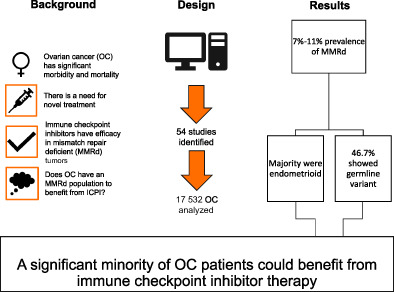
Abstract
Ovarian cancer (OC) is the least survivable gynecological malignancy and presents late. Five‐year survival for OC is around 45% increasing the need for innovative treatments. Checkpoint inhibitors have shown significant clinical efficacy in mismatch repair deficient (MMRd) cancers and could be a powerful treatment in OC. However, their application in OC is limited due to the lack of data on the prevalence of MMRd. The aim of our study was to conduct a systematic review of the literature and meta‐analysis to provide an accurate estimate of the prevalence of MMRd in OC. We followed PRISMA guidelines throughout. Studies were identified by electronic searches of Medline, Embase, Cochrane CENTRAL and Web of Science followed by citation searching. Studies not written in English were excluded. All studies were reviewed by at least two independent reviewers. Proportions of test positivity were calculated by random and fixed‐effects meta‐analysis models. I 2 score was used to assess heterogeneity across studies. In total 54 studies were included with 17 532 analyzed for MMRd. The overall proportions of MMRd by immunohistochemistry and microsatellite instability analysis were 6.7% and 10.4%, respectively. MMRd was reported in all histotypes of epithelial OC but was most common in endometrioid OC. We estimate that on average 46.7% (95% CI: 28.8‐65.4) of ovarian carcinomas showing MMRd by IHC had a germline path_MMR variant identified. OC in those with Lynch syndrome seems to present at an earlier age and stage. Studies however were generally of low quality and there was a high degree of heterogeneity. A significant minority (up to 16%) of OC displays MMRd and, therefore, could be amenable to checkpoint inhibition therapy. However, the current literature base is of limited quality and therefore high‐quality prospective studies exploring MMRd in OC with the use of multimodal testing are required. In addition, trials researching efficacy of checkpoint inhibition in MMRd OC are needed.
Keywords: biomarkers, checkpoint inhibition, germline testing, immune therapy, immunohistochemistry, Lynch syndrome, microsatellite instability, mismatch repair, ovarian cancer, somatic testing
What's new?
Despite gains in ovarian cancer survival, overall prognosis remains poor. Thus, to further improve patient outcomes', efforts are being made to identify cancers amenable to targeted therapeutics. Ovarian cancers with a mismatch repair deficiency (MMRd) phenotype could respond to immotherapy and therefore defining the prevelance of MMRd in ovarian cancer is important. In the present meta‐analysis examining the prevalence of MMRd in ovarian cancer we found a significant minority of ovarian cancers are MMRd. MMRd was observed in all histotypes but was more common in endometrioid tumors. Knowledge of MMRd prevalence in ovarian cancer could help guide therapeutic decisions, particularly surrounding the use of checkpoint inhibitors which are most effective in MMRd cancers.
Abbreviations
- FDA
US‐Federal Drug Administration
- ICPI
immune checkpoint inhibitors
- IHC
immunohistochemistry
- MMR
mismatch repair
- MMRd
mismatch repair deficient
- MSI
microsatellite instability analysis
- MSI‐H
microsatellite instability high
- MSS
microsatellite stable
- OC
ovarian cancer
- path_MMR
pathogenic variant in a mismatch repair gene
- QUADAS 2
quality and applicability of diagnostic accuracy studies
- TMA
tissue microarrays
1. INTRODUCTION
Ovarian cancer (OC) is the least survivable gynecological malignancy in developed nations. 1 It is associated with significant morbidity and mortality, with 230 000 women being diagnosed, and 150 000 women dying from OC annually. 1 Survival at 5 years is less than 50% with survival rates having only increased by 30% since the mid‐1970s. 2 The current treatment for OC consists of surgery to optimally debulk the disease alongside (neo)adjuvant platinum‐based chemotherapy with the selective addition of antiangiogenesis inhibitor bevacizumab and/or poly(ADP‐ribose) polymerase inhibitors. 1 Numerous factors contribute to the high mortality rate associated with OC. In the first instance, the symptoms of OC are vague, and women often present with advanced disease. 2 To date, there is no effective screening program. 3 Finally, until recently, effective treatment innovation has been lacking. 4
One such treatment innovation is the use of immune checkpoint inhibitors (ICPIs) in cancers with a mismatch repair deficient (MMRd) phenotype. MMRd cancers are highly immunogenic because of the production of numerous neopeptides due to their hypermutated genome. 5 Therefore, MMRd cancers must undergo immunoediting in order to escape the immune surveillance and destruction. 6 Immunoediting involves three steps: elimination, equilibrium and escape, with the later step in MMRd cancers often involving co‐signaling pathways; namely signaling via the PD‐1/PD‐L1 pathway. 7 This is a druggable pathway with the use of ICPIs.
ICPIs are monoclonal antibodies that reinvigorate the antitumor immune response by targeting co‐inhibitory receptors. 8 They bind directly to T cells inhibiting their ability to communicate with their immune checkpoint ligands. This instigates two outcomes, first, without the influence of inhibitory signals, the T cell can resume its effector functions and second, natural opsonization of antibody bound T‐cells allows for the expansion of new tumor‐specific T cells. 9 Trial data, most notably Dung et al, demonstrated the therapeutic affinity ICPIs possesses towards MMRd cancers. 10 Both objective response rates and progression free survival were significantly greater in MMRd cancers compared to MMR proficient cancers treated with ICPIs. These data led to the US‐Federal Drug Administration (FDA) approving Nivolumab and Pembrolizumab for use in MMRd tumors, regardless of histology and cancer site. 11 This was the first time a cancer treatment had been approved based on a molecular characteristic.
Data regarding use of ICPIs in OC is limited. Studies have been small and given mixed results as to ICPIs efficacy in OC. 12 , 13 , 14 , 15 , 16 However, these studies did not select for MMRd OC and therefore their inability to find treatment efficacy is understandable. Researchers were deterred for looking for MMRd OC as it was thought the prevalence of MMRd in OC would be too low as to be clinically meaningful. 17 Such an assumption could be made due to a lack of high quality MMRd prevalence data in OC. Therefore, a meta‐analysis of MMRd prevelance in OC is needed as to assess the utility of using MMRd as a biomarker for ICPIs in OC and to help inform any future ICPI treatment trials in OC.
2. OBJECTIVE
The aim of our study was to conduct a systematic review of the literature as to identify and appraise the current evidence base regarding the prevalence of MMRd in OC. In addition, we performed a meta‐analysis as to provide composite results. These data could inform the use of ICPIs in OC.
3. METHODS
3.1. Eligibility criteria, information sources and search strategy
A systematic review of literature, following PRIMSA guidelines, was performed. 18 Medline, Embase, Cochrane CENTRAL, NHS Health and Technology and Web of Science were searched from their inception to September 2021. Nonelectronic and gray literature were excluded. Search terms included “DNA mismatch repair,” “ovarian neoplasms” and “colorectal neoplasms, hereditary nonpolyposis” with associated Medical Subject Headings (MeSH); this search strategy was devised by a specialist medical librarian. A secondary search was carried out using “mismatch repair,” “ovarian cancer” and Lynch syndrome” as multipurpose search terms. Initial search results were supplemented by citation searching. The search strategy is detailed in Table S1.
3.2. Study selection
The protocol for this systematic review was preregistered with the PROSPERO database registration (ref: CRD42020220975). Studies investigating MMRd in both unselected and selected OC populations were included; that is studies that applied universal testing for MMRd in OC and studies in which the population of OC was selected based on a predefined criterion/criteria, for example histotype specific, were included. Interventional studies in which MMRd testing was carried out were also included. Searches were limited to English language, human adults (>18 years old) and female subjects. No restriction was placed on the date of publication. Studies that used immunohistochemistry (IHC), microsatellite instability analysis (MSI), MLH1 promotor hypermethylation testing, MMR germline mutation analysis and MMR somatic mutation analysis as diagnostic tools were included. Studies were excluded if they sampled less than 50 OCs or concentrated on synchronous ovarian tumors with other primary malignancies. These exclusions were to ensure high quality data 19 and prevent selection bias for Lynch syndrome respectively. In addition, articles found to have internal inconsistencies, such as different results reported within the study for the same outcome, were also removed.
