Abstract
Ankylosing spondylitis (AS) is an autoimmune disease affecting parts of the skeletal structure in particular. Previously increased levels of the inflammatory cell types Th17, Th22, Tc17 and Tc22 cells have been shown to be associated with AS. Here, we analysed the levels of inflammatory T cell subsets, related cytokines and clinical characteristics of AS patients vs controls from northern Sweden. Peripheral blood mononuclear cells (PBMCs) obtained from 50 AS patients and 50 matched controls were short term stimulated with PMA/Ionomycin, stained and analysed by flow cytometry. Plasma levels of Interleukin (IL)‐17, IL‐22, IL‐10 as well as clinically relevant markers were determined. Compared to male controls, male AS patients showed 1.5‐ to 2‐fold increases of Th17 (P = .013), Th22 (P = .003) and Tc22 (P = .024) among CD45+CD3+ lymphocytes. Plasma IL‐22 levels correlated with the Tc17 proportion in male patients (Rs = 0.499, P = .003) and plasma IL‐10 levels were inversely correlated with Tc17 among all patients (Rs = −0.276, P = .05). Male patients with syndesmophytes showed significantly higher Th17 proportions (P = .038). In female AS patients, Tc22 was negatively correlated with C‐reactive protein (high sensitivity) (hsCRP) (Rs = −0.573, P = .016). We confirmed increased proportions of inflammatory T cells and correlations with relevant cytokines from male AS patients. The correlation between Th17 and syndesmophytes supports a role of Th17 in the pathogenic process.
Keywords: ankylosing spondylitis, radiographic axial spondyloarthritis, T cells
1. INTRODUCTION
Ankylosing spondylitis (AS), also called radiographic axial spondyloarthritis (r‐axSpA), is part of the cluster of SpA diseases. AS is characterized by typical radiographic alterations in the sacroiliac joints due to sacroiliitis, and commonly by typical skeletal changes in the spine caused by the inflammatory process. 1 For unknown reasons, AS is more common in men than in women, and men generally develop more severe disease with more spinal radiographic alterations and a higher proportion of extra‐articular manifestations (EAM). 2 In about 85‐90% of cases, AS shows a genetic association with HLA‐B27. 3 , 4 However, the mechanisms underlying AS pathogenesis remain to be fully revealed. It has been suggested that AS may be related to functional alterations of antigen‐presenting cells that express HLA‐B27, which tends to misfold and dimerize, potentially triggering the interleukin‐23/interleukin‐17 (IL‐23/IL‐17) pathway. 5 , 6 , 7 , 8 , 9
Prior studies of AS patients demonstrate non‐lymphoid IL‐17‐expressing cells, 10 and relative increases of the proportions of inflammatory T‐cell subsets, such as Th17, Th22, Tc17 and Tc22. 11 , 12 , 13 The effects of these increased cytokines, including IL‐17, IL‐22 and IL‐23, 14 , 15 have been linked to bone remodelling. 16 , 17 , 18 , 19 Moreover, treatment with anti‐IL‐17A antibodies has clinical benefits, further supporting that the IL‐17/IL‐23 pathway is involved in the pathogenesis process. 20 Association between increased levels of particular T‐cell subsets and DAS28 has been shown, 21 but no studies have previously shown association with clinical parameters such as syndesmophytes. Moreover, little data are available regarding the influence of sex hormones in terms of inflammatory cellular subsets and/or clinical parameters. 22 One investigation showed increased levels of IL‐17 and TH17 cells among males with AS males, but not among females with AS. 23
The present study was conducted in a cohort of well‐phenotyped AS patients from northern Sweden. We aimed to test the hypothesis that particular inflammatory T cell subsets are increased in AS patients, and are associated with clinical characteristics of disease severity and some pro‐ and anti‐inflammatory cytokines.
2. MATERIAL AND METHODS
2.1. Patient cohort
The Backbone study was conducted in northern Sweden to investigate AS severity and comorbidities. This study included 155 patients with AS fulfilling the modified New York criteria. 24 Patients attending the rheumatology clinics at three hospitals in Region Västerbotten in northern Sweden with a diagnosis of AS (ICD‐10 M45.9) were identified through the digital administrative system (n = 523) in November 2015. The diagnosis of AS according to the modified New York criteria 24 was validated through the medical records leaving 346 patients. Two hundred and forty‐six patients between 18 and 75 years of age and without dementia, other inflammatory rheumatic diseases, pregnancy, or difficulties in understanding the Swedish language, were asked by postal invitation, between 2016 and 2017, to take part in the Backbone study. A detailed flow chart over the inclusion process has previously been published. 25
The mean age of these AS patents was 55.5 ± 11.4 years, and 107 (69.0%) were men. The patients with AS underwent clinical examination and answered questionnaires regarding medication and disease‐related data. Mobility was measured using the Bath Ankylosing Spondylitis Metrology Index (BASMI). Disease activity and physical function were assessed using the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI), the Ankylosing Spondylitis Disease Activity Score based on C‐reactive protein (ASDAS‐CRP) and the Bath Ankylosing Spondylitis Function Index (BASFI). 26 , 27 Spinal radiographic alterations were graded according to the modified Stoke Ankylosing Spondylitis Spinal Score (mSASSS), 27 on which the overall score ranges from 0 to 72, with 72 representing complete ankyloses. An mSASSS score of ≥2 at a vertebral corner was considered to indicate a syndesmophyte and higher mSASSS indicates a more severe AS disease.
Blood samples were drawn in the morning after overnight fasting, and used for consecutive analysis of erythrocyte sedimentation rate (ESR) and high‐sensitivity C‐reactive protein (hsCRP). HLA‐B27 was analysed using PCR‐SSP with amplification of exon 2. Plasma was stored and frozen at −80°C. Plasma levels of IL‐17A, IL‐22 and IL‐10 were measured on the Meso Scale Discovery U‐PLEX (custom made) platform following the manufacturer’s instructions (Meso Scale Discovery®). According to the manufacturer, the lower level of detection (LLOD) was 2.6 pg/mL for IL‐17A, 0.13 pg/mL for IL‐22 and 0.14 pg/mL for IL‐10. IL‐17A was below the LLOD in 90% of patients, and was thus not included in any further analyses.
