Abstract
Background
Little is known about the real-world effectiveness of oral antivirals against the SARS-CoV-2 omicron (B.1.1.529) variant. We aimed to assess the clinical effectiveness of two oral antiviral drugs among community-dwelling COVID-19 outpatients in Hong Kong.
Methods
In this observational study, we used data from the Hong Kong Hospital Authority to identify an unselected, territory-wide cohort of non-hospitalised patients with an officially registered diagnosis of SARS-CoV-2 infection between Feb 26 and June 26, 2022, during the period in which the omicron subvariant BA.2.2 was dominant in Hong Kong. We used a retrospective cohort design as primary analysis, and a case-control design as sensitivity analysis. We identified patients with COVID-19 who received either molnupiravir (800 mg twice daily for 5 days) or nirmatrelvir plus ritonavir (nirmatrelvir 300 mg and ritonavir 100 mg twice daily for 5 days, or nirmatrelvir 150 mg and ritonavir 100 mg if estimated glomerular filtration rate was 30–59 mL/min per 1·73 m2). Outpatient oral antiviral users were matched with controls using propensity score (1:10) according to age, sex, date of SARS-CoV-2 infection diagnosis, Charlson Comorbidity Index score, and vaccination status. Study outcomes were death, COVID-19-related hospitalisation, and in-hospital disease progression (in-hospital death, invasive mechanical ventilation, or intensive care unit admission). Hazard ratios (HRs) were estimated by Cox regression for the primary analysis, and odds ratios in oral antiviral users compared with non-users by logistic regression for the sensitivity analysis.
Findings
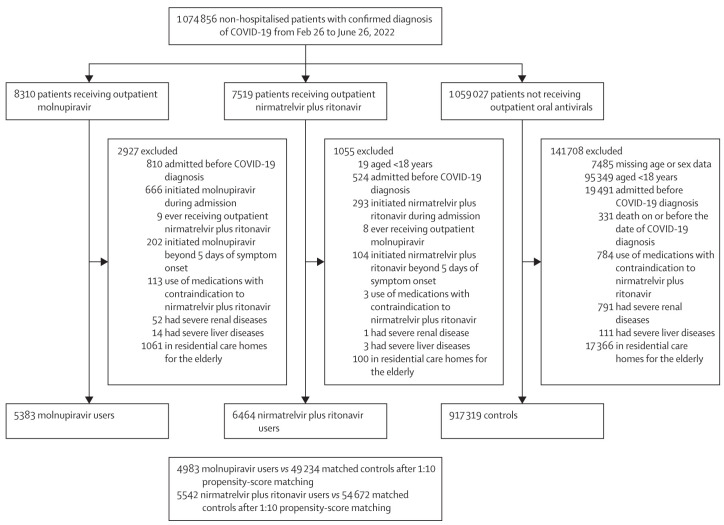
Among 1 074 856 non-hospitalised patients with COVID-19, 5383 received molnupiravir and 6464 received nirmatrelvir plus ritonavir in the community setting. Patients were followed up for a median of 103 days in the molnupiravir group and 99 days in the nirmatrelvir plus ritonavir group. Compared with nirmatrelvir plus ritonavir users, those on molnupiravir were older (4758 [85·9%] vs 4418 [88.7%] aged >60 years) and less likely to have been fully vaccinated (1850 [33·4%] vs 800 [16·1%]). Molnupiravir use was associated with lower risks of death (HR 0·76 [95% CI 0·61–0·95]) and in-hospital disease progression (0·57 [0·43–0·76]) than non-use was, whereas risk of hospitalisation was similar in both groups (0·98 [0·89–1·06]). Nirmatrelvir plus ritonavir use was associated with lower risks of death (0·34 [0·22–0·52]), hospitalisation (0·76 [0·67–0·86]), and in-hospital disease progression (0·57 [0·38–0·87]) than non-use was. We consistently found reduced risks of mortality and hospitalisation associated with early oral antiviral use among older patients. The findings from the case-control analysis broadly supported those from the primary analysis.
Interpretation
During Hong Kong's wave of SARS-CoV-2 omicron subvariant BA.2.2, among non-hospitalised patients with COVID-19, early initiation of novel oral antivirals was associated with reduced risks of mortality and in-hospital disease progression. Nirmatrelvir plus ritonavir use was additionally associated with a reduced risk of hospitalisation.
Funding
Health and Medical Research Fund, Health Bureau, Government of Hong Kong Special Administrative Region, China.
Translation
For the Chinese translation of the abstract see Supplementary Materials section.
Introduction
Oral antiviral drugs including molnupiravir and nirmatrelvir plus ritonavir are novel options for treating adults with COVID-19, which have been shown in clinical trials of unvaccinated patients before the emergence of the SARS-CoV-2 omicron (B.1.1.529) variant to reduce hospitalisation and mortality.1, 2, 3 Little evidence exists from contemporary real-world conditions.2, 4 Clinical trials, including the MOVe-OUT study,1 done in 20 countries, have shown that early administration of molnupiravir to non-hospitalised patients with mild-to-moderate COVID-19 accelerates viral clearance and reduces the relative risk of hospitalisation or mortality by 30%.1, 5 According to the global EPIC-HR trial, nirmatrelvir plus ritonavir reduced the rates of hospitalisation and mortality by 89% when the drug was initiated within 3 days of symptom onset, and consistently by 88% if initiated within 5 days of symptom onset, in non-hospitalised patients with mild-to-moderate COVID-19 who were at risk of progression to severe disease.6
Research in context.
Evidence before this study
Oral antivirals have been used in non-hospitalised patients with COVID-19 to reduce the risks of hospitalisation and death, and hence to reduce the burden on health-care systems. We searched Scopus and PubMed for studies published before Aug 4, 2022, using the search terms “SARS-CoV-2 OR COVID-19” AND “molnupiravir OR Lagevrio OR EIDD-2801” OR “nirmatrelvir OR Paxlovid OR PF-07321332”. Major studies examining the outpatient use of molnupiravir and nirmatrelvir plus ritonavir are MOVe-OUT and EPIC-HR trials. Both were done in unvaccinated, non-hospitalised patients with mild-to-moderate COVID-19, who were at risk of progression to severe disease, during a pandemic wave of the SARS-CoV-2 delta (B.1.617.2) variant. Early initiation of molnupiravir or nirmatrelvir plus ritonavir within 5 days of symptom onset has been associated with a relative risk reduction of hospitalisation or death by 30% for molnupiravir and 88% for nirmatrelvir plus ritonavir. A few observational studies have also shown similar clinical benefits in non-hospitalised patients infected with the omicron (B.1.1.529) variant. Understanding of the real-world effectiveness of oral antivirals is urgently needed to inform their clinical use in patients with COVID-19, considering patients’ vaccination status and the circulating variant of concern.
