Abstract
Traditionally, fermentation was used to preserve the shelf life of food. Currently, in addition to favouring food preservation, well standardized and controlled industrial processes are also aimed at improving the functional characteristics of the final product. In this regard, starter cultures have become an essential cornerstone of food production. The selection of robust microorganisms, well adapted to the food environment, has been followed by the development of microbial consortia that provide some functional characteristics, beyond their acidifying capacity, achieving safer, high‐quality foods with improved nutritional and health‐promoting properties. In addition to starters, adjunct cultures and probiotics, which normally do not have a relevant role in fermentation, are added to the food in order to provide some beneficial characteristics. This review focuses on highlighting the functional characteristics of food starters, as well as adjunct and probiotic cultures (mainly lactic acid bacteria and bifidobacteria), with a specific focus on the synthesis of metabolites for preservation and safety aspects (e.g. bacteriocins), organoleptic properties (e.g. exopolysaccharides), nutritional (e.g. vitamins) and health improvement (e.g. neuroactive molecules). Literature reporting the application of these functional cultures in the manufacture of foods, mainly those related to dairy production, such as cheeses and fermented milks, has also been updated.
Keywords: aroma, biopreservation, functional, lactic acid bacteria, nutritional, probiotic, vitamin
INTRODUCTION
In ancient times, the fermentation of raw materials took place spontaneously, although in the Neolithic era humans were able to empirically control these processes by using a kind of ‘basic’ technology. The first fermented dairy products for which there are bibliographic references, in the Bible among other documents, are yoghourts and fresh‐style cheeses originating from the spontaneous fermentation of milk (Ozzen & Dinleyici, 2015). However, archaeological remains of ‘drainage’ vessels with multiple holes, which have been shown to contain traces of milk fatty acids, is the first evidence of human technology controlling a process that led to the production of cheese (Salque et al., 2013). The remains of the oldest cheese found, to date, were obtained in excavations of the tomb of the nobleman Ptahmes (XIX dynasty, 3200 B.C.) in the Saqqara necropolis. Proteomic analysis of this residual ‘cheese’ shows the presence of peptides from cow’s milk with a mixture of goat and sheep´s milk. Interestingly, peptides were also detected corresponding to a bacterium, specifically Brucella melitensis the causative agent of brucellosis in humans, which is a pathogen of sheep and goats; this fact corroborates the presence of this milk type in the cheese residue (Greco et al., 2018).
When humans began to domesticate animal and plant species, eventually becoming breeders and farmers, they also initiated the ‘domestication’ of the microbiota that was found naturally in raw foods. In the same way that seeds or animal breeds were selected for their better production performance, foods spontaneously fermented from these raw materials allowed the selection of bacteria, moulds and yeasts that favoured the ‘reproducibility’ of the final product. The traditional use of the back‐slopping technique, or re‐inoculation with a previous curd, a sourdough, or a previously fermented product, allowed the selection of populations of microorganisms well‐adapted to these environmental conditions. These adaptations were fixed in the microbial genomes, which led to a genetic differentiation and specificity depending on the food matrix in which they were found (Li & Gänzle, 2020). This fact has led, in general, to a loss of natural microbial diversity in favour of a smaller number of well‐adapted microorganisms, but has also allowed control over the fermentation process to ensure reproducibility, quality and safety of fermented products (Gibbons & Rinker, 2015). In addition, it seems that this process of microbial domestication occurred relatively quickly, opening up the possibility of carrying out a ‘directed evolution’ in the laboratory to obtain strains which are better adapted to technological processes (Gibbons, 2019).
Nowadays, both spontaneous and non‐spontaneous (controlled) fermentations are used to obtain safer products, the first type of food being a suitable source for the isolation of novel strains with functional properties (Tamang et al., 2016). Lactic acid bacteria (LAB), which are the drivers of lactic fermentation, are widely used as cultures for a huge variety of traditionally fermented foods and functional foods. In addition, some specific species are also used as ‘cell factories’ for the synthesis of different compounds with multiple applications in food, pharma or cosmetics (Hatti‐Kaul et al., 2018). This versatility is, among other factors, due to the capability of this bacterial group to use a great variety of carbon sources (Sauer et al., 2017), which also makes them a valuable tool for the biotransformation of different residues in valuable by‐products, such as the lactic acid obtained by fermentation of the lactose from whey (Zandona et al., 2021). The diversity, physiology, taxonomy and/or applications of LAB have been extensively reviewed (for example, Holzapfel & Wood, 2014). It is worth noting that in taxonomy the genus Lactobacillus has recently been reclassified and the term ‘lactobacilli’ is now used to enclose bacteria belonging to the Lactobacillaceae family since 25 novel genera have been proposed (Zheng et al., 2020).
The aim of the current review is to give some indications about the functional properties that make LAB versatile bacterial cultures for multiple applications in food production, with a closer view to dairy products (Carminati et al., 2016; Leroy & De Vuyst, 2004; Linares et al., 2017). For this purpose, we have arbitrarily it into four sections (Figure 1) that, to our knowledge, underline the relevant role that this bacterial group plays in multiple facets of the food industry.
FIGURE 1.
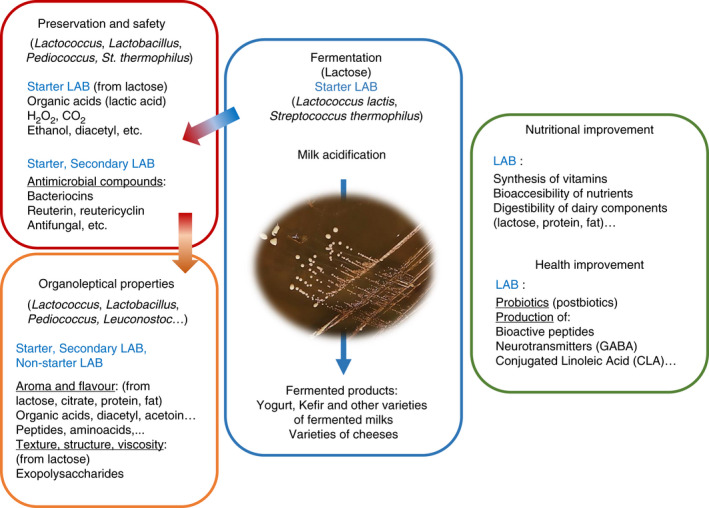
Type of lactic acid bacteria (LAB) cultures for the production of fermented dairy products
CULTURES FOR DAIRY SAFETY AND PRESERVATION
As stated in the introduction, the primary role of LAB in food production is the fermentation of carbon sources for the acidification of the raw material, by means of the lactic acid produced, thus reducing the presence of undesirable pathogenic and spoiling microorganisms. This activity will improve the safety and preservation time of the final product, while also modifying its sensorial properties (Barcenilla et al., 2021). Indeed, LAB is responsible for the production and preservation of a large variety of fermented foods and beverages widely consumed by humans, both from animal origin, such as dairy and meat products, and from plant origin such as pickles, olives and many traditional fermented foods from Asia, Africa and Latin America (Anyogu et al., 2021; Rebaza‐Cardenas et al., 2021). In fact, the long tradition of safe consumption of traditional fermented foods and beverages obtained from LAB fermentation prompt these bacteria to be considered as GRAS (Generally Recognized As Safe) microorganisms by the FDA of USA, some of the species also being included in the QPS (Qualified Presumption of Safety) list of EFSA in Europe (EFSA panel BIOHAZ, 2021). The bioprotective capability of the bacteria is driven by the production of specific metabolites from carbohydrate metabolism, such as different organic acids, diacetyl, ethanol or hydrogen peroxidase, as well as by metabolites acting against bacteria, as bacteriocins (Rouse & Van Sinderen, 2008), or metabolites with anti‐fungal properties (Chen et al., 2021; Leyva‐Salas et al., 2017).
Anti‐bacterial properties of LAB
LAB has an arsenal of strategies to fight against other bacteria that occupy a given ecological niche, among them are the small ribosomal synthetized peptides known as bacteriocins (Rodríguez et al., 2017). Due to their chemical nature, they have generally been considered as safe for consumption since they are degraded by gastric and pancreatic enzymes during food digestion, although some of them might present toxicity at high concentrations (Soltani et al., 2021). Currently, the use of bacteriocins as food additives in dairy production is still limited in Europe to nisin (E 234) as approved by EFSA (EFSA panel ANS, 2017). This bacteriocin, which is synthesized by strains of Lactococcus lactis, has antimicrobial activity against a large number of Gram‐positive bacteria such as Listeria, Staphylococcus, Bacillus or Clostridium among others. Nisin is mainly used in cheeses but it has proved its effectiveness against foodborne pathogens in other dairy products (Silva et al., 2018). The synthesis of bacteriocins by LAB is a complex process to be able to be scaled up to industrial level, since it is very much depends on the culture conditions which makes the process unprofitable (Sidooski et al., 2019). Therefore, the use of bacteriocin‐producing LAB as bioprotective cultures is still the most affordable application approach. There are different types of bacteriocins that have been proposed to be classified in different ways (Rodríguez et al., 2017; Soltani et al., 2021). This section will exclusively focus on some of those that might have an application in dairy production.
Bacteriocins are effective against Gram‐positive and Gram‐negative bacteria, their spectrum is highly dependent on their mode of action (Soltani et al., 2021). In the case of milk and cheeses, the main foodborne pathogens are Staphylococcus aureus and Listeria monocytogenes, the incidence of Salmonella enterica and Escherichia coli being much lower (Gonzales‐Barron et al., 2017). The bacteria causing spoilage in dairy products are spore formers, such as Clostridium tyrobutiricum and Bacillus cereus (Pancza et al., 2021). The application of bacteriocins and their producing LAB bacteria as bioprotective cultures in dairy products has been reviewed by Silva et al. (2018). These authors compiled comprehensive information in three tables, available from 2000 to 2018, about the application of purified or semi‐purified bacteriocins and their producing LAB for different dairy products such as raw, UHT and sterilized milk, skimmed milk powder, different varieties of cheeses and some fermented milk including yoghourt (Silva et al., 2018). More recently, Trejo‐González et al. (2021) conducted a bibliographic search in different databases for articles (from 2009 to 2021) dealing with bacteriocin‐producing LAB isolated from cheeses. They found that more than 30% of these LAB belong to Enterococcus and (former) Lactobacillus genera, followed by Lactococcus and Pediococcus (less than 14%), which were most often isolated from soft cheeses (Trejo‐González et al., 2021). The application of nisin, which is synthesized by Lactococcus, against spore‐forming bacteria in food production was also recently revisited since it is of special relevance for extending the shelf‐life of fermented products (Anumudu et al., 2021). The potential application of bacteriocins as a mechanism of action for probiotics against pathogens has also received some attention (Soltani et al., 2021). To cite an example, strains of Pediococcus pentosaceus are able to synthesize different types of bacteriocins, named pediocins, which could represent a positive advantage for the producing bacteria as an antagonistic mechanism to survive in the populated colonic niche (Jiang et al., 2021). The number of reviews about bacteriocins indicates that is a very active area of research and novel applications for existing or new bacteriocins are continuously being published. Several of these studies deal with the search for bacteriocins for the specific application against the dairy‐associated pathogen Lis. monocytogenes. This is the case of the co‐culture Streptococcus thermophilus B59671 (producing termophilin 110) and L. (Lactiplantibacillus) plantarum 076 (producing pediocin) tested in fermented milk and whey (Ceruso et al., 2021), or the Lactococcus lactis subsp. lactis BGBU1‐4 culture (producing lactolisterin) for the manufacture of fresh soft cheese (Mirkovic et al., 2020). Novel bacteriocins, such as a class III one produced by Lactobacillus acidophilus NX2‐6 with broad spectrum against Gram‐positive and Gram‐negative bacteria, are being characterized; this strain isolated from Chinese Koumiss was effective at reducing the viable number of Sal. enteritidis, Lis. monocytogenes, E. coli and S. aureus in contaminated milk and Mozzarella cheese (Meng et al., 2021). A novel IId bacteriocin was purified from the strain L. plantarum SHY 21–2 found in yoghourt from yak milk, which displayed bioprotective properties against S. aureus, among other pathogens (Peng et al., 2021). Finally, in the search for novel bioprotective strains, several studies have taken advantage of the currently available tools to search for bacteriocin production in LAB genomes and metagenomes of different dairy products (Bachtarzi et al., 2020; Suárez et al., 2020), which opens an avenue for the discovery of novel molecules with anti‐microbial properties for food applications.