Upon inclusion in Backbone, 106 (68.4%) patients donated samples for PBMC freezing and storage at −80°C, following the below‐described protocol. Based on previous findings, 21 we calculated that a sample size of 50 patients and controls would be sufficient for this project (i.e. power of 80%). The control group included blood donors fulfilling the Swedish criteria for blood donation. 28 The upper age limit for blood donors was 65 years; therefore, AS patients of >65 years of age were excluded, leaving 81 remaining patients. We also excluded HLA‐B27‐negative patients (n = 3), leaving 78 patients. To maintain a sex distribution similar to in Backbone, we further excluded 19 men and 9 women, evenly distributed according to age. Thus, our study finally included 50 AS patients (mean age, 52.1 ± 9.0 years; 66% men, 33/50; 34% women, 17/50) and 50 age‐ and sex‐matched blood donor controls (mean age, 54.6 ± 9.1 years; 66% men, 33/50; 34% women, 17/50). Cases and controls did not significantly differ in age (P = .1) or sex (P = 1). Ninety‐six percent of the AS patients were born in Sweden. We have no data on the ethnicity or smoking status of the blood donor controls.
PBMCs from the controls and AS patients were handled following the same protocol. All of the participants gave written informed consent. This study was approved by the Ethics Review Board at Umeå University, Umeå, Sweden (Dnr 2016/208–31, Forsblad‐d’Elia, PI), and was conducted in accordance with the Helsinki declaration.
2.2. Sample collection and freezing
Blood was collected from AS patients and controls in cell preparation tubes (BD Vacutainer® CPT, #362753). These tubes were centrifuged for 15 min at 1500 × g, and then 50% of the plasma was removed, followed by mixing of the buffy coat and remaining plasma. The cell suspension was transferred to a 50‐mL tube and kept on ice. Roswell Park Memorial Institute (RPMI) 1640 medium (Gibco, Thermo Fisher Scientific, #21875‐034) was added to a total volume of 40 ml, and then cell viability and concentration were assessed. Next, the cell suspension was centrifuged at 250 × g for 10 min. The supernatant was removed, and the cell pellet was resuspended in foetal bovine serum (FBS) (Gibco, Thermo Fisher Scientific, #10500‐064) to a concentration of 10 × 106 cells/mL FBS. Next, we carefully added freeze medium (90% FBS/10% DMSO), drop by drop, in the same amount as FBS. Cell samples were stored at −80°C.
2.3. Flow cytometry
Frozen cells were gently thawed at 37°C, transferred to ice‐cold Gibco™ PBS 1× pH 7.4 (Thermo Fisher scientific, #10010‐015), and then centrifuged at 300 × g for 10 min at 4°C. Next, the cells were washed in PBS, and 0.5‐2 × 106 cells were transferred to tubes. These cells were stimulated for 5 hours at 37°C in an 5% CO2 incubator in RPMI‐1640, l‐glutamine, PenStrep (Gibco, Thermo Fisher scientific) with PMA 50 ng/mL and Ionomycin 1 μg/mL (Sigma‐Aldrich, # P8139, #I0634) in the presence of Golgiplug (diluted 1/1000) (BD Biosciences, # 555029). After washing with PBS, the cells were stained with BD Horizon™ Fixable Viability Stain 510, following the manufacturer's instructions. After washing with FACS buffer (Gibco™ PBS 1× pH 7.4 containing 3% Gibco™ FBS; Thermo Fisher scientific) and 0.01% sodium azide (Sigma‐Aldrich, #S2002), the cells were surface stained using Brilliant Stain Buffer (BD Biosciences, #566385) with a mix of the following antibodies: anti‐CD4 (RPA‐T4, BV605, #562658), anti‐CD3 (APA1/1, FITC, #558261), anti‐CD8 (SK1, APC‐H7, #560179), anti‐CD161 (DX12, PE‐Cy5, #551138) (BD Biosciences) and anti‐ Valpha7.2 (3C10, APC, #351708, Biolegend). The cells were also intracellularly stained using BD Perm/Wash™ (#51‐2091KZ), anti‐IL‐17A (SCPL1362, PE, #560436) (BD Biosciences) and anti‐IL‐22 (IL22JOP, APC, #17‐7222‐82) (eBioscience™), according to the manufacturer's instructions.
Stained samples were resuspended in FACS buffer and analysed using an LSRII FACS machine (BD Biosciences). Data were analysed using FACSDiva Software (BD Biosciences) or FlowJo v10.4.2 (FlowJo, LLC) software. For this analysis, we applied doublet discrimination, that is, the exclusion of events in the flow cytometry that could potentially result from cells appearing in pairs rather than as single cells. Gates were set using fluorescent minus one (FMO) controls or on discrete populations.
2.4. Statistical methods
Descriptive statistics are presented as median and interquartile range (IQR) or as absolute number and percentages. Fisher's Exact Test or Chi‐Square Test was applied for categorical data. The Wilcoxon signed ranks samples test with paired samples was applied for comparing cases and controls, and for comparing groups with AS for continuous data. The Mann‐Whitney U test was used for continuous variables. Correlation analysis was performed using Spearman's rank correlation test. Correlation analysis included the following parameters: BASMI, BASFI, mSASSS, ASDAScrp, crp, ESR, age, symptom duration, BMI, presence or absence of syndesmophyte and smoking (ever or never). All tests were two‐tailed and P ≤ .05 was considered statistically significant. Multivariable logistic regression analyses were run with ≥1 syndesmophyte as dependent variables and percentage of TH17 cells and known risk factors for radiographic spinal changes; age, sex, smoking and hsCRP as independent variables. Due to its skewed distribution, hsCRP and percentage of TH17 cells were log‐transformed when used in the multiple logistic regression model. All data were analysed using IBM SPSS Statistics 25 (IBM).
3. RESULTS
3.1. Characteristics of the AS cohort
This study included 50 AS patients and 50 matched controls (blood donors). Table 1 displays the AS patients' characteristics. Compared to the women, the men with AS had significantly higher mSASSS, and more commonly exhibited syndesmophyte presence. Otherwise, the characteristics did not significantly differ.
TABLE 1.