Added value of this study
To the best of our knowledge, this is one of the first real-world studies exploring the clinical use of oral antivirals during a pandemic wave dominated by the SARS-CoV-2 omicron variant. We did a territory-wide, retrospective cohort study to examine the effectiveness of molnupiravir and nirmatrelvir plus ritonavir in community-dwelling patients with COVID-19. Risk of all-cause death was reduced by 24% with molnupiravir and by 66% with nirmatrelvir plus ritonavir initiated within 5 days of symptom onset, compared with not using any oral antivirals. Nirmatrelvir plus ritonavir use was also associated with a reduced risk of COVID-19-related hospitalisation by 24%. We consistently found reduced risks of death and hospitalisation associated with early oral antiviral use among older patients. Both oral antivirals were effective in reducing the risk of in-hospital death. Intriguingly, the need for invasive ventilation was reduced among molnupiravir users compared with that among matched controls.
Implications of all the available evidence
Guidelines prioritise nirmatrelvir plus ritonavir use over molnupiravir in community-dwelling patients with COVID-19 who are at high risk of hospitalisation or progression to severe disease, should the nirmatrelvir plus ritonavir be accessible and clinically appropriate. This study shows real-world effectiveness of oral antivirals in reducing the mortality risk of community-dwelling patients with COVID-19, consisting mostly of older patients and those who had not been fully vaccinated. These data extend the evidence from clinical trials of those infected with the delta variant and who were at risk of severe COVID-19 from being overweight. Several clinical trials (eg, RECOVERY and PANORAMIC) and observational studies of the two oral antivirals are ongoing, and further research is needed to confirm our results in other patient populations and health-care settings.
Although both oral antivirals have been authorised for the treatment of non-hospitalised patients with mild-to-moderate COVID-19, observational evidence investigating their real-world effectiveness is scarce.7, 8, 9, 10 In particular, the original trials were done mostly during the delta (B.1.617.2) wave, whereas their effectiveness against omicron and its subvariants remains unknown in different COVID-19 patient populations. Here, we assessed the clinical effectiveness of molnupiravir and nirmatrelvir plus ritonavir among community-dwelling COVID-19 outpatients in Hong Kong during the pandemic wave of SARS-CoV-2 omicron subvariant BA.2.2 in January to June, 2022.
Methods
Study design and data sources
We did a territory-wide observational study in non-hospitalised people, aged 18 years or older, with confirmed SARS-CoV-2 infection in Hong Kong, during the omicron wave. This fifth wave of the pandemic in Hong Kong has been reported to have been dominated by the omicron sublineage BA.2.2 in January to June, 2022.11
We analysed the electronic medical records of patients with confirmed SARS-CoV-2 infection (defined by laboratory-confirmed positive RT-PCR test, or positive rapid antigen test) from the Hong Kong Hospital Authority, a statutory provider of public inpatient services and primary public outpatient services in Hong Kong. We retrieved data on demographic characteristics, dates of registered deaths, hospital admissions, emergency department visits, diagnoses, prescription and drug dispensing records, procedures, laboratory tests, and COVID-19 vaccination records. The database has been widely used for observational cohort studies to investigate the effectiveness of drug treatments for COVID-19 at a population level.12, 13
Patients who had confirmed SARS-CoV-2 infection from Feb 26 to June 26, 2022, were eligible. Molnupiravir was locally available for prescription from Feb 26, 2022, and nirmatrelvir plus ritonavir from March 16, 2022. According to clinical management guidelines for COVID-19 from the Hong Kong Hospital Authority (appendix 2 p 11),14 patients who (1) had mild symptoms, (2) were at risk of progressing to severe disease (ie, with diabetes, obesity with body-mass index ≥30 kg/m2, aged ≥60 years, in an immunocompromised state, with underlying chronic illnesses, or not fully vaccinated), and (3) at early stage of disease (within 5 days of symptom onset) were recommended to receive molnupiravir or nirmatrelvir plus ritonavir. Later versions (since March 21, 2022, well after the wave peak) of the guidelines also added that nirmatrelvir plus ritonavir should be preferentially administered over molnupiravir, unless the patient was on any concomitant medications contraindicated for nirmatrelvir plus ritonavir. We excluded patients who were younger than 18 years, admitted to hospital before the SARS-CoV-2 infection diagnosis, dead on or before the SARS-CoV-2 infection diagnosis, or residents in residential care homes for the elderly. We excluded patients with drug contraindications to nirmatrelvir plus ritonavir (ie, use of amiodarone, apalutamide, enzalutamide, lumacaftor plus ivacaftor, ivosidenib, rifampicin, rifapentine, carbamazepine, St John's Wort [Hypericum perforatum], primidone, phenobarbital, or phenytoin in the 6 months before baseline), severe renal impairment 15 (estimated glomerular filtration rate [eGFR] <30 mL/min per 1·73 m2, dialysis, or renal transplantation), or severe liver impairment15 (cirrhosis, hepatocellular carcinoma, or liver transplantation) at baseline to further mitigate confounding by indication as much as possible. We also excluded those who were initiated on oral antivirals more than 5 days since symptom onset from the analysis.
Procedures
We used the retrospective cohort study design as primary analysis, and case-control study design as sensitivity analysis for internal validation. Oral antiviral users were allocated to the molnupiravir group (800 mg twice daily for 5 days) or the nirmatrelvir plus ritonavir group (nirmatrelvir 300 mg and ritonavir 100 mg twice daily for 5 days, or nirmatrelvir 150 mg and ritonavir 100 mg if eGFR was 30–59 mL/min per 1·73 m2) on the basis of their drug prescription and dispensing records. The index date within the cohort study was defined as the date of confirmed SARS-CoV-2 infection or symptom onset, whichever occurred earlier. The control cohort was selected from patients with confirmed SARS-CoV-2 infection before admission, and those who did not receive oral antiviral drugs in the outpatient setting during the observational period. Treatment cohorts were matched with the control cohort using the propensity score in a ratio of 1:10. We observed patients from the index date to death, outcome event occurrence, crossover of oral antiviral treatment, or the end of observational period (July 3, 2022), whichever came first.
