Abstract
Background and purpose
Nabiximols is a therapeutic option for patients with multiple sclerosis (MS) spasticity whose symptoms are poorly controlled by conventional oral first‐line medications. This study aimed to assess the relationship between changes in spasticity severity (measured on the 0–10 numeric rating scale [NRS]) and the presence of associated symptoms in patients treated with nabiximols, and to investigate the presence of the newly described ‘spasticity‐plus syndrome’.
Methods
We analyzed real‐world data from the Italian Medicines Agency e‐Registry on 1138 patients with MS spasticity who began treatment with nabiximols. Evaluation time points were baseline, 4 weeks, and 3, 6, 12 and 18 months after treatment start.
Results
Common symptoms associated with MS spasticity in this cohort were pain (38.4% at baseline), sleep disturbances (32.7%), and spasms/cramps (28.5%). Pain was frequently clustered with sleep disturbances (57.2% of pain cases) and spasms/cramps (43.9%). Approximately one‐third of patients with data at all evaluation time points maintained treatment at 18 months. Nabiximols reduced the baseline mean spasticity 0–10 NRS score by 24.6% at Week 4, and by 33.9% at 18 months in treatment continuers. Nabiximols resolved a range of MS spasticity‐associated symptoms at Week 4, and after 18 months in treatment continuers.
Conclusion
This real‐world analysis supports the concept of a spasticity‐plus syndrome and suggests that nabiximols can favorably impact a range of spasticity‐associated symptoms.
Keywords: e‐Registry analysis, multiple sclerosis, nabiximols, spasticity‐plus syndrome, spasticity‐related symptoms
This Agenzia Italiana del Farmaco (Italian Medicines Agency) e‐Registry analysis examined the presence of ‘spasticity‐plus syndrome’ in patients with multiple sclerosis (MS) spasticity treated with nabiximols oromucosal spray (n = 1138). The most common spasticity‐associated symptoms at baseline were pain (38.4%), sleep disturbances (32.7%), and spasms/cramps (28.5%). Spasticity‐associated symptoms were present in fewer patients who were still receiving nabiximols at 18 months and had available symptom data (n = 179). No relevant differences in symptom resolution rates were observed according to MS type, disease duration or gender. Along with relevant and durable improvement in MS spasticity, nabiximols responders can experience resolution of MS spasticity‐associated symptoms.
INTRODUCTION
Spasticity is one the most frequent complications of multiple sclerosis (MS), present with a least moderate severity in approximately one‐third of patients within 10 years of diagnosis and tending to worsen over time [1, 2]. Along with mobility restrictions, spasticity‐associated symptoms such as spasms, pain, sleep disturbances, and bladder dysfunction can interfere with patients' daily activities [3], profoundly impair autonomy and quality of life [4, 5, 6, 7], and increase healthcare resource consumption [2].
The traditional approach to management of MS‐related symptoms has been ‘organ‐oriented’, aiming to resolve complications at a local level, for example, muscle relaxants for spasticity, gabapentin and other agents for pain relief, and anticholinergics for bladder dysfunction [8, 9]. However, because an excess of medication can lead to interactions and intolerable adverse events, effective symptom management in MS continues to be an unmet need [10, 11]. The mediation of many MS symptoms in the brainstem, where there is also a high concentration of endocannabinoid receptors [10], points to a role for cannabinoids in symptom management.
Nabiximols (Sativex®, GW Pharmaceuticals) oromucosal spray is a complex botanical mixture containing balanced quantities of Δ‐9‐tetrahydrocannabinol and cannabidiol, along with other cannabinoid and non‐cannabinoid components [12]. Nabiximols' mechanism of action involves mimicking the effects of endogenous endocannabinoids at cannabinoid CB1 and CB2 receptors [13]. In Europe and other world regions, nabiximols is indicated as add‐on treatment for symptom improvement in adult patients with moderate to severe MS spasticity who have not responded adequately to conventional oral anti‐spasticity medications and who demonstrate clinically significant improvement in spasticity‐related symptoms during an initial trial of therapy [14]. European guidelines reflect the positioning of nabiximols for symptomatic management of MS spasticity [15, 16, 17, 18]. Evidence that nabiximols can ameliorate simultaneously occurring MS spasticity‐related symptoms would not only support the concept of the recently described ‘spasticity‐plus syndrome’ [10, 11], but also simplify the approach to symptomatic management of MS patients in daily practice, potentially reducing the risks associated with polypharmacy [19].
The spasticity 0–10 numeric rating scale (NRS) [20] is widely used to assess MS spasticity evolution during treatment with nabiximols. Improvement from baseline in NRS scores of ≥20% at 4 weeks (initial response) and ≥ 30% (clinically relevant response) at subsequent time points are the thresholds required to continue treatment. However, the NRS does not take into account spasticity‐associated symptoms, which may influence patients' overall perception of their muscle stiffness.
The Italian Medicines Agency (Agenzia Italiana del Farmaco [AIFA]) uses monitoring registries to track patient eligibility and enhance appropriate use of certain pharmaceutical products in their approved indications [21]. From 2013 to 2020, AIFA required that all MS patients eligible to begin add‐on treatment with nabiximols oromucosal spray be enrolled in an ad hoc prospective e‐Registry that reflected approved prescription criteria: age ≥18 years with moderate to severe MS spasticity (0–10 NRS score ≥ 4) not responding adequately to current anti‐spasticity medication. Recording of spasticity 0–10 NRS scores in the e‐Registry was required to initiate and renew prescriptions. Patients who failed to achieve ≥20% NRS improvement after a 4‐week trial period of nabiximols (non‐responders) were deemed to discontinue treatment.
Previous analyses of AIFA e‐Registry data have reported on the effectiveness and safety of nabiximols during the first 6 months of treatment [22], discontinuation rates by 6 weeks [23] and 18 months [24] after treatment start, and the effect of nabiximols on MS spasticity‐related symptoms during the first 4 weeks of treatment [25]. The current AIFA e‐Registry analysis was undertaken to assess the relationship between a change in MS spasticity severity (measured on the NRS) and the presence of spasticity‐associated symptoms for up to 18 months in nabiximols‐treated patients, and to investigate the existence of the spasticity‐plus syndrome.