The use of LAB as bioprotective cultures is an interesting approach that fulfils current food consumption trends consisting of a market with fewer chemical additives and preservatives. This fact, together with the huge number of LAB that produces a wide variety of molecules that can protect food from undesirable microorganisms, make these bacteria suitable for the development of natural food preservation cultures for use in the food industry.
CULTURES FOR OPTIMAL ORGANOLEPTIC PROPERTIES
LAB contributes to a high extent in the development of aroma and flavour, as well as other sensorial properties, of dairy products. This is due to their capacity to produce different metabolites, such as organic acids, volatile compounds and biopolymers derived from sugar metabolism, as well as to the conversion of amino acids, released after proteolysis, into aromatic compounds that give fermented products desirable organoleptic properties. LAB have a lesser effect on the conversion of the fat components of milk, an activity typically driven by yeast or moulds in cheese production (Thierry et al., 2016).
LAB involved in aroma and flavour development of dairy products
A wide range of metabolites is responsible for the ‘flavour,’ combination of aroma and flavour, development in dairy products and, particularly, cheeses. In fact, a new term ‘cheesomic’ has been proposed to compile all multi‐omic techniques that can currently be used to decipher the role that the microbial community plays in the identity attributes of a cheese (Afshari et al., 2020). The LAB‐biota present in cheese could have different origins and it is involved in different key events that result in the transformation of milk into a wide variety of cheeses. In the production of fermented dairy foods, the starter LAB is referred to as primary cultures, if they are responsible for the fast acidification of the milk; and secondary (or adjunct) cultures if they are involved in the ripening (development of aroma and flavour) of the cheese at later stages (Figure 2). Depending on the manufacturing practices for each specific cheese type, LAB is selected according to their optimal growth temperature: mesophilic (around 30–32°C) or thermophilic (around 42°C). Natural LAB starters are constituted by mixtures of non‐defined species and are mostly related to artisanal cheese production processes, using the back‐sloping technique. However, some of these ‘natural’ LAB mixtures can be reproduced, in a controlled way, by starter producing companies so they can be supplied to certain cheese producers; in this case, commercial LAB mixtures with no well‐defined composition are known as mixed‐strain starters (MSS). Natural LAB starters and the MSS are considered as ‘traditional’ cultures. On the other hand, some specific combinations of well‐defined strains, which have been selected based on their good performance on different attributes of cheese production, are commercialized by large starter production companies. These are defined strain starters (DSS) and currently, there are several combinations of different strains and/or very well‐known species included (Altieri et al., 2017; Fox et al., 2017). In addition to LAB starters, in fermented food production, a natural LAB‐biota is also present, which is named non‐starter lactic acid bacteria (NSLAB, Figure 2). This is acquired from the environment of the farm and cheese production facilities, as well as from the cheesemakers, and plays a pivotal role in the acquisition of sensorial properties of the product (Gobbetti et al., 2015, 2018). Finally, it should be noted that other microorganisms might be involved in the development of the specific organoleptic properties for certain cheese types. These can be added as functional starters, as they are also components of the microbial community (or microbiota) present in cheese. This could be the case of propionic acid bacteria (e.g. Propionibacterium freudenreichii subsp. shermanii), bacteria present in rind cheeses, such as Brevibacterium linens (Ruas‐Madiedo & Rodríguez, 2017), or eukaryotes, such as yeasts (e.g. Debaryomyces hansenii and Yarrowia lipolytica, Buzzini et al., 2017) and moulds (e.g. Penicillium camemberti and Penicillium roqueforti, Dantigny & Bevilacqua, 2017). In fact, cheese is a very complex ecosystem populated by a plethora of microorganisms differently organized in various microenvironments or niches (Mayo et al., 2021). As an example, the fascinating communities of cheese rinds, which can strongly contribute to certain organoleptic or sensorial attributes, but differ from the communities encountered in the cheese core (Wolfe et al., 2014). Microbial interactions among these actors i.e. the LAB starters, NSLAB and the natural microbiota of the cheese, have not been totally elucidated. However, it is clear that competition or cooperation in carbohydrate, protein or lipid metabolism takes place and shapes this dynamic but relatively small ecosystem (Blaya et al., 2018; Gobbetti et al., 2018). This fact underlines the relevance of choosing an adequate LAB starter and/or NSLAB for the production of cheeses with recognized characteristics, such as those with PDO (Protected Designation of Origin, in a reproducible way (Randazzo et al., 2021).
FIGURE 2.
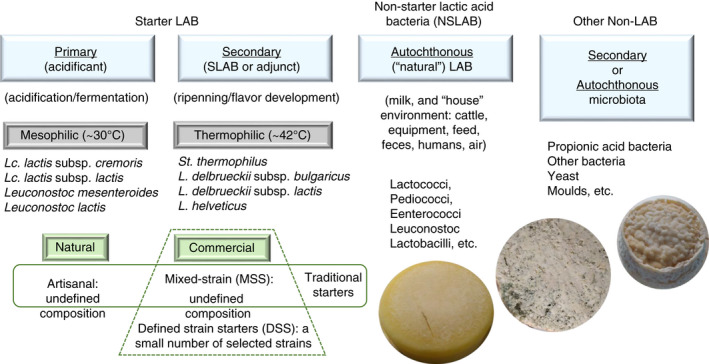
Different lactic acid bacteria (LAB) cultures used in milk transformation in fermented products. Information compiled from Altieri et al. (2017) and Fox et al. (2017)
EPS‐producing LAB to improve physical properties of dairy products
In addition to aroma and flavour development, other sensorial properties are influenced by LAB, such as the texture, structure or viscosity of fermented foods. Some particular LAB, both starter and NSLAB, which are able to synthesize natural biopolymers named exopolysaccharides (EPS), confer optimal physical properties on dairy‐fermented products (Hassan, 2008; Korcz & Varga, 2021; Prete et al., 2021). EPS are carbohydrate polymers of different chemical composition and structural complexity, which are present on the surface of the EPS‐producing LAB forming a slimy layer or a compact capsule (Ruas‐Madiedo, & de los Reyes‐Gavilan, C.G., 2005). In addition to their influence on the physical properties of fermented foods, these biopolymers also play a relevant role in the health‐promoting benefits of EPS‐producing LAB and bifidobacteria with probiotic traits. Bacterial EPS have the capability to modulate the intestinal microbiota and also the host immune response since they are able to directly interact with intestinal receptors; antagonism against the activity of pathogens or anti‐oxidant properties have also been reported (Castro‐Bravo et al., 2018; Hidalgo‐Cantabrana et al., 2014; Salazar et al., 2016). It is worth noting that, since bacterial EPS are extracellular components that might be released into the extracellular medium, or liberated after cellular lysis, they might play a relevant role in the health benefits attributed to fermented postbiotic foods (Molinero et al., 2022). With regard to cheese production, EPS synthesized by LAB are effective fat replacers due to their capability to interact with the network of caseins and to retain water, but these polymers also improve the mouth‐feel, consistency and texture of mainly fresh cheeses (Di Cagno et al., 2014; Hahn et al., 2014). In the case of fermented milk, EPS‐producing LAB starters have been traditionally used in the manufacturing of yoghourt, but they are also naturally present in many different fermented milks such as kefir, viili or långfil, among others (Ruas‐Madiedo et al., 2009). Some of the sensorial properties of yoghourt to be aware of are mouthfeel, creaminess, ropiness, appearance and hardness, which might be obtained by using different additives (pectin, carrageenan, etc.) acting as thickening and texturing agents. However, since there is a global trend for more natural and healthier products, the production in situ of EPS during milk fermentation offers a natural and consumer acceptable alternative to reach this milestone. Indeed, there is a wide catalogue of EPS‐producing LAB starters currently available for yoghourt production (Tiwari et al., 2021). In addition to the acidifying and flavour development capabilities of the EPS‐producing LAB, the intrinsic characteristics of the polymer, that is chemical composition, structure and stiffness among others, are crucial to determine their ability to influence viscosity and texture. In fact, for the same concentration, EPS with negative charges in their composition, stiffer structure of the repeating units, or having high molar mass are, in general, those able to confer suitable viscosifying and texturing properties to the fermented milk (Gentès et al., 2011; Ruas‐Madiedo et al., 2002; Surber et al., 2019). Therefore, the correct selection of the EPS‐producing LAB is of special relevance for obtaining a final product with the desired physical characteristics. As a recent illustrative example, Bachtarzi and co‐workers have studied the capability of different EPS‐producing L. plantarum strains, isolated from traditional dairy products from Algeria, to increase the viscosity of skimmed fermented milk obtaining variable results depending on the strain (Bachtarzi et al., 2019). A deeper characterization of three of these strains revealed that production of a high molecular mass polymer directly correlated with increased viscosity of the fermented milk; whereas, the acidification rate in combination with the high accumulation of synthesized EPS at earlier stages of fermentation, had a great influence on the microstructure of the milk gel and its water retention capability (Bachtarzi et al., 2020). In this way, it is known that EPS from LAB act as hydrocolloids and their water‐holding capability is a desirable property for set‐type yoghourts since it reduces the whey separation or syneresis (Xu et al., 2021).
Over recent decades, interest in developing fermented products with high sensorial standards has triggered the search for new LAB capable of producing optimal organoleptic properties and, thus, being used as starters in the food industry. Consumer demand for novel products has prompted the search for new LAB strains. In this context, traditional fermented foods constitute one of the possibilities for exploring the natural biodiversity of the LAB‐biota in them and evaluating their potential multiple biotechnological applications.
CULTURES FOR NUTRITIONAL IMPROVEMENT
Vitamins and minerals are essential micronutrients required to support many biological processes in all living organisms, however, most cannot be synthesized by humans who must acquire them externally from the diet. Some of these ingredients can also be destroyed during food processing and cooking, thus it is not surprising that their intake might not be sufficient sometimes. The deficiency of some of these micronutrients is a public health concern even in highly industrialized countries where imbalanced diets are common (FAO‐WHO, 2006).
Food fermentation can enhance the nutritional and functional properties of foods through various mechanisms linked to the specific activity of starter and adjunct bacterial cultures on the food matrix (Şanlier et al., 2019). These include, among others: (i) the in situ production of nutrients or other bioactive compounds, the generation of biofortified fermented foods that could be tailored to specific population groups with particular nutritional needs; (ii) the metabolism of otherwise indigestible substrates such as complex carbohydrates and dietary fibres, aiding an increase in bioavailability of certain nutrient types; or (iii) the reduction of the content in chemical components that might impede the intestinal absorption of certain nutrients, such as phytate or oxalate, which are common in cereals and plant‐based foods. In this scenario, the rational selection and inclusion in fermented foods of bacterial cultures technologically robust and capable of enhancing their nutritional properties are an attractive, economic, environmentally friendly and consumer acceptable approach to prevent nutritional deficiencies in large population segments without requiring radical changes in their dietary patterns (LeBlanc et al., 2011, 2013).
Micronutrients production
The inclusion in foodstuffs of cultures capable of producing nutrients can contribute to prevent nutritional deficiencies through two main approaches: the most direct is the in situ production of micronutrients in the fermented material leading to the production of biofortified foods. But, in some cases, bacteria included in fermented foods could also colonize the intestinal tract upon consumption, at least transiently, where they could continue synthesizing vitamins directly in the human intestine. Indeed, vitamin production is a relatively common trait in the intestinal microbiota, and fermented foods are a well‐known source of living microorganisms, some of which may transiently colonize our intestinal tract and beneficially impact our gut microbiota and overall wellbeing (Marco et al., 2017; Pompei et al., 2007; Rossi et al., 2011).
Some vitamins can be synthesized by microbial species of industrial interest for the food sector. The production of water‐soluble vitamins belonging to the B‐group, including riboflavin, folates, thiamine and cobalamin, have been the most extensively investigated in bacterial species including lactobacilli, bifidobacteria and other LAB species such as Lac. lactis and St. thermophilus among others (Table 1) (Acevedo‐Rocha et al., 2019; Crittenden et al., 2003; Gangadharan & Nampoothiri, 2011; Laiño et al., 2013). Fermented food biofortification through microbial production of micronutrients has been extensively investigated in fermented milks and yoghourts, other fermented food matrixes have also been investigated including vegetable‐based drinks, pasta and bread, fruit and cereal‐based foods and even kefir‐like cereal‐based beverages and dairy whey (Capozzi et al., 2012; Levit et al., 2021; Rad et al., 2016). Some specific examples are summarized in the following paragraphs and Table 1.