Within the case-control study design, patients with COVID-19 received molnupiravir or nirmatrelvir plus ritonavir treatment in the outpatient setting before the reference date, which was the date of outcome events for cases and 28 days after the SARS-CoV-2 infection diagnosis for controls. Up to ten control patients were randomly matched with each patient with COVID-19 according to age (within the same year), sex, date of SARS-CoV-2 infection diagnosis (within the same date), Charlson Comorbidity Index score, and full SARS-CoV-2 vaccination (with at least two doses of BNT162b2 [Fosun–BioNTech] or three doses of CoronaVac [Sinovac]13).
Our study followed STROBE guidelines. This study was approved by the institutional review board of the University of Hong Kong and Hospital Authority Hong Kong West Cluster (UW 20-493). Given the extraordinary nature of the COVID-19 pandemic, individual patient-informed consent was not required for this retrospective cohort study using anonymised data.
Outcomes
Outcomes of the cohort study were (1) all-cause mortality, (2) hospital admission due to COVID-19, (3) a composite outcome of in-hospital disease progression (in-hospital mortality, invasive mechanical ventilation [IMV], or intensive care unit [ICU] admission), and (4) individual in-hospital outcomes (in-hospital death, IMV initiation, and ICU admission).
The case-control study measured the same first three outcomes as in the cohort study. For the outcome of all-cause death, we defined cases as patients who died and controls as those who did not die within 28 days of confirmed SARS-CoV-2 infection during the observation period. For the outcome of COVID-19-related hospitalisation, cases were defined as patients admitted to hospital as a result of COVID-19 within 28 days of confirmed SARS-CoV-2 infection, and controls were defined as those without hospital admission within 28 days after the diagnosis. Only the first hospital admission after the diagnosis was used if a patient had multiple hospital admissions. The composite in-hospital outcome applied similar case and control definitions.
Statistical analysis
In the retrospective cohort design, we used propensity-score models conditional on age, sex, date of confirmed SARS-CoV-2 infection, Charlson Comorbidity Index, and vaccination status in a logistic regression model. We used propensity-score matching without replacement using a caliper width of 0·05. We calculated standardised mean differences (SMDs) of each covariate between the groups before and after the propensity-score matching, which was interpreted as balanced when the SMD was below the threshold of 0·1.16 Hazard ratios (HRs) with 95% CIs of each outcome between oral antiviral users and their respective matched non-users were estimated using Cox regression models. Subgroup analyses assessed the associations by vaccination status (fully vaccinated vs not fully vaccinated) and age groups (≤60 years vs >60 years). We assessed interactions between oral antiviral drug treatment and subgroups. In the case-control design as sensitivity analysis, we used conditional logistic regression to examine the association of receiving oral antiviral drug treatment with hospitalisation and all-cause mortality among patients with COVID-19. We estimated odds ratios in molnupiravir users and nirmatrelvir plus ritonavir users compared with non-users. We did post-hoc sensitivity analyses of treating oral antiviral use as a time-varying covariate in the Cox regression models, and which split the observation times for those in the oral antiviral group into two periods, an earlier one from the index date to the day before the antiviral treatment initiation, and a later one from the date of antiviral treatment initiation to the outcome occurrence, crossover of oral antiviral treatment, or the end of the observational period. All statistical analyses were done using Stata/MP (version 17). All significance tests were two-tailed, and a p value of less than 0·05 was considered statistically significant.
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
During the omicron BA.2.2 wave in Hong Kong, 1 074 856 patients with confirmed SARS-CoV-2 infection were identified in our study period, of whom 11 847 (1·1%) were initiated on either of the two novel oral antivirals in the community setting (5383 [45·4%] with molnupiravir, and 6464 [54·6%] with nirmatrelvir plus ritonavir; figure 1 ). In the retrospective cohort, we included 4983 molnupiravir users and 49 234 matched controls, in addition to 5542 nirmatrelvir plus ritonavir users and 54 672 matched controls, after excluding those who did not meet eligibility criteria or for whom we could not identify matched pairs. Treatment course completion rates were 99·7% for molnupiravir and 99·9% for nirmatrelvir plus ritonavir. Demographic and clinical characteristics of non-hospitalised patients with COVID-19 are presented in the appendix 2 (p 2) by oral antiviral use. After matching, patient characteristics were balanced between oral antiviral and control groups at baseline, with all SMDs below 0·1, except for age in the molnupiravir and matched control groups (SMD=0·12; table 1 ). The distribution of propensity scores in oral antiviral and matched control groups were highly overlapping, indicating an acceptable quality of matching for our propensity-score models (appendix 2 p 12). Overall, fewer than half of the cohort had been fully vaccinated, and most patients were older than 60 years (table 1). Among those who were fully vaccinated, the last dose of vaccination for molnupiravir users was received 1·68 months before baseline and 2·01 months before baseline for matched controls. For nirmatrelvir plus ritonavir users, the last dose was received 2·19 months before baseline and 2·25 months before baseline for matched controls. Patients with COVID-19 who were prescribed molnupiravir were older, with more pre-existing comorbidities, and less likely to be fully vaccinated, compared with those on nirmatrelvir plus ritonavir (appendix 2 p 2). At baseline, 176 (3·5%) of 4983 molnupiravir users and 76 (1·4%) of 5542 nirmatrelvir plus ritonavir users were identified as immunocompromised. Both oral antivirals were initiated in community-dwelling COVID-19 patients after a median of 2 days (IQR 1–3) since symptom onset.
Figure 1.
Study profile
Table 1.
