Abstract
Background
Whether intensity-modulated radiotherapy (IMRT) can enhance the efficacy of the programmed death (PD)-1 inhibitors combined with anti-angiogenic therapy for hepatocellular carcinoma (HCC) is unclear. Therefore, we conducted this multicenter retrospective study to investigate the efficacy of the combination of PD-1 inhibitors with anti-angiogenic therapy and IMRT.
Methods
From April 2019 to March 2022, a total of 197 patients with HCC [combination of PD-1 inhibitors with anti-angiogenic therapy and IMRT (triple therapy group), 54; PD-1 inhibitors plus anti-angiogenic therapy (control group), 143] were included in our study. Propensity score matching (PSM) was applied to identify two groups with similar baselines. The objective response rate (ORR), overall survival (OS), and progression-free survival (PFS) of the two groups were compared before and after matching.
Results
Prior to PSM, the triple therapy group had higher ORR (42.6% vs 24.5%, P = 0.013) and more superior median OS (mOS) (20.1 vs 13.3 months, P = 0.009) and median PFS (mPFS) (8.7 vs 5.4 months, P = 0.001) than the control group. Following PSM, the triple therapy group still exhibited better mPFS (8.7 vs 5.4 months, P = 0.013) and mOS (18.5 vs 12.6 months, P = 0.043) than the control group. However, the ORR of the two groups was similar (40% vs 25%, P = 0.152). No significant difference was observed in the treatment-related adverse events between the two groups (P < 0.05 for all).
Conclusions
The combination of PD-1 inhibitors with anti-angiogenic therapy and IMRT for HCC is a promising regimen.
Keywords: programmed death-1 inhibitors, anti-angiogenic therapy, intensity-modulated radiotherapy, hepatocellular carcinoma, propensity score matching
Introduction
Hepatocellular carcinoma (HCC) is the most common cause of cancer-related death (1). Despite the wide use of early detection techniques to diagnose HCC, most patients are diagnosed at an advanced stage (2). The overall survival (OS) of patients with HCC is extremely short, therefore, the prognosis of patients should be urgently improved (3).
Currently, the combination of programmed death 1/programmed death ligand 1 (PD-1/PD-L1) inhibitors and targeted drugs has become prominent in HCC research. Atezolizumab plus bevacizumab, the current first-line treatment option, extends median OS (mOS) to 19.2 months and objective response rate (ORR) to 27.3% in inoperable HCC (4, 5). Additionally, Ren et al. (6) reported an ORR of 21% and a median progression-free survival (mPFS) of 4.6 months in patients with inoperable HCC who received sintilimab plus bevacizumab. In the RESCUE study of camrelizumab plus apatinib for advanced HCC, the ORR was 34.3% and mPFS was 5.7 months (7). Despite breakthroughs in the combination therapy of PD-1/PD-L1 inhibitors and targeted drugs, its ORR was still low. The addition of other treatments that can improve local control of HCC has become a new research direction.
Intensity-modulated radiotherapy (IMRT), an external RT modality, is a local treatment method that uses radiation to irradiate malignant tumor cells. Abulimiti et al. (8) confirmed that IMRT plus sorafenib can improve the prognosis of advanced HCC, for which the mOS was observed to be 11.4 months and the mPFS was 6 months. Additionally, patients with advanced HCC who received IMRT in combination with apatinib had an mPFS of 7.8 months and an ORR of 15% (9). Radiotherapy can not only promote the generation and infiltration of T cells but also stimulate systemic anti-tumor immunity to control metastatic lesions, causing the “abscopal effect” (10). Furthermore, targeting vascular endothelial growth factor (VEGF) can normalize tumor vessels and enhance T cell infiltration, thus, providing a rationale for combining this therapy with immunotherapy (11).
Based on these results, the combination of PD-1 inhibitors with anti-angiogenic therapy and IMRT is a promising treatment modality. We conducted this multicenter retrospective study to investigate the efficacy of triple therapy.
Materials and methods
Patients
From April 2019 to March 2022, a total of 197 patients with HCC [combination of PD-1 inhibitors with anti-angiogenic therapy and IMRT (triple therapy group), 54; PD-1 inhibitors plus anti-angiogenic therapy (control group), 143] from three Chinese tertiary hospitals were included in our retrospective study.
The inclusion criteria were as follows: a) Pathologically diagnosed HCC; b) Barcelona Clinic Liver Cancer (BCLC) stage B/C; c) Eastern Cooperative Oncology Group performance status (ECOG PS) score of 0−2; d) Child-Pugh class A/B; e) at least one measurable lesion according to the modified Response Evaluation Criteria in Solid Tumors (mRECIST); f) administration of at least one cycle of PD-1 inhibitors plus anti-angiogenic therapy with or without IMRT; g) patients were able to undergo IMRT after evaluation. The exclusion criteria were as follows: a) Incomplete information; b) number of tumors >5 or diffuse lesions; c) presence of other malignancies; d) severe ascites or hepatic encephalopathy.