METHODS
Design and setting
This retrospective, multicenter, observational study in a routine outpatient setting analyzed prospective data reported in the AIFA e‐Registry for Italian adult patients with moderate to severe MS spasticity who were treated with nabiximols for symptom improvement. Exclusion criteria for nabiximols treatment were: severe cardiovascular diseases; history of psychiatric diseases; use of street cannabis and/or other psychoactive drugs; and/or a MS spasticity NRS score < 4. Patients were enrolled consecutively in the AIFA e‐Registry at the start of nabiximols treatment (baseline) and followed prospectively. Data collection time points were at scheduled clinic visits: baseline, 4 weeks, and 3, 6, 12 and 18 months after treatment start. At all centers, clinical evaluations were performed by highly experienced neurologists. Patients were treated in accordance with the approved nabiximols label [14] and standards of good clinical practice.
Baseline demographic and clinical data on patients' age, gender, MS type, MS duration, Expanded Disability Status Scale (EDSS) score, MS spasticity‐related symptoms, and nabiximols doses were provided by the e‐Registry and supplemented as required by patients' medical records. MS spasticity 0–10 NRS evolution and treatment discontinuations (numbers and reasons) were analyzed based on data recorded and stored in the e‐Registry at each scheduled clinical evaluation.
Study population
The study population was derived from the same AIFA e‐Registry database as reported in previously published analyses [22, 23, 24, 25].
Effectiveness analyses
Responder rates were calculated as the proportion of patients with an improvement of ≥20% or ≥30% from baseline in their MS spasticity 0–10 NRS score, as measured on the day of the clinic visit. Additional effectiveness measures assessed at each time point were: absolute mean ± standard deviation (SD) MS spasticity 0–10 NRS scores and relative (%) and absolute mean ± SD changes from baseline in MS spasticity 0–10 NRS scores; presence (yes/no) of MS spasticity‐associated symptoms (bladder dysfunction, clonic movements, mood disorders, pain, sleep disturbances, spasms/cramps, trigeminal neuralgia); and total number of MS spasticity‐associated symptoms.
Nabiximols dose
At each assessment time point, the mean ± SD number of daily sprays of nabiximols was calculated based on data recorded in the e‐Registry. The first few weeks of nabiximols treatment included the titration phase as per the approved label [14].
Statistical analyses
Data were analyzed for all patients enrolled in the AIFA e‐Registry who initiated treatment with nabiximols. No sample size calculation was necessary.
Continuous variables are described as mean ± SD or median and range (min–max), and categorical variables as counts (n) and percentages (%). The presence or absence of reported MS spasticity‐associated symptoms was summarized using shift (frequency) tables of baseline versus each assessment time point. An analysis of covariance model was used to estimate the change from baseline in total number of symptoms at Week 4 in non‐responders and responders to nabiximols using responder criterion rates (≥20% and ≥30%) as a factor and baseline number of symptoms as a covariate.
To account for missing data, the non‐responder imputation method was used for changes in MS spasticity 0–10 NRS scores when treatment had been discontinued due to lack of effectiveness or adverse events (imputed as 0% change), but not for cases where reasons for discontinuation were ‘other’ or ‘unknown’. The last observation carried forward (LOCF) method was used to impute missing data for frequency of MS spasticity‐associated symptoms.
All analyses were performed using SAS version 9.4 (SAS Institute Inc).
Ethics statement
The study was approved by the Policlinico‐Vittorio Emanuele (Catania, Italy) Ethics Committee (number 37/2015/PO) and by the Ethics Committees of other participating centers. As the study was observational and data were fully anonymized, no written patient consent was required (according to Italian law).
RESULTS
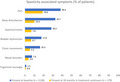
The AIFA e‐Registry data were retrieved and analyzed for 1138 patients who began treatment with nabiximols between January 2015 and June 2018 at 32 large MS centers across Italy. Baseline demographic and clinical characteristics are summarized in Table 1. Patients' mean age was 51.5 years, 54.6% were female and most (66.9%) had secondary progressive MS. The mean disease duration since MS diagnosis was 19.8 years, the mean spasticity 0–10 NRS score was 7.8 ± 1.25, and the mean EDSS score was 6.5 ± 1.16. The most common MS spasticity‐associated symptoms present at baseline were pain (38.4%), sleep disturbances (32.7%), spasms/cramps (28.5%), bladder dysfunction (17.8%), and tonic/clonic movements (16.3%).
TABLE 1.
Baseline demographic and clinical characteristics
| Characteristic | Total population (n = 1138) |
|---|---|
| Age, years | 51.5 ± 9.8 |
| Female n (%) | 621 (54.6) |
| Disease duration since MS diagnosis, years a | 19.8 ± 10.5 |
| Range, years | 1.0–67.0 |
| Disease duration a n (%) | |
| <5 years | 20 (2.0) |
| 5 to <10 years | 124 (12.4) |
| 10 to <15 years | 181 (18.1) |
| 15 to <20 years | 244 (24.4) |
| ≥20 years | 431 (43.1) |
| MS course type b n (%) | 1137 (100.0) |
| Relapsing–remitting | 193 (17.0) |
| Secondary progressive | 761 (66.9) |
| Primary progressive | 183 (16.1) |
| 0–10 Expanded Disability Status Scale score c | 6.5 ± 1.16 |
| Range (score) | 2.0–9.5 |
| Spasticity 0–10 NRS | 7.8 ± 1.25 |
| Median (range) | 8.0 (4.0–10.0) |
| MS spasticity‐associated symptoms at baseline, n (%) | |
| Pain | 437 (38.4) |
| Sleep disturbances | 372 (32.7) |
| Spasms/cramps | 324 (28.5) |
| Bladder dysfunction d | 203 (17.8) |
| Clonic movements | 185 (16.3) |
| Mood disorders e | 87 (7.6) |
| Trigeminal neuralgia | 17 (1.5) |
Data are expressed as mean ± SD, unless otherwise stated.
Abbreviations: N, number of patients; NRS, numeric rating scale; MS, multiple sclerosis; SD, standard deviation.
n = 1000.
n = 1137.
n = 1136.
Urinary incontinence/urgency or urinary retention.
Symptoms of affective instability (e.g., sadness, anxiety).
Over the 18‐month evaluation time frame, 633 patients (55.6% of the initial sample) discontinued nabiximols. Reasons for discontinuation were recorded for 588 (92.9%) of these cases and related mainly to ineffectiveness (63.6%) or adverse events (30.4%). Approximately one‐third (34.9%) of patients with data at all assessment time points continued nabiximols treatment at 18 months (Table 2).