Baseline characteristics of non-hospitalised patients with COVID-19 after 1:10 propensity-score matching
| Molnupiravir (n=4983) | Control (n=49 234) | Standardised mean difference | Nirmatrelvir plus ritonavi (n=5542) | Control (n=54 672) | Standardised mean difference | ||
|---|---|---|---|---|---|---|---|
| Age, years | |||||||
| 18–60 | 565 (11·3%) | 3819 (7·8%) | 0·12 | 784 (14·2%) | 8071 (14·8%) | 0·02 | |
| >60 | 4418 (88·7%) | 45 415 (92·2%) | .. | 4758 (85·9%) | 46 601 (85·2%) | .. | |
| Sex | |||||||
| Male | 2367 (47·5%) | 23 535 (47·8%) | 0·01 | 2566 (46·3%) | 25 490 (46·6%) | 0·01 | |
| Female | 2616 (52·5%) | 25 699 (52·2%) | .. | 2976 (53·7%) | 29 182 (53·4%) | .. | |
| Date of confirmed SARS-CoV-2 infection | |||||||
| February to March, 2022 | 4178 (83·9%) | 40 787 (82·8%) | 0·03 | 3895 (70·3%) | 39 477 (72·2%) | 0·04 | |
| April to June, 2022 | 805 (16·2%) | 8447 (17·2%) | .. | 1647 (29·7%) | 15 195 (27·8%) | .. | |
| Charlson Comorbidity Index score | |||||||
| 0–4 | 4475 (89·8%) | 44 984 (91·4%) | 0·05 | 5291 (95·5%) | 52 345 (95·7%) | 0·02 | |
| 5–6 | 353 (7·1%) | 2872 (5·8%) | .. | 206 (3·7%) | 1864 (3·4%) | .. | |
| 7–14 | 155 (3·1%) | 1378 (2·8%) | .. | 45 (0·8%) | 463 (0·9%) | .. | |
| Fully vaccinated* | 800 (16·1%) | 6115 (12·4%) | 0·10 | 1850 (33·4%) | 18 138 (33·2%) | 0·00 | |
Data are n (%), unless otherwise indicated.
Fully vaccinated patients were defined as those with at least two doses of BNT162b2 (Fosun–BioNTech) or three doses of CoronaVac (Sinovac).
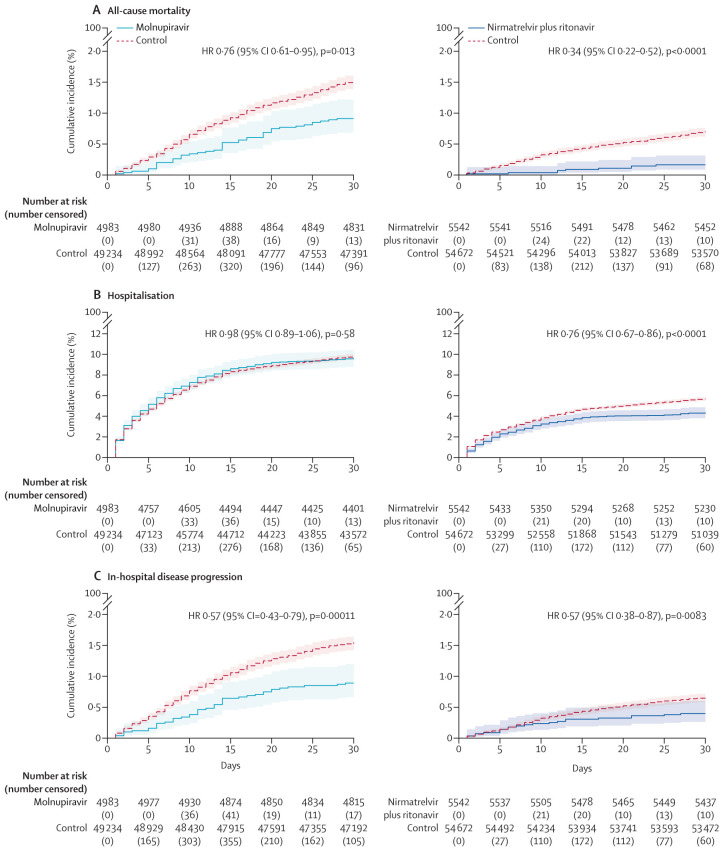
Overall, molnupiravir users were followed up for a median of 103 days (IQR 97–109) and nirmatrelvir plus ritonavir users for a median of 99 days (92–104). The cumulative incidences of all-cause mortality, COVID-19-related hospitalisation, and in-hospital disease progression between the oral antiviral and respective control groups are shown in figure 2 . The crude incidence rates of all-cause mortality were 17·9 per 100 000 person-days among molnupiravir users and 22·1 per 100 000 person-days among matched controls, and 4·2 per 100 000 person-days among nirmatrelvir plus ritonavir users and 11·6 per 100 000 person-days among matched controls (Table 2, Table 3 ). Molnupiravir use was associated with a significantly lower risk of all-cause mortality than non-use was (HR 0·76 [95% CI 0·61–0·95]; p=0·013), whereas the risk of hospitalisation was similar to that in the control group (0·98 [0·89–1·06]; p=0·58; figure 2). By contrast, nirmatrelvir plus ritonavir use was associated with significantly lower risks of both all-cause mortality (0·34 [0·22–0·52]; p<0·0001) and hospitalisation (0·76 [0·67–0·86]; p<0·0001) compared with non-use (figure 2).
Figure 2.
Cumulative incidence plots
All-cause mortality (A), hospitalisation (B), and in-hospital disease progression (C) for outpatient molnupiravir users and nirmatrelvir plus ritonavir versus their matched controls.
Table 2.
Outcomes for outpatient molnupiravir users versus matched controls
|
Molnupiravir (n=4983) |
Control (n=49 234) |
Molnupiravir vs control |
|||||
|---|---|---|---|---|---|---|---|
| Crude incidence rate (events per 100 000 person-days) |
Crude incidence rate (events per 100 000 person-days) |
HR (95% CI)* | p value | ||||
| Estimate (95% CI) | Person-days | Estimate (95% CI) | Person-days | ||||
| All-cause mortality | 17·9 (14·3–22·0) | 492 995 | 22·1 (20·9–23·4) | 5 134 524 | 0·76 (0·61–0·95) | 0·013 | |
| Hospitalisation | 107·6 (98·2–117·6) | 450 697 | 104·0 (101·1–107·0) | 4 740 249 | 0·98 (0·89–1·06) | 0·58 | |
| In-hospital disease progression | 10·2 (7·5–13·4) | 491 635 | 16·8 (15·7–18·0) | 5 115 217 | 0·57 (0·43–0·76) | 0·0001 | |
| In-hospital death | 7·3 (5·1–10·1) | 492 995 | 12·9 (11·9–13·9) | 5 134 524 | 0·53 (0·38–0·75) | 0·0002 | |
| Invasive mechanical ventilation | 1·4 (0·6–2·9) | 492 609 | 3·4 (2·9–3·9) | 5 127 257 | 0·40 (0·19–0·84) | 0·016 | |
| Intensive care unit admission | 3·5 (2·0–5·5) | 491 635 | 4·4 (3·8–5·0) | 5 116 681 | 0·74 (0·45–1·21) | 0·24 | |
HR=hazard ratio.
HRs greater than 1 indicate that molnupiravir users had higher risk of outcome compared with the matched control group.
