Abstract
Background
The extent of the relationship between age and the presence of breast cancer synchronous brain metastases (BCSBMs) and mortality has not yet been well-identified or sufficiently quantified. We aimed to examine the association of age with the presence of BCSBMs and all-cause and cancer-specific mortality outcomes using the SEER database.
Methods
Age-associated risk of the presence and survival of BCSBMs were evaluated on a continuous scale (restricted cubic spline, RCS) with logistic or Cox regression models. The main endpoints were the presence of BCSBMs and all-cause mortality or cancer-specific mortality. Cox proportional hazards regression and competing risk models were used in survival analysis.
Results
Among 374,132 adult breast cancer patients, 1,441 (0.38%) had BMs. The presence of BCSBMs displayed a U-shaped relationship with age, with the highest point of the curve occurring at the age of 62. In both the younger (age ≤ 61) and older (age ≥ 62) groups, the observed curve showed a nearly linear relationship between age and the presence of BCSBMs. The relationship between age and all-cause mortality (ASM) and cancer-specific mortality (CSM) was linear. Older age at diagnosis was associated with a higher risk of ASM (HR 1.019, 95% CI: 1.013–1.024, p < 0.001) and CSM (HR 1.016, 95% CI: 1.010–1.023, p < 0.001) in multivariable Cox models. Age (sHR 1.007, 95% CI 1–1.013, p = 0.049) was substantially related to a significantly increased risk of CSM in competing risk models.
Conclusion
Age had a non-linear U-shaped relationship with the presence of BCSBMs and a linear relationship with BCSBMs mortality.
Keywords: breast cancer, brain metastases (BMs), restricted cubic spline (RCS), linearity, non-linearity, prognosis, SEER
Introduction
The second most frequent solid tumor that can metastasize to the central nervous system is breast cancer (BC) (1, 2). Brain metastases (BMs) are expected to occur in 30–50% of people with metastatic BC (3–5). The brain microenvironment is vastly different from that of extracranial lesions, with its distinct cell types, architectural features, metabolic restrictions, and immunological milieu, which influence the metastatic process and treatment responses (6, 7). According to many important studies, HER2-positive disease, the existence of more than two metastatic sites at BC diagnosis, HR negative, and a more advanced stage of the original tumor were all linked to a greater risk of breast cancer BMs (4, 8, 9).
Despite this compelling evidence, there is less evidence about the associations between age and the presence of breast cancer synchronous brain metastases (BCSBMs). Studies on this topic found different results, with some studies showing that breast cancer BMs occurred more frequently among younger women (10, 11), one study considering that age had no impact on the presence of breast cancer BMs (12), some studies suggesting that patients with older age had greater odds of having breast cancer BMs (5, 9), and some studies regarding advanced age as a risk factor for the presence of BMs (13, 14). There are currently no reliable population-based estimations of the relationship between age and the occurrence of breast cancer BMs.
Prior studies establish that BMs confer a life-threatening prognosis for female BC patients (15–18). Diagnosed with BMs represents an independent risk factor for shorter survival time in a large cohort retrospective study, and it has been estimated that there is a 58% rise in the risk of death from all causes (15). Breast cancer patients previously had a median survival period of 3–6 months from the time their BMs were discovered (17). Another prospective study found that women with brain metastases have a median survival of 26.3 months, compared to 44.6 months for women who do not suffer from brain metastases (19). Furthermore, BMs have been shown to be a reliable predictor of bone metastases in patients with infiltrating duct carcinoma of the breast, which is associated with a worsening prognosis (20).
Age is a significant determinant in cancer incidence and survival, and it is also a considerable factor in BM survival (13, 14, 21). Studies have shown that increasing age has generally been associated with poorer survival of BMs (12–14). There were, however, some inconsistent results (11, 22). Furthermore, there is no credible data on the impact of age on the prognosis of breast cancer patients with BMs.
We aimed to examine in detail the association of age with the presence of synchronous BMs and all-cause and cancer-specific mortality outcomes at diagnosis of BC using a large, multicenter, contemporary, population-based cohort in the United States from the Surveillance, Epidemiology, and End Results (SEER) database.
Methods
Data sources and study population
We conducted a cohort study using data from the SEER program, which contains demographic, illness, and treatment-related information for 34.6 percent of cancer patients in the United States at the time of primary malignancy diagnosis (23). For patients diagnosed between 2010 and 2016, information about the presence or absence of brain metastases at the time of the initial systemic malignancy diagnosis was available. We examined 388,413 individuals aged 18 and above who were diagnosed with primary, invasive breast cancer between January 1, 2011, and December 31, 2016, in the SEER database.
We excluded individuals who were male (3 016 individuals), lacked data for education and household income (64 individuals), were diagnosed with carcinoma in situ (561 individuals), lacked information on bone metastases (9 380 individuals), liver metastases (487 individuals), lung metastases (443 individuals), and brain metastases (214 individuals). We also excluded cases if survival time was unknown (26 individuals). The final analytical cohort for the association of age and the presence of BCSBM included 374 132 participants. Of these, a total of 1,441 individuals were identified as having brain metastases. In addition, we excluded 6 participants whose diagnosis was based on an autopsy or death certificate, as well as one participant of undetermined race, leaving 1,434 participants eligible for survival analysis. Our institutional review board granted an exemption for this study since it is a secondary analysis of existing data (SEER).
Covariates
Demographic variables included patients' age, sex, median household income, high school education percentage, year of diagnosis, race/ethnicity, registry region, marital status, and insurance status were reported by the SEER program. Clinical covariates such as tumor site, histological subtypes, T-stage, lymph nodal positive rate (LNPRate), and metastases in other organs (bone, liver, lung, and brain) were also included in the research. All the above variables were treated as factors, with the exception of age, household income, and education proportion, which were treated as continuous variables and given as mean and standard deviation (SD). The presence or absence of other organ metastases was confirmed before the start of treatment.
Endpoints
In the logistic regression models, the primary endpoint was the presence of BMs at diagnosis. In Cox proportional hazard models, the primary endpoints were all-cause mortality (ASM) and cancer-specific mortality (CSM) based on the International Classification of Diseases, 10th revision (ICD-10) code recorded as the underlying cause of death. In the competing risk models that were analyzed using proportional subdistribution hazards models, the primary endpoint was cancer-specific mortality, while other causes of mortality were the competing risk (24, 25). The months to the event were calculated from the time of diagnosis to the end of follow-up or death.
Statistical analysis
The presence of BMs
Independent factors in demographic variables and clinical covariates were used to determine whether independent factors were associated with the presence of BMs at diagnosis. The associations between age and the presence of BMs at diagnosis were evaluated on a continuous scale with restricted cubic spline curves (RCSs) based on logistic regression models with 4 knots at the 5th, 45th, 65th, and 90th percentiles of age (26). The spline model was adjusted for variables that were found to have significance in univariable logistic analysis (p ≤ 0.05). Then, sensitivity analyses were carried out to see if the findings were reliable.
Based on the cut-off value from the result of RCS, we divided the cohort into two age groups. The mean and SD were calculated for continuous variables, and the proportion was calculated for categorical variables in each age group. The t-test or chi-square test was used to calculate statistical differences for continuous and categorical variables. As the associations between age and the presence of BMs were approximately linear below and above the cut-off value, we additionally used multivariable logistic regression models to calculate the odds ratio (OR) and 95% confidence interval (CI).
The survival of BCSBMs
The hazard ratios of mortality were calculated using univariable and multivariable Cox proportional hazards regression models adjusted for possible confounders (27). We used restricted cubic spline models fitted to Cox proportional hazards models with 4 knots at the 5th, 45th, 65th, and 90th percentiles of age (26). ASM and CSM spline models were further adjusted for significant variables in ASM and CSM univariable Cox regression models, respectively.
A competing mortality risk regression analysis on cumulative incidence functions was conducted using Fine and Gray models to better estimate breast CSM and better account for the high rate of competing events (28, 29). The researchers calculated unadjusted and adjusted subdistribution hazard ratios (sHR) with 95% CI. The Cumulative Incidence Function (CIF) allows for estimating the incidence of CSM while accounting for competing risk.
Statistical analyses were conducted using R language program version 4.0.3 released on 10-10-2020 and STATA software version 14 (StataCorp). The 2-tailed α values of <0.05 were considered statistically significant.
Results
Of data from 374 132 adult patients from the SEER database in the United States, Group A comprised 182,033 (48.6%) patients under the age of 61 (≤ 61), with a mean (SD) age of 50.49 (7.77), whereas Group B (age ≥ 62 years old) contained 192,099 (51.35 %) patients with a mean (SD) age of 72.60 (7.96). Among the entire cohort, 1,441 patients were diagnosed with BMs, accounting for 0.38% of the entire study population. Groups A and B had 775 (53.78%) and 666 (46.22%) BCSBMs, respectively, with the incidence proportion of BMs in Groups A and B being 0.43 percent and 0.35 percent. Tables 1, 3, 4 show the baseline characteristics of the entire cohort and the BMs cohort.
Table 1.