TABLE 2.
Treatment discontinuation at 18 months (N = 1138)
| Outcome, n (%) | |
|---|---|
| Treatment discontinuation | 633 (55.6) |
| Reason for discontinuation (n = 588 a ) | |
| Ineffectiveness | 374 (63.6) |
| Adverse event(s) | 179 (30.4) |
| Other | 35 (6.0) |
| Subjects (with no missed visits) maintaining treatment over time | |
| Baseline | 1138 (100.0) |
| 4 weeks | 1138 (100.0) |
| 3 months | 760 (66.8) |
| 6 months | 653 (57.4) |
| 12 months | 473 (41.6) |
| 18 months | 397 (34.9) |
Abbreviations: N, number of patients.
Reasons for discontinuation were not recorded (missing/unknown) for 45 (7.1%) discontinued cases.
The evolution of mean MS spasticity 0–10 NRS scores from baseline to 18 months is shown in Figure 1. The mean baseline 0–10 NRS score was reduced by 24.6% at 4 weeks. In treatment continuers, the mean baseline 0–10 NRS score was reduced by 31.6% at 3 months and by 33.8% at 6 months (33.8%), then remained stable. At 18 months, the mean MS spasticity 0–10 NRS score in treatment continuers was 5.1 ± 1.23. Absolute changes from baseline in mean MS spasticity 0–10 NRS scores were − 1.9 ± 1.24, −2.5 ± 1.04, −2.7 ± 1.08, −2.7 ± 1.17, and − 2.6 ± 1.24 at 4 weeks (n = 1138, responders and non‐responders), 3 months (n = 760), 6 months (n = 653), 12 months (n = 473), and 18 months (n = 397), respectively. Applying the non‐responder imputation method for discontinued cases due to lack of effectiveness or adverse events, the estimated changes from baseline in mean MS spasticity 0–10 NRS scores were − 1.9 ± 1.24, −1.7 ± 0.04, −1.6 ± 1.56, −1.3 ± 1.58, and − 1.1 ± 1.53 at 4 weeks (n = 1138, responders and non‐responders), 3 months (n = 1103), 6 months (n = 1081), 12 months (n = 996), and 18 months (n = 933), respectively.
FIGURE 1.
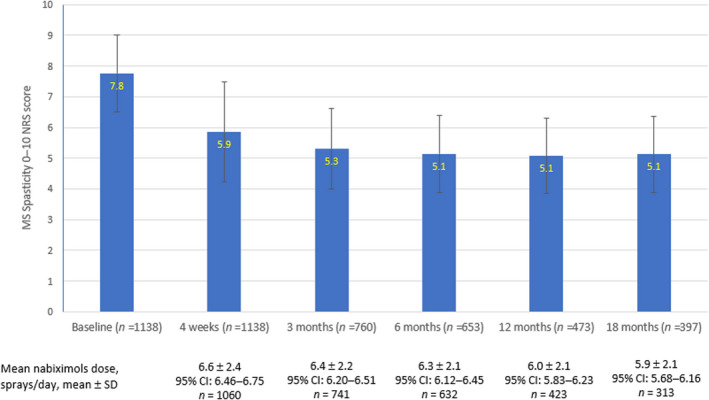
Evolution of mean multiple sclerosis (MS) spasticity 0–10 numeric rating scale (NRS) scores and mean dosages during nabiximols treatment [Colour figure can be viewed at wileyonlinelibrary.com]
The mean nabiximols dose was 6.6 sprays/day (95% confidence interval [CI] 6.46–6.75) at Week 4 (n = 1060) and decreased incrementally at successive time points in treatment continuers. At 18 months, the mean dose was 5.9 sprays/day (95% CI 5.68–6.16) in 313 patients with dosage data from baseline to month 18 (Figure 1).
MS spasticity responder rates at assessment time points up to 18 months are shown in Table 3. Approximately three‐quarters (76.8%) of patients were initial responders (≥20% NRS improvement) to nabiximols at Week 4. The ≥20% responder rate in treatment continuers exceeded 90% at 3, 6, 12 and 18 months. At 4 weeks, 36.1% of initial responders were also clinically relevant responders (≥30% NRS improvement). Corresponding ≥30% responder rates in treatment continuers were 53.7%, 60.5%, 62.0% and 60.2% at 3, 6, 12 and 18 months, respectively. Applying the non‐responder imputation method, an estimated 38.9% and 25.6% of evaluable patients (n = 933) achieved ≥20% and ≥30% NRS improvement, respectively, after 18 months' treatment with nabiximols. No relevant differences in mean spasticity NRS ≥ 20% and ≥ 30% response rates were observed according to MS type, MS disease duration or gender subgroups (data not shown).
TABLE 3.
Improvement (≥20% and ≥30%) in 0–10 multiple sclerosis spasticity numeric rating scale (NRS) scores during nabiximols treatment (observed cases)
| Evaluation timepoint | N | ≥20% NRS improvement | ≥30% NRS improvement |
|---|---|---|---|
| n (%) | |||
| 4 weeks | 1138 | 874 (76.8) | 411 (36.1) |
| 3 months | 760 | 711 (93.6) | 408 (53.7) |
| 6 months | 653 | 619 (94.8) | 395 (60.5) |
| 12 months | 473 | 443 (93.7) | 293 (62.0) |
| 18 months | 397 | 363 (91.4) | 239 (60.2) |
Abbreviations: N, number of patients; NRS, numeric rating scale.
Approximately one‐third of patients (n = 378) discontinued nabiximols between the first and third month of treatment (Table 3), but although fewer of those who had achieved 30% NRS improvement. At 18 months, the discontinuation rate was 31.1% for ≥30% responders compared with 55.6% for the overall sample.
