Abstract
Standard-of-care immunotherapy for non–muscle-invasive bladder cancer (NMIBC) with intravesical Bacillus Calmettte-Guérin (BCG) is associated with adverse events (AEs), disease recurrence/progression, and supply shortages. Preclinical data have shown that intravesical instillation of Ty21a/Vivotif, the oral vaccine against typhoid fever, may be an effective and safer alternative to BCG. We assessed the safety of intravesical Ty21a in NMIBC. For ethical reasons, patients with low- or intermediate-risk NMIBC not requiring BCG immunotherapy were enrolled. To determine the maximum tolerated dose, escalating doses of Ty21a/Vivotif were intravesically instilled in three patients once a week for 4 wk in phase 1a. In phase 1b, ten patients received the selected dose (1 × 108 CFU) once a week for 6 wk, as for standard BCG therapy. At this dose, all patients completed their treatment. Most patients experienced minor systemic AEs, while half reported mild local bladder AEs. AEs only occurred after one or two instillations for 40% of the patients. Ty21a bacteria were only recovered in three out of 72 urinary samples at 1 wk after instillation. Intravesical Ty21a might be well tolerated with no cumulative side effects, no fever >39 °C, and lower risk of bacterial persistence than with BCG. Ty21a treatment thus warrants clinical trials to explore its safety and antitumor efficacy in high-risk NMIBC. This trial is registered on ClinicalTrials.gov as NCT03421236.
Patient summary
We examined the safety of a new intra-bladder immunotherapy for non–muscle-invasive bladder cancer as an alternative to the standard BCG treatment. Our data show that the Ty21a vaccine might be well tolerated. Further studies are needed to determine the safety and antitumor efficacy of this treatment.
Keywords: Intravesical microbial immunotherapy, Non–muscle-invasive bladder cancer, Phase 1 trial, Salmonella Ty21a
Some 70% of bladder cancers are non–muscle-invasive bladder cancer (NMIBC) at diagnosis [1]. The gold standard treatment for reducing the recurrence and progression of high-risk lesions is intravesical Bacillus Calmette-Guérin (BCG) immunotherapy. However, repeated BCG treatments are associated with significant side effects [2] and treatment failure (30–50%) [3], and there are currently manufacturing shortages [4], so there is a need for alternative or complementary treatments. The highly attenuated Salmonella enterica serovar Typhi strain Ty21a was obtained almost 50 yr ago via mutagenesis [5]. Ty21a was included in a commercial oral vaccine against typhoid fever (Vivotif) that has an excellent safety profile confirmed worldwide in more than 200 million vaccinees over the last 30 yr [6]. We provided preclinical evidence [7], [8] of its safe intravesical use for induction of bladder tumor regression in an immunocompetent mouse model that closely mimics NMIBC [9]. Here we report on the safety of intravesical Ty21a in NMIBC patients. For ethical reasons, only patients with low- or intermediate-risk NMIBC not requiring BCG were enrolled. This trial is registered on ClinicalTrials.gov as NCT03421236.