TABLE 1.
Studies reporting the application of LAB for the bioenrichment of different fermented foodstuff matrixes
| Bacteria a | Food matrix evaluated | Other comments | Reference |
|---|---|---|---|
| Riboflavin producers | |||
| L. plantarum | Fermented soy beverage | Bacterial cells were immobilized in okara for the production | Feng et al. (2021) |
| L. plantarum | Fermented soy beverage | Bioenriched product exhibited increased nutritional and functional attributes | Zhu et al. (2020) |
| L. plantarum | Oat‐based products | A roseoflavin resistant overproducing derivative strain enable to bioenrich the fermented product | Russo et al. (2016) |
| L. plantarum | Bread and pasta | By using overproducing derivative strains riboflavin content increase from 2‐ to 3‐fold in the final products | Capozzi et al. (2011) |
| L. plantarum | Fermented soy beverage | Overproducing derivative strains | Ge et al. (2020) |
| L. plantarum | Soya beverage | The food reverts and prevents ariboflavinosis in murine models | Del Valle et al. (2016) |
| L. plantarum | Fermented soy curd | A combination of riboflavin‐producing L. plantarum strains resulted in a higher biofortification level than by using individual strains | Narayan et al. (2021) |
| L. acidophilus | Dairy whey and skimmed milk | Whey served as a better substrate for riboflavin bioenrichment | Guru and Viswanathan (2013) |
| L. fermentum | Bread | Tested a combination of yeast with riboflavin overproducing lactobacilli strains | Russo et al. (2014) |
| Folate producers | |||
| L. reuteri | Fruit fermentations | Combined production of folate: vitamin B12 (100:1) through genetic engineering. With the modified strain achieved folate bioenrichment levels significantly higher than those previously reported | Santos et al. (2008) |
| Lc. lactis | Fermented milk | Alleviates folate status in murine folate deficiency models | Jiao et al. (2020) |
| St. thermophilus and L. plantarum | Fermented milk | Tested several St. thermophilus strains combination with a L. plantarum strain to achieve highest vitamin production levels. Vitamin bioavailability and intestinal effects determined in mice model | Cucick et al. (2020) |
| L. plantarum, P. pentosaceus and C. tropicalis co‐culture | Fermented cereal gruel | Folate‐producing strains had been isolated from fermenting maize slurry. Up to 3‐fold increase in folate production during fermentation achieved with a combination of L. plantarum and C. tropicalis strains | Okoroafor et al. (2019) |
| Cobalamin producers | |||
| L. reuteri | Soybean based beverage | Consumption prevented development of B12 deficiency symptoms in pregnant mice and their offspring | Molina et al. (2012) |
| L. reuteri | Fruit fermentations | Combined production of folate: vitamin B12 (100:1) through genetic engineering. With the modified strain achieved folate bioenrichment levels significantly higher than those previously reported | Santos et al. (2008) |
| P. freudenreichii and L. brevis | Wheat bran | Optimized fermentation conditions with both species to maximize vitamin production while aiding to control Enterobacteriaceae and B. cereus | Xie et al. (2019) |
C. tropicalis (Candida tropicalis), Lc. lactis (Lactococcus lactis), L. acidophilus (Lactobacillus acidophilus), L. brevis (Levilactobacillus brevis), L. fermentum (Limosilactobacillus fermentum), L. plantarum (Lactiplantibacillus plantarum), L. reuteri (Limosilactobacillus reuteri), P. pentosaceus (Pedicocccus pentosaceus), P. freudenreichii (Propionibacterium freudenreichii), St. thermophilus (Streptococcus thermophilus).
Riboflavin, or vitamin B2, is a precursor of flavin mononucleotide (FMN) and flavin adenine dinucleotide (FAD) and is an essential cofactor in a variety of redox reactions. Most riboflavin synthesis for food fortification has been replaced by microbiological synthesis, mainly with Bacillus subtilis and the ascomycete Ashbya gossypii (Averianova et al., 2020). LAB species are frequently utilized during the production of fermented foods also have the capability to synthesize this vitamin, leading to the production of naturally biofortified foods. The genetic machinery required for its synthesis is present in strains of Lac. lactis, L. (Levilactobacillus) brevis, L. plantarum, Lactobacillus delbrueckii, Leuconostoc mesenteroides, Lactobacillus crispatus, L. (Limosilactobacillus) fermentum, L. (Limosilactobacillus) reuteri, L. (Ligilactobacillus) salivarius, Bifidobacterium longum subsp. infantis (Thakur et al., 2016). To date, riboflavin biofortification of fermented foods has been achieved through utilization of selected strains of L. acidophilus, L. plantarum, L. (Limosilactobacillus) mucosae, L. fermentum and P. freudenreichii in various food matrixes. Other producing bacteria of interest for the fermented foods industry, as those belonging to B. longum subsp. infantis, have been demonstrated as capable of producing this vitamin in vitro and could also possibly enhance this vitamin content in fermented foodstuffs (Levit et al., 2021; Solopova et al., 2020). An interesting aspect of riboflavin production by LAB is that the regulation of its genetic and enzymatic machinery has been extensively studied in some Gram positives. Strategies including genetic engineering and exposure to toxic analogous roseoflavin have enabled an enhancement of riboflavin bioproduction, leading to increase riboflavin production in representatives of various species (L. plantarum, Lac. lactis, Leuc. mesenteroides, P. freudenreichii and B. longum). This strategy represents an appealing and feasible alternative to developing fermented food products with increased riboflavin content as demonstrated on various food matrixes (Table 1) (Ge et al., 2020; Thakur et al., 2016), which could counteract riboflavin deficiency manifestations (LeBlanc et al., 2005, 2006). In some cases, riboflavin‐producing LAB strains have also demonstrated probiotic traits, thus they could be of interest from an industrial perspective, as multifunctional adjunct cultures, capable of producing biofortified fermented foods while also exerting other probiotic traits upon consumption (Arena et al., 2014; Russo et al., 2016).
Folate, or vitamin B11, is involved in many metabolic pathways and possesses anti‐oxidant capacity. Chemically produced folate used for fortification can have adverse effects and, thus, there exists interest in naturally produced folate for food and feed biofortification. Production of folate has been described in several LAB species and, in some strains, genetic engineering (Wegkamp et al., 2007) or exposure to the antagonist methrotexato has led to the generation of folate‐overproducing strains (Capozzi et al., 2012). Nevertheless, some issues impede their utilization for food biofortification, with the exception of L. plantarum, as production by most lactobacilli strains requires the presence of a precursor pABA (p‐aminobenzoate). Besides, some strains commonly present in fermented foods can also be folate consumers, thus reducing the bioavailability in the final product. This increases the need to conduct a rational and careful selection of bacterial strains to enable the efficient fortification of fermented foods. The combination of several folate‐producing strains (St. thermophilus in combination with Lac. lactis or Leuconostoc species) has significantly increased the content of this vitamin in fermented milks (LeBlanc et al., 2011). Also, natural folate‐overproducing strains have been isolated from artisanal Argentinean yoghourts. Their utilization has led to the production of yoghourts with a folate concentration 250% higher than the unfermented milk; and a combination of these strains with a Lactobacillus amylovorus further increased folate content by 44% (Laiño et al., 2012, 2014). While dairy products are an appealing matrix for folate fortification, as folate‐binding proteins present in milk may increase its bioavailability preventing its uptake by bacteria either in the fermented food or in the gut, St. thermophilus and L. reuteri folate‐producing strains have also been used to produce folate‐enriched oat and barley‐fermented foods, yet the highest production levels are achieved with yeast fermentation (Greppi et al., 2017; Kariluoto et al., 2014). Remarkably, administration of some LAB folate‐producing strains has demonstrated a capacity to increase faecal folate excretion (LeBlanc et al., 2013) and to ameliorate nutritional deficiency in in vivo models, suggesting its introduction in fermented foods can be an effective approach to prevent its deficiency (Tamene et al., 2019).
Production of other vitamins by microbial cultures of interest to the food production sector has been less studied. To highlight some examples, some thiamine‐producing LAB strains have been described, including representative strains of L. (Lacticaseibacillus) rhamnosus, L. plantarum, L. brevis and Lac. lactis. Remarkably, neuroprotective and immunomodulatory effects have been demonstrated in in vitro models for some of these thiamine‐producing strains (Teran et al., 2021). In any case, thiamine production in LAB strains usually occur at much lower levels than folate or riboflavin production; however, an overproduction, up to four‐fold, has been achieved through amprolium exposure (Masuda et al., 2012; Teran et al., 2021). Regarding cobalamin production, the genetic machinery required for its biosynthesis has been described in L. (Loigolactobacillus) coryniformis, L. reuteri and L. (Furfurilactobacillus) rossiae representative strains (De Angelis et al., 2014; Santos et al., 2008) and production has been demonstrated in a few LAB strains including L. coryniformis, L. reuteri, L. (Lactiplantibacillus) pentosus and P. freudenreichii (Deptula et al., 2017; Santos et al., 2008; Walhe et al., 2021). Yet, in most cases, their ability to produce the vitamin at nutritionally relevant doses remains to be shown. Despite the fact that the application of thiamine or cobalamine‐producing strains for fermented food biofortification has not been thoroughly investigated, some works have demonstrated their suitability to produce biofortified foods. For instance, supplementation with a cobalamin‐producing L. reuteri strain enabled the production of a soy‐yoghourt up to 18 μg/100 ml by optimizing the glycerol and fructose content of the matrix (Gu et al., 2015). Besides P. freudenreichii has been deemed capable of synthesizing vitamin B12 under growth conditions in food‐like conditions mimicking a cheese environment at nutritionally relevant doses (Deptula et al., 2017).
Overall, all these examples highlight the potential of some LAB cultures to achieve natural food biofortification. Combinations of cultures capable of leading to multivitamin production have also been demonstrated as an appealing approach to improve the nutritional properties of fermented cereals (Rajendran et al., 2017). Besides, genetic engineering strategies have been used to produce multivitamin‐producing cultures, yet their practical industrial application in food products might still face some regulatory obstacles (Sybesma et al., 2004).
Other metabolic traits from cultures to increase the nutritional value of fermented foods
Certain matrixes such as those derived from vegetables and cereals are quite complex (structurally) which might impede the proper activity of bacterial cultures during fermentation. The rational selection of appropriate starters capable of cooperatively fermenting the material and leading to the increase in bioactive or nutritional ingredients is crucial. As an example, in cereal‐based fermented porridge, the combination of folate‐producing with amylolytic strains enabled a significantly higher folate content to be achieved, as compared to the standard process (Bationo et al., 2019). In addition, the presence of some food components such as phytates or oxalates, commonly present in certain vegetable matrixes, such as cereal and pseudocereals, are known to negatively affect the bioaccessibility of some nutrients. For instance, phytate is the primary form of phosphate and inositol storage in plant seeds, and it is known to negatively impact mineral, protein and lipid digestion. Accordingly, phytase content increases through technological processing or exogenous supplementation can increase nutrient absorption and prevent nutritional deficiencies. Phytase activity is encountered in some LAB, yet it is not generally present in lactobacilli (Pradhan & Tamang, 2021). However, some investigations have demonstrated that phytase‐producing cultures are capable of improving the nutritional properties of fermented foods, such as quinoa sourdough or breads made from whole grains (Carrizo et al., 2020; García‐Mantrana et al., 2016). Thus, phytase activity might be a relevant trait when selecting cultures to improve the nutritional value of certain fermented vegetable matrixes. Undoubtedly, the potential of combining bacterial cultures capable of producing nutrients and phytase activities is a promising approach to improve the nutritional value of fermented foods and prevent mineral and vitamins deficiencies, as has been demonstrated in several in vitro and animal models (Carrizo et al., 2020). Synthetic ecology approaches supported by comprehensive genomic and metabolic characterization of cultures of interest would significantly aid towards this goal.