Baseline characteristics of study population.
| Variables | Categories | Patients with cancer (any stage): N (%) | Group A*N (%) | Group B*N (%) | Patients with brain metastases at diagnosis: N (%) | Incidence proportion of brain metastases |
|---|---|---|---|---|---|---|
| Age* | Continuous | 61.84 (13.56) | 50.49 (7.77) | 72.60 (7.96) | - | - |
| Year | 2011 | 59,276 (15.84%) | 29,711 (50.12) | 29,565 (49.88) | 230 (15.96) | 0.39% |
| 2012 | 60,510 (16.17%) | 29,901 (49.41) | 30,609 (50.59) | 206 (14.3) | 0.34% | |
| 2013 | 61,897 (16.54%) | 30,227 (48.83) | 31,670 (51.17) | 258 (17.9) | 0.42% | |
| 2014 | 62,904 (16.81%) | 30,597 (48.64) | 32,307 (51.36) | 255 (17.7) | 0.41% | |
| 2015 | 64,702 (17.29%) | 31,076 (48.03) | 33,626 (51.97) | 271 (18.81) | 0.42% | |
| 2016 | 64,843 (17.33%) | 30,521 (47.07) | 34,322 (52.93) | 221 (15.34) | 0.34% | |
| Race | NHW | 254,330 (67.98%) | 111,033 (43.66) | 143,297 (56.34) | 882 (61.21) | 0.35% |
| NHB | 41,359 (11.05%) | 23,175 (56.03) | 18,184 (43.97) | 262 (18.18) | 0.63% | |
| NHAI/AN | 2,078 (0.56%) | 1,154 (55.53) | 924 (44.47) | 8 (0.56) | 0.38% | |
| NHAPI | 31,764 (8.49%) | 18,884 (59.45) | 12,880 (40.55) | 109 (7.56) | 0.34% | |
| Hispanic | 42,459 (11.35%) | 26,711 (62.91) | 15748 (37.09) | 179 (12.42) | 0.42% | |
| Others | 2,142 (0.57%) | 1,076 (50.23) | 1,066 (49.77) | 1 (0.07) | 0.05% | |
| Region | Northeast | 61,753 (16.51%) | 30,181 (48.87) | 31,572 (51.13) | 265 (18.39) | 0.43% |
| Midwest | 32,796 (8.77%) | 15,234 (46.45) | 17,562 (53.55) | 120 (8.33) | 0.37% | |
| South | 81,533 (21.79%) | 39,795 (48.81) | 41,738 (51.19) | 381 (26.44) | 0.47% | |
| West | 198,050 (52.94%) | 96,823 (48.89) | 101,227 (51.11) | 675 (46.84) | 0.34% | |
| Marital status | Married | 200,221 (53.52%) | 110,259 (55.07) | 89,962 (44.93) | 601 (41.71) | 0.3% |
| Others | 173,911 (46.48%) | 71,774 (41.27) | 102,137 (58.73) | 840 (58.29) | 0.48% | |
| Insurance status | Insured | 316,864 (84.69%) | 146,250 (46.16) | 170,614 (53.84) | 986 (68.42) | 0.31% |
| Others | 57,268 (15.31%) | 35,783 (62.48) | 21,485 (37.52) | 455 (31.58) | 0.79% | |
| Primary tumor site | Central | 18,861 (5.04%) | 7,856 (41.65) | 11,005 (58.35) | 69 (4.79) | 0.37% |
| Upper-inner | 45,492 (12.16%) | 21,983 (48.32) | 23,509 (51.68) | 70 (4.86) | 0.15% | |
| Lower-inner | 20,571 (5.5%) | 9,354 (45.47) | 11,217 (54.53) | 40 (2.78) | 0.19% | |
| Upper-outer | 124,529 (33.28%) | 62,423 (50.13) | 62,106 (49.87) | 285 (19.78) | 0.23% | |
| Lower-outer | 27,880 (7.45%) | 13,788 (49.45) | 14,092 (50.55) | 58 (4.02) | 0.21% | |
| Axillary tail | 2,020 (0.54%) | 1,069 (52.92) | 951 (47.08) | 17 (1.18) | 0.84% | |
| Others | 134,779 (36.02%) | 65,560 (48.64) | 69,219 (51.36) | 902 (62.6) | 0.67% | |
| T-Stage | 1 | 216,452 (57.85%) | 98,866 (45.68) | 117,586 (54.32) | 183 (12.7) | 0.08% |
| 2 | 107,688 (28.78%) | 57,307 (53.22) | 50,381 (46.78) | 305 (21.17) | 0.28% | |
| 3 | 22,452 (6%) | 13,227 (58.91) | 9,225 (41.09) | 172 (11.94) | 0.77% | |
| 4 | 15,481 (4.14%) | 7,254 (46.86) | 8,227 (53.14) | 453 (31.44) | 2.93% | |
| Others | 12,059 (3.22%) | 5,379 (44.61) | 6,680 (55.39) | 328 (22.76) | 2.72% | |
| LNPRate | 0–20% | 221,250 (59.14%) | 106,721 (48.24) | 114,529 (51.76) | 37 (2.57) | 0.02% |
| 21–40% | 23,323 (6.23%) | 13,757 (58.98) | 9,566 (41.02) | 18 (1.25) | 0.08% | |
| 41–60% | 14,493 (3.87%) | 7,976 (55.03) | 6,517 (44.97) | 9 (0.62) | 0.06% | |
| 61–80% | 6,589 (1.76%) | 3,623 (54.99) | 2,966 (45.01) | 13 (0.9) | 0.2% | |
| 81–100% | 45,984 (12.29%) | 26,599 (57.84) | 19,385 (42.16) | 121 (8.4) | 0.26% | |
| Unexamined | 50,291 (13.44%) | 15,926 (31.67) | 34,365 (68.33) | 1,019 (70.71) | 2.03% | |
| Others | 12,202 (3.26%) | 7,431 (60.9) | 4,771 (39.1) | 224 (15.54) | 1.84% | |
| Subtype | HR+/HER2– | 256,413 (68.54%) | 116,506 (45.44) | 139,907 (54.56) | 550 (38.17) | 0.21% |
| HR+/HER2+ | 37,293 (9.97%) | 22,427 (60.14) | 14,866 (39.86) | 214 (14.85) | 0.57% | |
| HR–/HER2+ | 15,779 (4.22%) | 9,620 (60.97) | 6,159 (39.03) | 165 (11.45) | 1.05% | |
| Triple-negative | 38,558 (10.31%) | 21,842 (56.65) | 16,716 (43.35) | 256 (17.77) | 0.66% | |
| Unknown | 26,089 (6.97%) | 11,638 (44.61) | 14,451 (55.39) | 256 (17.77) | 0.98% | |
| Bone metastases | No | 360,946 (96.48%) | 175,577 (48.64) | 185,369 (51.36) | 522 (36.22) | 0.14% |
| Yes | 13,186 (3.52%) | 6,456 (48.96) | 6,730 (51.04) | 919 (63.78) | 6.97% | |
| Liver metastases | No | 369,081 (98.65%) | 179,181 (48.55) | 189,900 (51.45) | 957 (66.41) | 0.26% |
| Yes | 5,051 (1.35%) | 2,852 (56.46) | 2,199 (43.54) | 484 (33.59) | 9.58% | |
| Lung metastases | No | 367,815 (98.31%) | 179,264 (48.74) | 188,551 (51.26) | 759 (52.67) | 0.21% |
| Yes | 6,317 (1.69%) | 2,769 (43.83) | 3,548 (56.17) | 682 (47.33) | 10.8% | |
| Brain metastases | No | 372,691 (99.61%) | 181,258 (48.63) | 191,433 (51.37) | - | - |
| Yes | 1,441 (0.39%) | 775 (53.78) | 666 (46.22) | - | - | |
| Income* | Continuous | 0.66 (0.17) | 0.66 (0.17) | 0.65 (0.17) | - | - |
| Education* | Continuous | 13.66 (5.74) | 13.72 (5.76) | 13.61 (5.72) | - | - |
| Total | - | 374,132 (100) | 182,033 (48.65) | 192,099 (51.35) | 1,441 (100) | 0.38% |
Income*, median household income, increased by per $10 000 annual; Education*, high school education percent, increased by per 10%; NHW, Non-Hispanic White; NHB, Non-Hispanic Black; NHAI/AN, Non-Hispanic American Indian/Alaska Native; NHAPI, Non-Hispanic Asian or Pacific Islander. Continuous variables* are given as mean (standard deviation). Group A*, age ≤ 61; Group B*, age ≥ 62.
Age and the presence of BMs
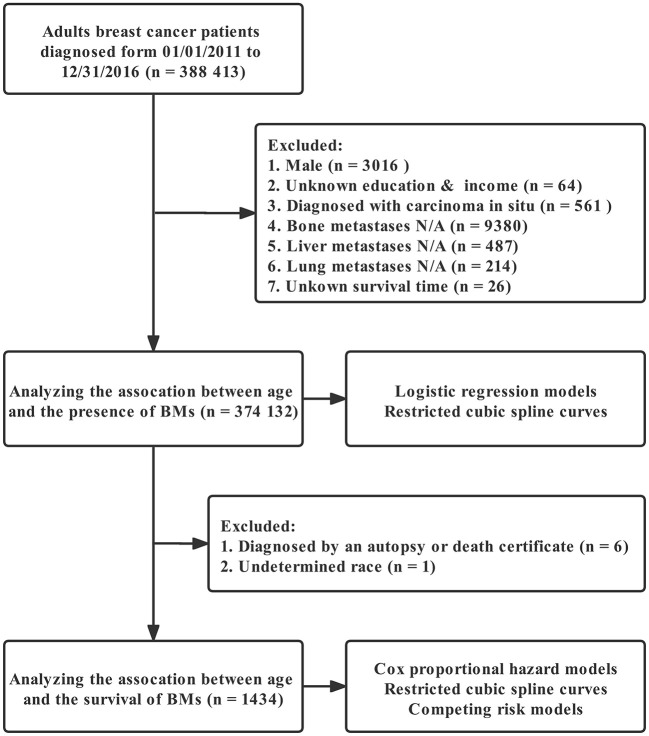
Figure 1 depicts the full analytical process. In univariable logistic regression, age (increased by per 1, odds ratio [OR], 0.99, 95%CI: 0.99–1, p < 0.001), non-Hispanic Black (NHB) (vs. non-Hispanic White [NHW], p < 0.001), Hispanic (vs. NHW, p = 0.017), West region (vs. Northeast, p = 0.001), other marital status (vs. married, p < 0.001), other insurance status (vs. insured, p < 0.001), tumor located in upper-inner or lower-inner or upper-outer or lower-outer or axillary tail of breast (vs. central, p < 0.001, p = 0.001, p < 0.001, p = 0.002, and p = 0.002, respectively), T-stage 2 or 3 or 4 (vs. 1, p < 0.001 for each one), LNPRate between 21 and 40% or between 41 and 60% or between 61 and 80% or between 81 and 100% (vs. 0–20%, p < 0.001 for each one), HR+/HER2+ or HR−/HER2+ or triple-negative subtype (vs. HR+/HER2− subtype, p < 0.001 for each one), and metastasized to bone or liver or lung at diagnosis (vs. Not to, p < 0.001 for each one) were related to significantly greater odds of having BMs at diagnosis. Median household income (increased by per $10,000 annual, p < 0.001) was at lower risk of presence of BMs at diagnosed. Restricted cubic spline revealed a U-shaped relationship between age and the presence of BMs after controlling for the aforementioned possible confounders (Figure 2). The risk of having BMs increased rapidly until approximately the age of 62, after which it began to decline rapidly (P for non-linearity < 0.001). The results for sensitivity analyses are shown in Supplementary Figure 1. In multivariable logistic regression models, each year of age conferred a 1% (95% CI: 1–1.02, p = 0.036, p for non-linearity = 0.163) increase in the OR to develop BMs in Group A (age ≤ 61) and a 4% (95% CI: 0.95–0.97, p < 0.001, p for non-linearity = 0.067) decrease in the OR to develop BMs in Group B (age ≥ 62). Detailed data is shown in Table 2.