3.3. Data extraction
Titles and abstracts were collated and screened using Rayyan software (https://rayyan.qcri.org/). Screening was done independently by three authors (A.S.A., M.C.D. and T.A.K.), with any discrepancies reviewed by a third party (N.A.J.R.). Studies that were identified as meeting the inclusion criteria underwent full article review and data extraction by two authors (A.S.A. and T.A.K.), with issues resolved through discussion with a senior author (N.A.J.R.). Articles excluded at full article review are detailed in Table S3. A bespoke data collection tool was designed to ensure complete capture of all primary and secondary outcome data points (available on request). This recorded demographic, pathological and clinical data, and the diagnostic method used to estimate the prevalence of MMRd in women with OC.
3.4. Assessment of risk of bias
Risk of bias was assessed using R, version 3.3.1 (https://cran.r-project.org) using the package “robvis”. 20 This package uses the Quality and Applicability of Diagnostic Accuracy Studies (QUADAS 2) tool for bias analysis. 21 All primary data is available by request.
3.5. Statistical analysis
We defined the primary outcome as the proportion of OC with MMRd defined by the author using either MSI, IHC or somatic sequencing. When estimating the proportion of OC with MMRd by MSI we did not include studies where MSI was only conducted on an unrepresentative sample of patients (eg, if MSI analysis was only performed in patients with MMRd by IHC). An equivalent approach was used when estimating the proportion of OC with MMRd by IHC. All analysis was performed using R version 4.1.0 (https://www.R-project.org/) and the package meta version 5.0. 22
Base case analyses used a generalized linear mixed model with logit transform and random intercepts at the study level (see Equations (1) and (2)). In addition, sensitivity analyses were conducted using a fixed effects approach with logit transform (Equations (1) and (3)) and using an inverse variance approach with Freeman‐Tukey double arcsine transforms (see Table S5). Confidence intervals for individual studies were produced by the Clopper‐Pearson method. Heterogeneity across studies was described with the use of an I 2 score (low: 25%, 50%: moderate, 75% high heterogeneity). Subgroup effects were tested using the Q‐statistic (ANOVA Q with estimated separately in each subgroup).
For the patient in the study , the outcome is equal to 1 in the event of a positive test (eg, when MMR IHC shows MMRd) and is equal to 0 in the event of a negative test.
| (1) |
| (2) |
| (3) |
4. RESULTS
4.1. Study selection
In total 826 articles were identified by the search with an additional seven titles found through citation searching. After abstract screening, 146 articles underwent full article review. During this process a further 78 titles were excluded. Data extraction was performed on 68 articles, however a further 14 articles were excluded due to either not meeting the inclusion criteria, an inability to extract results, internal inconsistencies or duplicate data (see Tables S1 and S2). Therefore 54 articles were included in the meta‐analysis. These data are summarized in Figure 1 and Tables 1 and S6.
FIGURE 1.
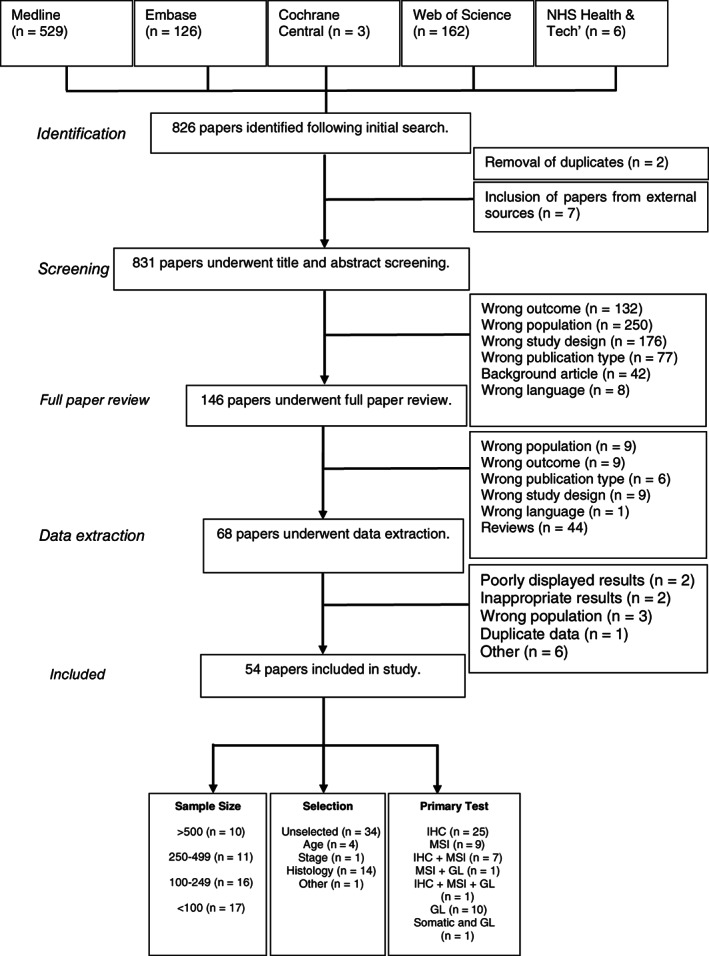
Prisma flow diagram. GL, germline analysis; IHC, immunohistochemistry; MSI, microsatellite instability; Tech, technology
TABLE 1.
Summary of studies
| Study feature | Number of studies | Proportion of studies (%) |
|---|---|---|
| Country | ||
| North America | 26 | 48.15 |
| Europe | 16 | 29.63 |
| Asia | 9 | 16.67 |
| Mixed continents | 2 | 3.70 |
| Australasia | 1 | 1.85 |
| Selection of patients | ||
| Unselected | 34 | 62.96 |
| Selected | 20 | 37.04 |
| Histology | 14 | 25.93 |
| EC | 4 | 7.40 |
| CCC | 4 | 7.40 |
| EC + CCC | 3 | 5.56 |
| Non‐HGS | 1 | 1.85 |
| Non‐CCC | 1 | 1.85 |
| Non‐serous + non‐mucinous | 1 | 1.85 |
| Age | 4 | 7.40 |
| Stage | 1 | 1.85 |
| Gene | 1 | 1.85 |
| Primary tests for MMRd conducted on unselected patients | ||
| IHC | 12 | 35.29 |
| MSI | 10 | 29.41 |
| GL | 7 | 20.59 |
| IHC + MSI | 2 | 5.88 |
| GL + MSI | 1 | 2.94 |
| GL + somatic | 1 | 2.94 |
| IHC + MSI + somatic | 1 | 2.94 |
4.2. Study characteristics
In total 17 532 OCs were reported in the 54 studies (see Table S4). 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 Geographically there was good representation with Europe (n = 16), Australasia (n = 1), North America (n = 26), Asia (n = 9) and multicontinental (n = 2) studies reported. No studies from South America were found. Twenty studies preselected their population on either age (n = 4), stage of disease (n = 1), histology (n = 14) or being path_BRCA negative and being <40 years old (n = 1). The mean age of participants was 52 years, however, not all articles (n = 36) reported this parameter. Narrative synthesis was performed for the remaining demographics. In total, 46 articles reported histology breakdown. articles in which all histoypes were reported (n = 33) of, on average, 53% were high grade serous, 18% were endometrioid, 14% were clear cell, 1% were low grade serous and 13% were of other histoypes. Regarding FIGO stage, 49% and 51% were stage I‐II and III‐IV, respectively. Too few articles (n = 3) reported ethnicity to draw a meaningful average. The risk of bias results are displayed in Figure S1, of note no study was free from a degree of bias.
4.3. Immunohistochemistry
In total 7086 OCs underwent IHC analysis. 23 , 24 , 25 , 31 , 32 , 37 , 38 , 39 , 42 , 45 , 46 , 47 , 49 , 50 , 53 , 54 , 55 , 56 , 58 , 60 , 61 , 66 , 72 , 75 , 76 The average age of MMRd OC by IHC was 48 years old; these data come from 13 studies. 24 , 32 , 33 , 36 , 37 , 39 , 43 , 45 , 49 , 61 , 66 , 75 , 76 This compares to an average age of 61 years in those whose OCs' were MMR proficient. Most studies (n = 23) used tissue microarrays (TMA) for analysis 23 , 24 , 25 , 31 , 32 , 37 , 38 , 39 , 42 , 45 , 46 , 47 , 49 , 50 , 53 , 54 , 55 , 56 , 58 , 60 , 61 , 66 , 72 , 75 , 76 or a combination of TMA and whole slides. 41 , 43 Only seven reported solely using whole slides in their analysis. 26 , 29 , 33 , 34 , 36 , 64 , 71 In two studies it was not clear if they used TMAs or whole slides. 50 , 75
Thirty studies were included in the IHC meta‐analysis as those studies that only performed IHC as a secondary test were excluded. 38 , 49 , 50 There was a wide variation in the rate of MMRd by IHC, ranging from 0.3% to 29% (see Figure S2). Overall, the estimated rate of OC that demonstrate MMRd by IHC was 6.7% (95% CI: 4.7%‐9.4%). Interestingly, the rates of MMRd by IHC was higher in unselected populations than selected at 7.4% vs 6.2%, respectively. These data are summarized in Figure 2. There was a significant degree of heterogeneity between studies (I 2 = 93%), which was not adequately explained by subgrouping according to selection into the study (Q = 0.26, df = 1, P = .61).