This study was approved by the Ethics Committee of the affiliated hospital of Southwest Medical University (approval number KY2020254). We waived individual informed consent since this was a retrospective study.
Treatment protocol
Imrt
IMRT was performed within 7 days of the administration of the first cycle of PD-1 inhibitors plus anti-angiogenic therapy. The radiologist used the radiation planning system to delineate the target volume with computed tomography (CT) guidance. Delineation of the clinical target volume (CTV) including a 4-mm margin of the primary liver tumor was accomplished through image technology. The planning target volume (PTV) was defined as a 5-10-cm peripheral expansion based on CTV. The total target radiation dose was 48 g with 3 Gy/fraction, and at least 95% of PTV received the prescribed dose. The dose constraints for the organs at risk were as follows: Spinal cord (maximum dose ≤45 Gy); normal liver (mean dose ≤30 Gy); stomach and duodenum (maximum dose ≤54 Gy); colon (maximum dose ≤55 Gy).
Administration of PD-1 inhibitors and targeted agents
All patients received PD-1 inhibitor injection once every three weeks as well as the antiangiogenic drug on daily basis until the appearance of intolerable toxic reactions or progressive disease. The doses of PD-1 inhibitors and targeted drugs were calculated based on the patient’s height and weight. Dosing delays were allowed when a serious treatment-related adverse event (TRAE) occurred.
Follow-up and data collection
The efficacy of patients was assessed by CT/Magnetic Resonance Imaging (MRI) performed every 2−3 months. Treatment response was divided into complete response (CR), partial response (PR), stable disease (SD), and progressive disease according to mRECIST. The time interval from treatment initiation to progressive disease was PFS. The time interval from the initiation of treatment to the death or last follow-up was OS.
Statistical analysis
χ2 test and McNemar analysis were used for categorical variables. Propensity score matching (PSM) was applied to identify two groups with similar baselines. Matching variables included age, sex, tumor size, alanine transaminase level, tumor number, platelet level, alkaline phosphatase level, Child-Pugh score, alpha-fetoprotein (AFP) level, leukocyte level, BCLC stage, portal vein invasion, hepatitis B virus infection, extrahepatic metastasis, and lymph node metastasis. PFS and OS were estimated using the Kaplan–Meier method and log-rank test. Cox analysis was used to identify prognostic factors affecting OS and PFS. Statistical analysis of this study was performed using SPSS for Windows version 26.0. Two-tailed P-value of <0.05 was considered significant.
Results
Patient characteristics prior to and following PSM
Between April 2019 and March 2022, a total of 197 patients who met the inclusion and exclusion criteria received the combination of PD-1 inhibitors with anti-angiogenic therapy and IMRT and PD-1 inhibitors plus anti-angiogenic therapy.
Prior to PSM, there were differences in gender, leukocyte level, BCLC stage, lymph node metastasis, and extrahepatic metastases between the two groups (P < 0.05 for all). Eighty patients were identified through PSM. In this matched cohort, no differences in any covariates at baseline were observed between the two groups ( Table 1 ).
Table 1.
Baseline characteristics of the patients before and after PSM.