Descriptive characteristics of 50 patients with ankylosing spondylitis, overall and stratified by sex
| Total, n = 50 | Men, n = 33 (66.0%) | Women, n = 17 (34.0%) | P value | |
|---|---|---|---|---|
| Age, years | 54.5 (45.5–60.0) | 53.0 (45.5–60.0) | 56.0 (44.5–60.5) | .6 |
| BMI, kg/m2 | 26.2 (23.4–31.1) | 27.0 (23.5–32.9) | 25.0 (23.1–28.0) | .1 |
| Ever smoker | 18 (36) | 11 (33.3) | 7 (41.2) | .6 |
| Symptom duration, years | 31.0 (21.0–39.0) | 32.0 (21.5–39.5) | 30.0 (20.5–38.0) | .7 |
| HLA‐B27 positive | 50 (100) | |||
| ESR, mm/h | 8.0 (3.8–15.2) | 6.0 (4.9–14.5) | 10.0 (3.0–18.5) | .5 |
| hsCRP, mg/L | 2.1 (0.8–5.2) | 1.8 (0.8–6.0) | 2.3 (0.7–3.8) | .8 |
| History of anterior uveitis | 26 (52.0) | 17 (51.5) | 9 (52.9) | .9 |
| History of peripheral arthritis | 30 (60.0) | 22 (66.7) | 8 (47.0) | .2 |
| ASDAS‐CRP, score | 1.8 (1.2–2.2) | 1.8 (1.3–2.3) | 1.8 (1.2–2.2) | .7 |
| BASDAI, score | 3.4 (2.2–5.5) | 3.1 (2.0–5.5) | 4.0 (3.2–5.4) | .2 |
| BASFI, score | 2.2 (0.9–3.9) | 2.1 (0.8–4.0) | 2.5 (1.0–4.0) | .7 |
| BASMI, score | 3.2 (2.4–5.2) | 4.0 (2.5–5.5) | 3.0 (2.4–4.1) | .2 |
| NSAID, regular use | 30 (60.0) | 19 (57.6) | 11 (64.7) | .6 |
| csDMARD | 7 (14.0) | 7 (21.1) | 0 (0) | .08 |
| bDMARD | 12 (24.0) | 10 (30.3) | 2 (11.8) | .2 |
| csDMARD and/or bDMARD | 14 (28.0) | 12 (36.4) | 2 (11.8) | .1 |
| mSASSS, score | 4 (0–31.0) | 18.0 (2.5–42.0) | 1.0 (0.0–5.5) | .006* |
| Syndesmophyte presence | 23 (46.0) | 19 (57.6) | 4 (23.5) | .02* |
Note: Values are presented as median with interquartile range (IQR) or number of patients and percent (%).
Abbreviations: ASDAS, Ankylosing Spondylitis Disease Activity Score; b, biologic (i.e. anti‐TNF antibodies); BASDAI, Bath Ankylosing Disease Activity Index; BASFI, Bath Ankylosing Spondylitis Functional Index; BASMI, Bath Ankylosing Spondylitis Metrology Index; BMI, body mass index; csDMARD, conventional synthetic disease‐modifying anti‐rheumatic drug; ESR, erythrocyte sedimentation rate; HLA‐B27, human leucocyte antigen B27; hsCRP, high‐sensitivity C‐reactive protein; mSASSS, Modified Stoke Ankylosing Spondylitis Score; NSAID, non‐steroidal anti‐inflammatory drug. *P ≤ .05.
3.2. Increased frequencies of TH17, TH22 and TC22 cells in male AS patients, and correlations with cytokines and clinical parameters
To reveal the potential contribution of inflammatory T‐cell subsets in our AS cohort, we performed flow cytometry analyses of peripheral blood. Intracellular staining of IL‐17 and IL‐22, and expressions of CD4 and CD8, were used to discriminate between Th17, Th22, Tc17 and Tc22. Figure S1 presents the full gating strategy.
The proportions of IL‐17‐ and IL‐22‐expressing cells were generally sparse, but consistently detectable by flow cytometry. Paired analysis revealed significantly higher proportions of Th17 cells (P = .005), Th22 cells (P = .008) and Tc22 cells (P = .025) in AS patients compared to controls. Next, we split the groups into females and males. In Figure 1, the observed distributions in different groups, including Tc17 cell frequencies, are displayed as truncated violin plots. Sex‐wise comparison revealed that compared to male controls, male AS patients showed significantly increased proportions of Th17 cells (0.34%, IQR 0.18%‐0.86% vs 0.16%, IQR 0.06%‐0.37%; P = .013) (Figure 1A), Th22 cells (0.25%, IQR 0.15%‐0.59% vs 0.14%, IQR 0.05%‐0.30%; P = .003) (Figure 1B) and Tc22 cells (0.07%, IQR 0.04%‐0.13% vs 0.06%, IQR 0.02%‐0.10%; P = .024) (Figure 1D). Compared to female controls, the female AS patients exhibited a similar trend of an increased median for all three T cell subsets, although these differences were not significant. We performed additional flow cytometry analyses including MAIT cells (CD3+CD4−CD8+/−CD161highvα7.2+), which have been previously reported to be altered in AS patients. 29 The results did not reveal any difference in the proportion of cells when comparing overall AS patients and controls (CD3+CD4−CD8+ CD161highvα7.2+ 0.6% ± 0.6 vs 0.9% ± 1.1; CD3+CD4−CD8− CD161highvα7.2+ 0.6% ± 0.6 vs 1.1% ± 1.7) or in sex‐stratified comparisons (data not shown).
FIGURE 1.