FIGURE 2.
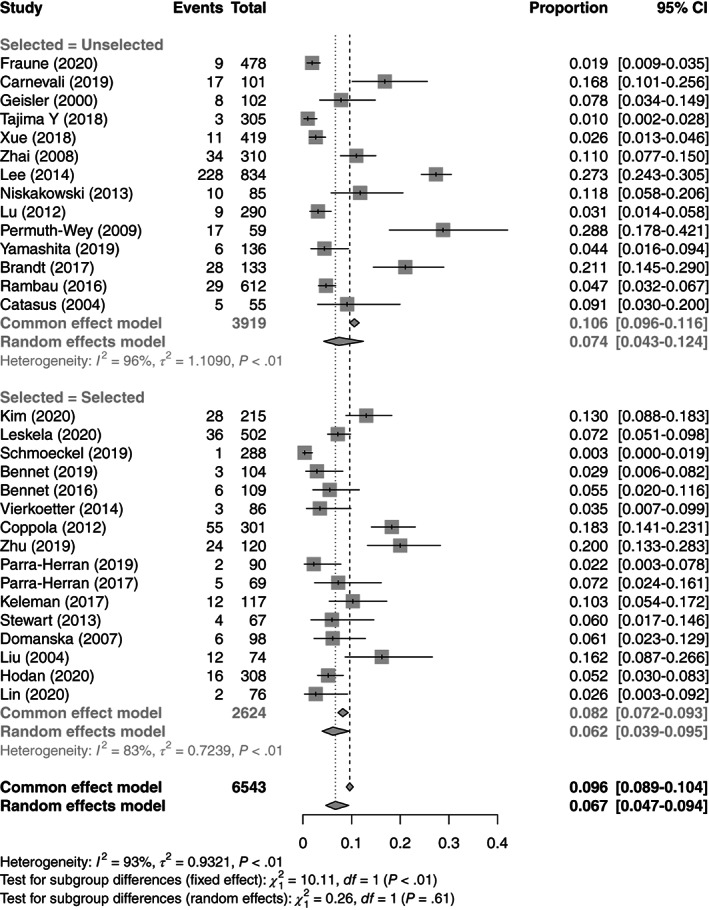
Forest plot for meta‐analysis of test positivity rate for IHC
There were 29 studies that gave information on the specific protein loss; however, 10 of these studies did not test for all four proteins. 25 , 29 , 37 , 38 , 41 , 47 , 50 , 56 , 71 , 72 Furthermore, two studies did not indicate if loss was isolated or in a dimeric pair. 39 , 60 Of note Zhu et al 53 reported isolated loss of MLH1 and MSH2 which is unusual given the literature on the ability to use a two‐antibody screen in MMRd using PMS2 and MSH6. 77 , 78 In addition, Xue et al reported isolated MLH1 loss, however it was not described as absent but as “low expression.” 34 Therefore 19 studies had sufficient information to describe MMR protein expression in OC. 23 , 24 , 26 , 31 , 32 , 33 , 34 , 36 , 42 , 45 , 49 , 53 , 54 , 58 , 61 , 64 , 66 , 75 , 76 These studies represent 3987 OCs of which 3986 had IHC testing of which 215 (5.4%) had MMRd. Of these 9 (4%) were reported as isolated MLH1, 82 (38%) had MLH1/PMS2 loss, 2 (1%) had isolated MSH2, 54 (25%) had isolated MSH6 loss and 68 (32%) had MSH2/MSH6 loss. Reflex MLH1 promotor hypermethylation data was reported in nine studies. 23 , 24 , 25 , 26 , 32 , 33 , 71 , 75 , 76 Of the 53 OCs with MLH1 or MLH1/PMS2 loss 40 (75%) were found to be hypermethylated. Eighteen studies provided information on the FIGO stage in their MMRd IHC cohort. 23 , 24 , 31 , 32 , 33 , 34 , 36 , 37 , 39 , 45 , 47 , 49 , 53 , 55 , 60 , 61 , 71 , 76 In total, 71 (38%) were stage I‐II and 118 (62%) were stage III‐IV. Regarding histotype, 10 unselected studies reported histological data. 25 , 33 , 34 , 42 , 45 , 47 , 49 , 55 , 61 , 71 MMRd was reported in endometrioid, clear cell, high grade serous, low grade serous and other histologies at 57%, 15%, 12%, 1% and 15%, respectively. We estimate that on average 46.7% (95% CI: 28.8‐65.4) of OCs showing MMRd by IHC had a germline path_MMR variant identified. If those not undergoing germline analysis are removed, this becomes 60.5% (95% CI: 39.5‐78.3). This is based on data from nine studies. 23 , 24 , 26 , 32 , 33 , 36 , 39 , 49 , 75 However, many of these studies which were restricted by histology to either clear cell only OCs (n = 2), nonhigh grade serous cancers (n = 2) or endometrioid OC (n = 1). 22 , 24 , 30 , 31 , 34 , 37 , 47 , 73
4.4. Microsatellite instability analysis
In total 5472 OCs underwent MSI analysis in 25 studies. 23 , 25 , 26 , 28 , 31 , 33 , 34 , 35 , 38 , 41 , 42 , 46 , 48 , 49 , 50 , 51 , 55 , 59 , 66 , 67 , 70 , 71 , 73 , 74 , 76 The average age of women with Microsatellite instability high (MSI‐H) OC was 40 years old; these data come from seven studies. 28 , 33 , 35 , 51 , 59 , 66 , 73 In total 16 studies were included in the MSI meta‐analysis as MSI was used as the primary tumor based test. 23 , 26 , 28 , 35 , 38 , 41 , 42 , 48 , 50 , 51 , 59 , 67 , 70 , 71 , 73 , 74 These studies reported a significant range of test positivity rates for MSI, from 0% to 68%. Overall, an estimated 10.4% (95% CI: 6.3‐16.8) of OCs demonstrate MMRd by MSI analysis. There was a significant degree of heterogeneity between studies (I 2 = 90%), which is not adequately explained by subgrouping (Q = 0.18, df = 2, P = .91). When the study by Shilpa et al 38 was excluded, the dropped to 54% and the estimated test positivity rate was 9.4%. Once more, preselection of sample populations had minimal effect on the proportion of MMRd OC. These data are summarized in Figures 3 and S3. Regarding stage and histotype, insufficient studies reported these outcomes limiting any meaningful synthesis. 21 , 24 , 33 We estimate that on average 34.0% (95% CI: 5.9‐81.0) of OCs showing MSI had a germline path_MMR variant identified.
FIGURE 3.
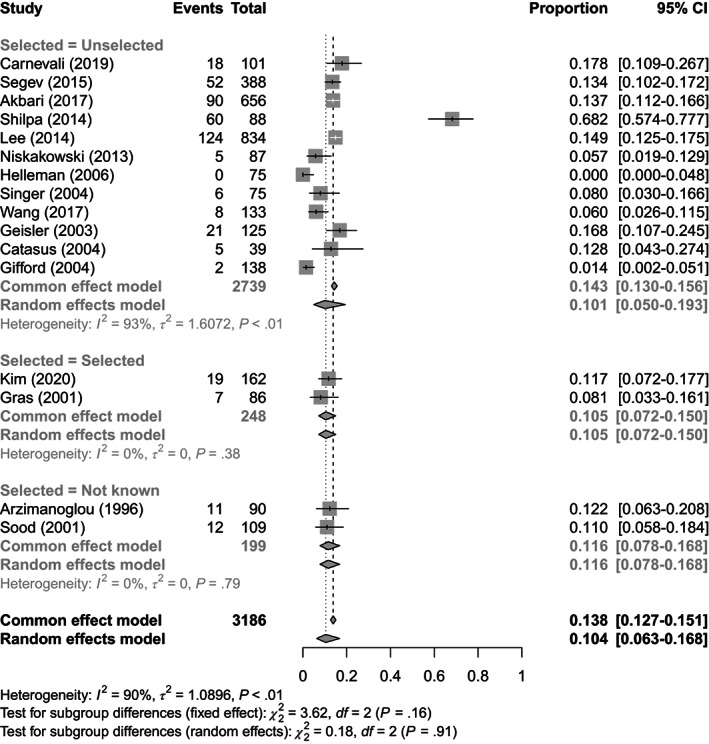
Forest plot for meta‐analysis of test positivity rate for MSI
4.5. Combined immunohistochemistry and microsatellite analysis
A small number of studies conducted IHC and MSI on all OCs (or an unselected subsample). 23 , 26 , 41 , 42 , 71 Of note, most of these studies were unselected. 26 , 41 , 42 , 71 These studies generally show similar test positivity rates, although the study by Lee et al 41 shows considerably more MMRd by IHC than MSI. Our study specifically found that of 270 discordant cases, 83 were MSI‐H with no loss of expression, while 187 were microsatellite stable (MSS) with loss of IHC expression. In studies with concurrent MSI and IHC, the proportions of MMRd OC were higher than in those studies in which MMRd was defined by one modality. Specifically, the proportion of MSI MMRd OC was 14% vs 10% and the proportion of IHC MMRd in OC was 15.7% vs 6.7%. These data are summarized in Figure 4.