Figure 1.
Analytical workflow.
Figure 2.
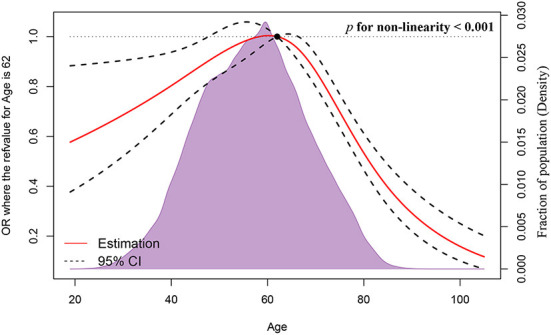
Association between age and the presence of BCSBMs using a restricted cubic spline regression model.
Table 2.
Univariable and multivariable logistic regression models for the presence of breast cancer synchronous brain metastases.
| Variables | Categories | Univariable | Multivariable | Multivariable | Multivariable | ||||
|---|---|---|---|---|---|---|---|---|---|
| Total | Group A* | Group B* | |||||||
| OR (95%CI) | p-Value | OR (95%CI) | p-Value | OR (95%CI) | p-Value | OR (95%CI) | p-Value | ||
| Age | Continuous | 0.99 (0.99, 1) | < 0.001 | 0.99 (0.98, 0.99) | < 0.001 | 1.01 (1, 1.02) | 0.036 | 0.96 (0.95, 0.97) | < 0.001 |
| Year | 2011 | Ref. | - | - | - | - | - | - | |
| 2012 | 0.88 (0.73, 1.06) | 0.172 | - | - | - | - | - | - | |
| 2013 | 1.07 (0.9, 1.28) | 0.429 | - | - | - | - | - | - | |
| 2014 | 1.04 (0.87, 1.25) | 0.63 | - | - | - | - | - | - | |
| 2015 | 1.08 (0.91, 1.29) | 0.393 | - | - | - | - | - | - | |
| 2016 | 0.88 (0.73, 1.06) | 0.168 | - | - | - | - | - | - | |
| Race | NHW | Ref. | Ref. | Ref. | Ref. | ||||
| NHB | 1.83 (1.6, 2.1) | < 0.001 | 0.98 (0.84, 1.15) | 0.836 | 0.95 (0.77, 1.17) | 0.632 | 1.04 (0.82, 1.32) | 0.733 | |
| NHAI/AN | 1.11 (0.55, 2.23) | 0.768 | 0.998 (0.475, 2.101) | 0.997 | 1.04 (0.4, 2.7) | 0.936 | 0.9 (0.27, 2.96) | 0.86 | |
| NHAPI | 0.99 (0.81, 1.21) | 0.917 | 0.97 (0.78, 1.2) | 0.764 | 1.17 (0.9, 1.53) | 0.239 | 0.63 (0.41, 0.96) | 0.031 | |
| Hispanic | 1.22 (1.04, 1.43) | 0.017 | 1.05 (0.88, 1.26) | 0.581 | 1.02 (0.8, 1.29) | 0.89 | 1.22 (0.91, 1.62) | 0.186 | |
| Others | 0.13 (0.02, 0.95) | 0.044 | 0.11 (0.01, 0.77) | 0.026 | 0.2 (0.03, 1.48) | 0.116 | 0 (0, 4.15*10,145) | 0.942 | |
| Region | Northeast | Ref. | Ref. | Ref. | Ref. | ||||
| Midwest | 0.85 (0.69, 1.06) | 0.147 | 1.08 (0.84, 1.37) | 0.559 | 1.22 (0.85, 1.74) | 0.995 (0.71, 1.39) | 0.978 | ||
| South | 1.09 (0.93, 1.27) | 0.286 | 1.28 (1.05, 1.56) | 0.014 | 1.5 (1.13, 1.99) | 1.11 (0.84, 1.47) | 0.469 | ||
| West | 0.79 (0.69, 0.91) | 0.001 | 1.0q (0.859, 1.179) | 0.935 | 1.19 (0.95, 1.51) | 0.86 (0.69, 1.08) | 0.191 | ||
| Marital status | Married | Ref. | Ref. | Ref. | Ref. | ||||
| Others | 1.61 (1.45, 1.79) | < 0.001 | 1.01 (0.894, 1.131) | 0.922 | 0.95 (0.81, 1.12) | 0.593 | 1.18 (0.99, 1.4) | 0.064 | |
| Insurance status | Insured | Ref. | Ref. | Ref. | Ref. | ||||
| Others | 2.57 (2.3, 2.87) | < 0.001 | 1.22 (1.07, 1.39) | 0.003 | 1.32 (1.12, 1.56) | 0.001 | 1.03 (0.83, 1.27) | 0.802 | |
| Primary tumor site | Central | Ref. | Ref. | Ref. | Ref. | ||||
| Upper-inner | 0.42 (0.3, 0.59) | < 0.001 | 0.97 (0.68, 1.37) | 0.85 | 1.41 (0.85, 2.34) | 0.19 | 0.7 (0.43, 1.15) | 0.155 | |
| Lower-inner | 0.53 (0.36, 0.78) | 0.001 | 1.04 (0.69, 1.57) | 0.841 | 1.46 (0.82, 2.62) | 0.201 | 0.79 (0.44, 1.42) | 0.428 | |
| Upper-outer | 0.62 (0.48, 0.81) | < 0.001 | 1.08 (0.82, 1.43) | 0.582 | 1.39 (0.91, 2.13) | 0.126 | 0.89 (0.61, 1.3) | 0.543 | |
| Lower-outer | 0.57 (0.4, 0.81) | 0.002 | 1.03 (0.71, 1.49) | 0.874 | 1.23 (0.71, 2.1) | 0.461 | 0.91 (0.55, 1.5) | 0.704 | |
| Axillary tail | 2.31 (1.36, 3.94) | 0.002 | 2.07 (1.17, 3.65) | 0.012 | 2.3 (0.99, 5.34) | 0.054 | 2.06 (0.95, 4.46) | 0.067 | |
| Others | 1.83 (1.44, 2.35) | < 0.001 | 1.37 (1.05, 1.77) | 0.019 | 1.72 (1.15, 2.56) | 0.009 | 1.16 (0.82, 1.63) | 0.405 | |
| T-Stage | 1 | Ref. | Ref. | Ref. | Ref. | ||||
| 2 | 3.36 (2.79, 4.03) | < 0.001 | 1.33 (1.1, 1.62) | 0.003 | 1.1 (0.85, 1.42) | 0.487 | 1.74 (1.3, 2.32) | < 0.001 | |
| 3 | 9.12 (7.41, 11.24) | < 0.001 | 1.66 (1.32, 2.08) | < 0.001 | 1.12 (0.82, 1.54) | 0.461 | 2.71 (1.94, 3.77) | < 0.001 | |
| 4 | 35.62 (29.98, 42.33) | < 0.001 | 1.97 (1.62, 2.41) | < 0.001 | 1.69 (1.3, 2.21) | < 0.001 | 2.27 (1.69, 3.07) | < 0.001 | |
| Others | 33.04 (27.55, 39.63) | < 0.001 | 2.47 (2.01, 3.03) | <0.001 | 2.06 (1.55, 2.73) | <0.001 | 3.02 (2.23, 4.09) | <0.001 | |
| LNPRate | 0–20% | Ref. | Ref. | Ref. | Ref. | ||||
| 21–40% | 4.62 (2.63, 8.11) | <0.001 | 3.45 (1.96, 6.08) | <0.001 | 4.9 (2.46, 9.75) | <0.001 | 1.81 (0.61, 5.41) | 0.288 | |
| 41–60% | 3.71 (1.79, 7.7) | <0.001 | 2.69 (1.3, 5.6) | 0.008 | 3.98 (1.68, 9.46) | 0.002 | 1.34 (0.31, 5.82) | 0.698 | |
| 61–80% | 11.82 (6.28, 22.24) | <0.001 | 6.61 (3.49, 12.53) | <0.001 | 8.94 (4.03, 19.85) | <0.001 | 4.42 (1.47, 13.29) | 0.008 | |
| 81–100% | 15.77 (10.91, 22.8) | <0.001 | 8.86 (6.09, 12.9) | <0.001 | 9.87 (5.97, 16.32) | <0.001 | 8.26 (4.69, 14.56) | <0.001 | |
| Unexamined | 123.64 (89.06, 171.67) | <0.001 | 27.52 (19.47, 38.89) | <0.001 | 30.85 (19.2, 49.57) | <0.001 | 26.98 (16.22, 44.88) | <0.001 | |
| Others | 111.81 (78.92, 158.39) | <0.001 | 20.66 (14.33, 29.77) | <0.001 | 21.97 (13.39, 36.06) | <0.001 | 22.01 (12.75, 38) | <0.001 | |
| Subtype | HR+/HER2− | Ref. | Ref. | Ref. | Ref. | ||||
| HR+/HER2+ | 2.68 (2.29, 3.15) | <0.001 | 1.51 (1.27, 1.79) | <0.001 | 1.57 (1.26, 1.97) | <0.001 | 1.48 (1.13, 1.94) | 0.004 | |
| HR−/HER2+ | 4.92 (4.13, 5.85) | <0.001 | 2.48 (2.04, 3.02) | <0.001 | 2.58 (2.01, 3.33) | <0.001 | 2.43 (1.78, 3.31) | <0.001 | |
| Triple-negative | 3.11 (2.68, 3.61) | <0.001 | 2.68 (2.28, 3.15) | <0.001 | 2.99 (2.4, 3.72) | <0.001 | 2.45 (1.92, 3.12) | <0.001 | |
| Unknown | 4.61 (3.97, 5.35) | <0.001 | 1.47 (1.25, 1.74) | <0.001 | 1.65 (1.29, 2.11) | <0.001 | 1.39 (1.11, 1.75) | 0.004 | |
| Bone metastases | No | Ref. | Ref. | Ref. | Ref. | ||||
| Yes | 51.73 (46.39, 57.68) | <0.001 | 5.2 (4.53, 5.96) | <0.001 | 6.33 (5.2, 7.7) | <0.001 | 3.94 (3.25, 4.78) | <0.001 | |
| No | Ref. | Ref. | Ref. | Ref. | |||||
| Yes | 40.77 (36.4, 45.65) | <0.001 | 1.88 (1.64, 2.15) | <0.001 | 1.68 (1.4, 2.02) | <0.001 | 2.18 (1.78, 2.66) | <0.001 | |
| Lung metastases | No | Ref. | Ref. | Ref. | Ref. | ||||
| Yes | 58.53 (52.61, 65.12) | <0.001 | 4.31 (3.79, 4.89) | <0.001 | 3.95 (3.3, 4.71) | <0.001 | 4.44 (3.7, 5.34) | <0.001 | |
| Income* | Continuous | 0.61 (0.45, 0.83) | <0.001 | 1.31 (0.89, 1.93) | 0.175 | 1.28 (0.75, 2.19) | 0.373 | 1.45 (0.82, 2.55) | 0.202 |
| Education* | Continuous | 1 (1.99, 1) | 0.724 | - | - | - | - | - | - |
Income*, median household income, increased by per $10 000 annual; Education*, high school education percent, increased by per 10%; NHW, Non-Hispanic White; NHB, Non-Hispanic Black; NHAI/AN, Non-Hispanic American Indian/Alaska Native; NHAPI, Non-Hispanic Asian or Pacific Islander. Group A*, age ≤ 61; Group B*, age ≥ 62.