| Variable | Before PSM | After PSM | ||||
|---|---|---|---|---|---|---|
| Triple therapy group | Control group | P | Triple therapy group | Control group | P | |
| Patients | 54 | 143 | 40 | 40 | ||
| Male sex | 51 (94.4) | 112 (78.3) | 0.008 | 37 (92.5) | 38 (95.0) | 1.000 |
| Age ≥ 65 years | 11 (20.4) | 29 (20.3) | 0.989 | 10 (25.0) | 6 (15.0) | 0.424 |
| Child–Pugh score | 0.735 | 0.568 | ||||
| 5 | 20 (37.0) | 62 (43.4) | 16 (40.0) | 15 (37.5) | ||
| 6 | 20 (37.0) | 39 (27.3) | 13 (32.5) | 13 (32.5) | ||
| 7 | 8 (14.8) | 27 (18.9) | 6 (15.0) | 8 (20.0) | ||
| 8 | 4 (7.4) | 10 (7.0) | 3 (7.5) | 2 (5.0) | ||
| 9 | 2 (3.7) | 5 (3.5) | 2 (5.0) | 2 (5.0) | ||
| Number of tumors ≥ 2 | 40 (74.1) | 118 (82.5) | 0.185 | 31 (77.5) | 28 (70.0) | 0.629 |
| Tumor diameter, cm | 0.243 | 0.937 | ||||
| < 3 | 3 (5.6) | 9 (6.3) | 2 (5.0) | 1 (2.5) | ||
| ≥ 3, < 5 | 6 (11.1) | 34 (23.8) | 6 (15.0) | 6 (15.0) | ||
| ≥ 5, < 10 | 30 (55.6) | 69 (48.3) | 21 (52.5) | 20 (50.0) | ||
| ≥ 10 | 15 (27.8) | 31 (21.7) | 11 (27.5) | 13 (32.5) | ||
| Serum AFP, ng/ml | 0.700 | 0.572 | ||||
| < 200 | 27 (50.0) | 79 (55.2) | 21 (52.5) | 17 (42.5) | ||
| ≥ 200, < 400 | 2 (3.7) | 7 (4.9) | 2 (5.0) | 4 (10.0) | ||
| ≥ 400 | 25 (46.3) | 57 (39.9) | 17 (42.5) | 19 (47.5) | ||
| ALP levels ≥ 125 U/L | 26 (48.1) | 87 (60.8) | 0.108 | 23 (57.5) | 23 (57.5) | 1.000 |
| Platelet count ≥ 100 × 109/L | 46 (85.2) | 109 (76.2) | 0.171 | 32 (80.0) | 35 (87.5) | 0.581 |
| ALT levels ≥ 40 U/L | 31 (57.4) | 74 (51.7) | 0.478 | 20 (50.0) | 20 (50.0) | 1.000 |
| Leukocyte ≥ 4 × 109/L | 41 (75.9) | 128 (89.5) | 0.015 | 30 (75.0) | 34 (85.0) | 0.388 |
| BCLC stage | 0.041 | 1.000 | ||||
| B | 3 (5.6) | 24 (16.8) | 3 (7.5) | 3 (7.5) | ||
| C | 51 (94.4) | 119 (83.2) | 37 (92.5) | 37 (92.5) | ||
| Portal vein invasion | 46 (85.2) | 91 (63.6) | 0.003 | 32 (80.0) | 32 (80.0) | 1.000 |
| HBV | 33 (61.1) | 77 (53.8) | 0.360 | 24 (60.0) | 21 (52.5) | 0.678 |
| Lymph node metastasis | 21 (38.9) | 80 (55.9) | 0.033 | 19 (47.5) | 19 (47.5) | 1.000 |
| Extrahepatic metastases | 11 (20.4) | 59 (41.3) | 0.006 | 9 (22.5) | 11 (27.5) | 0.774 |
| Lung | 4 (7.4) | 33 (23.1) | 3 (7.5) | 6 (15.0) | ||
| Bone | 6 (11.1) | 15 (10.5) | 5 (12.5) | 4 (10.0) | ||
| Other | 1 (1.9) | 28 (19.6) | 1 (2.5) | 5 (12.5) | ||
PSM, propensity score matching; AFP, alpha fetoprotein; ALP, alkaline phosphatase; ALT, alanine aminotransferase; BCLC, Barcelona Clinic Liver Cancer; HBV, hepatitis B virus.
The triple therapy group exhibited promising efficacy
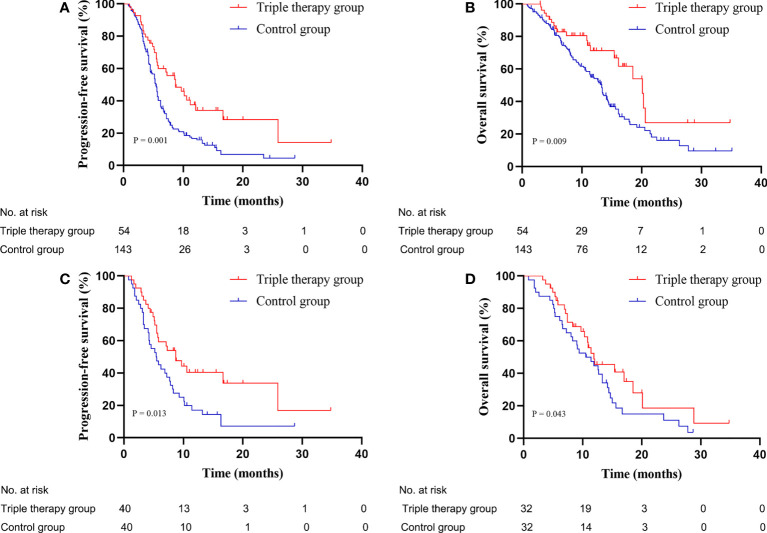
As of April 2022, before matching, a total of 91 (63.6%) and 19 (35.2%) patients died in the control group and the triple therapy group, respectively. The median follow-up time of the control group and triple therapy group was 15.5 and 12 months, respectively. Patients who received triple therapy had longer mPFS (8.7 vs 5.4 months, P = 0.001, Figure 1A ) and mOS (20.1 vs 13.3 months, P = 0.009, Figure 1B ) than those who received PD-1 inhibitors plus anti-angiogenic therapy. Following PSM, 14 patients (35%) in the triple therapy group and 27 patients (67.5%) in the control group died. Patients who received triple therapy had longer mPFS (8.7 vs 5.4 months, P = 0.013, Figure 1C ) and mOS (18.5 vs 12.6 months, P = 0.043, Figure 1D ) than those who received PD-1 inhibitors plus anti-angiogenic therapy.