At baseline, a total of 1625 MS spasticity‐associated symptoms were present in 625 patients (54.9% of the sample), equating to a mean of 1.43 symptoms/patient in the overall cohort and 2.6 symptoms/patient among those reporting one or more symptom. The mean number of MS spasticity‐associated symptoms per patient in the overall sample decreased from 1.43 ± 1.54 at baseline (n = 1138) to 0.96 ± 1.10 at 4 weeks (n = 1137) and was 1.02 ± 0.97 at 18 months in treatment continuers with available symptoms data (n = 179), corresponding to mean decreases of 33% and 29%, respectively. At baseline, pain was frequently clustered with sleep disturbances (250/437; 57.2%) and spasms/cramps (192/437; 43.9%). Among patients reporting symptoms at baseline, 52 (7.9%) had all four of the most common MS spasticity‐related symptoms (i.e., pain, sleep disturbances, spasms/cramps, and bladder dysfunction) present (Figure 2). Pain was the most common MS spasticity‐related symptom at Week 4 but, compared with baseline, was clustered with sleep disturbances (103/375; 27.5%) and spasms/cramps (68/375; 18.1%) in fewer patients (Figure 3).
FIGURE 2.
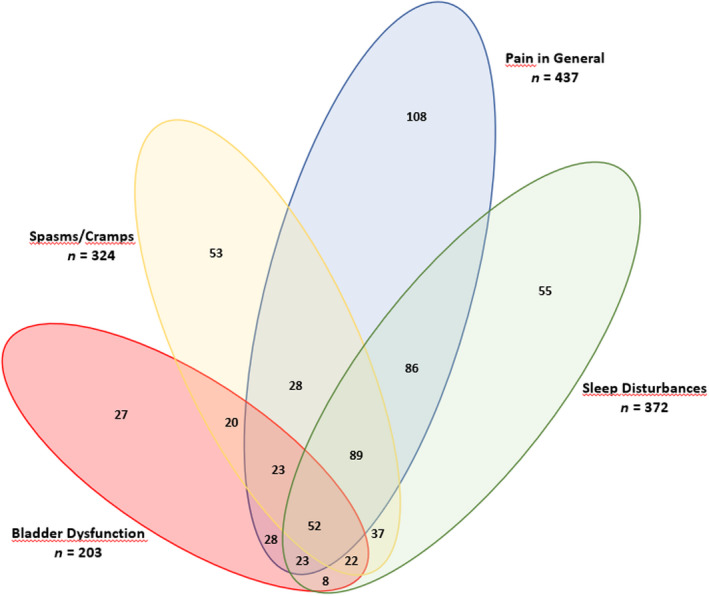
Venn diagram of common multiple sclerosis spasticity‐associated symptoms at baseline [Colour figure can be viewed at wileyonlinelibrary.com]
FIGURE 3.
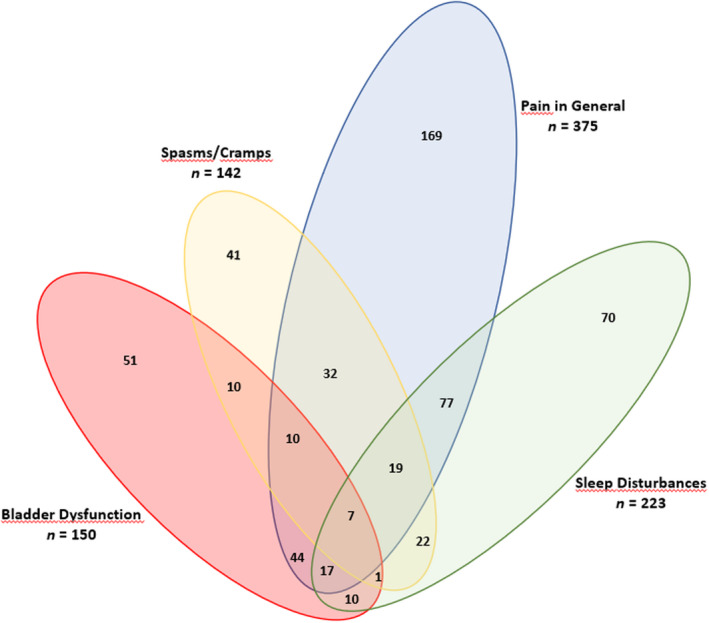
Venn diagram of common multiple sclerosis spasticity‐associated symptoms at Week 4 [Colour figure can be viewed at wileyonlinelibrary.com]
Resolution rates for MS spasticity‐associated symptoms present at baseline are shown in Table 4. At 4 weeks, symptom resolution rates were highest for spasms/cramps (182/324; 56.2%), clonic movements (87/185; 47.0%), and sleep disturbances (149/372; 40%). Symptom resolution rates at 18 months among treatment continuers with available symptoms data (n = 179) ranged from 75.3% for spasms/cramps to 48.4% for mood disorders. Applying LOCF methodology to this group, estimated symptom resolution rates at 18 months ranged from 61.3% (spasms/cramps) to 23.1% (mood disorders). No relevant differences in symptom resolution rates were observed in either analysis according to MS type, MS disease duration or gender subgroups (data not shown).
TABLE 4.
Resolution of multiple sclerosis spasticity‐associated symptoms at 4 weeks and 18 months
| MS spasticity‐associated symptom | Symptom present at baseline (n = 1138), n (%) | Symptom resolved at Week 4 a , n (%) | Symptom resolved at 18 months, observed cases (n = 179) b , n (%) | Symptom resolved at 18 months, LOCF analysis (n = 179) c , % |
|---|---|---|---|---|
| Pain | 437 (38.4) | 62 (14.2) | 53 (50.5) | 26.5 |
| Sleep disturbances | 372 (32.7) | 149 (40.0) | 61 (68.5) | 45.7 |
| Spasms/cramps | 324 (28.5) | 182 (56.2) | 64 (75.3) | 61.3 |
| Bladder dysfunction | 203 (17.8) | 53 (26.1) | 40 (62.5) | 43.0 |
| Clonic movements | 185 (16.3) | 87 (47.0) | 40 (66.7) | 55.1 |
| Mood disorders | 87 (7.6) | 0 (0.0) | 15 (48.4) | 23.1 |
| Trigeminal neuralgia | 17 (1.5) | 1 (6.3) | 3 (50.0) | 44.4 |
Abbreviations: LOCF, last observation carried forward; MS, multiple sclerosis, N, number of patients.
Calculated as the number (%) of patients in whom the symptom was present at baseline.
Calculated as the number (%) of treatment continuers at 18 months with available symptoms data in whom the symptom was present at baseline.
Calculated as the percent (%) of treatment continuers at 18 months with available symptoms data in whom the symptom was present at baseline.