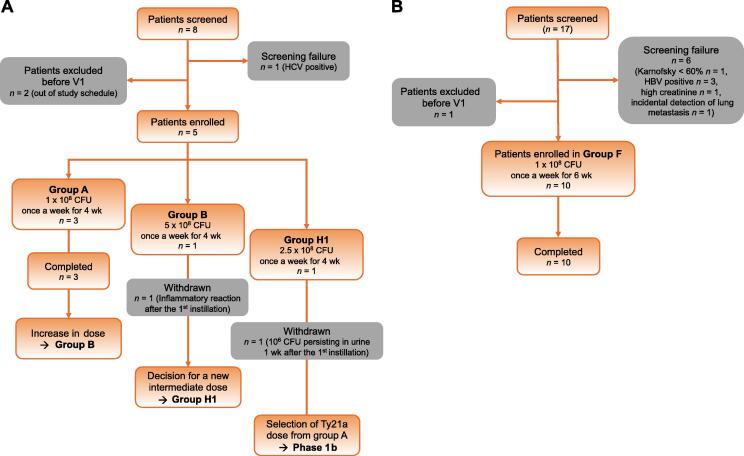
In this open-label phase 1 dose escalation study, 15 patients were recruited between May 2018 and May 2021 at our institution. Male and female patients with histological confirmation of low- or intermediate-risk NMIBC not requiring BCG treatment were included (Supplementary Table 1). A traditional 3 + 3 design for a dose escalation trial was adopted (Supplementary material) and adverse events (AEs) were graded using the Common Terminology Criteria for Adverse Events classification scheme. In phase 1a, eight patients were screened and five were enrolled (Fig. 1A). Among the first three patients (group A; Supplementary Table 1), who received the starting minimal dose of 1 × 108 colony-forming units (CFU) once weekly for 4 wk, two (67%) experienced mild AEs (grade 1; Table 1). A fivefold higher dose (5 × 108 CFU) was then instilled in a fourth patient (group B; Fig. 1A, Supplementary Table 1), who experienced a strong inflammatory syndrome (grade 2) 3 d later, with spontaneous remission within 48 h (Supplementary material). An intermediate dose was thus instilled in a fifth patient (2.5 × 108 CFU, group H1; Fig. 1A, Supplementary Table 1). At 1 wk after the first instillation, urinary culture revealed persistence of 1 × 106 CFU/ml of Ty21a bacteria and the patient reported mild (grade 1) local bladder and systemic AEs that all cleared after antibiotic treatment (Supplementary material). On the basis of these AEs, we considered 1 × 108 CFU of Ty21a as the maximum tolerated dose for phase 1b, which was instilled in ten patients once a week for 6 wk (group F; Fig. 1B, Supplementary Table 1). All patients completed the study. Most (n = 9, 90%) experienced minor systemic AEs (mainly general malaise and grade 1 AEs; Table 1) and half (n = 5, 50%) experienced mild (grade 1 or 2) local bladder AEs (mainly chemical cystitis and just one instance each of frequency and hematuria; Table 1). Four of the patients (40%) experienced AEs only after one or two instillations, while two patients (20%) reported AEs after five or six instillations. In addition, 30% of the patients reported AEs only after the fourth instillation.
Fig. 1.
Study flow diagram. Patients screened and included in (A) phase 1a and (B) phase 1b. CFU = colony-forming units; HBV = hepatitis B virus; HCV = hepatitis C virus; V1 = first visit.
Table 1.
Systemic and local bladder AEs per patienta
| Patients, n (%) |
||||||
|---|---|---|---|---|---|---|
| Group A (n = 3) |
Group F (n = 10) |
|||||
| Grade 1 | Grade 2 | Grades 1 + 2 |
Grade 1 | Grade 2 | Grades 1 + 2 |
|
| Systemic AEs | ||||||
| General malaiseb | 1 (33.3) | 0 | 1 (33.3) | 5 (50) | 1 (10) | 6 (60) |
| Fever >39 °C | 0 | 0 | 0 | 0 | 0 | 0 |
| Arthritis | 0 | 0 | 0 | 0 | 0 | 0 |
| Allergic reactions | 0 | 0 | 0 | 0 | 0 | 0 |
| Fever >38 °C c | 0 | 0 | 0 | 3 (30) | 0 | 3 (30) |
| Othersd | 1 (33.3) | 0 | 1 (33.3) | 3 (30) | 0 | 3 (30) |
| Any systemic AE | 2 (66.7) | 0 | 2 (66.7) | 8 (80) | 1 (10) | 9 (90) |
| Local bladder AEs | ||||||
| Bacterial cystitis | 1 (33.3) | 0 | 1 (33.3) | 1 (10) | 0 | 1 (10) |
| Chemical cystitise | 0 | 0 | 0 | 2 (20) | 1(10) | 3 (30) |
| Frequency >1/h | 0 | 0 | 0 | 0 | 1 (10) | 1 (10) |
| Hematuria | 0 | 0 | 0 | 1 (10) | 0 | 1 (10) |
| Ty21a cystitisf | 0 | 0 | 0 | 0 | 1 (10) | 1 (10) |
| Otherg | 0 | 0 | 0 | 0 | 1 (10) | 1 (10) |
| Any local bladder AE | 1 (33.3) | 0 | 1 (33.3) | 3 (30) | 2 (20) | 5 (50) |
| Any systemic or local bladder AE | 2 (66.7) | 0 | 2 (66.7) | 6 (60) | 3 (30) | 9 (90) |
| AEs after installations | ||||||
| After 1–2 instillations | 1 (33.3) | 0 | 1 (33.3) | 4 (40) | 0 | 4 (40) |
| After 3–4 instillations | 1 (33.3) | 0 | 1 (33.3) | 2 (20) | 1(10)i | 3 (30) |
| After 5 instillations | NA | NA | NA | 0 | 2 (20)j | 2 (20) |
| AEs only after the 4th dose | 0 | 0 | 0 | 3 (30) | 0 | 3 (30) |
| Asymptomatic urinary Ty21h | 0 | 2 (20) | ||||
AE = adverse event; NA = not applicable.