FIGURE 4.
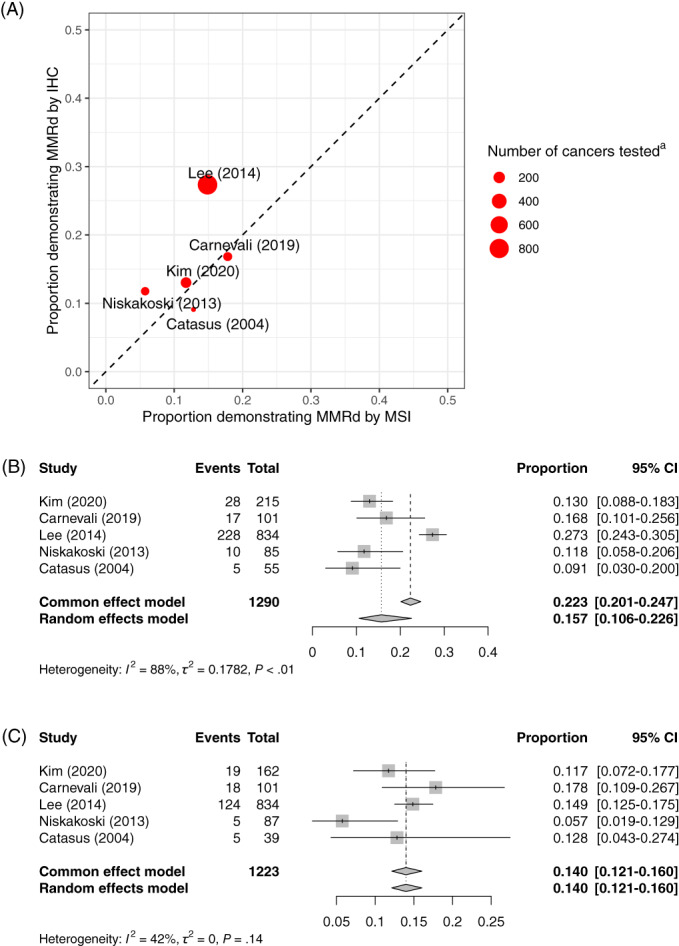
Combined IHC and MSI analysis. (A) Studies conducting MSI and IHC. (B) Forest plot for meta‐analysis of test positivity rate for IHC restricting to studies where unselected MSI was also conducted. (C) Forest plot for meta‐analysis of test positivity rate for MSI restricting to studies where unselected IHC was also conducted. aThis is the number of ovarian cancers tested by MSI or IHC (whichever is lower) [Color figure can be viewed at wileyonlinelibrary.com]
4.6. Somatic analysis
Due to the limited number data for somatic analysis, a descriptive synthesis was performed. Four studies conducted somatic MMR mutation analysis. 24 , 26 , 27 , 33 Carnevali et al 26 identified 3 (3.0%) OCs with somatic path_MMR out of 101 OC evaluated. Sugino et al 27 identified 11 (5.3%) OCs with somatic path_MMR out of 207 cancers evaluated. Tajima et al 33 identified 2 OCs with somatic path_MMR out of 3 OCs evaluated. Leskela et al 24 conducted somatic MMR mutation analysis on 17 ovarian cancers but results were not reported.
4.7. Germline analysis
In total 10 826 OCs in 21 studies underwent some form of germline analysis 23 , 24 , 26 , 27 , 30 , 32 , 33 , 35 , 36 , 39 , 40 , 44 , 49 , 52 , 57 , 62 , 63 , 65 , 68 , 69 , 75 ; of these, nine preselected their population. 23 , 24 , 30 , 32 , 36 , 39 , 44 , 65 , 75 These studies originated from Europe (n = 6), North America (n = 11), Asia (n = 3) and multicontinental (n = 1). In total 9114 underwent germline analysis in which 122 (1.3%) germline path_MMR were identified of which 19 (16%) were path_MLH1, 37 (30%) were path_MSH2, 52 (43%) were path_MSH6 and 14 (11%) were path_PMS2. Nine studies, representing 4993 OCs, reported variants of unknown significance. 23 , 33 , 39 , 44 , 52 , 62 , 65 , 68 , 75 In total 44 (0.9%) variants of unknown significance were found. A meta‐analysis of studies with near complete germline analysis in an unselected population is displayed in Figure 5. Among unselected OC cases the prevalence of germline path_MMR was 0.83% (95% CI: 0.52%‐1.3%). Considering studies in which MSI and/or IHC were conducted and where germline testing was not universal (ie, a situation more like current clinical practice with colorectal and endometrial cancer), we see a yield of germline path_MMR among those tested as shown in Figure S4. In these studies, there were generally not many cancers subjected to germline mutation testing, but around half of those tested were found to have germline path_MMR.
FIGURE 5.
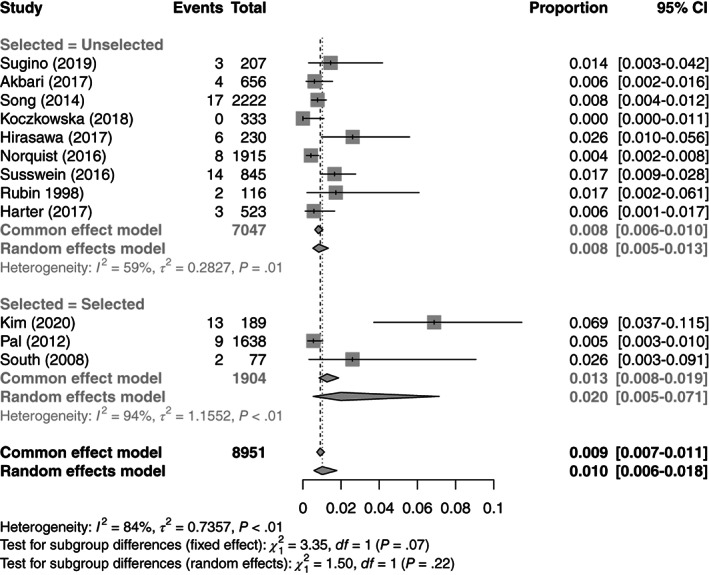
Meta‐analysis of the prevalence of germline path_MMR in studies including an unselected population of ovarian cancers and conducting (near‐) universal germline testing
Ten studies reported that those who had a germline path_MMR also had a significant family history. 23 , 33 , 35 , 36 , 40 , 44 , 49 , 57 , 65 , 68 The percentage of germline path_MMR ranged from 0% to 100% with an average of 50%. Twelve studies reported the FIGO stage of OCs with a germline path_MMR. 23 , 24 , 27 , 32 , 33 , 35 , 36 , 39 , 49 , 52 , 57 , 62 Of note, out of the 77 OCs with stage information and a germline path_MMR, 62 (81%) were FIGO stage I‐II. Eighteen studies reported the histotype of OCs with a germline path_MMR. 23 , 24 , 26 , 27 , 30 , 32 , 33 , 35 , 36 , 39 , 40 , 44 , 49 , 52 , 57 , 62 , 69 , 75 These articles included 268 high grade serous, 1629 endometrioid, 1097 clear cell and 1300 other histotypes. Out of 137 OCs with a germline path_MMR, 79 (58%) were endometroid 21 (15%) were high grade serous, 23 (17%) were clear cell and 14 (10%) were found in another histotype. In unselected studies with complete germline testing, 27 , 35 , 40 , 52 , 57 , 62 , 69 2% of endometrioid, 0.4% high grade serous and 1% of clear cell OCs had a germline path_MMR.