CULTURES TO IMPROVE HEALTH: PROBIOTICS AND POSTBIOTICS
In addition to the traditional use of LAB as food starter cultures for food fermentations and providers of sensorial properties, some of them, together with some members of the genus Bifidobacterium, have also been recognized as having health‐promoting effects in humans, either when administered included in foods or when consumed as food supplements. These bacteria are probiotics, which are defined as ‘live microorganisms that, when administered in adequate amounts, confer a health benefit on the host’ (Hill et al., 2014). The benefits of probiotics are extensively supported by current and past scientific literature and, since the middle of the last century (Gordon et al., 1957), there is clinical evidence of a variety of health effects, including an improvement in digestion, positive effects on our nervous system and consequently in our behaviour and cognition, immunomodulation, reinforcement of the epithelial barrier and protection against pathogenic microorganisms, among others (Sánchez et al., 2017). It is worth highlighting that non‐viable bacteria and their components and metabolites can also impact on human physiology. In this regard, the microorganisms that do not need to be alive to carry out their biological function, as well as their bioactive components, have recently been defined as postbiotics ‘preparation of inanimate microorganisms and/or their components that confer a health benefit on the host’ (Salminen et al., 2021). In relation to this, although the definition of probiotic was adopted by the scientific community with a wide consensus, the term postbiotic is still under debate, since it has not received unanimous support. Postbiotics had already been defined in the past as ‘any factor resulting from the metabolic activity of a probiotic or any released molecule capable of conferring beneficial effects to the host in a direct or indirect way’ (Tsilingiri & Rescigno, 2013); but also ‘inactivated, non‐viable microbial cells which after ingestion confer a health benefit’ had previously been defined as paraprobiotics (Taverniti & Guglielmetti, 2011). For this reason, there is currently some controversy regarding the definition of postbiotic. In any case, there is no doubt that these inactivated microorganisms can also have beneficial effects and they represent important advantages for the probiotic industry, since there is no risk of infection in immunodepresed individuals, and it is not necessary to maintain the cold chain to preserve the viability of the product during storage and delivery. However, we must emphasize that by using postbiotics, the physiological effects resulting from the colonization of the gut or derived from the metabolism of the bacteria in the gut will be missed.
The definition of probiotic or postbiotic does not necessarily imply that the product is orally administered. However, in this section we will only focus on those microorganisms that are consumed orally and whose beneficial effects are currently at the forefront of probiotic research, specifically: i) neuroactive metabolites produced by bacteria or bacteria triggering neurological effects through known molecular signals; ii) bioactive peptides and lipid metabolites resulting from its metabolic action on the food components.
LAB as bacterial mediators in the gut‐brain axis dialogue
Our gut is in continuous communication with our brain through molecular mediators, such as enteroendocrine signals, cytokines and neuropeptides. Currently, there is no doubt that bacterial by‐products and metabolites contribute to this two‐way dialogue. Animal studies have demonstrated that gut bacteria can change behaviour and cognition, and influence how nerve cells develop. In humans, an altered gut microbiome has been associated with impaired brain‐related conditions, which are often linked to intestinal symptoms (Collins et al., 2012; Morais et al., 2021). In this regard, some probiotics have shown the capacity to positively affect brain activity, either by synthesizing metabolites capable of directly interacting with our nervous system, or by stimulating different physiological responses. As an illustrative example, it has been proven by means of in vitro and animal models, that certain vitamins produced by LAB might have a neuroprotective effect that could prevent the development and progression of neurodegenerative diseases (Perez‐Visñuk et al., 2020; Teran et al., 2021).
Some lactobacilli and bifidobacteria produce neuroactive molecules that can partially trigger those effects. One of these molecules is gamma‐aminobutyric acid (GABA), the principal inhibitory neurotransmitter of the central nervous system. In bifidobacteria, the potential capacity to synthesize GABA has mainly been associated with the species Bifidobacterium adolescentis, a gut commensal with QPS status. A previous in silico screening of metagenomics datasets highlighted an association between B. adolescentis abundance and mental disorders (Duranti et al., 2020). Furthermore, a dietary intervention with a rat model demonstrated that B. adolescentis can stimulate the in vivo production of GABA (Duranti et al., 2020). In lactobacilli, the production of GABA exhibits considerable interspecies variations. Several works reported that L. brevis is among the most efficient GABA producers (Barrett et al., 2012). Using a mice model of metabolic syndrome, Patterson and co‐workers demonstrated that two strains of L. brevis showed potential to improve depressive symptoms and metabolic abnormalities associated with metabolic syndrome (Patterson et al., 2019). Also, a probiotic formulation including L. plantarum and B. adolescentis GABA‐producers was able to reduce depressive‐like behaviour in mice (Yunes et al., 2020). Recently, some strains of L. mucosae, L. reuteri and L. plantarum capable of synthesizing serotonin, a tryptophan‐derived metabolite playing a key role in mood, cognition, sleep regulation and other physiological processes, were reported. These serotonin‐producing lactobacilli have been proposed to treat disorders and diseases related to serotonin deficiency (Grasset et al., 2021). However, the physiological activity of these serotonin‐producing strains still needs to be confirmed in preclinical and clinical trials.
In addition to the direct effect through the synthesis of neuroactive compounds, other strains, alive or inactivated, have been shown to be capable of promoting different neuro‐related responses. For instance, oral administration of L. acidophilus NCFM and contact of the bacterium with intestinal epithelial cells modulates the perception of visceral pain in rats, by inducing the expression of opioid and cannabinoid receptors in the gut (Rousseaux et al., 2007). The analgesic functions of L. acidophilus NCFM were further studied in humans with mild to moderate abdominal pain, showing that it was able to modulate Mucosal Opioid Receptors (MOR) expression. Although L. acidophilus NCFM improved the intestinal symptoms, the authors concluded that the study did not have the sufficient power to draw solid conclusions (Ringel‐Kulka et al., 2014). Remarkably, some postbiotics composed of heat‐inactivated lactobacilli retain their neuro activity. In this regard, heat‐killed lactobacilli were able to favour sociability and decrease corticosterone levels in mice (Warda et al., 2019), and dietary intake of heat‐killed Lactobacillus gasseri CP2305 improved appetite in a mouse model and triggered an increase in the gene expression of neurotrophins in the hippocampus (Toyoda et al., 2020). In relation to bifidobacteria with a potential role in the gut‐brain axis, some studies have been focused on its potential to impact on sleep behaviour or depression, although the molecules that are behind the dialogue with the nervous system have not always been characterized. Moloney et al. (2020) evaluated the effect of B. longum 1714 on stress, cognition and mood in a randomized‐placebo controlled trial with a cross‐over design, in healthy human volunteers. Although B. longum did not display a significant effect on memory or attention, it was able to improve the quality and duration of sleep (Moloney et al., 2020). On the other hand, Bifidobacterium breve CCFM1025 reduced depression and anxiety behaviours in mice, in a process likely mediated by an increase in short‐chain fatty acids (SCFAs) and 5‐hydroxytryptophan levels. Likely, the capacity of this bacterium to adapt to intestinal conditions and its higher production levels of neuromodulatory metabolites contribute to its neuroactive potential (Tian et al., 2020, 2021).
In summary, functional cultures show promising evidence to modulate our physiological functions through bacterial metabolites able to interact with our brain. Not only the probiotics that confer mental health benefits to the host, the so‐called psychobiotics (Dinan et al., 2013), are able to do this job, since inactive bacteria have also shown promising results. Although the evidence behind the neuroactive capacity of functional cultures is still nascent, several preclinical studies support their effects, but more and properly designed intervention studies in humans are needed to confirm their suitability to treat and/or prevent neuro‐related disorders.
Bioactive peptides and lipid metabolites from food components
Some bioactive molecules released by LAB are the result of a metabolic action on different proteins or lipids present in food. LAB has an impressive proteolytic arsenal that can act on caseins, gluten and other proteins, releasing a wide spectrum of anti‐microbial, anti‐hypertensive and anti‐oxidant peptides, among other bioactive peptides. Indeed, the milk protein fraction is a source of encrypted anti‐microbial peptides acting on different spoilage and pathogenic microorganisms. Nebbia et al. (2021) found that Lac. lactis and Lactobacillus helveticus strains are able to release the peptide LEQLLRLKKY from α‐s1‐casein, a peptide showing inhibitory effects against E. coli and Ba. subtilis strains (Liu et al., 2015). On the other hand, L. rhamnosus and L. helveticus are able to release QKALNEINQF and TKKTKLTEEEKNRL from α‐S2 casein, two peptides with different mechanisms of anti‐microbial activity, the first disrupting the proton‐motive force of the cytoplasmic membrane, and the second with DNA‐binding capacity (Sistla, 2013). Interestingly, simulation models of intestinal digestion showed that some of these peptides produced as a consequence of the LAB metabolism in food can also be released in the human gastrointestinal tract. This is the case of YQEPVLGPVRGPFPI, a peptide produced by the action of L. rhamnosus 17D10 on β‐casein that is also generated by in vitro digestion with human gastric and duodenal juices, suggesting that some anti‐microbial peptides released from food proteins can have a double action, as a bioprotectant in the fermented food and as pathogen inhibition mechanisms in the human gut (Almaas et al., 2011; Nebbia et al., 2021).
A few reports have shown other promising activities encrypted in the food protein peptides. For instance, the anti‐oxidant activity of p10 peptide (QKALNEINQF) derived from α‐S2 casein was three times higher than ascorbic acid (Sistla, 2013), one of the main natural anti‐oxidants. Also, fermented milks selected on the basis of the proteolytic activity of lactobacilli strains showed a relevant radical‐scavenging activity, and L. (Lacticaseibacillus) casei strains were proposed to produce functional milks with anti‐oxidant activities (Shu et al., 2018; Solieri et al., 2015). In addition, vegetable material has also been used as a source of anti‐oxidant peptides. Peptides in soy flour fractions were assayed for high anti‐oxidative activity using human cell lines, showing that low and medium‐size peptides have potential anti‐oxidant activity (Cavaliere et al., 2021). However, we consider that it is worth highlighting that the majority of anti‐oxidant activity assays have been characterized under in vitro conditions, and the in vivo reproducibility of this activity is not easy to achieve.
Perhaps one of the most appealing characteristics of peptides derived from food fermentation is their anti‐hypertensive activity since several studies showed that various peptides derived from casein hydrolysis have this activity. Through the inhibition of angiotensin I‐converting enzyme (ACE), these peptides display anti‐hypertensive properties as was demonstrated using in vitro assays, mainly based on the degradation of synthetic substrates measured by fluorescence spectroscopy or UV–vis methods (de Oliveira et al., 2018). The proteolytic activities responsible for the generation of these peptides have been detected in some lactobacilli, such as L. helveticus and L. plantarum (de Oliveira et al., 2018; Xia et al., 2020). Furthermore, animal experiments, mainly carried out with spontaneously hypertensive rats, have shown promising results in reducing blood pressure (Beltrán‐Barrientos et al., 2016). However, human intervention trials are scarce and have reported contradictory results, with a mild to low reduction in blood pressure. In this regard, Lactobacilli‐fermented milks containing the IPP and VPP tripeptides displayed a slight reduction of blood pressure in hypertensive human individuals, this reduction being a dose‐dependent effect (Jauhiainen et al., 2007, 2012).
Another bioactive molecule that can be obtained by the activity of food‐grade bacteria (lactobacilli, bifidobacteria and propionibacteria) from the lipidic fraction of foods is the conjugated linoleic acid (CLA). CLA is attracting great interest due to its potential health‐promoting effects, such as anti‐carcinogenic and anti‐atherogenic effects, body fat modulation or reduction of inflammation (Yang et al., 2015). Among LAB, lactobacilli have shown an outstanding capacity in vitro to convert linoleic acid to CLA (Li et al., 2012; Macouzet et al., 2009). Although limited information exists regarding the molecular mechanisms that allow LAB to convert linoleic acid into CLA, it is stated that the linoleate isomerase enzyme (LAI) plays the most important role in this transformation (Liavonchanka & Feussner, 2008). The species L. plantarum has been the most widely assayed for its CLA‐producing ability, and several strains have been tested as adjunct cultures in different food fermentations (meat and dairy products) to increase CLA levels in the final product (Özer & Kılıçb, 2020; Renes et al., 2019). In the food system, conditions such as pH, temperature, amount of fatty acids or fermentation time could be critical for the conversion rates of these cultures (Özer & Kılıçb, 2021). Additionally, other food‐grade bacteria such as bifidobacteria have been tested for converting linoleic acid to CLA, with promising results on host health reported by different strains of B. breve in in vivo studies with animal models (Patterson et al., 2017). However, to exploit the CLA therapeutic effect in fermented food there is a need for further investigations.