Table 3.
Outcomes for outpatient nirmatrelvir plus ritonavir users versus matched controls
|
Nirmatrelvir plus ritonavir (n=5542) |
Control (n=54 672) |
Nirmatrelvir plus ritonavir vs control |
|||||
|---|---|---|---|---|---|---|---|
| Crude incidence rate (events per 100 000 person-days) |
Crude incidence rate (events per 100 000 person-days) |
HR (95% CI)* | p value | ||||
| Estimate (95% CI) | Person-days | Estimate (95% CI) | Person-days | ||||
| All-cause mortality | 4·2 (2·6–6·3) | 528 328 | 11·6 (10·8–12·6) | 5 471 588 | 0·34 (0·22–0·52) | <0·0001 | |
| Hospitalisation | 48·5 (42·6–54·9) | 507 655 | 61·0 (58·9–63·2) | 5 221 023 | 0·76 (0·67–0·86) | <0·0001 | |
| In-hospital disease progression | 4·6 (2·9–6·8) | 526 844 | 7·5 (6·8–8·3) | 5 462 351 | 0·57 (0·38–0·87) | 0·0083 | |
| In-hospital death | 1·5 (0·7–3·0) | 528 328 | 5·8 (5·2–6·4) | 5 471 588 | 0·25 (0·12–0·50) | 0·0001 | |
| Invasive mechanical ventilation | 0·8 (0·2–1·9) | 527 944 | 1·2 (0·9–1·5) | 5 468 815 | 0·62 (0·23–1·72) | 0·36 | |
| Intensive care unit admission | 3·2 (1·9–5·2) | 526 926 | 1·9 (1·6–2·3) | 5 463 019 | 1·58 (0·95–2·63) | 0·078 | |
HR=hazard ratio.
HRs greater than 1 indicate that nirmatrelvir plus ritonavir users had higher risk of outcome compared with the matched control group.
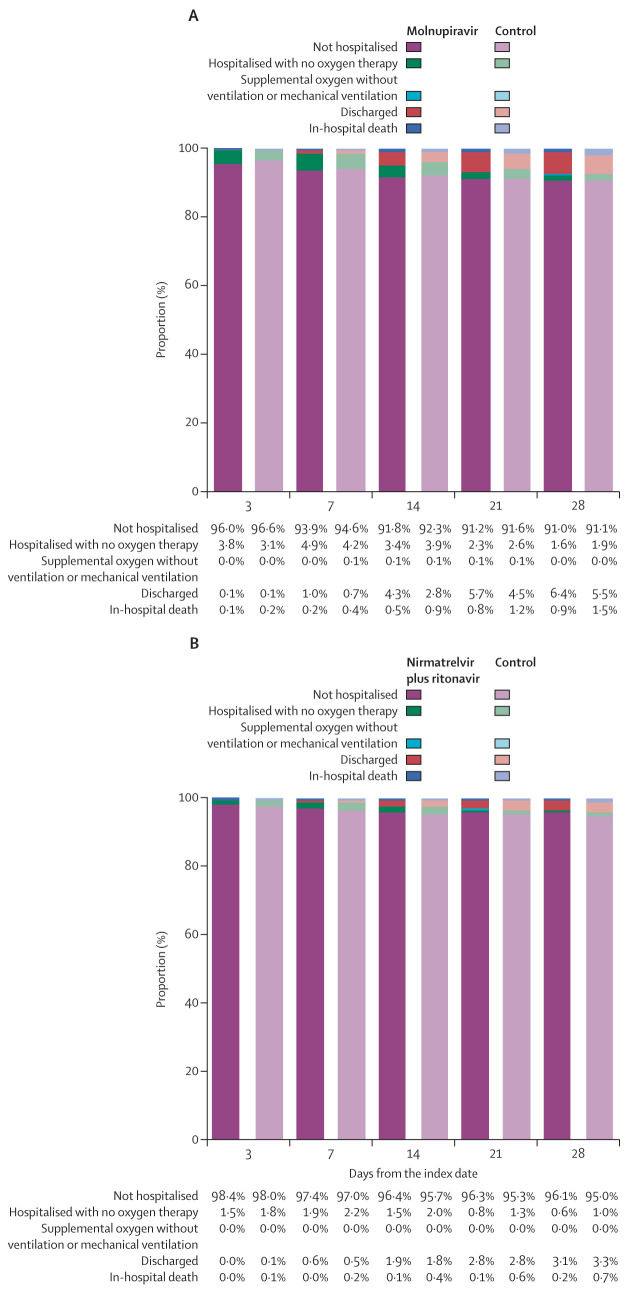
Concerning the composite outcome of in-hospital disease progression, molnupiravir use was associated with a significantly lower risk of disease progression than non-use was (HR 0·57 [95% CI 0·43–0·76]; p=0·0001), which was consistently observed for the individual outcomes of in-hospital death (0·53 [0·38–0·75]; p=0·0002) and IMV initiation (0·40 [0·19–0·84]; p=0·016; table 2). Risk of ICU admission was similar between the molnupiravir and control groups (0·74 [0·45–1·21; p=0·24; table 2). However, although the risk of the in-hospital composite outcome was also significantly reduced with nirmatrelvir plus ritonavir use compared with non-use (0·57 [0·38–0·87]; p=0·0083), this reduction was mainly driven by a substantial mortality benefit (0·25 [0·12–0·50]; p=0·0001) rather than reducing IMV initiation (0·62 [0·23-1·72]; p=0·36) or ICU admission (1·58 [0·95–2·63]; p=0·078; table 3). The proportion of community-dwelling patients with COVID-19 who were admitted to hospital, on respiratory support, discharged alive, or dead over the follow-up period are shown in figure 3 by oral antiviral use.
Figure 3.
Comparison of disease status at days 3, 7, 14, 21, and 28 after the index date
(A) Outpatient molnupiravir users versus matched controls. (B) Outpatient nirmatrelvir plus ritonavir users versus matched controls.
With respect to the sensitivity analysis using a case-control design, patient characteristics in case and control groups for each outcome after matching are reported in appendix 2 (p 3). Findings from sensitivity analyses (appendix 2 pp 4–5) were generally consistent with those of the primary analysis. In the subgroup analyses of study outcomes stratified by age and vaccination status (appendix 2 pp 6–7), results comparing molnupiravir use and non-use were significant for reduced risks of all-cause mortality and in-hospital disease progression among patients older than 60 years, in addition to a significantly reduced risk of COVID-19-related hospitalisation (0·89 [0·81–0·97]; p=0·0067). Results comparing nirmatrelvir plus ritonavir with non-use were generally consistent across subgroups in reducing the risks of both mortality and hospitalisation (appendix 2 pp 6–7).