4.8. Sensitivity analysis
Results of meta‐analyses using the fixed effects model are shown in Figures 2, 3, 4 as “Common effects.” For IHC and MSI these tend to show higher average prevalence of MMRd but given the high degree of heterogeneity it is unlikely that the fixed effects model is appropriate.
The results of meta‐analyses using the inverse variance approach and Freeman‐Tukey double arcsine transforms are shown in Table S2. These show minimal differences with the generalized linear modeling approach.
5. COMMENT
5.1. Main findings
To the authors' knowledge, we present the most comprehensive review of MMRd in OC. In total, 54 studies were included which detailed MMRd analysis in 17 532 OCs. These data indicate 7% or 10% of OCs are MMRd by IHC or MSI, respectively, although studies where both techniques are used do not suggest that one technique is superior. These data support the existing literature that both IHC and MSI can be used to define MMRd in OC. 79 This is clinically significant as these cancers would potentially be amenable to ICPIs; a treatment that has been shown be highly effective in solid cancers with MMRd. 10 Given the poor survival seen in OC, being able to target effective treatments is a clinical priority. In addition, these data would suggest around 1% to 5% of women have Lynch syndrome; although of note, those studies with universal germline testing estimate the prevalence of Lynch syndrome to be closer to 1%. Finding these women is important as they are at risk of synchronous and metachronous cancers; a risk the vast majority are unaware of. 80 Once diagnosed, those with Lynch syndrome can be enrolled in risk reducing strategies such as routine colonoscopy and aspirin prophylaxis. 81 Furthermore, cascade testing of the index case's relatives identifies, on average, a further three Lynch syndrome carriers. 82 These individuals can also benefit from risk reducing strategies that could prevent them from ever developing cancer. 83 Our data would suggest that 47% of those women found to have MMRd OC by either MSI or IHC went on to be diagnosed with Lynch syndrome. This high proportion could have implication for consenting; currently tumor testing does not require pretesting consent, 84 however given this high conversion rate clinicians may wish to mention Lynch syndrome testing before tumor analysis.
Of note, our data would, at first look, suggest that germline path_MSH6 carriers are at the most risk of OC, given it is the most common gene affected in our OC cohort. However, MSH6 is the gene most commonly affected in those with Lynch syndrome and therefore, the higher proportion of germline path_MSH6 in OC simply reflects its prevalence in those with Lynch syndrome. 85 This is demonstrated in population data in which, out of 50 703 healthy individuals tested for germline path_MMR, 9, 13, 23 and 36 had path_MLH1, path_MSH2, path_MSH6 and path_PMS2, respectively. 86 The low number of path_PMS2 in our data can be explained by the weaker association between this gene and OC when compared to other Lynch syndrome causative gene loci. 87
Our data would suggest the use of MSI or IHC leads to a similar rate of MMRd detection. The use of IHC does give additional information, namely the specific protein that is deficient, which can be useful in the interpretation of variant analysis. Furthermore, if maximizing MMRd yield is the priority, our data would not support the preselection of testing populations. It would seem MMRd is seen in all histotypes of OC, however, is common in endometrioid and rare in low grade serous cancers. Interestingly, the preselection of populations on clinical criteria, such as age or family history, did not significantly improve the yield of MMRd OCs and may miss a significant number of OCs that could be amenable to ICPIs. Therefore, population preselection does not seem beneficial.
5.2. Strengths and limitations
Our study has several key strengths. First, our search strategy was designed to capture all the relevant literature. This means our conclusions are based on the results of 54 studies and 17 532 OCs. The systematic review and meta‐analysis followed PRISMA guidelines. 18 Both article screening, risk of bias assessment and data extraction was performed by at least two independent reviewers. Meta‐analyses were conducted using appropriate methods for the type of data collected, and multiple sensitivity analyses were conducted to ensure the robustness of results.
However, there are certain limitations in our review approach and in the body of evidence identified. We took results as reported and did not seek to review original study data. We note a high degree of risk of bias within studies. Studies also used mixed methods such as TMAs vs whole slide analysis and different MSI assays. We have not attempted to identify publication bias (eg, smaller studies finding very low rates of MMRd in OC may not be published, or the rates of MMRd may not be reported when other characteristics are reported). In addition, incomplete testing led to incomplete data sets which were difficult to incorporate into our analysis. Furthermore, many studies were retrospective and used historical cohorts. The lack of a “gold standard” test for MMRd in OC makes accuracy estimates difficult. Our studies span a wide time frame (1998‐2020), during which diagnostic technologies have changed; this could impact on prevalence estimates. However, this would minimally affect IHC which has remained consistent. In addition, because most studies that use MSI, and sequencing technology are from 2015 or later, the impact should be limited. Finally, the data has a bias towards Western Caucasian populations which makes the generalizability to other populations less robust. This could have been further compounded by our pragmatic decision to only include studies written in English. Germline testing has implications for both the index case and their family. Due to the implications of a Lynch syndrome diagnosis in insurance‐based health care systems, women may have declined to take part in prospective studies in insurance‐based health care populations; this could potentially limit the validity of our germline results. 88 These issues are reflected in our wide confidence intervals around our estimated prevalence for MMRd in OC and in the high scores. Where possible we tried to mitigate this by performing subgroup analysis. This heterogeneity and the need to include low quality studies limits the strength of our conclusions.
5.3. Comparison with existing literature
Regarding those with a germline MMRd, our data found OCs in this population seem to be diagnosed at stage I or II disease. This has been reported before in Lynch syndrome populations. 89 This is an interesting finding as most OCs are diagnosed at a more advanced stage due to a diagnostic difficultly because of no screening test 90 and no pathognomonic symptom. 91 This finding could speak to Lynch syndrome associated OCs having a different biology. Indeed, it is known these cancers have an immunogenic profile, which limits their ability to metastasize. 92 These findings warrant more exploration in future studies.
Our data cannot speak to the effectiveness of ICPIs in MMRd OCs as this was not our aim. However, it is known these agents are highly effective in solid cancers with MMRd. 93 Their clinical utility in MMRd cancers is such that they were the first chemotherapeutic to receive FDA approval based on a molecular feature within a cancer as opposed to the anatomical origin of the cancer. 94 Yet these agents have been used in OC with limited success. 95 What is known is that ICPIs have a low toxicity profile in women with OC. 96 The JAVELIN ovarian 100 study explored the use of avelumab both in combination with standard chemotherapy and as a maintenance agent. 16 The study was stopped early as it failed to show any benefit for the use of avelumab in either arm; indeed, there was a suggestion of a detrimental effect. The NINJA study also compared an ICPI with standard chemotherapy; again, the authors failed to demonstrate an improvement in overall or progression free survival. 97 However, neither of these studies selected OC with MMRd and therefore it is not known if ICPIs would be of benefit in a MMRd OC population. The JAVELIN Ovarian 200 study also explored the potential benefit of ICPI use in OC and once more failed to demonstrate a survival benefit. 98 However, the authors did suggest that the use of biomarkers (CD8 and PD‐L1 expression) to select OC as candidates for ICPIs may aid in finding women who would gain a survival benefit from ICPI use; however, the study was underpowered to fully explore this. MMRd cancers are known to express higher levels of PD‐L1 and CD8. 92 Our data shows that MMRd testing in OC is possible. What is more, it is more prevalent than previously thought. Therefore, trials exploring checkpoint inhibition in MMRd OC should be considered.
5.4. Conclusions and implications
In summary, we present the most comprehensive systematic review and meta‐analysis exploring MMRd in OC. We found that a significant minority (up to 16%) of OC displays MMRd and therefore could be amenable to ICPIs. However, the current literature base is of limited quality and therefore high‐quality prospective studies exploring MMRd in OC with the use of multimodal testing are required. In addition, trials looking at the efficacy of check point inhibition in MMRd OC are needed.
AUTHOR CONTRIBUTIONS
Conception and design: Neil A. J. Ryan, Claire Newton, D. Gareth Evans and Emma J. Crosbie. Financial support: None. Collection and assembly of data: Neil A. J. Ryan, Amit Atwal, Thomas Krum and Marcus Cabrera Dandy. Data analysis and interpretation: Neil A. J. Ryan and Tristan Snowsill. Article writing: Neil A. J. Ryan and Amit Atwal. Final approval of article: All authors. The work reported in the article has been performed by the authors, unless clearly specified in the text.