CONCLUSION
Actual food trends demand more natural products with higher beneficial properties. LAB and other bacteria have been used in the food industry for a long time, but now, other LAB applications are being intentionally exploited or explored. Among those reviewed here, the use of anti‐microbial molecules, such as bacteriocins or anti‐microbial peptides encrypted in the milk matrix, as food preservatives might contribute to increase food safety. The search for novel LAB able to improve the sensorial properties of fermented foods is still a very active area since it might help to preserve the identity signature of specific products. Additionally, some LAB cultures also have an impact on our health by improving our nutritional status or by the probiotic benefits attributed to some specific strains or foods fermented with them. This positive effect on health could be due to the direct interaction of the bacteria, or their components, with the host and/or through the modulation of the intestinal microbiota which would need an additional revision. Therefore, LAB is still a promising way to obtain more natural, safer and high‐quality food products, using an environmental‐friendly biotechnology which is an increasing demand of 21st Century consumers.
González‐González, F. , Delgado, S. , Ruiz, L. , Margolles, A. & Ruas‐Madiedo, P. (2022) Functional bacterial cultures for dairy applications: Towards improving safety, quality, nutritional and health benefit aspects. Journal of Applied Microbiology, 133, 212–229. Available from: 10.1111/jam.15510
REFERENCES
- Acevedo‐Rocha, C.G. , Gronenberg, L.S. , Mack, M. , Commichau, F.M. & Genee, H.J. (2019) Microbial cell factories for the sustainable manufacturing of B vitamins. Current Opinion in Biotechnology, 56, 18–29. [DOI] [PubMed] [Google Scholar]
- Afshari, R. , Pillidge, C.J. , Dias, D.A. , Osborn, A.M. & Gill, H. (2020) Cheesomics: the future pathway to understanding cheese flavour and quality. Critical Reviews in Food Science and Nutrition, 60, 33–47. [DOI] [PubMed] [Google Scholar]
- Almaas, H. , Eriksen, E. , Sekse, C. , Comi, I. , Flengsrud, R. , Holm, H. et al. (2011) Antibacterial peptides derived from caprine whey proteins, by digestion with human gastrointestinal juice. The British Journal of Nutrition, 106, 896–905. [DOI] [PubMed] [Google Scholar]
- Altieri, C. , Ciuffreda, E. , Di Maggio, B. & Sinigaglia, M. (2017) Lactic acid bacteria as starter cultures. In: Speranza, B. , Bevilacqua, A. , Corbo, M.R. & Sinigaglia, M. (Eds.) Starter cultures in food production. Oxford: John Wiley & Sons, Ltd, pp. 1–15. [Google Scholar]
- Anumudu, C. , Hart, A. , Miri, T. & Onyeaka, H. (2021) Recent advances in the application of the antimicrobial peptide nisin in the inactivation of spore‐forming bacteria in foods. Molecules, 26, 5552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anyogu, A. , Olukorede, A. , Anumudu, C. , Onyeaka, H. , Areo, E. , Adewale, O. et al. (2021) Microorganisms and food safety risks associated with indigenous fermented foods from Africa. Food Control, 129, 108227. [Google Scholar]
- Arena, M.P. , Russo, P. , Capozzi, V. , Lopez, P. , Fiocco, D. & Spano, G. (2014) Probiotic abilities of riboflavin‐overproducing Lactobacillus strains: a novel promising application of probiotics. Applied Microbiology and Biotechnology, 98, 7569–7581. [DOI] [PubMed] [Google Scholar]
- Averianova, L.A. , Balabanova, L.A. , Son, O.M. , Podvolotskaya, A.B. & Tekutyeva, L.A. (2020) Production of vitamin B2 (riboflavin) by microorganisms: an overview. Frontiers in Bioengineering and Biotechnology, 8, 570828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachtarzi, N. , Kharroub, K. & Ruas‐Madiedo, P. (2019) Exopolysaccharide‐producing lactic acid bacteria isolated from traditional Algerian dairy products and their application for skim‐milk fermentations. LWT‐Food Science and Technology, 107, 117–124. [Google Scholar]
- Bachtarzi, N. , Speciale, I. , Kharroub, K. , De Castro, C. , Ruiz, L. & Ruas‐Madiedo, P. (2020) Selection of exopolysaccharide‐producing Lactobacillus plantarum (Lactiplantibacillus plantarum) isolated from Algerian fermented foods for the manufacture of skim‐milk fermented products. Microorganisms, 8, 1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barcenilla, C. , Ducic, M. , Lopez, M. , Prieto, M. & Alvarez‐Ordoñez, A. (2021) Application of lactic acid bacteria for the biopreservation of meat products: A systematic review. Meat Science, 183, 108661. [DOI] [PubMed] [Google Scholar]
- Barrett, E. , Ross, R.P. , O’Toole, P.W. , Fitzgerald, G.F. & Stanton, C. (2012) γ‐Aminobutyric acid production by culturable bacteria from the human intestine. Journal of Applied Microbiology, 113, 411–417. [DOI] [PubMed] [Google Scholar]
- Bationo, F. , Songré‐Ouattara, L.T. , Hemery, Y.M. , Hama‐Ba, F. , Parkouda, C. , Chapron, M. et al. (2019) Improved processing for the production of cereal‐based fermented porridge enriched in folate using selected lactic bacteria and a back‐slopping process. LWT‐Food Science and Technology, 106, 172–178. [Google Scholar]
- Beltrán‐Barrientos, L.M. , Hernández‐Mendoza, A. , Torres‐Llanez, M.J. , González‐Córdova, A.F. & Vallejo‐Córdoba, B. (2016) Invited review: fermented milk as antihypertensive functional food. Journal of Dairy Science, 99, 4099–4110. [DOI] [PubMed] [Google Scholar]
- Blaya, J. , Barzideh, Z. & LaPointe, G. (2018) Symposium review: interaction of starter cultures and nonstarter lactic acid bacteria in the cheese environment. Journal of Dairy Science, 101, 3611–3629. [DOI] [PubMed] [Google Scholar]
- Buzzini, P. , Di Mauro, S. & Turchetti, B. (2017) Yeasts as starter cultures. In: Speranza, B. , Bevilacqua, A. , Corbo, M.R. & Sinigaglia, M. (Eds.) Starter cultures in food production. Oxford: John Wiley & Sons, Ltd, pp. 16–49. [Google Scholar]
- Capozzi, V. , Menga, V. , Digesu, A.M. , De Vita, P. , van Sinderen, D. , Cattivelli, L. et al. (2011) Biotechnological production of vitamin B2‐enriched bread and pasta. Journal of Agricultural and Food Chemistry, 59, 8013–8020. [DOI] [PubMed] [Google Scholar]
- Capozzi, V. , Russo, P. , Dueñas, M.T. , López, P. & Spano, G. (2012) Lactic acid bacteria producing B‐group vitamins: a great potential for functional cereals products. Applied Microbiology and Biotechnology, 96, 1383–1394. [DOI] [PubMed] [Google Scholar]
- Carminati, D. , Meucci, A. , Tidona, F. , Zago, M. & Giraffa, G. (2016) Multifunctional lactic acid bacteria cultures to improve quality and nutritional benefits in dairy products. In: Ravishankar Rai, V. (Ed.) Advances in food biotechnology. West Sussex, UK: John Wiley & Sons, Ltd, pp. 263–275. [Google Scholar]
- Carrizo, S.L. , LeBlanc, A.M. , LeBlanc, J.G. & Rollán, G.C. (2020) Quinoa pasta fermented with lactic acid bacteria prevents nutritional deficiencies in mice. Food Research International, 2020(127), 108735. [DOI] [PubMed] [Google Scholar]
- Castro‐Bravo, N. , Wells, J.M. , Margolles, A. & Ruas‐Madiedo, P. (2018) Interactions of surface exopolysaccharides from Bifidobacterium and Lactobacillus within the intestinal environment. Frontiers in Microbiology, 9, 2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavaliere, C. , Montone, A.M.I. , Aita, S.E. , Capparelli, R. , Cerrato, A. , Cuomo, P. et al. (2021) Production and characterization of medium‐sized and short antioxidant peptides from soy flour‐simulated gastrointestinal hydrolysate. Antioxidants, 10, 734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceruso, M. , Liu, Y. , Gunther, N.W. , Pepe, T. , Anastasio, A. , Qi, P.X. et al. (2021) Anti‐listerial activity of thermophilin 110 and pediocin in fermented milk and whey. Food Control, 125, 107941. [Google Scholar]
- Chen, H. , Yan, X. , Du, G. , Guo, Q. , Shi, Y. , Chang, J. et al. (2021) Recent developments in antifungal lactic acid bacteria: application, screening methods, separation, purification of antifungal compounds and antifungal mechanisms. Critical Reviews in Food Science and Nutrition, 1–15. 10.1080/10408398.2021.1977610 [DOI] [PubMed] [Google Scholar]
- Collins, S.M. , Surette, M. & Bercik, P. (2012) The interplay between the intestinal microbiota and the brain. Nature Reviews. Microbiology, 10, 735–742. [DOI] [PubMed] [Google Scholar]
- Crittenden, R.G. , Martinez, N.R. & Playne, M.J. (2003) Synthesis and utilisation of folate by yoghurt starter cultures and probiotic bacteria. International Journal of Food Microbiology, 80, 217–222. [DOI] [PubMed] [Google Scholar]
- Cucick, A.C.C. , Gianni, K. , Todorov, S.D. , de Moreno de LeBlanc, A. , LeBlanc, J.G. and Franco, B.D.G.M. (2020) Evaluation of the bioavailability and intestinal effects of milk fermented by folate producing lactic acid bacteria in a depletion/repletion mice model. Journal of Functional Foods 66, 103785. [Google Scholar]
- Dantigny, P. & Bevilacqua, A. (2017) Fungal starters: an insight into the factors affecting the germination of conidia. In: Speranza, B. , Bevilacqua, A. , Corbo, M.R. & Sinigaglia, M. (Eds.) Starter cultures in food production. Oxford: John Wiley & Sons, Ltd, pp. 50–63. [Google Scholar]
- De Angelis, M. , Bottacini, F. , Fosso, B. , Kelleher, P. , Calasso, M. , Di Cagno, R. et al. (2014) Lactobacillus rossiae, a vitamin B12 producer, represents a metabolically versatile species within the genus Lactobacillus . PLoS One, 9, e107232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Valle, M.J. , Laiño, J.E. , de Moreno de LeBlanc, A. Savoy de Giori, G. & LeBlanc, J. G. (2016) Soyamilk fermented with riboflavin‐producing Lactobacillus plantarum CRL 2130 reverts and prevents ariboflavinosis in murine models. The British Journal of Nutrition 116, 1229–1235. [DOI] [PubMed] [Google Scholar]
- Deptula, P. , Chamlagain, B. , Edelmann, M. , Sangsuwan, P. , Nyman, T.A. , Savijoki, K. et al. (2017) Food‐like growth conditions support production of active vitamin B12 by Propionibacterium freudenreichii 2067 without DMBI, the lower ligand base, or cobalt supplementation. Frontiers in Microbiology, 8, 368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Cagno, R. , De Pasquale, I. , De Angelis, M. , Buchin, S. , Rizzello, C.G. & Gobbetti, M. (2014) Use of microparticulated whey protein concentrate, exopolysaccharide‐producing Streptococcus thermophilus, and adjunct cultures for making low‐fat Italian Caciotta‐type cheese. Journal of Dairy Science, 97, 72–84. [DOI] [PubMed] [Google Scholar]
- Dinan, T.G. , Stanton, C. & Cryan, J.F. (2013) Psychobiotics: a novel class of psychotropic. Biological Psychiatry, 74, 720–726. [DOI] [PubMed] [Google Scholar]
- Duranti, S. , Ruiz, L. , Lugli, G.A. , Tames, H. , Milani, C. , Mancabelli, L. et al. (2020) Bifidobacterium adolescentis as a key member of the human gut microbiota in the production of GABA. Scientific Reports, 10, 14112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Panel on Biological Hazards (BIOHAZ) . (2021) Update of the list of QPS‐recommended biological agents intentionally added to food or feed as notified to EFSA 14: suitability of taxonomic units notified to EFSA until march 2021. EFSA Journal, 19, 6689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS) . (2017) Safety of nisin (E 234) as a food additive in the light of new toxicological data and the proposed extension of use. EFSA Journal, 15, 5063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAO‐WHO . (2006) Guidelines on food fortification with micronutrients. Ed. Allen, L. , de Benoist, B. , Dary, O. and Hurrell, R . Switzerland: World Health Organization; (ISBN 92 4 159401 2). [Google Scholar]
- Feng, J.Y. , Thakur, K. , Ni, Z.J. , Zhu, Y.Y. , Hu, F. , Zhang, J.G. et al. (2021) Effects of okara and vitamin B2 bioenrichment on the functional properties and in vitro digestion of fermented soy milk. Food Research International, 145, 110419. [DOI] [PubMed] [Google Scholar]
- Fox, P. , Guinee, T.P. , Cogan, T.M. & McSweeney, P.L.H. (2017) Starter cultures. In: Fox, P. , Guinee, T.P. , Cogan, T.M. & McSweeney, P.L.H. (Eds.) Fundamentals of cheese science. New York: Springer Nature, pp. 121–183. [Google Scholar]
- Gangadharan, D. & Nampoothiri, M.K. (2011) Folate production using Lactococcus lactis ssp. cremoris with implications for fortification of skim milk and fruit juices. LWT‐Food Science and Technology, 44, 1859–1864. [Google Scholar]
- García‐Mantrana, I. , Yebra, M.J. , Haros, M. & Monedero, V. (2016) Expression of bifidobacterial phytases in Lactobacillus casei and their application in a food model of whole‐grain sourdough bread. International Journal of Food Microbiology, 216, 18–24. [DOI] [PubMed] [Google Scholar]
- Ge, Y.Y. , Zhang, J.R. , Corke, H. & Gan, R.Y. (2020) Screening and spontaneous mutation of pickle‐derived Lactobacillus plantarum with overproduction of riboflavin, related mechanism, and food application. Food, 9, 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentès, M.C. , St‐Gelais, D. & Turgeon, S.L. (2011) Gel formation and rheological properties of fermented milk with in situ exopolysaccharide production by lactic acid bacteria. Dairy Science & Technology, 91, 645–661. [Google Scholar]
- Gibbons, J.G. (2019) How to train your fungus. mBio, 10, e03031–e03019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons, J.G. & Rinker, D.C. (2015) The genomics of microbial domestication in the fermented food environment. Current Opinion in Genetics & Development, 35, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobbetti, M. , De Angelis, M. , Di Cagno, R. , Mancini, L. & Fox, P.F. (2015) Pros and cons for using non‐starter lactic acid bacteria (NSLAB) as secondary/adjunct starters for cheese ripening. Trends in Food Science and Technology, 45, 167–178. [Google Scholar]
- Gobbetti, M. , Di Cagno, R. , Calasso, M. , Neviani, E. , Fox, P.F. & De Angelis, M. (2018) Drivers that establish and assembly the lactic acid bacteria biota in cheeses. Trends in Food Science and Technology, 78, 244–254. [Google Scholar]
- Gonzales‐Barron, U. , Goncalves‐Tenorio, A. , Rodrigues, V. & Cadavez, C. (2017) Foodborne pathogens in raw milk and cheese of sheep and goat origin: a meta‐analysis approach. Current Opinion in Food Science, 18, 7–13. [Google Scholar]
- Gordon, D. , MacRae, J. & Wheather, D.M. (1957) A Lactobacillus preparation for use with antibiotics. Lancet, 272, 899–901. [DOI] [PubMed] [Google Scholar]
- Grasset, E. , Khan, M. , Möllstam, B. and Roos, S. (2021) Serotonin producing bacteria. WO2021091474.