AEs were graded using the Common Terminology Criteria for Adverse Events classification scheme.
Flu-like symptoms, shaking chills, headache, discomfort, and/or fatigue.
Three patients experienced 1-d fever (38.8 °C after the second instillation in patient 17F05, 38.9 °C after the first instillation in patient 22F07, and 38.5 °C after the second instillation in patient 23F08).
Erythema, arthralgia, loss of appetite, and/or diarrhea.
Urgency, urinary incontinence, urinary tract pain, and/or dysuria in the absence of urinary bacteria.
Cystitis symptoms with concomitant urinary Ty21a detection (104 CFU/ml 1 wk after the second instillation in patient 19F06).
Right hypochondrium pain.
Urinary 104 CFU/ml 1 wk after the third instillation in patient 12F03 and 103 CFU/ml 1 wk after the first instillation in patient 23F08.
experiened a Grade 2 AE after three instillations.
One patient experienced a grade 2 AE after one instillation and one patient experienced a grade 2 AE after four instillations.
Comparison of the frequency of instillations with systemic AEs (16.7% in group A and 41.7% in group F) and local bladder AEs (8.3% in group A and 18.3% in group F) shows that a majority of the Ty21a instillations were innocuous (Supplementary Table 2). Furthermore, no cumulative side effects were observed, as neither the number of patients reporting systemic or local bladder AEs nor the total number of systemic or local bladder AEs per instillation increased with successive intravesical Ty21a instillations (Supplementary Table 3), which differs from results observed after intravesical BCG instillations [10], [11]. In comparison to historical BCG side effects [12], [13], Ty21a appears to induce fewer local bladder AEs, but more systemic AEs, although no high fever (>39 °C) was reported and general malaise was the most common minor systemic AE. Quite unexpectedly in the context of the mild AEs observed with the selected Ty21a dose, first instillation of a fivefold or 2.5-fold higher dose resulted in AEs that dissuaded us from further testing these doses. Of note, the recovery of a relatively high number of live Ty21a bacteria in the urine of the patient in group H1 is in contrast to our preclinical study, in which Ty21a bacteria did not persist in bladder tissues [7]. Shedding of Ty21a bacteria in the stool of vaccinees is a rare event, only occurring within the first 24 h after oral vaccination [6]. Although ingestion of higher doses (3–10 × 1010 CFU, tenfold higher than the usual oral vaccine dose) results in excretion in the stools of approximately 30% of recipients after 1 d, no bacteria were recovered 3 d later, strongly suggesting the inability of Ty21a to proliferate in vivo, at least in the gastrointestinal tract [14]. It is also highly unlikely that Ty21a can replicate in the urinary tract given the harsh environment of urine [15], the metabolic impairments in Ty21a [14], and the rare recovery (mostly asymptomatic) of wild-type S. enterica serovar Typhi from urine, even in endemic areas of typhoid fever [16]. In our study, intravesical instillation of 1 × 108 CFU resulted in recovery of Ty21a bacteria (103–104 CFU/ml) in only three out of 72 urinary samples collected 7 d after instillation (Table 1), one from a symptomatic patient with cystitis and the other two from asymptomatic patients. This suggests that persistence of Ty21a in the urinary tract is also a rare event at the selected dose, which should not jeopardize safety. This is in contrast to BCG bacteria, which are found in approximately 30% of urine samples 7 d after intravesical instillation [17] and whose subsequent local or disseminated infection can be the cause of the most severe, albeit rare, AEs associated with BCG immunotherapy [2]. Overall, our data suggest that intravesical Ty21a might be well tolerated with no cumulative side effects, no fever >39 °C, and a lower risk of bacterial persistence than with BCG.