At Week 4, the mean change from baseline in total number of symptoms present was numerically but not significantly greater in ≥20% NRS responders (−0.54; 95% CI −0.60 to −0.48) than in non‐responders (−0.23; 95% CI −0.30 to −0.17); and in ≥30% NRS responders (−0.65; 95% CI −0.75 to −0.56) than in non‐responders (−0.37; 95% CI: −0.42 to −0.31). Patients who achieved ≥20% or ≥30% improvement in MS spasticity during nabiximols treatment reported more MS spasticity‐associated symptoms per patient at baseline than non‐responders (Table 5).
TABLE 5.
Change from baseline in total number of symptoms at Week 4 in non‐responders (<20% or <30% improvement in multiple sclerosis (MS) spasticity 0–10 numeric rating scale [NRS] score) and responders (≥20% or ≥30% improvement in MS spasticity 0–10 NRS score)
|
<20% NRS improvement (n = 264) |
≥20% NRS improvement (n = 874) |
<30% NRS improvement (n = 727) |
≥30% NRS improvement (n = 411) |
|
|---|---|---|---|---|
| Mean (95% CI) | ||||
| Baseline | 0.71 (0.59–0.83) | 1.65 (1.54–1.75) | 1.18 (1.08–1.28) | 1.86 (1.70–2.03) |
| 4 weeks | 0.48 (0.38–0.57) | 1.10 (1.02–1.18) a | 0.81 (0.74–0.88) b | 1.21 (1.09–1.33) |
| Change from baseline | −0.23 (−0.30 to −0.17) | −0.54 (−0.60 to −0.48) a | −0.37 (−0.42 to −0.31) b | −0.65 (−0.75 to −0.56) |
| p value | 0.1997 | 0.7128 | ||
Data were analyzed using analysis of covariance with responder rate (≥20% or ≥30% NRS improvement) as a factor and baseline number of symptoms as a covariate.
Abbreviations: CI, confidence interval, NRS, numeric rating scale.
n = 873.
n = 726.
DISCUSSION
This large real‐world evidence study based on an independent Italian e‐Registry of patients with MS spasticity starting treatment with nabiximols oromucosal spray aimed to assess the relationship between evolution in MS spasticity severity and the presence of spasticity‐associated symptoms. AIFA e‐Registry data were analyzed for 1138 patients at time points from 4 weeks up to 18 months after treatment start. The study extends the findings of a previous AIFA e‐Registry analysis which reported improvement in spasticity‐related symptoms at 4 weeks after the start of nabiximols treatment, including in patients who had not reached the ≥20% NRS response threshold [25].
Baseline demographic and clinical characteristics of patients were typical of advanced MS populations with regard to age, gender, MS disease duration and MS disease type. At baseline, patients had severe spasticity (mean 0–10 NRS score of 7.8) and moderately severe disability (EDSS score of 6.5), although the baseline prevalence of MS spasticity‐associated symptoms was lower than that reported in other European epidemiological studies [2, 3, 4, 5], possibly due to the unsolicited nature of real‐world data collection.
The discontinuation rate of 55.6% at 18 months was similar to that reported in a previous AIFA e‐Registry analysis involving 1502 patients (48.3% at 72 ± 3 weeks) [24], with most discontinuations occurring within 3 to 6 months of treatment start. Consistent with the findings of several other multicenter, observational studies of nabiximols for MS spasticity [26, 27, 28, 29, 30, 31, 32], the main reasons for treatment discontinuation in our analysis were lack of effectiveness and/or adverse events.
Most patients showed rapid improvement in spasticity severity, as indicated by the 24.6% reduction in the mean 0–10 NRS score (from 7.8 to 5.9) after 4 weeks of treatment. More than three‐quarters of patients (76.8%) achieved ≥20% improvement in MS spasticity at Week 4 (threshold for continued treatment), which is comparable to initial responder rates reported in the SAVANT (Sativex® as add‐on therapy versus further optimized first‐line ANTispastics) randomized controlled trial [33] and in several observational studies of nabiximols [26, 27, 29, 30, 31, 32]. Our clinical experience suggests that this level of improvement in spasticity severity can relieve muscle rigidity, spasms and associated symptoms while maintaining sufficient muscle tone to facilitate mobility and improve daily functioning, which is highly important from the patient's perspective [34]. By Week 4, 36.1% of patients had already achieved a clinically relevant ≥30% NRS improvement, and approximately 60% of treatment continuers maintained ≥30% NRS improvement from 6 months onwards. Not surprisingly, ≥30% NRS responders were less likely to discontinue treatment. No relevant differences were observed in mean spasticity NRS ≥20% and ≥30% response rates according to subgroup analyses by MS type, MS disease duration or gender.
The mean number of MS spasticity‐associated symptoms present at baseline decreased by 33% at Week 4 and by 29% at 18 months in treatment continuers. Pain, the most common associated symptom at baseline, was frequently clustered with sleep disturbances and spasms/cramps, supporting the concept of a spasticity‐plus syndrome. At 18 months, symptom resolution rates in treatment continuers with available symptoms data were ≥50% for all associated symptoms including pain. Although patients without full symptom resolution during nabiximols treatment may have experienced symptomatic improvement, these data are not available since the AIFA e‐Registry records only the presence of symptoms and not changes in symptom severity. Previous randomized clinical trials have documented improvements in spasticity‐associated symptoms such as pain severity, spasms severity, and sleep disruption concomitant to improvements in MS spasticity [33, 35, 36], and were the basis for the conceptual proposal of the spasticity‐plus syndrome. No relevant differences were observed for symptom resolution rates according to MS type, MS disease duration or gender.
At Week 4 of nabiximols treatment, the total number of MS‐spasticity associated symptoms was reduced irrespective of whether or not patients had achieved ≥20% or ≥30% improvement in their MS spasticity 0–10 NRS score, although reductions were numerically greater in NRS responders. It is interesting to consider that even patients whose skeletal muscle rigidity does not improve to the ≥20% or ≥30% threshold can experience improvement in MS spasticity‐related symptoms. Future investigations of nabiximols might consider evaluating progress with reference to the broader spasticity‐plus syndrome, which encompasses evolution in all MS spasticity‐associated symptoms [10, 11] rather than the single symptom of spasticity. Moreover, nabiximols‐related improvement of associated symptoms soon after treatment initiation may facilitate dose adjustments (reduction or discontinuation) of concomitant medications specifically targeting other spasticity‐associated symptoms, for example, oxybutynin for bladder dysfunction, although this requires confirmation in well‐controlled clinical trials.