Discussion
In this retrospective cohort of community-dwelling patients with COVID-19 during a pandemic period dominated by the SARS-CoV-2 omicron variant, early initiation of molnupiravir or nirmatrelvir plus ritonavir was associated with a significant reduction of all-cause mortality risk, compared with not having been administered either antiviral. Nirmatrelvir plus ritonavir use was additionally associated with a reduced risk of COVID-19-related hospitalisation. In terms of disease progression, the need for IMV was reduced among molnupiravir users. Reduced risks of mortality and hospitalisation associated with early oral antiviral use were consistently observed in patients older than 60 years.
Among non-hospitalised patients with mild-to-moderate COVID-19 who were unvaccinated and at risk of progression to severe disease, early initiation of molnupiravir within 5 days of symptom onset contributed to a relative reduction of hospitalisation or death by 30% in the MOVe-OUT trial,1 and with nirmatrelvir plus ritonavir by 88% in the EPIC-HR trial.6 The updated analysis of the EPIC-SR trial involving unvaccinated adults at standard risk of COVID-19 or fully vaccinated individuals with at least one risk factor showed a non-significant reduction of hospitalisation or death by 51% with nirmatrelvir plus ritonavir use in non-hospitalised patients.17 Although both oral antivirals have been shown to exhibit robust activity in substantially lowering the viral load relative to that of placebo,1, 6 the number needed to treat (NNT) was higher with molnupiravir than with nirmatrelvir plus ritonavir use, as based on the final results of MOVe-OUT and EPIC-HR trials.18, 19, 20 Notably, both estimated NNTs are expected to increase further in the current wave dominated by the omicron variant, which results in less hospitalisation and death compared with that of the delta variant that circulated during previous trials,21, 22 as is the increasing proportion of individuals who have been fully vaccinated.23 Our study adds to the literature on real-world effectiveness in specific contexts considering different variants of concern and population immune settings.
Our findings could be interpreted as showing a gradation of protection by the two novel antivirals by drawing parallels with the differential effectiveness of COVID-19 vaccines. Virtually all licensed vaccines offer very good protection against death and severe outcomes but differ considerably in protecting against infection and mild-to-moderate symptomatic disease. We found a similar pattern: good protection by both antivirals against in-hospital progression to severe outcomes including death (albeit with different effect sizes), whereas the superiority of nirmatrelvir plus ritonavir compared with molnupiravir extended into protection against hospitalisation. The differential effects between ICU admission and mechanical ventilation use could be explained by different ICU admission criteria and how different settings manage ventilated patients. Moreover, many studies reported a composite outcome for both mechanical ventilation and ICU admission because of small numbers or high correlation between the two outcomes. Incidentally, a secondary analysis of the MOVe-OUT trial also identified a reduced need for respiratory interventions among molnupiravir users compared with those on placebo.24 Notably, the interpretation of our results of the two oral antivirals should also take into consideration the potential differences in patient characteristics at baseline, whereby molnupiravir users and matched controls tended to be older, had more pre-existing comorbidities, and were less likely to be fully vaccinated than their counterparts on nirmatrelvir plus ritonavir. The significant drug–drug interactions of nirmatrelvir plus ritonavir, coupled with the later introduction of the drug (the so-called first-mover advantage of molnupiravir) are likely to explain these observations. These factors might have partially contributed to the inferior clinical outcomes with molnupiravir compared with nirmatrelvir plus ritonavir in this study.
Guidelines prioritise nirmatrelvir plus ritonavir use over molnupiravir in community-dwelling patients with COVID-19 who are at high risk of hospitalisation or progression to severe disease, should nirmatrelvir plus ritonavir be accessible and clinically appropriate.25, 26, 27 Although our results suggest similar trends to those of the MOVe-OUT and EPIC-HR trials, discrepancies in the respective effect sizes could possibly be attributed, at least partly, to differences in the risk profile of patients (as indicated by the older age of our patients vs overweight or obesity being the major risk factor in patients in the two clinical trials, and <20% of patients being aged >60 years or >65 years), or the circulating variant of concern (omicron in this study vs delta in previous trials).1, 6 Furthermore, our subgroup analyses reinforced the significant benefits of early oral antiviral use in reducing the risks of all-cause mortality and COVID-19-related hospitalisation among older patients in the community setting.7, 9 These benefits were also associated with nirmatrelvir plus ritonavir use in patients who had not been fully vaccinated, which was similarly observed in a population-based cohort study done during the omicron wave.8 Several clinical trials (eg, RECOVERY, PANORAMIC, NCT04746183, and NCT05011513) and an observational study (NCT05195060) of the two oral antivirals are ongoing, which will take into account the circulating omicron variant and vaccination status of patients.