Figure 1.
Kaplan-Meier plots: The triple therapy group exhibited longer mPFS (A, C) and mOS (B, D) than that of the control group before and after PSM. mPFS, median progression-free survival; mOS, median overall survival; PSM, propensity score matching.
PFS and OS in different subgroups
In the subgroup of patients with child-pugh class A and tumor diameter of ≥ 5 cm, the triple therapy group had longer mOS (not reach vs 14.4 months, P = 0.042, Supplementary Figure 1C ; 18.5 vs 11.4 months, P = 0.018, Supplementary Figure 1E ) and mPFS (25.9 vs 5.5 months, P = 0.005, Supplementary Figure 1H ; 8.7 vs 5.4 months, P = 0.009, Supplementary Figure 1J ) than the control group. However, in the subgroup analysis of patients with portal vein tumor thrombus (PVTT), child B, and extrahepatic metastases, there were no significant differences in OS and PFS between the two groups ( Supplementary Figure 1 ).
Tumor response
Prior to PSM, the ORR was 42.6% in the triple therapy group and 24.5% in the control group (P = 0.013). However, the disease control rates (DCR) of two groups were similar (90.7% vs 79.7%, P = 0.068). Following PSM, although the ORR and DCR of the triple therapy group were still slightly better than those of the control group, the differences were not significant (40% vs 25%, P = 0.152; 90% vs 77.5%, P = 0.130; respectively; Table 2 ).
Table 2.
Tumor response assessed by mRECIST.
| Best response | Before PSM | After PSM | ||||
|---|---|---|---|---|---|---|
| Triple therapy group | Control group | P | Triple therapy group | Control group | P | |
| Objective response | 23 (42.6) | 35 (24.5) | 0.013 | 16 (40.0) | 10 (25.0) | 0.152 |
| Disease control | 49 (90.7) | 114 (79.7) | 0.068 | 36 (90.0) | 31 (77.5) | 0.130 |
| Best overall response | ||||||
| Complete response | 1 (1.9) | 2 (1.4) | 1 (2.5) | 0 | ||
| Partial response | 22 (40.7) | 33 (23.1) | 15 (37.5) | 10 (25.0) | ||
| Stable disease | 26 (48.1) | 79 (55.2) | 20 (50.0) | 21 (52.5) | ||
| Progressive disease | 5 (9.3) | 29 (20.3) | 4 (10.0) | 9 (22.5) | ||
mRECIST, modified Response Evaluation Criteria in Solid Tumors.
Factors associated with PFS and OS following PSM
Univariate and multivariate Cox regression analyses were used to identify prognostic indicators affecting PFS and OS following PSM. Age, Child-Pugh class, AFP level, and triple therapy were determined to be influencing factors for PFS and OS (P < 0.05 for all). In the multivariate analysis, an AFP level of ≥400 ng/mL was an independent negative prognostic factor for PFS ( Table 3 ), whereas child B, lymph node metastasis, and treatment method were independent prognostic factors for OS ( Table 4 ).
Table 3.
Univariate and multivariate Cox regression analysis of progression-free survival after PSM.
| Variable | Univariable Cox regression | Multivariable Cox regression | ||||
|---|---|---|---|---|---|---|
| HR | 95%CI | P | HR | 95%CI | P | |
| Sex (male/female) | 2.121 | 0.657-6.853 | 0.209 | |||
| Age (≥65/<65 years) | 0.366 | 0.172-0.779 | 0.009 | 0.481 | 0.220-1.052 | 0.067 |
| Child-Pugh class (B/A) | 2.109 | 1.227-3.623 | 0.007 | 1.564 | 0.892-2.74 | 0.118 |
| Number of tumors (≥2/<2) | 1.584 | 0.859-2.922 | 0.141 | |||
| Tumor diameter (≥5/<5 cm) | 1.334 | 0.672-2.648 | 0.409 | |||
| AFP (≥400/<400 ng/ml) | 2.86 | 1.676-4.878 | <0.001 | 2.043 | 1.158-3.605 | 0.014 |
| ALP (≥125/<125 U/L) | 1.202 | 0.716-2.016 | 0.487 | |||
| Platelet (<100000/≥100000/μL) | 0.798 | 0.422-1.507 | 0.487 | |||
| ALT (≥40/<40U/L) | 1.129 | 0.676-1.887 | 0.642 | |||
| Leukocyte (<4000/≥4000/μL) | 0.730 | 0.400-1.333 | 0.305 | |||
| HBV (positive/negative) | 1.044 | 0.622-1.751 | 0.870 | |||
| Portal vein invasion (yes/no) | 1.291 | 0.669-2.490 | 0.446 | |||
| BCLC stage (C/B) | 1.008 | 0.363-2.798 | 0.987 | |||
| Lymph node metastasis (yes/no) | 1.287 | 0.767-2.159 | 0.340 | |||
| Extrahepatic metastases (yes/no) | 1.090 | 0.605-1.962 | 0.774 | |||
| Triple therapy (Yes/No) | 0.522 | 0.309-0.882 | 0.015 | 0.603 | 0.354-1.029 | 0.063 |
PSM, propensity score matching; HR, hazard ratio; AFP, alpha fetoprotein; ALP, alkaline phosphatase; ALT, alanine transaminase; HBV, hepatitis B virus; BCLC, Barcelona Clinic Liver Cancer.