Age and mortality
A median (interquartile range) of 16 (6–32) months was the time period of mortality ascertainment, corresponding to 1,042 deaths from all causes and 807 deaths caused by cancer. RCS indicated an ascending all-cause mortality risk with increasing age (Figure 3A, p for non-linearity = 0.264), controlled for median household income, race, region, marital status, tumor site, LNPRate, histological subtype, and metastasized to bone, liver, and lung. There was also an escalating cancer-specific mortality risk with increasing age (Figure 3B, p for non-linearity = 0.473), controlled for median household income, race, marital status, insurance status, T-stage, histological subtype, and metastasized to the liver and lung. Additionally, in multivariable Cox proportional-hazard models, the adjusted HRs of age for mortality due to all-cause and cancer-specific were HR = 1.019 (95% CI: 1.013–1.024, p < 0.001) and HR = 1.016 (95% CI: 1.010–1.023, p < 0.001), respectively. The corresponding detailed results of the unadjusted and adjusted Cox models are shown in Tables 3, 4.
Figure 3.
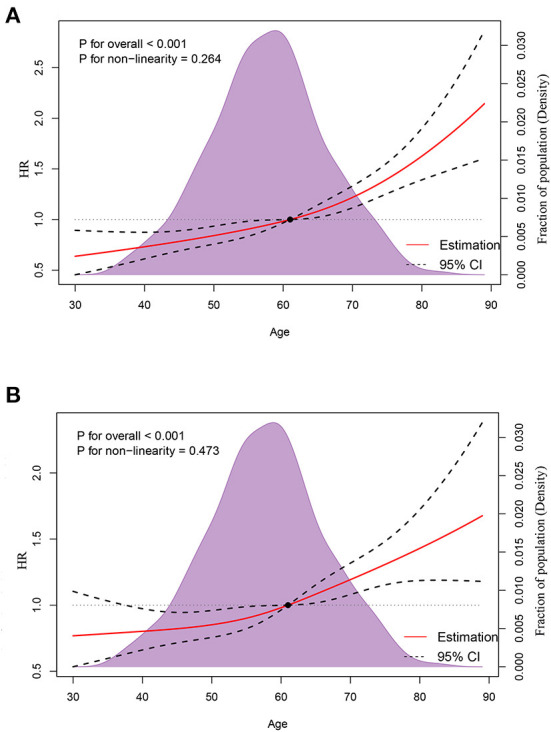
Association between age and all-cause mortality (A) and cancer-specific mortality (B) using restricted cubic spline regression models.
Table 3.
Distribution of breast cancer synchronous brain metastases all-cause mortality and hazard risk associated with various prognostic factors.
| Variables | Categories | Deaths N (%) | Univariable | Multivariable | ||
|---|---|---|---|---|---|---|
| HR (95%) | p-Value | HR (95%) | p-Value | |||
| Age | Continuous | 1,042 (100) | 1.018 (1.013, 1.024) | <0.001 | 1.019 (1.013, 1.024) | <0.001 |
| Year | 2011 | 208 (19.96) | Ref. | Ref. | ||
| 2012 | 178 (17.08) | 0.957 (0.782, 1.170) | 0.665 | |||
| 2013 | 210 (20.15) | 0.948 (0.780, 1.153) | 0.594 | |||
| 2014 | 199 (19.1) | 1.077 (0.882, 1.314) | 0.468 | |||
| 2015 | 170 (16.31) | 0.997 (0.809, 1.229) | 0.978 | |||
| 2016 | 77 (7.39) | 0.895 (0.683, 1.173) | 0.422 | |||
| Race | NHW | 637 (61.13) | Ref. | Ref. | ||
| NHB | 213 (20.44) | 1.301 (1.113, 1.519) | 0.001 | 1.266 (1.071, 1.496) | 0.006 | |
| NHAI/AN | 5 (0.48) | 0.603 (0.250, 1.453) | 0.259 | 0.783 (0.322, 1.905) | 0.590 | |
| NHAPI | 70 (6.72) | 0.968 (0.756, 1.239) | 0.793 | 1.180 (0.912, 1.527) | 0.208 | |
| Hispanic | 117 (11.23) | 0.859 (0.705, 1.046) | 0.131 | 0.909 (0.739, 1.118) | 0.366 | |
| Region | Northeast | 194 (18.62) | Ref. | Ref. | ||
| Midwest | 97 (9.31) | 1.214 (0.951, 1.549) | 0.119 | 1.008 (0.776, 1.310) | 0.951 | |
| South | 293 (28.12) | 0.956 (0.797, 1.146) | 0.628 | 0.915 (0.733, 1.143) | 0.434 | |
| West | 458 (43.95) | 0.840 (0.710, 0.993) | 0.042 | 0.927 (0.774, 1.110) | 0.407 | |
| Marital status | Married | 405 (38.87) | Ref. | Ref. | ||
| Others | 637 (61.13) | 1.350 (1.192, 1.530) | <0.001 | 1.211 (1.062, 1.380) | 0.004 | |
| Insurance status | Insured | 722 (69.29) | Ref. | Ref. | ||
| Others | 320 (30.71) | 1.026 (0.899, 1.170) | 0.704 | |||
| Primary tumor site | Central | 45 (4.32) | Ref. | Ref. | ||
| Upper-inner | 57 (5.47) | 1.593 (1.078, 2.356) | 0.020 | 1.591 (1.071, 2.363) | 0.022 | |
| Lower-inner | 26 (2.5) | 0.892 (0.550, 1.446) | 0.641 | 1.026 (0.629, 1.675) | 0.917 | |
| Upper-outer | 188 (18.04) | 1.113 (0.804, 1.541) | 0.520 | 1.340 (0.963, 1.865) | 0.082 | |
| Lower-outer | 41 (3.93) | 1.255 (0.822, 1.917) | 0.292 | 1.461 (0.952, 2.242) | 0.083 | |
| Axillary tail | 14 (1.34) | 1.495 (0.820, 2.725) | 0.189 | 1.766 (0.957, 3.262) | 0.069 | |
| Others | 671 (64.4) | 1.324 (0.979, 1.791) | 0.068 | 1.430 (1.052, 1.944) | 0.022 | |
| T-Stage | 1 | 131 (12.57) | Ref. | Ref. | ||
| 2 | 203 (19.48) | 0.968 (0.777, 1.207) | 0.774 | |||
| 3 | 126 (12.09) | 1.057 (0.828, 1.350) | 0.655 | |||
| 4 | 340 (32.63) | 1.190 (0.973, 1.456) | 0.091 | |||
| Others | 242 (23.22) | 1.137 (0.919, 1.407) | 0.236 | |||
| LNPRate | 0–20% | 21 (2.02) | Ref. | Ref. | ||
| 21–40% | 11 (1.06) | 0.775 (0.373, 1.607) | 0.493 | 0.875 (0.419, 1.827) | 0.722 | |
| 41–60% | 5 (0.48) | 0.694 (0.261, 1.841) | 0.463 | 0.782 (0.293, 2.086) | 0.624 | |
| 61–80% | 10 (0.96) | 1.087 (0.512, 2.309) | 0.829 | 1.267 (0.594, 2.704) | 0.541 | |
| 81–100% | 83 (7.97) | 1.177 (0.729,1.899) | 0.506 | 1.208 (0.744,1.964) | 0.445 | |
| Unexamined | 758 (72.74) | 1.599 (1.036, 2.468) | 0.034 | 1.505 (0.967, 2.343) | 0.070 | |
| Others | 154 (14.78) | 1.384 (0.877, 2.185) | 0.163 | 1.423 (0.894, 2.262) | 0.137 | |
| Subtype | HR+/HER2– | 369 (35.41) | Ref. | Ref. | ||
| HR+/HER2+ | 129 (12.38) | 0.766 (0.626, 0.936) | 0.009 | 0.770 (0.627, 0.945) | 0.012 | |
| HR–/HER2+ | 112 (10.75) | 1.090 (0.882, 1.346) | 0.427 | 1.076 (0.865, 1.338) | 0.513 | |
| Triple-negative | 215 (20.63) | 2.020 (1.702, 2.398) | <0.001 | 2.111 (1.763, 2.529) | <0.001 | |
| Unknown | 217 (20.83) | 1.986 (1.678, 2.351) | <0.001 | 1.882 (1.579, 2.242) | <0.001 | |
| Bone metastases | No | 388 (37.24) | Ref. | Ref. | ||
| Yes | 654 (62.76) | 0.878 (0.775, 0.996) | 0.043 | 0.899 (0.784, 1.030) | 0.123 | |
| Liver metastases | No | 672 (64.49) | Ref. | Ref. | ||
| Yes | 370 (35.51) | 1.372 (1.208, 1.559) | <0.001 | 1.543 (1.344, 1.770) | <0.001 | |
| Lung metastases | No | 538 (51.63) | Ref. | Ref. | ||
| Yes | 504 (48.37) | 1.217 (1.077, 1.374) | 0.002 | 1.119 (0.985, 1.273) | 0.085 | |
| Income* | Continuous | 1,042 (100) | 0.570 (0.405, 0.800) | 0.001 | 0.497 (0.324, 0.763) | 0.001 |
| Education* | Continuous | 1,042 (100) | 1.000 (0.990, 1.011) | 0.971 | - | - |
Income*, median household income, increased by per $10 000 annual; Education*, high school education percent, increased by per 10%; NHW, Non-Hispanic White; NHB, Non-Hispanic Black; NHAI/AN, Non-Hispanic American Indian/Alaska Native; NHAPI, Non-Hispanic Asian or Pacific Islander.
