bruno P, Aurigemma C, Trani C. Impella: pumps overview and access site management. Minerva Cardioangiol. 2018;66(5):606‐611. [DOI] [PubMed] [Google Scholar]
- 8. Pineton de Chambrun M, Bréchot N, Combes A. Venoarterial extracorporeal membrane oxygenation in cardiogenic shock: indications, mode of operation, and current evidence. Curr Opin Crit Care. 2019;25(4):397‐402. [DOI] [PubMed] [Google Scholar]
- 9. Thiele H, Freund A, Gimenez MR, et al. Extracorporeal life support in patients with acute myocardial infarction complicated by cardiogenic shock—design and rationale of the ECLS‐SHOCK trial. Am Heart J. 2021;234:1‐11. [DOI] [PubMed] [Google Scholar]
- 10. Banning AS, Adriaenssens T, Berry C, et al. Veno‐arterial extracorporeal membrane oxygenation (ECMO) in patients with cardiogenic shock: rationale and design of the randomised, multicentre, open‐label EURO SHOCK trial. EuroIntervention. 2021;16(15):e1227‐e1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Napp LC, Kühn C, Bauersachs J. ECMO bei Herz–Kreislauf–Stillstand und kardiogenem schock. Herz. 2017;42(1):27‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Baran DA, Grines CL, Bailey S, et al. SCAI clinical expert consensus statement on the classification of cardiogenic shock. Catheter Cardiovasc Interv. 2019;94(1):29‐37. [DOI] [PubMed] [Google Scholar]
- 13. Mehran R, Rao Sv, Bhatt DL, et al. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the bleeding academic research consortium. Circulation. 2011;123(23):2736‐2747. [DOI] [PubMed] [Google Scholar]
- 14. Wernly B, Karami M, Engström AE, et al. Impella versus extracorporal life support in cardiogenic shock: a propensity score adjusted analysis. ESC Heart Failure. 2021;8(2):953‐961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schrage B, Dabboura S, Yan I, et al. Application of the SCAI classification in a cohort of patients with cardiogenic shock. Catheter Cardiovasc Interv. 2020;96(3):E213‐E219. [DOI] [PubMed] [Google Scholar]
- 16. Thayer KL, Zweck E, Ayouty M, et al. Invasive hemodynamic assessment and classification of in‐hospital mortality risk among patients with cardiogenic shock. Circ Heart Fail. 2020;13(9):e007099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hanson ID, Tagami T, Mando R, et al. SCAI shock classification in acute myocardial infarction: insights from the National Cardiogenic Shock Initiative. Catheter Cardiovasc Interv. 2020;96(6):1137‐1142. [DOI] [PubMed] [Google Scholar]
- 18. Jentzer JC, van Diepen S, Barsness GW, et al. Cardiogenic shock classification to predict mortality in the cardiac intensive care unit. J Am Coll Cardiol. 2019;74(17):2117‐2128. [DOI] [PubMed] [Google Scholar]
- 19. Pazdernik M, Gramegna M, Bohm A, et al. Regional differences in presentation characteristics, use of treatments and outcome of patients with cardiogenic shock: results from multicenter, international registry. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2021;165(3):291‐297. [DOI] [PubMed] [Google Scholar]
- 20. Baran DA, Long A, Badiye AP, Stelling K. Prospective validation of the SCAI shock classification: single center analysis. Catheter Cardiovasc Interv. 2020;96(7):1339‐1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Thiele H, Akin I, Sandri M, et al. PCI strategies in patients with acute myocardial infarction and cardiogenic shock. N Engl J Med. 2017;377(25):2419‐2432. [DOI] [PubMed] [Google Scholar]
- 22. Petersen LT, Riddersholm S, Andersen DC, et al. Temporal trends in patient characteristics, presumed causes, and outcomes following cardiogenic shock between 2005 and 2017: a Danish registry‐based cohort study. Eur Heart J Acute Cardiovasc Care. 2021;10(9):1074‐1083. 10.1093/ehjacc/zuab084 [DOI] [PubMed] [Google Scholar]
- 23. Sieweke J‐T, Akin M, Beheshty J‐A, Flierl U, Bauersachs J, Schäfer A. Unloading in refractory cardiogenic shock after out‐of‐hospital cardiac arrest due to acute myocardial infarction—a propensity score‐matched analysis. Front Cardiovasc Med. 2021;8:704312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Takahashi K, Kubo S, Ikuta A, et al. Incidence, predictors, and clinical outcomes of mechanical circulatory support‐related complications in patients with cardiogenic shock. J Cardiol. 2021;79(2):163‐169. [DOI] [PubMed] [Google Scholar]