Despite renewed interest in microbial cancer immunotherapy, intravesical BCG is still the only bacterial cancer therapy approved for clinical use. Building on our promising preclinical data, although limited by the small population included, our study shows that another commercial bacterial vaccine, Ty21a, is promising when used for intravesical instillation in NMIBC, with few and mild AEs at the selected dose. Future clinical trials are warrant to evaluate the safety and efficacy of intravesical Ty21a in reducing NMIBC recurrence and progression in high-risk patients and to explore the mechanisms underlying its effects.
Author contributions: Denise Nardelli-Haefliger had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Derré, Jichlinski, Lucca, Nardelli-Haefliger.
Acquisition of data: Benmerzoug, Bohner, Cesson, Chevalier, Crettenand, Dartiguenave, Derré, Domingos-Pereira, Jichlinski, Masnada, Lucca, Nguyen, Polak, Rodrigues-Dias, Roth, Schneider, Texeira-Pereira.
Analysis and interpretation of data: Cesson, Derré, Lucca, Nardelli-Haefliger.
Drafting of the manuscript: Cesson, Derré, Lucca, Nardelli-Haefliger.
Critical revision of the manuscript for important intellectual content: Benmerzoug, Bohner, Cesson, Chevalier, Crettenand, Dartiguenave, Derré, Domingos-Pereira, Jichlinski, Masnada, Lucca, Nguyen, Polak, Rodrigues-Dias, Roth, Schneider, Texeira-Pereira.
Statistical analysis: Nardelli-Haefliger.
Obtaining funding: Jichlinski, Nardelli-Haefliger.
Administrative, technical, or material support: Cesson, Rodrigues-Dias, Dartiguenave.
Supervision: Derré, Jichlinski, Lucca, Nardelli-Haefliger, Roth.
Other: None.
Financial disclosures: Denise Nardelli-Haefliger certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (e.g. employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Denise Nardelli-Haefliger, Sonia Domingos-Pereira, and Patrice Jichlinski are inventors of patent PCT/EP2014/059392, “Salmonella strains for use in the treatment and/or prevention of cancer”, which is exclusively licensed to Prokarium. The company was not involved in this study. The remaining authors have nothing to disclose.
Funding/Support and role of the sponsor: This study was funded by the Swiss National Fund (FNS 320030-172991) and by the Technology Transfer Office of UNIL-CHUV via an InnoSTEP grant to Denise Nardelli-Haefliger. The sponsors played no direct role in the study.