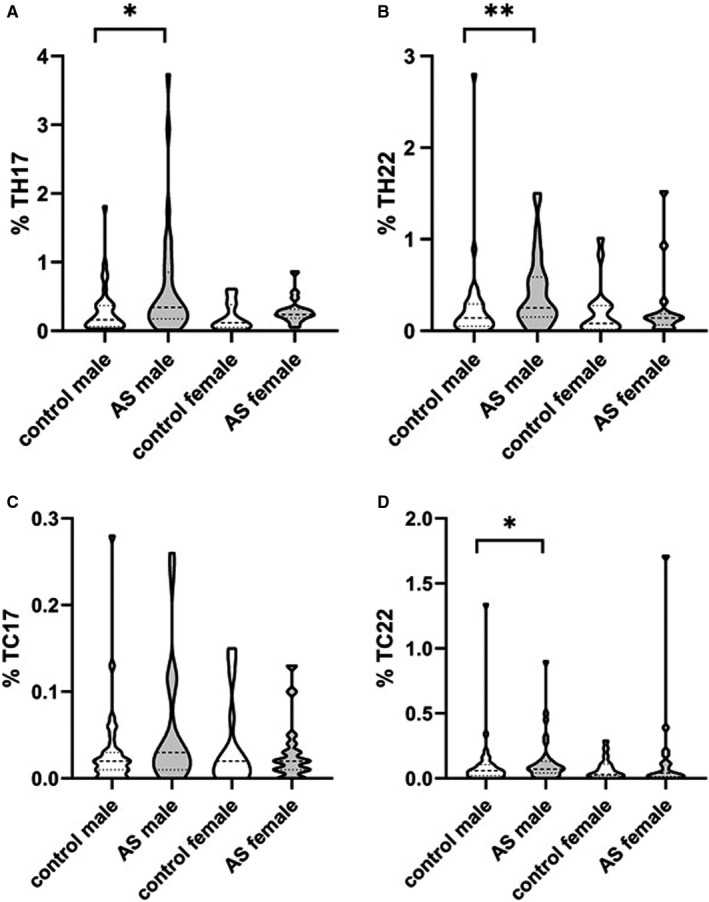
Sex‐wise comparison of the proportions of (A) Th17, (B) Th22, (C) Tc17 and (D) Tc22 cells among peripheral blood leucocytes gated for CD4+ or CD8+ cells according to this figure. Control male n = 33, AS male n = 33, Control female n = 17, AS female n = 17. Dashed lines indicate median and interquartile ranges. Mann‐Whitney U test. *P ≤ .05; **P ≤ .005
Plasma cytokine levels, that is, IL‐17, IL‐10 and IL‐22, have previously been correlated with the presence of particular T cell subsets. Measurement of plasma IL‐10 in the AS group as a whole (n = 49) showed 0.22 (median) (IQR 0.15‐0.30) pg/mL, with 0.23 (median) (IQR 0.18‐0.38) pg/mL in AS males (n = 32) and 0.17 (median) (IQR 0.13‐0.25) pg/mL in AS females (n = 17). Furthermore, plasma IL‐22 measurement in the AS group (n = 49) as a whole showed 0.32 (median) (IQR 0.22‐0.50) pg/mL, and 0.32 (median) (IQR 0.23‐0.50) pg/mL in AS males (n = 33) and 0.39 (median) (IQR 0.14‐0.53) pg/mL in AS females (n = 16). IL‐17 plasma levels were for 90% of the AS group under the detection limit and were therefore not further included in the study. No cytokine measurements were done for the control group.
When comparing AS patients and controls, we did not detect any difference in the proportion of Tc17, but we detected a correlation between the levels of Tc17 and IL‐22 (Rs = 0.283, P = .046), which was explained by the male AS patients (Rs = 0.499, P = .003). Moreover, we observed an inverse correlation between Tc17 and IL‐10 level within the whole group (Rs = −0.276, P = .05). Among male AS patients, Th17 was also negatively correlated with plasma levels of IL‐10 (Rs = −0.374, P = .035).
Next, we analysed the correlations between clinical parameters and the different T cell subsets. We observed a correlation between Th17 and syndesmophyte presence (yes/no) (Rs = 0.304, P = .032), which was explained by the male patients (Rs = 0.367, P = .036) and was non‐significant among females. Male patients with syndesmophyte presence (n = 19) showed a significantly higher proportion of Th17 cells compared to males without syndesmophytes (n = 14): 0.46% (0.26‐1.28) vs 0.19% (0.14‐0.40) (P = .038) (Figure 2). Multivariable logistic regression analyses with ≥1 syndesmophyte as the dependent variable and percentage of log Th17 cells as an independent variable adjusted for other known risk factors for radiographic changes in the spine in r‐axSpA; sex, smoking and hsCRP showed an OR of 7.17 (95% CI, 1.27‐40.46, P = .026) for the percentage of log Th17 cells, Table 3. Furthermore, we noted an inverse correlation between BMI and Tc22 (Rs = −0.286, P = .044), which was explained by the male patients (Rs = −0.430, P = .012). A full display of the correlation analyses, including all that we found to not correlate, is presented in Table S1.
FIGURE 2.
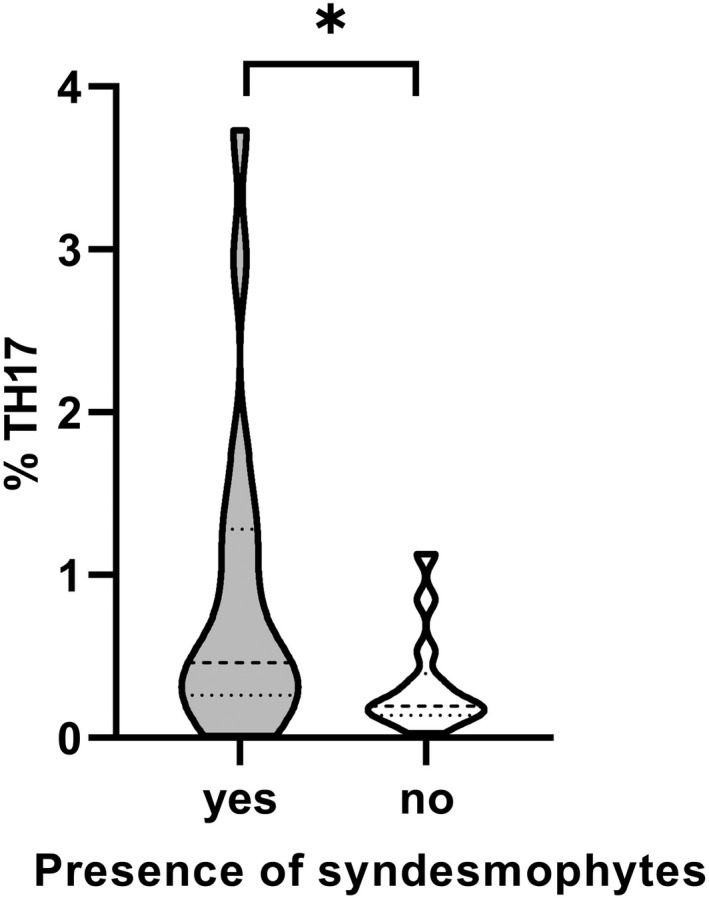
Comparison of Th17 levels in AS male with (yes) or without (no) the presence of syndesmophytes. Male patients with syndesmophyte presence (n = 19), males without syndesmophytes (n = 14). Dashed lines indicate median and interquartile ranges. Mann‐Whitney U test. *P ≤ .05
TABLE 3.