- Greco, E. , El‐Aguizy, O. , Ali, M.F. , Foti, S. , Cunsolo, V. , Saletti, R. et al. (2018) Proteomic analyses on an ancient Egyptian cheese and biomolecular evidence of brucellosis. Analytical Chemistry, 90, 9673–9676. [DOI] [PubMed] [Google Scholar]
- Greppi, A. , Saubde, F. , Botta, C. , Humblot, C. , Guyot, J.P. & Cocolin, L. (2017) Potential probiotic Pichia kudriavzevii strains and their ability to enhance folate content of traditional cereal‐based African fermented food. Food Microbiology, 62, 169–177. [DOI] [PubMed] [Google Scholar]
- Gu, Q. , Zhang, C. , Song, D. , Li, P. & Zhu, X. (2015) Enhancing vitamin B12 content in soy‐yogurt by Lactobacillus reuteri . International Journal of Food Microbiology, 206, 56–59. [DOI] [PubMed] [Google Scholar]
- Guru, V. & Viswanathan, K. (2013) Riboflavin production in milk whey using probiotic bacteria—Lactobacillus acidophilus and Lactococcus lactis . The Indian Journal of Fundamental and Applied Life Sciences, 3, 169–176. [Google Scholar]
- Hahn, C. , Müller, E. , Wille, S. , Weiss, J. , Atamer, Z. & Hinrichs, J. (2014) Control of microgel particle growth in fresh cheese (concentrated fermented milk) with an exopolysaccharide‐producing starter culture. International Dairy Journal, 36, 46–54. [Google Scholar]
- Hassan, A.N. (2008) ADSA foundation scholar award: possibilities and challenges of exopolysaccharide‐producing lactic cultures in dairy foods. Journal of Dairy Science, 91, 1282–1298. [DOI] [PubMed] [Google Scholar]
- Hatti‐Kaul, R. , Chen, L. , Dishisha, T. & El Enshasy, H. (2018) Lactic acid bacteria: from starter cultures to producers of chemicals. FEMS Microbiology Letters, 365, fny213. [DOI] [PubMed] [Google Scholar]
- Hidalgo‐Cantabrana, C. , Sánchez, B. , Milani, C. , Ventura, M. , Margolles, A. & Ruas‐Madiedo, P. (2014) Genomic overview and biological functions of exopolysaccharide biosynthesis in Bifidobacterium spp. Applied and Environmental Microbiology, 80, 9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill, C. , Guarner, F. , Reid, G. , Gibson, G.R. , Merenstein, D.J. , Pot, B. et al. (2014) The international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nature Reviews. Gastroenterology & Hepatology, 11, 506–515. [DOI] [PubMed] [Google Scholar]
- Holzapfel, W.H. & Wood, B.J.B. (2014) Lactic acid bacteria. Biodiversity and taxonomy. West Sussex, UK: John Wiley & Sons, Ltd. [Google Scholar]
- Jauhiainen, T. , Niittynen, L. , Orešič, M. , Järvenpää, S. , Hiltunen, T.P. , Rönnback, M. et al. (2012) Effects of long‐term intake of lactotripeptides on cardiovascular risk factors in hypertensive subjects. European Journal of Clinical Nutrition, 66, 843–849. [DOI] [PubMed] [Google Scholar]
- Jauhiainen, T. , Rönnback, M. , Vapaatalo, H. , Wuolle, K. , Kautiainen, H. & Korpela, R. (2007) Lactobacillus helveticus fermented milk reduces arterial stiffness in hypertensive subjects. International Dairy Journal, 17, 1209–1211. [Google Scholar]
- Jiang, S. , Cai, L. , Lv, L. & Li, L. (2021) Pediococcus pentosaceus, a future additive or probiotic candidate. Microbial Cell Factories, 20, 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Oliveira, M.R. , Silva, T.J. , Barros, E. , Guimarães, V.M. , Baracat‐Pereira, M.C. , Eller, M.R. et al. (2018) Anti‐hypertensive peptides derived from caseins: mechanism of physiological action, production bioprocesses, and challenges for dood applications. Applied Biochemistry and Biotechnology, 185, 884–908. [DOI] [PubMed] [Google Scholar]
- Jiao, W. , Wang, S. , Guan, J. , Shi, J. , Evivie, S.E. , Yan, F. et al. (2020) Milk fermented with Lactococcus lactis KLDS4.0325 alleviates folate status in deficient mice. Food & Function, 11, 4571–4581. [DOI] [PubMed] [Google Scholar]
- Kariluoto, S. , Edelmann, M. , Nystrom, L. , Sontag‐Stroh, T. , Salovaara, H. , Kivela, R. et al. (2014) In situ enrichment of folate by microorganisms in beta‐glucan rich oat and barley matrices. International Journal of Food Microbiology, 176, 38–48. [DOI] [PubMed] [Google Scholar]
- Korcz, E. & Varga, L. (2021) Exopolysaccharides from lactic acid bacteria: techno‐functional application in the food industry. Trends in Food Science and Technology, 110, 375–384. [Google Scholar]
- Laiño, J.E. , Leblanc, J.G. & Savoy de Giori, G. (2012) Production of natural folates by lactic acid bacteria starter cultures isolated from artisanal Argentinean yogurts. Canadian Journal of Microbiology, 58, 581–588. [DOI] [PubMed] [Google Scholar]
- Laiño, J.E. , Juarez del Valle, M. , Savoy de Giori, G. & LeBlanc, J.G. (2013) Development of a high folate concentration yogurt naturally bio‐enriched using selected lactic acid bacteria. LWT‐Food Science and Technology, 54, 1–5. [Google Scholar]
- Laiño, J.E. , Juarez del Valle, M. , Savoy de Giori, G. & LeBlanc, J.G. (2014) Applicability of a Lactobacillus amylovorus strain as co‐culture for natural folate bio‐enrichment of fermented milk. International Journal of Food Microbiology, 191, 10–16. [DOI] [PubMed] [Google Scholar]
- LeBlanc, J.G. , Burgess, C. , Sesma, F. , Savoy De Giori, G. & Van Sinderen, D. (2005) Ingestion of milk fermented by genetically modified Lactococcus lactis improves the riboflavin status of deficient rats. Journal of Dairy Science, 88, 3435–3442. [DOI] [PubMed] [Google Scholar]
- LeBlanc, J.G. , Rutten, G. , Bruinenberg, P. , Sesma, F. , De Giori, G.S. & Smid, E.J. (2006) A novel dairy product fermented with Propionibacterium freudenreichii improves the riboflavin status of deficient rats. Nutrition, 22, 645–651. [DOI] [PubMed] [Google Scholar]
- LeBlanc, J.G. , Laiño, J.E. , Juarez del Valle, M. , Vannini, V. , van Sinderen, D. , Taranto, M.P. et al. (2011) B‐group vitamin production by lactic acid bacteria‐current knowledge and potential applications. Journal of Applied Microbiology, 111, 1297–1309. [DOI] [PubMed] [Google Scholar]
- LeBlanc, J.G. , Milani, C. , Savoy de Giori, G. , Sesma, F. , van Sinderen, D. & Ventura, M. (2013) Bacteria as vitamin suppliers to their host: a gut microbiota perspective. Current Opinion in Biotechnology, 24, 160–168. [DOI] [PubMed] [Google Scholar]
- Leroy, F. & De Vuyst, L. (2004) Lactic acid bacteria as functional starter cultures for the food fermentation industry. Trends in Food Science and Technology, 15, 67–78. [Google Scholar]
- Levit, R. , Savoy de Giori, G. , de Moreno de LeBlanc, A. and LeBlanc, J.G. (2021) Recent update on lactic acid bacteria producing riboflavin and folates: application for food fortification and treatment of intestinal inflammation. Journal of Applied Microbiology 130, 1412–1424. [DOI] [PubMed] [Google Scholar]
- Leyva‐Salas, M. , Mounier, J. , Valence, F. , Coton, M. , Thierry, A. & Coton, E. (2017) Antifungal microbial agents for food biopreservation—a review. Microorganisms, 5, 1–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Q. & Gänzle, M. (2020) Host‐adapted lactobacilli in food fermentations: impact of metabolic traits of host adapted lactobacilli on food quality and human health. Current Opinion in Food Science, 31, 71–80. [Google Scholar]
- Li, H. , Liu, Y. , Bao, Y. , Liu, X. & Zhang, H. (2012) Conjugated linoleic acid conversion by six Lactobacillus plantarum strains cultured in MRS broth supplemented with sunfloweroOil and soymilk. Journal of Food Science, 77, M330–M336. [DOI] [PubMed] [Google Scholar]
- Liavonchanka, A. & Feussner, I. (2008) Biochemistry of PUFA double bond isomerases producing conjugated linoleic acid. ChemBioChem, 9, 1867–1872. [DOI] [PubMed] [Google Scholar]
- Linares, D.M. , Gómez, C. , Renes, E. , Fresno, J.M. , Tornadijo, M.E. , Ross, R.P. et al. (2017) Lactic acid bacteria and Bifidobacteria with potential to design natural biofunctional health‐promoting dairy foods. Frontiers in Microbiology, 8, 846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Y. , Eichler, J. & Pischetsrieder, M. (2015) Virtual screening of a milk peptide database for the identification of food‐derived antimicrobial peptides. Molecular Nutrition & Food Research, 59, 2243–2254. [DOI] [PubMed] [Google Scholar]
- Macouzet, M. , Lee, B.H. & Robert, N. (2009) Production of conjugated linoleic acid by probiotic Lactobacillus acidophilus La‐5. Journal of Applied Microbiology, 106, 1886–1891. [DOI] [PubMed] [Google Scholar]
- Marco, M.L. , Heeney, D. , Binda, S. , Cifelli, C.J. , Cotter, P.D. , Foligné, B. et al. (2017) Health benefits of fermented foods: microbiota and beyond. Current Opinion in Biotechnology, 44, 94–102. [DOI] [PubMed] [Google Scholar]
- Masuda, M. , Ide, M. Utsumi, H. Niiro, T. Shimamura, Y. & Murata, M. (2012) Production potency of folate, vitamin B(12), and thiamine by lactic acid bacteria isolated from Japanese pickles. Bioscience, Biotechnology, and Biochemistry, 76, 2061–2067. [DOI] [PubMed] [Google Scholar]
- Mayo, B. , Rodríguez, J. , Vázquez, L. & Flórez, A.B. (2021) Microbial interactions within the cheese ecosystem and their application to improve quality and safety. Food, 10, 602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng, F. , Zhu, X. , Zhao, H. , Nie, T. , Lu, F. , Lu, Z. et al. (2021) Class III bacteriocin with broad‐spectrum antibacterial activity from Lactobacillus acidophilus NX2‐6 and its preservation in milk and cheese. Food Control, 121, 107597. [Google Scholar]
- Mirkovic, N. , Kulas, J. , Miloradovic, Z. , Miljkovic, M. , Tucovic, D. , Miocinovic, J. et al. (2020) Lactolisterin BU‐producer Lactococcus lactis subsp. lactis BGBU1‐4: bio‐control of listeria monocytogenes and Staphylocococcus aureus in fresh soft cheese and effect on immunological response of rats. Food Control, 111, 107076. [Google Scholar]
- Molina, V. , Médici, M. , Font de Valdez, G. & Taranto, M.P. (2012) Soybean‐based functional food with vitamin B12‐producing lactic acid bacteria. Journal of Functional Foods, 4, 831–836. [Google Scholar]
- Molinero, N. , Sabater, C. , Calvete, I. , Delgado, S. , Ruas‐Madiedo, P. , Ruiz, L. et al. (2022) Mechanisms of gut microbiota modulation by food, probiotics, prebiotics and more. In: Glibetic, M. (Ed.) Comprehensive Gut Microbiota. Amsterdam, Netherlands: Elsevier Inc., pp. 84–101. 10.1016/B978-0-12-819265-8.00095-4 [DOI] [Google Scholar]