FUNDING INFORMATION
No specific funding was used for our study. Emma J. Crosbie is supported by the NIHR Manchester Biomedical Research Centre (IS‐BRC‐1215‐20007) and an NIHR Advanced Fellowship (NIHR300650). D Gareth Evans is an NIHR Senior Investigator (NF‐SI‐0513‐10076).
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
ETHICS STATEMENT
All studies were approved by the respective institutional review boards and conducted with appropriate ethical criteria in each country and in accordance with the Declaration of Helsinki.
Supporting information
Appendix S1 Supporting Information.
ACKNOWLEDGEMENTS
We would like to thank the University of Bristol's Medical School library for helping devise and conduct the search that informed this work.
Atwal A, Snowsill T, Dandy MC, et al. The prevalence of mismatch repair deficiency in ovarian cancer: A systematic review and meta‐analysis. Int. J. Cancer. 2022;151(9):1626‐1639. doi: 10.1002/ijc.34165
Funding information NIHR, Grant/Award Number: NF‐SI‐0513‐10076; NIHR Advanced Fellowship, Grant/Award Number: NIHR300650; NIHR Manchester Biomedical Research Centre, Grant/Award Number: IS‐BRC‐1215‐20007
DATA AVAILABILITY STATEMENT
All data contained here within this article is available on request from the corresponding author.
REFERENCES
- 1. Lheureux S, Gourley C, Vergote I, Oza AM. Epithelial ovarian cancer. Lancet. 2019;393:1240‐1253. [DOI] [PubMed] [Google Scholar]
- 2. Torre LA, Trabert B, DeSantis CE, et al. Ovarian cancer statistics, 2018. CA Cancer J Clin. 2018;68:284‐296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jacobs IJ, Menon U, Ryan A, et al. Ovarian cancer screening and mortality in the UKcollaborative trial of ovarian cancer screening (UKCTOCS): a randomised controlled trial. Lancet. 2016;387:945‐956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lheureux S, Braunstein M, Oza AM. Epithelial ovarian cancer: evolution of management in the era of precision medicine. CA Cancer J Clin. 2019;69:280‐304. [DOI] [PubMed] [Google Scholar]
- 5. Ramchander NC, Ryan NAJ, Walker TDJ, et al. Distinct immunological landscapes characterize inherited and sporadic mismatch repair deficient endometrial cancer. Front Immunol. 2020;10:3023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Swann JB, Smyth MJ. Immune surveillance of tumors. J Clin Invest. 2007;117:1137‐1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Darvin P, Toor SM, Nair VS, Elkord E. Immune checkpoint inhibitors: recent progress and potential biomarkers. Exp Mol Med. 2018;50:165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Liu J, Chen Z, Li Y, Zhao W, Wu J, Zhang Z. PD‐1/PD‐L1 checkpoint inhibitors in tumor immunotherapy. Front Pharmacol. 2021;12:731798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wilden SM, Lang BM, Mohr P, Grabbe S. Immune checkpoint inhibitors: a milestone in the treatment of melanoma: immune checkpoint inhibitors in the treatment of melanoma. J Dtsch Dermatol Ges. 2016;14:685‐695. [DOI] [PubMed] [Google Scholar]
- 10. Le DT, Uram JN, Wang H, et al. PD‐1 blockade in tumors with mismatch‐repair deficiency. N Engl J Med. 2015;372:2509‐2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Marcus L, Lemery SJ, Keegan P, Pazdur R. FDA approval summary: pembrolizumab for the treatment of microsatellite instability‐high solid tumors. Clin Cancer Res. 2019;25:3753‐3758. [DOI] [PubMed] [Google Scholar]
- 12. Hamanishi J, Mandai M, Ikeda T, et al. Safety and antitumor activity of anti‐PD‐1 antibody, nivolumab, in patients with platinum‐resistant ovarian cancer. J Clin Oncol. 2015;33:4015‐4022. [DOI] [PubMed] [Google Scholar]
- 13. Varga A, Piha‐Paul S, Ott PA, et al. Pembrolizumab in patients with programmed death ligand 1‐positive advanced ovarian cancer: analysis of KEYNOTE‐028. Gynecol Oncol. 2018;152:243‐250. [DOI] [PubMed] [Google Scholar]
- 14. Disis ML, Taylor MH, Kelly K, et al. Efficacy and safety of avelumab for patients with recurrent or refractory ovarian cancer: phase 1b results from the JAVELIN solid tumor trial. JAMA Oncol. 2019;5:393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Matulonis UA, Shapira‐Frommer R, Santin AD, et al. Antitumor activity and safety of pembrolizumab in patients with advanced recurrent ovarian cancer: results from the phase II KEYNOTE‐100 study. Ann Oncol. 2019;30:1080‐1087. [DOI] [PubMed] [Google Scholar]
- 16. Monk BJ, Colombo N, Oza AM, et al. Chemotherapy with or without avelumab followed by avelumab maintenance versus chemotherapy alone in patients with previously untreated epithelial ovarian cancer (JAVELIN ovarian 100): an open‐label, randomised, phase 3 trial. Lancet Oncol. 2021;22:1275‐1289. [DOI] [PubMed] [Google Scholar]
- 17. Rubinstein MM, Makker V. Optimizing immunotherapy for gynecologic cancers. Curr Opin Obstet Gynecol. 2020;32:1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Turner RM, Bird SM, Higgins JPT. The impact of study size on meta‐analyses: examination of underpowered studies in Cochrane reviews. PLoS One. 2013;8:e59202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. McGuinness LA, Higgins JPT. Risk‐of‐bias VISualization (robvis): an R package and shiny web app for visualizing risk‐of‐bias assessments. Res Synth Methods. 2020;12:55‐61. [DOI] [PubMed] [Google Scholar]
- 21. Whiting PF, Rutjes AWS, Westwood ME, et al. Group Q‐2. QUADAS‐2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155:529. [DOI] [PubMed] [Google Scholar]
- 22. Balduzzi S, Rücker G, Schwarzer G. How to perform a meta‐analysis with R: a practical tutorial. Evid Based Ment Health. 2019;22:153‐160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kim SR, Tone A, Kim RH, et al. Performance characteristics of screening strategies to identify Lynch syndrome in women with ovarian cancer. Cancer. 2020;126:4886‐4894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Leskela S, Romero I, Cristobal E, et al. Mismatch repair deficiency in ovarian carcinoma: frequency, causes, and consequences. Am J Surg Pathol. 2020;44:649‐656. [DOI] [PubMed] [Google Scholar]
- 25. Fraune C, Rosebrock J, Simon R, et al. High homogeneity of MMR deficiency in ovarian cancer. Gynecol Oncol. 2020;156:669‐675. [DOI] [PubMed] [Google Scholar]
- 26. Carnevali I, Riva C, Chiaravalli AM, et al. Inherited cancer syndromes in 220 Italian ovarian cancer patients. Cancer Genet. 2019;237:55‐62. [DOI] [PubMed] [Google Scholar]
- 27. Sugino K, Tamura R, Nakaoka H, et al. Germline and somatic mutations of homologous recombination‐associated genes in Japanese ovarian cancer patients. Sci Rep. 2019;9:17808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Segev Y, Zhang S, Akbari MR, et al. Survival in women with ovarian cancer with and without microsatellite instability. Eur J Gynaecol Oncol. 2015;36:681‐684. [PubMed] [Google Scholar]
- 29. Geisler JP, Geisler HE, Miller GA, Wiemann MC, Zhou Z, Crabtree W. Immunohistochemical staining of the mismatch repair gene, hMSH2, and survival in patients with ovarian carcinoma. Eur J Gynaecol Oncol. 2000;21:237‐240. [PubMed] [Google Scholar]
- 30. Stratton JF, Thompson D, Bobrow L, et al. The genetic epidemiology of early‐onset epithelial ovarian cancer: a population‐based study. Am J Hum Genet. 1999;65:1725‐1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Schmoeckel E, Hofmann S, Fromberger D, et al. Comprehensive analysis of PD‐L1 expression, HER2 amplification, ALK/EML4 fusion, and mismatch repair deficiency as putative predictive and prognostic factors in ovarian carcinoma. Virchows Arch. 2019;474:599‐608. [DOI] [PubMed] [Google Scholar]
- 32. Bennett JA, Pesci A, Morales‐Oyarvide V, Silva AD, Nardi V, Oliva E. Incidence of mismatch repair protein deficiency and associated clinicopathologic features in a cohort of 104 ovarian endometrioid carcinomas. Am J Surg Pathol. 2019;43:235‐243. [DOI] [PubMed] [Google Scholar]