The mean nabiximols dose was 6.6 sprays/day at Week 4 and decreased gradually but progressively to 5.9 sprays/day at 18 months in treatment continuers. Other longer‐term observational clinical practice studies have reported doses in the same range (~6–7 sprays/day) [28, 32, 37], which is approximately 1–2 sprays/day lower than daily doses reported in randomized clinical trials of nabiximols which used a trial of therapy design and set the maximum dosage at 12 sprays/day [33, 38].
The study is limited by its retrospective daily‐life practice design and frame, although this is mitigated to some extent by having used the prospective AIFA e‐Registry as the primary data source. Mandatory documentation of scheduled clinic visits at 4 weeks and every 3 months after the start of nabiximols treatment allowed us to collect data for the same primary endpoint from each center at similar time points. To minimize the impact of missing data at assessment time points, we applied non‐responder imputation or LOCF methods. Nevertheless, data quality is a core consideration in observational studies. Unlike NRS spasticity evolution, reporting of MS spasticity‐associated symptoms in the e‐Registry was not compulsory. This may have resulted in under‐reporting, especially if symptoms were mild or moderate, as suggested by the relatively low baseline prevalence of certain symptoms (e.g., bladder dysfunction) in our patient cohort despite considerable disability (EDSS score 6.5). Other limitations are the lack of data on comorbidities (not collected in the e‐Registry) and the lack of data about the type, dose and duration of other symptomatic treatments (e.g., pregabalin, benzodiazepines, gabapentin, alpha blockers, antidepressants) besides nabiximols. As such, we cannot confirm whether symptom amelioration was due to nabiximols or to a change in concomitant medications. Moreover, dichotomous reporting of the presence/absence of MS spasticity‐associated symptoms offers no insight into possible changes in the severity of symptoms still present after starting nabiximols treatment. Consideration should be given to quantifying the severity of spasticity‐associated symptoms in future research. The disappearance (or amelioration) of symptoms such as spasms, pain, sleep disturbances, and bladder dysfunction during nabiximols treatment does not prove but rather suggests an association with spasticity. This relationship, and the ability of nabiximols to alleviate symptoms along with spasticity, remains to be demonstrated in well‐controlled clinical trials.
In conclusion, this analysis of real‐world AIFA e‐Registry data in a large number of patients showed that nabiximols improves MS spasticity often in conjunction with amelioration of spasticity‐associated symptoms, supporting the notion of a syndrome. Publications which introduced the concept of a spasticity‐plus syndrome described a frequent clustering of spasticity, spasms/cramps and pain, which was also observed in our patient cohort.
Overall, the analysis indicated that nabiximols responders can achieve a relevant and durable improvement in MS spasticity as well as resolution or improvement of many MS spasticity‐associated symptoms at stable doses of around 6 sprays/day. The finding that symptom resolution may be more pronounced in patients with a higher symptom burden at treatment start is worthy of further investigation. As improvement in MS spasticity and associated symptoms resolution did not appear to depend on MS type, MS disease duration or gender, nabiximols is suitable for use in a wide range of MS patients. It is interesting to consider that early detection of spasticity‐plus syndrome components may simplify management and potentially limit polypharmacy in MS patients. Further prospective and, where possible, randomized controlled studies with detailed tracking of MS spasticity‐associated symptom severity are warranted to advance the symptomatic management of MS. Importantly, additional insight from the patient's perspective must be gathered about the effectiveness of nabiximols on spasticity‐associated symptoms.
AUTHOR CONTRIBUTIONS
Francesco Patti: Conceptualization (equal); data curation (equal); formal analysis (equal); funding acquisition (equal); investigation (equal); methodology (equal); project administration (equal); resources (equal); software (equal); supervision (equal); validation (equal); visualization (equal); writing – original draft (equal); writing – review and editing (equal). Clara Chisari: Conceptualization (equal); data curation (equal); formal analysis (equal); funding acquisition (equal); investigation (equal); methodology (equal); project administration (equal); resources (equal); software (equal); supervision (equal); validation (equal); visualization (equal); writing – original draft (equal); writing – review and editing (equal). Óscar Fernández: Conceptualization (equal); data curation (equal); formal analysis (equal); funding acquisition (equal); investigation (equal); methodology (equal); project administration (equal); resources (equal); software (equal); supervision (equal); validation (equal); visualization (equal); writing – original draft (equal); writing – review and editing (equal). Jorge Sarroca: Conceptualization (equal); data curation (equal); formal analysis (equal); funding acquisition (equal); investigation (equal); methodology (equal); project administration (equal); resources (equal); software (equal); supervision (equal); validation (equal); visualization (equal); writing – original draft (equal); writing – review and editing (equal). Elena Ferrer‐Picón: Conceptualization (equal); data curation (equal); formal analysis (equal); funding acquisition (equal); investigation (equal); methodology (equal); project administration (equal); resources (equal); software (equal); supervision (equal); validation (equal); visualization (equal); writing – original draft (equal); writing – review and editing (equal). Francisco Hernández Vicente: Conceptualization (equal); data curation (equal); formal analysis (equal); funding acquisition (equal); investigation (equal); methodology (equal); project administration (equal); resources (equal); software (equal); supervision (equal); validation (equal); visualization (equal); writing – original draft (equal); writing – review and editing (equal). Carlos Vila: Conceptualization (equal); data curation (equal); formal analysis (equal); funding acquisition (equal); investigation (equal); methodology (equal); project administration (equal); resources (equal); software (equal); supervision (equal); validation (equal); visualization (equal); writing – original draft (equal); writing – review and editing (equal).