Table 4.
Distribution of breast cancer synchronous brain metastases cancer-specific mortality and hazard risk associated with various prognostic factors.
| Variables | Categories | Deaths N (%) | Univariable | Multivariable | ||
|---|---|---|---|---|---|---|
| HR (95%) | p-Value | HR (95%) | p-Value | |||
| Age | 807 (100%) | 1.013 (1.007, 1.019) | <0.001 | 1.016 (1.010, 1.023) | <0.001 | |
| Year | 2011 | 167 (20.69%) | Ref. | Ref. | ||
| 2012 | 132 (16.36%) | 0.886 (0.704, 1.11) | 0.300 | - | - | |
| 2013 | 172 (21.31%) | 0.972 (0.782, 1.207) | 0.797 | - | - | |
| 2014 | 148 (18.34%) | 0.996 (0.794, 1.249) | 0.970 | - | - | |
| 2015 | 127 (15.74%) | 0.923 (0.727, 1.171) | 0.507 | - | - | |
| 2016 | 61 (7.56%) | 0.889 (0.656 1.204) | 0.447 | - | - | |
| Race | NHW | 493 (61.09%) | Ref. | Ref. | ||
| NHB | 165 (20.45%) | 1.302 (1.091, 1.553) | 0.003 | 1.153 (0.959, 1.387) | 0.131 | |
| NHAI/AN | 5 (0.62%) | 0.790 (0.327, 1.906) | 0.599 | 1.094 (0.449, 2.665) | 0.843 | |
| NHAPI | 55 (6.82%) | 0.983 (0.743, 1.299) | 0.901 | 1.071 (0.803, 1.427) | 0.642 | |
| Hispanic | 89 (11.03%) | 0.846 (0.675, 1.06) | 0.147 | 0.843 (0.669, 1.063) | 0.149 | |
| Region | Northeast | 148 (18.34%) | Ref. | Ref. | ||
| Midwest | 75 (9.29%) | 1.231 (0.932, 1.625) | 0.143 | - | - | |
| South | 233 (28.87%) | 1.000 (0.814, 1.229) | 0.999 | - | - | |
| West | 351 (43.49%) | 0.847 (0.699, 1.026) | 0.090 | - | - | |
| Marital status | Married | 304 (37.67%) | Ref. | Ref. | ||
| Others | 503 (62.33%) | 1.418 (1.229, 1.635) | 0.001 | 1.266 (1.090, 1.472) | 0.002 | |
| Insurance status | Insured | 536 (66.42%) | Ref. | Ref. | ||
| Others | 271 (33.58%) | 1.175 (1.015, 1.36) | 0.030 | 1.165 (0.994, 1.365) | 0.060 | |
| Primary tumor site | Central | 36 (4.46%) | Ref. | Ref. | ||
| Upper-inner | 43 (5.33%) | 1.493 (0.958, 2.325) | 0.076 | - | - | |
| Lower-inner | 16 (1.98%) | 0.679 (0.376, 1.224) | 0.197 | - | - | |
| Upper-outer | 149 (18.46%) | 1.0994 (0.764, 1.582) | 0.610 | - | - | |
| Lower-outer | 31 (3.84%) | 1.181 (0.731, 1.910) | 0.497 | - | - | |
| Axillary tail | 6 (0.74%) | 0.783 (0.330, 1.859) | 0.579 | - | - | |
| Others | 526 (65.18%) | 1.289 (0.919, 1.806) | 0.142 | - | - | |
| T-Stage | 1 | 82 (10.16%) | Ref. | Ref. | ||
| 2 | 156 (19.33%) | 1.191 (0.911, 1.556) | 0.202 | 1.234 (0.942, 1.618) | 0.127 | |
| 3 | 102 (12.64%) | 1.378 (1.030, 1.843) | 0.031 | 1.340 (0.998, 1.801) | 0.052 | |
| 4 | 287 (35.56%) | 1.615 (1.263, 2.064) | <0.001 | 1.536 (1.197, 1.972) | 0.001 | |
| Others | 180 (22.3%) | 1.353 (1.042, 1.757) | 0.023 | 1.331 (1.022, 1.733) | 0.034 | |
| LNPRate | 0–20% | 19 (2.35%) | Ref. | Ref. | ||
| 21–40% | 10 (1.24%) | 0.779 (0.362, 1.676) | 0.522 | - | - | |
| 41–60% | 5 (0.62%) | 0.771 (0.288, 2.066) | 0.605 | - | - | |
| 61–80% | 8 (0.99%) | 0.965 (0.422, 2.206) | 0.933 | - | - | |
| 81–100% | 64 (7.93%) | 0.999 (0.598, 1.667) | 0.995 | - | - | |
| Unexamined | 577 (71.5%) | 1.334 (0.844, 2.109) | 0.217 | - | - | |
| Others | 124 (15.37%) | 1.230 (0.758, 1.993) | 0.404 | - | - | |
| Subtype | HR+/HER2– | 289 (35.81%) | Ref. | Ref. | ||
| HR+/HER2+ | 110 (13.63%) | 0.836 (0.670, 1.041) | 0.109 | 0.865 (0.693, 1.081) | 0.202 | |
| HR–/HER2+ | 90 (11.15%) | 1.118 (0.883, 1.417) | 0.355 | 1.072 (0.842, 1.364) | 0.573 | |
| Triple-negative | 169 (20.94%) | 2.014 (1.659, 2.444) | <0.001 | 2.185 (1.792, 2.665) | <0.001 | |
| Unknown | 149 (18.46%) | 1.715 (1.406, 2.092) | <0.001 | 1.777 (1.448, 2.181) | <0.001 | |
| Bone metastases | No | 286 (35.44%) | Ref. | Ref. | ||
| Yes | 521 (64.56%) | 0.952 (0.824, 1.100) | 0.507 | - | - | |
| Liver metastases | No | 506 (62.7%) | Ref. | Ref. | ||
| Yes | 301 (37.3%) | 1.480 (1.282, 1.707) | <0.001 | 1.616 (1.389, 1.880) | <0.001 | |
| Lung metastases | No | 405 (50.19%) | Ref. | Ref. | ||
| Yes | 402 (49.81%) | 1.288 (1.122, 1.479) | <0.001 | 1.169 (1.012, 1.350) | 0.033 | |
| Income* | Continuous | 807 (100%) | 0.403 (0.273, 0.596) | <0.001 | 0.408 (0.271, 0.615) | <0.001 |
| Education* | Continuous | 807 (100%) | 1.006 (0.994, 1.018) | 0.317 | - | - |
Income*, median household income, increased by per $10 000 annual; Education*, high school education percent, increased by per 10%; NHW, Non-Hispanic White; NHB, Non-Hispanic Black; NHAI/AN, Non-Hispanic American Indian/Alaska Native; NHAPI, Non-Hispanic Asian or Pacific Islander.
In addition, we evaluated CSM using a competing risk model. The CIF curves for the observed risk of CSM are shown in Supplementary Figure 2. Table 5 shows the results of multivariable competing-risk regression analyses predicting the time to CSM. Age (increased by per 1, sHR 1.007, 95% CI 1–1.013, p = 0.049), other marital status (vs. married, sHR 1.191, 95% CI 1.016–1.397, p < 0.001), other insurance status (vs. insured, sHR 1.221, 95% CI 1.034–1.442, p < 0.001), T-stage 3 (vs. 1, sHR 1.438, 95% CI 1.057–1.956, p = 0.021), T-stage 4 (vs. 1, sHR 1.612, 95% CI 1.242–2.091, p < 0.001), triple-negative subtype (vs. HR+/HER2− subtype, sHR 1.693, 95% CI 1.374–2.086, p < 0.001), and metastasized to liver (vs. not to, sHR 1.367, 95% CI 1.163–1.607, p < 0.001) were significantly associated with an increased risk for CSM.
Table 5.