Table 4.
Univariate and multivariate Cox regression analysis of overall survival after PSM.
| Variable | Univariable Cox regression | Multivariable Cox regression | ||||
|---|---|---|---|---|---|---|
| HR | 95%CI | P | HR | 95%CI | P | |
| Sex (male/female) | 1.924 | 0.460-8.048 | 0.37 | |||
| Age (≥65/<65 years) | 0.364 | 0.142-0.934 | 0.036 | 0.460 | 0.170-1.242 | 0.125 |
| Child-Pugh class (B/A) | 3.638 | 1.919-6.897 | <0.001 | 3.114 | 1.538-6.305 | 0.002 |
| Number of tumors (≥2/<2) | 2.035 | 0.931-4.449 | 0.075 | |||
| Tumor diameter (≥5/<5 cm) | 1.334 | 0.605-2.939 | 0.475 | |||
| AFP (≥400/<400 ng/ml) | 2.539 | 1.344-4.797 | 0.004 | 1.856 | 0.919-3.748 | 0.084 |
| ALP (≥125/<125 U/L) | 1.300 | 0.693-2.439 | 0.413 | |||
| Platelet (<100000/≥100000/μL) | 0.877 | 0.400-1.924 | 0.744 | |||
| ALT (≥40/<40U/L) | 0.927 | 0.501-1.716 | 0.809 | |||
| Leukocyte (<4000/≥4000/μL) | 0.72 | 0.343-1.511 | 0.385 | |||
| HBV (positive/negative) | 1.017 | 0.545-1.899 | 0.957 | |||
| Portal vein invasion (yes/no) | 2.091 | 0.819-5.339 | 0.123 | |||
| BCLC stage (C/B) | 3.172 | 0.434-23.157 | 0.255 | |||
| Lymph node metastasis (yes/no) | 1.928 | 1.014-3.665 | 0.045 | 2.002 | 1.036-3.871 | 0.039 |
| Extrahepatic metastases (yes/no) | 0.963 | 0.470-1.975 | 0.919 | |||
| Triple therapy (Yes/No) | 0.520 | 0.272-0.993 | 0.048 | 0.511 | 0.262-0.996 | 0.049 |
PSM, propensity score matching; HR, hazard ratio; AFP, alpha fetoprotein; ALP, alkaline phosphatase; ALT, alanine transaminase; HBV, hepatitis B virus; BCLC, Barcelona Clinic Liver Cancer.
Safety
We further investigated the TRAEs of the two groups. Treatment was interrupted in 55 patients (triple therapy group, 18; control group, 37) secondary to serious TRAEs. The addition of IMRT did not significantly increase the TRAEs of PD-1 inhibitors plus anti-angiogenic therapy (P < 0.05 for all). There were no treatment-related deaths ( Table 5 ).
Table 5.
Treatment-related adverse events in the two groups.