Logistics and distribution issues of oral antivirals should be adequately addressed by governments and the health-care sector to facilitate drug initiation soon after symptom onset for maximal efficacy, and promote equitable access amid limited supplies.18, 23 For instance, a validated risk-prediction tool or evidence-based scoring system can be developed to guide physicians to identify and prioritise patients with COVID-19 who would most likely benefit from the use of oral antivirals.18, 27 Further, several research gaps remain in the investigation of oral antiviral use, namely the safety of nirmatrelvir plus ritonavir in children, pregnant or breastfeeding women, efficacy of oral antivirals in patients with COVID-19 by serostatus at baseline, and risk of emergence of new viral variants attributed to genetic mutations induced by molnupiravir.23, 27, 28 Active pharmacovigilance programmes are crucial to monitoring the long-term safety of oral antivirals, especially for the mutational risk and potential genotoxicity associated with molnupiravir use in light of conflicting experimental evidence.29, 30, 31, 32 Moreover, given the high mutation rates of SARS-CoV-2 and selective pressure induced by the widespread use of antiviral monotherapy, concerns about the development of antiviral resistance have been raised.23, 27 Further clinical studies are needed to examine the feasibility of combination therapy in accelerating the elimination of the virus and minimising drug resistance—eg, molnupiravir plus nirmatrelvir plus ritonavir or favipiravir, or oral antivirals plus anti-SARS-CoV-2 monoclonal antibodies.23, 27, 30, 33
Using public health-care databases that encompass all reported cases of confirmed SARS-CoV-2 infection during the observation period, our study covers a non-selective patient population in the local region amid a pandemic wave of the SARS-CoV-2 omicron variant. Alongside the introduction of both oral antivirals in the public health-care system during this outbreak, their clinical effectiveness could be assessed in a real-world setting. Further, we found consistent results from both the retrospective cohort and case-control analyses of our study, hence supporting the robustness of our findings. Nevertheless, several study limitations should be acknowledged. First, residents at residential care homes for the elderly were excluded from the analysis because of substantial missing records, complex referral patterns between different levels and categories of treatment facilities, or prolonged delays in oral antiviral prescription during the peak of this pandemic wave. Further studies are needed to examine the real-world safety and effectiveness of oral antivirals in specific health-care settings—eg, in nursing homes and residential care facilities. Second, indication bias could not be eliminated in the prescription of oral antivirals, as reflected by the considerably older age and lower percentage of patients who had been fully vaccinated in the oral antiviral groups than in the matched control groups at baseline. Residual confounding by indication could still be present in the clinical decision to prescribe molnupiravir versus nirmatrelvir plus ritonavir, because nirmatrelvir plus ritonavir has risk of drug–drug interactions, thus might be less preferred by clinicians for treating those maintained on multiple medications. After excluding patients with drug contraindications to nirmatrelvir plus ritonavir and applying propensity-score matching, patient characteristics between the oral antiviral and respective control groups were well balanced at baseline. Third, although we captured all reported patients in Hong Kong, we cannot rule out self-ascertainment bias, in that many patients with SARS-CoV-2 infection might not be aware of having been infected (because of not testing or suboptimal sensitivity of rapid antigen tests, etc) or not having reported infection status to health authorities thus becoming eligible to be offered the novel antivirals. Fourth, we did not have complete sequence data on all cases in terms of omicron subvariants, although 98·4% of all cases belonged to the prevailing BA.2.2 strain during the period of observation.11 Fifth, we did not further differentiate all-cause mortality into deaths directly caused by COVID-19 as opposed to other causes. Sixth, booster doses were not included in the vaccination status of patients with COVID-19 in our analysis because of the low proportion of patients who had been fully vaccinated with the primary series. Finally, the usual caveats of residual and unmeasured confounding about observational studies apply.
In conclusion, during Hong Kong's SARS-CoV-2 wave in which the omicron sublineage BA.2.2 was dominant, among non-hospitalised patients with COVID-19, early initiation of either molnupiravir or nirmatrelvir plus ritonavir was associated with reduced risks of death and in-hospital disease progression. Nirmatrelvir plus ritonavir use was additionally associated with a reduced risk of hospitalisation. Further research is needed to confirm our results in specific patient populations and other health-care settings, and a mechanistic explanation should be investigated in future laboratory studies.
Data sharing
The clinical outcome data and vaccination records were extracted from the Hospital Authority database in Hong Kong and data on confirmed cases of SARS-CoV-2 infection were extracted from the eSARS data provided by the Centre for Health Protection. Restrictions apply to the availability of these data, which were used under licence for this study.
For more on PANORAMIC see https://www.panoramictrial.org/
Declaration of interests
BJC reports honoraria from AstraZeneca, Fosun Pharma, GlaxoSmithKline, Moderna, Pfizer, Roche, and Sanofi Pasteur. BJC has provided scientific advice to Pfizer and AstraZeneca on issues related to disease burden and vaccine effectiveness. He has not provided scientific advice to either company related to COVID-19 antiviral effectiveness, and he has not received any funding from Pfizer or AstraZeneca for any research on antiviral effectiveness including the current work. All other authors declare no competing interests.
Acknowledgments
Acknowledgments
Authors would like to thank Kathy Leung for her input to oral antiviral drug prescription patterns and ascertainment rate of COVID-19 in Hong Kong, Eric Tang for statistical advice, and Xi Xiong for administrative and technical support. This study was supported by the Health and Medical Research Fund (COVID190210), Health Bureau, Government of Hong Kong Special Administrative Region, China.
Acknowledgments
Contributors
The study was designed by CKHW, GML, and BJC. The underlying data were accessed and verified by CKHW, ICHA, and EHYL, and data analyses were done by CKHW and ICHA. CKHW and KTKL wrote the first draft of the manuscript, which was revised by GML and BJC. All authors interpreted data, provided critical review and revision of the text, and approved the final version of the manuscript. All authors had access to the data.
Supplementary Material
References
- 1.Jayk Bernal A, Gomes da Silva MM, Musungaie DB, et al. Molnupiravir for oral treatment of COVID-19 in nonhospitalized patients. N Engl J Med. 2022;386:509–520. doi: 10.1056/NEJMoa2116044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singh AK, Singh A, Singh R, Misra A. Molnupiravir in COVID-19: a systematic review of literature. Diabetes Metab Syndr. 2021;15 doi: 10.1016/j.dsx.2021.102329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wen W, Chen C, Tang J, et al. Efficacy and safety of three new oral antiviral treatment (molnupiravir, fluvoxamine and Paxlovid) for COVID-1: a meta-analysis. Ann Med. 2022;54:516–523. doi: 10.1080/07853890.2022.2034936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.US Food and Drug Administration Coronavirus (COVID-19) update: FDA authorizes first oral antiviral for treatment of COVID-19. Dec 22, 2021. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-first-oral-antiviral-treatment-covid-19