Proportional subdistribution hazards models for breast cancer synchronous brain metastases.
| Variables | Categories | Univariable | Multivariable | ||
|---|---|---|---|---|---|
| sHR (95%CI) | p-Value | sHR (95%CI) | p-Value | ||
| Age | Continuous | 1.004 (0.997, 1.009) | 0.226 | 1.007 (1.000, 1.013) | 0.049 |
| Year | 2011 | Ref. | . | Ref. | - |
| 2012 | 0.834 (0.665, 1.047) | 0.117 | 0.792 (0.628, 0.999) | 0.049 | |
| 2013 | 0.868 (0.700, 1.077) | 0.198 | 0.804 (0.648, 0.998) | 0.047 | |
| 2014 | 0.819 (0.654, 1.026) | 0.083 | 0.753 (0.596, 0.951) | 0.017 | |
| 2015 | 0.715 (0.560, 0.915) | 0.008 | 0.634 (0.491, 0.818) | < 0.001 | |
| 2016 | 0.732 (0.525, 1.019) | 0.064 | 0.664 (0.473, 0.933) | 0.018 | |
| Race | NHW | Ref. | Ref. | ||
| NHB | 1.247 (1.035, 1.503) | 0.02 | 1.082 (0.877, 1.335) | 0.464 | |
| NHAI/AN | 0.843 (0.430, 1.655) | 0.62 | 1.122 (0.621, 2.028) | 0.702 | |
| NHAPI | 0.881 (0.657, 1.180) | 0.396 | 0.976 (0.716, 1.332) | 0.88 | |
| Hispanic | 0.852 (0.677, 1.072) | 0.173 | 0.856 (0.669, 1.094) | 0.214 | |
| Region | Northeast | Ref. | Ref. | ||
| Midwest | 1.131 (0.833, 1.537) | 0.43 | 0.889 (0.633, 1.248) | 0.495 | |
| South | 1.089 (0.877, 1.350) | 0.44 | 0.822 (0.623, 1.084) | 0.165 | |
| West | 0.881 (0.719, 1.079) | 0.221 | 0.831 (0.663, 1.042) | 0.108 | |
| Marital status | Married | Ref. | Ref. | ||
| Others | 1.316 (1.137, 1.525) | <0.001 | 1.191 (1.016, 1.397) | 0.031 | |
| Insurance status | Insured | Ref. | Ref. | ||
| Others | 1.267 (1.089, 1.475) | 0.002 | 1.221 (1.034, 1.442) | 0.018 | |
| Primary tumor site | Central | Ref. | Ref. | ||
| Upper-inner | 1.252 (0.809, 1.940) | 0.313 | 1.226 (0.771, 1.952) | 0.389 | |
| Lower-inner | 0.589 (0.320, 1.083) | 0.089 | 0.689 (0.364, 1.306) | 0.254 | |
| Upper-outer | 1.103 (0.772, 1.575) | 0.591 | 1.195 (0.810, 1.763) | 0.369 | |
| Lower-outer | 1.126 (0.691, 1.836) | 0.634 | 1.337 (0.806, 2.217) | 0.26 | |
| Axillary tail | 0.514 (0.187, 1.413) | 0.197 | 0.555 (0.188, 1.639) | 0.286 | |
| Others | 1.175 (0.843, 1.638) | 0.34 | 1.183 (0.821, 1.704) | 0.367 | |
| T-Stage | 1 | Ref. | Ref. | ||
| 2 | 1.227 (0.934, 1.610) | 0.141 | 1.270 (0.961, 1.678) | 0.093 | |
| 3 | 1.445 (1.081, 1.930) | 0.013 | 1.438 (1.057, 1.956) | 0.021 | |
| 4 | 1.689 (1.321, 2.160) | <0.001 | 1.612 (1.242, 2.091) | <0.001 | |
| Others | 1.244 (0.950, 1.627) | 0.112 | 1.235 (0.929, 1.642) | 0.146 | |
| LNPRate | 0–20% | Ref. | Ref. | ||
| 21–40% | 0.846 (0.432, 1.657) | 0.626 | 0.893 (0.455, 1.750) | 0.741 | |
| 41–60% | 0.824 (0.414, 1.642) | 0.583 | 0.997 (0.441, 2.255) | 0.994 | |
| 61–80% | 0.991 (0.448, 2.193) | 0.982 | 0.923 (0.442, 1.927) | 0.83 | |
| 81–100% | 0.894 (0.549, 1.456) | 0.653 | 0.815 (0.508, 1.308) | 0.397 | |
| Unexamined | 1.023 (0.656, 1.595) | 0.919 | 0.962 (0.626, 1.478) | 0.861 | |
| Others | 0.998 (0.624, 1.595) | 0.993 | 1.031 (0.654, 1.625) | 0.894 | |
| Subtype | HR+/HER2– | Ref. | Ref. | ||
| HR+/HER2+ | 0.950 (0.764, 1.181) | 0.643 | 0.949 (0.755, 1.193) | 0.655 | |
| HR–/HER2+ | 1.030 (0.813, 1.305) | 0.804 | 0.954 (0.744, 1.222) | 0.708 | |
| Triple-negative | 1.581 (1.299, 1.924) | <0.001 | 1.693 (1.374, 2.086) | <0.001 | |
| Unknown | 1.117 (0.887, 1.406) | 0.346 | 1.135 (0.890, 1.447) | 0.308 | |
| Bone metastases | No | Ref. | Ref. | ||
| Yes | 1.023 (0.879, 1.191) | 0.771 | 1.001 (0.848, 1.181) | 0.994 | |
| Liver metastases | No | Ref. | Ref. | ||
| Yes | 1.361 (1.170, 1.583) | <0.001 | 1.367 (1.163, 1.607) | <0.001 | |
| Lung metastases | No | Ref. | Ref. | ||
| Yes | 1.242 (1.075, 1.435) | 0.003 | 1.114 (0.957, 1.298) | 0.163 | |
| Income* | Continuous | 0.367 (0.245, 0.552) | <0.001 | 0.320 (0.169, 0.608) | 0.001 |
| Education* | Continuous | 1.010 (0.998, 1.021) | 0.113 | 0.998 (0.982, 1.014) | 0.767 |
Income*, median household income, increased by per $10 000 annual; Education*, high school education percent, increased by per 10%; NHW, Non-Hispanic White; NHB, Non-Hispanic Black; NHAI/AN, Non-Hispanic American Indian/Alaska Native; NHAPI, Non-Hispanic Asian or Pacific Islander.
Discussion
In a large population-based retrospective cohort study, we examined the complicated association between age and the presence and survival of BCSBMs. As far as we know, this is an epidemiologic study with great interest and novelty based on the SEER program that will generally offer a superior understanding of variation in the onset and prognosis of BCSBMs. The presence of BCSBMs was shown to have a U-shaped relationship with age, with the maximum HR occurring at the age of 62. The association between age and all-cause and cause-specific mortality, on the other hand, resembled linear behavior.
Comparison with other studies
In recent years, there has been an increasing amount of literature on the epidemiology of BMs at the diagnosis of systemic malignancy (12–14, 30). Using data from the SEER database from 2010 to 2013, Martin et al. (13) and Cagney et al. (14) found that 0.41 percent of adult breast cancer patients have synchronous BMs in the United States. In earlier research assessing the 2014–2016 SEER data, we found that 0.42 percent of midlife breast cancer diagnosed in the United States have synchronous BMs. The current study found similar results, and the incidence fraction of BCSBMs remained generally steady from 2011 to 2016.
In reviewing the above-mentioned literature, no solid evidence was found for the association between age and the presence of BMs. As mentioned in the research of Cagney et al. (14) among all patients with cancer, age 41–60 years (vs. age 18–40 y, OR 1.55, 95% CI: 1.39–1.71, p < 0.05) was associated with higher risk of having synchronous BMs, whereas age > 80 years (vs. age 18–40 y, OR 0.60, p < 0.05) had significantly lower odds and age 61–80 years had non-significantly greater odds (vs. age 18–40 y, OR 1.08, p > 0.05). According to another study concentrating on BCSBMs, the relationship between age and the occurrence of synchronous BMs was more elusive and harder to reconcile because OR values of age 41–60 years, 61–80 years, and > 80 years (vs. age 18–40 years) were all non-applicable (13). We reason that these seemingly misleading results might be due to the use of age as a categorical variable in the adjusted logistic regression model. Naturally, we assumed that treating age as a continuous variable would help to ameliorate the situation and lead to a positive conclusion. In this case, however, age (increased by per 1, OR 1, 95% CI: 0.99–1.00, p = 0.49) was not related to the presence of BMs (12). Another report discovered that age ≤ 40 years (vs. age > 40 years, HR 2.10, 95% CI 1.02–4.36) was associated with an increased risk of developing metachronous BMs in HER2-positive breast cancer (31). Additional research showed no information on the effect of age on the occurrence of BCSBMs (11, 32, 33).
A possible explanation for this might be that prior research relied on the essential assumption that the presence of BMs was related linearly to age (12–14, 33). For ordinal or continuous factors, the linearity assumption may be inappropriate, and more elaborate interactions may be necessary (34). Conversely, cubic splines are commonly used because they offer a lot of flexibility when it comes to fitting data, are visually smooth due to their continuous first and second derivatives, and have fewer fit constants than higher degree splines (35–39). In this study, we discovered that using a regression spline to solve such situations is a preferable option. Our findings on age were not in line with the previous findings, whereby we observed a U-shaped association (p for non-linearity <0.001) with the presence of BMs after accounting for potential confounders. Further research revealed that the relationships between age and the occurrence of BCSBMs are approximately linear in both the younger (p for non-linearity = 0.163) and older (p for non-linearity = 0.068) age groups. Consequently, we were able to convert a complex non-linear association into a linear one. Undoubtedly, therefore, age may represent a clinical marker for early identification of a population at high risk for having BCSBMs.
The National Comprehensive Cancer Network (NCCN), American Society of Clinical Oncology (ASCO), and European School of Oncology-Metastatic Breast Cancer guidelines for breast cancer do not recommend routine imaging assessment or continued imaging reassessment of BMs for breast cancer because the overall incidence of BMs is relatively low in the general BC population and there is no proven benefit from non-randomized retrospective studies (40–42). Imaging assessment of the brain is recommended only after the appearance of central nervous system symptoms (43, 44). Nonetheless, timely identification of BMs is critical for BC patients since it may allow better therapeutic responses than delays in diagnosis (45, 46). Research finds that the combination of early detection and advanced therapies (both local and systemic) ultimately leads to more favorable outcomes (47). Although routine screening of all BC patients is not justified, screening people who are at high risk might be beneficial.
For all malignancies, age is a well-validated prognosticator related to survival (21, 48, 49). And previous studies have found that being younger at the time of BCSBMs diagnosis is one of the characteristics that predicts a better outcome (12–14, 50, 51). To determine the association between age and the survival of BCSBMs, many researchers used Cox proportional hazards models (13, 22, 52). To our knowledge, none of them has carried out a comprehensive analysis to determine whether a Cox model would be appropriate in this circumstance. Our current study has significant strengths that compensate for the lack of information. Linear associations between age and ASM (p for non-linearity = 0.264) and CSM (p for non-linearity = 0.473) were discovered in this investigation. Those results suggest that the HR and 95%CI of age obtained by Cox models are reasonable.