| Adverse Event | Triple therapy group | Control group | |||
|---|---|---|---|---|---|
| Grade 1-2 | Grade ≥3 | Grade 1-2 | Grade ≥3 | P | |
| Leukopenia | 29 (53.7) | 4 (7.4) | 58 (40.6) | 8 (5.6) | 0.173 |
| Thrombocytopenia | 24 (44.4) | 3 (5.6) | 52 (36.4) | 7 (4.9) | 0.541 |
| Decreased appetite | 15 (27.8) | 3 (5.6) | 32 (22.4) | 7 (4.9) | 0.699 |
| Neutropenia | 14 (25.9) | 1 (1.9) | 26 (18.2) | 2 (1.4) | 0.461 |
| Fatigue | 6 (11.1) | 2 (3.7) | 14 (9.8) | 5 (3.5) | 0.959 |
| Nausea | 8 (14.8) | 3 (5.6) | 16 (11.2) | 5 (3.5) | 0.612 |
| Anemia | 7 (13.0) | 1 (1.9) | 9 (6.3) | 1 (0.7) | 0.232 |
| Increased alanine aminotransferase | 10 (18.5) | 2 (3.7) | 18 (12.6) | 1 (0.7) | 0.160 |
| Rash | 4 (7.4) | 2 (3.7) | 8 (5.6) | 1 (0.7) | 0.268 |
| Pruritus | 4 (7.4) | 0 | 9 (6.3) | 1 (0.7) | 0.798 |
| Fever | 3 (5.6) | 0 | 5 (3.5) | 0 | 0.514 |
| Increased aspartate aminotransferase | 9 (16.7) | 2 (3.7) | 14 (9.8) | 3 (2.1) | 0.314 |
| Hypothyroidism | 3 (5.6) | 0 | 5 (3.5) | 0 | 0.514 |
| Hypertension | 2 (3.7) | 0 | 3 (2.1) | 0 | 0.523 |
| Headache | 1 (1.9) | 0 | 1 (0.7) | 1 (0.7) | 0.640 |
Discussion
Currently, although atezolizumab plus bevacizumab is the first recommendation for treating advanced HCC, its ORR of 27.3% remains unsatisfactory (4, 5). Therefore, it is necessary to explore other therapeutic methods that can improve the local control of advanced HCC. This was the first study on PD-1 inhibitors with anti-angiogenic therapy and IMRT vs PD-1 inhibitors plus anti-angiogenic therapy for the treatment of advanced HCC.
Prior to PSM, the triple therapy group had higher ORR (42.6% vs 24.5%, P = 0.013) and longer mOS (20.1 vs 13.3 months, P = 0.009) and mPFS (8.7 vs 5.4 months, P = 0.001) than those of the control group. Following PSM, the triple therapy group revealed better efficacy than the control group. This may be owing to strong local control of radiotherapy (12, 13). It not only induces immunogenic death but also modulates the tumor microenvironment to stimulate the production of antitumor T cells (14, 15). Moreover, radiotherapy increases the production of cell adhesion molecules, and targeting VEGF can promote the normalization of the vascular endothelium. This further enhances antitumor T cell infiltration (11, 16, 17).
Currently, new techniques such as stable homogeneous iodinated formulation technology hold good potential for surgical resection after arterial embolization in clinical practice (18). However, many HCC patients have already lost the opportunity for surgery. Immunotherapy plus targeted therapy for advanced HCC has been the focus of research (4–7), whereas the research on the combination of radiotherapy and immunotherapy is in its infancy. In a retrospective study of patients with HCC receiving stereotactic body radiotherapy (SBRT) plus PD-1 inhibitors, the mPFS was 19.6 months and ORR was 71% (19). Zhong et al. (20) observed that patients with advanced HCC treated with SBRT combined with PD-1 inhibitors had a higher ORR of 40%, mPFS of 3.8 months, and mOS of 21.2 months. Additionally, Ricke et al. reported that the mOS of patients with HCC receiving selective internal radiation therapy plus sorafenib was 12.1 months (21). Further, satisfactory results were also obtained with nivolumab plus ipilimumab for advanced HCC (mOS = 22.8 months, ORR = 32%) (22). In our study, the triple therapy group revealed better efficacy than the control group.
The safety of other methods based on PD-1 inhibitors plus anti-angiogenic therapy has been questioned. Liu et al. (23) confirmed that patients with HCC treated with hepatic artery infusion chemotherapy, tyrosine kinase inhibitors, and anti-PD-1 antibodies exhibited good efficacy (mPFS = 10.6 months, ORR = 63%) and safety. Furthermore, among patients with unresectable HCC, transarterial chemoembolization-lenvatinib-pembrolizumab sequential therapy exhibited promising efficacy (mPFS = 9.2 months, mOS = 18.1 months), with a well-characterized safety profile (24). In our research, we confirmed that the addition of IMRT did not significantly increase the TRAEs of PD-1 inhibitors plus anti-angiogenic therapy. Based on these findings, combining radiotherapy with immune and targeted therapies is a promising combination modality.
In the subgroups of patients with child A and tumor diameter ≥5 cm, the triple therapy group had more superior mOS and mPFS than the control group. However, in the other subgroups, there were no significant differences in OS and PFS between the two groups. Additionally, we observed that the ORR of the triple therapy group prior to PSM was better than that of the control group (42.6% vs 24.5%, P = 0.013) whereas the ORR of the two groups of patients following PSM was similar (40% vs 25%, P = 0.152). These may be owing to the smaller sample size.
Further, we explored prognostic factors affecting PFS and OS. The AFP level of ≥400 ng/mL is a risk factor for disease progression. However, for child A, without lymph node metastasis, triple therapy was an independent prognostic factor causing longer OS. Moreover, previous studies have also reported that these indicators were associated with prognosis (25–27).