- 33. Tajima Y, Eguchi H, Chika N, et al. Prevalence and molecular characteristics of defective mismatch repair epithelial ovarian cancer in a Japanese hospital‐based population. Jpn J Clin Oncol. 2018;48:728‐735. [DOI] [PubMed] [Google Scholar]
- 34. Xiao X, Dong D, He W, et al. Mismatch repair deficiency is associated with MSI phenotype, increased tumor‐infiltrating lymphocytes and PD‐L1 expression in immune cells in ovarian cancer. Gynecol Oncol. 2018;149:146‐154. [DOI] [PubMed] [Google Scholar]
- 35. Akbari MR, Zhang S, Cragun D, et al. Correlation between germline mutations in MMR genes and microsatellite instability in ovarian cancer specimens. Fam Cancer. 2017;16:351‐355. [DOI] [PubMed] [Google Scholar]
- 36. Bennett JA, Morales‐Oyarvide V, Campbell S, Longacre TA, Oliva E. Mismatch repair protein expression in clear cell carcinoma of the ovary: incidence and morphologic associations in 109 cases. Am J Surg Pathol. 2016;40:656‐663. [DOI] [PubMed] [Google Scholar]
- 37. Zhai QJ, Rosen DG, Lu K, Liu J. Loss of DNA mismatch repair protein hMSH6 in ovarian cancer is histotype‐specific. Int J Clin Exp Pathol. 2008;1:502‐509. [PMC free article] [PubMed] [Google Scholar]
- 38. Shilpa V, Bhagat R, Premalata S, Pallavi R, Krishnamoorthy L. Microsatellite instability, promoter methylation and protein expression of the DNA mismatch repair genes in epithelial ovarian cancer. Genomics. 2014;104:257‐263. [DOI] [PubMed] [Google Scholar]
- 39. Vierkoetter KR, Ayabe AR, VanDrunen M, Ahn HJ, Shimizu DM, Terada KY. Lynch syndrome in patients with clear cell and endometrioid cancers of the ovary. Gynecol Oncol. 2014;135:81‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Song H, Cicek MS, Dicks E, et al. The contribution of deleterious germline mutations in BRCA1, BRCA2 and the mismatch repair genes to ovarian cancer in the population. Hum Mol Genet. 2014;23:4703‐4709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lee J‐H, Cragun D, Thompson Z, et al. Association between IHC and MSI testing to identify mismatch repair‐deficient patients with ovarian cancer. Genet Test Mol Biomarkers. 2014;18:229‐235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Niskakoski A, Kaur S, Renkonen‐Sinisalo L, et al. Distinct molecular profiles in Lynch syndrome‐associated and sporadic ovarian carcinomas. Int J Cancer. 2013;133:2596‐2608. [DOI] [PubMed] [Google Scholar]
- 43. Coppola D, Nicosia SV, Doty A, et al. Uncertainty in the utility of immunohistochemistry in mismatch repair protein expression in epithelial ovarian cancer. Anticancer Res. 2012;32:4963‐4969. [PMC free article] [PubMed] [Google Scholar]
- 44. Pal T, Akbari MR, Sun P, et al. Frequency of mutations in mismatch repair genes in a population‐based study of women with ovarian cancer. Br J Cancer. 2012;107:1783‐1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lu F‐I, Gilks CB, Mulligan A‐M, et al. Prevalence of loss of expression of DNA mismatch repair proteins in primary epithelial ovarian tumors. Int J Gynecol Pathol. 2012;31:524‐531. [DOI] [PubMed] [Google Scholar]
- 46. Fuseya C, Horiuchi A, Hayashi A, et al. Involvement of pelvic inflammation‐related mismatch repair abnormalities and microsatellite instability in the malignant transformation of ovarian endometriosis. Hum Pathol. 2012;43:1964‐1972. [DOI] [PubMed] [Google Scholar]
- 47. Permuth‐Wey J, Boulware D, Valkov N, et al. Sampling strategies for tissue microarrays to evaluate biomarkers in ovarian cancer. Cancer Epidemiol Biomarkers Prev. 2009;18:28‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Helleman J, van Staveren IL, Dinjens WN, et al. Mismatch repair and treatment resistance in ovarian cancer. BMC Cancer. 2006;6:201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Malander S, Rambech E, Kristoffersson U, et al. The contribution of the hereditary nonpolyposis colorectal cancer syndrome to the development of ovarian cancer. Gynecol Oncol. 2006;101:238‐243. [DOI] [PubMed] [Google Scholar]
- 50. Singer G, Kallinowski T, Hartmann A, et al. Different types of microsatellite instability in ovarian carcinoma. Int J Cancer. 2004;112:643‐646. [DOI] [PubMed] [Google Scholar]
- 51. Arzimanoglou II, Lallas T, Osborne M, Barber H, Gilbert F. Microsatellite instability differences between familial and sporadic ovarian cancers. Carcinogenesis. 1996;17:1799‐1804. [DOI] [PubMed] [Google Scholar]
- 52. Koczkowska M, Krawczynska N, Stukan M, et al. Spectrum and prevalence of pathogenic variants in ovarian cancer susceptibility genes in a group of 333 patients. Cancer. 2018;10:442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zhu J, Ke G, Bi R, Wu X. Clinicopathological and survival characteristic of mismatch repair status in ovarian clear cell carcinoma. J Surg Oncol. 2020;122:538‐546. [DOI] [PubMed] [Google Scholar]
- 54. Parra‐Herran C, Bassiouny D, Lerner‐Ellis J, et al. p53, mismatch repair protein, and POLE abnormalities in ovarian clear cell carcinoma: an outcome‐based clinicopathologic analysis. Am J Surg Pathol. 2019;43:1591‐1599. [DOI] [PubMed] [Google Scholar]
- 55. Yamashita H, Nakayama K, Ishikawa M, et al. Relationship between microsatellite instability, immune cells infiltration, and expression of immune checkpoint molecules in ovarian carcinoma: immunotherapeutic strategies for the future. Int J Mol Sci. 2019;20:5129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Brandt S, Samartzis EP, Zimmermann A‐K, et al. Lack of MRE11‐RAD50‐NBS1 (MRN) complex detection occurs frequently in low‐grade epithelial ovarian cancer. BMC Cancer. 2017;17:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hirasawa A, Imoto I, Naruto T, et al. Prevalence of pathogenic germline variants detected by multigene sequencing in unselected Japanese patients with ovarian cancer. Oncotarget. 2017;8:112258‐112267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Parra‐Herran C, Lerner‐Ellis J, Xu B, et al. Molecular‐based classification algorithm for endometrial carcinoma categorizes ovarian endometrioid carcinoma into prognostically significant groups. Mod Pathol. 2017;30(12):1748‐1759. [DOI] [PubMed] [Google Scholar]
- 59. Wang YK, Bashashati A, Anglesio MS, et al. Genomic consequences of aberrant DNA repair mechanisms stratify ovarian cancer histotypes. Nat Genet. 2017;49:856‐865. [DOI] [PubMed] [Google Scholar]
- 60. Kelemen LE, Rambau PF, Koziak JM, Steed H, Köbel M. Synchronous endometrial and ovarian carcinomas: predictors of risk and associations with survival and tumor expression profiles. Cancer Causes Control. 2017;28:447‐457. [DOI] [PubMed] [Google Scholar]
- 61. Rambau PF, Duggan MA, Ghatage P, et al. Significant frequency of MSH2/MSH6 abnormality in ovarian endometrioid carcinoma supports histotype‐specific Lynch syndrome screening in ovarian carcinomas. Histopathology. 2016;69:288‐297. [DOI] [PubMed] [Google Scholar]
- 62. Norquist BM, Harrell MI, Brady MF, et al. Inherited mutations in women with ovarian carcinoma. JAMA Oncol. 2016;2:482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Susswein LR, Marshall ML, Nusbaum R, et al. Pathogenic and likely pathogenic variant prevalence among the first 10,000 patients referred for next‐generation cancer panel testing. Genet Med. 2015;18:823‐832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Stewart CJR, Walsh MD, Budgeon CA, Crook ML, Buchanan DB. Immunophenotypic analysis of ovarian endometrioid adenocarcinoma: correlation with KRAS mutation and the presence of endometriosis. Pathology. 2013;45:559‐566. [DOI] [PubMed] [Google Scholar]
- 65. South SA, Vance H, Farrell C, et al. Consideration of hereditary nonpolyposis colorectal cancer in BRCA mutation‐negative familial ovarian cancers. Cancer. 2009;115:324‐333. [DOI] [PubMed] [Google Scholar]
- 66. Domanska K, Malander S, Måsbäck A, Nilbert M. Ovarian cancer at young age: the contribution of mismatch‐repair defects in a population‐based series of epithelial ovarian cancer before age 40. Int J Gynecol Cancer. 2007;17:789‐793. [DOI] [PubMed] [Google Scholar]