CONFLICT OF INTEREST
Francesco Patti has received honoraria for speaking activities from Bayer Schering, Biogen, Merck Serono, Novartis and Sanofi Aventis, has served as an advisory board member for Bayer Schering, Biogen Idec, Merck Serono and Novartis, has received funding from the Italian Multiple Sclerosis Foundation (FISM) and Pfizer for epidemiological studies, and has received grants for congress participation from Bayer Schering, Biogen Idec, Merck Serono, Novartis, Roche, Sanofi Aventis and Teva. CCG has received grants for congress participation from Almirall, Biogen, Merck Serono, Novartis, Roche, Sanofi and Teva. Óscar Fernández has received honoraria as consultant in advisory boards, as chair/lecturer in meetings, from participation in clinical trials and other research projects promoted by Actelion, Allergan, Almirall, Araclon, Bayer‐Schering, Biogen, Merck Serono, Novartis, Orizon, Sanofi Genzyme, Roche and Teva, and research support from the Hospital Foundation FIMABIS. Jorge Sarroca, Elena Ferrer‐Picón, Francisco Hernández Vicente, and Carlos Vila Silván. are fulltime employees of Almirall S.A. (Barcelona, Spain).
ACKNOWLEDGMENTS
Collaborators: Pasquale Annunziata, Assunta Bianco, Simona Bonavita, Elisabetta Capello, Paola Cavalla, Diego Centonze, Gianfranco Costantino, Federica Esposito, Claudio Gasperini, Alberto Gajofatto, Giacomo Lus, Giorgia Teresa Maniscalco, Manuela Matta, Damiano Paolicelli, Carlo Pozzilli, Marco Rovaris, Claudio Solaro, Gabriella Spinicci, Daniele Spitaleri and Paola Valentino. Writing assistance for this article was provided by Rob Furlong and Kerry Dechant on behalf of Content Ed Net (Madrid, Spain) with funding from Almirall S.A. (Barcelona, Spain). Statistical analyses were performed by the department of Global Clinical Statistics, Almirall S.A., Barcelona Spain. Open Access Funding provided by Universita degli Studi di Catania within the CRUI‐CARE Agreement.
Patti F, Chisari CG, Fernández Ó, et al. A real‐world evidence study of nabiximols in multiple sclerosis patients with resistant spasticity: Analysis in relation to the newly described ‘spasticity‐plus syndrome’. Eur J Neurol. 2022;29:2744‐2753. doi: 10.1111/ene.15412
Francesco Patti and Clara Grazia Chisari contributed equally.
Funding information
Funding from Almirall S.A. (Barcelona, Spain).
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Kister I, Bacon TE, Chamot E, et al. Natural history of multiple sclerosis symptoms. Int J MS Care. 2013;15(3):146‐158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Oreja‐Guevara C, González‐Segura D, Vila C. Spasticity in multiple sclerosis: results of a patient survey. Int J Neurosci. 2013;123(6):400‐408. [DOI] [PubMed] [Google Scholar]
- 3. Bethoux F, Marrie RA. A cross‐sectional study of the impact of spasticity on daily activities in multiple sclerosis. Patient. 2016;9(6):537‐546. [DOI] [PubMed] [Google Scholar]
- 4. Arroyo R, Massana M, Vila C. Correlation between spasticity and quality of life in patients with multiple sclerosis: the CANDLE study. Int J Neurosci. 2013;123(12):850‐858. [DOI] [PubMed] [Google Scholar]
- 5. Flachenecker P, Henze T, Zettl UK. Spasticity in patients with multiple sclerosis‐‐clinical characteristics, treatment and quality of life. Acta Neurol Scand. 2014;129(3):154‐162. [DOI] [PubMed] [Google Scholar]
- 6. Milinis K, Tennant A, Young CA. Spasticity in multiple sclerosis: associations with impairments and overall quality of life. Mult Scler Relat Disord. 2016;5:34‐39. [DOI] [PubMed] [Google Scholar]
- 7. Barin L, Salmen A, Disanto G, et al. The disease burden of multiple sclerosis from the individual and population perspective: which symptoms matter most? Mult Scler Relat Disord. 2018;25:112‐121. [DOI] [PubMed] [Google Scholar]
- 8. Otero‐Romero S, Sastre‐Garriga J, Comi G, et al. Pharmacological management of spasticity in multiple sclerosis: systematic review and consensus paper. Mult Scler. 2016;22:1386‐1396. [DOI] [PubMed] [Google Scholar]
- 9. Nicholas RS, Friede T, Hollis S, Young CA. Anticholinergics for urinary symptoms in multiple sclerosis. Cochrane Database Syst Rev. 2009;(1):CD004193. [DOI] [PubMed] [Google Scholar]
- 10. Fernández Ó, Costa‐Frossard L, Martínez‐Ginés M, Montero P, Prieto JM, Ramió L. The broad concept of “spasticity‐plus syndrome” in multiple sclerosis: a possible new concept in the management of multiple sclerosis symptoms. Front Neurol. 2020;11:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fernández Ó, Costa‐Frossard L, Martínez‐Ginés ML, Montero P, Prieto‐González JM, Ramió‐Torrentà L. Integrated management of multiple sclerosis spasticity and associated symptoms using the spasticity‐plus syndrome concept: results of a structured specialists' discussion using the Workmat® methodology. Front Neurol. 2021;12:722801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Russo E, Guy GW. A tale of two cannabinoids: the therapeutic rationale for combining tetrahydrocannabinol and cannabidiol. Med Hypotheses. 2006;66(2):234‐246. [DOI] [PubMed] [Google Scholar]
- 13. Pertwee RG. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: delta9‐tetrahydrocannabinol, cannabidiol and delta9‐tetrahydrocannabivarin. Br J Pharmacol. 2008;153(2):199‐215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Electronic Medicines Compendium . Sativex oromucosal spray. Summary of product characteristics. 25 August 2020. https://www.medicines.org.uk/emc/product/602/smpc#gref. Accessed April 22, 2022.