This study had some limitations. First, although PSM was performed to minimize the effects of observed confounding factors, the effects of selectivity bias and various potential defects were not excluded. Second, despite this being the largest study reported to date, the number of patients in the triple therapy group remained less. Last, although our study confirms that IMRT further improves the efficacy of the combination of PD-1 inhibitors and anti-angiogenic therapy, it is still affected by the underlying heterogeneity of different therapeutic agents.
Conclusions
Conclusively, this study confirmed that the combination of PD-1 inhibitors with anti-angiogenic therapy and IMRT is a promising combination regimen. Our study provides a theoretical basis for studying combination therapy for HCC. Future prospective studies with larger sample sizes are needed to determine the efficacy of triple therapy.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by the Ethics Committee of the affiliated hospital of Southwest Medical University (approval number KY2020254). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
KS, LG, WM, JW, YX, MR, JZ, XL, LW, BL, XY, YS, WH, HC, TG, KX, YL, JC, ZW, YJ, HL, HZ, PW, XF, SC, BY, HJ, KH, and YH collected the data. YH and KH designed the research study. KS, LG, WM, and YH wrote the manuscript and analyzed the data. All authors approved the final version of the manuscript.
Funding
This work was supported by the Project of Science and Technology Department of Sichuan Province (2020JDTD0036) and the Nuclear Medicine and Molecular Imaging Key Laboratory of Sichuan Province (HYX18001).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2022.972503/full#supplementary-material
References
- 1. Sung H, Ferlay J, Siegel R, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 2. Llovet J, Zucman-Rossi J, Pikarsky E, Sangro B, Schwartz M, Sherman M, et al. Hepatocellular carcinoma. Nat Rev Dis Primers (2016) 2:16018. doi: 10.1038/nrdp.2016.18 [DOI] [PubMed] [Google Scholar]
- 3. Llovet J, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc J, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med (2008) 359(4):378–90. doi: 10.1056/NEJMoa0708857 [DOI] [PubMed] [Google Scholar]
- 4. Finn R, Qin S, Ikeda M, Galle P, Ducreux M, Kim T, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med (2020) 382(20):1894–905. doi: 10.1056/NEJMoa1915745 [DOI] [PubMed] [Google Scholar]
- 5. Cheng A, Qin S, Ikeda M, Galle P, Ducreux M, Kim T, et al. Updated efficacy and safety data from IMbrave150: Atezolizumab plus bevacizumab vs. sorafenib unresectable hepatocellular carcinoma. J Hepatol (2022) 76(4):862–73. doi: 10.1016/j.jhep.2021.11.030 [DOI] [PubMed] [Google Scholar]
- 6. Ren Z, Xu J, Bai Y, Xu A, Cang S, Du C, et al. Sintilimab plus a bevacizumab biosimilar (IBI305) versus sorafenib in unresectable hepatocellular carcinoma (ORIENT-32): a randomised, open-label, phase 2-3 study. Lancet Oncol (2021) 22(7):977–90. doi: 10.1016/s1470-2045(21)00252-7 [DOI] [PubMed] [Google Scholar]
- 7. Xu J, Shen J, Gu S, Zhang Y, Wu L, Wu J, et al. Camrelizumab in combination with apatinib in patients with advanced hepatocellular carcinoma (RESCUE): A nonrandomized, open-label, phase II trial. Clin Cancer Res (2021) 27(4):1003–11. doi: 10.1158/1078-0432.Ccr-20-2571 [DOI] [PubMed] [Google Scholar]
- 8. Abulimiti M, Li Z, Wang H, Apiziaji P, Abulimiti Y, Tan YJ. Combination intensity-modulated radiotherapy and sorafenib improves outcomes in hepatocellular carcinoma with portal vein tumor thrombosis. J Oncol (2021) 2021:1–10. doi: 10.1155/2021/9943683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Qiu H, Ke S, Cai G, Wu Y, Wang J, Shi W, et al. An exploratory clinical trial of apatinib combined with intensity-modulated radiation therapy for patients with unresectable hepatocellular carcinoma. Cancer Med (2022) 11(6):35. doi: 10.1002/cam4.4900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Herrera F, Bourhis J, Coukos GJ. Radiotherapy combination opportunities leveraging immunity for the next oncology practice. CA Cancer J Clin (2017) 67(1):65–85. doi: 10.3322/caac.21358 [DOI] [PubMed] [Google Scholar]