Associate Editor: M. Carmen Mir
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.euros.2022.09.004.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Kamat A.M., Hahn N.M., Efstathiou J.A., et al. Bladder cancer. Lancet. 2016;388:2796–2810. doi: 10.1016/S0140-6736(16)30512-8. [DOI] [PubMed] [Google Scholar]
- 2.Gontero P., Bohle A., Malmstrom P.U., et al. The role of bacillus Calmette-Guerin in the treatment of non–muscle-invasive bladder cancer. Eur Urol. 2010;57:410–429. doi: 10.1016/j.eururo.2009.11.023. [DOI] [PubMed] [Google Scholar]
- 3.Babjuk M., Bohle A., Burger M., et al. EAU guidelines on non–muscle-invasive urothelial carcinoma of the bladder: update 2016. Eur Urol. 2017;71:447–461. doi: 10.1016/j.eururo.2016.05.041. [DOI] [PubMed] [Google Scholar]
- 4.Mostafid A.H., Palou Redorta J., Sylvester R., Witjes J.A. Therapeutic options in high-risk non–muscle-invasive bladder cancer during the current worldwide shortage of bacille Calmette-Guerin. Eur Urol. 2015;67:359–360. doi: 10.1016/j.eururo.2014.11.031. [DOI] [PubMed] [Google Scholar]
- 5.Germanier R., Fürer E. Isolation and characterization of galE mutant Ty 21a of Salmonella typhi. A candidate strain for a live, oral typhoid vaccine. J Infect Dis. 1975;131:553–558. doi: 10.1093/infdis/131.5.553. [DOI] [PubMed] [Google Scholar]
- 6.Guzman C.A., Borsutzky S., Griot-Wenk M., et al. Vaccines against typhoid fever. Vaccine. 2006;24:3804–3811. doi: 10.1016/j.vaccine.2005.07.111. [DOI] [PubMed] [Google Scholar]
- 7.Domingos-Pereira S., Cesson V., Chevalier M.F., Derre L., Jichlinski P., Nardelli-Haefliger D. Preclinical efficacy and safety of the Ty21a vaccine strain for intravesical immunotherapy of non-muscle-invasive bladder cancer. Oncoimmunology. 2017;6:e1265720. doi: 10.1080/2162402X.2016.1265720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Domingos-Pereira S., Sathiyanadan K., La Rosa S., et al. Intravesical Ty21a vaccine promotes dendritic cells and T cell-mediated tumor regression in the MB49 bladder cancer model. Cancer Immunol Res. 2019;7:621–629. doi: 10.1158/2326-6066.CIR-18-0671. [DOI] [PubMed] [Google Scholar]
- 9.Summerhayes I.C., Franks L.M. Effects of donor age on neoplastic transformation of adult mouse bladder epithelium in vitro. J Natl Cancer Inst. 1979;62:1017–1023. [PubMed] [Google Scholar]
- 10.Orihuela E., Herr H.W., Pinsky C.M., Whitmore W.F., Jr. Toxicity of intravesical BCG and its management in patients with superficial bladder tumors. Cancer. 1987;60:326–333. doi: 10.1002/1097-0142(19870801)60:3<326::aid-cncr2820600309>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 11.Berry D.L., Blumenstein B.A., Magyary D.L., Lamm D.L., Crawford E.D. Local toxicity patterns associated with intravesical bacillus Calmette-Guerin: a Southwest Oncology Group study. Int J Urol. 1996;3:98–100. doi: 10.1111/j.1442-2042.1996.tb00490.x. [DOI] [PubMed] [Google Scholar]
- 12.van der Meijden A.P., Sylvester R.J., Oosterlinck W., Hoeltl W., Bono A.V. EORTC Genito-Urinary Tract Cancer Group. Maintenance bacillus Calmette-Guerin for Ta T1 bladder tumors is not associated with increased toxicity: results from a European Organisation for Research and Treatment of Cancer Genito-Urinary Group phase III trial. Eur Urol. 2003;44:429–434. doi: 10.1016/s0302-2838(03)00357-9. [DOI] [PubMed] [Google Scholar]
- 13.Koch G.E., Smelser W.W., Chang S.S. Side effects of intravesical BCG and chemotherapy for bladder cancer: what they are and how to manage them. Urology. 2021;149:11–20. doi: 10.1016/j.urology.2020.10.039. [DOI] [PubMed] [Google Scholar]
- 14.Gilman R.H., Hornick R.B., Woodard W.E., et al. Evaluation of a UDP-glucose-4-epimeraseless mutant of Salmonella typhi as a liver oral vaccine. J Infect Dis. 1977;136:717–723. doi: 10.1093/infdis/136.6.717. [DOI] [PubMed] [Google Scholar]
- 15.Ipe D.S., Horton E., Ulett G.C. The basics of bacteriuria: strategies of microbes for persistence in urine. Front Cell Infect Microbiol. 2016;6:14. doi: 10.3389/fcimb.2016.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mathai E., John T.J., Rani M., et al. Significance of Salmonella typhi bacteriuria. J Clin Microbiol. 1995;33:1791–1792. doi: 10.1128/jcm.33.7.1791-1792.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Durek C., Richter E., Basteck A., et al. The fate of bacillus Calmette-Guerin after intravesical instillation. J Urol. 2001;165:1765–1768. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.