However, one study found that in multivariate Cox regression analysis, younger age at first diagnosis of BMs in breast cancer patients was a predictor of shorter OS (52). We believe what the authors stated was a mistake after reviewing the original findings in the Supplementary material, which indicated that the risk of all-cause death rose with age (increased by per 1, HR = 1.02, 95% CI 1.01–1.03, p < 0.001). Another study found that in a univariable Cox model, age did not correlate with breast cancer BMs survival time (22). A possible explanation for this might be the potentially inappropriate classification of age groups. The research of Martin et al., on the other hand, performs better (13). Martin's study has strength in using Fine and Gray's competing risk regression models to analyze BCSBMs CSM (13). Yet, the study did not include CIF curves or sHR values for each variable. Here, we presented the sHR for the occurrence of CSM and gave plots of all cumulative incidences for categorical variables, as indicated by a prior study (24). Mortality risk tends to increase with age in all regression models, and age-related increases in the risk of CSM are substantially overestimated by the standard Cox model (53).
Age is commonly utilized as a covariate in investigations to build prognostic models for predicting the survival of BC patients with BMs, such as a recursive partitioning analysis (RPA) strategy (54) and graded prognostic assessment (GPA) (55, 56). In the era of individualized therapy, the accurate prediction of BC patients with BMs is critical for optimizing care (18, 57). To some extent, the current study is significant in determining the ability of prognostic tools in future research and an improved prognosis for those individuals.
Strengths and limitations
The discovery of a U-shaped association between age and the presence of cancer, as well as approximate linear behaviors between age and ASM and CSM, in a large, nationally representative sample of US general cancer patients from the SEER database with rigorous capture of death events, which was undetected by previous excellent work, is a major strength of our study. At the same time, the results of our competing risk model help to compensate for the lack of survival analyses.
Our research, however, has several limitations. First, in the current research, only the presence or absence of BMs at the diagnosis of the study population was provided. Data on whether metastases develop in the brain in the subsequent course of the disease is not available at this time for the SEER program. Our study did not include the data of some patients who later acquired brain metastases, which might have influenced the accuracy of the results (58). Second, the SEER program is a population-based study being carried out primarily in the United States, which has concerns for generalizability outside of the United States (59, 60). Third, we did not report sHR for the competing event, which may have resulted in a bias toward a better understanding of BCSBMs survival (61, 62).
Conclusion
In conclusion, utilizing a nationally representative database from the United States, this study discovered that age had a non-linear U-shaped relationship with the presence of BCSBMs and a linear relationship with BCSBMs mortality. This article lays the groundwork for future studies. And a better understanding of the complex association might aid in developing age-appropriate public health guidelines.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found at: https://seer.cancer.gov.
Ethics statement
This study was reviewed and approved by the First Affiliated Hospital of Jinan University. Written informed consent for participation was not required for this study in accordance with the national legislation and institutional requirements.
Author contributions
WC had the initial idea, analyzed the data, and wrote the paper. WC, YW, XW, and JL contributed to study design and commenting on drafts and revisions. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We heartily thank all of the employees and scientists at each of the SEER registry locations.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2022.1000415/full#supplementary-material
References
- 1.Tabouret E, Chinot O, Metellus P, Tallet A, Viens P, Goncalves AJAr. Recent trends in epidemiology of brain metastases: an overview. Anticancer Res. (2012) 32:4655–62. [PubMed] [Google Scholar]
- 2.Pellerino A, Internò V, Mo F, Franchino F, Soffietti R, Rudà R. Management of brain and leptomeningeal metastases from breast cancer Int J Mol Sci. (2020) 21:8534. 10.3390/ijms21228534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brosnan EM, Anders CKJAotm. Understanding patterns of brain metastasis in breast cancer and designing rational therapeutic strategies. Ann Transl Med. (2018) 6:163. 10.21037/atm.2018.04.35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pasquier D, Darlix A, Louvel G, Fraisse J, Jacot W, Brain E, et al. Treatment and outcomes in patients with central nervous system metastases from breast cancer in the real-life ESME MBC cohort. Eur J Cancer. (2020) 125:22–30. 10.1016/j.ejca.2019.11.001 [DOI] [PubMed] [Google Scholar]
- 5.Garcia-Alvarez A, Papakonstantinou A, Oliveira MJC. Brain metastases in HER2-positive breast cancer: current and novel treatment strategies. Cancers. (2021) 13:2927. 10.3390/cancers13122927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boire A, Brastianos PK, Garzia L, Valiente M. Brain metastasis. Nat Rev Cancer. (2020) 20:4–11. 10.1038/s41568-019-0220-y [DOI] [PubMed] [Google Scholar]
- 7.Bailleux C, Eberst L, Bachelot T. Treatment strategies for breast cancer brain metastases. Br J Cancer. (2021) 124:142–55. 10.1038/s41416-020-01175-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pestalozzi BC, Zahrieh D, Price K, Holmberg S, Lindtner J, Collins J, et al. Identifying breast cancer patients at risk for central nervous system (CNS) metastases in trials of the international breast cancer study group (IBCSG). Ann Oncol. (2006) 17:935–44. 10.1093/annonc/mdl064 [DOI] [PubMed] [Google Scholar]
- 9.Sperduto PW, Mesko S, Cagney D, Nesbit E, Chan J, Lee J, et al. Tumor subtype and other prognostic factors in breast cancer patients with brain metastases: the updated graded prognostic assessment (Breast-GPA). J Clin Oncol. (2019) 37(15_Suppl) 1079. 10.1200/JCO.2019.37.15_suppl.1079 [DOI] [Google Scholar]
- 10.Yeh RH, Yu JC, Chu CH, Ho CL, Kao HW, Liao GS, et al. Distinct MR imaging features of triple-negative breast cancer with brain metastasis. J Neuroimaging. (2015) 25:474–81. 10.1111/jon.12149 [DOI] [PubMed] [Google Scholar]
- 11.Rostami R, Mittal S, Rostami P, Tavassoli F, Jabbari BJ. Brain metastasis in breast cancer: a comprehensive literature review. J Neurooncol. (2016) 127:407–14. 10.1007/s11060-016-2075-3 [DOI] [PubMed] [Google Scholar]
- 12.Che W, Wang Y, Wang X, Lyu J. Midlife brain metastases in the United States: is male at risk? Cancer Med. (2022) 11:1202–16. 10.1002/cam4.4499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martin AM, Cagney DN, Catalano PJ, Warren LE, Bellon JR, Punglia RS, et al. Brain metastases in newly diagnosed breast cancer: a population-based study. JAMA Oncol. (2017) 3:1069–77. 10.1001/jamaoncol.2017.0001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cagney DN, Martin AM, Catalano PJ, Redig AJ, Lin NU, Lee EQ, et al. Incidence and prognosis of patients with brain metastases at diagnosis of systemic malignancy: a population-based study. Neurooncology. (2017) 19:1511–21. 10.1093/neuonc/nox077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jung SY, Rosenzweig M, Sereika SM, Linkov F, Brufsky A, Weissfeld JL. Factors associated with mortality after breast cancer metastasis. Cancer Causes Control. (2012) 23:103–12. 10.1007/s10552-011-9859-8 [DOI] [PubMed] [Google Scholar]
- 16.Gori S, Rimondini S, De Angelis V, Colozza M, Bisagni G, Moretti G, et al. Central nervous system metastases in HER-2–positive metastatic breast cancer patients treated with trastuzumab: incidence, survival, and risk factors. Oncologist. (2007) 12:766–73. 10.1634/theoncologist.12-7-766 [DOI] [PubMed] [Google Scholar]
- 17.Mahmoud-Ahmed AS, Suh JH, Lee S-Y, Crownover RL, Barnett G. Results of whole brain radiotherapy in patients with brain metastases from breast cancer: a retrospective study. Int J Radiat Oncol Biol Phys. (2002) 54:810–7. 10.1016/S0360-3016(02)02967-X [DOI] [PubMed] [Google Scholar]
- 18.Marko NF, Xu Z, Gao T, Kattan MW, Weil RJ. Predicting survival in women with breast cancer and brain metastasis: a nomogram outperforms current survival prediction models. Cancer. (2012) 118:3749–57. 10.1002/cncr.26716 [DOI] [PubMed] [Google Scholar]
- 19.Brufsky AM, Mayer M, Rugo HS, Kaufman PA, Tan-Chiu E, Tripathy D, et al. Central nervous system metastases in patients with HER2-positive metastatic breast cancer: incidence, treatment, and survival in patients from registHER. Clin Cancer Res. (2011) 17:4834–43. 10.1158/1078-0432.CCR-10-2962 [DOI] [PubMed] [Google Scholar]
- 20.Huang Z, Hu C, Liu K, Yuan L, Li Y, Zhao C, et al. Risk factors, prognostic factors, and nomograms for bone metastasis in patients with newly diagnosed infiltrating duct carcinoma of the breast: a population-based study. BMC Cancer. (2020) 20:1–17. 10.1186/s12885-020-07635-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang G-M, Cioffi G, Patil N, Waite KA, Lanese R, Ostrom QT, et al. Importance of the intersection of age and sex to understand variation in incidence and survival for primary malignant gliomas. Neurooncology. (2022) 24:302–10. 10.1093/neuonc/noab199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Niu L, Lv H, Zhang M, Zeng H, Wang L, Cui S, et al. Clinical diagnosis and treatment of breast cancer with brain metastases and establishment of a prognostic model: a 10-year, single-center, real-world study of 559 cases. Ann Transl Med. (2021) 9:1331. 10.21037/atm-21-3734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang J, Li Y, Liu Q, Li L, Feng A, Wang T, et al. Brief introduction of medical database and data mining technology in big data era. J Evid Based Med. (2020) 13:57–69. 10.1111/jebm.12373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Latouche A, Allignol A, Beyersmann J, Labopin M. Fine J. A competing risks analysis should report results on all cause-specific hazards and cumulative incidence functions. J Clin Epidemiol. (2013) 66:648–53. 10.1016/j.jclinepi.2012.09.017 [DOI] [PubMed] [Google Scholar]