- 11. Missiaen R, Mazzone M, Bergers GJ. The reciprocal function and regulation of tumor vessels and immune cells offers new therapeutic opportunities in cancer. Semin Cancer Biol (2018) 52:107–16. doi: 10.1016/j.semcancer.2018.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chen B, Wu J, Cheng S, Wang L, Rong W, Wu F, et al. Phase 2 study of adjuvant radiotherapy following narrow-margin hepatectomy in patients with HCC. Hepatology (2021) 74(5):2595–604. doi: 10.1002/hep.31993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chen Z, Zhang X, Feng S, Feng J, Chai Z, Guo W, et al. Liver resection versus intensity-modulated radiation therapy for treatment of hepatocellular carcinoma with hepatic vein tumor thrombus: A propensity score matching analysis. Hepatobiliary Surg Nutr (2021) 10(5):646–60. doi: 10.21037/hbsn.2020.03.20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Levy A, Chargari C, Marabelle A, Perfettini J, Magné N, Deutsch EJ. Can immunostimulatory agents enhance the abscopal effect of radiotherapy? Eur J Cancer (2016) 62:36–45. doi: 10.1016/j.ejca.2016.03.067 [DOI] [PubMed] [Google Scholar]
- 15. Paris F, Fuks Z, Kang A, Capodieci P, Juan G, Ehleiter D, et al. Endothelial apoptosis as the primary lesion initiating intestinal radiation damage in mice. Science (2001) 293(5528):293–7. doi: 10.1126/science.1060191 [DOI] [PubMed] [Google Scholar]
- 16. Hallahan D, Kuchibhotla J, Wyble CJ. Cell adhesion molecules mediate radiation-induced leukocyte adhesion to the vascular endothelium. Cancer Res (1996) 56(22):5150–5. doi: [DOI] [PubMed] [Google Scholar]
- 17. Carvalho H, Villar RJC. Radiotherapy and immune response: The systemic effects of a local treatment. Clinics (2018) 73:e557s. doi: 10.6061/clinics/2018/e557s [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chen H, Cheng H, Dai Q, Cheng Y, Zhang Y, Li D, et al. A superstable homogeneous lipiodol-ICG formulation for locoregional hepatocellular carcinoma treatment. J Control Release (2020) 323:635–43. doi: 10.1016/j.jconrel.2020.04.021 [DOI] [PubMed] [Google Scholar]
- 19. Xiang Y, Wang K, Zheng Y, Feng S, Yu H, Li X, et al. Effects of stereotactic body radiation therapy plus PD-1 inhibitors for patients with transarterial chemoembolization refractory. Front Oncol (2022) 12:839605. doi: 10.3389/fonc.2022.839605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhong L, Wu D, Peng W, Sheng H, Xiao Y, Zhang X, et al. Safety of PD-1/PD-L1 inhibitors combined with palliative radiotherapy and anti-angiogenic therapy in advanced hepatocellular carcinoma. Front Oncol (2021) 11:686621. doi: 10.3389/fonc.2021.686621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ricke J, Klümpen H, Amthauer H, Bargellini I, Bartenstein P, de Toni E, et al. Impact of combined selective internal radiation therapy and sorafenib on survival in advanced hepatocellular carcinoma. J Hepatol (2019) 71(6):1164–74. doi: 10.1016/j.jhep.2019.08.006 [DOI] [PubMed] [Google Scholar]
- 22. Yau T, Kang Y, Kim T, El-Khoueiry A, Santoro A, Sangro B, et al. Efficacy and safety of nivolumab plus ipilimumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib: The CheckMate 040 randomized clinical trial. JAMA Oncol (2020) 6(11):e204564. doi: 10.1001/jamaoncol.2020.4564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Liu B, Gao S, Zhu X, Guo J, Kou F, Liu S, et al. Real-world study of hepatic artery infusion chemotherapy combined with anti-PD-1 immunotherapy and tyrosine kinase inhibitors for advanced hepatocellular carcinoma. Immunotherapy (2021) 13(17):1395–405. doi: 10.2217/imt-2021-0192 [DOI] [PubMed] [Google Scholar]
- 24. Chen S, Wu Z, Shi F, Mai Q, Wang L, Wang F, et al. Lenvatinib plus TACE with or without pembrolizumab for the treatment of initially unresectable hepatocellular carcinoma harbouring PD-L1 expression: A retrospective study. J Cancer Res Clin Oncol (2022) 148(8):2115–25. doi: 10.1007/s00432-021-03767-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mehta N, Dodge J, Roberts J, Yao FJ. A novel waitlist dropout score for hepatocellular carcinoma - identifying a threshold that predicts worse post-transplant survival. J Hepatol (2021) 74(4):829–37. doi: 10.1016/j.jhep.2020.10.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vitale A, Burra P, Frigo A, Trevisani F, Farinati F, Spolverato G, et al. Survival benefit of liver resection for patients with hepatocellular carcinoma across different Barcelona clinic liver cancer stages: A multicentre study. J Hepatol (2015) 62(3):617–24. doi: 10.1016/j.jhep.2014.10.037 [DOI] [PubMed] [Google Scholar]
- 27. Xia F, Wu L, Lau W, Li G, Huan H, Qian C, et al. Positive lymph node metastasis has a marked impact on the long-term survival of patients with hepatocellular carcinoma with extrahepatic metastasis. PloS One (2014) 9(4):e95889. doi: 10.1371/journal.pone.0095889 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.