- Moloney, G.M. , Long‐Smith, C.M. , Murphy, A. , Dorland, D. , Hojabri, S.F. , Ramirez, L.O. et al. (2020) Improvements in sleep indices during exam stress due to consumption of a Bifidobacterium longum . Brain, Behavior, and Immunity‐Health, 10, 100174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morais, L.H. , Schreiber, H.L. & Mazmanian, S.K. (2021) The gut microbiota‐brain axis in behaviour and brain disorders. Nature Reviews. Microbiology, 19, 241–255. [DOI] [PubMed] [Google Scholar]
- Narayan, K.S. , Gaurkhede, S. , Sharma, V. , Kumar, A. , Bhushan, B. & Mishra, V. (2021) Technological and functional assessment of riboflavin enriched probiotic soycurd. Fermentation, 7, 47. [Google Scholar]
- Nebbia, S. , Lamberti, C. , Lo Bianco, G. , Cirrincione, S. , Laroute, V. , Cocaign‐Bousquet, M. et al. (2021) Antimicrobial potential of food lactic acid bacteria: bioactive peptide decrypting from caseins and bacteriocin production. Microorganisms, 9, 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okoroafor, I. , Banwo, K. , Olanbiwoninu, A.A. & Odunfa, S.A. (2019) Folate enrichment of Ogi (a fermented cereal gruel) using folate producing starter cultures. Advances in Microbiology, 9, 177–193. [Google Scholar]
- Özer, C.O. & Kılıçb, B. (2020) Utilization of optimized processing conditions for high yield synthesis of conjugated linoleic acid by L. plantarum AB20‐961 and L. plantarum DSM2601 in semi‐dry fermented sausage. Meat Science, 169, 108218. [DOI] [PubMed] [Google Scholar]
- Özer, C.O. & Kılıçb, B. (2021) Optimization of pH, time, temperature, variety and concentration of the added fatty acid and the initial count of added lactic acid bacteria strains to improve microbial conjugated linoleic acid production in fermented ground beef. Meat Science, 171, 108303. [DOI] [PubMed] [Google Scholar]
- Ozzen, M. & Dinleyici, E.C. (2015) The history of probiotics: the untold story. Beneficial Microbes, 6, 159–165. [DOI] [PubMed] [Google Scholar]
- Pancza, B. , Szathmary, M. , Gyurjan, I. , Bankuti, B. , Tudos, C. , Szathmary, S. et al. (2021) A rapid and efficient DNA isolation method for qPCR‐based detection of pathogenic and spoilage bacteria in milk. Food Control, 130, 108236. [Google Scholar]
- Patterson, E. , Wall, R. , Lisai, S. , Ross, R.P. , Dinan, T.G. , Cryan, J.F. et al. (2017) Bifidobacterium breve with α‐linolenic acid alters the composition, distribution and transcription factor activity associated with metabolism and absorption of fat. Scientific Reports, 7, 43300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson, E. , Ryan, P.M. , Wiley, N. , Carafa, I. , Sherwin, E. , Moloney, G. et al. (2019) Gamma‐aminobutyric acid‐producing lactobacilli positively affect metabolism and depressive‐like behaviour in a mouse model of metabolic syndrome. Scientific Reports, 9, 16323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng, P. , Song, J. , Zeng, W. , Wang, H. , Zhang, Y. , Xin, J. et al. (2021) A broad‐spectrum novel bacteriocin produced by Lactobacillus plantarum SHY 21–2 from yak yogurt: purification, antimicrobial characteristics and antibacterial mechanism. LWT‐Food Science and Technology, 142, 110955. [Google Scholar]
- Perez‐Visñuk, D. , Savoy de Giori, G. & LeBlanc, J.G. (2020) Neuroprotective effects associated with immune modulation by selected lactic acid bacteria in a Parkinson’s disease model. Nutrition, 79‐80, 110995. [DOI] [PubMed] [Google Scholar]
- Pompei, A. , Cordisco, L. , Amaretti, A. , Zanoni, S. , Matteuzzi, D. & Rossi, M. (2007) Folate production by bifidobacteria as a potential probiotic property. Applied and Environmental Microbiology, 73, 179–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradhan, P. & Tamang, J.P. (2021) Probiotic properties of lactic acid bacteria isolated from traditionally prepared dry starters of the eastern Himalayas. World Journal of Microbiology and Biotechnology, 37, 7. [DOI] [PubMed] [Google Scholar]
- Prete, R. , Alam, M.K. , Perpetuini, G. , Perla, C. , Pittia, P. & Corsetti, A. (2021) Lactic acid bacteria exopolysaccharides producers: a sustainable tool for functional foods. Food, 10, 1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rad, A.H. , Khosroushahi, A.Y. , Khalili, M. & Jafarzadeh, S. (2016) Folate bio‐fortification of yoghurt and fermented milk: a review. Dairy Science & Technology, 96, 427–471. [Google Scholar]
- Rajendran, S.C.C. , Chamlagain, B. , Kariluoto, S. , Piironen, V. & Saris, P.E.J. (2017) Biofortification of riboflavin and foalte in Idli batter, based on fermented cereal and pulse, by Lactococcus lactis N8 and saccharomyces boulardii SAA655. Journal of Applied Microbiology, 122, 1663–1671. [DOI] [PubMed] [Google Scholar]
- Randazzo, C.L. , Liotta, L. , De Angelis, M. , Celano, G. , Russo, N. , Van Hoorde, K. et al. (2021) Adjunct culture of non‐starter lactic acid bacteria for the production of Provola Dei Nebrodi PDO cheese: in vitro screening and pilot‐scale cheese‐making. Microorganisms, 9, 179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebaza‐Cardenas, T.D. , Silva‐Cajaleón, K. , Sabater, C. , Delgado, S. , Montes‐Villanueva, N.D. & Ruas‐Madiedo, P. (2021) “Masato de yuca” and “Chicha de Siete Semillas” two traditional vegetable fermented beverages from Peru as source for the isolation of potential probiotic bacteria. Probiotics and Antimicrobial Proteins, 1–12. 10.1007/s12602-021-09836-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renes, E. , Gómez‐Cortés, P. , de la Fuente, M.A. , Linares, D.M. , Tornadijo, M.E. & Fresno, J.M. (2019) CLA‐producing adjunct cultures improve the nutritional value of sheep cheese fat. Food Research International, 116, 819–826. [DOI] [PubMed] [Google Scholar]
- Ringel‐Kulka, T. , Goldsmith, J.R. , Carroll, I.M. , Barros, S.P. , Palsson, O. , Jobin, C. et al. (2014) Lactobacillus acidophilus NCFM affects colonic mucosal opioid receptor expression in patients with functional abdominal pain—a randomised clinical study. Alimentary Pharmacology & Therapeutics, 40, 200–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez, A. , Martínez, B. , García, P. , Ruas‐Madiedo, P. & Sánchez, B. (2017) New trends in dairy microbiology: towards safe and healthy products. In: Speranza, B. , Bevilacqua, A. , Corbo, M.R. & Sinigaglia, M. (Eds.) Starter cultures in food production. Oxford: John Wiley & Sons, Ltd, pp. 299–323. [Google Scholar]
- Rouse, S. & Van Sinderen, D. (2008) Bioprotective potential of lactic acid bacteria in malting and brewing. Journal of Food Protection, 71, 1724–1733. [DOI] [PubMed] [Google Scholar]
- Rossi, M. , Amaretti, A. & Raimondi, S. (2011) Folate production by probiotic bacteria. Nutrients, 3, 118–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruas‐Madiedo, P. , Hugenholtz, J. & Zoon, P. (2002) An overview of the functionality of exopolysaccharides produced by lactic acid bacteria. International Dairy Journal, 12, 163–171. [Google Scholar]
- Ruas‐Madiedo, P. & de los Reyes‐Gavilan C.G. (2005) Invited review: methods for the screening, isolation, and characterization of exopolysaccharides produced by lactic acid bacteria. Journal of Dairy Science, 88, 843–856. [DOI] [PubMed] [Google Scholar]
- Ruas‐Madiedo, P. , Salazar, N. & de los Reyes‐Gavilan C.G. (2009) Exopolysaccharides produced by lactic acid bacteria in food and probiotic applications. In: Moran, A. , Holst, O. , Brennan, P. & von Itzstein, M. (Eds.) Microbial glycobiology structures, relevance and applications. San Diego: Academic Press, Elsevier Inc., pp. 887–902. [Google Scholar]
- Ruas‐Madiedo, P. & Rodríguez, A. (2017) Non‐starter bacteria ‘functional’ cultures. In: Speranza, B. , Bevilacqua, A. , Corbo, M.R. & Sinigaglia, M. (Eds.) Starter cultures in food production. Oxford: John Wiley & Sons, Ltd, pp. 64–78. [Google Scholar]
- Rousseaux, C. , Thuru, X. , Gelot, A. , Barnich, N. , Neut, C. , Dubuquoy, L. et al. (2007) Lactobacillus acidophilus modulates intestinal pain and induces opioid and cannabinoid receptors. Nature Medicine, 13, 35–37. [DOI] [PubMed] [Google Scholar]
- Russo, P. , Capozzi, V. , Arena, M.A. , Spadaccino, G. , Dueñas, M.T. , López, P. et al. (2014) Riboflavin‐overproducing strains of Lactobacillus fermentum for riboflavin‐enriched bread. Applied Microbiology and Biotechnology, 98, 3691–3700. [DOI] [PubMed] [Google Scholar]
- Russo, P. , De Chiara, M.L.V. , Capozzi, V. , Arena, M.P. , Amodio, M.L. , Rascón, A. et al. (2016) Lactobacillus plantarum strains for multifunctional oat‐based foods. LWT‐Food Science and Technology, 68, 288–294. [Google Scholar]
- Salazar, N. , Gueimonde, M. , de los Reyes‐Gavilán, C.G. & Ruas‐Madiedo, P. (2016) Exopolysaccharides produced by lactic acid bacteria and bifidobacteria as fermentable substrates by the intestinal microbiota. Critical Reviews in Food Science and Nutrition, 56, 1440–1453. [DOI] [PubMed] [Google Scholar]
- Salminen, S. , Collado, M.C. , Endo, A. , Hill, C. , Lebeer, S. , Quigley, E.M.M. et al. (2021) The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nature Reviews. Gastroenterology & Hepatology, 18, 649–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez, B. , Delgado, S. , Blanco‐Míguez, A. , Lourenço, A. , Gueimonde, M. & Margolles, A. (2017) Probiotics, gut microbiota, and their influence on host health and disease. Molecular Nutrition & Food Research, 61, 201600240. [DOI] [PubMed] [Google Scholar]
- Şanlier, N. , Gökcen, B.B. & Sezgin, A.C. (2019) Health benefits of fermented foods. Critical Reviews in Food Science and Nutrition, 59, 506–527. [DOI] [PubMed] [Google Scholar]
- Santos, F. , Vera, J.L. , van der Heijden, R. , Valdez, G. , de Vos, W.M. , Sesma, F. et al. (2008) The complete coenzyme B12 biosynthesis gene cluster of Lactobacillus reuteri CRL 1098. Microbiology, 154, 81–93. [DOI] [PubMed] [Google Scholar]