Multivariable logistic regression analysis with presence of ≥1 syndesmophyte as dependent variable
| ≥Syndesmophyte | |||
|---|---|---|---|
| Covariates | OR | 95% CI | P‐value |
| sex | 5.51 | 0.98–31.12 | .053 |
| Symptom duration, years | 1.19 | 1.07–1.32 | .002 ** |
| Ever smoker | 2.71 | 0.47–15.63 | .26 |
| Log hsCRP, mg/L | 9.59 | 1.50–61.50 | .017* |
| Log Th17 cells, % | 7.17 | 1.27–40.46 | .026* |
Note: Female sex was coded 0 and male sex 1.
Abbreviation: hsCRP, high‐sensitivity C‐reactive protein. *P ≤ .05; **P ≤ .005.
3.3. Clinical parameters are associated with T cell subsets and cytokines, differently between premenopausal versus post‐menopausal female AS patients
Analysis of all female AS patients revealed that Tc22 was negatively correlated with the hsCRP level (Rs = −0.573, P = .016) and with ever smoking (Rs = −0.586, P = .013). Female AS patients who were current or previous smokers displayed significantly lower proportions of Tc22 compared with female patients who had never been smokers: 0.020% (0.00‐0.035) vs. 0.098% (0.028‐0.25) (P = .019). Since the group of female AS patients included women with ages ranging from 38.5‐63 years, we split the group according to menopausal status. Table 2 shows the characteristics for these two subgroups. Repeated analysis of the correlation between Tc22 and hsCRP revealed that this association was mainly explained by the postmenopausal group (Rs = −0.635, P = .036, n = 11). Further analysis of these two subgroups in terms of the T‐cell subsets revealed a strong reverse correlation between Tc17 and IL‐10 (Rs = −0.688, P = .019) among the postmenopausal females, and a correlation between Tc22 and BASFI (Rs = 0.930, P < .008) among the premenopausal females (n = 6). To exclude that our observations was merely reflecting an age effect, that is, age in the pre‐ and post‐menopausal group respectively, we analysed the correlations between age and clinical as well as cellular parameters. In this analysis, no significant correlation was found.
TABLE 2.
Descriptive characteristics of 17 women with ankylosing spondylitis, stratified by menopausal status
| Characteristics | Postmenopausal women, n = 11 (64.7%) | Premenopausal women, n = 6 (35.3%) | P‐value |
|---|---|---|---|
| Age, years | 58.0 (53.0–63.0) | 42.5 (38.5–47.5) | <.001** |
| BMI, kg/m2 | 26.4 (22.8–29.8) | 23.8 (23.0–24.8) | .1 |
| Ever smoker | 6 (54.5) | 1 (16.7) | .3 |
| Symptom duration, years | 34.0 (30.0–39.0) | 19.5 (17.8–22.8) | <.001** |
| HLA‐B27‐positive | 11 (100) | 6 (100) | NA |
| ESR, mm/h | 16.0 (8.0–28.0) | 4.5 (1.8–10.8) | .03* |
| hsCRP, mg/L | 2.8 (0.8–7.0) | 2.2 (0.6–2.9) | .4 |
| IL‐22, pg/mL (n = 16) | 0.47 (0.18–0.64) | 0.22 (0.12–0.49) | .2 |
| IL‐10, pg/mL (n = 17) | 0.23 (0.14–0.26) | 0.15 (0.11–0.19) | .1 |
| History of anterior uveitis | 6 (54.5) | 3 (50.0) | 1.0 |
| History of peripheral arthritis | 6 (54.5) | 4 (66.7) | .6 |
| ASDAS‐CRP, score | 1.9 (1.5–2.2) | 1.1 (0.9–1.9) | .05* |
| BASDAI, score | 4.5 (3.4–5.6) | 3.0 (1.8–4.4) | .05* |
| BASFI, score | 2.6 (1.2–4.2) | 1.4 (0.8–3.4) | .3 |
| BASMI, score | 3.4 (2.6–4.4) | 2.5 (2.0–3.0) | .03* |
| NSAID, regular use | 6 (54.5) | 5 (83.3) | .3 |
| csDMARD | 0 (0) | 0 (0) | NA |
| bDMARD | 0 (0) | 2 (33.3) | .1 |
| csDMARD and/or bDMARD | 0 (0) | 2 (33.3) | .1 |
| mSASSS, score | 3.0 (0.0–8.0) | 0.0 (0.0–1.2) | .05* |
| Syndesmophyte presence | 4 (36.4) | 0 (0) | .2 |
Note: Values presented as median with interquartile range (IQR) or number of patients and percent (%).
Abbreviations: ASDAS, Ankylosing Spondylitis Disease Activity Score; b, biologic; BASDAI, Bath Ankylosing Disease Activity Index; BASFI, Bath Ankylosing Spondylitis Functional Index; BASMI, Bath Ankylosing Spondylitis Metrology Index; BMI, body mass index; csDMARD, conventional synthetic disease‐modifying anti‐rheumatic drug; ESR, erythrocyte sedimentation rate; HLA‐B27, human leucocyte antigen B27; hsCRP, high‐sensitivity C‐reactive protein; mSASSS, Modified Stoke Ankylosing Spondylitis Score; NSAID, non‐steroidal anti‐inflammatory drug. *P ≤ .05; **P ≤ .005.