- 25.Austin PC, Lee DS, Fine JPJC. Introduction to the analysis of survival data in the presence of competing risks. Circulation. (2016) 133:601–9. 10.1161/CIRCULATIONAHA.115.017719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frank E. Harrell J. Regression Modeling Strategies: With Applications to Linear Models, Logistic and Ordinal Regression, and Survival Analysis. New York, NY: Springer; (2001). [Google Scholar]
- 27.Wu W-T, Li Y-J, Feng A-Z, Li L, Huang T, Xu A-D, et al. Data mining in clinical big data: the frequently used databases, steps, and methodological models. Mil Med Res. (2021) 8:1–12. 10.1186/s40779-021-00338-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scrucca L, Santucci A, Aversa F. Regression modeling of competing risk using R: an in depth guide for clinicians. Bone Marrow Transplant. (2010) 45:1388–95. 10.1038/bmt.2009.359 [DOI] [PubMed] [Google Scholar]
- 29.Massa ST, Osazuwa-Peters N, Christopher KM, Arnold LD, Schootman M, Walker RJ, et al. Competing causes of death in the head and neck cancer population. Oral Oncology. (2017) 65:8–15. 10.1016/j.oraloncology.2016.12.006 [DOI] [PubMed] [Google Scholar]
- 30.Lamba N, Wen PY, Aizer A. Epidemiology of brain metastases and leptomeningeal disease. Neuro Oncol. (2021) 23:1447–56. 10.1093/neuonc/noab101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maurer C, Tulpin L, Moreau M, Dumitrescu C, de Azambuja E, Paesmans M, et al. Risk factors for the development of brain metastases in patients with HER2-positive breast cancer. ESMO Open. (2018) 3:e000440. 10.1136/esmoopen-2018-000440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Laakmann E, Witzel I, Neunhöffer T, Weide R, Schmidt M, Park-Simon T-W, et al. Characteristics and clinical outcome of breast cancer patients with asymptomatic brain metastases. Cancers. (2020) 12:2787. 10.3390/cancers12102787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ascha MS, Ostrom QT, Wright J, Kumthekar P, Bordeaux JS, Sloan AE, et al. Lifetime occurrence of brain metastases arising from lung, breast, and skin cancers in the elderly: a SEER-Medicare study. Cancer Epidemiol Biomarkers Prev. (2019) 28:917–25. 10.1158/1055-9965.EPI-18-1116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Durrleman S, Simon RJ. Flexible regression models with cubic splines. Stat Med. (1989) 8:551–61. 10.1002/sim.4780080504 [DOI] [PubMed] [Google Scholar]
- 35.Bhaskaran K, dos-Santos-Silva I, Leon DA, Douglas IJ, Smeeth L. Association of BMI with overall and cause-specific mortality: a population-based cohort study of 3·6 million adults in the UK. Lancet Diabetes Endocrinol. (2018) 6:944–53. 10.1016/S2213-8587(18)30288-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Inoue K, Ritz B, Brent GA, Ebrahimi R, Rhee CM. Association of subclinical hypothyroidism and cardiovascular disease with mortality. JAMA Netw Open. (2020) 3:e1920745. 10.1001/jamanetworkopen.2019.20745 [DOI] [PubMed] [Google Scholar]
- 37.Ho AS, Luu M, Zalt C, Morris LG, Chen I, Melany M, et al. Mortality risk of nonoperative papillary thyroid carcinoma: a corollary for active surveillance. Thyroid. (2019) 29:1409–17. 10.1089/thy.2019.0060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Johannesen CDL, Langsted A, Mortensen MB, Nordestgaard B. Association between low density lipoprotein and all cause and cause specific mortality in Denmark: prospective cohort study. BMJ. (2020) 371:m4266. 10.1136/bmj.m4266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee DH, Keum N, Hu FB, Orav EJ, Rimm EB, Willett WC, et al. Predicted lean body mass, fat mass, and all cause and cause specific mortality in men: prospective US cohort study. BMJ. (2018) 362:k2575. 10.1136/bmj.k2575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gradishar WJ, Anderson BO, Abraham J, Aft R, Agnese D, Allison KH, et al. Breast cancer, version 3.2020, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. (2020) 18:452–78. 10.6004/jnccn.2020.0016 [DOI] [PubMed] [Google Scholar]
- 41.Ramakrishna N, Temin S, Chandarlapaty S, Crews JR, Davidson NE, Esteva FJ, et al. Recommendations on disease management for patients with advanced human epidermal growth factor receptor 2–positive breast cancer and brain metastases: American society of clinical oncology clinical practice guideline. J Clin Oncol. (2014) 32:2100. 10.1200/JCO.2013.54.0955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cardoso F, Costa A, Norton L, Senkus E, Aapro M, Andre F, et al. 2nd international consensus guidelines for advanced breast cancer (ABC2). Breast. (2014) 23:489–502. 10.1016/j.breast.2014.08.009 [DOI] [PubMed] [Google Scholar]
- 43.Canney P, Murray E, Dixon-Hughes J, Lewsley L-A, Paul J. A prospective randomised phase III clinical trial testing the role of prophylactic cranial radiotherapy in patients treated with trastuzumab for metastatic breast cancer—Anglo Celtic VII. Clin Oncol. (2015) 27:460–4. 10.1016/j.clon.2015.04.033 [DOI] [PubMed] [Google Scholar]
- 44.Cagney DN, Martin AM, Catalano PJ, Brown PD, Alexander BM, Lin NU. Implications of screening for brain metastases in patients with breast cancer and non–small cell lung cancer. JAMA Oncol. (2018) 4:1001–3. 10.1001/jamaoncol.2018.0813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mahner S, Schirrmacher S, Brenner W, Jenicke L, Habermann C, Avril N. Comparison between positron emission tomography using 2-[fluorine-18] fluoro-2-deoxy-D-glucose, conventional imaging and computed tomography for staging of breast cancer. Ann Oncol. (2008) 19:1249–54. 10.1093/annonc/mdn057 [DOI] [PubMed] [Google Scholar]
- 46.Hodgson NC, Gulenchyn K. Is there a role for positron emission tomography in breast cancer staging? J Clin Oncol. (2008) 26:712–20. 10.1200/JCO.2007.13.8412 [DOI] [PubMed] [Google Scholar]
- 47.Soerjomataram I, Louwman MW, Ribot JG, Roukema JA. An overview of prognostic factors for long-term survivors of breast cancer. Breast Cancer Res Treat. (2008) 107:309–30. 10.1007/s10549-007-9556-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ostrom QT, Gittleman H, Fulop J, Liu M, Blanda R, Kromer C, et al. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2008-2012. Neuro-oncology. (2015) 17(Suppl. 4):iv1–62. 10.1093/neuonc/nov189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ostrom QT, Patil N, Cioffi G, Waite K, Kruchko C, Barnholtz-Sloan J. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2013–2017. Neuro-oncology. (2020) 22(Supplement_1):iv1–96. 10.1093/neuonc/noaa200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lamba N, Kearney RB, Catalano PJ, Hassett MJ, Wen PY, Haas-Kogan DA. Population-based estimates of survival among elderly patients with brain metastases. Neuro-oncology. (2021) 23:661–76. 10.1093/neuonc/noaa233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ho V, Gijtenbeek J, Brandsma D, Beerepoot L, Sonke GJ. Survival of breast cancer patients with synchronous or metachronous central nervous system metastases. Eur J Cancer. (2015) 51:2508–16. 10.1016/j.ejca.2015.07.040 [DOI] [PubMed] [Google Scholar]
- 52.Witzel I, Laakmann E, Weide R, Neunhoeffer T, Park-Simon T-J, Schmidt M, et al. Treatment and outcomes of patients in the brain metastases in breast cancer network registry. Eur J Cancer. (2018) 102:1–9. 10.1016/j.ejca.2018.07.004 [DOI] [PubMed] [Google Scholar]
- 53.Wolbers M, Koller MT, Witteman JC, Steyerberg EWJE. Prognostic models with competing risks: methods and application to coronary risk prediction. Epidemiology. (2009) 20:555–61. 10.1097/EDE.0b013e3181a39056 [DOI] [PubMed] [Google Scholar]
- 54.Gaspar L, Scott C, Rotman M, Asbell S, Phillips T, Wasserman T, et al. Recursive partitioning analysis (RPA) of prognostic factors in three radiation therapy oncology group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys. (1997) 37:745–51. 10.1016/S0360-3016(96)00619-0 [DOI] [PubMed] [Google Scholar]
- 55.Sperduto PW, Berkey B, Gaspar LE, Mehta M, Curran W. A new prognostic index and comparison to three other indices for patients with brain metastases: an analysis of 1,960 patients in the RTOG database. Int J Radiat Oncol Biol Phys. (2008) 70:510–4. 10.1016/j.ijrobp.2007.06.074 [DOI] [PubMed] [Google Scholar]
- 56.Sperduto CM, Watanabe Y, Mullan J, Hood T, Dyste G, Watts C, et al. A validation study of a new prognostic index for patients with brain metastases: the graded prognostic assessment. J Neurosurg. (2008) 109(Supplement):87–9. 10.3171/JNS/2008/109/12/S14 [DOI] [PubMed] [Google Scholar]
- 57.Huang Z, Sun B, Wu S, Meng X, Cong Y, Shen G, et al. nomogram for predicting survival in patients with breast cancer brain metastasis. Oncol Lett. (2018) 15:7090–6. 10.3892/ol.2018.8259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pestalozzi BC, Holmes E, de Azambuja E, Metzger-Filho O, Hogge L, Scullion M, et al. CNS relapses in patients with HER2-positive early breast cancer who have and have not received adjuvant trastuzumab: a retrospective substudy of the HERA trial (BIG 1-01). Lancet Oncol. (2013) 14:244–8. 10.1016/S1470-2045(13)70017-2 [DOI] [PubMed] [Google Scholar]
- 59.Du XL, Lairson DR, Begley CE, Fang S. Temporal and geographic variation in the use of hematopoietic growth factors in older women receiving breast cancer chemotherapy: findings from a large population-based cohort. J Clin Oncol. (2005) 23:8620. 10.1200/JCO.2005.02.6252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Warren JL, Butler EN, Stevens J, Lathan CS, Noone A-M, Ward KC. Receipt of chemotherapy among Medicare patients with cancer by type of supplemental insurance. J Clin Oncol. (2015) 33:312. 10.1200/JCO.2014.55.3107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen D, Liu Z, Liu W, Fu M, Jiang W, Xu S, et al. Predicting postoperative peritoneal metastasis in gastric cancer with serosal invasion using a collagen nomogram. Nat Commun. (2021) 12:1–11. 10.1038/s41467-020-20429-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sun W, Cheng M, Zhou H, Huang W, Qiu Z. Nomogram predicting cause-specific mortality in nonmetastatic male breast cancer: a competing risk analysis. J Cancer. (2019) 10:583. 10.7150/jca.28991 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Publicly available datasets were analyzed in this study. This data can be found at: https://seer.cancer.gov.