- 25. Schrage B, Becher PM, Bernhardt A, et al. Left ventricular unloading is associated with lower mortality in patients with cardiogenic shock treated with venoarterial extracorporeal membrane oxygenation: results from an International, Multicenter Cohort Study. Circulation. 2020;142(22):2095‐2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. O'Neill WW, Schreiber T, Wohns DHW, et al. The current use of impella 2.5 in acute myocardial infarction complicated by cardiogenic shock: results from the USpella Registry. J Interv Cardiol. 2014;27(1):1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Iannaccone M, Albani S, Giannini F, et al. Short term outcomes of Impella in cardiogenic shock: a review and meta‐analysis of observational studies. Int J Cardiol. 2021;324:44‐51. [DOI] [PubMed] [Google Scholar]
- 28. Choi KH, Yang JH, Hong D, et al. Optimal timing of venoarterial‐extracorporeal membrane oxygenation in acute myocardial infarction patients suffering from refractory cardiogenic shock. Circ J. 2020;84(9):1502‐1510. [DOI] [PubMed] [Google Scholar]
- 29. Alonso‐Fernandez‐Gatta M, Merchan‐Gomez S, Toranzo‐Nieto I, et al. Short‐term mechanical circulatory support in elderly patients. Artificial Organs. 2021;46(5):867‐877. 10.1111/aor.14117 [DOI] [PubMed] [Google Scholar]
- 30. Kowalewski M, Zielinski K, Maria Raffa G, et al. Mortality predictors in elderly patients with cardiogenic shock on venoarterial extracorporeal life support. Analysis from the Extracorporeal Life Support Organization Registry*. Crit Care Med. 2021;49(1):7‐18. [DOI] [PubMed] [Google Scholar]
- 31. Kanwar M, Thayer KL, Garan AR, et al. Impact of age on outcomes in patients with cardiogenic shock. Front Cardiovasc Med. 2021;8:688098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Marashly Q, Taleb I, Kyriakopoulos CP, et al. Predicting mortality in cardiogenic shock secondary to ACS requiring short‐term mechanical circulatory support: the ACS‐MCS score. Catheter Cardiovasc Interven. 2021;98(7):1275‐1284. 10.1002/ccd.29581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Freund A, Pöss J, de Waha‐Thiele S, et al. Comparison of risk prediction models in infarct‐related cardiogenic shock. Eur Heart J Acute Cardiovasc Care. 2021;10(8):890‐897. 10.1093/ehjacc/zuab054 [DOI] [PubMed] [Google Scholar]
- 34. Pöss J, Köster J, Fuernau G, et al. Risk stratification for patients in cardiogenic shock after acute myocardial infarction. J Am Coll Cardiol. 2017;69(15):1913‐1920. [DOI] [PubMed] [Google Scholar]
- 35. Venkatason P, Zubairi YZ, Wan Ahmad WA, et al. In‐hospital mortality of cardiogenic shock complicating ST‐elevation myocardial infarction in Malaysia: a retrospective analysis of the Malaysian National Cardiovascular Database (NCVD) registry. BMJ Open. 2019;9(5):e025734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Papolos AI, Kenigsberg BB, Berg DD, et al. Management and outcomes of cardiogenic shock in cardiac ICUs with versus without shock teams. J Am Coll Cardiol. 2021;78(13):1309‐1317. [DOI] [PubMed] [Google Scholar]
- 37. Shiva A, Michele E, Sudeep K, et al. TCT‐513 the Impella micro‐axial flow catheter is and effective for Treatmenttreatment of Myocarditis Complicated by Cardiogenic Shock: an Analysis from the Global cVAD registry. J Am Coll Cardiol. 2017;70(18_suppl):B212. 10.1016/j.jacc.2017.09.629 [DOI] [Google Scholar]
- 38. Chang J‐J, Lin M‐S, Chen T‐H, et al. Heart failure and mortality of adult survivors from acute myocarditis requiring intensive care treatment—a Nationwide Cohort Study. Int J Med Sci. 2017;14(12):1241‐1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Okada Y, Narumiya H, Kobayashi N, et al. Survival after cardiac arrest with instantaneous rigorlike stiffness: a case report. Ann Emerg Med. 2018;730(3):393‐396. [DOI] [PubMed] [Google Scholar]
- 40. Lebreton G, Pozzi M, Luyt C‐E, et al. Out‐of‐hospital extra‐corporeal life support implantation during refractory cardiac arrest in a half‐marathon runner. Resuscitation. 2011;82:1239‐1242. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.
Supporting information.
Supporting information.
Supporting information.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