4. DISCUSSION
It has been proposed that inflammatory‐related, that is, IL‐17‐ and IL‐22‐producing lymphoid cells may be involved in AS pathogenesis, and increased proportions of Th17 have been found in blood from AS patients. 11 , 12 , 13 In our present analyses of well‐characterized AS patients from northern Sweden, our findings are in line with these previous findings. We observed significantly increased Th17 among male AS patients. Among female AS patients, we observed a similar trend of increased levels of Th17 cells, although this difference was not significant. We could not examine a possible correlation between Th17 and IL‐17 levels, since IL‐17 was unfortunately below the detection limit in the majority of patient samples. However, it has been previously reported that Th17 is correlated with increased IL‐17 levels. 14 , 15 Th17 cells can produce IL‐17, and to some extent IL‐22, which is in contrast to the Th22 subset that exclusively produces IL‐22. 30 , 31 , 32 It has been suggested that Th22 and Tc22 play a pathogenic role in inflammatory diseases, including AS and psoriasis, 21 , 33 , 34 and IL‐22 has convincingly been shown to be associated with autoimmune disease. 35 Within our cohort, male AS patients displayed significant increases of both Th22 and Tc22 cells. Additionally, we detected a correlation between Tc17 and IL‐22 levels, which could be either a direct association (e.g. increased levels of IL‐22‐producing Tc17 cells) or an indirect association (e.g. as this cytokine is produced by several cell types and intricate cross regulation likely occurs).
Regulation of the differentiation and effector functions of the above‐mentioned inflammatory cells is promoted by several cytokines, such as IL‐23, IL‐1β, IL‐6 and IL‐21. 36 In contrast, the cytokine IL‐10 reportedly down‐regulates inflammation by inducing Th17 cells to differentiate into IL‐10‐producing Tregs. 37 , 38 The microbiota also seem to play a role in this situation. We and other researchers have previously found signs of an altered microbiota in patients with AS. 39 , 40 In our present analysis, we also detected an inverse correlation between IL‐10 and Tc17, implicating cross‐regulation of Tc17 similar to that previously observed for Th17. Additionally, it has been demonstrated that Th22 and IL‐10 show an inverse correlation with the development of Tregs, including immune responses in the gut, highlighting the intricate crosstalk between all of these subsets and the environment. 41 In line with this a decrease of Tregs in AS patients has been demonstrated by several studies and was recently supported by a meta‐analysis of these findings. 42
It has been proposed that microbial dysbiosis, as one key initiating factor in AS, including IL‐23 production by gut epithelial cells, may contribute to Th17 and Th22 differentiation and dysfunctional IL‐10‐mediated regulation. Additionally, the strong contribution of HLA‐B27 to AS development has recently been attributed to protein misfolding in antigen‐presenting cells, leading to autophagy and subsequent IL‐23 production. 43 The fact that two independent molecular causes could potentially promote the same disease‐related mechanism, that is, Type 3 immunity, is in line with the multifactorial nature of AS. 36 , 38
In the present study, we found that Th17 levels were associated with syndesmophyte presence in male patients. To our knowledge, this is the first study to examine correlations between clinical parameters and increased Th17, thus corroborating the role of Th17 in AS pathogenesis. Due to the much smaller size of the female group, and the fact that very few within that group (n = 4) displayed syndesmophytes, the power was likely too low to be able to detect an association in this group. In multivariable logistic regression analyses, we found that log Th17 cells were significantly associated with the presence of ≥ syndesmophyte adjusted for other risk factors for spinal radiographic changes. However, the confidence interval was broad 1.27‐40.46. Thus, one needs to interpret the results with caution, also since the number of patents was limited and the observation needs to be confirmed in other studies.
Interestingly, among female AS patients, we found a strong correlation between the Tc22 subset and BASFI within the premenopausal group. As this association was not found among male AS patients or among female post‐menopausal AS patients, we speculate that female sex hormones influence this trait. The influence of sex hormones in rheumatic diseases has been extensively investigated and demonstrated for rheumatoid arthritis and SLE. 44 The observed correlation between Tc22 and BASFI only in premenopausal females supports the concept that sex hormones affect AS disease activity. This highlights the importance of considering this factor when studying AS initiation and progression.
Lastly, it has been suggested that BMI may influence AS disease activity and the progression of AS‐related radiographic spinal alterations, both through mechanical factors and adipose‐related inflammation. 2 , 45 , 46 In our study, we found that BMI, as well as ever having smoked and hsCRP, which have also been shown to correlate with AS, exhibited negative correlations with the level of Tc22 cells. Further studies, including of T cell subset dynamics in relation to lifestyle factors, could elucidate the underlying mechanisms.
Limitations of this study include the relatively small numbers of patients and controls, which was mainly due to the limited population size in this part of Sweden. This was particularly evident for the group of females. This drawback may be compensated to some extent by the uniform characterization and clinical evaluation of our cohort. Furthermore, many statistical comparisons are presented which needs to be considered, also emphasizing that replication studies in independent and larger cohorts of patients are required to confirm and expand our observations. Moreover, the cellular origin of the plasma cytokines could be of interest to further understand the underlying mechanisms of our observed correlations. Another limitation is that we do not have any data on cytokines or other characteristics of the blood‐donor controls to compare with the AS patients.
AS pathogenesis is multifactorial and involves altered immune reactions as well as non‐immunological factors. As we have demonstrated in the current study, the contribution of inflammatory T cells in this process should be noted as one piece in this complex puzzle.
AUTHOR CONTRIBUTIONS
The study was designed by KL, UH and HFdE. Sample‐ and patient‐related data collection was performed by AK and HFD. Laboratory work and data collection were performed by KL, UH, LD and AK. Data analysis was performed by KL, UH and HFdE. Data interpretation and writing of the manuscript were done by KL, UH and HFdE. All authors read and approved the final manuscript.
CONFLICT OF INTEREST
The authors declare no conflict of interests.
ETHICS STATEMENT
All of the participants gave written informed consent. This study was approved by the Ethics Review Board at Umeå University, Umeå, Sweden (Dnr 2016/208–31, Forsblad‐d’Elia, PI), and was conducted in accordance with the Helsinki declaration.