- 15. Oreja‐Guevara C, Montalban X, de Andrés C, et al. Documento de consenso sobre la espasticidad en pacientes con esclerosis multiple [Consensus document on spasticity in patients with multiple sclerosis. Grupo de Enfermedades Desmielinizantes de la Sociedad Española de Neurología]. Rev Neurol. 2013;57(8):359‐373. [PubMed] [Google Scholar]
- 16. Solari A, Giordano A, Sastre‐Garriga J, et al. EAN guideline on palliative care of people with severe, progressive multiple sclerosis. J Palliat Med. 2020;23(11):1426‐1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Comi G, Solari A, Leocani L, Centonze D, Otero‐Romero S. Italian Consensus Group on treatment of spasticity in multiple sclerosis. Italian consensus on treatment of spasticity in multiple sclerosis. Eur J Neurol. 2020;27(3):445‐453. [DOI] [PubMed] [Google Scholar]
- 18. Hemmer B, Bayas A, Berthele A, Faßhauer E, Flachenecker P, Haghikia A. Diagnose und Therapie der Multiplen Sklerose, Neuromyelitis‐optica‐SpektrumErkrankungen und MOG‐IgG‐assoziierten Erkrankungen, S2k‐Leitlinie. In: Deutsche Gesellschaft für Neurologie , ed. Leitlinien für Diagnostik und Therapie in der Neurologie; 2021. https://dgn.org/wp‐content/uploads/2021/04/030050_LL_Multiple_Sklerose_2021.pdf [Google Scholar]
- 19. Zanghì A, D'Amico E, Lo Fermo S, Patti F. Exploring polypharmacy phenomenon in newly diagnosed relapsing‐remitting multiple sclerosis: a cohort ambispective single‐centre study. Ther Adv Chronic Dis. 2021;12:2040622320983121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Farrar JT, Troxel AB, Stott C, Duncombe P, Jensen MP. Validity, reliability, and clinical importance of change in a 0‐10 numeric rating scale measure of spasticity: a post hoc analysis of a randomized, double‐blind, placebo‐controlled trial. Clin Ther. 2008;30(5):974‐985. [DOI] [PubMed] [Google Scholar]
- 21. Montilla S, Xoxi E, Russo P, Cicchetti A, Pani L. Monitoring registries at Italian Medicines Agency: fostering access, guaranteeing sustainability. Int J Technol Assess Health Care. 2015;31(4):210‐213. [DOI] [PubMed] [Google Scholar]
- 22. Patti F, Messina S, Solaro C, et al. Efficacy and safety of cannabinoid oromucosal spray for multiple sclerosis spasticity. J Neurol Neurosurg Psychiatry. 2016;87(9):944‐951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Messina S, Solaro C, Righini I, et al. Sativex in resistant multiple sclerosis spasticity: discontinuation study in a large population of Italian patients (SA.FE. study). PLoS One. 2017;12(8):e0180651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chisari CG, Solaro C, Annunziata P, et al. Nabiximols discontinuation rate in a large population of patients with multiple sclerosis: a 18‐month multicentre study. J Neurol Neurosurg Psychiatry. 2020;91(9):914‐920. [DOI] [PubMed] [Google Scholar]
- 25. Patti F, Chisari CG, Solaro C, et al. Effects of THC/CBD oromucosal spray on spasticity‐related symptoms in people with multiple sclerosis: results from a retrospective multicenter study. Neurol Sci. 2020;41(10):2905‐2913. [DOI] [PubMed] [Google Scholar]
- 26. Flachenecker P, Henze T, Zettl UK. Nabiximols (THC/CBD oromucosal spray, Sativex®) in clinical practice—results of a multicenter, non‐interventional study (MOVE 2) in patients with multiple sclerosis spasticity. Eur Neurol. 2014;71:271‐279. [DOI] [PubMed] [Google Scholar]
- 27. Ferrè L, Nuara A, Pavan G, et al. Efficacy and safety of nabiximols (Sativex[®]) on multiple sclerosis spasticity in a real‐life Italian monocentric study. Neurol Sci. 2016;37(2):235‐242. [DOI] [PubMed] [Google Scholar]
- 28. Paolicelli D, Direnzo V, Manni A, et al. Long‐term data of efficacy, safety, and tolerability in a real‐life setting of THC/CBD oromucosal spray‐treated multiple sclerosis patients. J Clin Pharmacol. 2016;56(7):845‐851. [DOI] [PubMed] [Google Scholar]
- 29. Vermersch P, Trojano M. Tetrahydrocannabinol:cannabidiol oromucosal spray for multiple sclerosis‐related resistant spasticity in daily practice. Eur Neurol. 2016;76:216‐226. [DOI] [PubMed] [Google Scholar]
- 30. Grimaldi AE, De Giglio L, Haggiag S, et al. The influence of physiotherapy intervention on patients with multiple sclerosis‐related spasticity treated with nabiximols (THC:CBD oromucosal spray). PLoS One. 2019;14(7):e0219670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Carotenuto A, Costabile T, De Lucia M, et al. Predictors of nabiximols (Sativex®) discontinuation over long‐term follow‐up: a real‐life study. J Neurol. 2020;267(6):1737‐1743. [DOI] [PubMed] [Google Scholar]
- 32. D'hooghe M, Willekens B, Delvaux V, et al. Sativex® (nabiximols) cannabinoid oromucosal spray in patients with resistant multiple sclerosis spasticity: the Belgian experience. BMC Neurol. 2021;21(1):227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Markovà J, Essner U, Akmaz B, et al. Sativex® as add‐on therapy vs. further optimized first‐line ANTispastics (SAVANT) in resistant multiple sclerosis spasticity: a double‐blind, placebo‐controlled randomised clinical trial. Int J Neurosci. 2019;129:119‐128. [DOI] [PubMed] [Google Scholar]
- 34. Bhimani R, Anderson L. Clinical understanding of spasticity: implications for practice. Rehabil Res Pract 2014;2014:279175, 1, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Collin C, Ehler E, Waberzinek G, et al. A double‐blind, randomized, placebo‐controlled, parallel‐group study of Sativex, in subjects with symptoms of spasticity due to multiple sclerosis. Neurol Res. 2010;32:451‐459. [DOI] [PubMed] [Google Scholar]
- 36. Wade DT, Makela P, Robson P, House H, Bateman C. Do cannabis‐based medicinal extracts have general or specific effects on symptoms in multiple sclerosis? A double‐blind, randomized, placebo‐controlled study on 160 patients. Mult Scler. 2004;10(4):434‐441. [DOI] [PubMed] [Google Scholar]
- 37. Flachenecker P, Henze T, Zettl UK. Long‐term effectiveness and safety of nabiximols (tetrahydrocannabinol/cannabidiol oromucosal spray) in clinical practice. Eur Neurol. 2014;72(1–2):95‐102. [DOI] [PubMed] [Google Scholar]
- 38. Novotna A, Mares J, Ratcliffe S, et al. A randomized, double‐blind, placebo‐controlled, parallel‐group, enriched‐design study of nabiximols* (Sativex[®]), as add‐on therapy, in subjects with refractory spasticity caused by multiple sclerosis. Eur J Neurol. 2011;18:1122‐1131. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