- 67. Geisler JP, Goodheart MJ, Sood AK, Holmes RJ, Hatterman‐Zogg MA, Buller RE. Mismatch repair gene expression defects contribute to microsatellite instability in ovarian carcinoma. Cancer. 2003;98:2199‐2206. [DOI] [PubMed] [Google Scholar]
- 68. Rubin SC, Blackwood MA, Bandera C, et al. BRCA1, BRCA2, and hereditary nonpolyposis colorectal cancer gene mutations in an unselected ovarian cancer population: relationship to family history and implications for genetic testing. Am J Obstet Gynecol. 1998;178:670‐677. [DOI] [PubMed] [Google Scholar]
- 69. Harter P, Hauke J, Heitz F, et al. Prevalence of deleterious germline variants in risk genes including BRCA1/2 in consecutive ovarian cancer patients (AGO‐TR‐1). PLoS One. 2017;12:e0186043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Gras E, Catasus L, Argüelles R, et al. Microsatellite instability, MLH‐1 promoter hypermethylation, and frameshift mutations at coding mononucleotide repeat microsatellites in ovarian tumors. Cancer. 2001;92:2829‐2836. [DOI] [PubMed] [Google Scholar]
- 71. Catasús L, Bussaglia E, Rodríguez I, et al. Molecular genetic alterations in endometrioid carcinomas of the ovary: similar frequency of beta‐catenin abnormalities but lower rate of microsatellite instability and PTEN alterations than in uterine endometrioid carcinomas. Hum Pathol. 2004;35:1360‐1368. [DOI] [PubMed] [Google Scholar]
- 72. Liu J, Albarracin CT, Chang K‐H, et al. Microsatellite instability and expression of hMLH1 and hMSH2 proteins in ovarian endometrioid cancer. Mod Pathol. 2003;17:75‐80. [DOI] [PubMed] [Google Scholar]
- 73. Sood AK, Holmes R, Hendrix MJ, Buller RE. Application of the National Cancer Institute international criteria for determination of microsatellite instability in ovarian cancer. Cancer Res. 2001;61:4371‐4374. [PubMed] [Google Scholar]
- 74. Gifford G, Paul J, Vasey PA, Kaye SB, Brown R. The acquisition of hMLH1 methylation in plasma DNA after chemotherapy predicts poor survival for ovarian cancer patients. Clin Cancer Res. 2004;10:4420‐4426. [DOI] [PubMed] [Google Scholar]
- 75. Hodan R, Kingham K, Cotter K, et al. Prevalence of Lynch syndrome in women with mismatch repair‐deficient ovarian cancer. Cancer Med. 2020;10:1012‐1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Lin S‐Y, Hang J‐F, Lin Y‐Y, Lai C‐R, Ho H‐L, Chou T‐Y. Diffuse Intratumoral stromal inflammation in ovarian clear cell carcinoma is associated with loss of mismatch repair protein and high PD‐L1 expression. Int J Gynecol Pathol. 2021;40:148‐155. [DOI] [PubMed] [Google Scholar]
- 77. Crim AK, Perkins VB, Husain S, Ding K, Holman LL. Feasibility of two‐antibody vs four‐antibody mismatch repair protein immunohistochemistry as initial screening for Lynch syndrome in patients with endometrial adenocarcinoma. Gynecol Oncol. 2017;145:44. [Google Scholar]
- 78. Mojtahed A, Schrijver I, Ford JM, Longacre TA, Pai RK. A two‐antibody mismatch repair protein immunohistochemistry screening approach for colorectal carcinomas, skin sebaceous tumors, and gynecologic tract carcinomas. Mod Pathol. 2011;24:1004‐1014. [DOI] [PubMed] [Google Scholar]
- 79. Crosbie EJ, Ryan NAJ, McVey RJ, et al. Assessment of mismatch repair deficiency in ovarian cancer. J Med Genet. 2020;58:687‐691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Ryan NA, McMahon RF, Ramchander NC, Seif MW, Evans DG, Crosbie EJ. Lynch syndrome for the gynaecologist. Obstet Gynaecol. 2021;23:9‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Seppälä TT, Latchford A, Negoi I, et al. European guidelines from the EHTG and ESCP for Lynch syndrome: an updated third edition of the Mallorca guidelines based on gene and gender. Br J Surg. 2021;108:484‐498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Ryan NAJ, McMahon R, Tobi S, et al. The proportion of endometrial tumours associated with Lynch syndrome (PETALS): a prospective cross‐sectional study. PLoS Med. 2020;17:e1003263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Schmeler KM, Lynch HT, Chen L, et al. Prophylactic surgery to reduce the risk of gynecologic cancers in the Lynch syndrome. N Engl J Med. 2006;354:261‐269. [DOI] [PubMed] [Google Scholar]
- 84. Crosbie EJ, Ryan NAJ, Arends MJ, et al. The Manchester international consensus group recommendations for the management of gynecological cancers in Lynch syndrome. Genet Med. 2019;21:2390‐2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Ryan NAJ, Morris J, Green K, et al. Association of mismatch repair mutation with age at cancer onset in Lynch syndrome: implications for stratified surveillance strategies. JAMA Oncol. 2017;3:1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Consortium BCA , Dorling L, Carvalho S, et al. Breast cancer risk genes—association analysis in more than 113,000 women. N Engl J Med. 2021;384:428‐439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Møller P, Seppälä TT, Bernstein I, et al. Cancer risk and survival in path_MMR carriers by gene and gender up to 75 years of age: a report from the prospective Lynch syndrome database. Gut. 2017;67:1306‐1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Armstrong K, Calzone K, Stopfer J, Fitzgerald G, Coyne J, Weber B. Factors associated with decisions about clinical BRCA1/2 testing. Cancer Epidemiol Biomarkers Prev. 2000;9:1251‐1254. [PubMed] [Google Scholar]
- 89. Ryan NAJ, Evans DG, Green K, Crosbie EJ. Pathological features and clinical behavior of Lynch syndrome‐associated ovarian cancer. Gynecol Oncol. 2017;144:491‐495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Menon U, Gentry‐Maharaj A, Burnell M, et al. Ovarian cancer population screening and mortality after long‐term follow‐up in the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS): a randomised controlled trial. Lancet. 2021;397:2182‐2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Funston G, O'Flynn H, Ryan NAJ, Hamilton W, Crosbie EJ. Correction to: recognizing gynecological cancer in primary care: risk factors, red flags, and referrals. Adv Ther. 2018;35:590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Rasmussen M, Lim K, Rambech E, et al. Lynch syndrome‐associated epithelial ovarian cancer and its immunological profile. Gynecol Oncol. 2021;162:686‐693. [DOI] [PubMed] [Google Scholar]
- 93. Le DT, Durham JN, Smith KN, et al. Mismatch repair deficiency predicts response of solid tumors to PD‐1 blockade. Science. 2017;357:409‐413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Beaver JA, Tzou A, Blumenthal GM, et al. An FDA perspective on the regulatory implications of complex signatures to predict response to targeted therapies. Clin Cancer Res. 2016;23:1368‐1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. James NE, Woodman M, DiSilvestro PA, Ribeiro JR. The perfect combination: enhancing patient response to PD‐1‐based therapies in epithelial ovarian cancer. Cancer. 2020;12:2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Borella F, Ghisoni E, Giannone G, et al. Immune checkpoint inhibitors in epithelial ovarian cancer: an overview on efficacy and future perspectives. Diagnostics. 2020;10:146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Hamanishi J, Takeshima N, Katsumata N, et al. Nivolumab versus gemcitabine or pegylated liposomal doxorubicin for patients with platinum‐resistant ovarian cancer: open‐label, randomized trial in Japan (NINJA). J Clin Oncol. 2021;39:3671‐3681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Pujade‐Lauraine E, Fujiwara K, Ledermann JA, et al. Avelumab alone or in combination with chemotherapy versus chemotherapy alone in platinum‐resistant or platinum‐refractory ovarian cancer (JAVELIN ovarian 200): an open‐label, three‐arm, randomised, phase 3 study. Lancet Oncol. 2021;22:1034‐1046. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Supporting Information.
Data Availability Statement
All data contained here within this article is available on request from the corresponding author.