Supporting information
Figure S1
Table S1
ACKNOWLEDGMENT
We thank the research nurses at the University Hospital of Umeå, Viktoria von Zweigbergk and Jeanette Beckman Rehnman, for assisting with the project. We also acknowledge all participants in the study. This work was supported by Umeå Universitet, The Swedish Research Council [2016‐02035], the County of Västerbotten [ALFVLL‐640251], King Gustaf Vth 80‐year Foundation [FAI‐2017‐0454], Västerbotten’s Association Against Rheumatism, The Swedish Association Against Rheumatism, The Norrland’s Heart Foundation and Mats Kleberg’s Foundation. The authors declare no conflicts of interest and all authors have approved the final version of the manuscript.
Lejon K, Hellman U, Do L, Kumar A, Forsblad‐d’Elia H. Increased proportions of inflammatory T cells and their correlations with cytokines and clinical parameters in patients with ankylosing spondylitis from northern Sweden. Scand J Immunol. 2022;96:e13190. doi: 10.1111/sji.13190
DATA AVAILABILITY STATEMENT
The data sets analyzed during the current study are not publicly available due to Swedish legislation (the Personal Data Act). A limited and fully anonymized data set, which supports the main analyses, is available from the corresponding author upon request.
REFERENCES
- 1. Braun J, Sieper J. Ankylosing spondylitis. Lancet. 2007;21(369):1379‐1390. [DOI] [PubMed] [Google Scholar]
- 2. Deminger A, Klingberg E, Geijer M, et al. A five‐year prospective study of spinal radiographic progression and its predictors in men and women with ankylosing spondylitis. Arthritis Res Ther. 2018;3(20):162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brown MA, Kenna T, Wordsworth BP. Genetics of ankylosing spondylitis—insights into pathogenesis. Nat Rev Rheumatol. 2016;12:81‐91. [DOI] [PubMed] [Google Scholar]
- 4. Bowness P. Hla‐B27. Annu Rev Immunol. 2015;33:29‐48. [DOI] [PubMed] [Google Scholar]
- 5. Taurog JD, Chhabra A, Colbert RA. Ankylosing spondylitis and axial spondyloarthritis. N Engl J Med. 2016;30(374):2563‐2574. [DOI] [PubMed] [Google Scholar]
- 6. DeLay ML, Turner MJ, Klenk EI, Smith JA, Sowders DP, Colbert RA. HLA‐B27 misfolding and the unfolded protein response augment interleukin‐23 production and are associated with Th17 activation in transgenic rats. Arthritis Rheum. 2009;60:2633‐2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bowness P, Ridley A, Shaw J, et al. Th17 cells expressing KIR3DL2+ and responsive to HLA‐B27 homodimers are increased in ankylosing spondylitis. J Immunol. 2011;15(186):2672‐2680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Loll B, Fabian H, Huser H, et al. Increased conformational flexibility of HLA‐B*27 subtypes associated with ankylosing spondylitis. Arthritis Rheumatol. 2016;68:1172‐1182. [DOI] [PubMed] [Google Scholar]
- 9. Colbert RA, DeLay ML, Layh‐Schmitt G, Sowders DP. HLA‐B27 misfolding and spondyloarthropathies. Prion. 2009;3(1):15‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Appel H, Maier R, Wu P, et al. Analysis of IL‐17(+) cells in facet joints of patients with spondyloarthritis suggests that the innate immune pathway might be of greater relevance than the Th17‐mediated adaptive immune response. Arthritis Res Ther. 2011;20(13):R95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shen H, Goodall JC, Hill Gaston JS. Frequency and phenotype of peripheral blood Th17 cells in ankylosing spondylitis and rheumatoid arthritis. Arthritis Rheum. 2009;60:1647‐1656. [DOI] [PubMed] [Google Scholar]
- 12. Jandus C, Bioley G, Rivals JP, Dudler J, Speiser D, Romero P. Increased numbers of circulating polyfunctional Th17 memory cells in patients with seronegative spondylarthritides. Arthritis Rheum. 2008;58:2307‐2317. [DOI] [PubMed] [Google Scholar]
- 13. Smith JA, Colbert RA. Review: the interleukin‐23/interleukin‐17 axis in spondyloarthritis pathogenesis: Th17 and beyond. Arthritis Rheumatol. 2014;66:231‐241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mei Y, Pan F, Gao J, et al. Increased serum IL‐17 and IL‐23 in the patient with ankylosing spondylitis. Clin Rheumatol. 2011;30:269‐273. [DOI] [PubMed] [Google Scholar]
- 15. Wendling D, Cedoz JP, Racadot E, Dumoulin G. Serum IL‐17, BMP‐7, and bone turnover markers in patients with ankylosing spondylitis. Joint Bone Spine. 2007;74:304‐305. [DOI] [PubMed] [Google Scholar]
- 16. Adamopoulos IE, Bowman EP. Immune regulation of bone loss by Th17 cells. Arthritis Res Ther. 2008;10:225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sato K, Suematsu A, Okamoto K, et al. Th17 functions as an osteoclastogenic helper T cell subset that links T cell activation and bone destruction. J Exp Med. 2006;27(203):2673‐2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhang JR, Pang DD, Tong Q, Liu X, Su DF, Dai SM. Different modulatory effects of IL‐17, IL‐22, and IL‐23 on osteoblast differentiation. Mediators Inflamm. 2017;2017:5950395‐5950311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. El‐Zayadi AA, Jones EA, Churchman SM, et al. Interleukin‐22 drives the proliferation, migration and osteogenic differentiation of mesenchymal stem cells: a novel cytokine that could contribute to new bone formation in spondyloarthropathies. Rheumatology (Oxford). 2017;1(56):488‐493. [DOI] [PubMed] [Google Scholar]
- 20. Baeten D, Baraliakos X, Braun J, et al. Anti‐interleukin‐17A monoclonal antibody secukinumab in treatment of ankylosing spondylitis: a randomised, double‐blind, placebo‐controlled trial. Lancet. 2013;23(382):1705‐1713. [DOI] [PubMed] [Google Scholar]