- Santos, F. , Wegkamp, A. , de Vos, W.M. , Smid, E.J. & Hugenholtz, J. (2008) High‐level folate production in fermented foods by the B12 producer Lactobacillus reuteri JCM1112. Applied and Environmental Microbiology, 74, 3291–3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salque, M. , Bogucki, P.I. , Pyzel, J. , Sobkowiak‐Tabaka, I. , Grygiel, R. , Szmyt, M. et al. (2013) Earliest evidence for cheese making in the sixth millennium BC in northern Europe. Nature, 493, 522–525. [DOI] [PubMed] [Google Scholar]
- Sauer, M. , Russmayer, H. , Grabherr, R. , Peterbauer, C.K. & Marx, H. (2017) The efficient clade: lactic acid bacteria for industrial chemical production. Trends in Biotechnology, 35, 756–769. [DOI] [PubMed] [Google Scholar]
- Shu, G. , Shi, X. , Chen, L. , Kou, J. , Meng, J. & Chen, H. (2018) Antioxidant peptides from goat milk fermented by Lactobacillus casei L61: preparation, optimization, and stability evaluation in simulated gastrointestinal fluid. Nutrients, 10, 797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidooski, T. , Brandelli, A. , Bertoli, S.L. , Krebs de Souza, C. & Fernandes de Carvalho, L. (2019) Physical and nutritional conditions for optimized production of bacteriocins by lactic acid bacteria—a review. Critical Reviews in Food Science and Nutrition, 59, 2839–2849. [DOI] [PubMed] [Google Scholar]
- Silva, C.C.G. , Silva, S.P.M. & Ribeiro, S.C. (2018) Application of bacteriocins and protective cultures in dairy food preservation. Frontiers in Microbiology, 9, 594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sistla, S. (2013) Structure–activity relationships of α‐casein peptides with multifunctional biological activities. Molecular and Cellular Biochemistry, 384, 29–38. [DOI] [PubMed] [Google Scholar]
- Solieri, L. , Rutella, G.S. & Tagliazucchi, D. (2015) Impact of non‐starter lactobacilli on release of peptides with angiotensin‐converting enzyme inhibitory and antioxidant activities during bovine milk fermentation. Food Microbiology, 51, 108–116. [DOI] [PubMed] [Google Scholar]
- Solopova, A. , Bottacini, F. , Venturi Degli Esposti, E. , Amaretti, A. , Raimondi, S. , Rossi, M. et al. (2020) Riboflavin biosynthesis and overproduction by a derivative of the human gut commensal Bifidobacterium longum subsp. infantis ATCC 15697. Frontiers in Microbiology, 11, 573335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltani, S. , Hammami, R. , Cotter, P.D. , Rebuffat, S. , Ben Said, L. , Gaudreau, H. et al. (2021) Bacteriocins as a new generation of antimicrobials: toxicity aspects and regulations. FEMS Microbiology Reviews, 45, 1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suárez, N. , Weckx, S. , Minahk, C. , Heberta, E.M. & Saavedraa, L. (2020) Metagenomics‐based approach for studying and selecting bioprotective strains from the bacterial community of artisanal cheeses. International Journal of Food Microbiology, 335, 108894. [DOI] [PubMed] [Google Scholar]
- Surber, G. , Mende, S. , Jaros, D. & Rohm, H. (2019) Clustering of Streptococcus thermophilus strains to establish a relation between exopolysaccharide characteristics and gel properties of acidified milk. Food, 8, 146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sybesma, W. , Burgess, C. , Starrenburg, M. , van Sinderen, D. & Hugenholtz, J. (2004) Multivitamin production in Lactococcus lactis using metabolic engineering. Metabolic Engineering, 6(2), 109–115. [DOI] [PubMed] [Google Scholar]
- Tamang, J.P. , Watanabe, K. & Holzapfel, W.H. (2016) Review: diversity of microorganisms in global fermented foods and beverages. Frontiers in Microbiology, 7, 377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamene, A. , Baye, K. , Kariluoto, S. , Edelmann, M. , Bationo, F. , Leconte, N. et al. (2019) Lactobacillus plantarum P2R3FA isolated from traditional cereal‐based fermented food increase folate status in deficient rats. Nutrients, 11, 2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taverniti, V. & Guglielmetti, S. (2011) The immunomodulatory properties of probiotic microorganisms beyond their viability (ghost probiotics: proposal of paraprobiotic concept). Genes & Nutrition, 6, 261–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teran, M.M. , de Moreno de LeBlanc, A. , Savoy de Giori, G. and LeBlanc, J.G. (2021) Thiamine‐producing lactic acid bacteria and their potential use in the prevention of neurodegenerative diseases. Applied Microbiology and Biotechnology 105, 2097–2107. [DOI] [PubMed] [Google Scholar]
- Thakur, K. , Kumar Tomar, S. , Brahma, B. & De, S. (2016) Screening of riboflavin‐producing lactobacilli by a polymerase‐chain‐reaction‐based approach and microbiological assay. Journal of Agricultural and Food Chemistry, 64, 1950–1956. [DOI] [PubMed] [Google Scholar]
- Thierry, A. , Pogačic, T. , Weber, M. & Lortal, S. (2016) Production of flavor compounds by lactic acid bacteria in fermented foods. In: Mozzi, F. , Raya, R.R. & Vignolo, G.M. (Eds.) Biotechnology of lactic acid bacteria: novel applications, 2nd edition. West Sussex: John Wiley & Sons, Ltd, pp. 314–340. [Google Scholar]
- Tian, P. , Bastiaanssen, T.F.S. , Song, L. , Jiang, B. , Zhang, X. , Zhao, J. et al. (2021) Unraveling the microbial mechanisms underlying the psychobiotic potential of a Bifidobacterium breve strain. Molecular Nutrition & Food Research, 65, e2000704. [DOI] [PubMed] [Google Scholar]
- Tian, P. , O’Riordan, K.J. , Lee, Y.K. , Wang, G. , Zhao, J. , Zhang, H. et al. (2020) Towards a psychobiotic therapy for depression: Bifidobacterium breve CCFM1025 reverses chronic stress‐induced depressive symptoms and gut microbial abnormalities in mice. Neurobiology of Stress, 12, 100216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari, S. , Kavitake, D. , Devi, P.B. & Shetty, P.H. (2021) Bacterial exopolysaccharides for improvement of technological, functional and rheological properties of yoghurt. International Journal of Biological Macromolecules, 183, 1585–1595. [DOI] [PubMed] [Google Scholar]
- Trejo‐González, L. , Gutiérrez‐Carrillo, A.E. , Rodríguez‐Hernández, A.I. , López‐Cuellar, M.R. & Chavarría‐Hernández, N. (2021) Bacteriocins produced by LAB isolated from cheeses within the period 2009–2021: a review. Probiotics and Antimicrobial Proteins. 10.1007/s12602-021-09825-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsilingiri, K. & Rescigno, M. (2013) Postbiotics: what else? Beneficial Microbes, 4, 101–107. [DOI] [PubMed] [Google Scholar]
- Toyoda, A. , Kawase, T. & Tsukahara, T. (2020) Effects of dietary intake of heat‐inactivated Lactobacillus gasseri CP2305 on stress‐induced behavioral and molecular changes in a subchronic and mild social defeat stress mouse model. Biomedical Research, 41, 101–111. [DOI] [PubMed] [Google Scholar]
- Walhe, R.A. , Diwanay, S.S. , Patole, M.S. , Sayyed, R.Z. , AL‐Shwaiman, H.A. , Alkhulaifi, M.M. et al. (2021) Cholesterol reduction and vitamin B12 production study on enterococcus faecium and Lactobacillus pentosus isolated from yoghurt. Sustainability, 13, 5853. [Google Scholar]
- Warda, A.K. , Rea, K. , Fitzgerald, P. , Hueston, C. , Gonzalez‐Tortuero, E. , Dinan, T.G. et al. (2019) Heat‐killed lactobacilli alter both microbiota composition and behaviour. Behavioural Brain Research, 362, 213–223. [DOI] [PubMed] [Google Scholar]
- Wegkamp, A. , van Oorschot, W. , de Vos W. & Smid, E.J. (2007) Characterization of the role of para‐aminobenzoic acid biosynthesis in folate production by Lactococcus lactis . Applied and Environmental Microbiology, 73, 2673–2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe, B.E. , Button, J.E. , Santarelli, M. & Dutton, R.J. (2014) Cheese rind communities provide tractable systems for in situ and in vitro studies of microbial diversity. Cell, 158, 422–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia, Y. , Yu, J. , Xu, W. & Shuang, Q. (2020) Purification and characterization of angiotensin‐I‐converting enzyme inhibitory peptides isolated from whey proteins of milk fermented with Lactobacillus plantarum QS670. Journal of Dairy Science, 103, 4919–4928. [DOI] [PubMed] [Google Scholar]
- Xie, C. , Coda, R. , Chamlagain, B. , Varmanen, P. , Piironen, B. & Katina, K. (2019) Co‐fermentation of Propionibacterium freudenreichii and Lactobacillus brevis in wheat bran for in situ production of vitamin B12. Frontiers in Microbiology, 10, 1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, Z. , Guoc, Q. , Zhang, H. , Xiong, Z. , Zhang, X. & Ai, L. (2021) Structural characterisation of EPS of S‐3 and its Streptococcus thermophilus application in milk fermentation. International Journal of Biological Macromolecules, 178, 263–269. [DOI] [PubMed] [Google Scholar]
- Yang, B. , Chen, H.Q. , Stanton, C. , Ross, R.P. , Zhang, H. , Chen, Y.Q. et al. (2015) Review of the roles of conjugated linoleic acid in health and disease. Journal of Functional Foods, 15, 314–325. [Google Scholar]
- Yunes, R.A. , Poluektova, E.U. , Vasileva, E.V. , Odorskaya, M.V. , Marsova, M.V. , Kovalev, G.I. et al. (2020) A multi‐strain potential probiotic formulation of GABA‐producing Lactobacillus plantarum 90sk and Bifidobacterium adolescentis 150 with antidepressant effects. Probiotics and Antimicrobial Proteins, 12, 973–979. [DOI] [PubMed] [Google Scholar]
- Zandona, E. , Blažić, M. & Režek Jambrak, A. (2021) Whey utilisation: sustainable uses and environmental approach. Food Technology and Biotechnology, 59, 147–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng, J. , Wittouck, S. , Salvetti, E. , Franz, C.M.A.P. , Harris, G.M.B. , Mattarelli, P. et al. (2020) A taxonomic note on the genus Lactobacillus: description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae . International Journal of Systematic and Evolutionary Microbiology, 70, 2782–2858. [DOI] [PubMed] [Google Scholar]
- Zhu, Y.Y. , Thakur, K. , Feng, J.Y. , Cai, J.S. , Zhang, J.G. , Hu, F. et al. (2020) Riboflavin‐overproducing lactobacilli for the enrichment of fermented soymilk: insights into improved nutritional and functional attributes. Applied Microbiology and Biotechnology, 104, 5759–5772. [DOI] [PubMed] [Google Scholar]