- 5.Fischer WA, 2nd, Eron JJ, Jr, Holman W, et al. A phase 2a clinical trial of molnupiravir in patients with COVID-19 shows accelerated SARS-CoV-2 RNA clearance and elimination of infectious virus. Sci Transl Med. 2022;14 doi: 10.1126/scitranslmed.abl7430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hammond J, Leister-Tebbe H, Gardner A, et al. Oral nirmatrelvir for high-risk, nonhospitalized adults with COVID-19. N Engl J Med. 2022;386:1397–1408. doi: 10.1056/NEJMoa2118542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arbel R, Sagy YW, Hoshen M, et al. Nirmatrelvir use and severe Covid-19 outcomes during the omicron surge. NEJM. 2022;387:790–798. doi: 10.1056/NEJMoa2204919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dryden-Peterson S, Kim A, Kim AY, et al. Nirmatrelvir plus ritonavir for early COVID-19 and hospitalization in a large US health system. medRxiv. 2022 doi: 10.1101/2022.06.14.22276393. published online June 17. (preprint). [DOI] [Google Scholar]
- 9.Najjar-Debbiny R, Gronich N, Weber G, et al. Effectiveness of paxlovid in reducing severe COVID-19 and mortality in high risk patients. Clin Infect Dis. 2022 doi: 10.1093/cid/ciac443. published online June 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vena A, Traman L, Bavastro M, et al. Early clinical experience with molnupiravir for mild to moderate breakthrough COVID-19 among fully vaccinated patients at risk for disease progression. Vaccines. 2022;10 doi: 10.3390/vaccines10071141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen L-L, Abdullah SMU, Chan W-M, et al. Contribution of low population immunity to the severe omicron BA.2 outbreak in Hong Kong. Nat Commun. 2022;13 doi: 10.1038/s41467-022-31395-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wong CKH, Wan EYF, Luo S, et al. Clinical outcomes of different therapeutic options for COVID-19 in two Chinese case cohorts: a propensity-score analysis. EClinicalMedicine. 2021;32 doi: 10.1016/j.eclinm.2021.100743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McMenamin ME, Nealon J, Lin Y, et al. Vaccine effectiveness of one, two, and three doses of BNT162b2 and CoronaVac against COVID-19 in Hong Kong: a population-based observational study. Lancet Infect Dis. 2022;22:1435–1443. doi: 10.1016/S1473-3099(22)00345-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hospital Authority Task Force on Clinical Management on Infection . Hospital Authority Central Committee on Infectious Diseases and Emergency Response; Hong Kong: 2022. Interim recommendation on clinical management of adult cases with COVID-19. Version 1.12. [Google Scholar]
- 15.US Food and Drug Administration Fact sheet for healthcare providers: emergency use authorization for paxlovid. July 6, 2022. https://www.fda.gov/media/155050/download
- 16.Austin PC. Some methods of propensity-score matching had superior performance to others: results of an empirical investigation and Monte Carlo simulations. Biom J. 2009;51:171–184. doi: 10.1002/bimj.200810488. [DOI] [PubMed] [Google Scholar]
- 17.Pfizer Pfizer reports additional data on PAXLOVID supporting upcoming new drug application submission to US FDA. June 14, 2022. https://www.pfizer.com/news/press-release/press-release-detail/pfizer-reports-additional-data-paxlovidtm-supporting
- 18.Dal-Ré R, Becker SL, Bottieau E, Holm S. Availability of oral antivirals against SARS-CoV-2 infection and the requirement for an ethical prescribing approach. Lancet Infect Dis. 2022;22:e231–e238. doi: 10.1016/S1473-3099(22)00119-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee TC, Morris AM, Grover SA, Murthy S, McDonald EG. Outpatient therapies for COVID-19: how do we choose? Open Forum Infect Dis. 2022;9 doi: 10.1093/ofid/ofac008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Singh AK, Singh A, Singh R, Misra A. An updated practical guideline on use of molnupiravir and comparison with agents having emergency use authorization for treatment of COVID-19. Diabetes Metab Syndr. 2022;16 doi: 10.1016/j.dsx.2022.102396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nyberg T, Ferguson NM, Nash SG, et al. Comparative analysis of the risks of hospitalisation and death associated with SARS-CoV-2 omicron (B.1.1.529) and delta (B.1.617.2) variants in England: a cohort study. Lancet. 2022;399:1303–1312. doi: 10.1016/S0140-6736(22)00462-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ulloa AC, Buchan SA, Daneman N, Brown KA. Estimates of SARS-CoV-2 omicron variant severity in Ontario, Canada. JAMA. 2022;327:1286–1288. doi: 10.1001/jama.2022.2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sendi P, Razonable RR, Nelson SB, Soriano A, Gandhi RT. First-generation oral antivirals against SARS-CoV-2. Clin Microbiol Infect. 2022;28:1230–1235. doi: 10.1016/j.cmi.2022.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johnson MG, Puenpatom A, Moncada PA, et al. Effect of molnupiravir on biomarkers, respiratory interventions, and medical services in COVID-19 : a randomized, placebo-controlled trial. Ann Intern Med. 2022;175:1126–1134. doi: 10.7326/M22-0729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Infectious Diseases Society of America IDSA guidelines on the treatment and management of patients with COVID-19. June 29, 2022. https://www.idsociety.org/practice-guideline/covid-19-guideline-treatment-and-management/
- 26.US National Institutes of Health Therapeutic management of nonhospitalized adults with COVID-19. April 8, 2022. https://www.covid19treatmentguidelines.nih.gov/management/clinical-management/nonhospitalized-adults--therapeutic-management/
- 27.WHO . World Health Organization; Geneva: 2022. Therapeutics and COVID-19: living guideline, July 14, 2022. [PubMed] [Google Scholar]
- 28.Dyer O. Covid-19: FDA expert panel recommends authorising molnupiravir but also voices concerns. BMJ. 2021;375 doi: 10.1136/bmj.n2984. [DOI] [PubMed] [Google Scholar]
- 29.Githaka JM. Molnupiravir does not induce mutagenesis in host lung cells during SARS-CoV-2 treatment. Bioinform Biol Insights. 2022;16 doi: 10.1177/11779322221085077. 11779322221085077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tian L, Pang Z, Li M, et al. Molnupiravir and its antiviral activity against COVID-19. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.855496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Waters MD, Warren S, Hughes C, Lewis P, Zhang F. Human genetic risk of treatment with antiviral nucleoside analog drugs that induce lethal mutagenesis: the special case of molnupiravir. Environ Mol Mutagen. 2022;63:37–63. doi: 10.1002/em.22471. [DOI] [PubMed] [Google Scholar]
- 32.Zhou S, Hill CS, Sarkar S, et al. β-d-N4-hydroxycytidine inhibits SARS-CoV-2 through lethal mutagenesis but is also mutagenic to mammalian cells. J Infect Dis. 2021;224:415–419. doi: 10.1093/infdis/jiab247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li P, Wang Y, Lavrijsen M, et al. SARS-CoV-2 omicron variant is highly sensitive to molnupiravir, nirmatrelvir, and the combination. Cell Res. 2022;32:322–324. doi: 10.1038/s41422-022-00618-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The clinical outcome data and vaccination records were extracted from the Hospital Authority database in Hong Kong and data on confirmed cases of SARS-CoV-2 infection were extracted from the eSARS data provided by the Centre for Health Protection. Restrictions apply to the availability of these data, which were used under licence for this study.
For more on PANORAMIC see https://www.panoramictrial.org/