- 21. Zhang L, Li YG, Li YH, et al. Increased frequencies of Th22 cells as well as Th17 cells in the peripheral blood of patients with ankylosing spondylitis and rheumatoid arthritis. PLoS One. 2012;7:e31000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rusman T, van Vollenhoven RF, van der Horst‐Bruinsma IE. Gender differences in axial spondyloarthritis: women are not so lucky. Curr Rheumatol Rep. 2018;20(6):35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gracey E, Yao Y, Green B, et al. Sexual dimorphism in the Th17 signature of ankylosing spondylitis. Arthritis Rheumatol. 2016;68:679‐689. [DOI] [PubMed] [Google Scholar]
- 24. van der Linden S, Valkenburg HA, Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum. 1984;27:361‐368. [DOI] [PubMed] [Google Scholar]
- 25. Forsblad‐d’Elia H, Law L, Bengtsson K, et al. Biomechanical properties of common carotid arteries assessed by circumferential 2D strain and beta stiffness index in patients with ankylosing spondylitis. J Rheumatol. 2021;48:352‐360. [DOI] [PubMed] [Google Scholar]
- 26. Speier J, Rudwaleit M, Baraliakos X, et al. The Assessment of SpondyloArthritis international Society (ASAS) handbook: a guide to assess spondyloarthritis. Ann Rheum Dis. 2009;68(suppl 2):ii1‐ii44. [DOI] [PubMed] [Google Scholar]
- 27. Creemers MC, Franssen MJ, van't Hof MA, Gribnau FW, van de Putte LB, van Riel PL. Assessment of outcome in ankylosing spondylitis: an extended radiographic scoring system. Ann Rheum Dis. 2005;64:127‐129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Socialstyrelsen . Regler för blodgivning. 2020. [cited 2020 Sept 29th]; Available from: https://www.socialstyrelsen.se/stod‐i‐arbetet/organ‐och‐vavnadsdonation/blodgivning/
- 29. Hayashi E, Chiba A, Tada K, et al. Involvement of mucosal‐associated invariant T cells in ankylosing spondylitis. J Rheumatol. 2016;43:1695‐1703. [DOI] [PubMed] [Google Scholar]
- 30. Duhen T, Geiger R, Jarrossay D, Lanzavecchia A, Sallusto F. Production of interleukin 22 but not interleukin 17 by a subset of human skin‐homing memory T cells. Nat Immunol. 2009;10:857‐863. [DOI] [PubMed] [Google Scholar]
- 31. Trifari S, Kaplan CD, Tran EH, Crellin NK, Spits H. Identification of a human helper T cell population that has abundant production of interleukin 22 and is distinct from T(H)‐17, T(H)1 and T(H)2 cells. Nat Immunol. 2009;10:864‐871. [DOI] [PubMed] [Google Scholar]
- 32. Eyerich S, Eyerich K, Pennino D, et al. Th22 cells represent a distinct human T cell subset involved in epidermal immunity and remodeling. J Clin Invest. 2009;119:3573‐3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Res PC, Piskin G, de Boer OJ, et al. Overrepresentation of IL‐17A and IL‐22 producing CD8 T cells in lesional skin suggests their involvement in the pathogenesis of psoriasis. PLoS One. 2010;24(5):e14108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Luan L, Ding Y, Han S, Zhang Z, Liu X. An increased proportion of circulating Th22 and Tc22 cells in psoriasis. Cell Immunol. 2014;290:196‐200. [DOI] [PubMed] [Google Scholar]
- 35. Pan HF, Li XP, Zheng SG, Ye DQ. Emerging role of interleukin‐22 in autoimmune diseases. Cytokine Growth Factor Rev. 2013;24:51‐57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ranganathan V, Gracey E, Brown MA, Inman RD, Haroon N. Pathogenesis of ankylosing spondylitis ‐ recent advances and future directions. Nat Rev Rheumatol. 2017;13:359‐367. [DOI] [PubMed] [Google Scholar]
- 37. Ohnmacht C, Park JH, Cording S, et al. MUCOSAL IMMUNOLOGY. The microbiota regulates type 2 immunity through RORgammat(+) T cells. Science. 2015. Aug;28(349):989‐993. [DOI] [PubMed] [Google Scholar]
- 38. Sefik E, Geva‐Zatorsky N, Oh S, et al. MUCOSAL IMMUNOLOGY. Individual intestinal symbionts induce a distinct population of RORgamma(+) regulatory T cells. Science. 2015;28(349):993‐997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Breban M, Tap J, Leboime A, et al. Faecal microbiota study reveals specific dysbiosis in spondyloarthritis. Ann Rheum Dis. 2017;76:1614‐1622. [DOI] [PubMed] [Google Scholar]
- 40. Klingberg E, Magnusson MK, Strid H, et al. A distinct gut microbiota composition in patients with ankylosing spondylitis is associated with increased levels of fecal calprotectin. Arthritis Res Ther. 2019;27(21):248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ouyang W, Rutz S, Crellin NK, Valdez PA, Hymowitz SG. Regulation and functions of the IL‐10 family of cytokines in inflammation and disease. Annu Rev Immunol. 2011;29:71‐109. [DOI] [PubMed] [Google Scholar]
- 42. Liu D, Liu B, Lin C, Gu J. Imbalance of peripheral lymphocyte subsets in patients with ankylosing spondylitis: a meta‐analysis. Front Immunol. 2021;12:696973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Navid F, Colbert RA. Causes and consequences of endoplasmic reticulum stress in rheumatic disease. Nat Rev Rheumatol. 2017. Jan;13:25‐40. [DOI] [PubMed] [Google Scholar]
- 44. Bove R. Autoimmune diseases and reproductive aging. Clin Immunol. 2013;149:251‐264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Durcan L, Wilson F, Conway R, Cunnane G, O’Shea FD. Increased body mass index in ankylosing spondylitis is associated with greater burden of symptoms and poor perceptions of the benefits of exercise. J Rheumatol. 2012;39:2310‐2314. [DOI] [PubMed] [Google Scholar]
- 46. Liew JW, Huang IJ, Louden DN, Singh N, Gensler LS. Association of body mass index on disease activity in axial spondyloarthritis: systematic review and meta‐analysis. RMD Open. 2020;6:e001225. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1
Table S1
Data Availability Statement
The data sets analyzed during the current study are not publicly available due to Swedish legislation (the Personal Data Act). A limited and fully anonymized data set, which supports the main analyses, is available from the corresponding author upon request.


